Influence of Impurity Dissolution on Surface Properties and NH3-SCR Catalytic Activity of Rare Earth Concentrate
Abstract
:1. Introduction
2. Experiment
2.1. Materials
2.2. Sample Preparation
2.3. Analyses
2.3.1. Sample Analysis
2.3.2. Catalysis Activity Testing
3. Results and Discussion
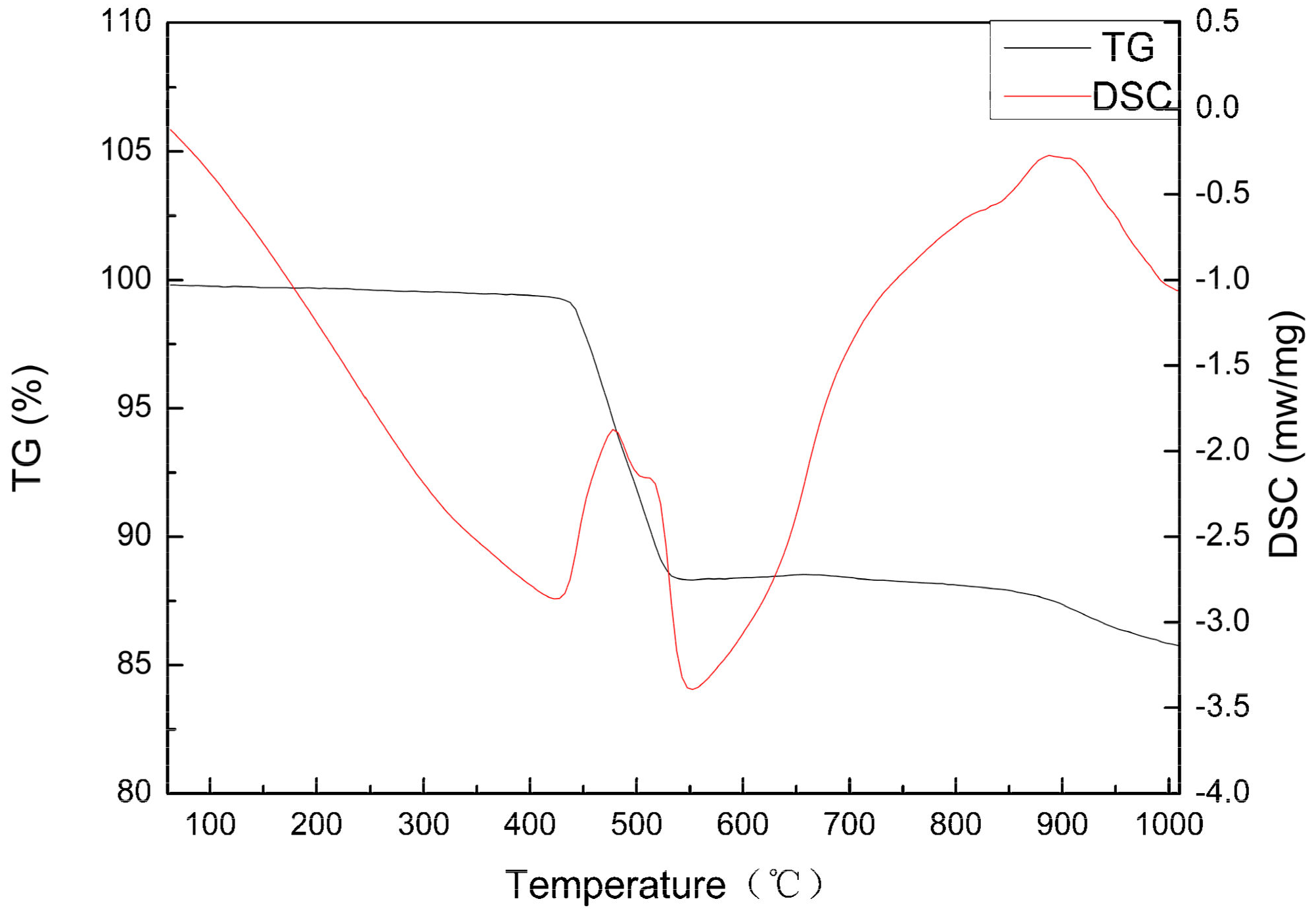
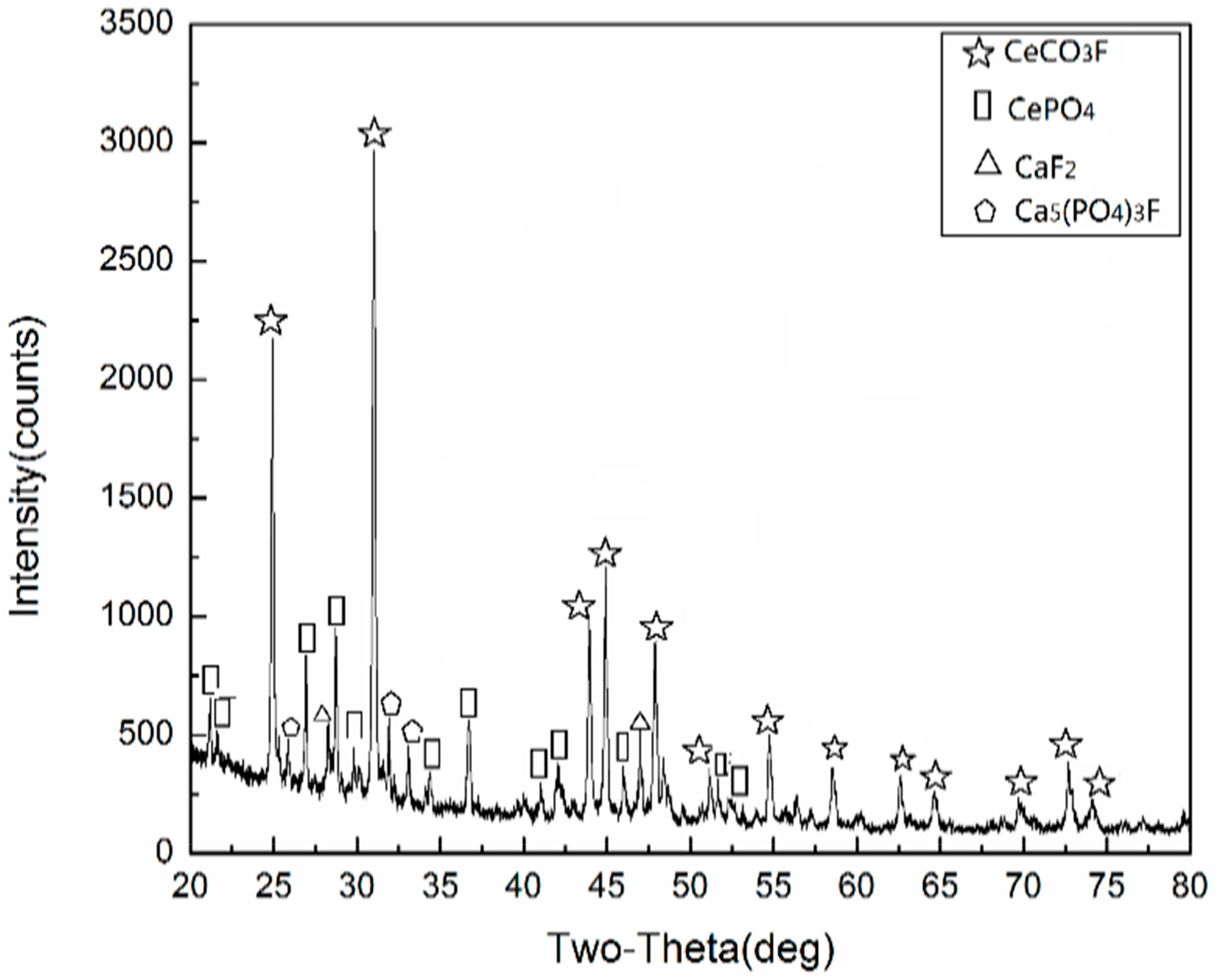
3.1. Characterization and Thermogravimetric Analysis of Rare Earth Concentrate
3.2. Catalytic Activity of Rare Earth Concentrate
3.3. Activity Test for Different Treatments of Concentrate
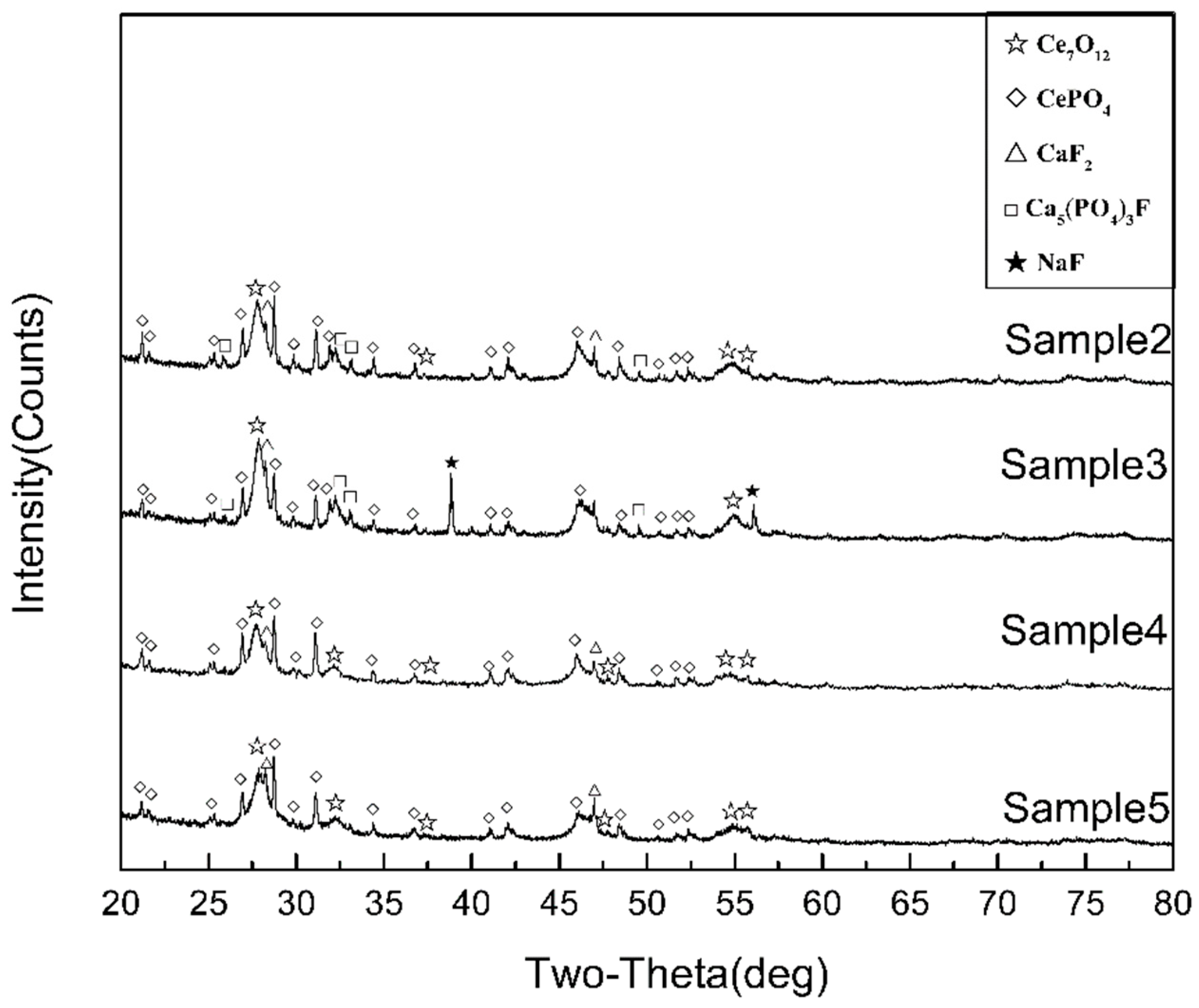
3.4. Properties and Micromorphology of Modified Rare Earth Concentrates
3.4.1. Properties of Modified Rare Earth Concentrates
Surface Properties
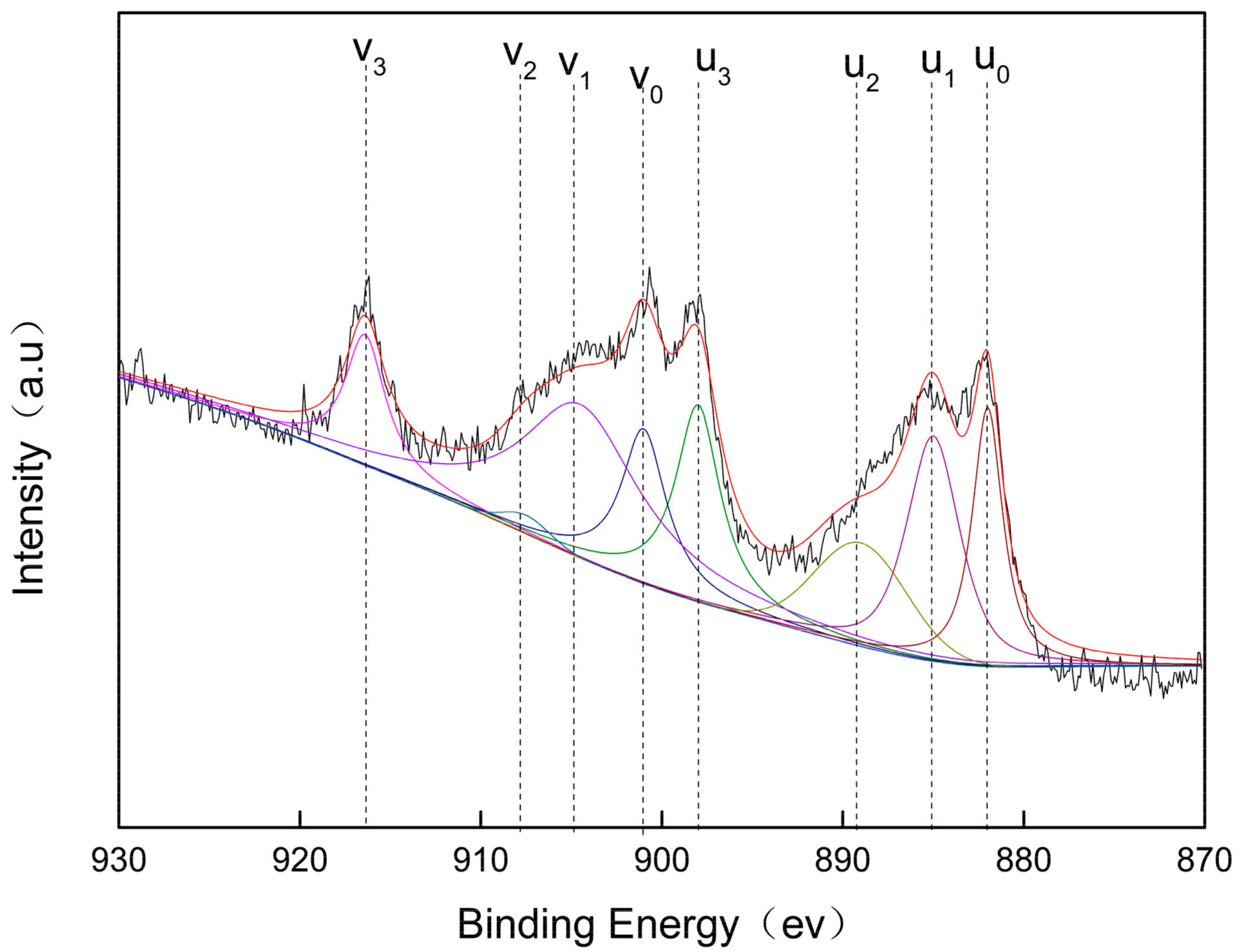
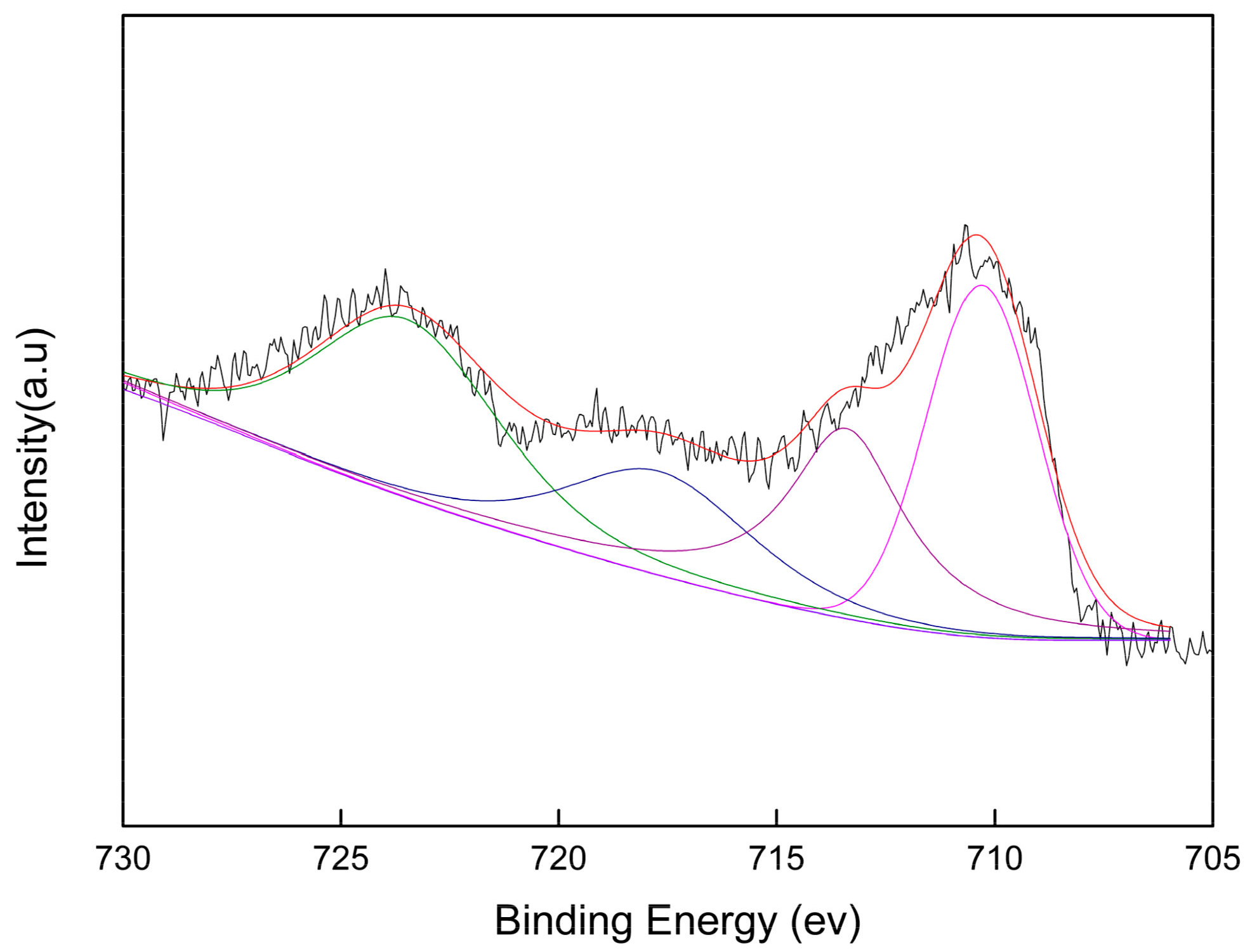
Valences of Ce and Fe Elements
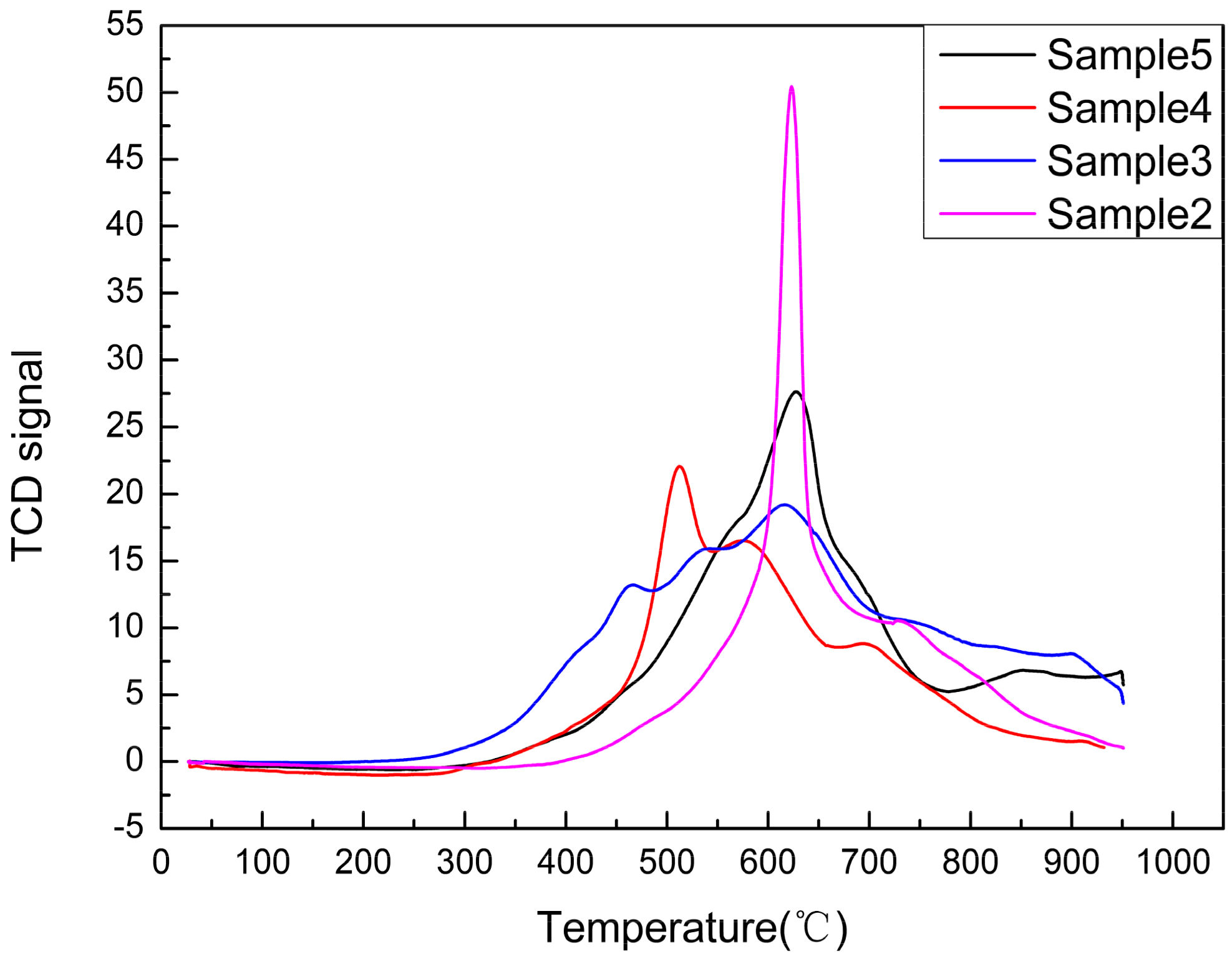
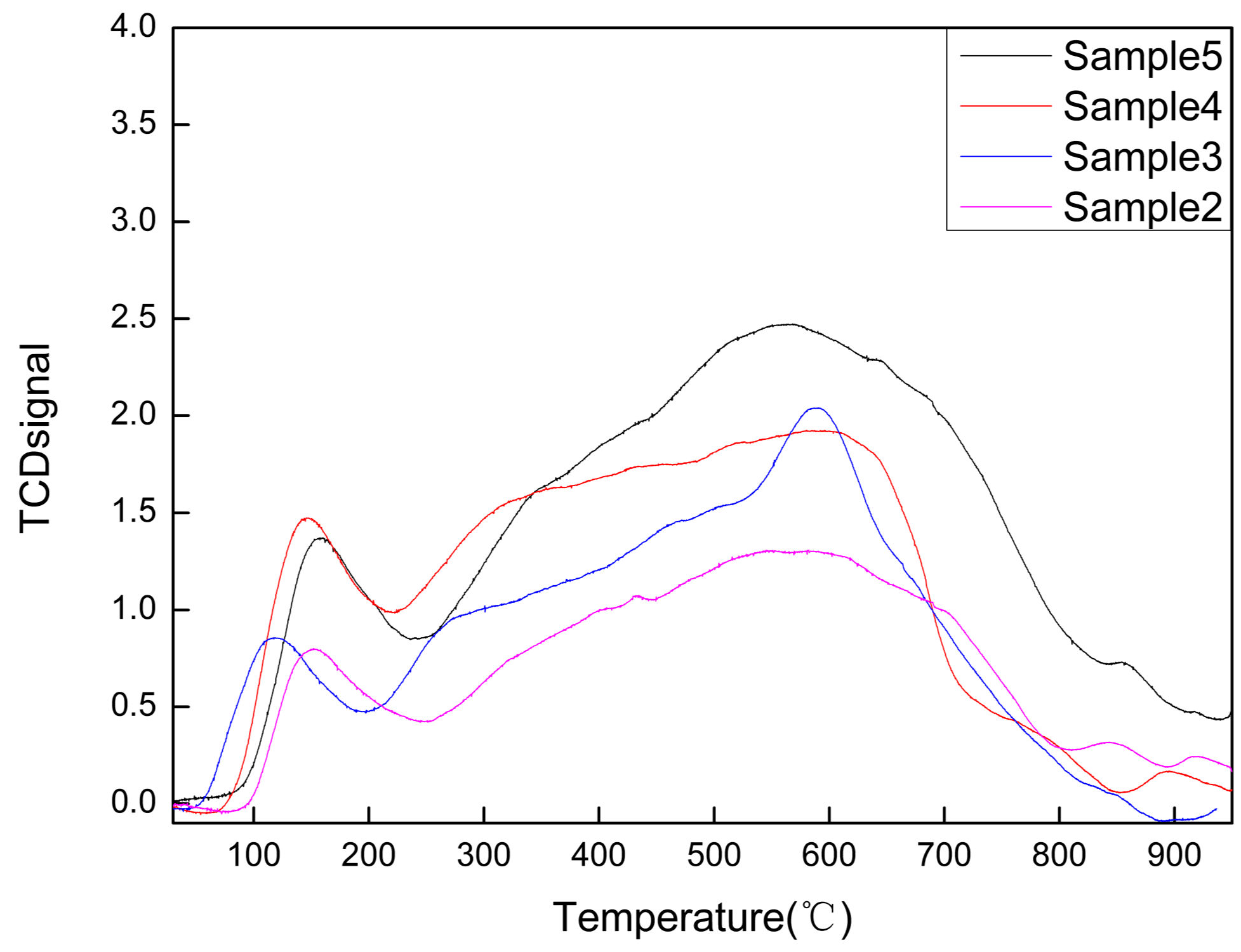
Surface Reduction Ability Characterization
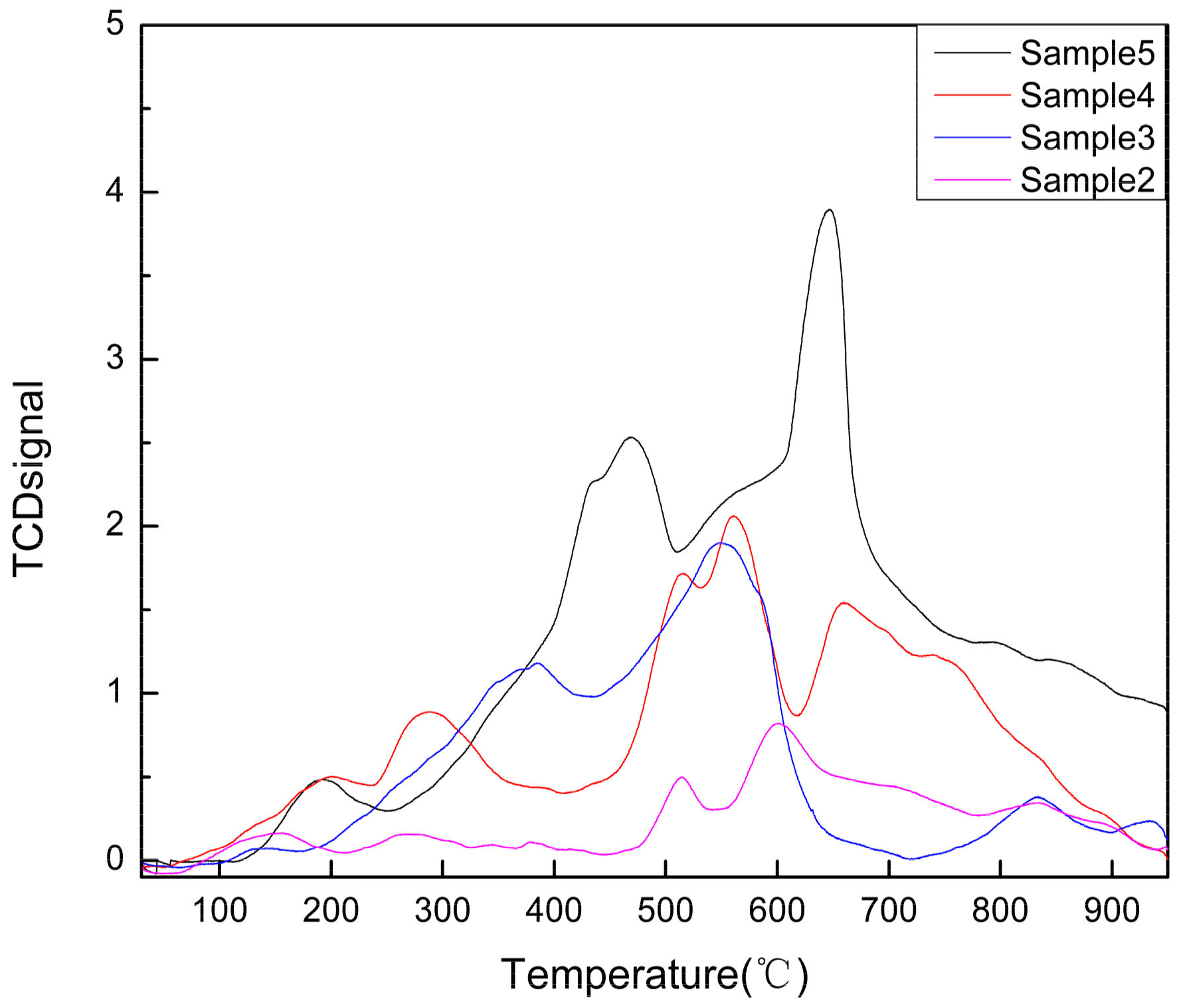
Surface Acidity Characterization
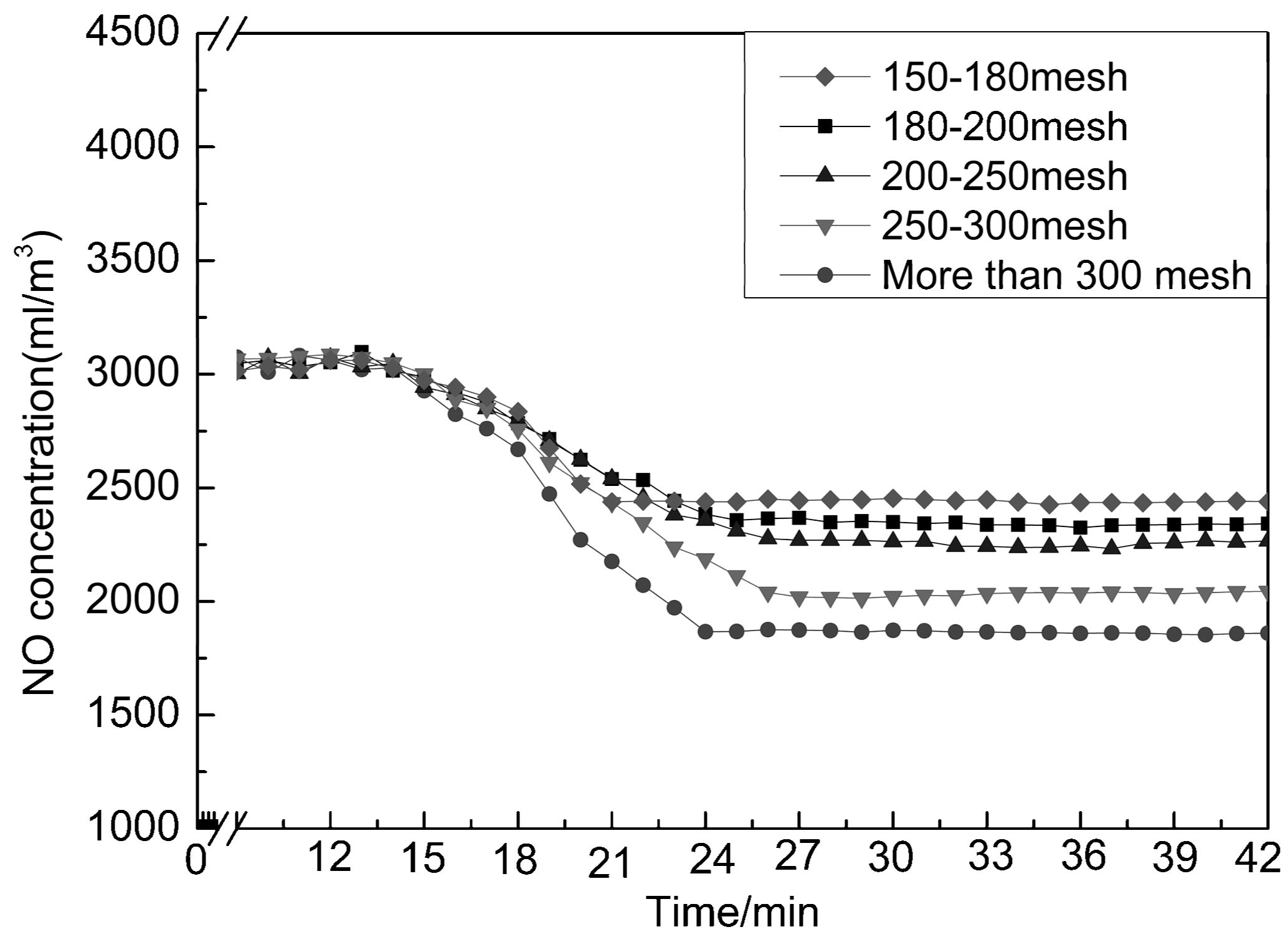
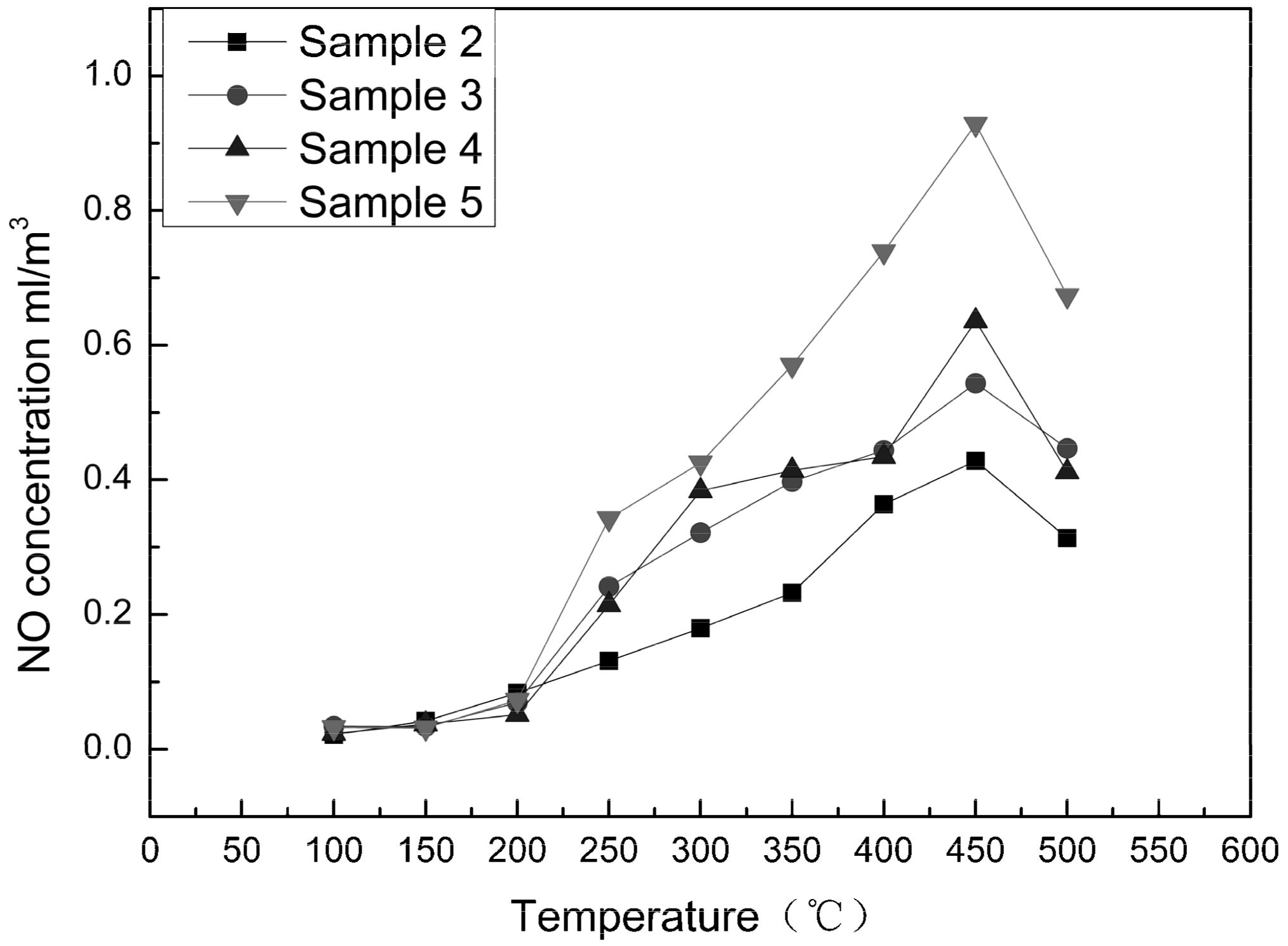
Characterization of Adsorption Capacity of NO
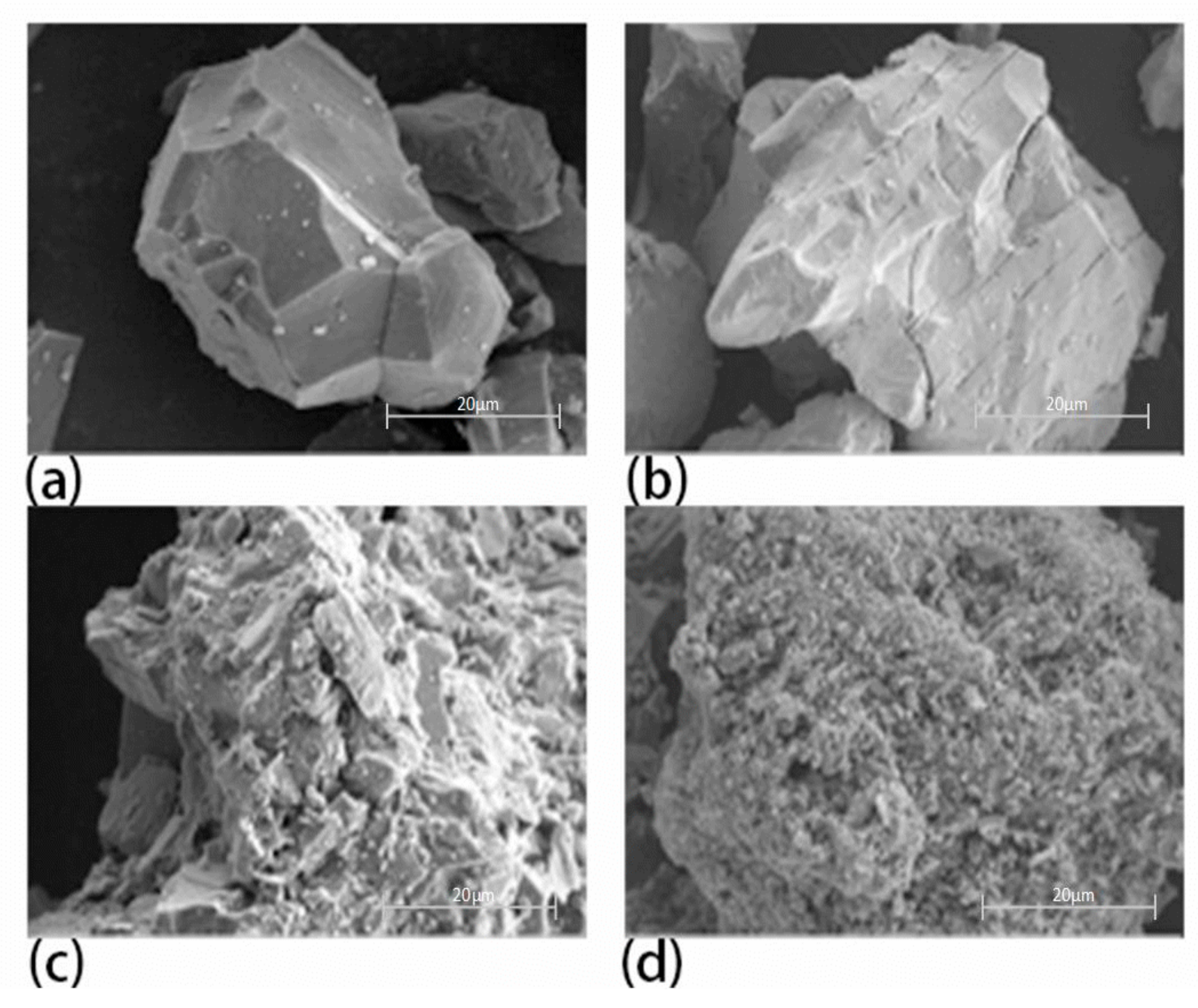
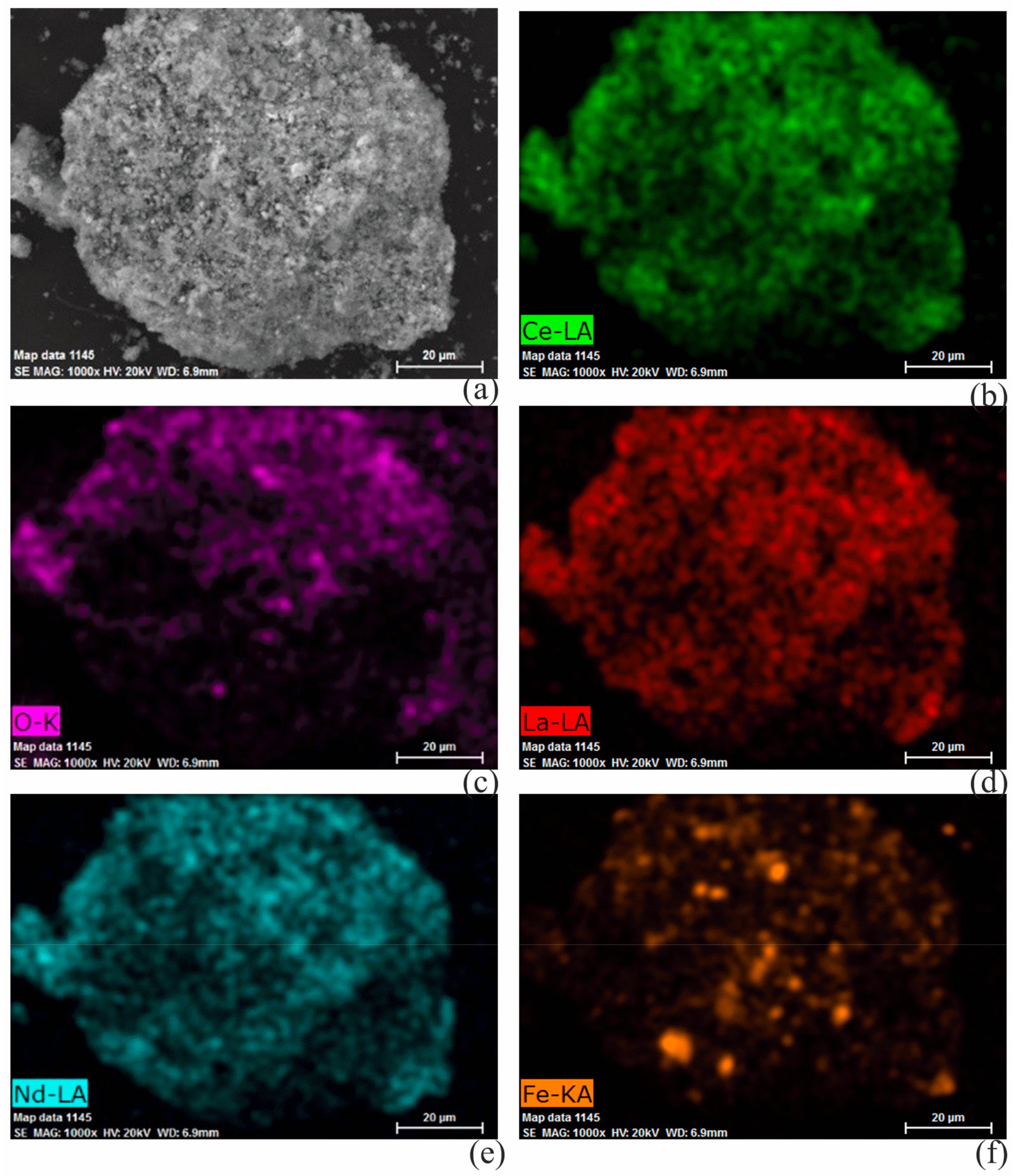
3.4.2. Microscopic Appearance of the Modified Rare Earth Concentrate
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shi, Z.; Yang, X.; Yan, S. Photocatalytic activity of cerium-doped mesoporous TiO2 coated Fe3O4 magnetic composite under UV and visible light. J. Rare Earths 2012, 30, 355–360. [Google Scholar] [CrossRef]
- Al-Musa, A.; Al-Saleh, M.; Ioakeimidis, Z.C.; Ouzounidou, M.; Yentekakis, I.V.; Konsolakis, M.; Marnellos, G.E. Hydrogen production by iso-octane steam reforming over Cu catalysts supported on rare earth oxides (REOs). Int. J. Hydrogen Energy 2014, 39, 1350–1363. [Google Scholar] [CrossRef]
- Thirupathi, B.; Smirniotis, P.G. Co-doping a metal (Cr, Fe, Co, Ni, Cu, Zn, Ce, and Zr) on Mn/TiO2 catalyst and its effect on the selective reduction of NO with NH3 at low-temperatures. Appl. Catal. B Environ. 2011, 110, 195–206. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kwon, H.J.; Heo, I.; Nam, I.-S.; Cho, B.K.; Choung, J.W.; Cha, M.-S.; Yeo, G.K. Mn–Fe/ZSM5 as a low-temperature SCR catalyst to remove NOx from diesel engine exhaust. Appl. Catal. B Environ. 2012, 126, 9–21. [Google Scholar] [CrossRef]
- Yang, S.; Qi, F.; Xiong, S.; Dang, H.; Liao, Y.; Keung, W.; Li, J. MnOX supported on Fe–Ti spinel: A novel Mn based low temperature SCR catalyst with a high N2 selectivity. Appl. Catal. B Environ. 2016, 181, 570–580. [Google Scholar] [CrossRef]
- Casanova, M.; Schermanz, K.; Llorca, J.; Trovarelli, A. Improved high temperature stability of NH3-SCR catalysts based on rare earth vanadates supported on TiO2–WO3–SiO2. Catal. Today 2012, 184, 227–236. [Google Scholar] [CrossRef]
- Wei, Y.; Chen, Y.; Wang, R. Rare earth salt of 12-tungstophosphoric acid supported on iron oxide as a catalyst for selective catalytic reduction of NOX. Fuel Process. Technol. 2018, 178, 262–270. [Google Scholar] [CrossRef]
- Zhao, K.; Meng, J.; Lu, J.; He, Y.; Huang, H.; Tang, Z.; Zhen, X. Sol-gel one-pot synthesis of efficient and environmentally friendly iron-based catalysts for NH3-SCR. Appl. Surf. Sci. 2018, 445, 454–461. [Google Scholar] [CrossRef]
- Shi, A.; Wang, X.; Yu, T.; Shen, M. The effect of zirconia additive on the activity and structure stability of V2O5/WO3–TiO2 ammonia SCR catalysts. Appl. Catal. B Environ. 2011, 106, 359–369. [Google Scholar] [CrossRef]
- Vuong, T.H.; Radnik, J.; Rabeah, J.; Bentrup, U.; Scheneider, M.; Atia, H.; Armbruster, U.; Grunert, W.; Bruckner, A. Efficient VOX/Ce–XTiXO2 Catalysts for Low-Temperature NH3-SCR: Reaction Mechanism and Active Sites Assessed by in Situ/Operando Spectroscopy. Acs Catal. 2017, 7, 1693–1705. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, Y.; Wang, X.; Xu, H.; Sun, K.; Shen, K. Influence of the addition of transition metals (Cr, Zr, Mo) on the properties of MnOX–FeOX catalysts for low-temperature selective catalytic reduction of NOX by Ammonia. J. Colloid. Interface Sci. 2013, 392, 319–324. [Google Scholar] [CrossRef]
- Yue, B.; Zhou, R.; Wang, Y.; Zheng, X. Influence of transition metals (Cr, Mn, Fe, Co and Ni) on the methane combustion over Pd/Ce–Zr/Al2O3 catalyst. Appl. Surf. Sci. 2006, 252, 5820–5828. [Google Scholar] [CrossRef]
- Fernandez-Garcia, M.; Martinez-Arias, A.; Iglesias-Juez, A.; Hungria, A.B.; Anderson, J.A.; Conesa, J.C.; Soria, J. Behavior of bimetallic Pd/Cr/Al2O3 and Pd/Cr/(Ce,Zr)OX/Al2O3 catalysts for CO and NO elimination. J. Catal. 2003, 214, 220–233. [Google Scholar] [CrossRef]
- He, H.; Dai, H.X.; Au, C.T. Defective structure, oxygen mobility, oxygen storage capacity, and redox properties of RE-based (RE = Ce, Pr) solid solutions. Catal. Today 2004, 90, 245–254. [Google Scholar] [CrossRef]
- Tran, U.P.N.; Le, K.K.A.; Phan, N.T.S. Expanding Applications of Metal−Organic Frameworks: Zeolite Imidazolate Framework ZIF-8 as an Efficient Heterogeneous Catalyst for the Knoevenagel Reaction. ACS Catal. 2011, 1, 120–127. [Google Scholar] [CrossRef]
- Lan, X.; Gao, J.; Du, Y.; Guo, Z. Mineral evolution and separation of rare-earth phases from Bayan Obo rare-earth concentrate in a super-gravity field. J. Alloy. Compd. 2018, 731, 873–880. [Google Scholar] [CrossRef]
- Wang, L.; Liang, T.; Zhang, Q.; Li, K. Rare earth element components in atmospheric particulates in the Bayan Obo mine region. Environ. Res. 2014, 131, 64–70. [Google Scholar] [CrossRef]
- Xu, C.; Campbell, L.H.; Kynicky, J.; Allen, C.M.; Chen, Y.; Huang, Z.; Qi, L. Comparison of the Daluxiang and Maoniuping carbonatitic REE deposits with Bayan Obo REE deposit, China. Lithos 2008, 106, 12–24. [Google Scholar] [CrossRef]
- Wu, Z.; Jiang, B.; Liu, Y. Effect of transition metals addition on the catalyst of manganese/titania for low-temperature selective catalytic reduction of nitric oxide with ammonia. Appl. Catal. B Environ. 2008, 79, 347–355. [Google Scholar] [CrossRef]
- Gao, G.; Shi, J.-W.; Liu, C.; Cao, C.; Fan, Z.; Niu, C. Mn/CeO2 catalysts for SCR of NOX with NH3: Comparative study on the effect of supports on low-temperature catalytic activity. Appl. Surf. Sci. 2017, 411, 338–346. [Google Scholar] [CrossRef]
- Yang, J.; Ma, H.; Yanamoto, Y.; Yu, J.; Xu, G.; Zhang, Z.; Suzuki, Y. SCR catalyst coated on low-cost monolith support for flue gas denitration of industrial furnaces. Chem. Eng. J. 2013, 230, 513–521. [Google Scholar] [CrossRef]
- Yao, X.; Kong, T.; Yu, S.; Li, L.; Yang, F.; Dong, L. Influence of different supports on the physicochemical properties and denitration performance of the supported Mn-based catalysts for NH3-SCR at low temperature. Appl. Surf. Sci. 2017, 402, 208–217. [Google Scholar] [CrossRef]
- Xu, C.; Liu, J.; Zhao, Z.; Yu, F.; Cheng, K.; Wei, Y.; Duan, A.; Jiang, G. NH3-SCR denitration catalyst performance over vanadium-titanium with the addition of Ce and Sb. J. Environ. Sci. 2015, 31, 74–80. [Google Scholar] [CrossRef]
- Jiang, B.; Li, Z.; Lee, S.-C. Mechanism study of the promotional effect of O2 on low-temperature SCR reaction on Fe–Mn/TiO2 by DRIFT. Chem. Eng. J. 2013, 225, 52–58. [Google Scholar] [CrossRef]
- Klukowski, D.; Ballea, P.; Geigera, B.; Wagloehnera, S.; Kuretia, S.; Kimmerleb, B.; Baikerb, A.; Grunwaldtc, J.-D. On the mechanism of the SCR reaction on Fe/HBEA zeolite. Appl. Catal. B Environ. 2009, 93, 185–193. [Google Scholar] [CrossRef]
- Priolkar, K.R.; Parthasarathi Bera, P.R.; Sarode, M.S.; Hegde, S.; Emura, S.; Kumashiro, R.; Lalla, N. Formation of Ce1-xPdxO2-δ solid solution in combustion-synthesized Pd/CeO2 catalyst: XRD, XPS, and EXAFS investigation. Chem. Mater. 2002, 14, 2120–2128. [Google Scholar] [CrossRef]
- Yamashita, T.; Hayes, P. Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials. Appl. Surf. Sci. 2008, 254, 2441–2449. [Google Scholar] [CrossRef]
| Composition | Sample 1 (%) | Sample 3 (%) | Sample 4 (%) | Sample 5 (%) |
|---|---|---|---|---|
| Ce | 24.200 | 23.894 | 24.616 | 31.509 |
| O | 22.600 | 31.319 | 35.596 | 26.298 |
| Ca | 16.500 | 5.162 | 5.434 | 5.612 |
| La | 12.600 | 12.614 | 10.857 | 15.513 |
| Fe | 7.530 | 7.490 | 7.894 | 10.824 |
| Nd | 7.100 | 4.125 | 3.424 | 5.285 |
| P | 2.190 | 2.400 | 1.643 | 1.478 |
| Pr | 2.070 | 1.921 | 1.544 | 1.401 |
| Ba | 1.490 | 0.219 | 0.201 | 0.159 |
| Si | 0.732 | 0.508 | 0.514 | 0.322 |
| S | 0.559 | 0.412 | 0.157 | 0.142 |
| Mn | 0.512 | 0.527 | 0.631 | 0.465 |
| Zn | 0.210 | 0.030 | 0.109 | 0.116 |
| Ti | 0.201 | 0.119 | 0.087 | 0.067 |
| Gd | 0.190 | 1.275 | 1.156 | 0.059 |
| Th | 0.166 | 0.043 | 0.034 | 0.037 |
| Y | 0.155 | 0.046 | 0.037 | 0.091 |
| Sr | 0.132 | 0.032 | 0.032 | 0.037 |
| Nb | 0.120 | 0.021 | 0.018 | 0.019 |
| Pb | 0.081 | 0.150 | 0.109 | 0.279 |
| Mg | 0.065 | 0.857 | 0.974 | 0.547 |
| K | 0.061 | 0.030 | 0.029 | 0.018 |
| Al | 0.029 | 0.158 | 0.119 | 0.101 |
| Cu | 0.015 | 0.011 | 0.013 | 0.009 |
| Sm | 0.454 | 0.427 | 0.431 | 0.361 |
| Sample Serial Number | Raw Material | Sample Preparation | Sample | Test Methods | ||
|---|---|---|---|---|---|---|
| Drying Temperature | Calcination Temperature | Roasting Time | ||||
| Sample 1 | Concentrate | 110 °C | - | - | Concentrate | XRD/TG-DSC/SEM |
| Sample 2 | Concentrate | 110 °C | 500 °C | 4 h | Direct roasting concentrate | XRD/BET/H2-TPR/NH3-TPD/NO-TPD/SEM |
| Sample 3 | NaHCO3 (4 g) + Concentrate (10 g) + H2O (50 mL) | 110 °C | 500 °C | 4 h | Alkali treatment concentrate | XRD/BET/H2-TPR/NH3-TPD/NO-TPD/SEM |
| Sample 4 | H2C2O4 (4 g) + Concentrate (10 g) + H2O (50 mL) | 110 °C | 500 °C | 4 h | Acid treated concentrate | XRD/BET/H2-TPR/NH3-TPD/NO-TPD |
| Sample 5 | NaHCO3 (4 g) + H2C2O4 (4 g) + Concentrate (10 g) + H2O (50 mL) | 110 °C | 500 °C | 4 h | Acid-base co-processing concentrate | XRD/BET/XPS/H2-TPR/NH3-TPD/NO-TPD/SEM |
| Physical Properties | Sample 2 | Sample 3 | Sample 4 | Sample 5 |
|---|---|---|---|---|
| Specific surface area (m2/g) | 154.9 | 190.5 | 212.4 | 208.0 |
| Pore volume (mL/g) | 0.4 | 0.4 | 0.57 | 0.6 |
| Average aperture (nm) | 12.7 | 11.9 | 10.1 | 10.7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, K.; Zhu, J.; Zhang, S.; Li, N.; Luo, H.; Li, B.; Zhao, Z.; Jin, G.; Wu, W. Influence of Impurity Dissolution on Surface Properties and NH3-SCR Catalytic Activity of Rare Earth Concentrate. Minerals 2019, 9, 246. https://doi.org/10.3390/min9040246
Zhang K, Zhu J, Zhang S, Li N, Luo H, Li B, Zhao Z, Jin G, Wu W. Influence of Impurity Dissolution on Surface Properties and NH3-SCR Catalytic Activity of Rare Earth Concentrate. Minerals. 2019; 9(4):246. https://doi.org/10.3390/min9040246
Chicago/Turabian StyleZhang, Kai, Jinhao Zhu, Shuning Zhang, Na Li, Huijuan Luo, Baowei Li, Zengwu Zhao, Guang Jin, and Wenfei Wu. 2019. "Influence of Impurity Dissolution on Surface Properties and NH3-SCR Catalytic Activity of Rare Earth Concentrate" Minerals 9, no. 4: 246. https://doi.org/10.3390/min9040246
APA StyleZhang, K., Zhu, J., Zhang, S., Li, N., Luo, H., Li, B., Zhao, Z., Jin, G., & Wu, W. (2019). Influence of Impurity Dissolution on Surface Properties and NH3-SCR Catalytic Activity of Rare Earth Concentrate. Minerals, 9(4), 246. https://doi.org/10.3390/min9040246






