Enhanced Potential Toxic Metal Removal Using a Novel Hierarchical SiO2–Mg(OH)2 Nanocomposite Derived from Sepiolite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Preparation of the SiO2–Mg(OH)2 Nanocomposite
2.3. Characterizations
2.4. Heavy Metal Removal
3. Results and Discussion
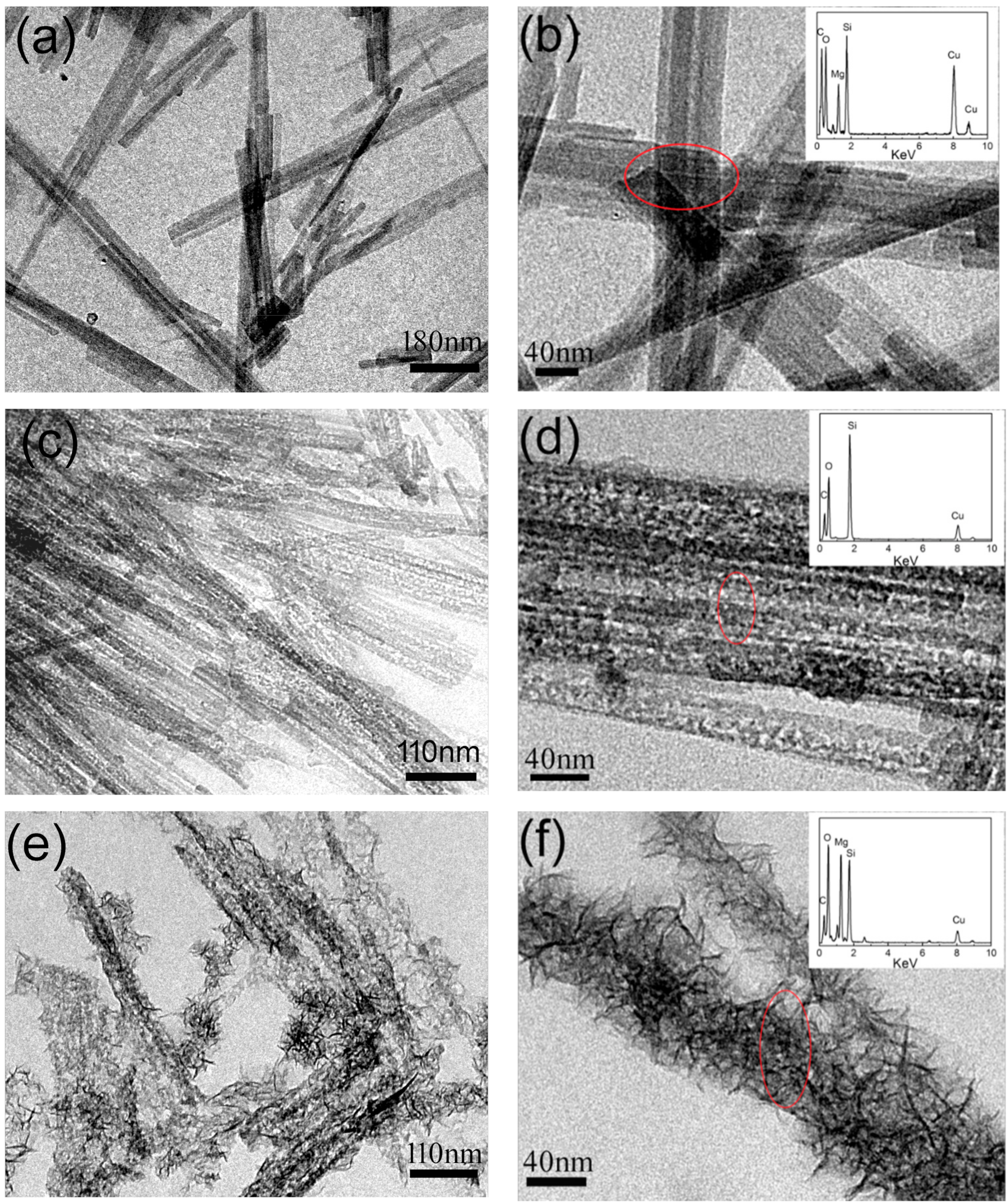
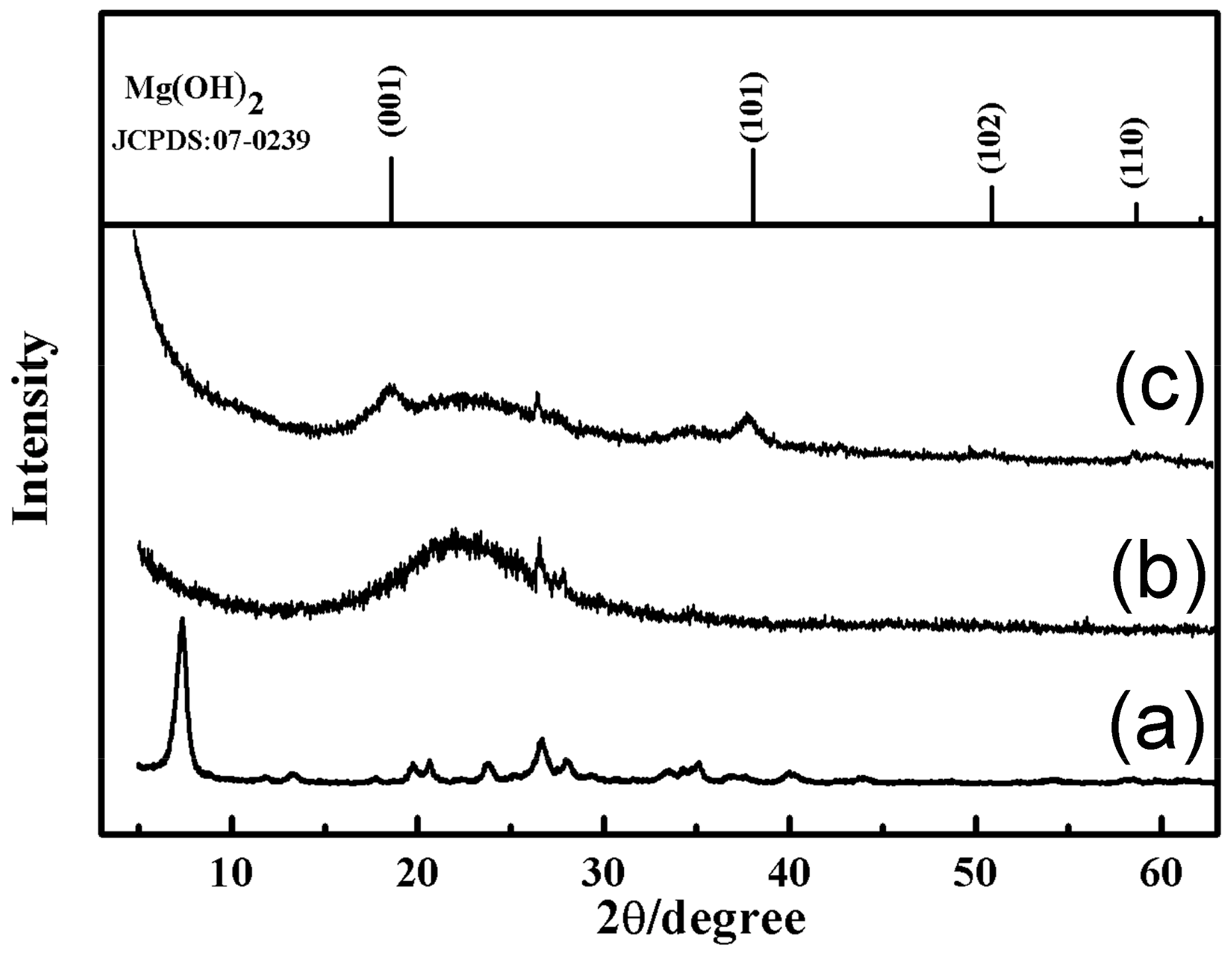
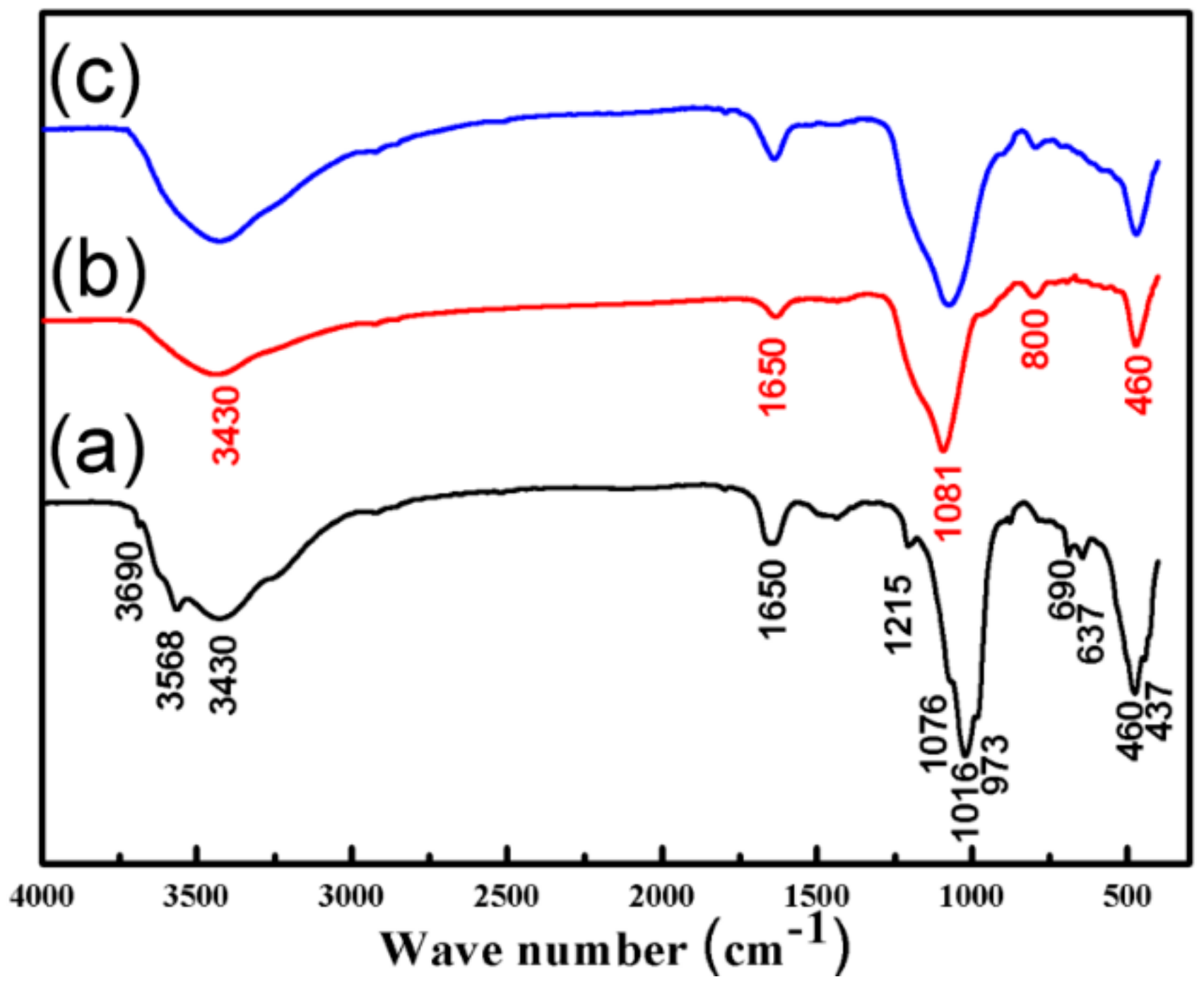
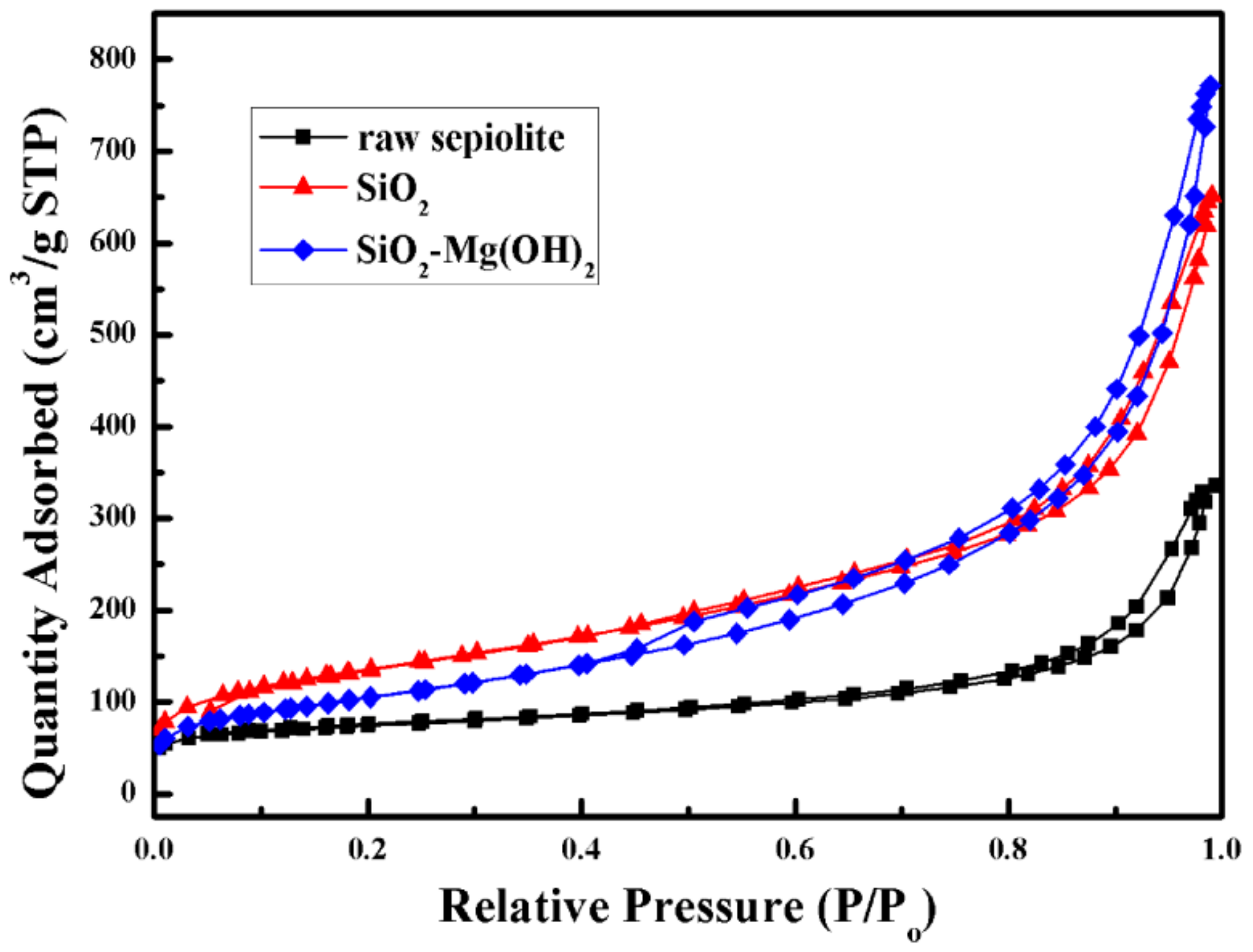
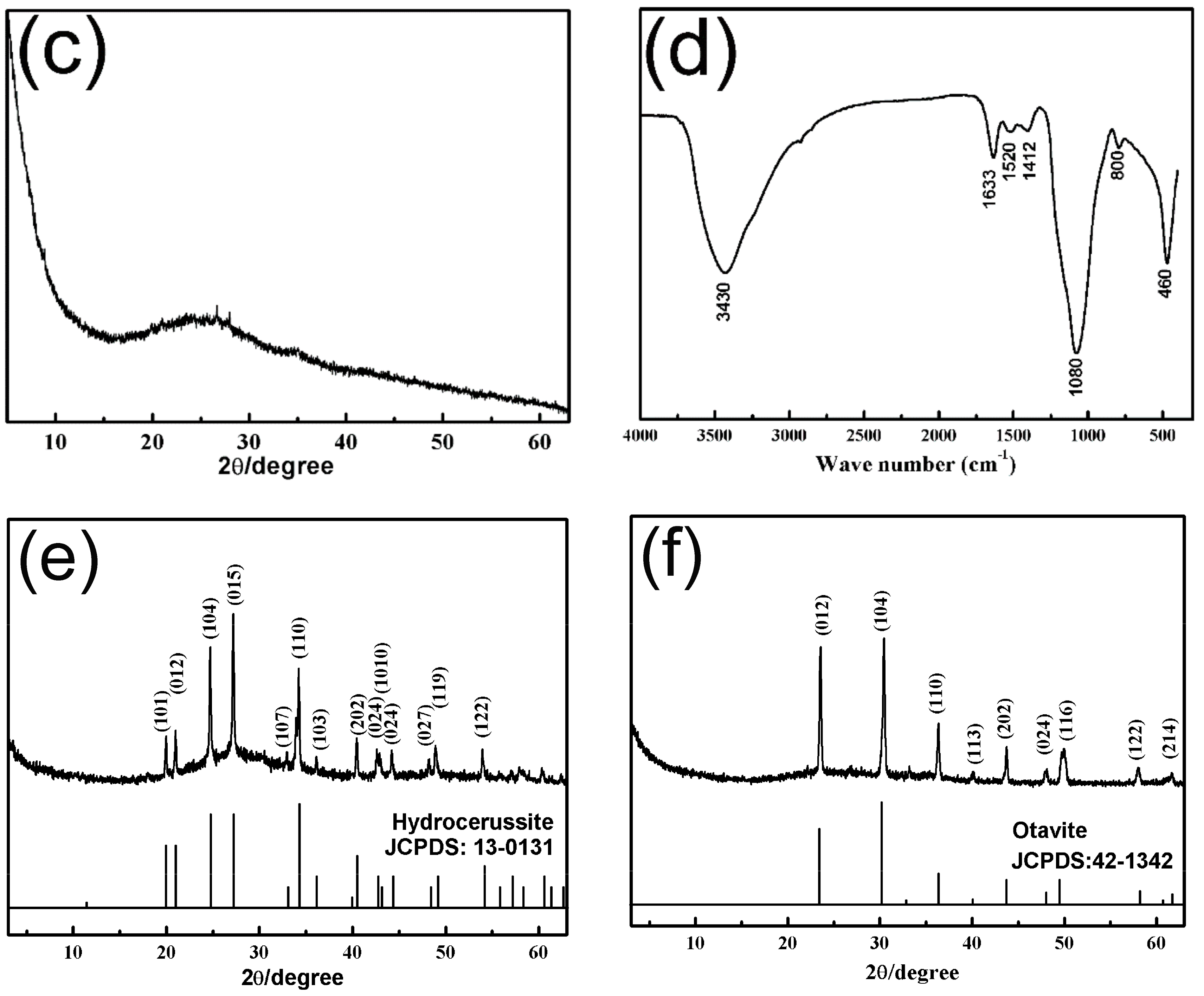
3.1. Characterization of the SiO2–Mg(OH)2 Nanocomposite
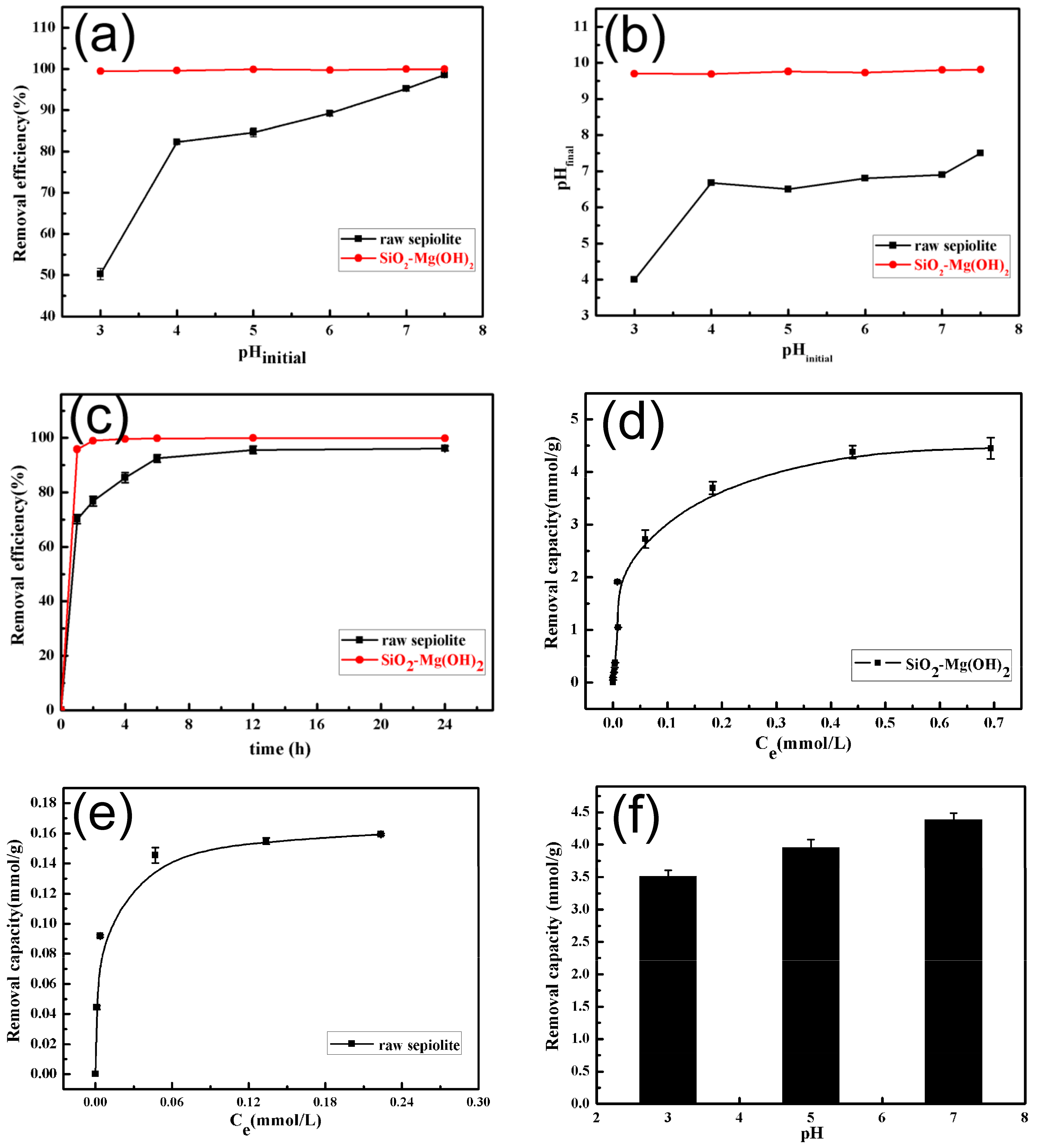
3.2. Removal of Gd(III) by SiO2–Mg(OH)2 Nanocomposite
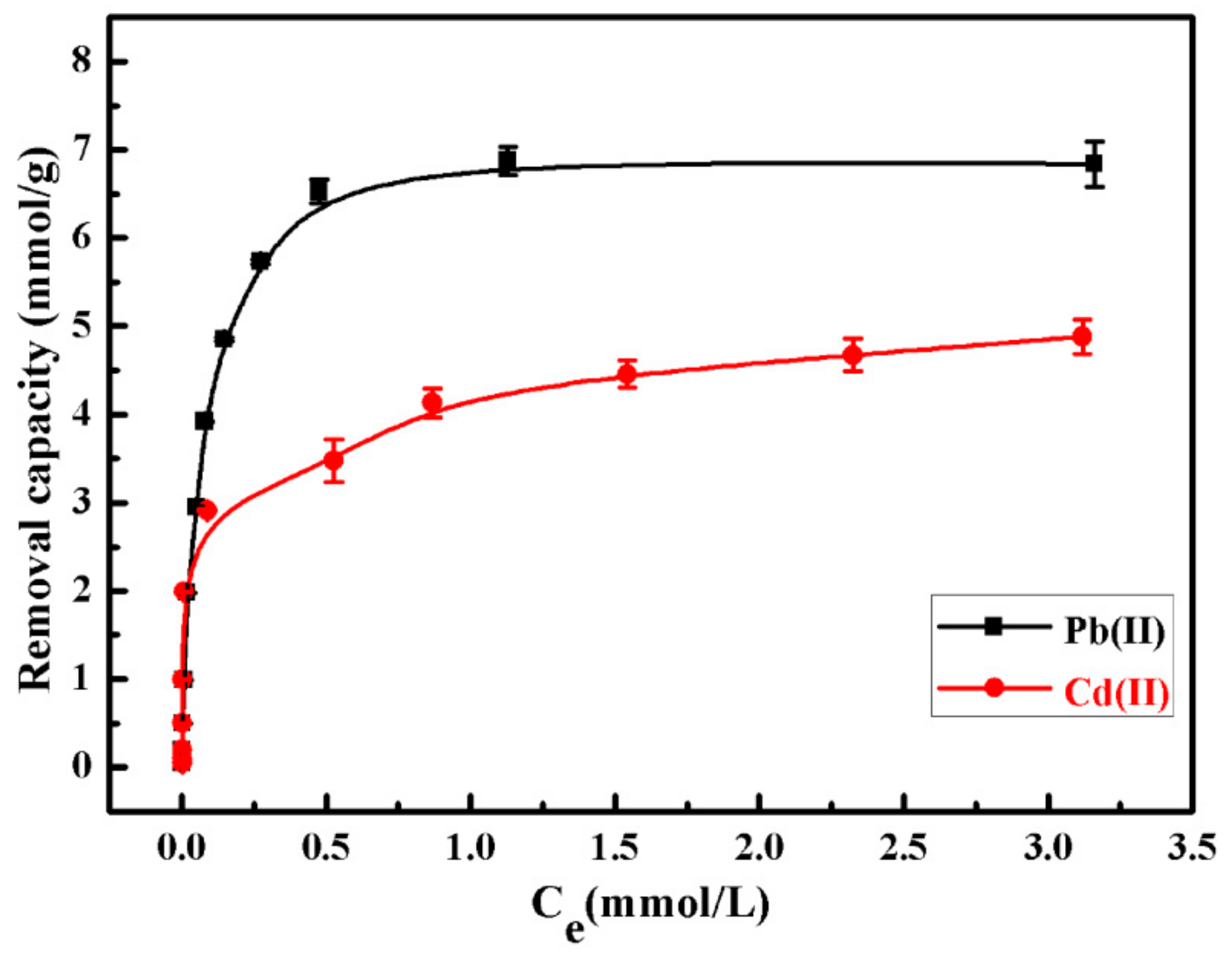
3.3. Removal Abilities of SiO2–Mg(OH)2 Nanocomposite for Pb(II) and Cd(II)
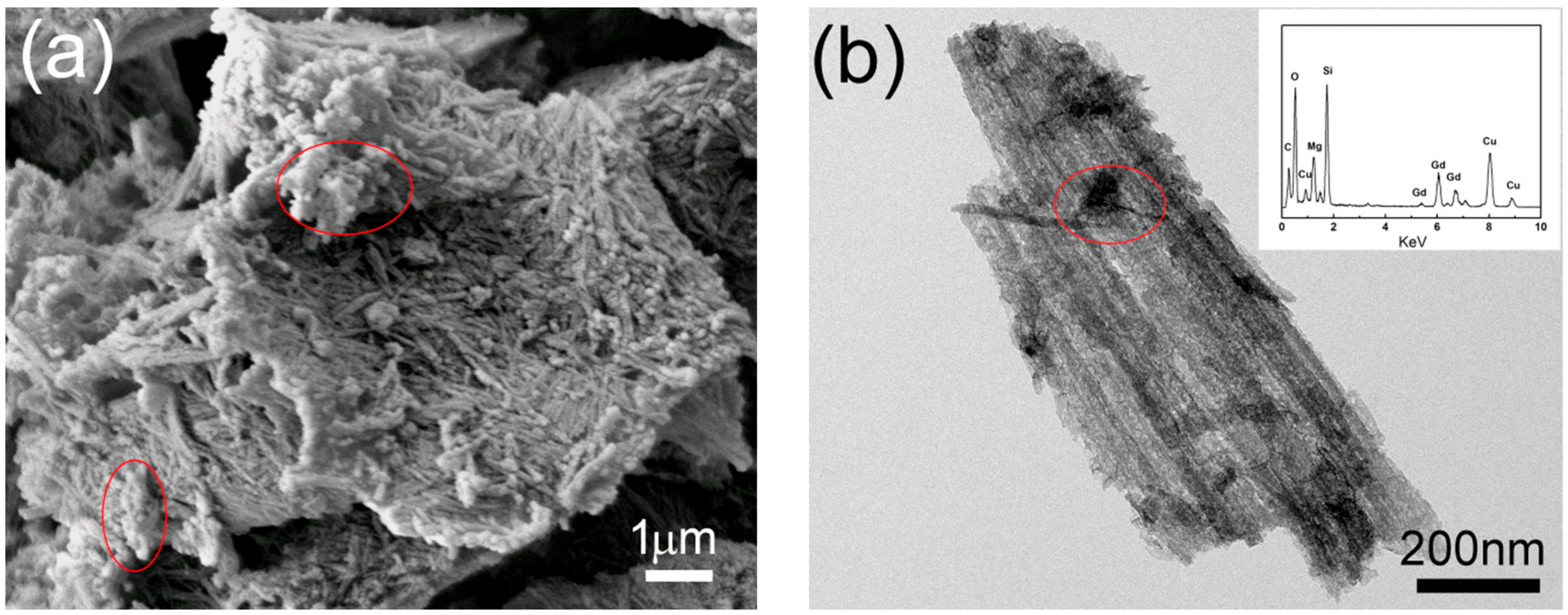
3.4. Removal Mechanism of SiO2–Mg(OH)2 Nanocomposite for Heavy Metals
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Nagajyoti, P.C.; Lee, K.D.; Sreekanth, T.V.M. Heavy metals, occurrence and toxicity for plants: A review. Environ. Chem. Lett. 2010, 8, 199–216. [Google Scholar] [CrossRef]
- Kocaoba, S. Adsorption of Cd(II), Cr(III) and Mn(II) on natural sepiolite. Desalination 2009, 244, 24–30. [Google Scholar] [CrossRef]
- Fabbricino, M.; Ferraro, A.; Luongo, V.; Pontoni, L.; Race, M. Soil washing optimization, recycling of the solution, and ecotoxicity assessment for the remediation of Pb-Contaminated sites using EDDS. Sustainability 2018, 10, 636. [Google Scholar] [CrossRef]
- Uddin, M.K. A review on the adsorption of heavy metals by clay minerals, with special focus on the past decade. Chem. Eng. J. 2017, 308, 438–462. [Google Scholar] [CrossRef]
- Kaji, M. Role of experts and public participation in pollution control: the case of Itai-itai disease in Japan. Ethics Sci. Environ. Polit. 2012, 12, 99–111. [Google Scholar] [CrossRef]
- Ali, O.I.M.; Osman, H.H.; Sayed, S.A.; Shalabi, M.E.H. The removal of some rare earth elements from their aqueous solutions on by-pass cement dust (BCD). J. Hazard. Mater. 2011, 195, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, Z.; Huang, F.; Wu, Z.; Hong, Y.; Lin, Z. Recycling rare earth elements from industrial wastewater with flowerlike nano-Mg(OH)2. ACS Appl. Mater. Interfaces 2013, 5, 9719–9725. [Google Scholar] [CrossRef] [PubMed]
- Charalampides, G.; Vatalis, K.I.; Apostoplos, B.; Ploutarch-Nikolas, B. Rare earth elements: Industrial applications and economic dependency of Europe. Procedia Econ. Finance 2015, 24, 126–135. [Google Scholar] [CrossRef]
- Aghayan, H.; Mahjoub, A.R.; Khanchi, A.R. Samarium and dysprosium removal using 11-molybdo-vanadophosphoric acid supported on Zr modified mesoporous silica SBA-15. Chem. Eng. J. 2013, 225, 509–519. [Google Scholar] [CrossRef]
- Pałasz, A.; Czekaj, P. Toxicological and cytophysiological aspects of lanthanides Action. Acta Biochim. Pol. 2000, 47, 1107–1114. [Google Scholar] [PubMed]
- Chen, Z.Y.; Zhu, X.Z. Accumulation of rare earth elements in bone and its toxicity and potential hazard to health. J. Ecol. Rural Environ. 2008, 24, 88–91. [Google Scholar]
- Tan, X.; Fan, Q.; Wang, X.; Grambow, B. Eu(III) sorption to TiO2 (anatase and rutile): batch, XPS, and EXAFS studies. Environ. Sci. Technol. 2009, 43, 3115–3121. [Google Scholar] [CrossRef] [PubMed]
- Yesiller, S.U.; Eroğlu, A.E.; Shahwan, T. Removal of aqueous rare earth elements (REEs) using nano-iron based materials. J. Ind. Eng. Chem. 2013, 19, 898–907. [Google Scholar] [CrossRef]
- Yu, S.H.; Yao, Q.Z.; Zhou, G.T.; Fu, S.Q. Preparation of hollow core/shell microspheres of hematite and its adsorption ability for samarium. ACS Appl. Mater. Interfaces 2014, 6, 10556–10565. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.J. Global rare earth resources and scenarios of future rare earth industry. J. Rare Earth 2011, 2, 1–6. [Google Scholar] [CrossRef]
- Celis, R.; Hermosin, M.C.; Cornejo, J. Heavy metal adsorption by functionalized clays. Environ. Sci. Technol. 2000, 34, 4593–4599. [Google Scholar] [CrossRef]
- Lothenbach, B.; Furrer, G.; Schulin, R. Immobilization of heavy metals by polynuclear aluminium and montmorillonite compounds. Environ. Sci. Technol. 1997, 31, 1452–1462. [Google Scholar] [CrossRef]
- Bailey, S.E.; Olin, T.J.; Bricka, R.M.; Adrian, D.D. A review of potentially low-cost sorbents for heavy metals. Water Res. 1999, 33, 2469–2479. [Google Scholar] [CrossRef]
- Yuan, P.; Li, D.; Fan, M.; Yang, D.; Zhu, R.; Ge, F.; Zhu, J.; He, H. Removal of hexavalent chromium [Cr(VI)] from aqueous solutions by the diatomite-supported/unsupported magnetite nanoparticles. J. Hazard. Mater. 2010, 173, 614–621. [Google Scholar] [CrossRef]
- Arancibia-Miranda, N.; Baltazar, S.E.; García, A.; Muñoz-Lira, D.; Sepúlveda, P.; Rubio, M.A.; Altbir, D. Nanoscale zero valent supported by Zeolite and Montmorillonite: Template effect of the removal of lead ion from an aqueous solution. J. Hazard. Mater. 2016, 301, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Wang, L.; Xu, Y. Chitosan and surfactant co-modified montmorillonite: A multifunctional adsorbent for contaminant removal. Appl. Clay Sci. 2017, 146, 35–42. [Google Scholar] [CrossRef]
- Doğan, M.; Turhan, Y.; Alkan, M.; Namli, H.; Turan, P.; Demirbaş, Ö. Functionalized sepiolite for heavy metal ions adsorption. Desalination 2008, 230, 248–268. [Google Scholar] [CrossRef]
- García, N.; Guzmán, J.; Benito, E.; Esteban-Cubillo, A.; Aguilar, E.; Santarén, J.; Tiemblo, P. Surface modification of sepiolite in aqueous gels by using methoxysilanes and its impact on the nanofiber dispersion ability. Langmuir 2011, 27, 3952–3959. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Hu, Q.; Chen, C.; Sun, Y.; Xu, D.; Sheng, G. New insights into Th(IV) speciation on sepiolite: Evidence for EXAFS and modeling investigation. Chem. Eng. J. 2017, 322, 66–72. [Google Scholar] [CrossRef]
- Kara, M.; Yuzer, H.; Sabah, E.; Celik, M.S. Adsorption of cobalt from aqueous solutions onto sepiolite. Water Res. 2003, 37, 224–232. [Google Scholar] [CrossRef]
- Sheng, G.; Xu, S.; Boyd, S.A. A dual function organoclay sorbent for lead and chlorobenzene. Soil Sci. Soc. Am. J. 1999, 63, 73–78. [Google Scholar] [CrossRef]
- Mercier, L.; Detellier, C. Preparation, characterization, and applications as heavy metals sorbents of covalently grafted thiol functionalities on the interlamellar surface of montmorillonite. Environ. Sci. Technol. 1995, 29, 1318–1323. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yu, K.; Liu, G. Chromium immobilization in soil using quaternary ammonium cations modified montmorillonite: Characterization and mechanism. J. Hazard. Mater. 2017, 321, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Mercier, L.; Pinnavaia, T.J. Heavy metal ion adsorbents formed by the grafting of a thiol functionality to mesoporous silica molecular sieves: Factors affecting Hg(II) uptake. Environ. Sci. Technol. 1998, 32, 2749–2754. [Google Scholar] [CrossRef]
- Theng, B.K.G.; Churchman, G.J.; Gates, W.P.; Yuan, G.D. Organically Modified Clays for Pollutant Uptake and Environmental Protection. In Soil Mineral Microbe-Organic Interactions; Huang, Q., Huang, P., Violante, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 145–174. [Google Scholar]
- Ma, L.; Chen, Q.; Zhu, J.; Xi, Y.; He, H.; Zhu, R.; Tao, Q.; Ayoko, G.A. Adsorption of phenol and Cu(II) onto cationic and zwitterionic surfactant modified montmorillonite in single and binary systems. Chem. Eng. J. 2016, 283, 880–888. [Google Scholar] [CrossRef]
- Liang, X.; Xu, Y.; Sun, G.; Wang, L.; Sun, Y.; Sun, Y.; Qin, X. Preparation and characterization of mercapto functionalized sepiolite and their application for sorption of lead and cadmium. Chem. Eng. J. 2011, 174, 436–444. [Google Scholar] [CrossRef]
- Al-Ani, A.; Gertisser, R.; Zholobenko, V. Structural features and stability of Spanish sepiolite as a potential catalyst. Appl. Clay Sci. 2018, 162, 297–304. [Google Scholar] [CrossRef]
- Wan, C.Y.; Chen, B.Q. Synthesis and characterization of biomimetic hydroxyapatite/sepiolite nanocomposites. Nanoscale 2011, 3, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.H.; Li, H.; Yao, Q.Z.; Zhou, G.T.; Fu, S.Q. Microwave-assisted preparation of sepiolite-supported magnetite nanoparticles and their ability to remove low concentrations of Cr(VI). RSC Adv. 2015, 5, 84471–84482. [Google Scholar] [CrossRef]
- Aznar, A.J.; Gutiérrez, E.; Díaz, P.; Alvarez, A.; Poncelet, G. Silica from sepiolite: Preparation, textural properties, and use as support to catalysts. Microporous Mater. 1996, 6, 105–114. [Google Scholar] [CrossRef]
- Lazarević, S.; Janković-Častvan, I.; Jovanović, D.; Milonjić, S.; Janaćković, D.; Petrović, R. Adsorption of Pb2+, Cd2+ and Sr2+ ions onto natural and acid-activated sepiolites. Appl. Clay Sci. 2007, 37, 47–57. [Google Scholar] [CrossRef]
- Myriam, M.; Suaárez, M.; Martín-Pozas, J.M. Structural and textural modifications of palygorskite and sepiolite under acid treatment. Clay Clay Miner. 1998, 46, 225–231. [Google Scholar] [CrossRef]
- Franco, F.; Pozo, M.; Cecilia, J.A.; Benítez-Guerrrero, M.; Pozo, E.; Martín-Rubí, J.A. Microwave assisted acid treatment of sepiolite: The role of composition and crystallinity. Appl. Clay Sci. 2014, 102, 15–27. [Google Scholar] [CrossRef]
- Liu, W.; Huang, F.; Wang, Y.; Zou, T.; Zheng, J.; Lin, Z. Recycling Mg(OH)2 nanoadsorbent during treating the low concentration of CrVI. Environ. Sci. Technol. 2011, 45, 1955–1961. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Yu, S.H.; Yao, Q.Z.; Fu, S.Q.; Zhou, G.T. One-step synthesis of Ag2O@Mg(OH)2 nanocomposite as an efficient scavenger for iodine and uranium. J. Colloid. Interf. Sci. 2018, 510, 280–291. [Google Scholar] [CrossRef] [PubMed]
- Venkatathri, N.; Srivastava, R.; Yun, D.S.; Yoo, J.W. Synthesis of a novel class of mesoporous hollow silica from organic templates. Micropor. Mesopor. Mat. 2008, 112, 147–152. [Google Scholar] [CrossRef]
- Shi, J.Y.; Yao, Q.Z.; Li, X.M.; Zhou, G.T.; Fu, S.Q. Formation of asymmetrical structured silica controlled by a phase separation process and implication for Biosilicification. PLoS ONE 2013, 8, e61164. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.C.; Chen, W.; Liu, C.; Liu, Y.; Dong, C.L. Magnetic mesoporous clay adsorbent: Preparation, characterization and adsorption capacity for atrazine. Microporous Mesoporous Mater. 2014, 194, 72–78. [Google Scholar] [CrossRef]
- Pokrovsky, O.S.; Schott, J. Experimental study of brucite dissolution and precipitation in aqueous solutions: Surface speciation and chemical affinity control. Geochim. Cosmochim. Acta 2004, 68, 31–45. [Google Scholar] [CrossRef]
- Moeller, T.; Fogel, N. Observations on the rare earths. LXI. precipitation of hydrous oxides or hydroxides from perchlorate solutions. JACS 1951, 73, 4481. [Google Scholar] [CrossRef]
- Babel, S.; Kurniawan, T.A. Low-cost adsorbents for heavy metals uptake from contaminated water: A review. J. Hazard. Mater. 2003, 97, 219–243. [Google Scholar] [CrossRef]
- Yanagihara, N.; Vemulapalli, K.; Fernando, Q.; Dyke, J.T. Synthesis of lanthanide carbonates. J. Less Common Met. 1991, 167, 223–232. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, X.; Tang, Q.; Li, Y. A new type of silica-coated Gd2(CO3)3: Tb nanoparticle as a bifunctional agent for magnetic resonance imaging and fluorescent imaging. Nanotechnology 2012, 23, 205103. [Google Scholar] [CrossRef]
- Nasution, E.Y.; Ahab, A.; Nuryadin, B.W.; Haryanto, F.; Arif, I.; Iskandar, F. Synthesis of gadolinium carbonate-conjugated-poly(ethylene)glycol (Gd2(CO3)3@PEG) particles via a modified solvothermal method. AIP Conf. Proc. 2016, 1710, 030001–030007. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, Q.-Z.; Yu, S.-H.; Zhao, T.-L.; Qian, F.-J.; Li, H.; Zhou, G.-T.; Fu, S.-Q. Enhanced Potential Toxic Metal Removal Using a Novel Hierarchical SiO2–Mg(OH)2 Nanocomposite Derived from Sepiolite. Minerals 2019, 9, 298. https://doi.org/10.3390/min9050298
Yao Q-Z, Yu S-H, Zhao T-L, Qian F-J, Li H, Zhou G-T, Fu S-Q. Enhanced Potential Toxic Metal Removal Using a Novel Hierarchical SiO2–Mg(OH)2 Nanocomposite Derived from Sepiolite. Minerals. 2019; 9(5):298. https://doi.org/10.3390/min9050298
Chicago/Turabian StyleYao, Qi-Zhi, Sheng-Hui Yu, Tian-Lei Zhao, Fei-Jin Qian, Han Li, Gen-Tao Zhou, and Sheng-Quan Fu. 2019. "Enhanced Potential Toxic Metal Removal Using a Novel Hierarchical SiO2–Mg(OH)2 Nanocomposite Derived from Sepiolite" Minerals 9, no. 5: 298. https://doi.org/10.3390/min9050298
APA StyleYao, Q.-Z., Yu, S.-H., Zhao, T.-L., Qian, F.-J., Li, H., Zhou, G.-T., & Fu, S.-Q. (2019). Enhanced Potential Toxic Metal Removal Using a Novel Hierarchical SiO2–Mg(OH)2 Nanocomposite Derived from Sepiolite. Minerals, 9(5), 298. https://doi.org/10.3390/min9050298



