Effects of Single and Mixed Energy Sources on Intracellular Nanoparticles Synthesized by Acidithiobacillus ferrooxidans
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Enrichment
2.2. Experimental Design
2.3. Analytical Methods
2.4. TEM Analysis
2.5. XANES Analysis
3. Results and Discussion
3.1. Growth Characteristics
3.2. Morphology of Bacteria
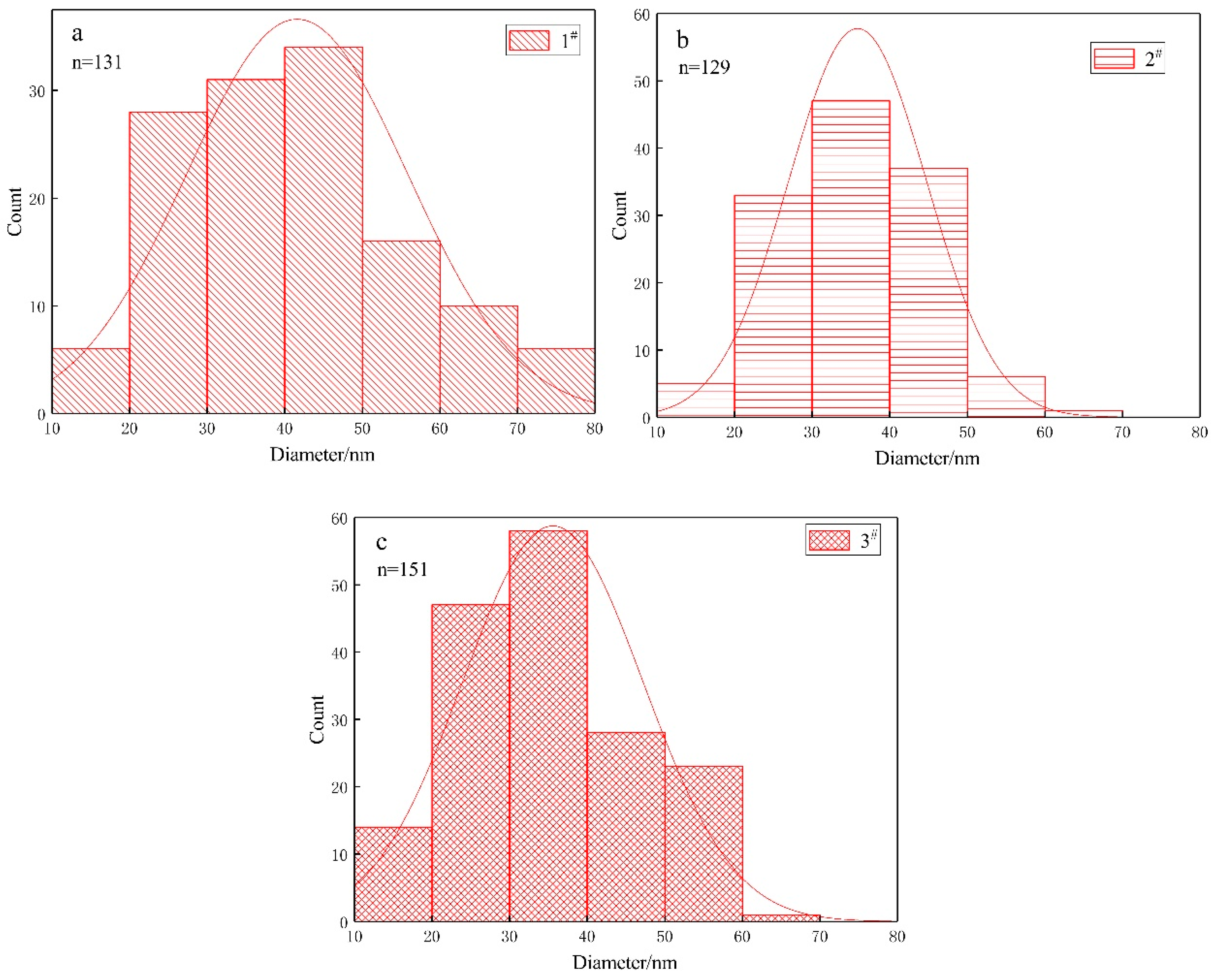
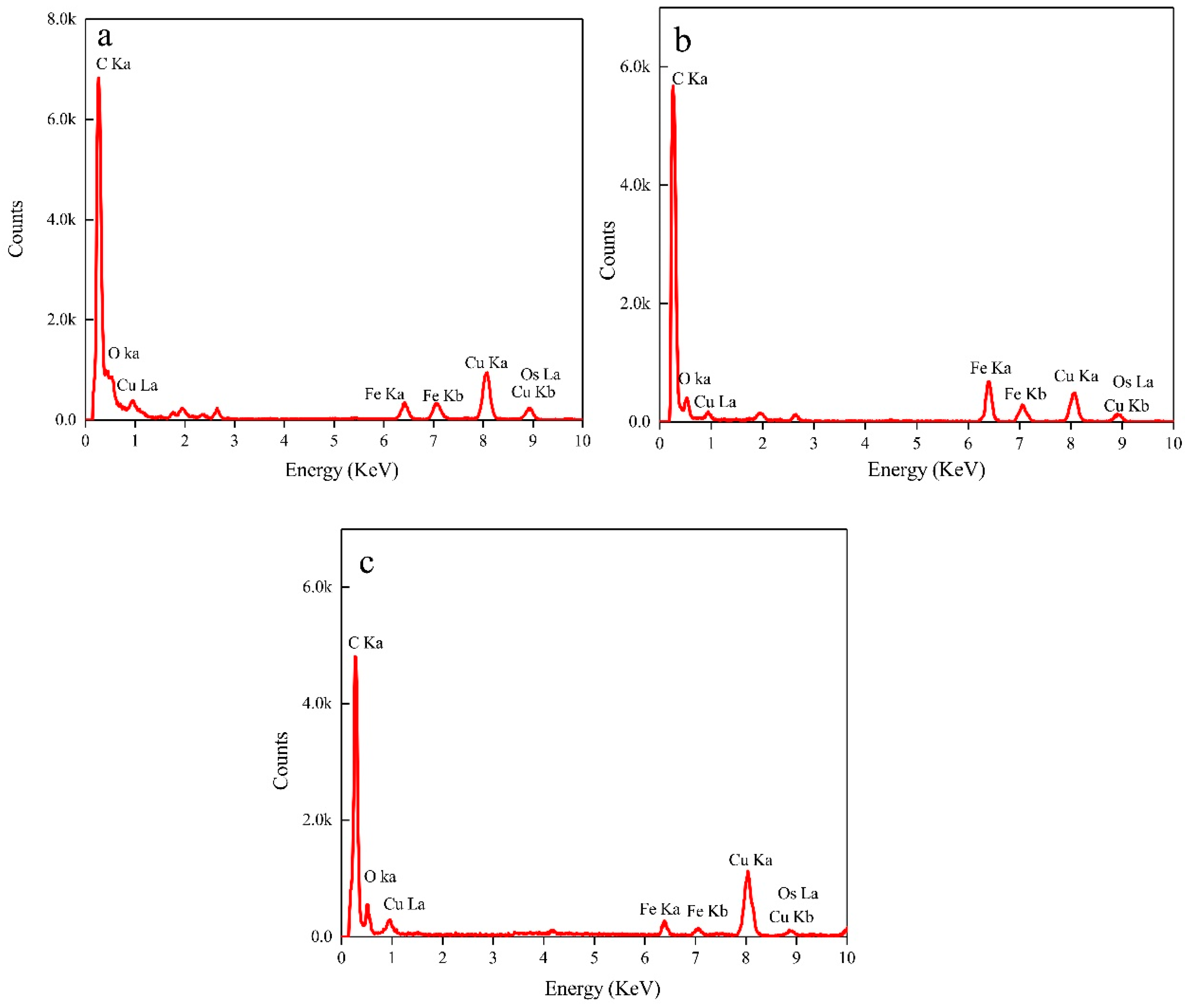
3.3. Nanoparticles
4. Conclusions
- In the ferrous-containing medium, more elemental sulphur in the medium resulted in an increased delay in microbial growth and ferrous iron oxidation.
- Different ratios of ferrous and sulphur had no significant effect on the morphology of bacteria or the size of nanoparticles synthesized by A. ferroxidans.
- EDX, HRTEM, and XANES results showed that the nanoparticles were composed of magnetite.
Author Contributions
Funding
Conflicts of Interest
References
- Duan, X.P.; Li, Y.P. Physicochemical Characteristics of Nanoparticles Affect Circulation, Biodistribution, Cellular Internalization, and Trafficking. Small 2013, 9, 1521–1532. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, K.B.; Sakthivel, N. Biological synthesis of metal nanoparticles by microbes. Adv. Colloid Interface Sci. 2010, 156, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Verma, P.; Maheshwari, S.K. Applications of Silver nanoparticles in diverse sectors. Int. J. Nano Dimens. 2019, 10, 18–36. [Google Scholar]
- De Matteis, V.; Cascione, M.; Toma, C.; Leporatti, S. Silver nanoparticles: Synthetic routes, in vitro toxicity and theranostic applications for cancer disease. Nanomaterials 2018, 8, 319. [Google Scholar] [CrossRef] [PubMed]
- Tartaj, P.; del Puerto Morales, M.; Veintemillas-Verdaguer, S.; González-Carreño, T.; Serna, C.J. The preparation of magnetic nanoparticles for applications in biomedicine. J. Phys. D Appl. Phys. 2003, 36, R182. [Google Scholar] [CrossRef]
- Blakemore, R. Magnetotactic bacteria. Science 1975, 190, 377–379. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.W.; Zheng, Y.; Hong, Z.L.; Cai, K.; Zhao, F.; Han, H.Y. Microbial synthesis of highly dispersed PdAu alloy for enhanced electrocatalysis. Sci. Adv. 2016, 2, 8. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.L.; Li, Y.C.; Yin, H.J.; Liu, Z.Z.; Luan, E.X.; Zhao, F.; Tang, Z.Y.; Liu, S.Q. Three-dimensional graphene/Pt nanoparticle composites as freestanding anode for enhancing performance of microbial fuel cells. Sci. Adv. 2015, 1, 8. [Google Scholar] [CrossRef] [PubMed]
- Lefèvre, C.T.; Trubitsyn, D.; Abreu, F.; Kolinko, S.; Jogler, C.; de Almeida, L.G.P.; de Vasconcelos, A.T.R.; Kube, M.; Reinhardt, R.; Lins, U. Comparative genomic analysis of magnetotactic bacteria from the D eltaproteobacteria provides new insights into magnetite and greigite magnetosome genes required for magnetotaxis. Environ. Microbiol. 2013, 15, 2712–2735. [Google Scholar]
- Kolinko, S.; Richter, M.; Glöckner, F.O.; Brachmann, A.; Schüler, D. Single-cell genomics of uncultivated deep-branching magnetotactic bacteria reveals a conserved set of magnetosome genes. Environ. Microbiol. 2016, 18, 21–37. [Google Scholar] [CrossRef]
- Uebe, R.; Schüler, D. Magnetosome biogenesis in magnetotactic bacteria. Nat. Rev. Microbiol. 2016, 14, 621. [Google Scholar] [CrossRef] [PubMed]
- Jacob, J.J.; Suthindhiran, K. Magnetotactic bacteria and magnetosomes–Scope and challenges. Mater. Sci. Eng. C 2016, 68, 919–928. [Google Scholar] [CrossRef]
- Faivre, D.; Schuler, D. Magnetotactic bacteria and magnetosomes. Chem. Rev. 2008, 108, 4875–4898. [Google Scholar] [CrossRef] [PubMed]
- Keim, C.N.; Lins, U.; Farina, M. Manganese in biogenic magnetite crystals from magnetotactic bacteria. FEMS Microbiol. Lett. 2009, 292, 250–253. [Google Scholar] [CrossRef] [PubMed]
- Mathuriya, A.S. Magnetotactic bacteria: Nanodrivers of the future. Crit. Rev. Biotechnol. 2016, 36, 788–802. [Google Scholar] [CrossRef] [PubMed]
- Yoza, B.; Matsumoto, M.; Matsunaga, T. DNA extraction using modified bacterial magnetic particles in the presence of amino silane compound. J. Biotechnol. 2002, 94, 217–224. [Google Scholar] [CrossRef]
- Herborn, C.; Papanikolaou, N.; Reszka, R.; Grünberg, K.; Schüler, D.; Debatin, J. Magnetosomes as biological model for iron binding: Relaxivity determination with MRI. RoFo Fortschr. Geb. Rontgenstrahlen Nukl. 2003, 175, 830–834. [Google Scholar]
- Schuler, D.; Frankel, R.B. Bacterial magnetosomes: Microbiology, biomineralization and biotechnological applications. Appl. Microbiol. Biotechnol. 1999, 52, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.T.; Guo, W.S.; Le, W.J.; Lv, G.X.; Zhang, F.H.; Shi, L.; Wang, X.L.; Wang, J.; Wang, S.; Chang, J.; et al. Albumin-Bioinspired Gd:CuS Nanotheranostic Agent for In Vivo Photoacoustic/Magnetic Resonance Imaging-Guided Tumor-Targeted Photothermal Therapy. Acs Nano 2016, 10, 10245–10257. [Google Scholar] [CrossRef]
- Bazylinski, D.A.; Frankel, R.B. Magnetosome formation in prokaryotes. Nat. Rev. Microbiol. 2004, 2, 217. [Google Scholar] [CrossRef]
- Bazylinski, D.A.; Lefevre, C.T. Magnetotactic Bacteria from Extreme Environments. Life-Basel 2013, 3, 295–307. [Google Scholar] [CrossRef]
- Yan, L.; Da, H.Y.; Zhang, S.; Lopez, V.M.; Wang, W.D. Bacterial magnetosome and its potential application. Microbiol. Res. 2017, 203, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Araujo, A.C.V.; Abreu, F.; Silva, K.T.; Bazylinski, D.A.; Lins, U. Magnetotactic Bacteria as Potential Sources of Bioproducts. Mar. Drugs 2015, 13, 389–430. [Google Scholar] [CrossRef] [PubMed]
- Brierley, C.L.; Brierley, J.A. Progress in bioleaching: Part B: Applications of microbial processes by the minerals industries. Appl. Microbiol. Biotechnol. 2013, 97, 7543–7552. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wang, J.; Hu, M.; Qin, W.; Zhang, Y.; Qiu, G. Synergistic bioleaching of chalcopyrite and bornite in the presence of Acidithiobacillus ferrooxidans. Bioresour. Technol. 2013, 149, 71–76. [Google Scholar] [CrossRef]
- Olson, G.J.; Brierley, J.A.; Brierley, C.L. Bioleaching review part B: Progress in bioleaching: Applications of microbial processes by the minerals industries. Appl. Microbiol. Biotechnol. 2003, 63, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Jafari, M.; Shafaei, S.Z.A.; Abdollahi, H.; Gharabaghi, M.; Chelgani, S.C. A Comparative Study on the Effect of Flotation Reagents on Growth and Iron Oxidation Activities of Leptospirillum ferrooxidans and Acidithiobacillus ferrooxidans. Minerals 2017, 7, 2. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Qin, W.Q.; Wang, J.; Zhen, S.J.; Yang, C.R.; Zhang, J.W.; Nai, S.S.; Qiu, G.Z. Bioleaching of chalcopyrite by pure and mixed culture. Trans. Nonferrous Met. Soc. China 2008, 18, 1491–1496. [Google Scholar] [CrossRef]
- Wei, D.P.; Liu, T.; Zhang, Y.M.; Cai, Z.L.; He, J.T.; Xu, C.B. Vanadium Bioleaching Behavior by Acidithiobacillus ferrooxidans from a Vanadium-Bearing Shale. Minerals 2018, 8, 24. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Q.F.; Zhu, J.Y.; Zhou, S.; Gan, M.; Jiang, H.; Sand, W. Effect of Extracellular Polymeric Substances on Surface Properties and Attachment Behavior of Acidithiobacillus ferrooxidans. Minerals 2016, 6, 100. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, H.B.; Qin, W.Q.; Qiu, G.Z. Bioleaching of complex polymetallic sulfide ores by mixed culture. J. Cent. South Univ. 2014, 21, 2633–2637. [Google Scholar] [CrossRef]
- Zhao, H.; Huang, X.; Wang, J.; Li, Y.; Liao, R.; Wang, X.; Qiu, X.; Xiong, Y.; Qin, W.; Qiu, G. Comparison of bioleaching and dissolution process of p-type and n-type chalcopyrite. Miner. Eng. 2017, 109, 153–161. [Google Scholar] [CrossRef]
- Liu, W.B.; Wu, H.Y.; Liu, X.D.; Liu, X.X. Influence of different Fe sources and concentrations on formation of magnetosomes in Acidithiobacillus ferrooxidans. Trans. Nonferrous Met. Soc. China 2008, 18, 1379–1385. [Google Scholar] [CrossRef]
- Yan, L.; Zhang, S.; Liu, H.; Wang, W.; Chen, P.; Li, H. Optimization of magnetosome production by Acidithiobacillus ferrooxidans using desirability function approach. Mater. Sci. Eng. C 2016, 59, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Yue, X.X.; Zhang, S.; Chen, P.; Xu, Z.L.; Li, Y.; Li, H.Y. Biocompatibility evaluation of magnetosomes formed by Acidithiobacillus ferrooxidans. Mat. Sci. Eng. C-Mater. 2012, 32, 1802–1807. [Google Scholar] [CrossRef]
- Zhang, S.; Yan, L.; Li, H.; Liu, H. Optimal conditions for growth and magnetosome formation of Acidithiobacillus ferrooxidans. Afr. J. Microbiol. Res. 2012, 6, 6142–6151. [Google Scholar] [CrossRef]
- Wang, L.; Li, H.X.; Guo, Y.C.; Li, A. Explore the effects of magnetic field intensity change on Acidithiobacillus ferrooxidans growth and magnetism. In New Materials And Advanced Materials, Pts 1 And 2; Jiang, Z.Y., Han, J.T., Liu, X.H., Eds.; Trans Tech Publications Ltd.: Stafa-Zurich, Switzerland, 2011; Volume 152, pp. 1783–1787. [Google Scholar]
- Li, L.L.; Lv, Z.S.; Zuo, Z.Y.; Yang, Z.H.; Yuan, X.L. Effect of energy source and leaching method on bio-leaching of rock phosphates by Acidithiobacillus ferrooxidans. Hydrometallurgy 2016, 164, 238–247. [Google Scholar] [CrossRef]
- Chen, P.; Yan, L.; Leng, F.F.; Nan, W.B.; Yue, X.X.; Zheng, Y.N.; Feng, N.; Li, H.Y. Bioleaching of realgar by Acidithiobacillus ferrooxidans using ferrous iron and elemental sulfur as the sole and mixed energy sources. Bioresour. Technol. 2011, 102, 3260–3267. [Google Scholar] [CrossRef]
- Govender, E.; Bryan, C.G.; Harrison, S.T. Effect of physico-chemical and operating conditions on the growth and activity of Acidithiobacillus ferrooxidans in a simulated heap bioleaching environment. Miner. Eng. 2015, 75, 14–25. [Google Scholar] [CrossRef]
- Fang, J.-h.; Yong, L.; He, W.-l.; Qin, W.-q.; Qiu, G.-z.; Jun, W. Transformation of iron in pure culture process of extremely acidophilic microorganisms. Trans. Nonferrous Met. Soc. China 2017, 27, 1150–1155. [Google Scholar] [CrossRef]
- Yan, L.; Zhang, S.; Chen, P.; Wang, W.D.; Wang, Y.J.; Li, H.Y. Magnetic properties of Acidithiobacillus ferrooxidans. Mat. Sci. Eng. C-Mater. 2013, 33, 4026–4031. [Google Scholar] [CrossRef] [PubMed]
- Fdez-Gubieda, M.L.; Muela, A.; Alonso, J.; Garcia-Prieto, A.; Olivi, L.; Fernandez-Pacheco, R.; Barandiaran, J.M. Magnetite Biomineralization in Magnetospirillum gryphiswaldense: Time-Resolved Magnetic and Structural Studies. Acs Nano 2013, 7, 3297–3305. [Google Scholar] [CrossRef] [PubMed]
- Pham, A.L.-T.; Lee, C.; Doyle, F.M.; Sedlak, D.L. A Silica-Supported Iron Oxide Catalyst Capable of Activating Hydrogen Peroxide at Neutral pH Values. Environ. Sci. Technol. 2009, 43, 8930–8935. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.T.; Martell, A.E. Metal ion and metal chelate catalyzed oxidation of ascorbic acid by molecular oxygen. I. Cupric and ferric ion catalyzed oxidation. J. Am. Chem. Soc. 1967, 89, 4176–4185. [Google Scholar] [CrossRef] [PubMed]
- Ravel, B.; Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: Data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrot. Radiat. 2005, 12, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.K.; Das, A.; Rao, K.H.; Forssberg, K.S.E. Surface characterization of Acidithiobacillus ferrooxidans cells grown under different conditions. Hydrometallurgy 2003, 71, 285–292. [Google Scholar] [CrossRef]
- Harneit, K.; Sand, W. Influence of growth substrate and attachment substratum on EPS and biofilm formation by Acidithiobacillus ferrooxidans. Adv. Mat. Res. 2007, 20, 385. [Google Scholar]
- Lizama, H.; Zielinski, P.; Kerby, L.; Abraham, C. Comparison of biooxidation with carbon dioxide assimilation during bacterial growth on ferrous ion or elemental sulfur. Biotechnol. Bioeng. 2002, 77, 111–117. [Google Scholar] [CrossRef]
- Nagpal, S.; Dahlstrom, D.; Oolman, T. Effect of carbon dioxide concentration on the bioleaching of a pyrite–arsenopyrite ore concentrate. Biotechnol. Bioeng. 1993, 41, 459–464. [Google Scholar] [CrossRef]
- Brierley, C.L.; Le Roux, N.W. Bacterial leaching. CRC Crit. Rev. Microbiol. 1978, 6, 207–262. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Yang, Y.; Liu, J.S.; Qiu, G.Z. Isolation and characterization of Acidithiobacillus ferrooxidans strain QXS-1 capable of unusual ferrous iron and sulfur utilization. Hydrometallurgy 2013, 136, 51–57. [Google Scholar] [CrossRef]
- Zhang, S.; Yan, L.; Xing, W.J.; Chen, P.; Zhang, Y.; Wang, W.D. Acidithiobacillus ferrooxidans and its potential application. Extremophiles 2018, 22, 563–579. [Google Scholar] [CrossRef] [PubMed]
- Moskowitz, B.M.; Frankel, R.B.; Bazylinski, D.A. Rock magnetic criteria for the detection of biogenic magnetite. Earth Planet. Sci. Lett. 1993, 120, 283–300. [Google Scholar] [CrossRef]
- Lefevre, C.T.; Abreu, F.; Lins, U.; Bazylinski, D.A. A Bacterial Backbone: Magnetosomes in Magnetotactic Bacteria; Springer: Berlin/Heidelberger, Germany, 2011; pp. 75–102. [Google Scholar] [CrossRef]
- Faivre, D.; Menguy, N.; Pósfai, M.; Schuler, D. Environmental parameters affect the physical properties of fast-growing magnetosomes. Am. Mineral. 2008, 93, 463–469. [Google Scholar] [CrossRef]
- Baumgartner, J.; Morin, G.; Menguy, N.; Gonzalez, T.P.; Widdrat, M.; Cosmidis, J.; Faivre, D. Magnetotactic bacteria form magnetite from a phosphate-rich ferric hydroxide via nanometric ferric (oxyhydr) oxide intermediates. Proc. Natl. Acad. Sci. 2013, 110, 14883–14888. [Google Scholar] [CrossRef] [PubMed]
- Voegelin, A.; Kaegi, R.; Frommer, J.; Vantelon, D.; Hug, S.J. Effect of phosphate, silicate, and Ca on Fe (III)-precipitates formed in aerated Fe (II)-and As (III)-containing water studied by X-ray absorption spectroscopy. Geochim. Cosmochim. Acta 2010, 74, 164–186. [Google Scholar] [CrossRef]
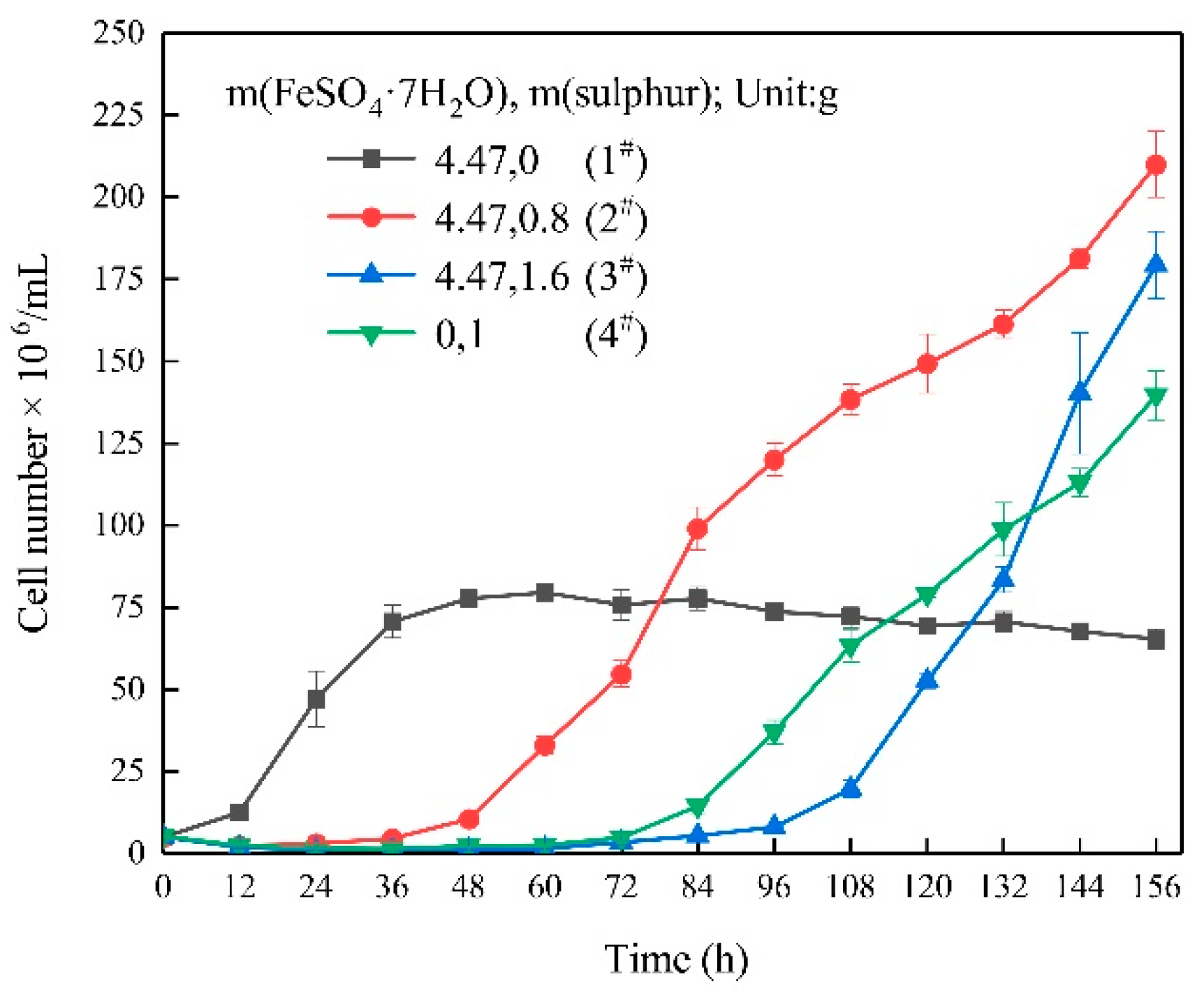
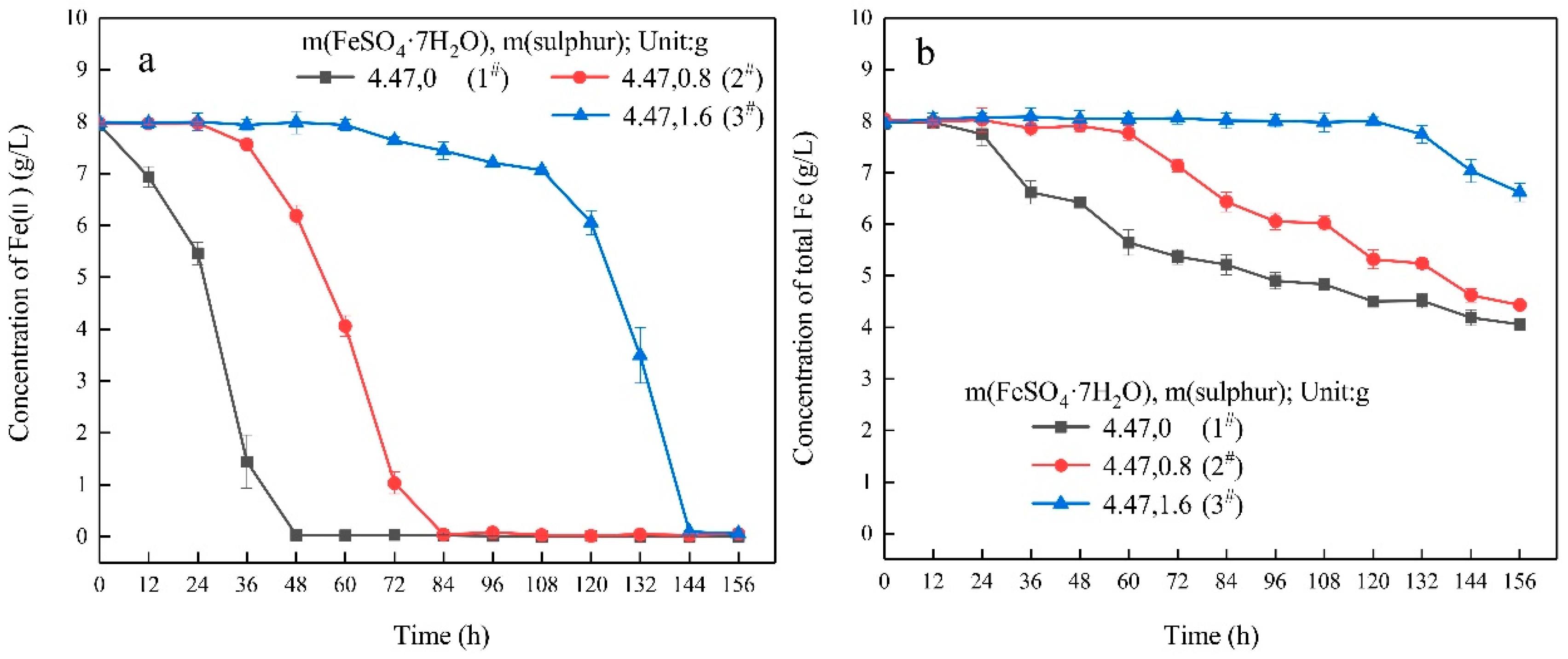


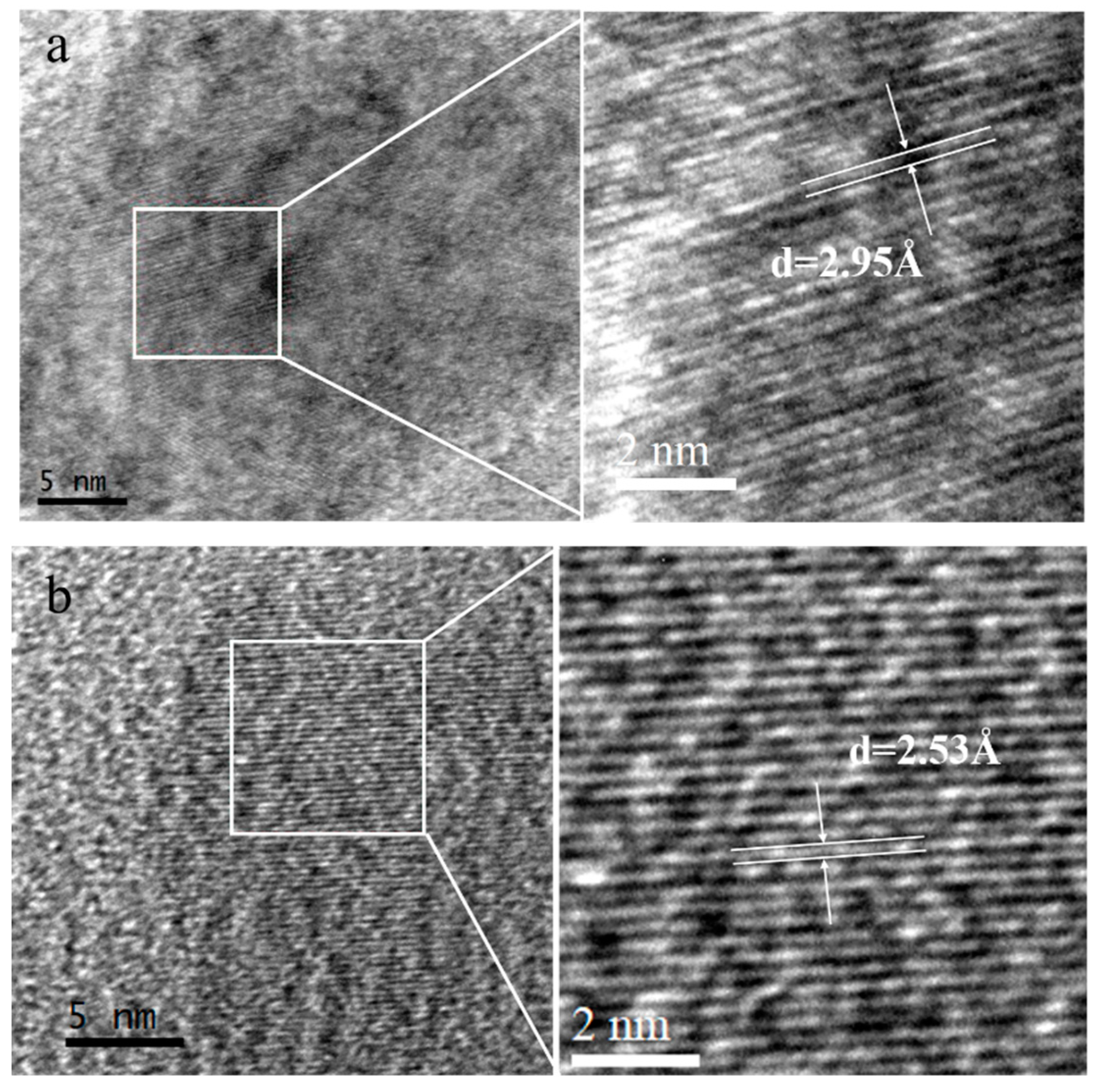

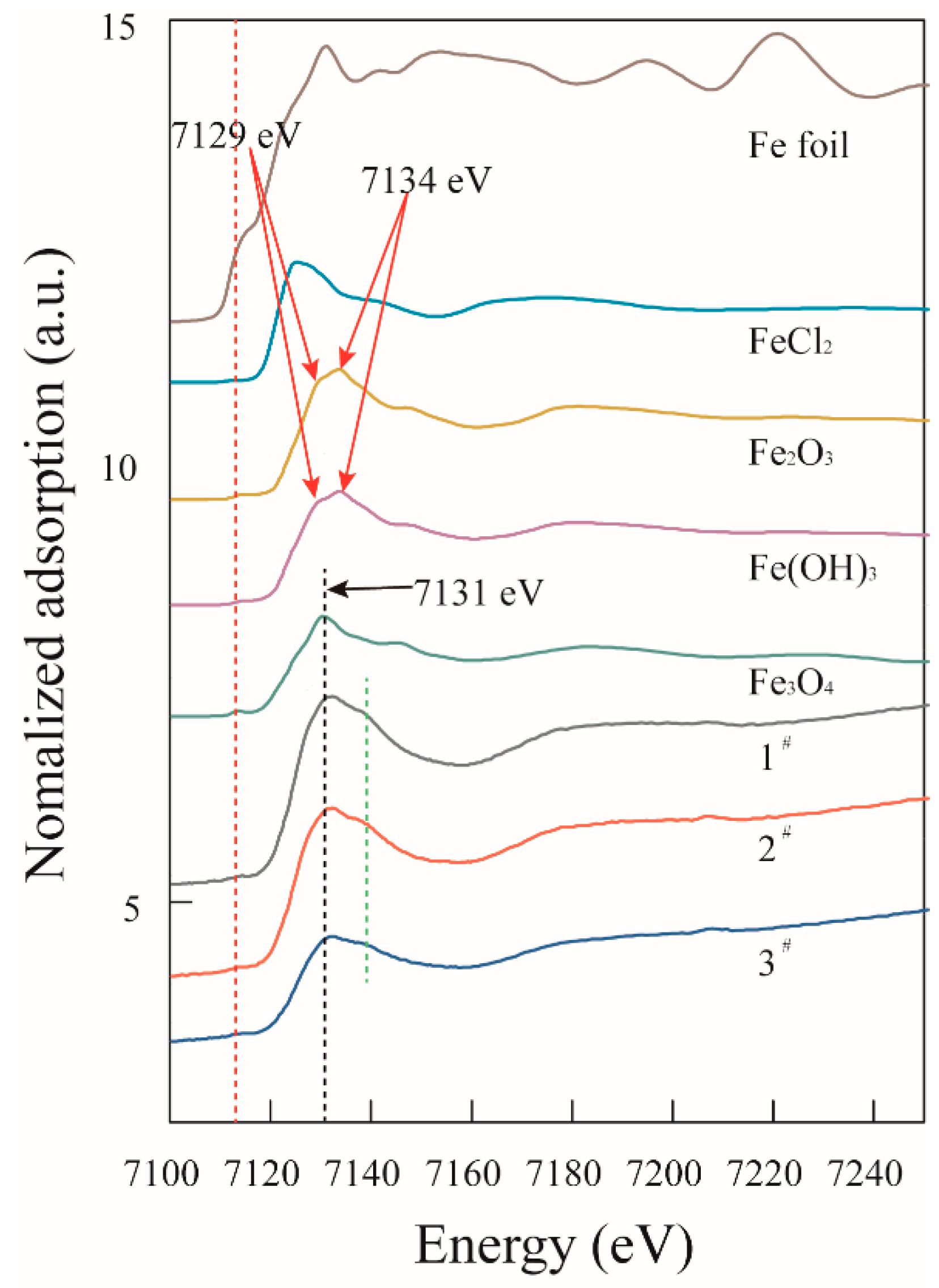
| Experimental Systems | FeSO4·7H2O and Sulphur Composition Ratios (FeSO4·7H2O: Sulphur; Unit: g) |
|---|---|
| 1# | 4.47:0 |
| 2# | 4.47:0.8 |
| 3# | 4.47:1.6 |
| 4# | 0:1 |
| Name of Strain | Component | Concentration (g/100 mL) in Growth Medium | Size of Bacterium (μm) | Size of Nanoparticles (nm) | |
|---|---|---|---|---|---|
| A. ferrooxidans | 1# | ferrous | 4.47 | (0.80 ± 0.21) × (0.36 ± 0.05) | (41.5 ± 14.2) |
| 2# | ferrous | 4.47 | (0.82 ± 0.32) × (0.32 ± 0.04) | (38.1 ± 9.3) | |
| sulphur | 0.8 | ||||
| 3# | ferrous | 4.47 | (0.84 ± 0.31) × (0.34 ± 0.05) | (36.1 ± 12.6) | |
| sulphur | 1.6 | ||||
| 4# | sulphur | 1 | (1.03 ± 0.40) × (0.35 ± 0.05) | None | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, L.; Yang, B.; Wang, X.; Wu, B.; He, W.; Gan, M.; Qiu, G.; Wang, J. Effects of Single and Mixed Energy Sources on Intracellular Nanoparticles Synthesized by Acidithiobacillus ferrooxidans. Minerals 2019, 9, 163. https://doi.org/10.3390/min9030163
Wu L, Yang B, Wang X, Wu B, He W, Gan M, Qiu G, Wang J. Effects of Single and Mixed Energy Sources on Intracellular Nanoparticles Synthesized by Acidithiobacillus ferrooxidans. Minerals. 2019; 9(3):163. https://doi.org/10.3390/min9030163
Chicago/Turabian StyleWu, Lingbo, Baojun Yang, Xingxing Wang, Baiqiang Wu, Wanli He, Min Gan, Guanzhou Qiu, and Jun Wang. 2019. "Effects of Single and Mixed Energy Sources on Intracellular Nanoparticles Synthesized by Acidithiobacillus ferrooxidans" Minerals 9, no. 3: 163. https://doi.org/10.3390/min9030163
APA StyleWu, L., Yang, B., Wang, X., Wu, B., He, W., Gan, M., Qiu, G., & Wang, J. (2019). Effects of Single and Mixed Energy Sources on Intracellular Nanoparticles Synthesized by Acidithiobacillus ferrooxidans. Minerals, 9(3), 163. https://doi.org/10.3390/min9030163





