Clay Mineralogical Characteristics of Sediments Deposited during the Late Quaternary in the Larsen Ice Shelf B Embayment, Antarctica
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Location
2.2. Analysis of Sedimentary Facies and Grain Size
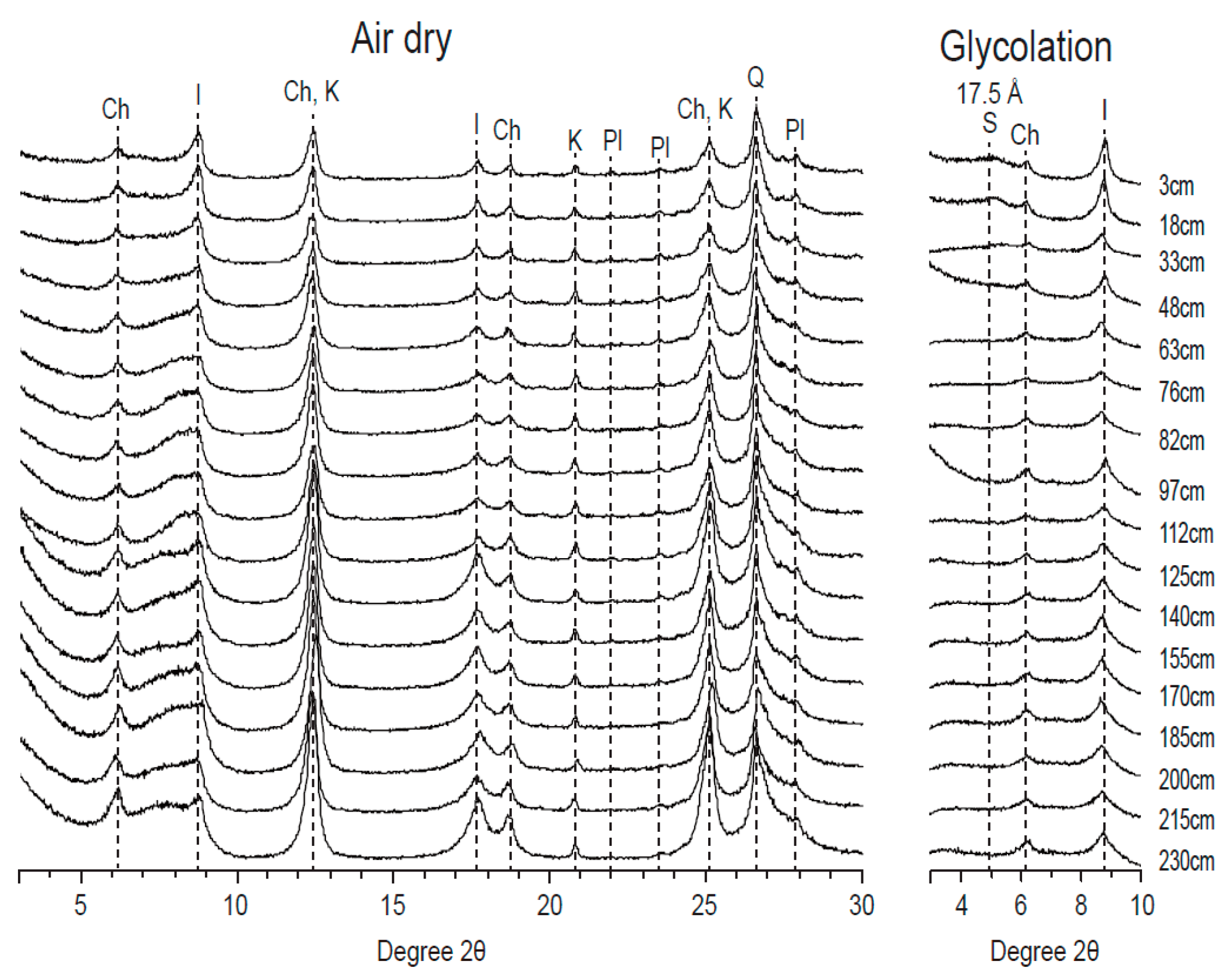
2.3. X-ray Diffraction
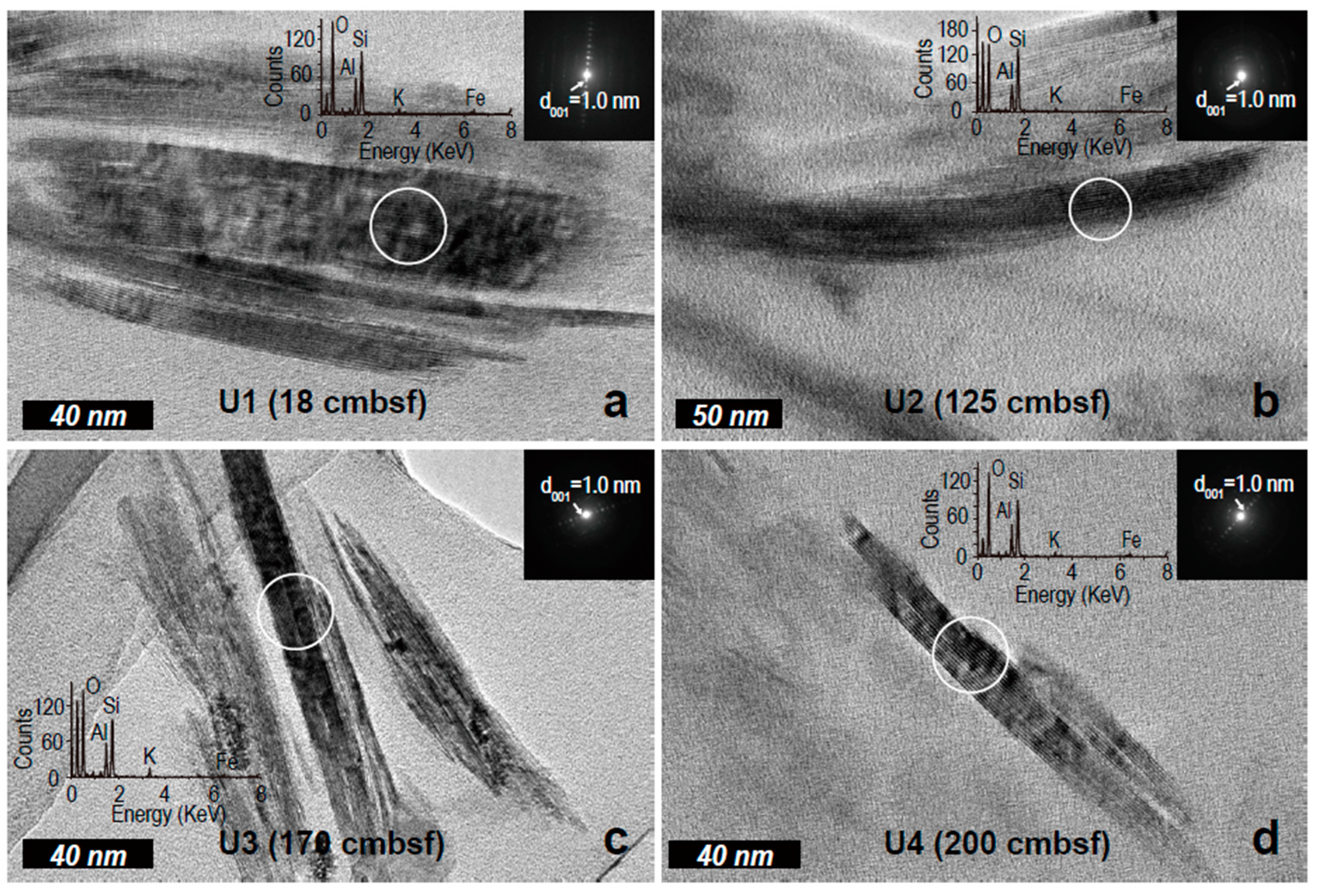
2.4. Transmission Electron Microscopy
2.5. Isotopic Composition (δ13C and δ15N)
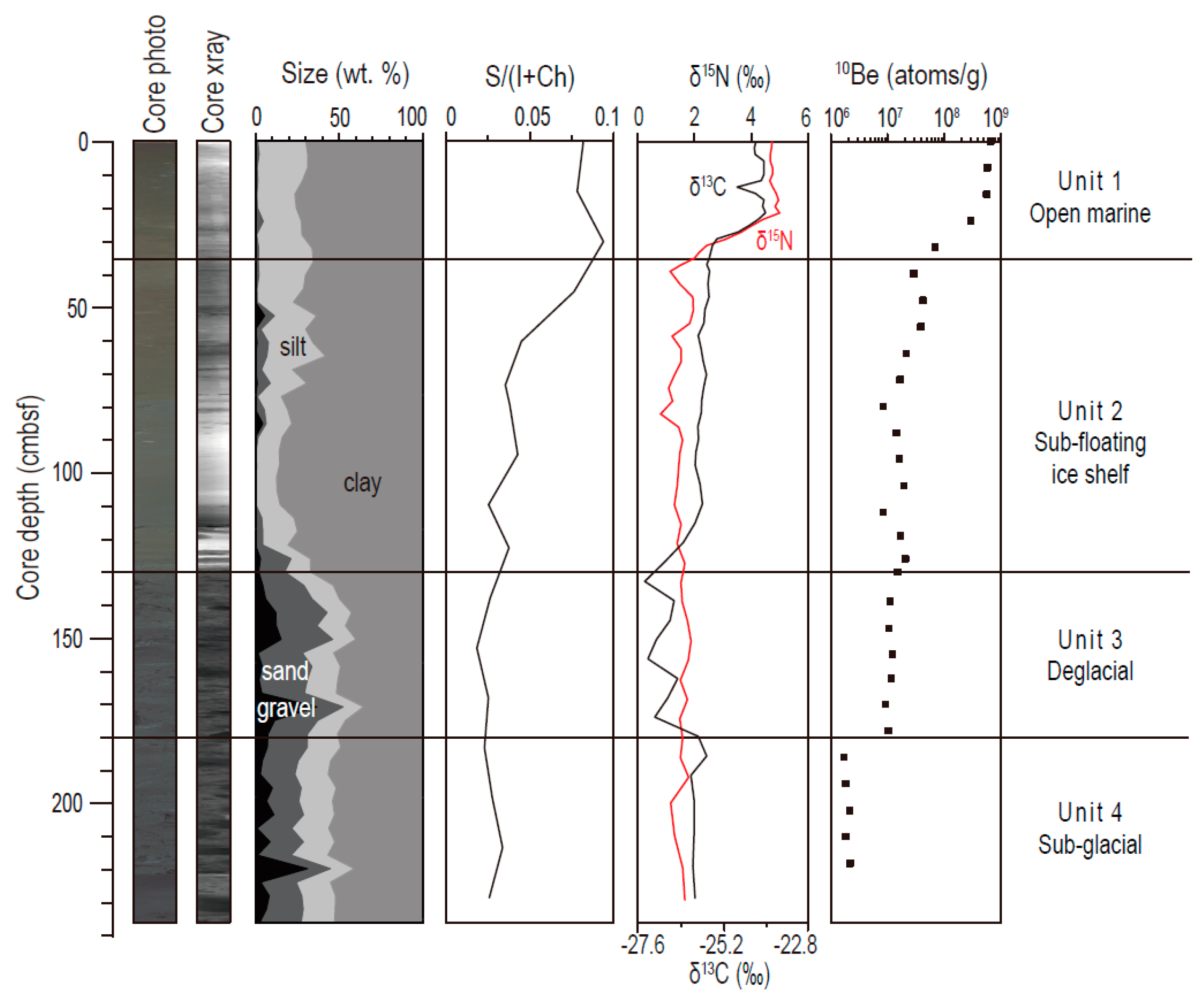
3. Results
3.1. Lithological Units and Isotope Data
3.2. Semi-Quantification of Clay Minerals and Mineralogy
3.3. Electron Microscopic Measurement
4. Discussion
4.1. Depositional Environments
4.2. Illite Structure Responding to Depositional Environments
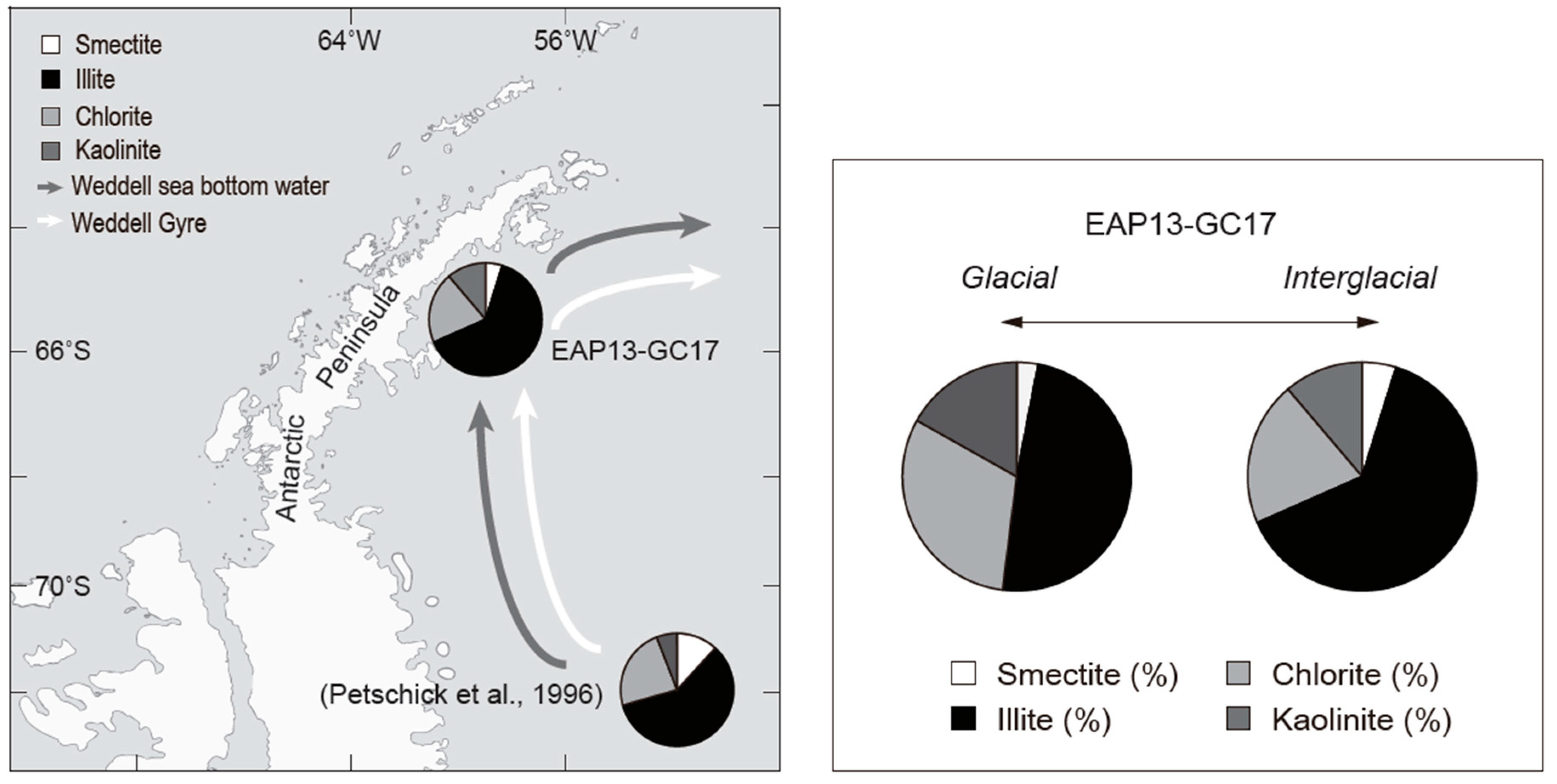
4.3. Provenance of Late Quaternary Glacial and Interglacial Sediments
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Domack, E.W.; Ishman, S.E.; Stein, A.B.; McClennen, C.E.; Jull, A.T. Late holocene advance of the müller ice shelf, antarctic peninsula: Sedimentological, geochemical and palaeontological evidence. Antarct. Sci. 1995, 7, 159–170. [Google Scholar] [CrossRef]
- Domack, E.W.; Burnett, A.; Leventer, A. Environmental setting of the antarctic peninsula. Antarct. Penins. Clim. Var. Hist. Paleoenviron. Perspect. 2003, 79. [Google Scholar] [CrossRef]
- Petschick, R.; Kuhn, G.; Gingele, F. Clay mineral distribution in surface sediments of the south Atlantic: Sources, transport, and relation to oceanography. Mar. Geol. 1996, 130, 203–229. [Google Scholar] [CrossRef]
- Pandarinath, K. Clay minerals in SW Indian continental shelf sediment cores as indicators of provenance and palaeomonsoonal conditions: A statistical approach. Int. Geol. Rev. 2009, 51, 145–165. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, S. Clay mineralogy indicates the Holocene monsoon climate in the Changjiang (Yangtze River) Catchment, China. Appl. Clay Sci. 2013, 74, 28–36. [Google Scholar] [CrossRef]
- Griffin, J.J.; Windom, H.; Goldberg, E.D. The Distribution of clay Minerals in the World Ocean, Deep Sea Research and Oceanographic Abstracts; Elsevier: Amsterdam, The Netherlands, 1968; pp. 433–459. [Google Scholar]
- Domack, E.W.; Jacobson, E.A.; Shipp, S.; Anderson, J.B. Late Pleistocene–Holocene retreat of the West Antarctic Ice-Sheet system in the Ross Sea: Part 2—Sedimentologic and stratigraphic signature. Geol. Soc. Am. Bull. 1999, 111, 1517–1536. [Google Scholar] [CrossRef]
- Rosenheim, B.E.; Santoro, J.A.; Gunter, M.; Domack, E.W. Improving Antarctic Sediment 14C Dating Using Ramped Pyrolysis: An Example from the Hugo Island Trough. Radiocarbon 2013, 55, 115–126. [Google Scholar] [CrossRef]
- Johnsson, M.J. The System Controlling the Composition of Clastic Sediments; Special papers; Geological Society of America: Boulder, CO, USA, 1993. [Google Scholar]
- Weaver, C.E. Clays, Muds, and Shales; Elsevier: Amsterdam, The Netherlands, 1989; Volume 44. [Google Scholar]
- Tsipursky, S.I.; Drits, V. The distribution of octahedral cations in the 2:1 layers of dioctahedral smectites studied by oblique-texture electron diffraction. Clay Miner. 1984, 19, 177–193. [Google Scholar] [CrossRef]
- Jeong, G.; Yoon, H. The origin of clay minerals in soils of King George Island, South Shetland Islands, West Antarctica, and its implications for the clay-mineral compositions of marine sediments. J. Sediment. Res. 2001, 71, 833–842. [Google Scholar] [CrossRef]
- Lee, J.I.; Yoon, H.I.; Yoo, K.-C.; Lim, H.S.; Lee, Y.I.; Kim, D.; Bak, Y.-S.; Itaki, T. Late quaternary glacial–interglacial variations in sediment supply in the southern drake passage. Quat. Res. 2012, 78, 119–129. [Google Scholar] [CrossRef]
- Hillenbrand, C.-D.; Grobe, H.; Diekmann, B.; Kuhn, G.; Fütterer, D.K. Distribution of clay minerals and proxies for productivity in surface sediments of the Bellingshausen and Amundsen seas (West Antarctica)—Relation to modern environmental conditions. Mar. Geol. 2003, 193, 253–271. [Google Scholar] [CrossRef]
- Hillenbrand, C.-D.; Ehrmann, W.; Larter, R.D.; Benetti, S.; Dowdeswell, J.; Cofaigh, C.Ó.; Graham, A.G.; Grobe, H. Clay mineral provenance of sediments in the southern Bellingshausen Sea reveals drainage changes of the West Antarctic Ice Sheet during the Late Quaternary. Mar. Geol. 2009, 265, 1–18. [Google Scholar] [CrossRef]
- Delmonte, B.; Petit, J.; Maggi, V. Glacial to Holocene implications of the new 27,000-year dust record from the EPICA Dome C (East Antarctica) ice core. Clim. Dyn. 2002, 18, 647–660. [Google Scholar]
- Gearing, J.; Gearing, P.; Rudnick, D.; Requejo, A.; Hutchins, M. Isotopic variability of organic carbon in a phytoplankton-based, temperate estuary. Geochim. Cosmochim. Acta 1984, 48, 1089–1098. [Google Scholar] [CrossRef]
- Sweeney, R.E.; Kaplan, I. Natural abundances of 15N as a source indicator for near-shore marine sedimentary and dissolved nitrogen. Mar. Chem. 1980, 9, 81–94. [Google Scholar] [CrossRef]
- Quan, T.M.; Wright, J.D.; Falkowski, P.G. Co-variation of nitrogen isotopes and redox states through glacial–interglacial cycles in the Black Sea. Geochim. Cosmochim. Acta 2013, 112, 305–320. [Google Scholar] [CrossRef]
- Jeong, A.; Lee, J.I.; Seong, Y.B.; Balco, G.; Yoo, K.-C.; Yoon, H.I.; Domack, E.; Rhee, H.H.; Yu, B.Y. Late Quaternary deglacial history across the Larsen B embayment, Antarctica. Quat. Sci. Rev. 2018, 189, 134–148. [Google Scholar] [CrossRef]
- Rumolo, P.; Barra, M.; Gherardi, S.; Marsella, E.; Sprovieri, M. Stable isotopes and C/N ratios in marine sediments as a tool for discriminating anthropogenic impact. J. Environ. Monit. 2011, 13, 3399–3408. [Google Scholar] [CrossRef] [PubMed]
- Waldron, S.; Tatner, P.; Jack, I.; Arnott, C. The impact of sewage discharge in a marine embayment: A stable isotope reconnaissance. Estuar. Coast. Shelf Sci. 2001, 52, 111–115. [Google Scholar] [CrossRef]
- Costanzo, S.D.; Udy, J.; Longstaff, B.; Jones, A. Using nitrogen stable isotope ratios (δ15N) of macroalgae to determine the effectiveness of sewage upgrades: Changes in the extent of sewage plumes over four years in Moreton Bay, Australia. Mar. Pollut. Bull. 2005, 51, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Wada, E.; Minagawa, M.; Mizutani, H.; Tsuji, T.; Imaizumi, R.; Karasawa, K. Biogeochemical studies on the transport of organic matter along the Otsuchi River watershed, Japan. Estuar. Coast. Shelf Sci. 1987, 25, 321–336. [Google Scholar] [CrossRef]
- Scherer, R.P.; Aldahan, A.; Tulaczyk, S.; Possnert, G.; Engelhardt, H.; Kamb, B. Pleistocene collapse of the West Antarctic ice sheet. Science 1998, 281, 82–85. [Google Scholar] [CrossRef] [PubMed]
- Mulvaney, R.; Abram, N.J.; Hindmarsh, R.C.; Arrowsmith, C.; Fleet, L.; Triest, J.; Sime, L.C.; Alemany, O.; Foord, S. Recent Antarctic peninsula warming relative to Holocene climate and ice-shelf history. Nature 2012, 489, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Cook, A.; Fox, A.; Vaughan, D.; Ferrigno, J. Retreating glacier fronts on the Antarctic peninsula over the past half-century. Science 2005, 308, 541–544. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, D.G.; Marshall, G.J.; Connolley, W.M.; Parkinson, C.; Mulvaney, R.; Hodgson, D.A.; King, J.C.; Pudsey, C.J.; Turner, J. Recent rapid regional climate warming on the Antarctic Peninsula. Clim. Chang. 2003, 60, 243–274. [Google Scholar] [CrossRef]
- Domack, E.; Leventer, A.; Gilbert, R.; Brachfeld, S.; Ishman, S.; Camerlenghi, A.; Gavahan, K.; Carlson, D.; Barkoukis, A. Cruise reveals history of Holocene Larsen ice shelf. Eos Trans. Am. Geophys. Union 2001, 82, 13–17. [Google Scholar] [CrossRef]
- Vaughan, D.; Doake, C. Recent atmospheric warming and retreat of ice shelves on the Antarctic Peninsula. Nature 1996, 379, 328–331. [Google Scholar] [CrossRef]
- Brachfeld, S.; Domack, E.; Kissel, C.; Laj, C.; Leventer, A.; Ishman, S.; Gilbert, R.; Camerlenghi, A.; Eglinton, L.B. Holocene history of the Larsen-A Ice Shelf constrained by geomagnetic Paleointensity dating. Geology 2003, 31, 749–752. [Google Scholar] [CrossRef]
- Evans, J.; Pudsey, C.J.; ÓCofaigh, C.; Morris, P.; Domack, E. Late Quaternary glacial history, flow dynamics and sedimentation along the eastern margin of the Antarctic Peninsula Ice Sheet. Quat. Sci. Rev. 2005, 24, 741–774. [Google Scholar] [CrossRef]
- Heroy, D.C.; Anderson, J.B. Ice-sheet extent of the Antarctic Peninsula region during the Last Glacial Maximum (LGM)—Insights from glacial geomorphology. Geol. Soc. Am. Bull. 2005, 117, 1497–1512. [Google Scholar] [CrossRef]
- Bentley, M.J.; Hodgson, D.; Smith, J.; Cofaigh, C.; Domack, E.; Larter, R.; Roberts, S.; Brachfeld, S.; Leventer, A.; Hjort, C. Mechanisms of Holocene palaeoenvironmental change in the Antarctic Peninsula region. Holocene 2009, 19, 51–69. [Google Scholar] [CrossRef]
- Davies, B.J.; Hambrey, M.J.; Smellie, J.L.; Carrivick, J.L.; Glasser, N.F. Antarctic Peninsula ice sheet evolution during the Cenozoic Era. Quat. Sci. Rev. 2012, 31, 30–66. [Google Scholar] [CrossRef]
- Domack, E.; Duran, D.; Leventer, A.; Ishman, S.; Doane, S.; McCallum, S.; Amblas, D.; Ring, J.; Gilbert, R.; Prentice, M. Stability of the Larsen B ice shelf on the Antarctic Peninsula during the Holocene epoch. Nature 2005, 436, 681–685. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.; McCave, I.; Patel, D. A computer-interfaced sedigraph for modal size analysis of fine-grained sediment. Sedimentology 1988, 35, 163–172. [Google Scholar] [CrossRef]
- Yang, K.; Kim, J.-W.; Kogure, T.; Dong, H.; Baik, H.; Hoppie, B.; Harris, R. Smectite, illite, and early diagenesis in South Pacific Gyre subseafloor sediment. Appl. Clay Sci. 2016, 134, 34–43. [Google Scholar] [CrossRef]
- Biscaye, P. Distinction between kaolinite and chlorite in recent sediments by X-ray diffraction. Am. Mineral. 1964, 49, 1281–1289. [Google Scholar]
- Biscaye, P.E. Mineralogy and sedimentation of recent deep-sea clay in the Atlantic Ocean and adjacent seas and oceans. Geol. Soc. Am. Bull. 1965, 76, 803–832. [Google Scholar] [CrossRef]
- Liu, Z.; Trentesaux, A.; Clemens, S.C.; Colin, C.; Wang, P.; Huang, B.; Boulay, S. Clay mineral assemblages in the northern South China Sea: Implications for East Asian monsoon evolution over the past 2 million years. Mar. Geol. 2003, 201, 133–146. [Google Scholar] [CrossRef]
- Diekmann, B.; Hofmann, J.; Henrich, R.; Fütterer, D.K.; Röhl, U.; Wei, K.-Y. Detrital sediment supply in the southern Okinawa Trough and its relation to sea-level and Kuroshio dynamics during the late Quaternary. Mar. Geol. 2008, 255, 83–95. [Google Scholar] [CrossRef]
- Ehrmann, W.U.; Melles, M.; Kuhn, G.; Grobe, H. Significance of clay mineral assemblages in the Antarctic ocean. Mar. Geol. 1992, 107, 249–273. [Google Scholar] [CrossRef]
- Kuhn, G.; Hillenbrand, C.-D.; Kasten, S.; Smith, J.A.; Nitsche, F.O.; Frederichs, T.; Wiers, S.; Ehrmann, W.; Klages, J.P.; Mogollón, J.M. Evidence for a palaeo-subglacial lake on the Antarctic continental shelf. Nat. Commun. 2017, 8, 15591. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-W.; Peacor, D.R.; Tessier, D.; Elsass, F. A technique for maintaining texture and permanent expansion of smectite interlayers for TEM observations. Clays Clay Miner. 1995, 43, 51–57. [Google Scholar] [CrossRef]
- Li, G.; Andrews, J.; Riding, R.; Dennis, P.; Dresser, Q. Possible microbial effects of stable carbon isotopes in hot-spring travertines. J. Sediment. Res. 1996, 66, 468–473. [Google Scholar] [CrossRef]
- Meksumpun, S.; Meksumpun, C. Stable carbon and nitrogen isotope ratios of sediment in ban don bay: Evidence for understanding sources of organic matters in the coastal environment. Kasetsart J. (Nat. Sci.) 2002, 36, 75–82. [Google Scholar]
- Shepherd, A.; Wingham, D.; Payne, T.; Skvarca, P. Larsen ice shelf has progressively thinned. Science 2003, 302, 856–859. [Google Scholar] [CrossRef] [PubMed]
- Koo, T.-H.; Jang, Y.-N.; Kogure, T.; Kim, J.H.; Park, B.C.; Sunwoo, D.; Kim, J.-W. Structural and chemical modification of nontronite associated with microbial Fe (III) reduction: Indicators of “illitization”. Chem. Geol. 2014, 377, 87–95. [Google Scholar] [CrossRef]
- Dong, H.; Kukkadapu, R.K.; Fredrickson, J.K.; Zachara, J.M.; Kennedy, D.W.; Kostandarithes, H.M. Microbial reduction of structural Fe (III) in illite and goethite. Environ. Sci. Technol. 2003, 37, 1268–1276. [Google Scholar] [CrossRef]
- Hernández-Molina, F.; Larter, R.; Rebesco, M.; Maldonado, A. Miocene reversal of bottom water flow along the pacific margin of the Antarctic Peninsula: Stratigraphic evidence from a contourite sedimentary tail. Mar. Geol. 2006, 228, 93–116. [Google Scholar] [CrossRef]
- Pudsey, C.J. Late quaternary changes in Antarctic bottom water velocity inferred from sediment grain size in the northern Weddell sea. Mar. Geol. 1992, 107, 9–33. [Google Scholar] [CrossRef]
- Kuhn, G.; Weber, M.E. Acoustical characterization of sediments by Parasound and 3.5 kHz systems: Related sedimentary processes on the southeastern Weddell Sea continental slope, Antarctica. Mar. Geol. 1993, 113, 201–217. [Google Scholar] [CrossRef]
- Esquevin, J. Influence de la composition chimique des illites sur leur cristallinité. Bull. Cent. Rech. Pau-SNPA 1969, 3, 147–153. [Google Scholar]
- Tingey, R. The regional geology of Archean and Proterozoic rocks in Antractica. Geol. Antarct. 1991, 1–73. [Google Scholar]
- Weaver, C.E.; Pollard, L.D. The Chemistry of Clay Minerals (Developments in Sedimentology); Elsevier: Amsterdam, The Netherlands, 1973; Volume 15. [Google Scholar]

| Unit. | Depth (cm) | Smectite (%) | Illite (%) | Chlorite (%) | Kaolinite (%) |
|---|---|---|---|---|---|
| 1 | 3 | 4.5 ± 0.1 | 61.2 ± 1.4 | 23.4 ± 0.6 | 12.7 ± 0.4 |
| 18 | 5.3 ± 0.1 | 68.4 ± 1.8 | 18.1 ± 0.5 | 10.2 ± 0.3 | |
| 33 | 8.2 ± 0.5 | 52.1 ± 3.0 | 28.3 ± 1.6 | 15.9 ± 0.9 | |
| 2 | 48 | 2.5 ± 0.0 | 61.5 ± 0.9 | 23.7 ± 0.4 | 13.5 ± 0.2 |
| 63 | 2.9 ± 0.1 | 55.8 ± 1.1 | 28.6 ± 0.6 | 14.2 ± 0.3 | |
| 76 | 2.5 ± 0.1 | 49.3 ± 0.9 | 32.7 ± 0.6 | 16.8 ± 0.3 | |
| 82 | 2.7 ± 0.0 | 41.7 ± 0.8 | 37.0 ± 0.7 | 20 ± 0.4 | |
| 97 | 3.1 ± 0.1 | 48.3 ± 1.1 | 32.8 ± 0.7 | 17.5 ± 0.4 | |
| 112 | 2.1 ± 0.0 | 47.3 ± 0.7 | 33.6 ± 0.6 | 18.1 ± 0.3 | |
| 125 | 3.1 ± 0.1 | 46.5 ± 0.9 | 33.9 ± 0.7 | 18.3 ± 0.4 | |
| 3 | 140 | 3.0 ± 0.1 | 55.0 ± 1.1 | 27.7 ± 0.6 | 15.9 ± 0.4 |
| 155 | 1.6 ± 0.0 | 56.0 ± 0.6 | 28.0 ± 0.4 | 15.3 ± 0.1 | |
| 170 | 2.4 ± 0.1 | 48.9 ± 0.8 | 33.1 ± 0.6 | 16.9 ± 0.3 | |
| 4 | 185 | 1.7 ± 0.1 | 52.7 ± 0.7 | 29.9 ± 0.4 | 16.6 ± 0.1 |
| 200 | 2.8 ± 0.1 | 48.6 ± 0.9 | 33.7 ± 0.6 | 16.5 ± 0.4 | |
| 215 | 3.9 ± 0.1 | 48.9 ± 1.3 | 31.7 ± 0.9 | 17.6 ± 0.5 | |
| 230 | 4.2 ± 0.4 | 48.9 ± 1.5 | 31.3 ± 0.7 | 17.9 ± 0.6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, J.; Yoo, K.-C.; Lee, K.-H.; Park, Y.K.; Lee, J.I.; Kim, J. Clay Mineralogical Characteristics of Sediments Deposited during the Late Quaternary in the Larsen Ice Shelf B Embayment, Antarctica. Minerals 2019, 9, 153. https://doi.org/10.3390/min9030153
Jung J, Yoo K-C, Lee K-H, Park YK, Lee JI, Kim J. Clay Mineralogical Characteristics of Sediments Deposited during the Late Quaternary in the Larsen Ice Shelf B Embayment, Antarctica. Minerals. 2019; 9(3):153. https://doi.org/10.3390/min9030153
Chicago/Turabian StyleJung, Jaewoo, Kyu-Cheul Yoo, Kee-Hwan Lee, Young Kyu Park, Jae Il Lee, and Jinwook Kim. 2019. "Clay Mineralogical Characteristics of Sediments Deposited during the Late Quaternary in the Larsen Ice Shelf B Embayment, Antarctica" Minerals 9, no. 3: 153. https://doi.org/10.3390/min9030153
APA StyleJung, J., Yoo, K.-C., Lee, K.-H., Park, Y. K., Lee, J. I., & Kim, J. (2019). Clay Mineralogical Characteristics of Sediments Deposited during the Late Quaternary in the Larsen Ice Shelf B Embayment, Antarctica. Minerals, 9(3), 153. https://doi.org/10.3390/min9030153







