Geochemical Characteristics of Dolomitic Phosphorite Containing Rare Earth Elements and Its Weathered Ore
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
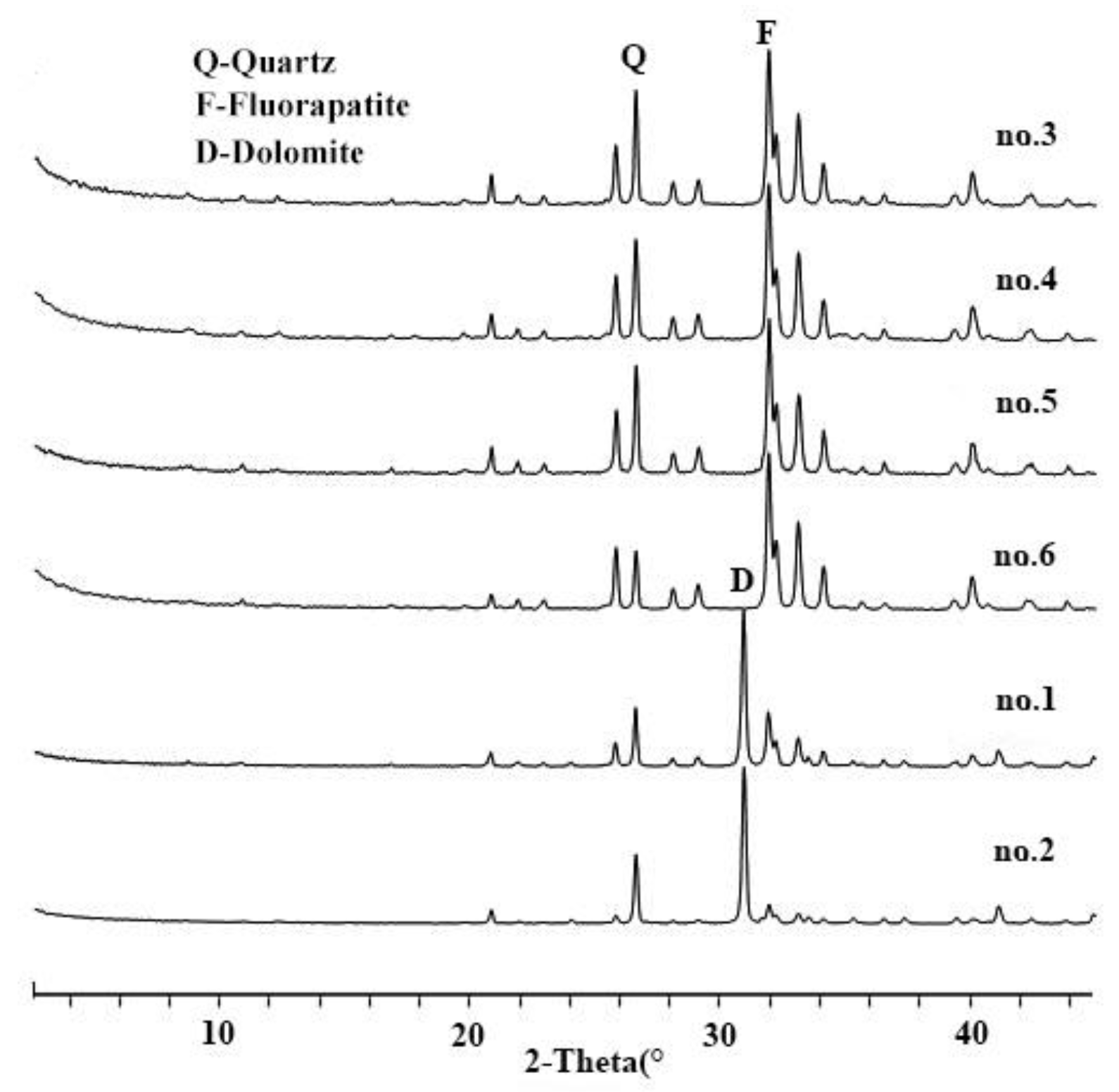
2.2.1. X-ray Diffraction (XRD) Analysis
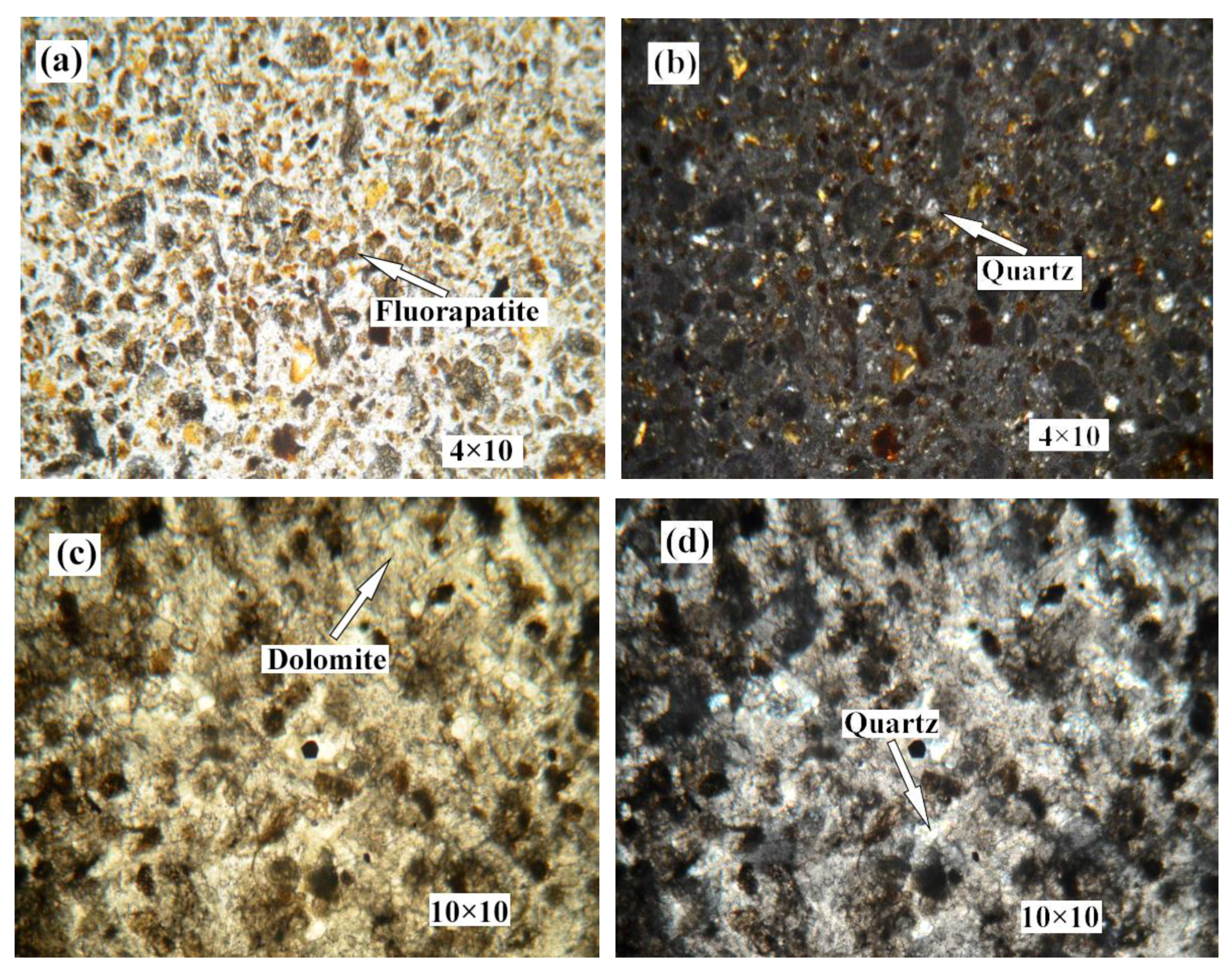
2.2.2. Optical Microscopy
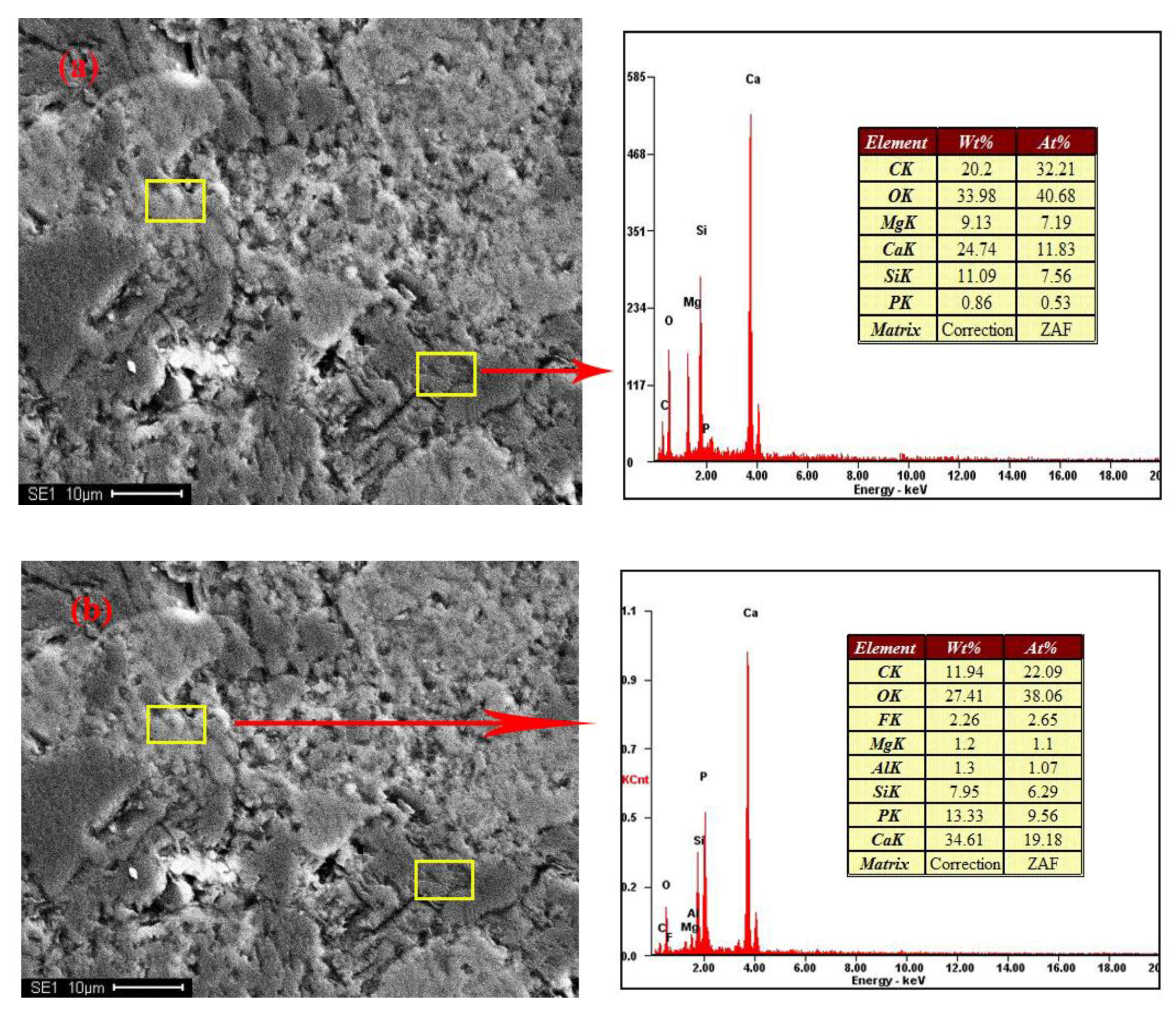
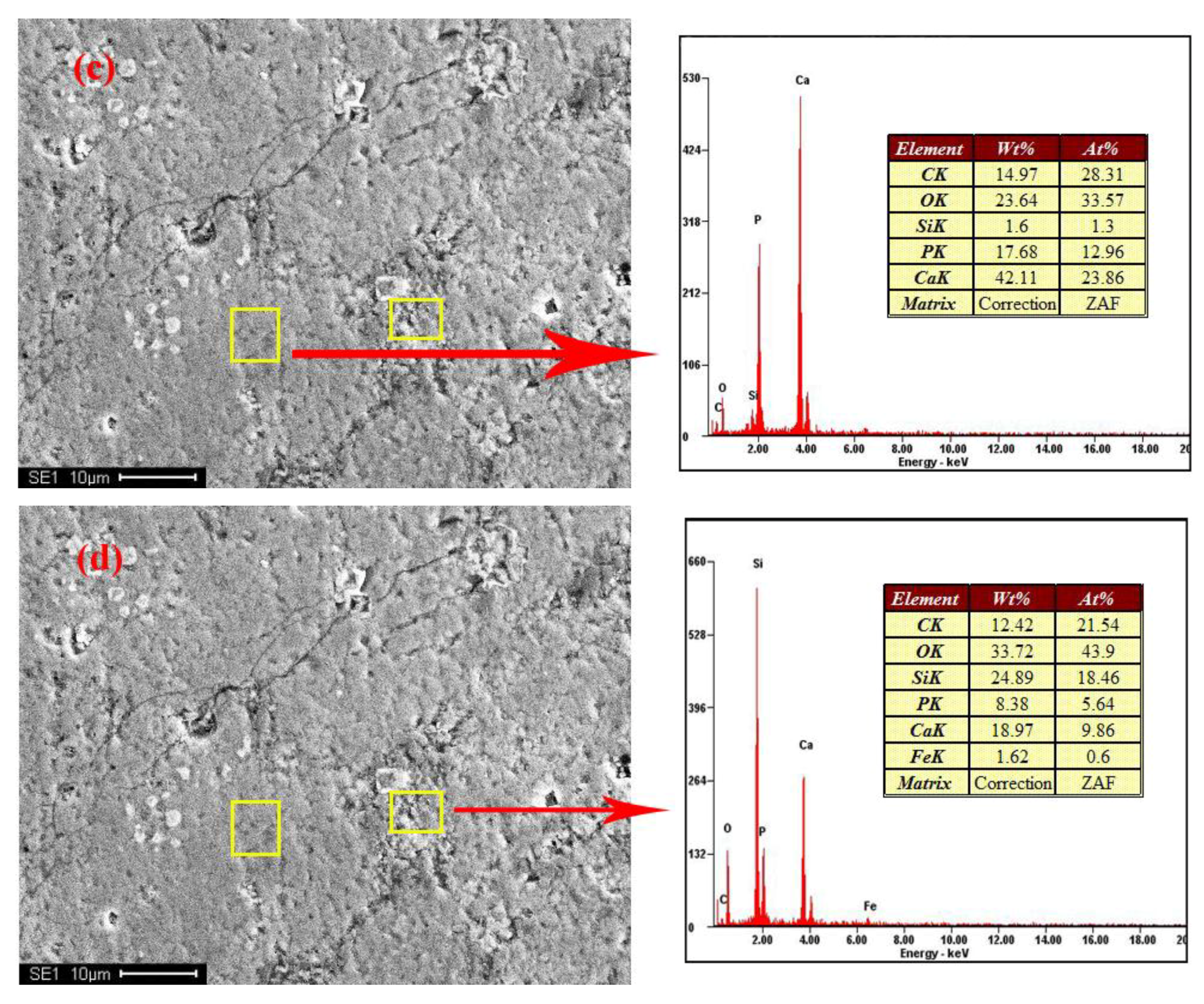
2.2.3. SEM-EDX
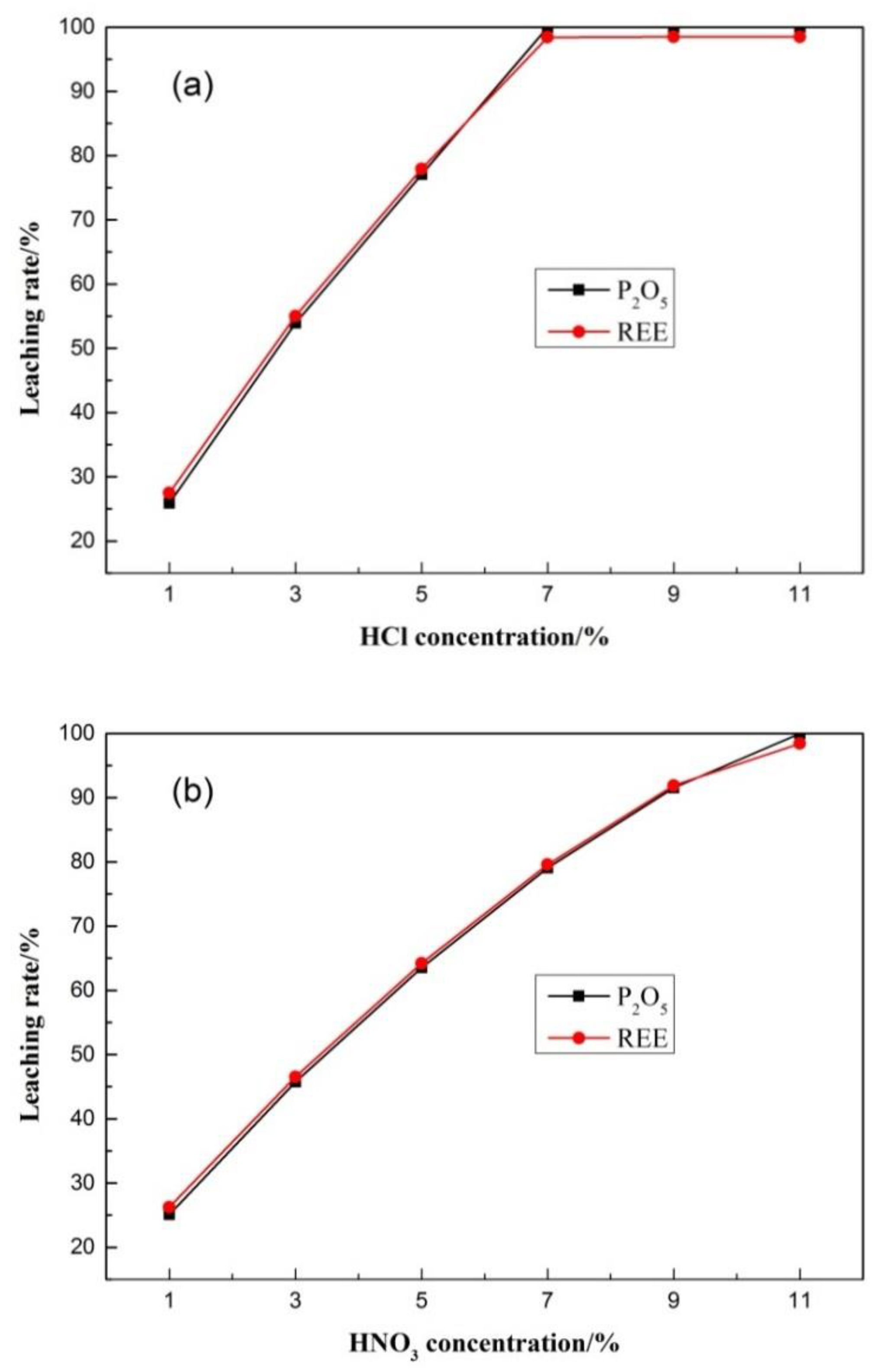
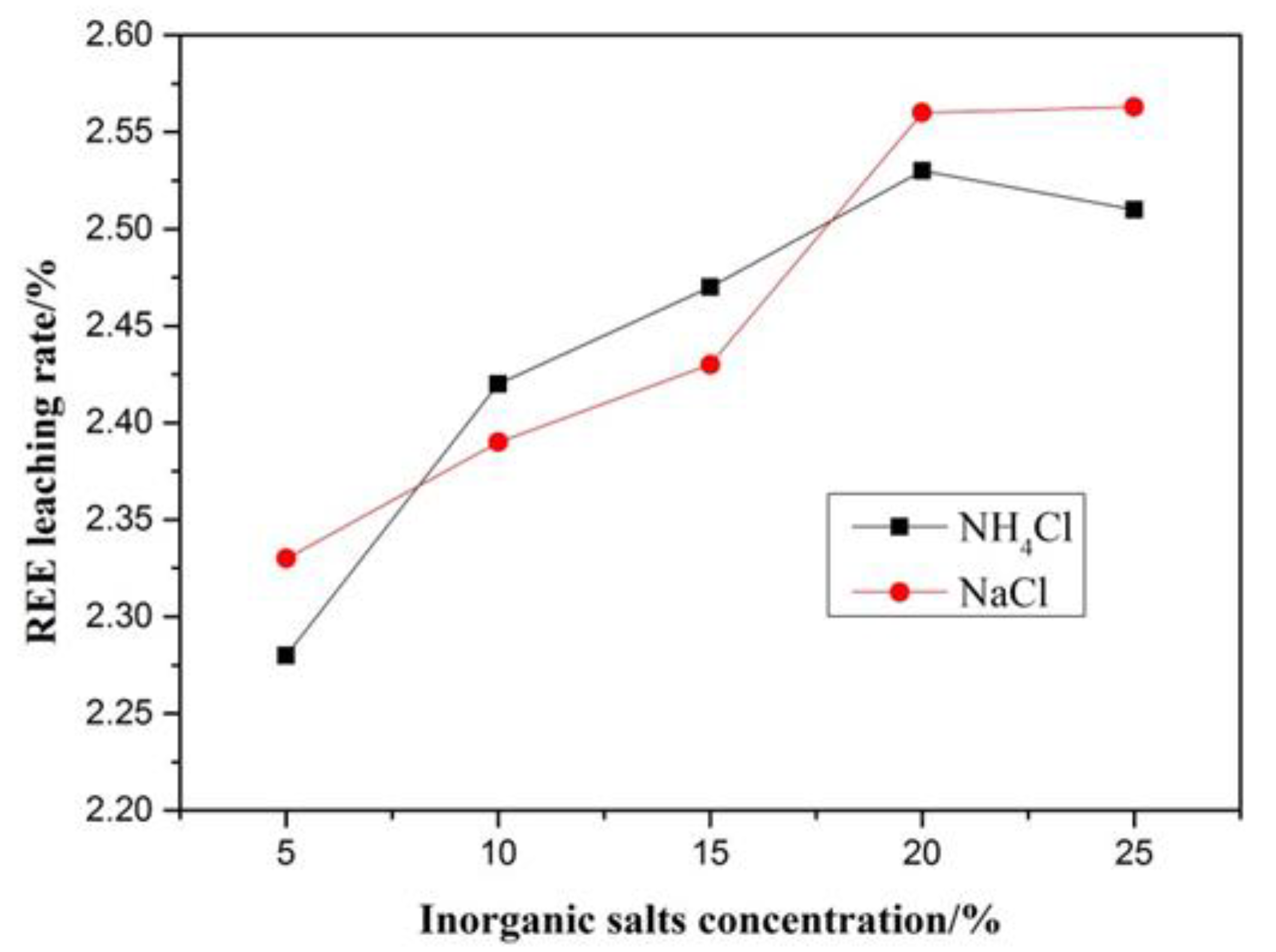
2.3. Leaching Test
2.4. Mathematical Method for Judging Independent Minerals
3. Results and Discussion
3.1. Comparion of Mineral Composition between Primary Weathered Phosphorites
3.2. Ore Texture Differences
3.3. Comparison of Trace Elements
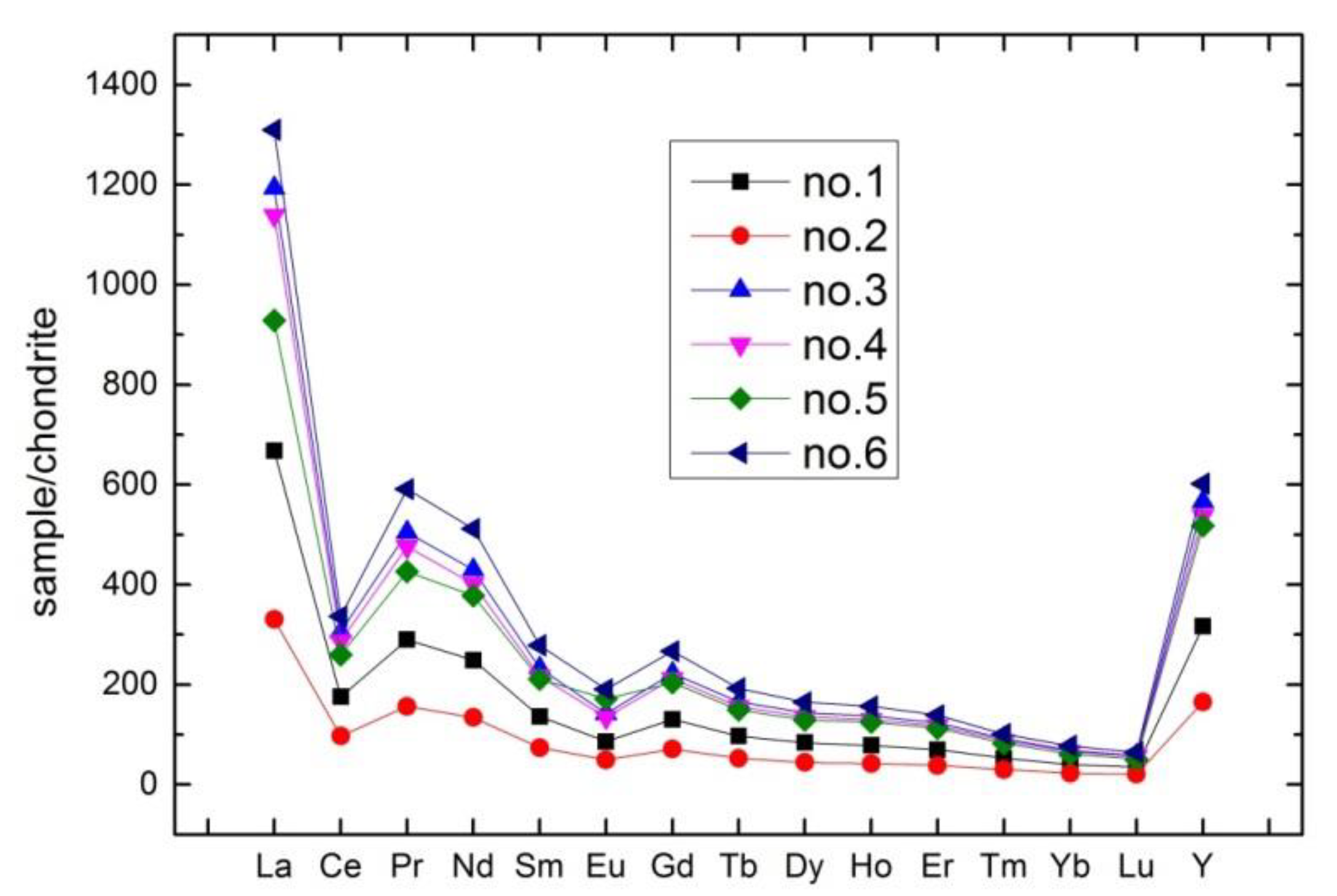
3.4. Comparison of Rare Earth Elements
3.5. Analysis of Rare Earth Elements (REE) Occurrence State
3.5.1. Analysis of Independent Form
3.5.2. Analysis of Isomorphic Form
3.5.3. Analysis of Ion Adsorption Form
4. Conclusions
Author Contributions
Acknowledgments
Funding
Conflicts of Interest
References
- Abouzeid, A.Z.A. Physical and thermal treatment of phosphate ores—An overview. Int. J. Miner. Process. 2008, 85, 59–84. [Google Scholar] [CrossRef]
- dos Santos, M.A.; Santana, R.C.; Capponi, F.; Ataide, C.H.; Barrozo, M.A.S. Effect of ionic species on the performance of apatite flotation. Sep. Purif. Technol. 2010, 76, 15–20. [Google Scholar] [CrossRef]
- Gharabaghi, M.; Irannajad, M.; Noaparast, M. A review of the beneficiation of calcareous phosphate ores using organic acid leaching. Hydrometallurgy 2010, 103, 96–107. [Google Scholar] [CrossRef]
- Lin, Y. The Construction and Application of Phosphorus Resource Development and Utilization Model in China; Hefei University of Tecnology: Hefei, China, 2010. [Google Scholar]
- Zhang, W.; Ma, W.; Zhang, F.; Ma, J. Comparative analysis of the superiority of China’s phophate rock and development strategies with that of the United States and Morocco. J. Nat. Resour. 2005, 20, 378–386. [Google Scholar]
- Li, T.; Wen, S.; Tian, P.; Tang, Z.; Huang, Y. Weathered phosphorites in south China. Geol. Chem. Miner. 1996, 3, 53–57. [Google Scholar]
- Wang, J. Study on Geology and Geochemistry of Wheathered Phosphate Deposits, South China; China University of Geosciences: Beijing, China, 2011. [Google Scholar]
- Chang, S.; Zhu, J.; Liu, Y.; He, J. Study status and trends of weathered phosphate rocks in Yunnan, Guizhou. Ind. Miner. Process. 2010, 39, 41–44. [Google Scholar]
- Tian, S.; Wang, Q.; Zhu, H.; Zhu, Y.; Zhou, J.; Wang, J. Fixed quantity exploration and assessment of weathered phosphoric ore in southwest of China. Geol. Chem. Miner. 2004, 4, 205–209. [Google Scholar]
- Wei, D.; Cui, X. Study on process characteristic of heavy rare earths in phosphorite in Guizhou province. Conserv. Util. Miner. Resour. 2003, 2, 38–40. [Google Scholar]
- Yang, R.D.; Gao, H.; Wang, Q.; Bao, M. REE enrichment in Early Cambrian Gezhongwu formation phosphorous rock series in Sanjia, Zhijun County, Guizhou Province, China. J. Rare Earths 2005, 23, 760–767. [Google Scholar]
- Ross, J.; Gao, L.; Meouch, O.; Anthony, E.; Sutarwala, D.; Mamo, H. Carbonate Apatite Precipitation from Synthetic Municipal Wastewater. Minerals 2017, 7, 129. [Google Scholar] [CrossRef]
- Salama, W.; Khirekesh, Z.; Amini, A.; Bafti, B.S. Diagenetic evolution of the upper Devonian phosphorites, Alborz for Mountain Range, northern Iran. Sediment. Geol. 2018, 376, 90–112. [Google Scholar] [CrossRef]
- Zhang, J.; Shun, C.; Yang, G.; Xie, F. Separation and enrichment of rare earth elements in phosphorite in Xinhua, Zhijin, Guizhou. J. Rare Earths 2006, 24, 413. [Google Scholar]
- Shi, C.; Hu, R.; Wang, G. Study on REE geochemistry of Zhijin phosphorites, Guizhou province. J. Mineral. Petrol. 2004, 24, 71–75. [Google Scholar]
- Wang, M.; Sun, X.; Ma, M. Rare earth elements geochemistry and genesis of Xinhua large-size phosphorite deposit in western Guizhou. Miner. Depos. 2004, 23, 484–493. [Google Scholar]
- Jin, H.-X.; Wu, F.-Z.; Mao, X.-H.; Wang, M.-L.; Xie, H.-Y. Leaching isomorphism rare earths from phosphorite ore by sulfuric acid and phosphoric acid. Rare Met. 2017, 36, 840–850. [Google Scholar] [CrossRef]
- Lednev, A.V.; Lozhkin, A.V. Remediation of agrosoddy-podzolic soils contaminated with cadmium. Eurasian Soil Sci. 2017, 50, 620–629. [Google Scholar] [CrossRef]
- Li, J.; Jin, H.; Chen, Y.; Mao, X. Rare earth elements in Zhijin phosphorite and distribution in two-stage flotation process. J. Rare Earths 2007, 25, 85–90. [Google Scholar]
- Zhang, J. The weathered ore criterion of Dianchi phosphorite. Yunnan Metall. 1992, 24–30. [Google Scholar]
- Li, X.; Zhang, Q.; Hou, B.; Ye, J.; Mao, S.; Li, X. Flotation separation of quartz from collophane using an amine collector and its adsorption mechanisms. Powder Technol. 2017, 318, 224–229. [Google Scholar] [CrossRef]
- Gao, P.; He, Z.; Li, S.; Lash, G.G.; Li, B.; Huang, B.; Yan, D. Volcanic and hydrothermal activities recorded in phosphate nodules from the Lower Cambrian Niutitang Formation black shales in South China. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2018, 505, 381–397. [Google Scholar] [CrossRef]
- Huggett, J.; Hooker, J.N.; Cartwright, J. Very early diagenesis in a calcareous, organic-rich mudrock from Jordan. Arab. J. Geosci. 2017, 10, 270. [Google Scholar] [CrossRef]
- Breiland, A.A.; Flood, B.E.; Nikrad, J.; Bakarich, J.; Husman, M.; Rhee, T.; Jones, R.S.; Bailey, J.V. Polyphosphate-Accumulating Bacteria: Potential Contributors to Mineral Dissolution in the Oral Cavity. Appl. Environ. Microbiol. 2018, 84. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yang, R.; Wei, H.; Gao, J. Rare earth element geochemistry of Cambrian phosphorites from the Yangtze Region. J. Rare Earths 2013, 31, 101–112. [Google Scholar] [CrossRef]
- Gamez Vintaned, J.A.; Linan, E.; Navarro, D.; Zhuravlev, A.Y. The oldest Cambrian skeletal fossils of Spain (Cadenas Ibericas, Aragon). Geol. Mag. 2018, 155, 1465–1474. [Google Scholar] [CrossRef]
- Walawalkar, M.; Nichol, C.K.; Azimi, G. Process investigation of the acid leaching of rare earth elements from phosphogypsum using HCl, HNO3, and H2SO4. Hydrometallurgy 2016, 166, 195–204. [Google Scholar] [CrossRef]
- Linsy, P.; Nath, B.N.; Mascarenhas-Pereira, M.B.L.; Vinitha, P.V.; Ray, D.; Babu, C.P. Benthic cycling of phosphorus in the Eastern Arabian Sea: Evidence of present day phosphogenesis. Mar. Chem. 2018, 199, 53–66. [Google Scholar] [CrossRef]
- Sinirkaya, M.; Ozer, A.K.; Gulaboglu, M.S. Kinetics of dissolution of Mardin-Mazidagi (Turkey) phosphate ore in dilute phosphoric acid solutions. Miner. Metall. Process. 2010, 27, 110–115. [Google Scholar] [CrossRef]
- Boynton, W.V. Cosmochemistry of the Rare Earth Elements. In Rare Earth Element Geochemistry; Henderson, P., Ed.; Elsevier: Amsterdam, The Netherlands, 1984. [Google Scholar]
- Xie, F.; Zhang, T.A.; Dreisinger, D.; Doyle, F. A critical review on solvent extraction of rare earths from aqueous solutions. Miner. Eng. 2014, 56, 10–28. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Q.; Chen, D. REE geochemistry of the ore-bearing REE in Xinhua phosphorite, Zhijin, Guizhou. J. Mineral. Petrol. 2003, 23, 35–38. [Google Scholar]
- Baghdady, A.R.; Howari, F.M.; Al-Wakeel, M.I. On the mineral characteristics and geochemistry of the Florida phosphate of Four Corners and Hardee County mines. Sediment. Geol. 2016, 342, 1–14. [Google Scholar] [CrossRef]
- Chen, J.; Yang, R.; Zhang, J. Mode of occurrence of rare earth elements in posphorite in Zhijin county, Guizhou province, China. Acta Mineral. Sin. 2010, 30, 123–129. [Google Scholar]
- Gall, Q.; Davis, W.J.; Lowe, D.G.; Dabros, Q. Diagenetic apatite character and in situ ion microprobe U-Pb age, Keeseville Formation, Potsdam Group, New York State. Can. J. Earth Sci. 2017, 54, 785–797. [Google Scholar] [CrossRef]
- Huang, X.-W.; Long, Z.-Q.; Wang, L.-S.; Feng, Z.-Y. Technology development for rare earth cleaner hydrometallurgy in China. Rare Met. 2015, 34, 215–222. [Google Scholar] [CrossRef]

| Oxides | Phosphorite | |||||
|---|---|---|---|---|---|---|
| Primary | Weathered | |||||
| Sample No. | ||||||
| 1 | 2 | 3 | 4 | 5 | 6 | |
| P2O5 | 19.36 | 9.42 | 30.12 | 29.21 | 31.01 | 35.18 |
| SiO2 | 13.61 | 24.95 | 17.59 | 18.29 | 17.69 | 10.15 |
| Al2O3 | 1.57 | 1.71 | 4.25 | 4.75 | 2.91 | 2.23 |
| Fe2O3 | 0.99 | 0.95 | 3.28 | 3.49 | 2.74 | 1.98 |
| CaO | 35.75 | 26.98 | 38.73 | 37.59 | 39.51 | 44.72 |
| MgO | 7.79 | 10.36 | 0.55 | 0.61 | 0.54 | 0.27 |
| Na2O | 0.06 | 0.07 | 0.08 | 0.08 | 0.06 | 0.09 |
| K2O | 0.42 | 0.53 | 1.13 | 1.27 | 0.90 | 0.57 |
| TiO2 | 0.08 | 0.05 | 0.12 | 0.13 | 0.12 | 0.11 |
| L.O.I | 18.50 | 23.70 | 3.90 | 4.06 | 3.33 | 2.78 |
| Total | 98.13 | 98.72 | 99.75 | 99.48 | 98.81 | 98.08 |
| Trace Elements (ppm) | ||||||
| Ba | 183 | 3200 | 433 | 479 | 732 | 455 |
| Ce | 142 | 78 | 247 | 232 | 210 | 272 |
| Cr | 20 | 20 | 40 | 40 | 50 | 40 |
| Cs | 1 | 1 | 2 | 2 | 2 | 1 |
| Dy | 27 | 14 | 46 | 44 | 42 | 53 |
| Er | 15 | 8 | 26 | 25 | 24 | 29 |
| Eu | 6 | 4 | 10 | 10 | 13 | 14 |
| Ga | 4 | 3 | 9 | 10 | 7 | 6 |
| Gd | 34 | 18 | 58 | 55 | 53 | 69 |
| Hf | 1 | 1 | 2 | 2 | 1 | 2 |
| Ho | 6 | 3 | 10 | 9 | 9 | 11 |
| La | 207 | 103 | 370 | 353 | 288 | 406 |
| Lu | 1 | 1 | 2 | 2 | 2 | 2 |
| Nb | 2 | 2 | 4 | 4 | 2 | 2 |
| Nd | 150 | 81 | 258 | 241 | 227 | 307 |
| Pr | 35 | 19 | 62 | 58 | 52 | 72 |
| Rb | 9 | 8 | 27 | 28 | 19 | 14 |
| Sm | 27 | 14 | 45 | 42 | 41 | 54 |
| Sn | 1 | 1 | 1 | 1 | 1 | 1 |
| Sr | 679 | 335 | 677 | 722 | 698 | 1305 |
| Tb | 5 | 2 | 8 | 7 | 7 | 9 |
| Th | 7 | 5 | 9 | 9 | 5 | 9 |
| Tm | 2 | 1 | 3 | 3 | 3 | 3 |
| U | 5 | 3 | 10 | 9 | 18 | 11 |
| V | 19 | 8 | 33 | 32 | 72 | 24 |
| W | 2 | 2 | 2 | 2 | 1 | 3 |
| Y | 317 | 166 | 566 | 538 | 518 | 602 |
| Yb | 8 | 5 | 14 | 14 | 13 | 16 |
| Zr | 49 | 37 | 74 | 66 | 44 | 54 |
| U/Th | 0.64 | 0.57 | 1.03 | 1.01 | 3.55 | 1.11 |
| Sr/Ba | 3.72 | 0.10 | 1.56 | 1.51 | 0.95 | 2.87 |
| Rb/Cs | 14.92 | 10.25 | 15.46 | 14.71 | 10.32 | 16.63 |
| Zr/Hf | 37.69 | 37.00 | 35.24 | 31.43 | 33.85 | 31.76 |
| REE | Primary Phosphorite | Weathered Phosphorite | ||||
|---|---|---|---|---|---|---|
| Sample No. | ||||||
| 1 | 2 | 3 | 4 | 5 | 6 | |
| La | 207 | 102.5 | 370 | 353 | 288 | 406 |
| Ce | 142 | 78.3 | 247 | 232 | 210 | 272 |
| Pr | 35.4 | 19.1 | 61.6 | 58.1 | 52 | 72.1 |
| Nd | 149.5 | 80.6 | 258 | 241 | 227 | 307 |
| Sm | 26.6 | 14.5 | 45.4 | 42.2 | 41.1 | 54.3 |
| Eu | 6.34 | 3.65 | 10.45 | 9.89 | 12.6 | 14 |
| Gd | 33.8 | 18.45 | 57.6 | 54.8 | 52.8 | 69.1 |
| Tb | 4.55 | 2.48 | 7.78 | 7.35 | 7.02 | 9.05 |
| Dy | 27 | 14.3 | 46.2 | 43.7 | 41.7 | 53 |
| Ho | 5.63 | 3.04 | 9.86 | 9.33 | 9 | 11.25 |
| Er | 14.65 | 8.05 | 26 | 24.7 | 23.7 | 29.1 |
| Tm | 1.71 | 0.97 | 2.98 | 2.85 | 2.69 | 3.28 |
| Yb | 8.43 | 4.76 | 14.35 | 13.75 | 12.8 | 16.25 |
| Lu | 1.13 | 0.67 | 1.87 | 1.81 | 1.65 | 2.08 |
| Y | 317 | 165.5 | 566 | 538 | 518 | 602 |
| ΣREE | 980.74 | 516.82 | 1725.09 | 1632.48 | 1500.06 | 1920.51 |
| LREE | 566.84 | 298.6 | 992.45 | 936.19 | 830.7 | 1125.4 |
| HREE | 413.9 | 218.22 | 732.64 | 696.29 | 669.36 | 795.11 |
| LREE/HREE | 1.37 | 1.37 | 1.35 | 1.34 | 1.24 | 1.42 |
| (La/Yb)N | 16.37 | 14.36 | 17.19 | 17.12 | 15 | 16.66 |
| δEu | 0.64 | 0.68 | 0.62 | 0.63 | 0.82 | 0.70 |
| δCe | 0.36 | 0.39 | 0.35 | 0.35 | 0.38 | 0.35 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Zhang, J.; Wang, H.; Wang, C. Geochemical Characteristics of Dolomitic Phosphorite Containing Rare Earth Elements and Its Weathered Ore. Minerals 2019, 9, 416. https://doi.org/10.3390/min9070416
Li S, Zhang J, Wang H, Wang C. Geochemical Characteristics of Dolomitic Phosphorite Containing Rare Earth Elements and Its Weathered Ore. Minerals. 2019; 9(7):416. https://doi.org/10.3390/min9070416
Chicago/Turabian StyleLi, Shuai, Jie Zhang, Huaifa Wang, and Caili Wang. 2019. "Geochemical Characteristics of Dolomitic Phosphorite Containing Rare Earth Elements and Its Weathered Ore" Minerals 9, no. 7: 416. https://doi.org/10.3390/min9070416
APA StyleLi, S., Zhang, J., Wang, H., & Wang, C. (2019). Geochemical Characteristics of Dolomitic Phosphorite Containing Rare Earth Elements and Its Weathered Ore. Minerals, 9(7), 416. https://doi.org/10.3390/min9070416





