Constraints from Geochemistry and Field Relationships for the Origin of Kornerupine-Bearing Gneiss from the Grenvillian New Jersey Highlands and Implications for the Source of Boron
Abstract
1. Introduction
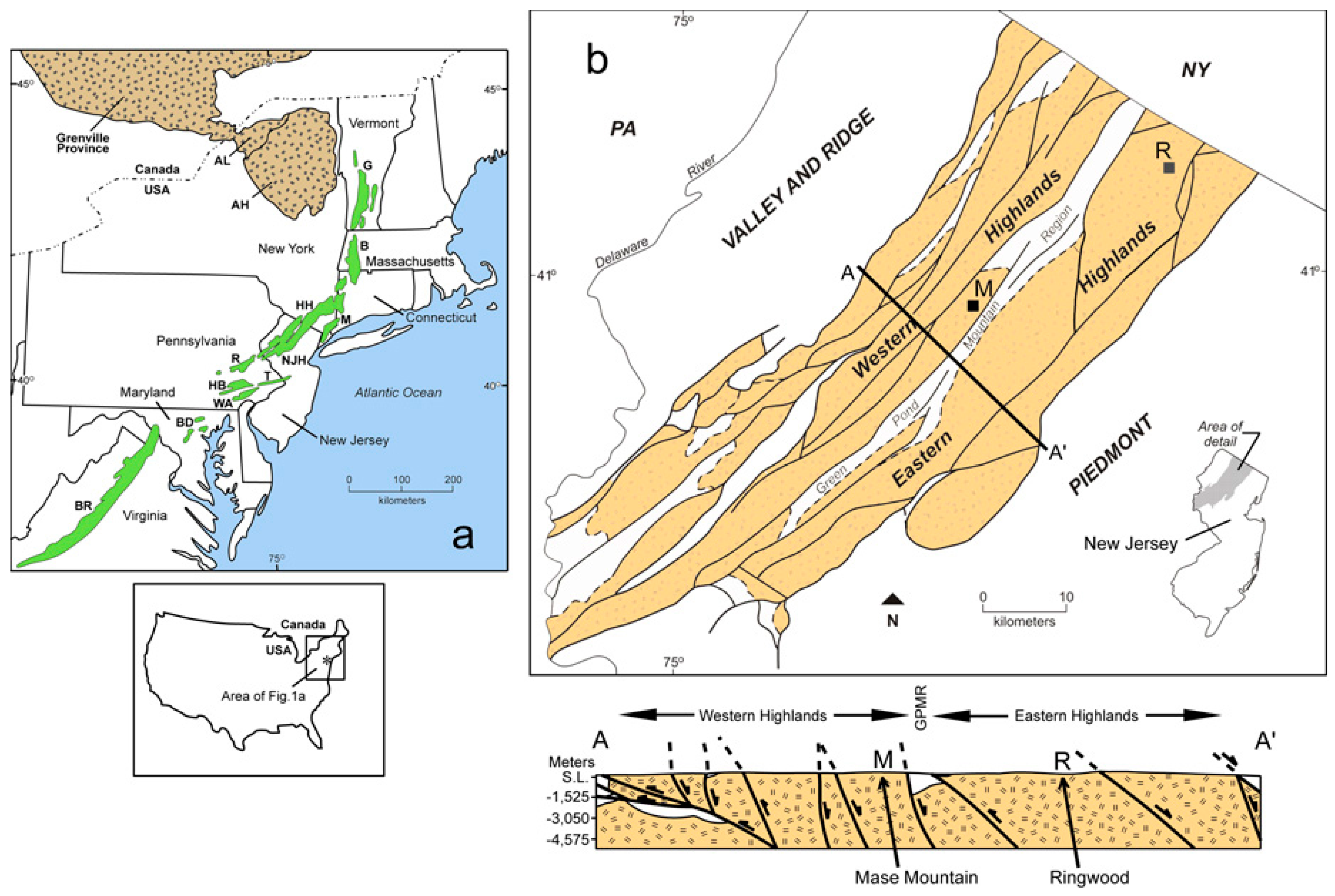
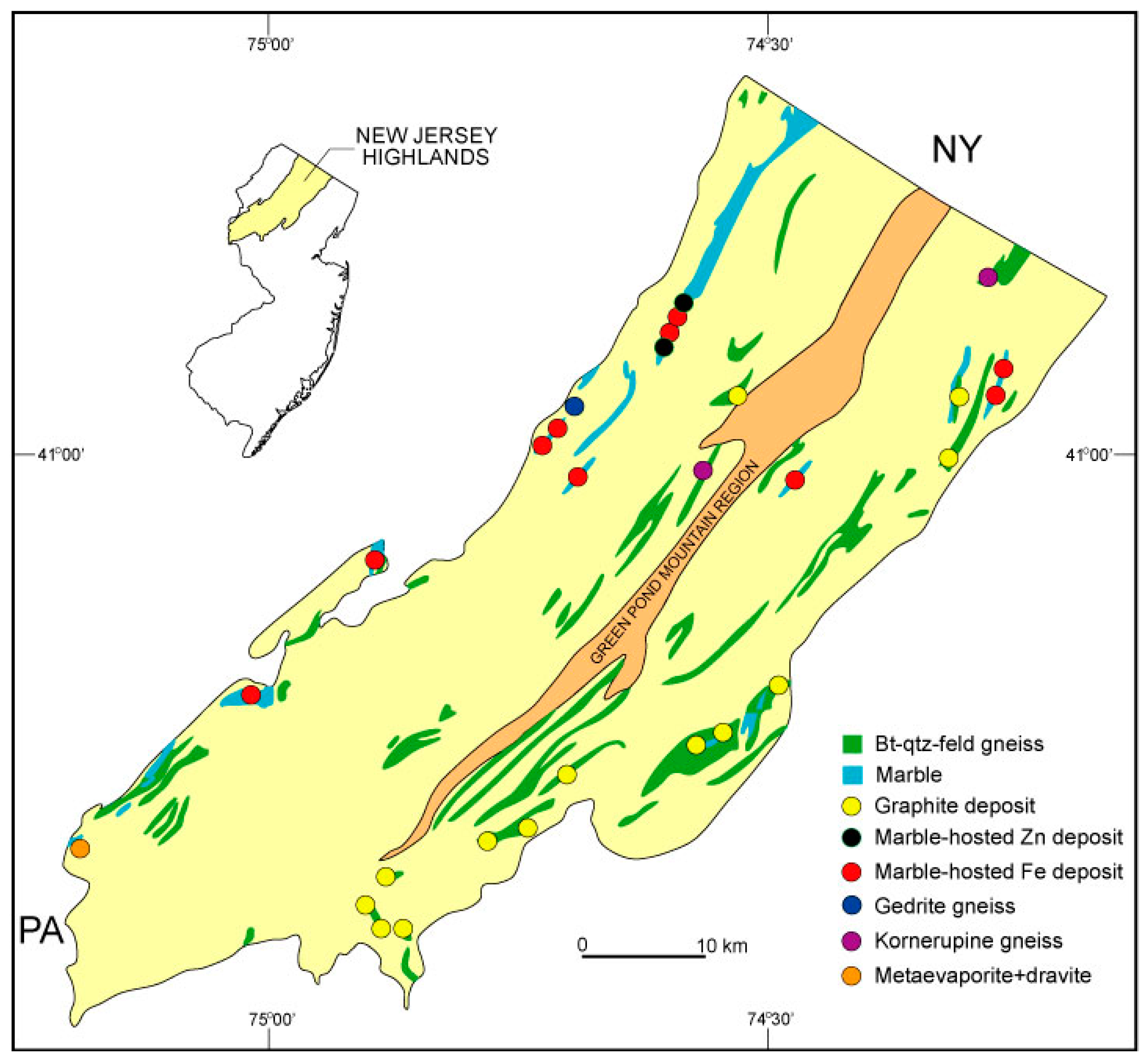
2. Geologic Setting
3. Ottawan Metamorphic Conditions
4. Kornerupine-Bearing Gneiss
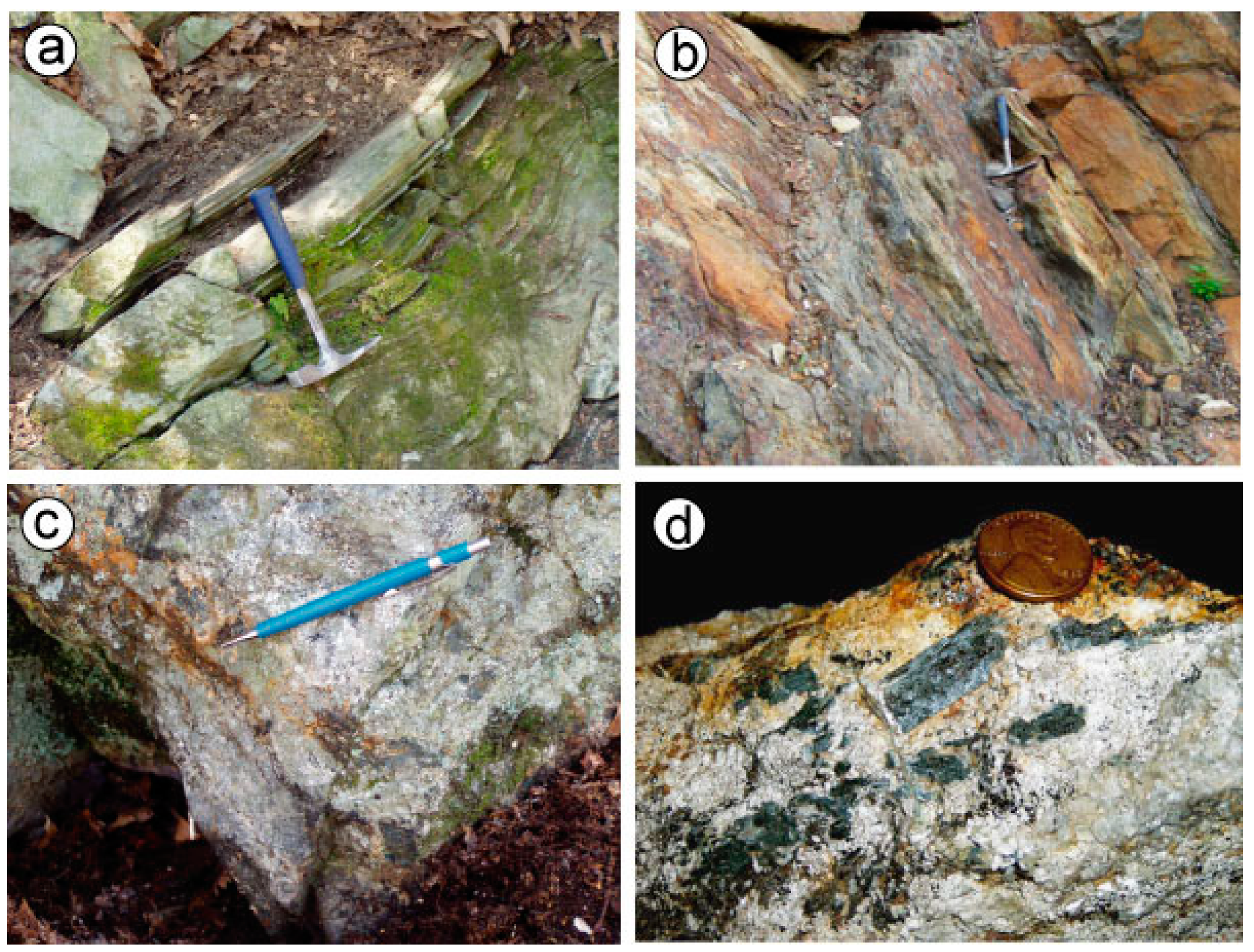
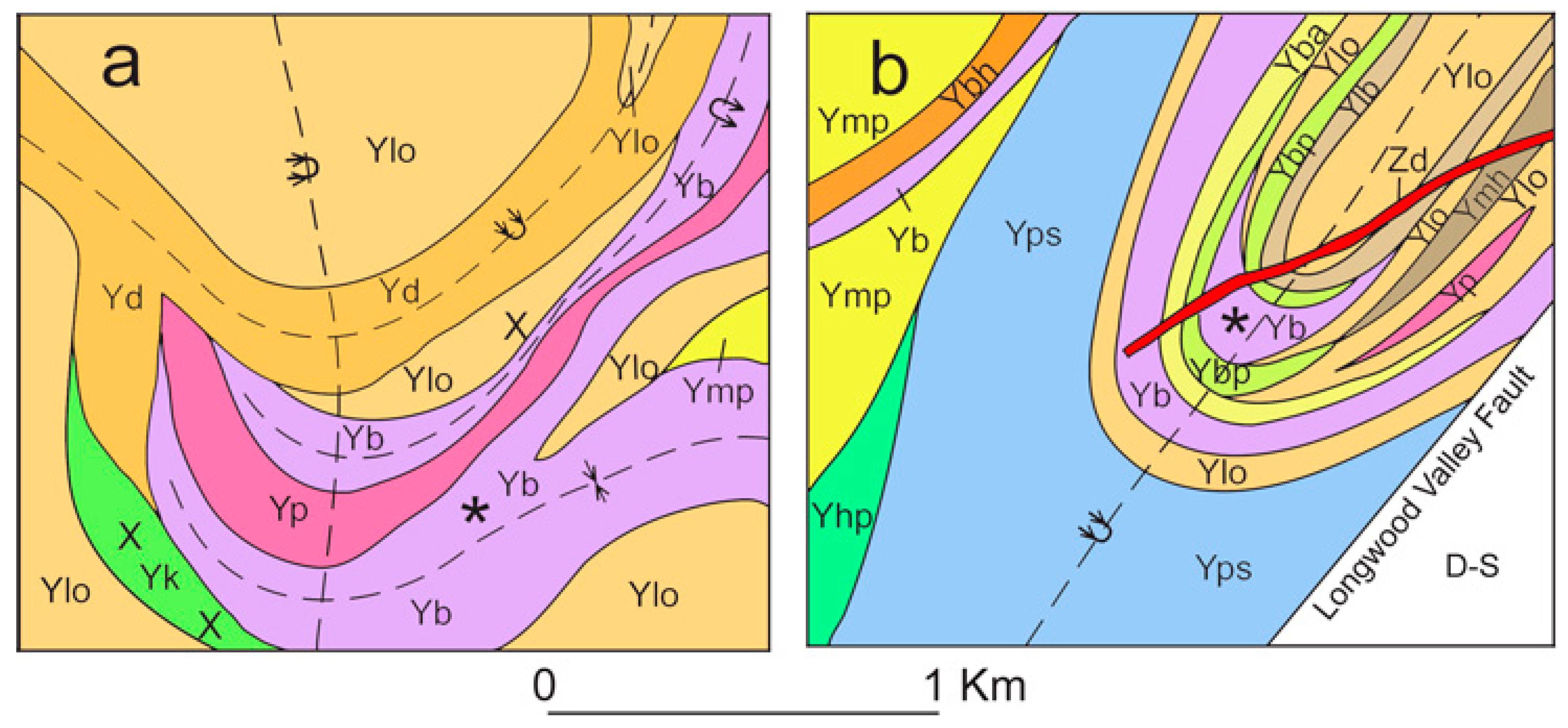
4.1. Field Relationships and Stratigraphy
4.1.1. Ringwood Location
4.1.2. Mase Mountain Location
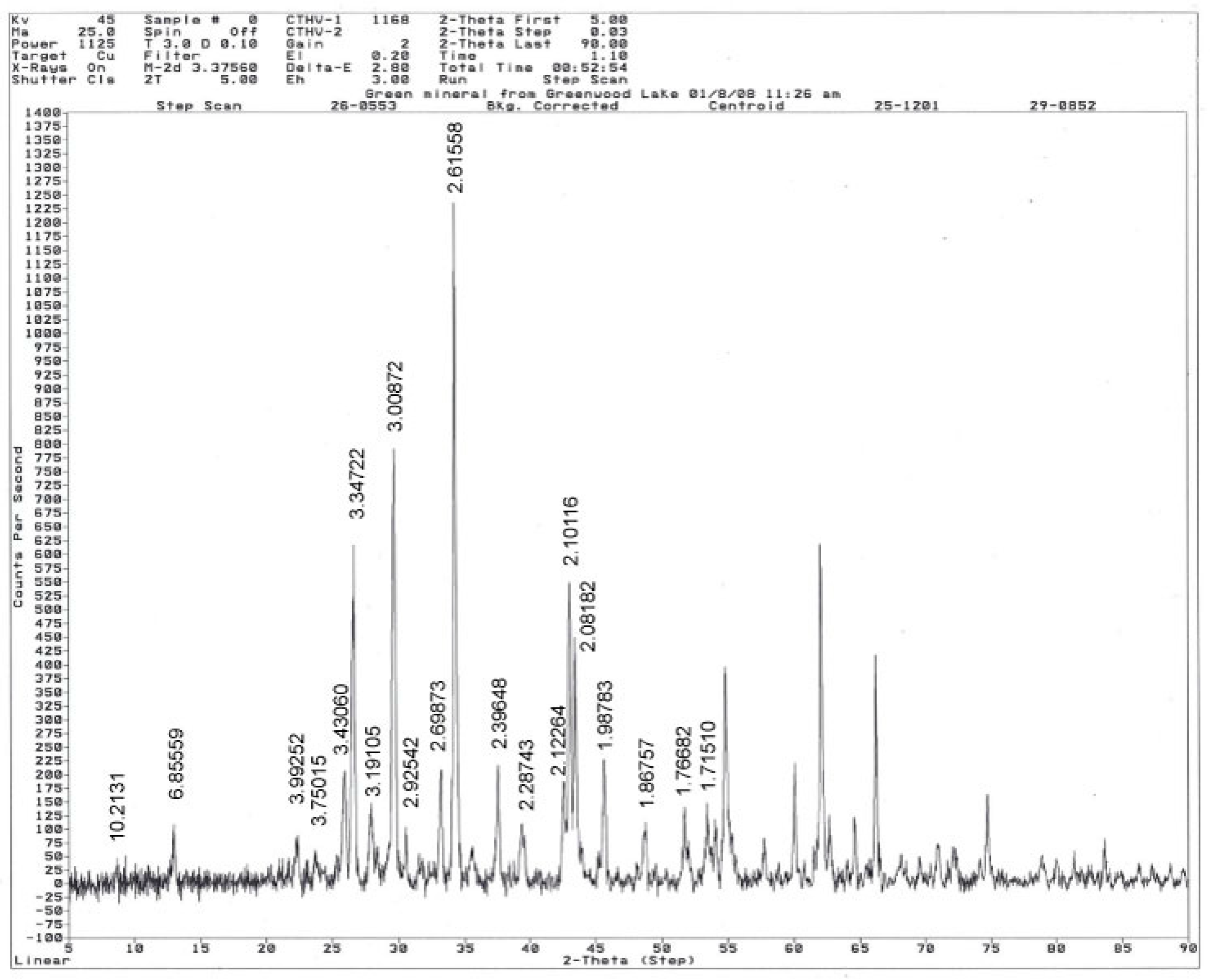
4.2. Mineralogy
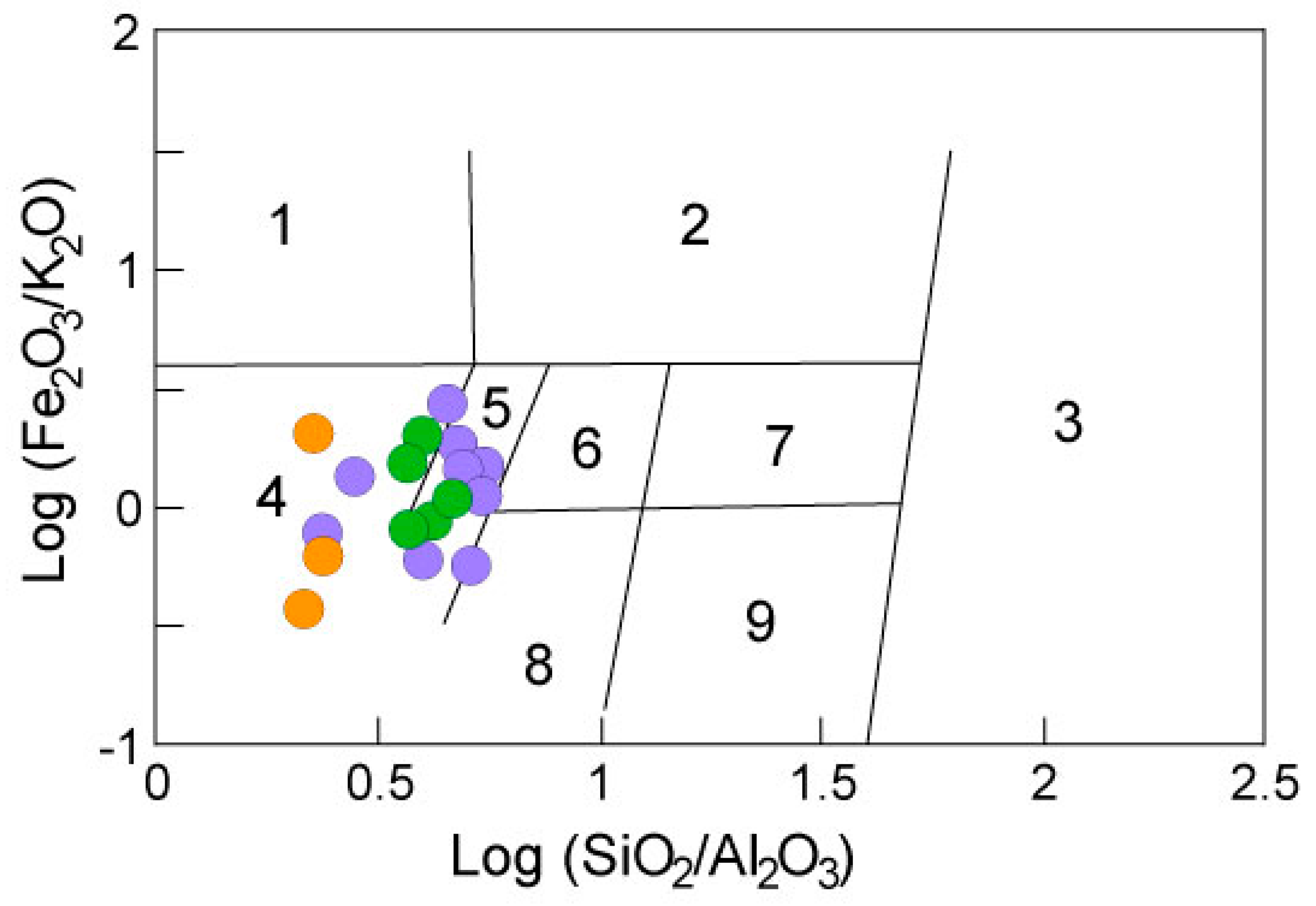
4.3. Protolith Geochemistry
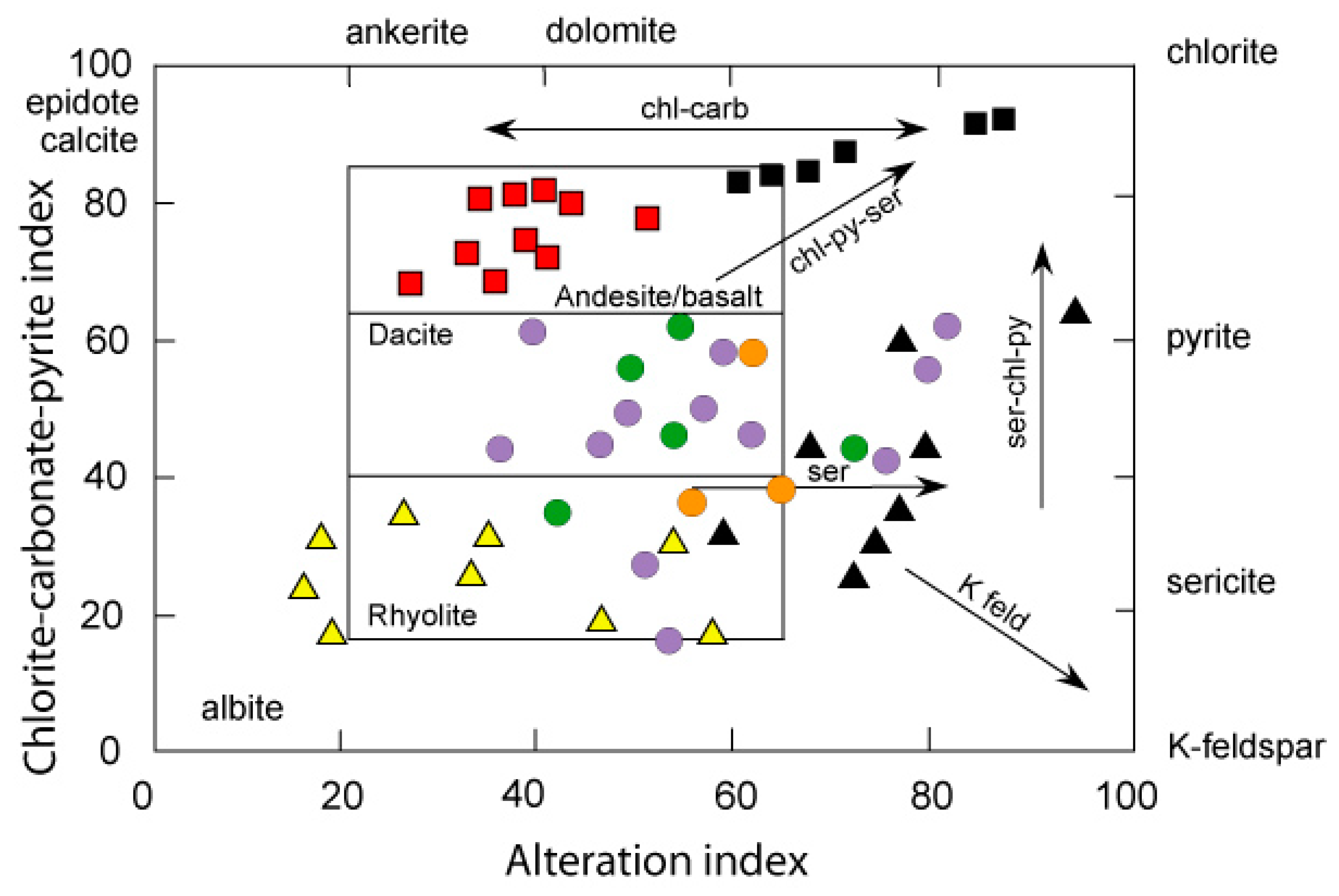
Weathering and Alteration
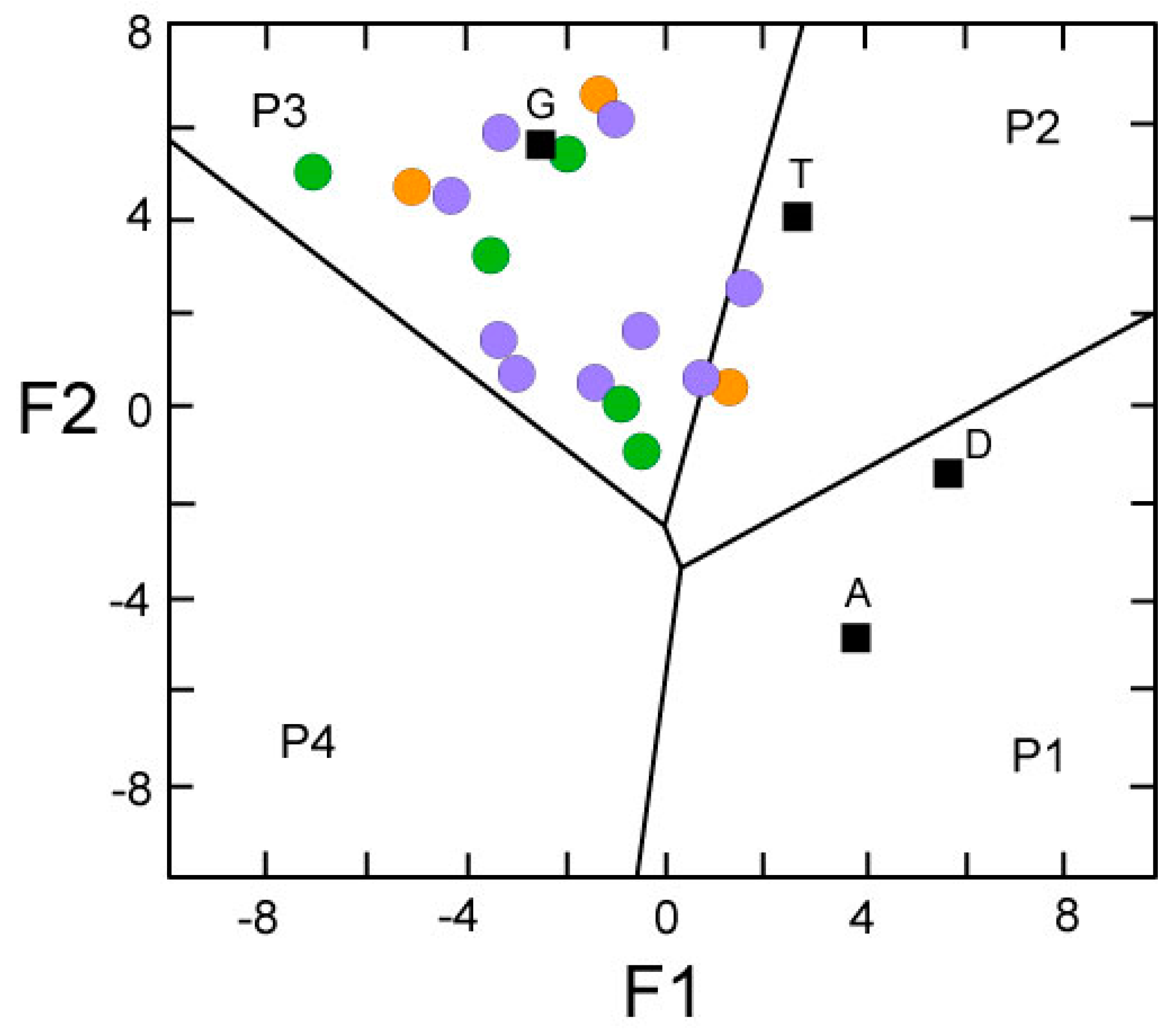
4.4. Provenance
4.5. Depositional Environment
5. Implications for the Source of Boron
5.1. Metamorphism of a Marine Evaporite Protolith
5.2. Replacement by B-Rich Hydrothermal Fluid
5.3. Breakdown of B-Rich Sediments in the Protoliths during Metamorphism
6. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Grew, E.S.; Cooper, M.A.; Hawthorne, F.C. Prismatine: Revalidation for boron-rich compositions in the kornerupine group. Miner. Mag. 1996, 60, 483–491. [Google Scholar] [CrossRef]
- Lonker, S.W. An occurrence of grandidierite, kornerupine, and tourmaline in southeastern Ontario, Canada. Contrib. Miner. Petrol. 1988, 98, 502–516. [Google Scholar] [CrossRef]
- Sharma, I.N.; Prakash, D. Occurrence of kornerupine-bearing granulite from Karimnagar, Andhra, Pradesh. Curr. Sci. India 2006, 91, 678–683. [Google Scholar]
- Warren, R.G.; McColl, D.H. Occurrences of boron-bearing kornerupine in the western Harts Range and near Mount Baldwin, Arunta Block, central Australia. J. Aust. Geol. Geophys. 1983, 8, 93–96. [Google Scholar]
- Ackermand, D.; Windley, B.F.; Razafiniparany, A.H. Kornerupine breakdown reactions in paragneisses from southern Madagascar. Miner. Mag. 1991, 55, 71–80. [Google Scholar] [CrossRef]
- Carson, C.J.; Hand, M.; Dirks, P.H.G.M. Stable coexistence of grandidierite and kornerupine during medium pressure granulite facies metamorphism. Miner. Mag. 1995, 59, 327–339. [Google Scholar] [CrossRef]
- Young, D.A. Kornerupine-group minerals in Grenville granulite-facies paragneiss, Reading Prong, New Jersey. Can. Miner. 1995, 33, 1255–1262. [Google Scholar]
- Drake, A.A., Jr.; Volkert, R.A.; Monteverde, D.H.; Herman, G.C.; Houghton, H.F.; Parker, R.A.; Dalton, R.F. Bedrock Geologic Map of New Jersey; Misc. Inves. Series Map I-2540-A; U.S. Geological Survey: Reston, VA, USA, 1996. [Google Scholar]
- Volkert, R.A.; Aleinikoff, J.N.; Fanning, C.M. Tectonic, magmatic, and metamorphic history of the New Jersey Highlands: New insights from SHRIMP U-Pb geochronology. In From Rodinia to Pangea: The Lithotectonioc Record of the Appalachian Region; Tollo, R.P., Bartholomew, M.J., Hibbard, J.P., Karabinos, P.M., Eds.; The Geological Society of America: Boulder, CO, USA, 2010; Volume 206, pp. 307–346. [Google Scholar]
- Volkert, R.A. Mesoproterozoic rocks of the New Jersey Highlands, north-central Appalachians: Petrogenesis and tectonic history. In Proterozoic Tectonic Evolution of the Grenville Orogen in North America; Tollo, R.P., Corriveau, L., McLelland, J., Bartholomew, M.J., Eds.; The Geological Society of America: Boulder, CO, USA, 2004; Volume 197, pp. 697–728. [Google Scholar]
- Volkert, R.A.; Zartman, R.E.; Moore, P.B. U-Pb zircon geochronology of Mesoproterozoic postorogenic rocks and implications for post-Ottawan magmatism and metallogenesis, New Jersey Highlands and contiguous areas, USA. Precambrian Res. 2005, 139, 1–19. [Google Scholar] [CrossRef]
- Peck, W.H.; Volkert, R.A.; Meredith, M.T.; Rader, E.L. Calcite-graphite thermometry of the Franklin Marble, New Jersey Highlands. J. Geol. 2006, 114, 485–499. [Google Scholar] [CrossRef]
- Henry, D.J.; Guidotti, C.V.; Thomson, J.A. The Ti-saturation surface for low-to-medium pressure metapelitic biotites: Implications for geothermometry and Ti-substitution mechanisms. Am. Miner. 2005, 90, 316–328. [Google Scholar] [CrossRef]
- Sonnenwald, E.W. Updated Pressure and Temperature Determinations from the Metapelites of the Western Hudson Highlands. Master’s Thesis, Montclair State University, Montclair, NJ, USA, 2013; 115p. [Google Scholar]
- Volkert, R.A.; Drake, A.A., Jr. Geochemistry and Stratigraphic Relations of Middle Proterozoic Rocks of the New Jersey Highlands; Professional Paper 1565-C; U.S. Geological Survey: Reston, VA, USA, 1999; 77p. [Google Scholar]
- Volkert, R.A. Bedrock Geologic Map of the New Jersey Parts of the Greenwood Lake and Sloatsburg Quadrangles Sussex, Passaic, and Bergen Counties, New Jersey; OFM 106; New Jersey Geological and Water Survey: Ewing Township, NJ, USA, 2015. [Google Scholar]
- Volkert, R.A. Bedrock Geologic Map of the Dover Quadrangle Morris and Sussex Counties, New Jersey; OFM 91; New Jersey Geological and Water Survey: Ewing Township, NJ, USA, 2012. [Google Scholar]
- Volkert, R.A.; Johnson, C.A.; Tamashausky, A.V. Mesoproterozoic graphite deposits, New Jersey Highlands: Geologic and stable isotope evidence for possible algal origins. Can. J. Earth Sci. 2000, 37, 1665–1675. [Google Scholar] [CrossRef]
- Matt, P.; Powell, W.; Volkert, R.; Gorring, M.; Johnson, A. Sedimentary exhalative magnetite deposits of the New Jersey Highlands. Can. J. Earth Sci. 2017, 54, 1008–1023. [Google Scholar] [CrossRef]
- Dallmeyer, R.D.; Dodd, R.T. Distribution and significance of cordierite in paragneiss of the Hudson Highlands, southeastern New York. Contrib. Miner. Petrol. 1977, 33, 289–308. [Google Scholar] [CrossRef]
- Hammerli, J.; Spandler, C.; Oliver, N.H.S. Element redistribution and mobility during upper crustal metamorphism of metasedimentary rocks: An example from the eastern Mount Lofty Ranges, South Australia. Contrib. Miner. Petrol. 2016, 171, 36. [Google Scholar] [CrossRef]
- Roser, B.P.; Nathan, S. An evaluation of element mobility during metamorphism of a turbidite sequence (Greenland Group, New Zealand). Geol. Mag. 1997, 134, 219–234. [Google Scholar] [CrossRef]
- Rudnick, R.L.; Gao, S. Composition of the continental crust. In The Crust; Rudnick, R.L., Ed.; Elsevier: Amsterdam, The Netherlands, 2003; pp. 1–64. [Google Scholar]
- Taylor, S.R.; McLennan, S.M. The Continental Crust: Its Composition and Evolution; Blackwell Scientific Publications: Oxford, UK, 1985; 312p. [Google Scholar]
- Herron, M.M. Geochemical classification of terrigenous sands and shales from core or log data. J. Sediment. Petrol. 1988, 58, 820–829. [Google Scholar]
- Nesbitt, H.W.; Young, G.M. Early Proterozoic climates and plate motions inferred from major element chemistry of lutites. Nature 1982, 299, 715–717. [Google Scholar] [CrossRef]
- Leising, J.F.; Tyler, S.W.; Miller, W.W. Convection of saline brines in enclosed lacustrine basins: A mechanism for potassium metasomatism. Geol. Soc. Am. Bull. 1995, 107, 1157–1163. [Google Scholar] [CrossRef]
- Bhatia, M.R.; Crook, K.A.W. Trace element characteristics of graywackes and tectonic setting discrimination of sedimentary basins. Contrib. Miner. Petrol. 1986, 92, 181–193. [Google Scholar] [CrossRef]
- Roser, B.P.; Korsch, R.J. Provenance signatures of sandstone-mudstone suites determined using discriminant function analysis of major element data. Chem. Geol. 1988, 67, 119–139. [Google Scholar] [CrossRef]
- Hayashi, K.-I.; Fujisawa, H.; Holland, H.D.; Ohmoto, H. Geochemistry of ~1.9 Ga sedimentary rocks from northeastern Labrador, Canada. Geochim. Cosmochim. Acta 1997, 61, 4115–4137. [Google Scholar] [CrossRef]
- Cox, R.; Lowe, D.R.; Cullers, R.L. The influence of sediment recycling and basement composition on evolution of mudrock chemistry in the southwestern United States. Geochim. Cosmochim. Acta 1995, 59, 2919–2940. [Google Scholar] [CrossRef]
- Peck, W.H.; Quinan, M.P.; Selleck, B.W. Detrital zircon constraints on Grenville sedimentation at the margin of Laurentia. Precambrian Res. 2019, 331, 105342. [Google Scholar] [CrossRef]
- Peck, W.H.; Volkert, R.A.; Mansur, A.T.; Doverspike, B.A. Stable isotope and petrologic evidence for the origin of regional marble-hosted magnetite deposits and the zinc deposits at Frankin and Sterling Hill, New Jersey Highlands, United States. Econ. Geol. 2009, 104, 1037–1054. [Google Scholar] [CrossRef]
- Trabucho-Alexandre, J.; Hay, W.W.; de Boer, P.L. Phanerozoic environments of black shale deposition and the Wilson Cycle. Solid Earth 2012, 3, 29–42. [Google Scholar] [CrossRef]
- Peng, Q.M.; Palmer, M.R. The Paleoproterozoic boron deposits in eastern Liaoning, China: A metamorphosed evaporite. Precambrian Res. 1995, 3–4, 185–197. [Google Scholar] [CrossRef]
- Volkert, R.A. Geologic setting of Proterozoic iron, zinc, and graphite deposits, New Jersey Highlands. In Proterozoic Iron and Zinc Deposits of the Adirondack Mountains of New York and the New Jersey Highlands; Guidebook Ser. 35; Slack, J.F., Ed.; Society of Economic Geologists: Littleton, CO, USA, 2001; pp. 59–73. [Google Scholar]
- Swihart, G.H.; Moore, P.B. A reconnaissance of the boron isotope composition of tourmaline. Geochim. Cosmochim. Acta 1989, 53, 911–916. [Google Scholar] [CrossRef]
- Swihart, G.H.; Moore, P.B.; Callis, E.L. Boron isotope composition of marine and nonmarine evaporates. Geochim. Cosmochim. Acta 1986, 50, 1297–1301. [Google Scholar] [CrossRef]
- Volkert, R.; Aleinikoff, J. 1.3 Ga continental-margin magmatic arc and back arc in the New Jersey Highlands and implications for the origin of zinc + iron deposits. Geol. Soc. Am. Abstr. Prog. 2007, 39, 37. [Google Scholar]
- Johnson, C.A.; Rye, D.M.; Skinner, B.J. Petrology and stable isotope geochemistry of the metamorphosed zinc-iron-manganese deposit at Sterling Hill, New Jersey. Econ. Geol. 1990, 85, 1133–1161. [Google Scholar] [CrossRef]
- Volkert, R.A.; Peck, W.H. Constraints from geochemistry and oxygen isotopes for the hydrothermal origin of orthoamphibole mafic gneiss in the New Jersey Highlands, north-central Appalachians, USA. Lithos 2017, 294–295, 184–197. [Google Scholar] [CrossRef]
- Slack, J.F. Tourmaline associations with hydrothermal ore deposits. Boron: Mineralogy, Petrology and Geochemistry. Rev. Miner. 1996, 33, 559–643. [Google Scholar]
- Boström, K. The origin and fate of ferromanganoan active ridge sediments. Stockholm Contrib. Geol. 1973, 27, 147–243. [Google Scholar]
- Peter, J.M.; Goodfellow, W.D. Hydrothermal sedimentary rocks of the Heath Steele Belt, Bathurst Mining Camp, New Brunswick: Part 3. Application of mineralogy and mineral and bulk compositions to massive sulfide exploration. Econ. Geol. 2003, 11, 417–433. [Google Scholar]
- Large, R.R.; Gemmell, J.B.; Paulick, H.; Huston, D.L. The alteration box plot: A simple approach to understanding the relationship between alteration mineralogy and lithogeochemistry associated with volcanic-hosted massive sulfide deposits. Econ. Geol. 2001, 96, 957–971. [Google Scholar] [CrossRef]
- Plank, T.; Langmuir, C.H. The chemical composition of subducting sediment and its consequences for the crust and mantle. Chem. Geol. 1998, 145, 325–394. [Google Scholar] [CrossRef]
- Moran, A.E.; Sisson, V.B.; Leeman, W.P. Boron depletion during progressive metamorphism: Implications for subduction processes. Earth Planet. Sci. Lett. 1992, 111, 331–349. [Google Scholar] [CrossRef]
- Marschall, H.R.; Jiang, S.-Y. Tourmaline isotopes: No element left behind. Elements 2011, 7, 313–319. [Google Scholar] [CrossRef]



| Sample | R1 | R16 | 12 | 30 | 13 | T1 | T2 | T5 | UCC | PAAS |
|---|---|---|---|---|---|---|---|---|---|---|
| wt. % | ||||||||||
| SiO2 | 62.30 | 63.68 | 64.30 | 65.90 | 67.60 | 53.35 | 53.80 | 56.00 | 66.60 | 62.80 |
| TiO2 | 0.72 | 0.87 | 0.86 | 0.72 | 0.71 | 1.81 | 1.26 | 1.07 | 0.64 | 1.00 |
| Al2O3 | 15.80 | 16.23 | 16.30 | 16.10 | 13.80 | 26.14 | 24.20 | 23.80 | 15.40 | 18.90 |
| Fe2O3* | 5.74 | 5.86 | 7.52 | 2.81 | 4.63 | 2.18 | 7.32 | 3.33 | 5.00 | 7.22 |
| MnO | 0.03 | 0.06 | 0.04 | 0.01 | 0.02 | 0.01 | 0.05 | 0.01 | 0.10 | 0.11 |
| MgO | 2.44 | 2.94 | 2.86 | 2.61 | 1.86 | 3.95 | 3.75 | 2.67 | 2.48 | 2.20 |
| CaO | 1.24 | 1.69 | 2.06 | 1.66 | 2.00 | 1.93 | 1.03 | 1.05 | 3.59 | 1.30 |
| Na2O | 2.47 | 3.87 | 2.77 | 6.37 | 3.34 | 3.25 | 3.64 | 4.87 | 3.27 | 1.20 |
| K2O | 7.37 | 2.65 | 3.15 | 3.32 | 4.34 | 5.89 | 3.66 | 5.28 | 2.80 | 3.70 |
| P2O5 | 0.16 | 0.09 | 0.08 | 0.13 | 0.18 | n.d. | n.d. | n.d. | 0.15 | 0.16 |
| LOI | 1.10 | 0.77 | 0.47 | 0.31 | 0.54 | 0.46 | 0.90 | 1.20 | n.d. | 6.00 |
| Total | 99.79 | 98.71 | 100.41 | 99.94 | 99.02 | 98.29 | 98.58 | 97.75 | 100.05 | 104.59 |
| ppm | ||||||||||
| Ba | 1549 | 623 | 570 | 440 | 710 | n.d. | 707 | 1030 | 628 | 650 |
| Rb Sr | 237 69 | 100 94 | 150 90 | 130 100 | 160 120 | n.d. n.d. | 141 138 | 234 156 | 82 320 | 160 200 |
| Nb Cr Y | 15 60 41 | 11 70 42 | <10 89 50 | 20 84 60 | <10 70 10 | n.d. n.d. n.d. | 36 n.d. 116 | 23 n.d. 87 | 12 92 21 | 18 100 27 |
| Zr | 212 | 248 | 210 | 200 | 180 | n.d. | 347 | 243 | 193 | 210 |
| B | n.d. | n.d. | n.d. | n.d. | n.d. | 276 | 519 | 416 | 17 | 114 |
| SiO2/Al2O3 Al2O3/TiO2 K2O/Al2O3 | 3.94 21.94 0.47 | 3.92 18.65 0.16 | 3.94 18.95 0.19 | 4.09 22.36 0.21 | 4.90 19.44 0.31 | 2.04 14.44 0.22 | 2.22 19.21 0.15 | 2.35 22.24 0.22 | 4.32 24.06 0.18 | 3.32 18.90 0.19 |
| ICV CIA | 1.27 52 | 1.11 57 | 1.18 58 | 1.09 49 | 1.22 50 | 0.72 63 | 0.82 67 | 0.75 60 | n.d. n.d. | n.d. n.d. |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Volkert, R.A. Constraints from Geochemistry and Field Relationships for the Origin of Kornerupine-Bearing Gneiss from the Grenvillian New Jersey Highlands and Implications for the Source of Boron. Minerals 2019, 9, 431. https://doi.org/10.3390/min9070431
Volkert RA. Constraints from Geochemistry and Field Relationships for the Origin of Kornerupine-Bearing Gneiss from the Grenvillian New Jersey Highlands and Implications for the Source of Boron. Minerals. 2019; 9(7):431. https://doi.org/10.3390/min9070431
Chicago/Turabian StyleVolkert, Richard A. 2019. "Constraints from Geochemistry and Field Relationships for the Origin of Kornerupine-Bearing Gneiss from the Grenvillian New Jersey Highlands and Implications for the Source of Boron" Minerals 9, no. 7: 431. https://doi.org/10.3390/min9070431
APA StyleVolkert, R. A. (2019). Constraints from Geochemistry and Field Relationships for the Origin of Kornerupine-Bearing Gneiss from the Grenvillian New Jersey Highlands and Implications for the Source of Boron. Minerals, 9(7), 431. https://doi.org/10.3390/min9070431




