Crystal Chemistry and High-Temperature Behaviour of Ammonium Phases NH4MgCl3·6H2O and (NH4)2Fe3+Cl5·H2O from the Burned Dumps of the Chelyabinsk Coal Basin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Occurrence
2.2. Chemical Composition
2.3. Single-Crystal XRD
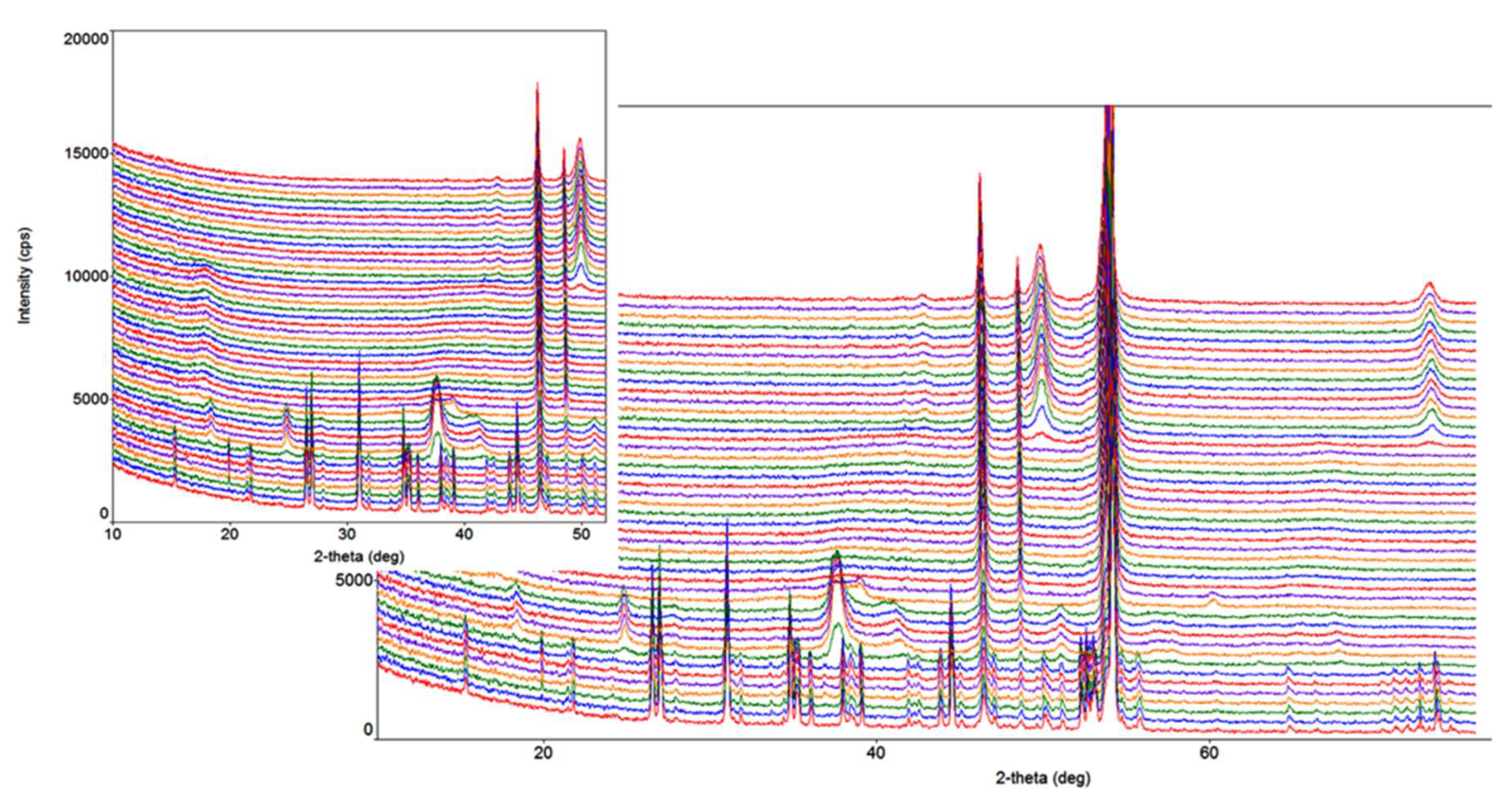
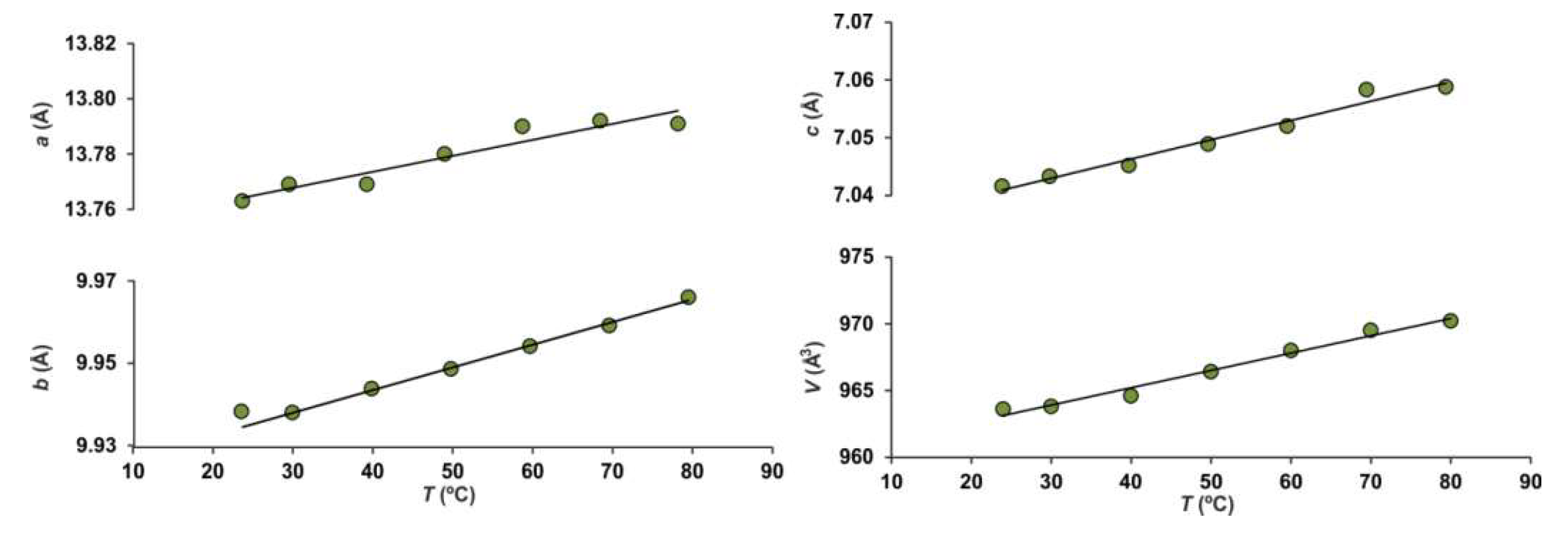
2.4. High-Temperature Powder X-Ray Diffraction
3. Results
3.1. Chemical Composition
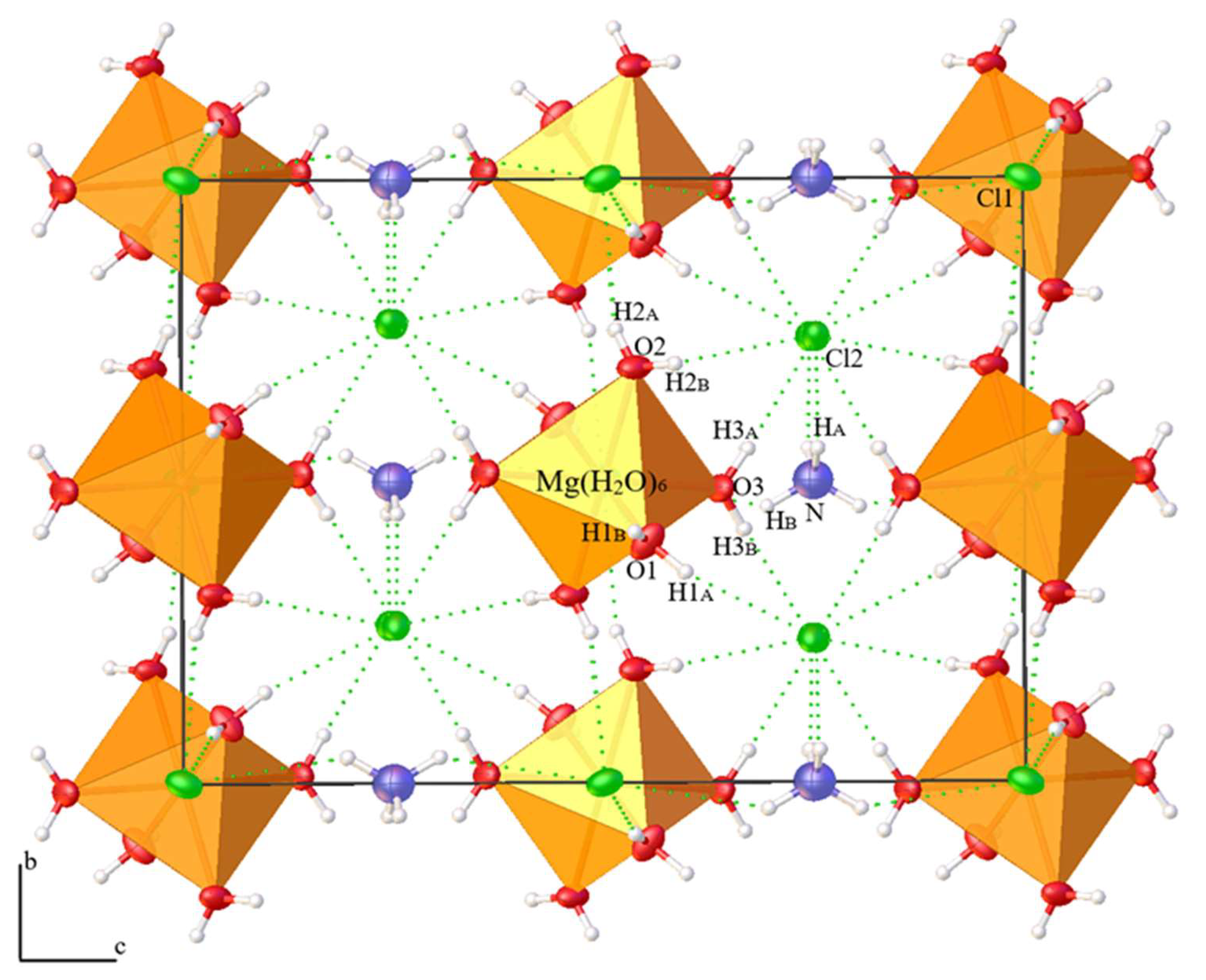
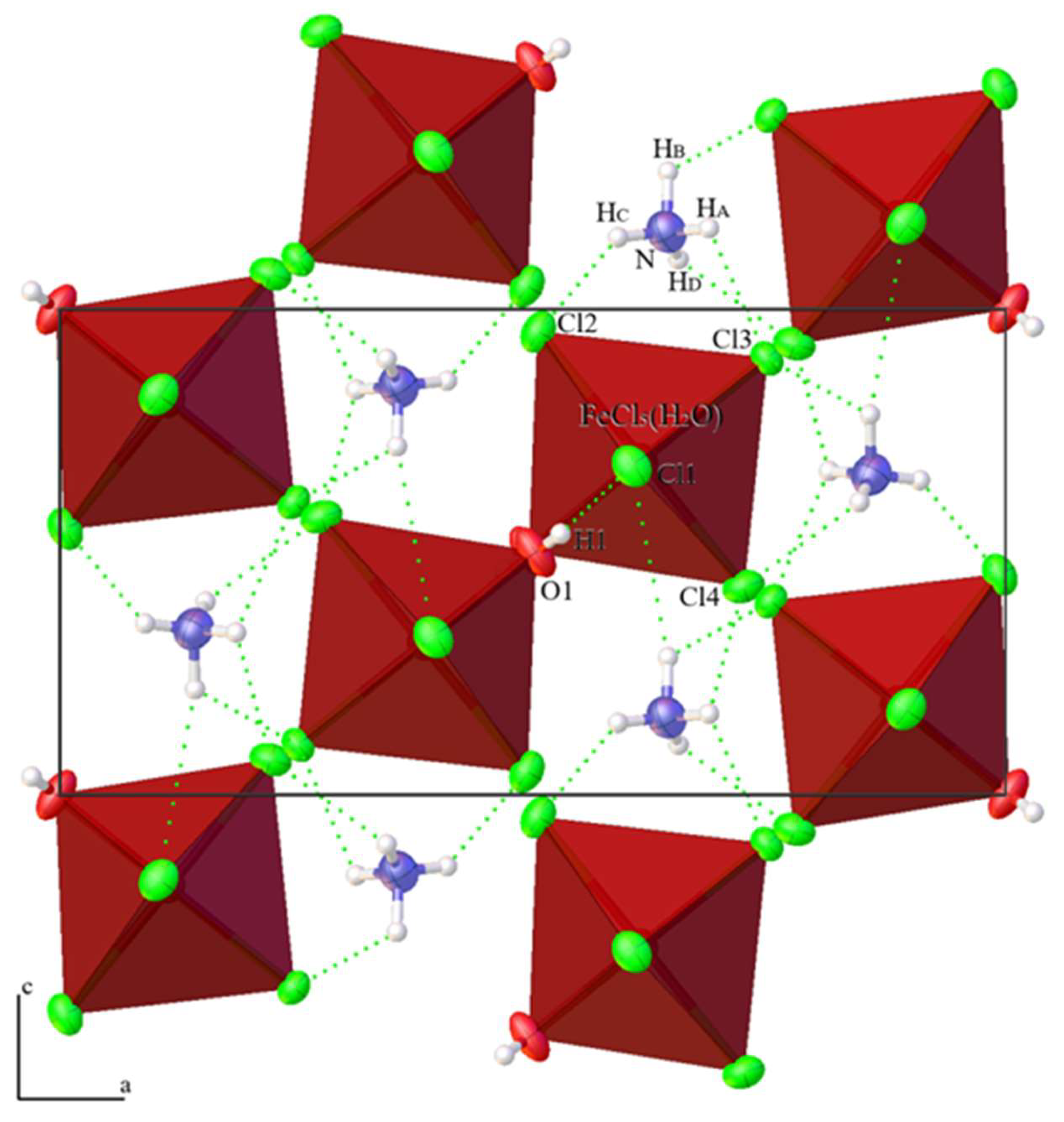
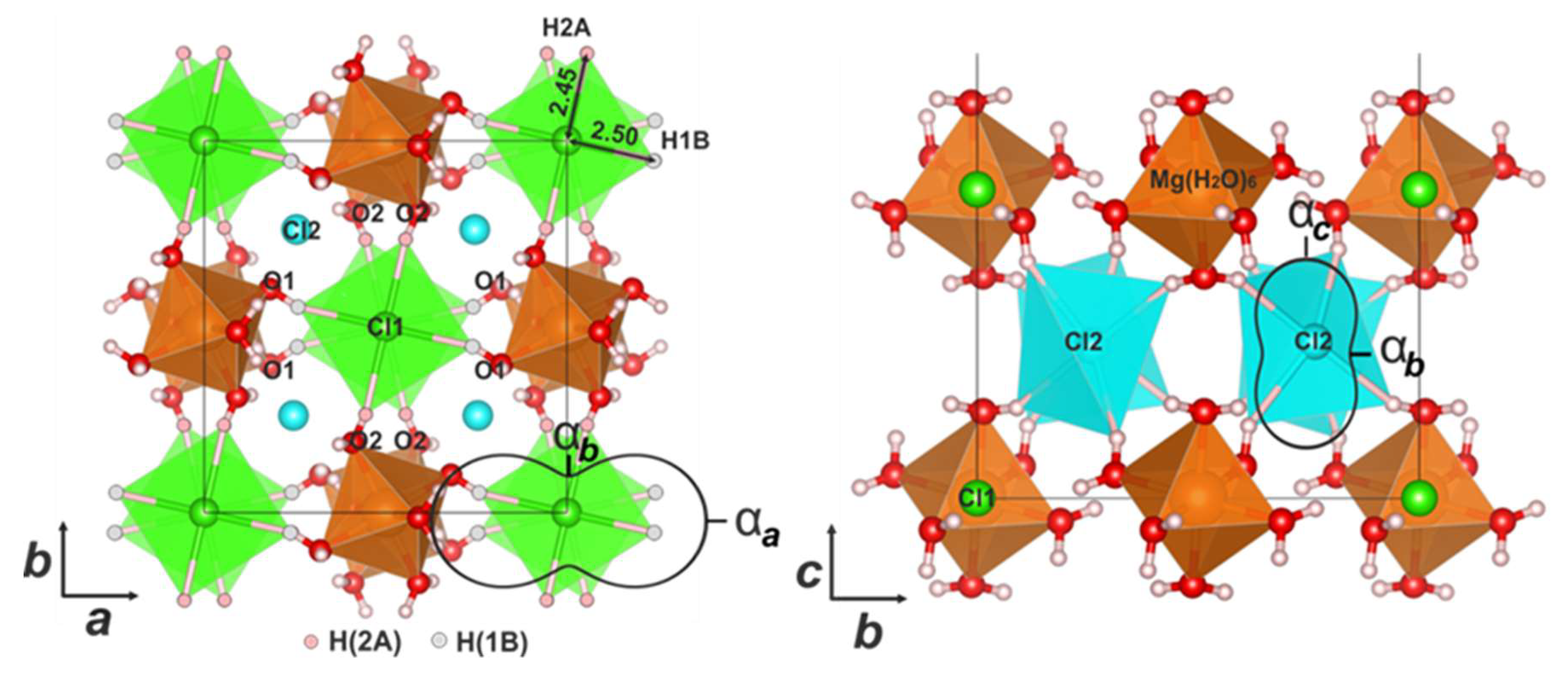
3.2. Crystal Structures
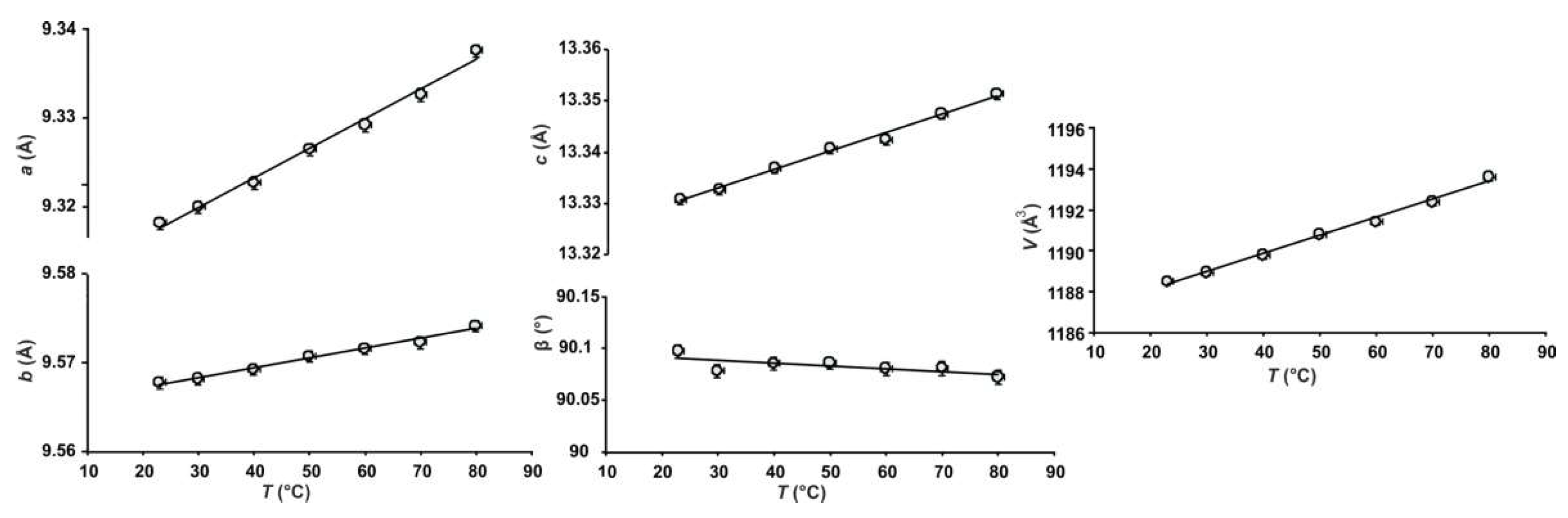
3.3. HTXRD
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chesnokov, B.V.; Polyakov, V.O.; Bushmakin, A.F. Bazhenovite CaS5·CaS2O3·6Ca(OH)2·20H2O—A new mineral. Zap. Vseross. Miner. Obs. 1987, 116, 737–743. (In Russian) [Google Scholar]
- Shcherbakova, Y.P.; Bazhenova, L.F.; Chesnokov, B.V. Godovikovite—NH4(Al,Fe)(SO4)2 a new ammonium-bearing sulfate. Zap. Vseross. Miner. Obs. 1988, 117, 208–211. (In Russian) [Google Scholar]
- Chesnokov, B.V.; Lotova, E.V.; Nigmatullina, E.N.; Pavlutchenko, V.S.; Bushmakin, A.F. Dmisteinbergite CaAl2Si2O8 (hexagonal)—A new mineral. Zap. Vseross. Miner. Obs. 1990, 119, 43–46. (In Russian) [Google Scholar]
- Chesnokov, B.V.; Lotova, E.V.; Pavlyuchenko, V.S.; Usova, L.V.; Bushmakin, A.F.; Nishanbayev, T.P. Svyatoslavite CaAl2Si2O8 (orthorhombic)—A new mineral. Zap. Vseross. Miner. Obs. 1989, 118, 111–114. (In Russian) [Google Scholar]
- Chesnokov, B.V.; Nishanbaev, T.P.; Bazhenova, L.F. Rorisite CaFCl—A new mineral. Zap. Vseross. Miner. Obs. 1990, 119, 73–76. (In Russian) [Google Scholar]
- Shcherbakova, Y.P.; Bazhenova, L.F. Efremovite (NH4)2Mg2(SO4)3—Ammonium analogue of langbeinite—A new mineral. Zap. Vseross. Miner. Obs. 1989, 118, 84–87. (In Russian) [Google Scholar]
- Chesnokov, B.V.; Bazhenova, L.F. Srebrodolskite Ca2Fe2O5—A new mineral. Zap. Vseross. Miner. Obs. 1985, 114, 195–199. (In Russian) [Google Scholar]
- Chesnokov, B.V.; Bazhenova, L.F.; Bushmakin, A.F. Fluorellestadite Ca10[(SO4),(SiO4)]6F2—A new mineral. Zap. Vseross. Miner. Obs. 1987, 116, 743–746. (In Russian) [Google Scholar]
- Chesnokov, B.V.; Shcherbakova, E.P.; Nishanbaev, T.P. Minerals of Burnt Dumps of the Chelyabinsk Coal Basin; Ural branch of RAS: Miass, Russia, 2008; pp. 1–139. (In Russian) [Google Scholar]
- Okrugin, V.M.; Kudaeva, S.S.; Karimova, O.V.; Yakubovich, O.V.; Belakovskiy, D.I.; Chukanov, N.V.; Zolotarev, A.A.; Gurzhiy, V.V.; Zinovieva, N.G.; Shiryaev, A.A.; et al. The new mineral novograblenovite, (NH4,K)MgCl3·6H2O from the Tolbachik volcano, Kamchatka, Russia: Mineral information and crystal structure. Miner. Mag. 2019, 83, 223–231. [Google Scholar] [CrossRef]
- Demartin, F.; Gramaccioli, C.M.; Campostrini, I. Pyracmonite, (NH4)3Fe(SO4)3, a new ammonium iron sulfate from La Fossa crater, Vulcano, Aeolian Islands, Italy. Can. Miner. 2010, 48, 307–313. [Google Scholar] [CrossRef]
- Galuskina, I.O.; Lazic, B.; Armbruster, T.; Galuskin, E.V.; Gazeev, V.M.; Zadov, A.E.; Pertsev, N.N.; Jezak, L.; Wrzalik, R.; Gurbanov, A.G. Kumtyubeite Ca5(SiO4)2F2—A new calcium mineral of the humite group from Northern Caucasus, Kabardino-Balkaria, Russia. Am. Miner. 2009, 94, 1361–1370. [Google Scholar] [CrossRef]
- Galuskina, I.O.; Vapnik, Y.; Lazic, B.; Armbruster, T.; Murashko, M.; Galuskin, E.V. Harmunite CaFe2O4: A new mineral from the Jabel Harmun, West Bank, Palestinian Autonomy, Israel. Am. Miner. 2014, 99, 965–975. [Google Scholar] [CrossRef]
- Rossi, M.; Nestola, F.; Zorzi, F.; Lanza, A.; Peruzzo, L.; Guastoni, A.; Kasatkin, A. Ghiaraite: A new mineral from Vesuvius volcano, Naples (Italy). Am. Miner. 2014, 99, 519–524. [Google Scholar] [CrossRef]
- Murashko, M.N.; Pekov, I.V.; Krivovichev, S.V.; Chernyatyeava, A.P.; Yapaskurt, V.O.; Zadov, A.E.; Zelensky, M.E. Steklite, KAl(SO4)2: The find at Tolbachik volcano (Kamchatka, Russia). Validation as a mineral species and crystal structure. Zap. Vseross. Min. Obs. 2012, 141, 36–44. (In Russian) [Google Scholar]
- Galuskina, I.O.; Galuskin, E.V.; Pakhomova, A.S.; Widmer, R.; Armbruster, T.; Krüger, B.; Grew, E.S.; Vapnik, Y.; Dzierażanowski, P.; Murashko, M. Khesinite, Ca4Mg2Fe3+10O4[(Fe3+10Si2)O36], a new rhönite-group (sapphirine supergroup) mineral from the Negev Desert, Israel—Natural analogue of the SFCA phase. Eur. J. Miner. 2017, 29, 101–116. [Google Scholar] [CrossRef]
- Galuskin, E.V.; Galuskina, I.O.; Lazic, B.; Armbruster, T.; Zadov, A.E.; Krzykawski, T.; Banasik, K.; Gazeev, V.M.; Pertsev, N.N. Rusinovite, Ca10(Si2O7)3Cl2: A new skarn mineral from the Upper Chegem caldera, Kabardino-Balkaria, Northern Caucasus, Russia. Eur. J. Miner. 2011, 23, 837–844. [Google Scholar] [CrossRef]
- Rose, H. Ueber den carnallit. Ann. der Phys. und Chem. 1856, 98, 161–163. (In Russian) [Google Scholar] [CrossRef]
- Chesnokov, B.V.; Bazhenova, L.F.; Shcherbakova, E.P.; Michal, T.A.; Deriabina, T.N. New minerals from the burned dumps of the Chelyabinsk coal basin, in Mineralogy, Technogenesis, and Mineral-Resource Complexes of the Urals. Akad. Nauk SSSR-Uralskoe Otdel. 1988, 5, 31. (In Russian) [Google Scholar]
- Witzke, T. Die Minerale der brennenden Halde der Steinkohlengrube “Deutschlandschacht” in Oelsnitz bei Zwickau. Aufschluss 1996, 47, 41–48. (In German) [Google Scholar]
- Stachowicz, M.; Parafiniuk, J.; Baginski, B.; Macdonald, R.; Wozniak, K. Structural analysis of new mineral phases. Acta Cryst. 2011, A67, C573. [Google Scholar] [CrossRef]
- Kremers, P. Ueber die aschenbestandtheile und die producte der trocknen destillation bei Braun und Steinkohlen. Ann. der Phys. und Chem. 1851, 84, 67–80. (In German) [Google Scholar] [CrossRef]
- Scacchi, A. Notizie preliminari di alcune specie mineralogiche rinvenute nel Vesuvio dopo l’incendio di aprile. Rend. Sci. Fis. Mat. Napoli 1872, 11, 210–213. (In Italian) [Google Scholar]
- Ackermann, M.; Brüning, D.; Lorenz, T.; Becker, P.; Bohatý, L. Thermodynamic properties of the new multiferroic material (NH4)2[FeCl5(H2O)]. New J. Phys. 2013, 15, 3001. [Google Scholar] [CrossRef]
- Ackermann, M.; Lorenz, T.; Becker, P.; Bohat ý, L. Magnetoelectric properties of A2[FeCl5(H2O)] with A = K., Rb, Cs. J. Phys-Condens. Mat. 2014, 26, 506002. [Google Scholar] [CrossRef] [PubMed]
- Demartin, F.; Castellano, C.; Campostrini, I. Acmonidesite, a new ammonium sulfate chloride from La Fossa crater, Vulcano, Aeolian Islands, Italy. Miner. Mag. 2019, 83, 137–142. [Google Scholar] [CrossRef]
- Kampf, A.R.; Nash, B.P.; Adams, P.M.; Marty, L.; Hughes, J.M. Ammoniolasalite, [(NH4)2Mg2(H2O)20][V10O28], A New Decavanadate Species from the Burro Mine, Slick Rock District, Colorado. Can. Miner. 2018, 56, 859–869. [Google Scholar] [CrossRef]
- Kampf, A.R.; Plášil, J.; Nash, B.P.; Marty, J. Ammoniomathesiusite, a new uranyl sulfate–vanadate mineral from the Burro mine, San Miguel County, Colorado, USA. Mineral. Mag. 2019, 83, 115–121. [Google Scholar] [CrossRef]
- Kampf, A.R.; Nash, B.P.; Hughes, J.M.; Marty, J. Burroite, Ca2(NH4)2(V10O28)·15H2O, a New Decavanadate Mineral from the Burro Mine, San Miguel County, Colorado. Can. Mineral. 2017, 55, 473–481. [Google Scholar] [CrossRef]
- Zhitova, E.S.; Siidra, O.I.; Belakovsky, D.I.; Shilovskikh, V.V.; Nuzhdaev, A.A.; Ismagilova, R.M. Ammoniovoltaite, (NH4)2Fe2+5Fe3+3Al(SO4)12(H2O)18, a new mineral from the Severo-Kambalny geothermal field, Kamchatka, Russia. Mineral. Mag. 2018, 82, 1057–1077. [Google Scholar] [CrossRef]
- Kampf, A.R.; Plášil, J.; Olds, T.A.; Nash, B.P.; Marty, J. Ammoniozippeite, a New Uranyl Sulfate Mineral from the Blue Lizard Mine, San Juan County, Utah, and the Burro Mine, San Miguel County, Colorado, USA. Can. Mineral. 2018, 56, 235–245. [Google Scholar] [CrossRef]
- Olds, T.A.; Plášil, J.; Kampf, A.R.; Burns, P.C.; Nash, B.P.; Marty, J.; Rose, T.P.; Carlson, S.M. Redcanyonite, (NH4)2Mn[(UO2)4O4(SO4)2](H2O)4, a new zippeite-group mineral from the Blue Lizard mine, San Juan County, Utah, USA. Mineral. Mag. 2018, 82, 1261–1275. [Google Scholar] [CrossRef]
- Kampf, A.R.; Chukanov, N.V.; Möhn, G.; Dini, M.; Donoso, A.A.; Friis, H. Cuatrocapaite-(NH4) and cuatrocapaite-(K), two new minerals from the Torrecillas mine, Iquique Province, Chile, related to lucabindiite and gajardoite. Mineral. Mag. 2019, 1–24. [Google Scholar] [CrossRef]
- Kampf, A.R.; Cooper, M.A.; Rossman, G.R.; Nash, B.P.; Hawthorne, F.C.; Marty, J. Davidbrownite, IMA 2018-129. CNMNC Newsletter No. 47, February 2019, page 203. Eur. J. Mineral. 2019, 31, 199–204. [Google Scholar]
- Kampf, A.R.; Celestian, A.J.; Nash, B.P.; Marty, J. Phoxite, (NH4)2Mg2(C2O4)(PO3OH)2(H2O)4, the first phosphate-oxalate mineral. Am. Mineral. 2019, 104, 973–979. [Google Scholar] [CrossRef]
- Kampf, A.R.; Cooper, M.A.; Nash, B.P.; Cerling, T.E.; Marty, J.; Hummer, D.R.; Celestian, A.J.; Rose, T.P.; Trebisky, T.J. Rowleyite, [Na(NH4,K)9Cl4][V25+,4+(P,As)O8]6·n[H2O,Na,NH4,K,Cl], a new mineral with a microporous framework structure. Am. Mineral. 2017, 102, 1037–1044. [Google Scholar]
- Chukanov, N.V.; Pekov, I.V.; Sejkora, J.; Plášil, J.; Belakovskiy, D.I.; Britvin, S.N. Ferrierite-NH4, (NH4,Mg0.5)5(Al5Si31O72)·22H2O, A New Zeolite From Northern Bohemia, Czech Republic. Can. Mineral. 2019, 55, 81–90. [Google Scholar] [CrossRef]
- Kampf, A.R.; Plášil, J.; Nash, B.P.; Marty, J. Greenlizardite, (NH4)Na(UO2)2(SO4)2(OH)2·4H2O, a new mineral with phosphuranylite-type uranyl sulfate sheets from Red Canyon, San Juan County, Utah, USA. Mineral. Mag. 2018, 82, 401–411. [Google Scholar] [CrossRef]
- Kampf, A.R.; Plášil, J.; Nash, B.P.; Marty, J. Meitnerite, (NH4)(UO2)(SO4)(OH)·2H2O, a new uranyl-sulfate mineral with a sheet structure. Eur. J. Mineral. 2018, 30, 999–1006. [Google Scholar] [CrossRef]
- Chukanov, N.V.; Pekov, I.V.; Belakovskiy, D.I.; Britvin, S.N.; Stergiou, V.; Voudouris, P.; Magganas, A. Katerinopoulosite, (NH4)2Zn(SO4)2·6H2O, a new mineral from the Esperanza mine, Lavrion, Greece. Eur. J. Mineral. 2018, 30, 821–826. [Google Scholar] [CrossRef]
- Campostrini, I.; Demartin, F.; Scavini, M. Russoite, NH4ClAs23+O3(H2O)0.5, a new phylloarsenite mineral from Solfatara Di Pozzuoli, Napoli, Italy. Mineral. Mag. 2019, 83, 89–94. [Google Scholar] [CrossRef]
- Parafiniuk, J.; Kruszewski, Ł. Ammonium minerals from burning coal-dumps of the Upper Silesian Coal Basin (Poland). Geol. Quart. 2009, 53, 341–356. [Google Scholar]
- Siidra, O.I.; Lukina, E.A.; Nazarchuk, E.V.; Depmeier, W.; Bubnova, R.S.; Agakhanov, A.A.; Avdontseva, E.Y.; Filatov, S.K.; Kovrugin, V.M. Saranchinaite, Na2Cu(SO4)2, a new ehalative mineral from Tolbachik volcano, Kamchatka, Russia, and a product of the reversible dehydration of krönkeite, Na2Cu(SO4)2(H2O)2. Mineral. Mag. 2018, 82, 257–274. [Google Scholar] [CrossRef]
- Siidra, O.I.; Borisov, A.S.; Lukina, E.A.; Depmeier, W.; Platonova, N.V.; Colmont, M.; Nekrasova, D.O. Reversible hydration/dehydration and thermal expansion of euchlorine, ideally KNaCu3O(SO4)3. Phys. Chem. Miner. 2019, 46, 403–416. [Google Scholar] [CrossRef]
- Zhitova, E.S.; Sergeeva, A.V.; Nuzhdaev, A.A.; Krzhizhanovskaya, M.G.; Chubarov, V.M. Tschermigite from thermal fields of Southern Kamchatka: high-temperature transformation and peculiarities of IR-spectrum. Zap. Ross. Mineral. Obs. 2019, 148. [Google Scholar]
- Bruker-AXS. APEX2; Version 2014.11-0; Bruker-AXS: Madison, WI, USA, 2014. [Google Scholar]
- Sheldrick, G.M. SADABS; University of Goettingen: Goettingen, Germany, 2007. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- CrysAlisPro Software System, version 1.171.39.44; Rigaku Oxford Diffraction: Oxford, UK, 2015.
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Topas V.4.2. General Profile and Structure Analysis Software for Powder Diffraction Data; Bruker-AXS: Karlsruhe, Germany, 2009.
- Belousov, R.; Filatov, S.K. Algorithm for Calculating the Thermal Expansion Tensor and Constructing the Thermal Expansion Diagram for Crystals. Glass Phys. Chem. 2007, 33, 271–275. [Google Scholar] [CrossRef]
- Bubnova, R.S.; Firsova, V.A.; Filatov, S.K. Software for determining the thermal expansion tensor and the graphic representation of its characteristic surface (theta to tensor-TTT). Glass Phys. Chem. 2013, 39, 347–350. [Google Scholar] [CrossRef]
- Langreiter, T.; Kahlenberg, V. TEV—A Program for the Determination of the Thermal Expansion Tensor from Diffraction Data. Crystals 2015, 5, 143–153. [Google Scholar] [CrossRef]
- Jensen, S.J.; Lehmann, M.S. Neutron diffraction study of β-RbMnCl3·2H2O. Acta Chem. Scand. 1970, 24, 3422–3424. [Google Scholar] [CrossRef]
- Solans, X.; Font-Altaba, M.; Aguiló, M.; Solans, J.; Domenech, V. Crystal form and structure of ammonium hexaaquamagnesium trichloride, NH4[Mg(H2O)6]Cl3. Acta Crystallogr. 1983, C39, 1488–1490. [Google Scholar] [CrossRef]
- Marsh, R.E. Structure of MgCl2.RbCl·6H2O. Corrigendum. Acta Crystallogr. 1992, C48, 218–219. [Google Scholar] [CrossRef]
- Brown, I.D.; Altermatt, D. Bond-valence parameters obtained from a systematic analysis of the Inorganic Crystal Structure Database. Acta Crystallogr. 1985, B41, 244–247. [Google Scholar] [CrossRef]
- Brese, N.E.; O’Keeffe, M. Bond-valence parameters for solids. Acta Crystallogr. 1991, B47, 192–197. [Google Scholar] [CrossRef]
- Malcherek, T.; Schlueter, J. Cu3MgCl2(OH)6 and the bond-valence parameters of the OH–Cl bond. Acta Crystallogr. 2007, B63, 157–160. [Google Scholar] [CrossRef]
- Brown, D.I. The Chemical Bond in Inorganic Chemistry: the bond valence model. In International Union of Crystallography Monographs on Crystallography; Oxford University Press: Oxford, UK, 2002; pp. 1–230. [Google Scholar]
- Figgis, B.N.; Raston, C.L.; Sharma, R.P.; White, A.H. Crystal structure of diammonium aquapentachloroferrate (III). Aust. J. Chem. 1978, 31, 2717–2720. [Google Scholar] [CrossRef]
- Waizumi, K.; Masuda, H.; Ohtaki, H.; Scripkin, M.Y.; Burkov, K.A. Crystallographic investigations of [Mg(H2O)6]XCl3 double salts (X+=K+,Rb+,Cs+,NH4+): Crystal structure of [Mg(H2O)6]XCl3. Am. Mineral. 1991, 76, 1884–1888. [Google Scholar]
- Schlemper, E.O.; Sen Gupta, P.K.; Zoltai, T. Refinement of the structure of carnallite, Mg(H2O)6KCl3. Am. Mineral. 1985, 70, l309–l313. [Google Scholar]
- Schmidt, H.; Euler, B.; Voigta, W.; Heide, G. Lithium carnallite, LiCl·MgCl2·7H2O. Acta Crystallogr. 2009, C65, 57–59. [Google Scholar] [CrossRef]
- O’Connor, C.J.; Deaver, B.S., Jr.; Sinn, E. Crystal structures of A2FeCl5·H2O (A=Rb+, Cs+) and field dependent superconducting susceptometer measurements. J. Chem. Phys. 1979, 70, 5161. [Google Scholar] [CrossRef]
- Bellanca, A. La struttura dell’eritrosiderite. Period. Mineral. 1948, 17, 59–72. (In Italian) [Google Scholar]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. 1976, A32, 751–767. [Google Scholar] [CrossRef]
- Sidey, V. On the effective ionic radii for ammonium. Acta Crystallogr. 2016, B72, 626–633. [Google Scholar] [CrossRef]
| Mineral | Locality, Scenarios of Formation | Reference |
|---|---|---|
| Acmonidesite (NH4,K,Pb2+,Na)9Fe42+(SO4)5Cl8 | La Fossa Crater, Vulcano, Aeolian Islands, Sicily, Italy. Fumarolic phase, (T ~ 250 °C). | [26] |
| Ammoniolasalite [(NH4)2Mg2(H2O)20]·[V10O28] | Burro Mine, San Miguel County, Utah, USA. Secondary phases. | [27] |
| Ammoniomathesiusite (NH4)5(UO2)4(SO4)4(VO5)·4H2O | [28] | |
| Burroite Ca2(NH4)2(V10O28)·15H2O | [29] | |
| Ammoniovoltaite (NH4)2Fe2+5Fe3+3Al(SO4)12(H2O)18 | Severo-Kambalny geothermal field, southern Kamchatka, Russia. Efflorescence around gas-steam hydrothermal vents. | [30] |
| Ammoniozippeite (NH4)2[(UO2)2(SO4)O2]·H2O | Blue Lizard Mine, San Juan County, Utah, and the Burro Mine, San Miguel County, Colorado, USA. Low-temperature, secondary phase within organic-rich beds. | [31] |
| Redcanyonite (NH4)2Mn[(UO2)4O4(SO4)2](H2O)4 | [32] | |
| Cuatrocapaite-(NH4) (NH4)3(NaM☐)(As2O3)6Cl6·16H2O | Torrecillas Mine, Salar Grande, Iquique Province, Tarapacá Region, Chile. | [33] |
| Davidbrownite-(NH4) (NH4)5(V4+O)2(C2O4)[PO2.75(OH)1.25]4·3H2O | Rowley Mine, Painted Rock Mts, Arizona, USA. Low-temperature, apparently post-mining suite of phases that include various vanadates, phosphates, oxalates and chlorides, some containing NH4+. | [34] |
| Phoxite (NH4)2Mg2(C2O4)(PO3OH)2(H2O)4 | [35] | |
| Rowleyite [Na(NH4,K)9Cl4][V5+,4+2(P,As)O8]6·n[H2O,Na, NH4,K,Cl] | [36] | |
| Ferrierite-NH4 (NH4,Mg0.5)5(Al5Si31O72)·22H2O | Northern Bohemia, Czech Republic. | [37] |
| Greenlizardite (NH4)Na(UO2)2(SO4)2(OH)2·4H2O | Green Lizard Mine, Red Canyon, San Juan County, Utah, USA. Secondary alteration phase. | [38] |
| Meitnerite (NH4)(UO2)(SO4)(OH)·2H2O | [39] | |
| Katerinopoulosite (NH4)2Zn(SO4)2·6H2O | Esperanza Mine, Lavrion District, Attikí Prefecture, Greece. Oxidation zone of a sphalerite-rich orebody. | [40] |
| Novograblenovite (NH4,K)MgCl3·6H2O | 2012–2013 Tolbachik effusive eruption basalts, Plosky Tolbachik volcano, Kamchatka Oblast, Far-Eastern Region, Russia. Exhalation due to volcanic gas exposure. | [10] |
| Russoite (NH4)ClAs2O3(H2O)0.5 | Solfatara di Pozzuoli, Pozzuoli, Napoli, Italy. Fumarolic phase. | [41] |
| Ideal Chemical Formula | Mineral Analogue If Known | Chesnakov’s Name |
|---|---|---|
| NH4Cl | salammoniac | |
| NH4MgCl3·6H2O | novograblenovite | redikortsevite |
| (NH4)2Fe3+Cl5·H2O | kremersite | kopeiskite |
| (NH4)2Mg2(SO4)3 | efremovite 1 | efremovite |
| (NH4)Al(SO4)2 | godovikovite 1 | godovikovite |
| NH4Fe3+3(SO4)2(OH)6 | ammoniojarosite | |
| (NH4)2Mg(SO4)2·6H2O | boussingaultite | |
| (NH4)2Ca(SO4)2·H2O | koktaite | |
| (NH4)Fe3+(SO4)2 | sabieite | terriconite |
| (NH4)2SO4 | mascagnite | |
| (NH4)3Fe3+(SO4)3 | pyracmonite | (NH4)3Fe3+(SO4)3 |
| (NH4)2Fe2+(SO4)2·6H2O | mohrite | |
| NH4Al(SO4)2·12H2O | tschermigite | |
| NH4Fe3+(SO4)2·12H2O | lonecreekite | |
| NH4Al(SO4)2·4H2O | - | NH4Al(SO4)2·4H2O |
| (NH4)2Mg(SO4)2·4H2O | - | ammonioleonite |
| NH4MgCl3·6H2O | (NH4)2Fe3+Cl5·(H2O) | Standard | ||||
|---|---|---|---|---|---|---|
| Constituent | Wt. % | Atoms Per Formula Unit 3 | Constituent | Wt. % | Atoms Per Formula Unit 4 | |
| (NH4)2O 1 | 9.68 | 0.96 | (NH4)2O 1 | 15.96 | 1.70 | BN (N) |
| MgO | 15.61 | 1.00 | Fe2O3 | 27.19 | 0.94 | MgO (Mg) FeS2 (Fe) |
| Cl | 42.34 | 3.08 | Cl | 64.64 | 5.06 | NaCl (Cl) |
| H2O 2 | 41.93 | 6.00 | H2O (2) | 6.50 | 1.00 | |
| Cl2=O | –9.55 | Cl2=O | −14.29 | |||
| Total | 100.00 | Total | 100.00 | |||
| Compound | NH4MgCl3·6H2O | (NH4)2FeCl5·H2O |
|---|---|---|
| Crystal system | monoclinic | orthorhombic |
| Space group | C2/c | Pnma |
| a, Å | 9.3091(9) | 13.725(2) |
| b, Å | 9.5353(7) | 9.9365(16) |
| c, Å | 13.2941(12) | 7.0370(11) |
| β, ° | 90.089(8) | - |
| V, Å3 | 1180.05(18) | 959.7(3) |
| Z | 4 | 4 |
| ρcalc, g/cm3 | 1.445 | 1.988 |
| μ, mm−1 | 0.822 | 2.900 |
| F(000) | 536.0 | 572.0 |
| Radiation | MoKα (λ = 0.71073) | MoKα (λ = 0.71073) |
| 2θ range, deg. | 8.55–59.99 | 5.936–71.776 |
| Index ranges | −13 ≤ h ≤ 12, −13 ≤ k ≤ 13, −18 ≤ l ≤ 18 | −18 ≤ h ≤ 21, −13 ≤ k ≤ 16, −11 ≤ l ≤ 11 |
| Reflections collected | 5504 | 11813 |
| Independent reflections | 1678 (Rint = 0.066, Rsigma = 0.037) | 2256 (Rint = 0.0230, Rsigma = 0.0173) |
| Data/restraints/parameters | 1678/4/84 | 2256/7/65 |
| Goodness of Fit | 1.243 | 1.091 |
| Final R indexes (I ≥ 2σ(I)) | R1 = 0.0783, wR2 = 0.1847 | R1 = 0.0229, wR2 = 0.0660 |
| Final R indexes (all data) | R1 = 0.0883, wR2 = 0.1907 | R1 = 0.0292, wR2 = 0.0690 |
| Largest diff. peak/hole / e Å−3 | 0.53/−0.32 | 0.34/−0.80 |
| Atom | x | y | z | Ueq |
|---|---|---|---|---|
| NH4MgCl3·6H2O | ||||
| Cl1 | 0.5 | 0.5 | 0.5 | 0.0414(3) |
| Cl2 | 0.7542(1) | 0.73838(9) | 0.74664(6) | 0.0401(3) |
| Mg | 0 | 0.5 | 0.5 | 0.0225(3) |
| N | 0.5 | 0.5012(6) | 0.75 | 0.061(1) |
| HA | 0.496(4) | 0.454(2) | 0.8028(7) | 0.053 |
| HB | 0.429(4) | 0.560(3) | 0.749(3) | 0.053 |
| O1 | 0.1795(3) | 0.6010(3) | 0.4481(2) | 0.0420(6) |
| O2 | 0.4099(3) | 0.1877(3) | 0.5379(2) | 0.0398(6) |
| O3 | 0.9089(3) | 0.5131(3) | 0.3603(2) | 0.0403(6) |
| H1A | 0.198(5) | 0.652(5) | 0.404(4) | 0.06(1) |
| H1B | 0.250(5) | 0.58105(4) | 0.463(3) | 0.04(1) |
| H2A | 0.440(6) | 0.248(5) | 0.515(4) | 0.05(1) |
| H2B | 0.360(5) | 0.193(5) | 0.586(4) | 0.05(1) |
| H3A | 0.890(6) | 0.447(6) | 0.329(4) | 0.06(2) |
| H3B | 0.885(5) | 0.579(5) | 0.332(3) | 0.04(1) |
| (NH4)2FeCl5·H2O | ||||
| N | 0.14167(9) | 0.0006(1) | 0.6594(2) | 0.0360(2) |
| HA | 0.191(1) | 0.050(2) | 0.671(2) | 0.054 |
| HB | 0.114(1) | 0.05(2) | 0.771(2) | 0.054 |
| HC | 0.119(1) | 0.053(2) | 0.585(2) | 0.054 |
| HD | 0.148(1) | −0.075(2) | 0.614(2) | 0.054 |
| Fe | 0.11623(2) | 0.25 | 0.18959(2) | 0.02252(6) |
| Cl1 | 0.10486(2) | 0.01065(3) | 0.17604(4) | 0.03113(8) |
| Cl2 | 0.00609(3) | 0.25 | 0.45303(6) | 0.03264(9) |
| Cl3 | 0.24790(3) | 0.25 | 0.39805(5) | 0.02664(8) |
| Cl4 | 0.22324(3) | 0.25 | −0.07176(6) | 0.0340(1) |
| O1 | −0.0033(1) | 0.25 | 0.0011(2) | 0.0388(3) |
| H1 | −0.028(2) | 0.184(2) | −0.033(3) | 0.072(7) |
| Atom | U11 | U22 | U33 | U23 | U13 | U12 |
|---|---|---|---|---|---|---|
| NH4MgCl3·6H2O | ||||||
| Cl1 | 0.0346(6) | 0.0335(6) | 0.0561(8) | 0.0098(4) | −0.0010(5) | −0.0015(4) |
| Cl2 | 0.0503(5) | 0.0378(5) | 0.0323(5) | 0.0002(3) | −0.0001(3) | 0.0102(3) |
| Mg | 0.0214(7) | 0.0227(6) | 0.0234(7) | 0.0006(4) | −0.0011(5) | −0.0007(4) |
| N | 0.058(3) | 0.063(4) | 0.062(4) | 0 | −0.003(3) | 0 |
| O1 | 0.023(1) | 0.056(2) | 0.048(2) | 0.020(1) | −0.002(1) | −0.008(1) |
| O2 | 0.049(2) | 0.025(1) | 0.046(2) | 0.002(1) | 0.018(1) | 0.006(1) |
| O3 | 0.057(2) | 0.033(1) | 0.031(1) | 0.002(1) | −0.020(1) | 0.002(1) |
| (NH4)2FeCl5·H2O | ||||||
| N | 0.0369(5) | 0.0319(6) | 0.0392(6) | 0.0037(4) | −0.0035(3) | −0.0013(4) |
| Fe | 0.0229(1) | 0.0197(1) | 0.0250(1) | 0 | −0.00326(7) | 0 |
| Cl1 | 0.0353(1) | 0.0186(1) | 0.0395(2) | −0.00229(9) | −0.0073(1) | −0.00072(9) |
| Cl2 | 0.0282(2) | 0.0307(2) | 0.0390(2) | 0 | 0.0085(1) | 0 |
| Cl3 | 0.0244(1) | 0.0293(2) | 0.0263(2) | 0 | −0.0054(1) | 0 |
| Cl4 | 0.0398(2) | 0.0372(2) | 0.0250(2) | 0 | 0.0054(1) | 0 |
| O1 | 0.0370(6) | 0.0240(6) | 0.0553(9) | 0 | −0.0237(6) | 0 |
| NH4MgCl3·6H2O | |||||
| Mg–O | Cl–N | ∠ H–N–H in NH4 | |||
| Mg–O1 | 2.052(2) ×2 | Cl1–N | 3.3235(4) ×2 | HA–N–HB | 110(2) |
| Mg–O2 | 2.040(2) ×2 | Cl2–N | 3.273(5) ×2 | HA–N–HA 1 | 115(3) |
| Mg–O3 | 2.044(2) ×2 | Cl2 2–N | 3.394(5) ×2 | HB–N–HB 1 | 100(6) |
| <Mg–O> | 2.045 | <Cl–N> | 3.330 | HA–N–HB 1 | 111(2) |
| Di4 | 0.00153 | Di4 | 0.01269 | ||
| ∠ H–O–H in H2O | |||||
| H1A–O1–H1B | 106(4) | ||||
| H2A–O2–H2B | 120(5) | ||||
| H3A–O3–H3B | 110(5) | ||||
| (NH4)2FeCl5·H2O | |||||
| Fe–Cl,O | ∠ H–O–H in H2O | ∠ H–N–H in NH4 | |||
| Fe–Cl1 | 2.3853(5) ×2 | H1–O1–H1 3 | 115(3) | HA–N–HB | 104(1) |
| Fe–Cl2 | 2.3920(5) | HA–N–HC | 90(2) | ||
| Fe–Cl3 | 2.3276(5) | HA–N–HD | 118(2) | ||
| Fe–Cl4 | 2.3537(5) | HB–N–HC | 113(2) | ||
| Fe–O1 | 2.109(1) | HB–N–HD | 116(2) | ||
| <Fe–Cl,O> | 2.325 | HC–N–HD | 112(2) | ||
| Di4 | 0.03097 | ||||
| NH4MgCl3·6H2O | β-Rb(MnCl3)·2H2O 1 (Isotypic to HT Modification of NH4MgCl3·6H2O) | (NH4)2FeCl5·H2O | |
|---|---|---|---|
| Symmetry | Monoclinic | Triclinic | Orthorhombic |
| Space group | C2/c | P−1 | Pnma |
| a (Å) | 9.3091(9) | 6.65 | 13.725(2) |
| b (Å) | 9.5353(7) | 7.01 | 9.9365(16) |
| c (Å) | 13.2941(12) | 9.03 | 7.0370(11) |
| α (°) | 90 | 92.3 | 90 |
| β (°) | 90.089(8) | 109.4 | 90 |
| γ (°) | 90 | 112.9 | 90 |
| V (Å3) | 1180.05(18) | 358.74 | 959.7(3) |
| Reference | this work | [56] | this work |
| D–H | d(D–H) (Å) | d(H..A) (Å) | <DHA (°) | d(D..A) (Å) | A |
|---|---|---|---|---|---|
| NH4MgCl3·6H2O | |||||
| O1–H1A | 0.783 | 2.395 | 166.88 | 3.164 | Cl2 1 |
| O1–H1B | 0.701 | 2.514 | 171.80 | 3.209 | Cl1 |
| O2–H2A | 0.710 | 2.472 | 156.34 | 3.135 | Cl1 |
| O2–H2B | 0.794 | 2.390 | 167.42 | 3.169 | Cl2 2 |
| O3–H3A | 0.773 | 2.435 | 161.92 | 3.178 | Cl2 3 |
| O3–H3B | 0.762 | 2.411 | 164.46 | 3.152 | Cl2 4 |
| N–HA | 0.834 | 2.659 | 137.70 | 3.224 | Cl1 5 |
| N–HB | 0.863 | 2.414 | 174.62 | 3.274 | Cl2 5 |
| (NH4)2FeCl5·H2O | |||||
| O1–H1 | 0.775 | 2.424 | 173.29 | 3.195 | Cl1 6 |
| N–HA | 0.842 | 2.862 | 132.12 | 3.483 | Cl1 7 |
| N–HA | 0.842 | 2.873 | 123.80 | 3.414 | Cl3 |
| N–HA | 0.842 | 2.726 | 128.12 | 3.313 | Cl4 8 |
| N–HB | 0.872 | 2.855 | 156.62 | 3.672 | Cl1 8 |
| N–HC | 0.806 | 2.907 | 125.71 | 3.440 | Cl1 |
| N–HC | 0.806 | 2.662 | 158.11 | 3.423 | Cl2 |
| N–HC | 0.806 | 2.947 | 119.25 | 3.414 | Cl3 |
| N–HD | 0.818 | 2.784 | 123.53 | 3.307 | Cl2 9 |
| N–HD | 0.818 | 2.802 | 145.15 | 3.505 | Cl4 7 |
| Atom | NH4 | Mg | H1A | H1B | H2A | H2B | H3A | H3B | Total |
|---|---|---|---|---|---|---|---|---|---|
| O1 | 0.38↓ ×2 | 0.80 | 0.87 | 2.05 | |||||
| O2 | 0.39↓ ×2 | 0.86 | 0.79 | 2.04 | |||||
| O3 | 0.39↓ ×2 | 0.81 | 0.82 | 2.02 | |||||
| Cl1 | 0.16↓ → ×2 | 0.11 → × 2 | 0.12 → × 2 | 0.78 | |||||
| Cl2 | 0.18↓ ×2 0.13↓ ×2 | 0.13 | 0.14 | 0.13 | 0.13 | 0.84 | |||
| Total | 0.96 | 2.32 | 0.93 | 0.98 | 0.98 | 0.93 | 0.94 | 0.95 |
| NH4MgCl3·6H2O | HT Phase 1 | (NH4)2Fe3+Cl5·H2O | |
|---|---|---|---|
| α11 | 36.3 | −30.7 | 40.8 |
| α22 | 11.5 | 161.1 | 45.9 |
| α33 | 25.2 | 275.2 | 47.0 |
| <α11a | 12.8 | 70.7 | - |
| <α22b | - | 24.3 | - |
| <α33c | 12.7 | 35.8 | - |
| αa | 36(1) | 182(9) | 41(6) |
| αb | 11.5(6) | 159(22) | 46(6) |
| αc | 26(1) | 172(7) | 47(4) |
| αα | - | 73(9) | - |
| αβ | −3(1) | 97(12) | - |
| αγ | - | −10(8) | - |
| αV | 73(2) | 406(25) | 134(9) |
| Formula | Symmetry | Cation X+ | Ionic Radii 1 of X+, Å | Reference |
|---|---|---|---|---|
| XMgCl3·6H2O | ||||
| CsMgCl3·6H2O | Monoclinic C2/c | Cs | 1.81 | [64] |
| RbMgCl3·6H2O | Monoclinic C2/c | Rb | 1.52 | [58] |
| NH4MgCl3·6H2O | Monoclinic C2/c | NH4 | 1.48 | [57], this work |
| KMgCl3·6H2O | Orthorhombic Pnna | K | 1.38 | [65] |
| LiMgCl3·7H2O | Trigonal R3 | Li | 0.76 | [66] |
| (X)2FeCl5·H2O | ||||
| Cs2FeCl5·H2O | Orthorhombic Cmcm | Cs | 1.81 | [67] |
| Rb2FeCl5·H2O | Orthorhombic Pnma | Rb | 1.52 | [67] |
| (NH4)2FeCl5·H2O | Orthorhombic Pnma | NH4 | 1.48 | [63], this work |
| K2FeCl5·H2O | Orthorhombic Pnma | K | 1.38 | [68] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zolotarev, A.A., Jr.; Zhitova, E.S.; Krzhizhanovskaya, M.G.; Rassomakhin, M.A.; Shilovskikh, V.V.; Krivovichev, S.V. Crystal Chemistry and High-Temperature Behaviour of Ammonium Phases NH4MgCl3·6H2O and (NH4)2Fe3+Cl5·H2O from the Burned Dumps of the Chelyabinsk Coal Basin. Minerals 2019, 9, 486. https://doi.org/10.3390/min9080486
Zolotarev AA Jr., Zhitova ES, Krzhizhanovskaya MG, Rassomakhin MA, Shilovskikh VV, Krivovichev SV. Crystal Chemistry and High-Temperature Behaviour of Ammonium Phases NH4MgCl3·6H2O and (NH4)2Fe3+Cl5·H2O from the Burned Dumps of the Chelyabinsk Coal Basin. Minerals. 2019; 9(8):486. https://doi.org/10.3390/min9080486
Chicago/Turabian StyleZolotarev, Andrey A., Jr., Elena S. Zhitova, Maria G. Krzhizhanovskaya, Mikhail A. Rassomakhin, Vladimir V. Shilovskikh, and Sergey V. Krivovichev. 2019. "Crystal Chemistry and High-Temperature Behaviour of Ammonium Phases NH4MgCl3·6H2O and (NH4)2Fe3+Cl5·H2O from the Burned Dumps of the Chelyabinsk Coal Basin" Minerals 9, no. 8: 486. https://doi.org/10.3390/min9080486
APA StyleZolotarev, A. A., Jr., Zhitova, E. S., Krzhizhanovskaya, M. G., Rassomakhin, M. A., Shilovskikh, V. V., & Krivovichev, S. V. (2019). Crystal Chemistry and High-Temperature Behaviour of Ammonium Phases NH4MgCl3·6H2O and (NH4)2Fe3+Cl5·H2O from the Burned Dumps of the Chelyabinsk Coal Basin. Minerals, 9(8), 486. https://doi.org/10.3390/min9080486







