Hydrothermal Aluminum-Phosphate-Sulfates in Ash from the 2014 Hydrothermal Eruption at Ontake Volcano, Central Honshu, Japan
Abstract
1. Introduction
2. Background of Ontake Volcano, Japan
2.1. Geological Setting
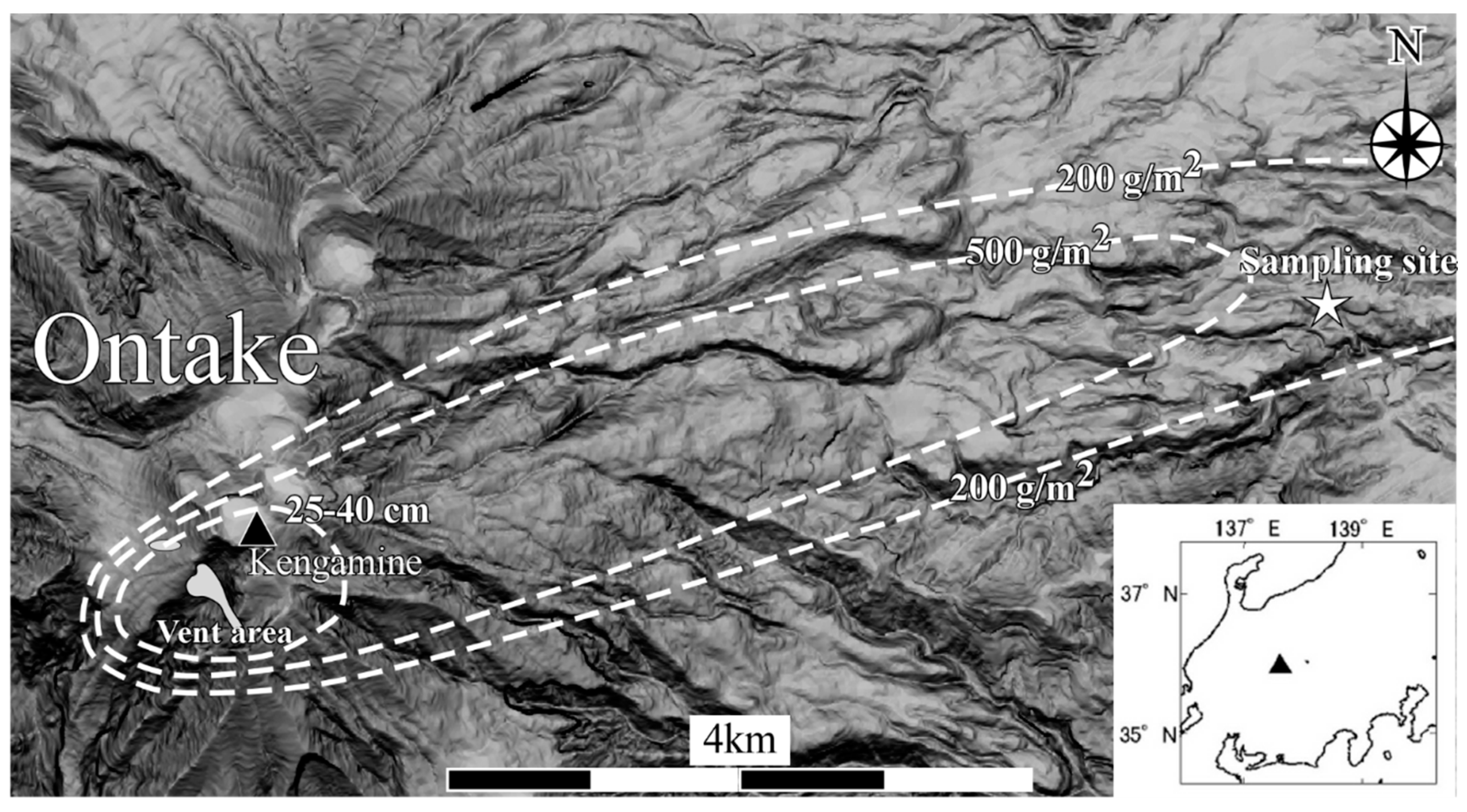
2.2. Volcanic Ash from the 2014 Hydrothermal Eruption
3. Methodology
4. Results
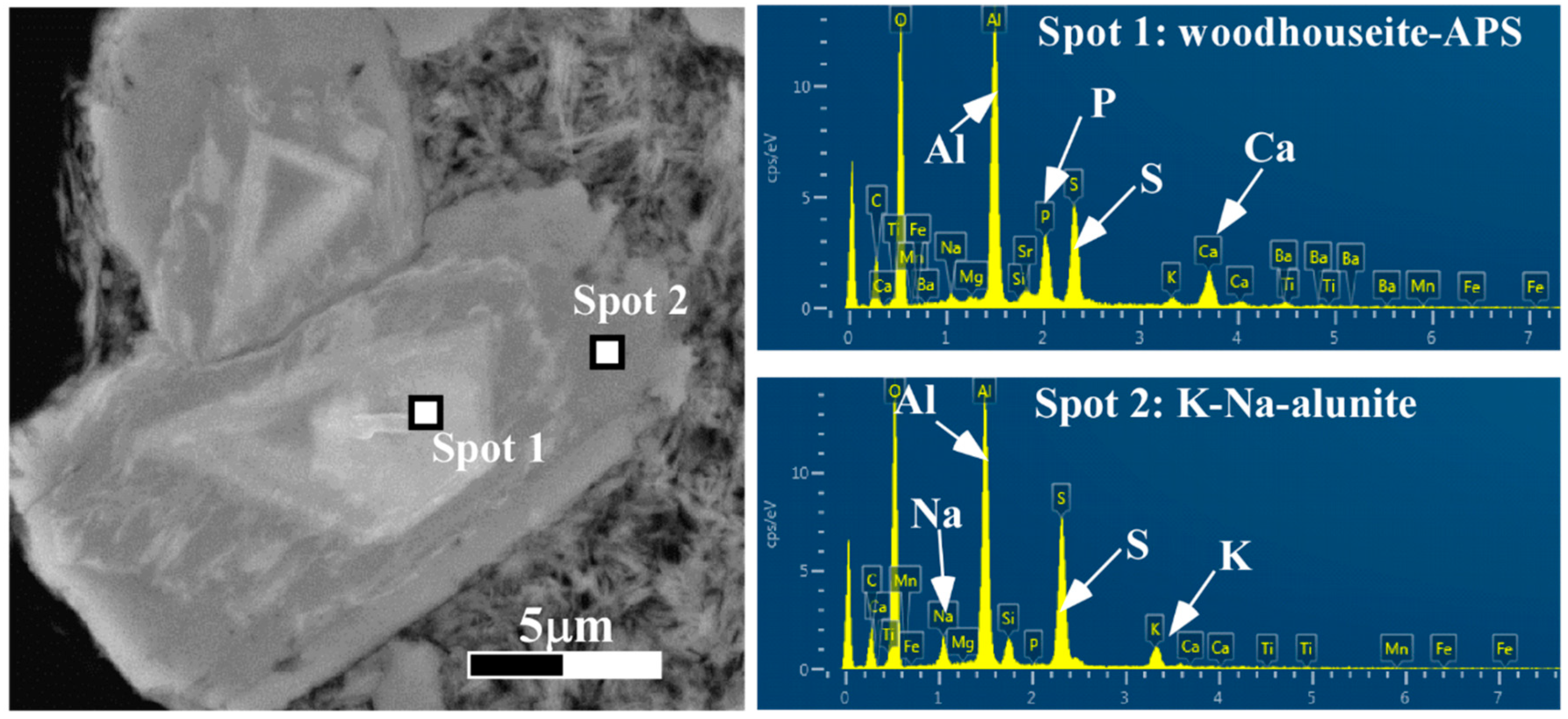
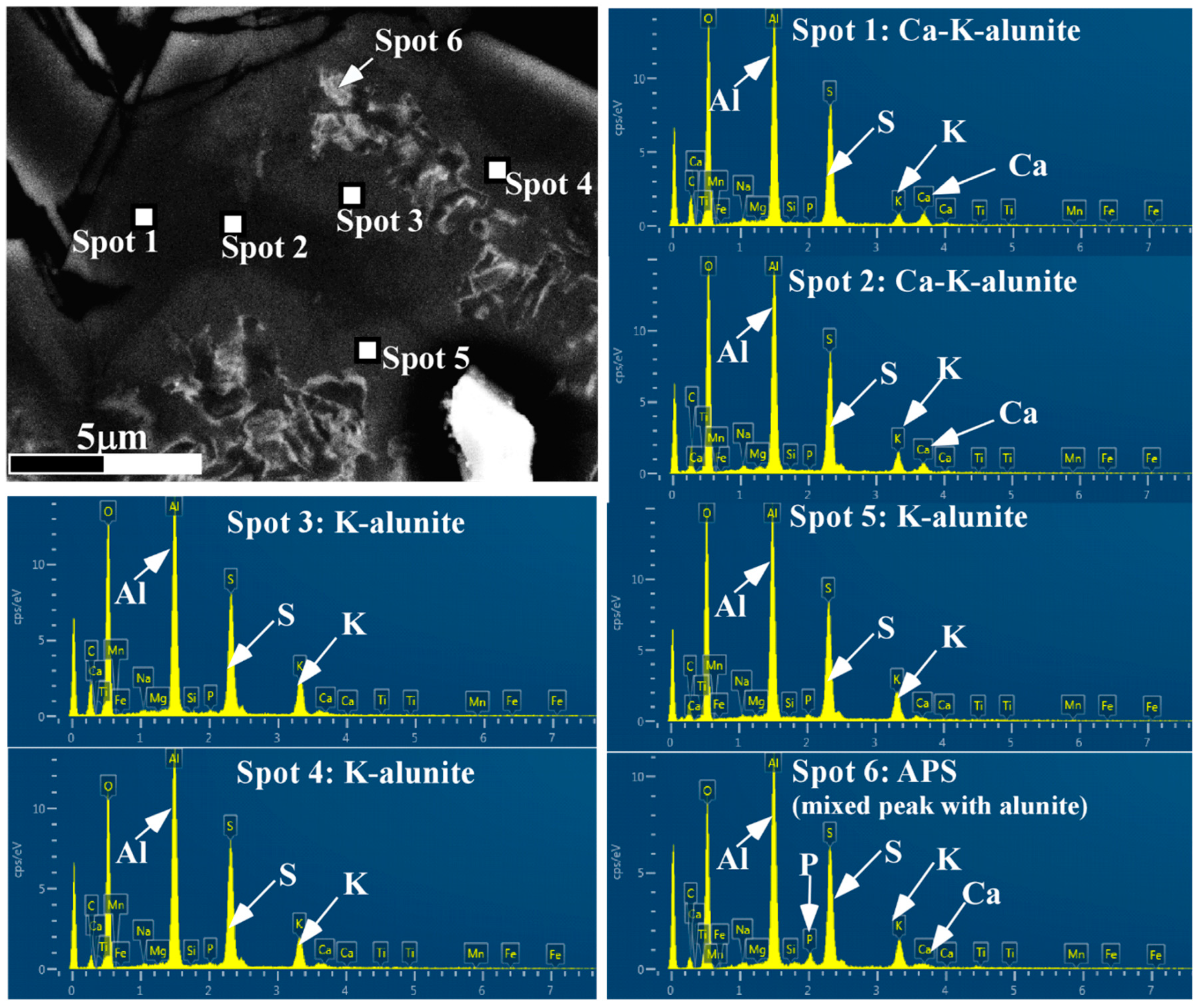
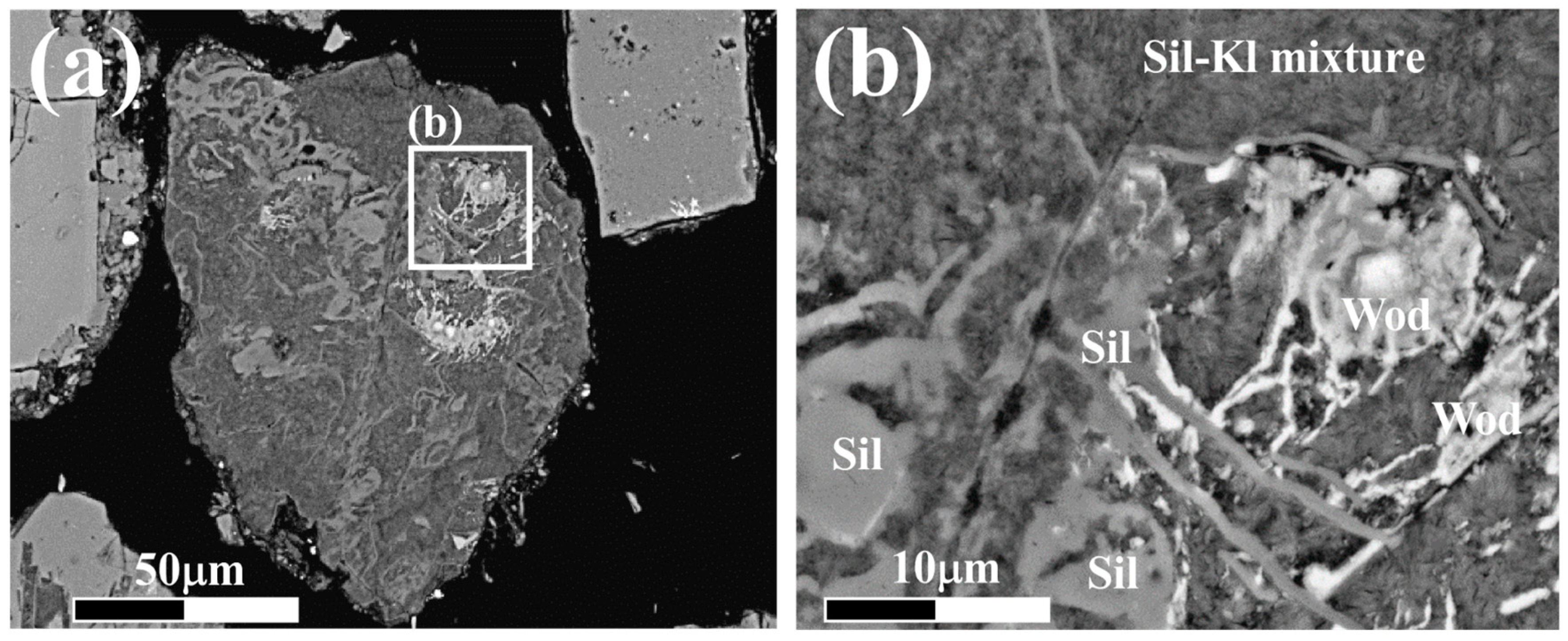
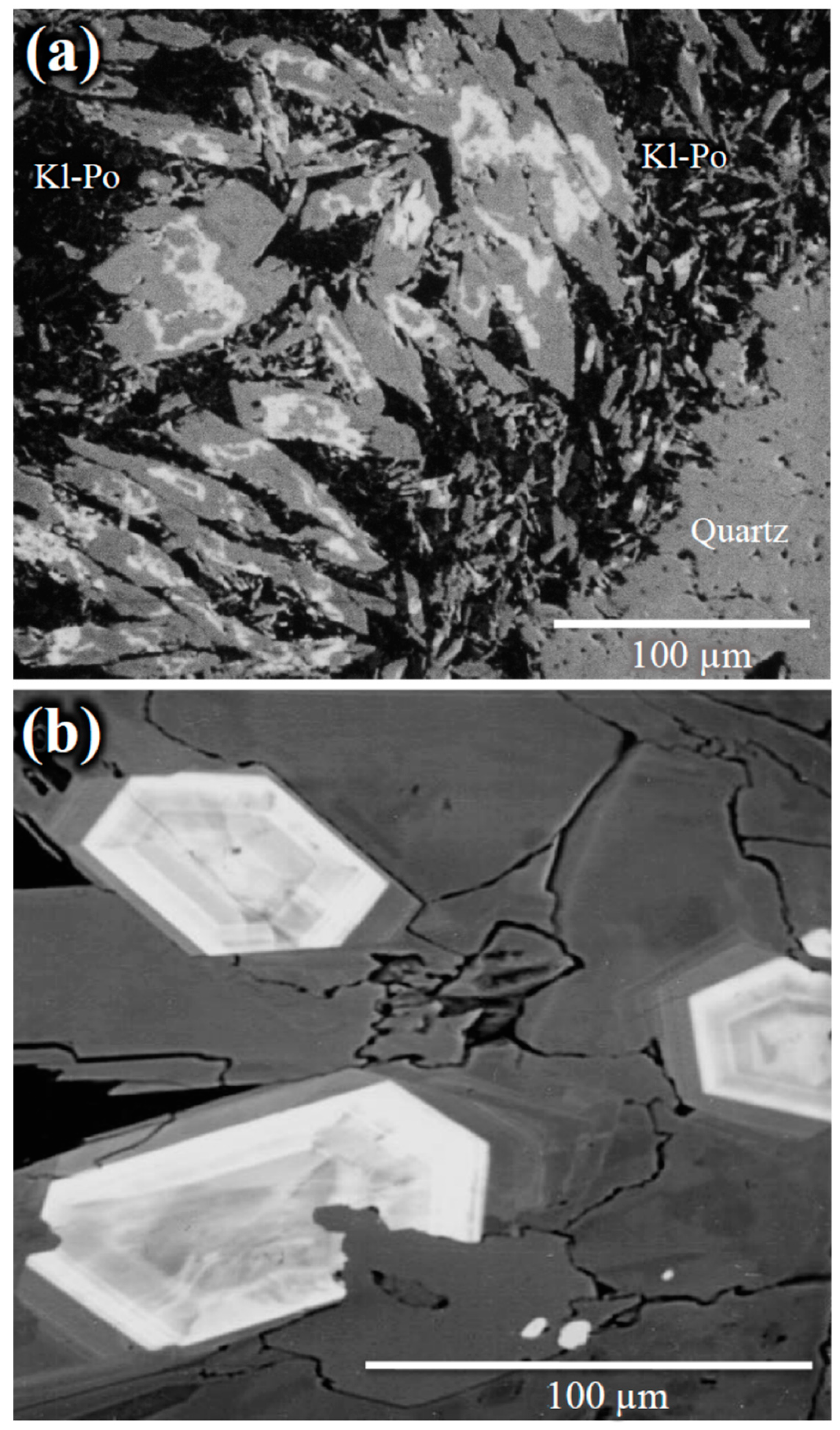
4.1. Mineral Identification
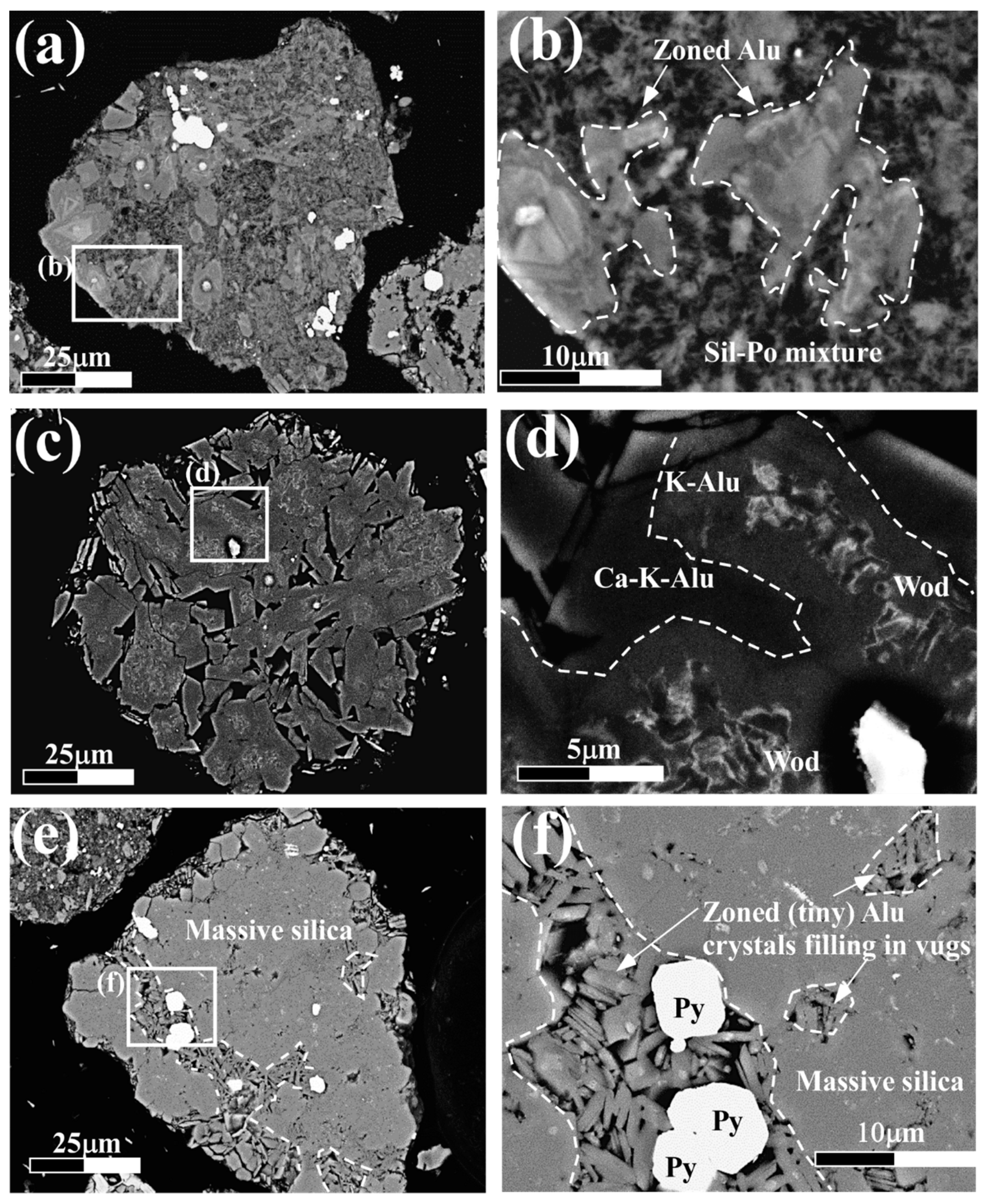
4.2. Petrography of Woodhouseite-APS-Bearing Volcanic Ash Grains
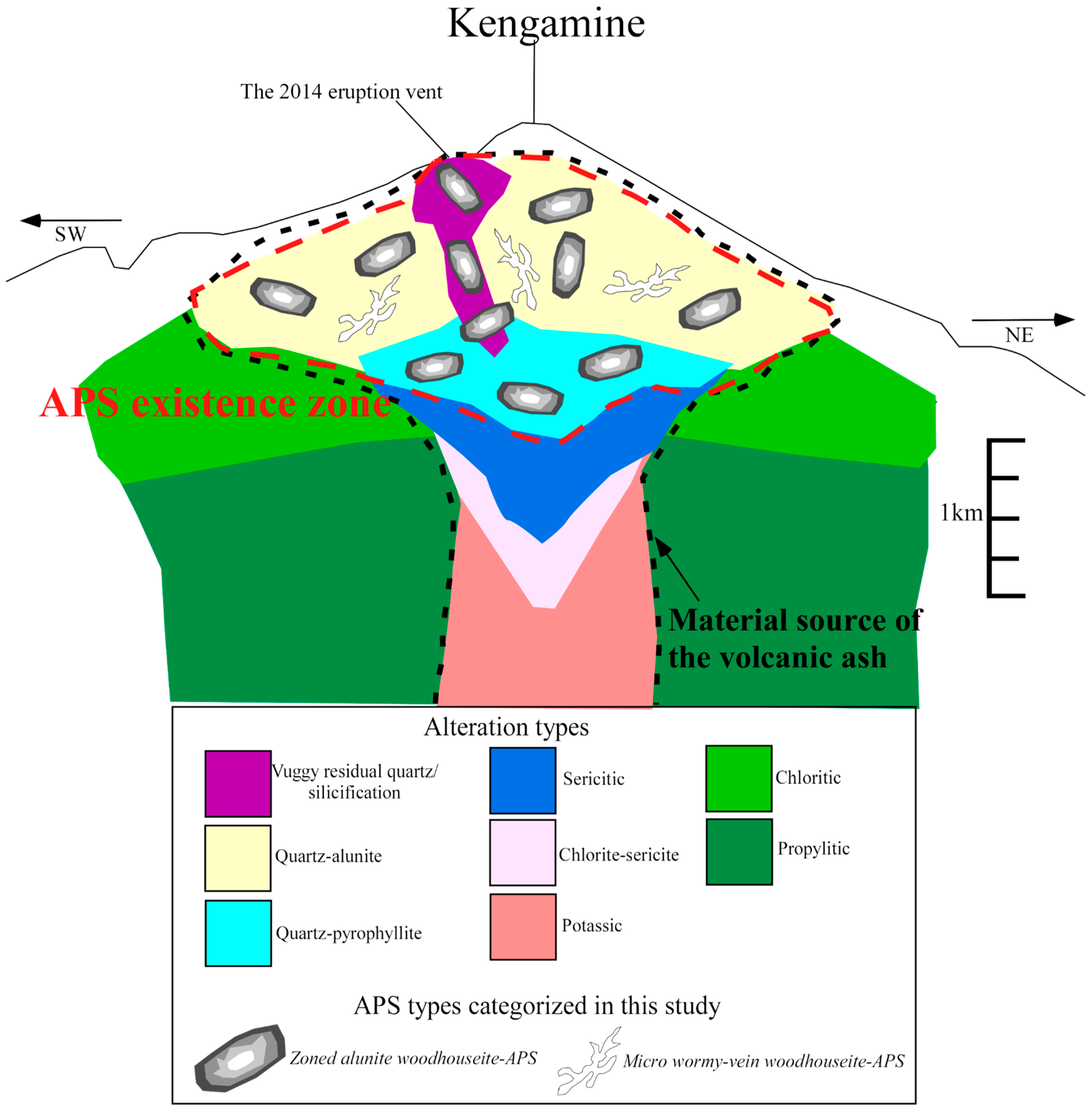
5. Discussion
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mindat.org. Nickel-Strunz Classification-Primary Groups 10th Edition. 2019. Available online: https://www.mindat.org/strunz.php (accessed on 29 July 2019).
- Scott, K.M. Solid solution in, and classification of gossan-derived members of the alunite―Jarosite family, Northwest Queensland, Australia. Am. Mineral. 1987, 72, 178–187. [Google Scholar]
- Scott, K.M. The mineralogical distribution of pathfinder elements in gossans derived from dolomitic shale-hosted Pb-Zn deposits, Northwest Queensland, Australia. Chem. Geol. 1987, 64, 295–306. [Google Scholar] [CrossRef]
- Stoffregen, R.E.; Alpers, C.N. Woodhouseite and svanbergite in hydrothermal ore deposits: products of apatite destruction during advanced argillic alteration. Can. Mineral. 1987, 25, 201–211. [Google Scholar]
- Smith, D.K.; Roberts, A.C.; Bayliss, P.; Liebau, F. A systematic approach to general and structure-type formulas for minerals and other inorganic phases. Am Mineral. 1998, 83, 126–132. [Google Scholar] [CrossRef]
- Mills, S.J.; Hatert, F.; Nickel, E.H.; Ferraris, G. The standardisation of mineralgroup hierarchies: Application to recent nomenclature proposals. Eur. J. Miner. 2009, 21, 1073–1080. [Google Scholar] [CrossRef]
- Bayliss, P.; Kolitsch, U.; Pring, A. Alunite supergroup: Recommended nomenclature. Miner. Mag. 2010, 74, 919–927. [Google Scholar] [CrossRef]
- Dill, H.G. The geology of aluminum phosphate and sulphates of the alunite group minerals: A review. Earth-Sci. Rev. 2001, 53, 35–93. [Google Scholar] [CrossRef]
- Heald, P.; Foley, N.K.; Hyaba, D.O. Comparative anatomy of volcanic-hosted epithermal deposits: Acid-sulphate and adularia-sulfate types. Econ. Geol. 1987, 82, 11–26. [Google Scholar] [CrossRef]
- Stoffregen, R.E.; Cygan, G.L. An experimental study of Na-K exchange between alunite and aqueous sulfate solutions. Am. Mineral. 1990, 75, 209–220. [Google Scholar]
- Stoffregen, R.E.; Rye, R.O.; Wasserman, M.D. Experimental studies of aluntie: I 18O-16O and DH fractionation factors between alunite and water at 250–450 °C. Geochim. Cosmochim. Acta 1994, 58, 903–916. [Google Scholar] [CrossRef]
- Beaufort, D.; Patrier, P.; Laverret, E.; Bruneton, P.; Mondy, J. Clay alteration associated with proterozoic unconformity-type uranium deposits in the East Alligator Rivers Uranium Field, Northern Territory, Australia. Econ. Geol. 2005, 100, 515–536. [Google Scholar] [CrossRef]
- Gaboreau, S.; Beaufort, D.; Vieillard, P.; Partrier, P. Aluminum phosphate-sulfate minerals associated with Proterozoic unconformity-type uranium deposits in the East Alligator River Uranium Field, Northern Territories, Australia. Can. Mineral. 2005, 43, 813–827. [Google Scholar] [CrossRef]
- Gaboreau, S.; Cuney, M.; Quirt, D.; Beaufort, D.; Patrier, P.; Mathieu, D. Significance of aluminum phosphate-sulfate minerals associated with U unconformity-type deposits: The Athabasca basin, Canada. Am. Mineral. 2007, 92, 267–280. [Google Scholar] [CrossRef]
- Aoki, M.; Comsti, E.C.; Lazo, F.B. Advanced argillic alteration and geochemistry of alunite in an evolving hydrothermal system at Baguio, Northern Luzon, Philippines. Resour. Geol. 1993, 43, 155–164. [Google Scholar]
- Arribas, A., Jr.; Cunningham, C.G.; Rytuba, J.J.; Rye, R.O.; Kelly, W.C.; Podwysocki, M.H.; McKee, E.H.; Tosdal, R.M. Geology, geochemistry, fluid inclusions and isotope geochemistry of the Rodalquilar gold alunite deposit, Spain. Econ. Geol. 1995, 90, 795–822. [Google Scholar] [CrossRef]
- Hedenquist, J.W.; Matsuhisa, Y.; Izawa, E.; White, N.C.; Giggenbach, W.F.; Aoki, M. Geology, geochemistry, and origin of high sulphidation Cu-Au mineralization in the Nansatsu District, Japan. Econ. Geol. 1994, 89, 1–30. [Google Scholar] [CrossRef]
- Matsubara, S.; Matsuyama, F.; Kiyota, K.; Kato, A. Huangite from okumanza, gunnma prefecture, Japan. Mineral. Mag. 1998, 20, 123–135. [Google Scholar]
- Dill, H.G.; Fricke, A.; Hening, K.-H. The origin of Ba- and REE-bearing aluminium-phosphate-sulphate minerals from the Lohrheim kaolinitic clay deposit (Rheinisches Schiefergebirge, Germany). Appl. Clay. Sci. 1995, 10, 231–245. [Google Scholar] [CrossRef]
- Dill, H.G.; Bosse, H.-R.; Henning, K.-H.; Fricke, A.; Ahrend, H. Mineralogical and chemical variations in hypogene and supergene kaolin deposits in a mobile fold belt—The Central Andes of northwestern Peru. Miner. Depos. 1997, 32, 149–163. [Google Scholar] [CrossRef]
- Dill, H.G.; Bosse, H.-R.; Kassbohm, J. Mineralogical and chemical studies of volcanic-related argillaceous industrial minerals of the Central American Cordillera (western El Salvador). Econ. Geol. 2000, 95, 517–538. [Google Scholar] [CrossRef]
- Ando, Y.; Tsutsumi, S. Acid alteration and geochemistry of alunite in the western Izu Peninsula, Shizuoka Prefecture. Jpn. Mag. Miner. Petrol. Sci. 2005, 34, 59–68, (In Japanese with English abstract). [Google Scholar]
- Hedenquist, J.W.; Arribas, A., Jr.; Aoki, M. Zonation of sulfate and sulfide minerals and isotopic composition inc the Far Southeast Porphyry and Lepanto epithermal Cu-Au deposits, Philippines. Resour. Geol. 2017, 67, 174–196. [Google Scholar] [CrossRef]
- Stoppa, F.; Scordari, F.; Mesto, E.; Sharygin, V.V.; Bortolozzi, G. Calcium-aluminum-silicate-hydrate “cement” phases and rare Ca-zeolite association at Colle Fabbri, Central Italy. Open Geosci. 2010, 2, 175–187. [Google Scholar] [CrossRef]
- Stoppa, F.; Schiazza, M. Extreme chemical conditions of crystallization of Umbrian Melilitolites and wealth of rare, late stage/hydrothermal minerals. Cent. Eur. J. Geosci. 2014, 6, 549–564. [Google Scholar] [CrossRef]
- Takano, B.; Watanuki, K. Monitoring of volcanic eruptions at Yugama crater lake by aqueous sulphur oxyanions. J. Volcanol. Geotherm. Res. 1990, 40, 71–87. [Google Scholar] [CrossRef]
- Tomita, K.; Kawano, M.; Kobayashi, T. Minerals in the volcanic ash erupted from Shin-dake in Kuchinoerabu Island in 1980—Report of the Faculty of Science, Kagoshima University. Earth Sci. Biol. 1994, 27, 1–10. [Google Scholar]
- Ohba, T.; Kitade, Y. Subvolcanic hydrothermal systems: Implications from hydrothermal minerals in hydrovolcanic ash. J. Volcanol. Geotherm. Res. 2005, 145, 249–262. [Google Scholar] [CrossRef]
- Minami, Y.; Imura, T.; Hayashi, S.; Ohba, T. Mineralogical study on volcanic ash of the eruption on September 27, 2014 at Ontake volcano, central Japan: Correlation with porphyry copper systems. Earth Planets Space 2016, 68, 67. [Google Scholar] [CrossRef]
- Sugimoto, T. Kashimir 3D Ver 9.2.8 23819 (9.2.8). Available online: http://www.kashmir3d.com/ (accessed on 3 July 2018).
- Earthquake Research Institute at the University of Tokyo (ERI): Eruption of the Ontakesan. 27 September 2014. Available online: http://www.eri.u-tokyo.ac.jp/en/2014/09/30/eruption-of-the-ontakesan-27th-september-2014/ (accessed on 6 July 2018).
- Yamada, N.; Kobayashi, T. Geology of the Ontakesan District. Geological Sheet Map at 1:50,000; Geological Survey of Japan: Tsukuba, Japan, 1988; (In Japanese with English abstract). [Google Scholar]
- Takeuchi, M.; Nakano, S.; Harayama, S.; Otuska, T. Geology of the Kiso-Fukushima District. With Geological Sheet Map at 1:50,000; Geological Survey of Japan: Tsukuba, Japan, 1998; (In Japanese with English abstract). [Google Scholar]
- Matsumoto, A.; Kobayashi, T. K-Ar age determination of late Quaternary volcanic rocks using the “mass fractionation correction procedure”: Application to the Younger Ontake Volcano, central Japan. Chem. Geol. 1995, 125, 123–135, (In Japanese with English abstract). [Google Scholar] [CrossRef]
- Matsumoto, A.; Kobayashi, T. K-Ar ages of the older Ontake volcanic products, Ontake volcano, central Japan: Reappraisal of the volcanic history based on the radiometric data. Bull. Volcanol. Soc. Japan 1999, 44, 1–12, (In Japanese with English abstract). [Google Scholar]
- Kioka, H.; Furuyama, K.; Miyake, Y.; Sakai, I.; Nagao, K.; Ikemoto, M.; Noiri, H.; Oda, K. K-Ar chronology of the Middle Pleistocene lavas at Ontake Volcano, central Japan. Earth. Sci. (Chikyu Kagaku) 1998, 52, 464–474. [Google Scholar]
- Oikawa, T.; Suzuki, Y.; Chiba, T. Eruptions of Ontake-san: History and 2014 eruption. Kagaku 2014, 84, 1218–1225. (In Japanese) [Google Scholar]
- Oikawa, T.; Yamaoka, K.; Yoshimoto, M.; Nakada, S.; Takeshita, Y.; Maeno, F.; Ishizuka, Y.; Komori, J.; Shimano, T.; Nakano, S. The 2014 eruption of Ontake volcano, Central Japan. Bull. Volcanol. Soc. Japan 2015, 60, 411–415, (In Japanese with English abstract). [Google Scholar]
- Japan Meteorological Agency (JMA). Ontake volcano. Bull. Volcanol. Soc. Japan 1991, 36, 385. (In Japanese) [Google Scholar]
- Nakamichi, H.; Kumagai, H.; Nakano, M.; Okubo, M.; Kimata, F.; Ito, Y.; Obara, K. Source mechanism of very-long-period event at Mt. Ontake, central Japan: Response of a hydrothermal system to magma intrusion beneath the summit. J. Volcanol. Geotherm. Res. 2009, 187, 167–177. [Google Scholar] [CrossRef]
- Oikawa, T. Reinvestigation of the historical eruption and fumarolic activity records at Ontake Volcano, central Japan—Misunderstanding reports about the 774 AD and 1892 AD eruptions. Bull. Geol. Surv. Japan 2008, 59, 203–210. [Google Scholar] [CrossRef]
- Takarada, S.; Oikawa, T.; Furukawa, R.; Hoshizumi, H.; Itoh, J.; Geshi, N.; Miyagi, I. Estimation of total discharged mass from the phreatic eruption of Ontake Volcano, central Japan, on September 27, 2014. Earth Planets Space 2016, 68, 138. [Google Scholar] [CrossRef]
- Japan Meteorological Agency (JMA)—Report of Coordinating Committee for Prediction of Volcanic Eruption. 2014. Available online: http://www.data.jma.go.jp/svd/vois/data/tokyo/STOCK/kaisetsu/CCPVE/shiryo/130/130_no01.pdf (accessed on 6 July 2018). (In Japanese).
- Maeno, F.; Nakada, S.; Oikawa, T.; Yoshimoto, M.; Komori, J.; Ishizuka, Y.; Takeshita, Y.; Shimano, T.; Kaneko, T.; Nagai, M. Reconstruction of a phreatic eruption on 27 September 2014 at Ontake volcano, central Japan, based on proximal pyroclastic density current and fallout deposits. Earth Planets Space 2016, 68, 82. [Google Scholar] [CrossRef]
- Oikawa, T.; Yoshimoto, M.; Nakada, S.; Maeno, F.; Komori, J.; Shimano, T.; Takeshita, Y.; Ishizuka, Y.; Ishimine, Y. Reconstruction of the 2014 eruption sequence of Ontake Volcano from recorded images and interviews. Earth Planets Space 2016, 68, 79. [Google Scholar] [CrossRef]
- Kato, A.; Terakawa, T.; Yamanaka, Y.; Maeda, Y.; Horikawa, S.; Matsuhiro, K.; Okuda, T. Preparatory and precursory processes leadingup to the 2014 phreatic eruption of Mount Ontake, Japan. Earth Planets Space 2015, 67, 111. [Google Scholar] [CrossRef]
- Maeda, Y.; Kato, A.; Terakawa, T.; Yamanaka, Y.; Horikawa, Y.; Matsuhiro, K.; Okuda, T. Source mechanism of a VLP event immediately before the 2014 eruption of Mt. Ontake, Japan. Earth Planets Space 2015, 67, 187. [Google Scholar] [CrossRef]
- Ogiso, M.; Matsubayashi, H.; Yamamoto, T. Descent of tremor source locations before the 2014 phreatic eruption of Ontake volcano, Japan. Earth Planets Space 2015, 67, 206. [Google Scholar] [CrossRef]
- Miyagi, I.; Geshi, N.; Hamasaki, S.; Tomiya, A. Volcanic ash particles from Ontake volcano on September 2014. In Emergency academic session in the Volcanological Society of Japan 2014 fall meeting, Supplement: Emergency Academic Session; Fukuoka University: Fukuoka, Japan, 2014; pp. 2–4. [Google Scholar]
- Sillitoe, R.H. Porphyry copper systems. Econ. Geol. 2010, 105, 3–41. [Google Scholar] [CrossRef]
- Ikehata, K.; Maruoka, T. Sulfur isotopic characteristics of volcanic products from September 2014 Mount Ontake eruption, Japan. Earth Planets Space 2016, 68, 116. [Google Scholar] [CrossRef]
- Browne, P.R.L.; Lawless, J.V. Characteristics of hydrothermal eruptions, with examples from New Zealand and elsewhere. Earth-Sci. Rev. 2001, 52, 299–331. [Google Scholar] [CrossRef]
- Gustafson, L.B.; Vidal, C.E.; Pinto, R.; Noble, D.C. Porphyry-epithermal transition, Cajamarca region, Northern Peru. Soc. Eco. Geo. Spc. Pub. 2004, 11, 279–299. [Google Scholar]
- Hedenquist, J.W.; Richard, W.H. Hydrothermal eruptions in the Waiotapu geothermal system, New Zealand: Their origin, associated breccias, and relation to previous metal mineralization. Econ. Geol. 1985, 80, 1640–1668. [Google Scholar] [CrossRef]
- Ohba, T.; Taniguchi, H.; Miyamoto, T.; Hayashi, S.; Hasenaka, T. Mud plumbing system of an isolated phreatic eruption at Akita Yakeyama volcano, northern Honshu, Japan. J. Volcanol. Geotherm. Res. 2007, 161, 35–46. [Google Scholar] [CrossRef]
- John, D.A.; Sisson, T.W.; Breit, G.N.; Rye, R.O.; Vallence, J.W. Characteristics, extent and origin of hydrothermal alteration at Mount Rainier Volcano, Cascades Arc, USA: Implications for debris-flow hazards and mineral deposits. J. Volcanol. Geotherm. Res. 2007, 161, 35–46. [Google Scholar] [CrossRef]
- Browne, P.R.L. Hydrothermal alteration in active geothermal fields. Annu. Rev. Earth. Pl. Sc. 1978, 6, 229–250. [Google Scholar] [CrossRef]
- Hayashi, M. Hydrothermal alteration in the Otake geothermal area, Kyushu. J. Jpn. Geoth. Energy Assoc. 1973, 10, 9–46, (In Japanese with English abstract). [Google Scholar]
- Arribas, A., Jr. Characteristics of high sulfidation deposits, and their relation to magmatic fluid. Mineral. Soc. Can. 1995, 23, 419–454. [Google Scholar]
- Hedenquist, J.W.; Arribas, A., Jr.; Reynolds, T.J. Evolution of an intrusion-centered hydrothermal system: Far southeast-Lepanto porphyry and epithermal Cu-Au deposits, Philippines. Econ. Geol. 1998, 93, 373–404. [Google Scholar] [CrossRef]
- Hedenquist, J.W.; Lowenstern, J.B. The role of magmas in the formation of hydrothermal ore deposits. Nature 1994, 370, 519–527. [Google Scholar] [CrossRef]

| APS Endmembers | Alunite Subgroup Endmembers | ||
|---|---|---|---|
| Svanbergite | SrAl3(PO4)(SO4)(OH)6 | Alunite | KAl3(SO4)2(OH)6 |
| Woodhouseite | CaAl3(PO4)(SO4)(OH)6 | Huangite | Ca0.5Al3(SO4)2(OH)6 |
| Hinsdalite | PbAl3(PO4)(SO4)(OH)6 | Natroalunite | NaAl3(SO4)2(OH)6 |
| Goyazite | SrAl3(PO4)(PO3OH)(OH)6 | ||
| Crandallite | CaAl3(PO4)(PO3OH)(OH)6 | ||
| Gorceixide | BaAl3(PO4)(PO3OH)(OH)6 | ||
| Florencite | CeAl3(PO4)2(OH)6 | ||
| Alunite | KAl3(SO4)2(OH)6 | ||
| Huangite | Ca0.5Al3(SO4)2(OH)6 | ||
| Natroalunite | NaAl3(SO4)2(OH)6 | ||
| Ash Grain ID | Minerals in Ash Grains a | Alteration b | APS Type c | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Sil | Kl | Po | Alu | Wod | Py | Ti | |||
| ONTK-VA-001 | + | + | + | + | + | RS | ZA | ||
| ONTK-VA-002 | + | + | + | + | RS | ZA | |||
| ONTK-VA-003 | + | + | AA | n.d. | |||||
| ONTK-VA-004 | + | + | AA | ZA | |||||
| ONTK-VA-005 | + | + | + | + | + | AA | ZA | ||
| ONTK-VA-006 | + | + | + | AA-RS | n.d. | ||||
| ONTK-VA-007 | + | + | + | AA-RS | n.d. | ||||
| ONTK-VA-008 | + | + | + | AA-RS | ZA | ||||
| ONTK-VA-009 | + | + | + | + | AA-RS | MW | |||
| ONTK-VA-010 | + | + | AA | ZA | |||||
| ONTK-VA-011 | + | AA | ZA | ||||||
| ONTK-VA-012 | + | + | + | + | AA | ZA | |||
| ONTK-VA-013 | + | + | + | AA-RS | n.d. | ||||
| ONTK-VA-014 | + | + | + | + | RS | n.d. | |||
| ONTK-VA-015 | + | + | + | AA | MW | ||||
| ONTK-VA-016 | + | + | AA | n.d. | |||||
| ONTK-VA-017 | + | + | + | + | + | AA | ZA | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imura, T.; Minami, Y.; Ohba, T.; Matsumoto, A.; Arribas, A.; Nakagawa, M. Hydrothermal Aluminum-Phosphate-Sulfates in Ash from the 2014 Hydrothermal Eruption at Ontake Volcano, Central Honshu, Japan. Minerals 2019, 9, 462. https://doi.org/10.3390/min9080462
Imura T, Minami Y, Ohba T, Matsumoto A, Arribas A, Nakagawa M. Hydrothermal Aluminum-Phosphate-Sulfates in Ash from the 2014 Hydrothermal Eruption at Ontake Volcano, Central Honshu, Japan. Minerals. 2019; 9(8):462. https://doi.org/10.3390/min9080462
Chicago/Turabian StyleImura, Takumi, Yusuke Minami, Tsukasa Ohba, Akiko Matsumoto, Antonio Arribas, and Mitsuhiro Nakagawa. 2019. "Hydrothermal Aluminum-Phosphate-Sulfates in Ash from the 2014 Hydrothermal Eruption at Ontake Volcano, Central Honshu, Japan" Minerals 9, no. 8: 462. https://doi.org/10.3390/min9080462
APA StyleImura, T., Minami, Y., Ohba, T., Matsumoto, A., Arribas, A., & Nakagawa, M. (2019). Hydrothermal Aluminum-Phosphate-Sulfates in Ash from the 2014 Hydrothermal Eruption at Ontake Volcano, Central Honshu, Japan. Minerals, 9(8), 462. https://doi.org/10.3390/min9080462




