Micro-Laser-Induced Breakdown Spectroscopy: A Novel Approach Used in the Detection of Six Rare Earths and One Transition Metal
Abstract
:1. Introduction
2. Materials and Methods
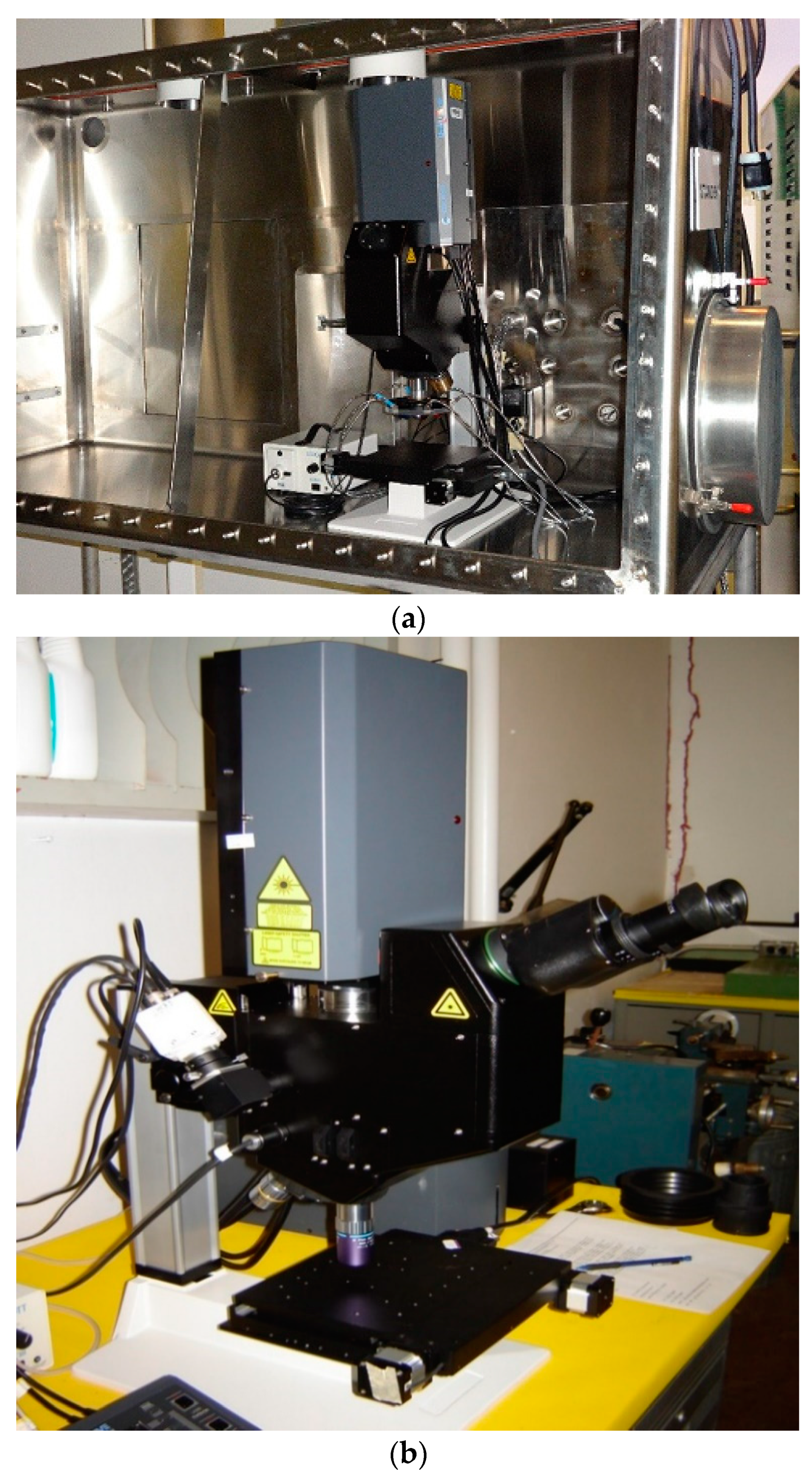
2.1. Laser Scribing System
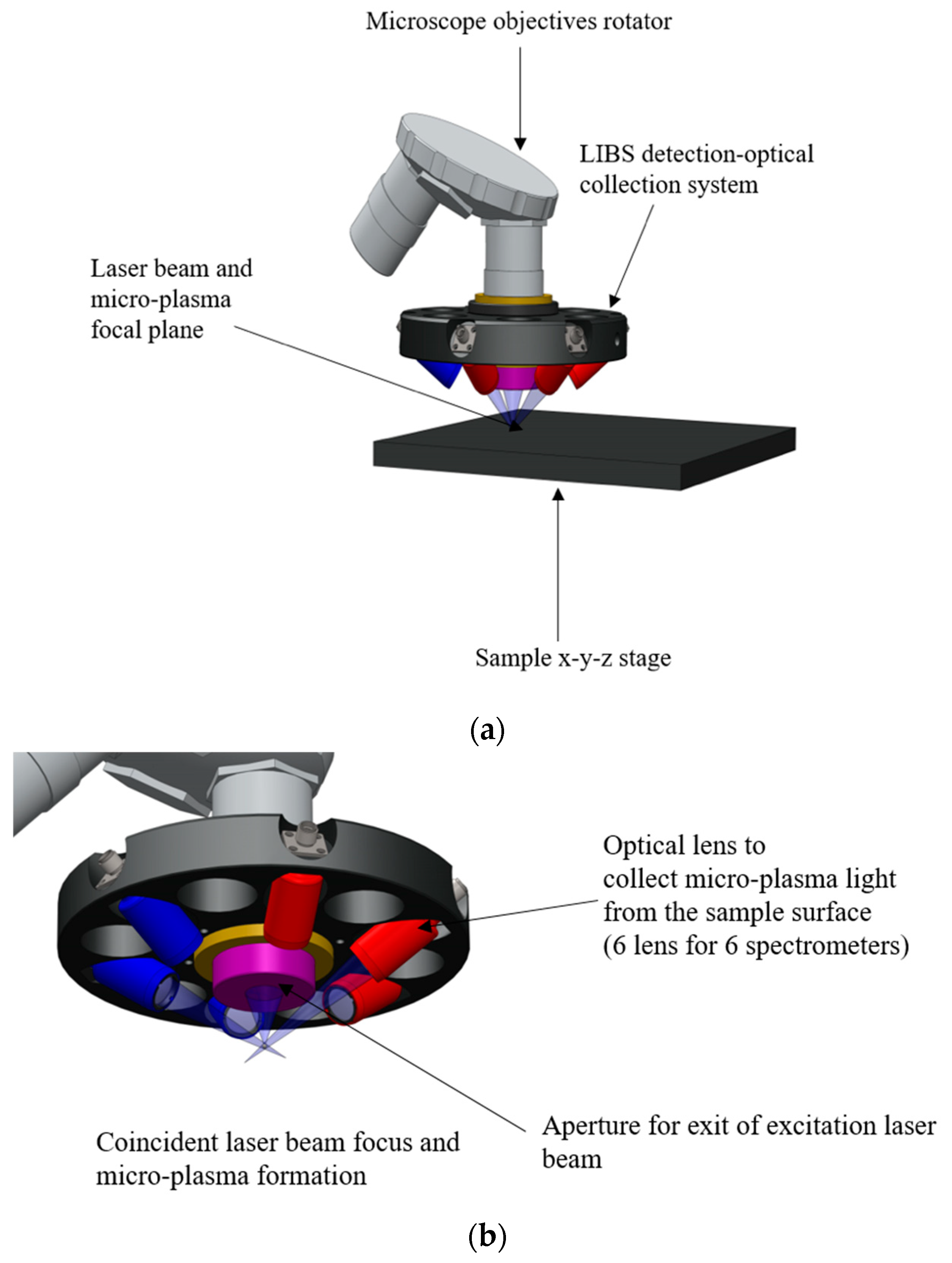
2.2. LIBS Detection Coupled to the Laser Scribing System
2.3. Materials and Preparation
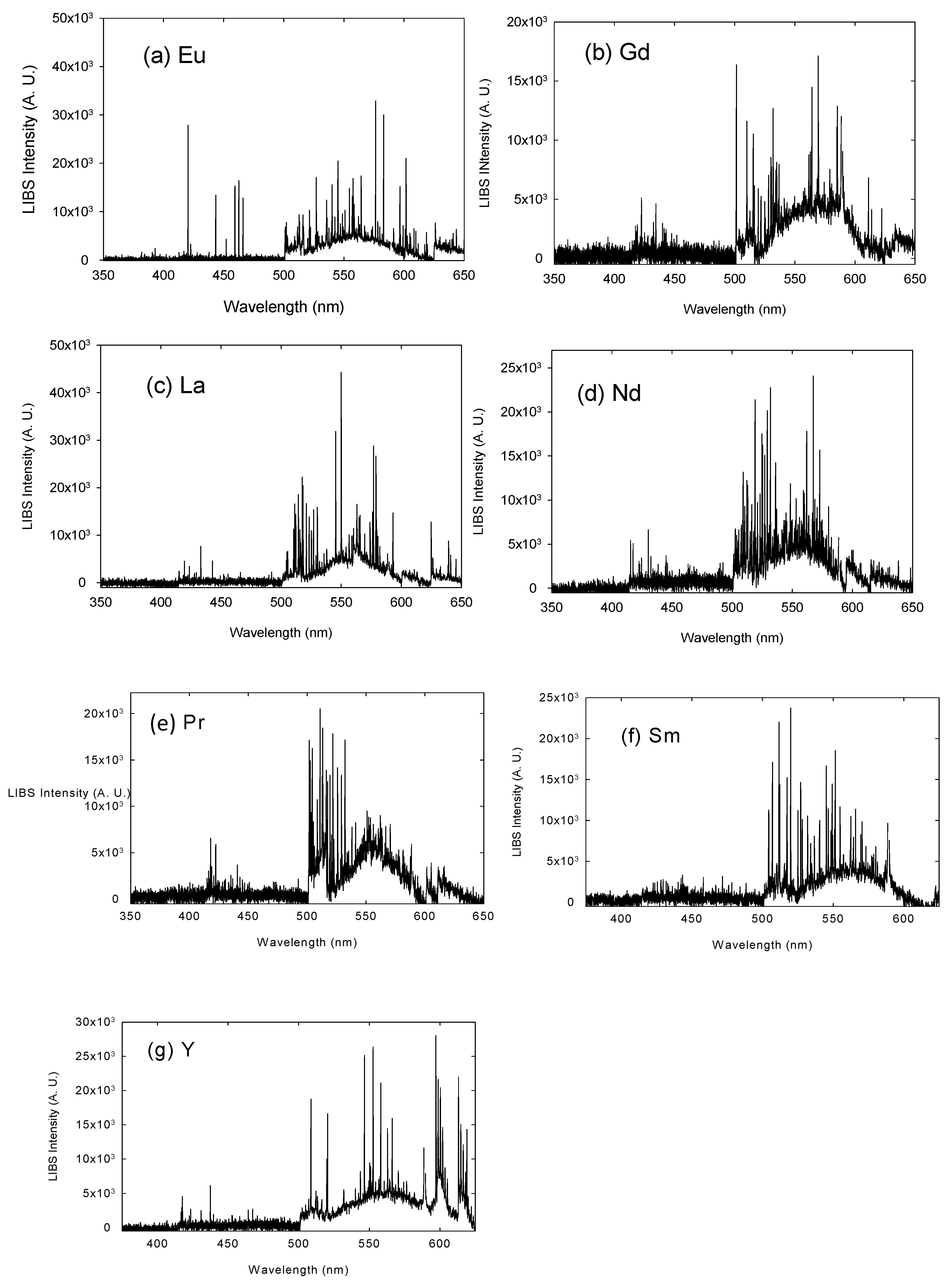
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yun, J.; Klenze, R.; Kim, J. Laser-induced breakdown spectroscopy for the on-line multielement analysis of highly radioactive glass melt. Part I: Characterization and evaluation of the method. Appl. Spectrosc. 2002, 56, 437–448. [Google Scholar] [CrossRef]
- Yun, J.; Klenze, R.; Kim, J. Laser-induced breakdown spectroscopy for the on-line multielement analysis of highly radioactive glass melt. Part II: Analyses of molten glass samples. Appl. Spectrosc. 2002, 56, 852–858. [Google Scholar] [CrossRef]
- Smith, C.A.; Martinez, M.A.; Veirs, D.K.; Cremers, D.A. Pu-239/Pu-240 isotope ratios determined using high-resolution emission spectroscopy in a laser-induced plasma. Spectrochim. Acta Part B Atomic Spectrosc. 2002, 57, 929–937. [Google Scholar] [CrossRef]
- Cremers, D.A.; Beddingfield, A.; Smithwick, R.; Chinni, R.; Jones, C.R.; Beardsley, B.; Karch, L. Monitoring uranium, hydrogen, and lithium and their isotopes using a compact laser-induced breakdown spectroscopy (LIBS) probe high-resolution spectrometer. Appl. Spectrosc. 2012, 66, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Barefield, J.E., II; Judge, E.J.; Berg, J.M.; Willson, S.P.; Le, L.A.; Lopez, L.N. Analysis and spectral assignments of mixed actinide oxide samples using laser-induced breakdown spectroscopy (LIBS). Appl. Spectrosc. 2013, 67, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Judge, E.J.; Barefield, J.E., II; Berg, J.M.; Clegg, S.M.; Havrilla, G.J.; Montoya, V.M.; Le, L.A.; Lopez, L.N. Laser-induced breakdown spectroscopy measurements of uranium and thorium powders and uranium ore. Spectrochim. Acta Part B Atomic Spectrosc. 2013, 83, 28–36. [Google Scholar] [CrossRef]
- Benedict, M.; Pigford, T.H.; Levi, H.W. Nuclear Chemical Engineering, 2nd ed.; McGraw-Hill Book Company: New York, NY, USA, 1981; p. 457. [Google Scholar]
- Martin, M.Z.; Martin, R.C.; Allman, S.; Brice, D.J.; Wymore, A.; Andre, N.O. Quantification of rare earth elements using laser-induced breakdown spectroscopy. Spectrochim. Acta Part B Atomic Spectrosc. 2015, 114, 65–73. [Google Scholar] [CrossRef]
- Bundschuh, T.; Yun, J.; Knopp, R. Determination of size, concentration and elemental composition of colloids with laser-induced breakdown detection/spectroscopy (LIBD/S.). Fresenius J. Anal. Chem. 2001, 371, 1063–1069. [Google Scholar] [CrossRef] [PubMed]
- Alamelu, D.; Sarkar, A.; Aggarwal, S.K. Laser-induced breakdown spectroscopy for simultaneous determination of Sm, Eu, and Gd in aqueous solutions. Talanta 2008, 77, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.; Bundschuh, T.; Neck, V.; Kim, J. Selective determination of europium (III) oxide and hydroxide colloids in aqueous solution by laser-induced breakdown spectroscopy. Appl. Spectrosc. 2001, 55, 273–278. [Google Scholar] [CrossRef]
- Jung, E.C.; Lee, D.H.; Yun, J.; Kim, J.G.; Yeon, J.W.; Song, K. Quantitative determination of uranium and europium in glass matrix by laser-induced breakdown spectroscopy. Spectrochim. Acta Part B Atomic Spectrosc. 2011, 66, 761–764. [Google Scholar] [CrossRef]
- Hotokezaka, H.; Tanaka, S.; Suzuki, A.; Nagasaki, S. Speciation analysis on europium (III) using laser-induced breakdown spectroscopy. Radiochim. Acta 2000, 88, 645–648. [Google Scholar] [CrossRef]
- Menut, D.; Mauchien, P.; Gif, Y.S.; Descostes, M.; Meier, P.; Radwan, J.; Poinssot, C. Eu migration in Callovo-Oxfodianargillite: On the use of micro laser-induced breakdown spectroscopy (micro LIBS) as a microanalysis tool. In Proceedings of the International Conference on Chemistry and Migration Behavior of Actinides and Fission Products in the Geosphere, Avignon, France, 1 September 2005; Volume 38. [Google Scholar]
- Zikratov, G.; Vasudev, R.; Yueh, F.Y.; Singh, J.P.; Marra, J.C. Laser-induced breakdown spectroscopy of hafnium doped vitrified glass. Glass Technol. 1999, 40, 84–88. [Google Scholar]
- Song, K.; Kim, D.; Cha, H.K.; Kim, Y.; Jung, E.C.; Choi, I.; Yoo, H.S.; Oh, S. Characterization of laser-induced plasma in a vacuum using laser ablation mass spectrometry, and laser-induced breakdown spectrometry. Microchem. J. 2004, 76, 95–103. [Google Scholar] [CrossRef]
- Oba, M.; Maruyama, Y.; Akaoka, K.; Miyabe, M.; Wakaida, I. Double-pulse LIBS of gadolinium oxide ablated by femto- and nano-secondlaser pulses. Appl. Phys. A 2010, 101, 545–549. [Google Scholar] [CrossRef]
- Kim, S.H.; Ju, J.S.; Lee, Y.H.; Jeong, J.H.; Sin, H.S. Semi-quantitative analysis of the neodymium binary alloys using laser induced breakdown spectroscopy. In Proceedings of the Transactions of Korean Nuclear Society, Autumn Meeting, Pyeongchang, Korea, 30–31 October 2008. [Google Scholar]
- Rehse, S.J.; Ryder, C.A. Laser-induced breakdown spectroscopy for branching ratio and atomic lifetime measurements in singly-ionizing neodymium and gallium. Spectrochim. Acta Part B Atomic Spectrosc. 2009, 64, 974–980. [Google Scholar] [CrossRef]
- Haider, A.F.M.Y.; Rony, M.A.; Lubna, R.S.; Abedin, K.M. Detection of multiple elements in coal samples from Bangladesh by laser-induced breakdown spectroscopy. Opt. Laser Technol. 2011, 43, 1405–1410. [Google Scholar] [CrossRef]
- Kim, S.H.; Hong, J.S.; Ju, J.S.; Sin, H.S. A quantitative analysis for Lanthanum series element using Laser-induced breakdown spectroscopy. In Proceedings of the Transactions of Korean Nuclear Society, Spring Meeting, Gyeongju, Korea, 29–30 May 2008. [Google Scholar]
- Unnikrishnan, V.K.; Nayak, R.; Devangad, P.; Tamboli, M.M.; Santhosh, C.; Kumar, G.A.; Sardar, D.K. Calibration-based laser-induced breakdown spectroscopy (LIBS) for quantitative analysis of doped rare earth elements in phosphors. Mat. Lett. 2013, 107, 322–324. [Google Scholar] [CrossRef]
- Ciucci, A.; Palleschi, V.; Rastelli, S.; Barbini, R.; Colao, F.; Fontini, R.; Palucci, A.; Ribezzo, S.; van der Steen, H.J.L. Trace pollutants analysis in soil by a time-resolved laser-induced breakdown spectroscopy technique. Appl. Phys. B 1996, 63, 185–190. [Google Scholar] [CrossRef]
- Kim, S.H.; Ju, J.S.; Jeong, J.-H.; Sin, H.-S.; Kim, H.-D. Semi-quantitative analysis for samarium alloys sample using laser-induced breakdown spectroscopy. In Proceedings of the Transactions of Korean Nuclear Society, Spring Meeting, Jeju, Korea, 22 May 2009. [Google Scholar]
- Pigosova, J.; Kilianova, A.; Vojtek, P.; Kopcok, M.; Cigan, A. Preparation of bismuth-doped yttrium iron garnets and their characterization. In Proceedings of the SPIE 6609, M1-M7 (2007). 15th Czech-Polish-Slovak Conference on Wave and Quantum Aspects of Contemporary Optics, 66091M, Liberec, Czech Republic, 12 April 2007. [Google Scholar]
- Buckley, S.G.; Johnson, H.A.; Hencken, K.R.; Hahn, D.W. Implementation of laser-induced breakdown spectroscopy as a continuous emissions monitor for toxic metals. Waste Manag. 2002, 20, 455–462. [Google Scholar] [CrossRef]
- Zhang, H.; Yueh, F.-Y.; Singh, J.P. Laser-induced breakdown spectrometry as a multimetal continuous-emission monitor. Appl. Opt. 1999, 38, 1459–1466. [Google Scholar] [CrossRef] [PubMed]
- Abedin, K.M.; Haider, A.F.M.Y.; Rony, M.A.; Khan, Z.H. Identification of multiple rare earths and associated elements in ras monazite sands by laser-induced breakdown spectroscopy. Opt. Laser Technol. 2011, 43, 45–49. [Google Scholar] [CrossRef]
- Martin, M.Z.; Allman, S.; Brice, D.J.; Martin, R.C.; Andre, N.O. Exploring laser-induced breakdown spectroscopy for nuclear materials analysis and in-situ applications. Spectrochim. Acta Part B Atomic Spectrosc. 2012, 74–75, 177–183. [Google Scholar] [CrossRef]
- Martin, M.Z.; Labbe, N.; Rials, T.G.; Wullschleger, S.D. Analysis of preservative-treated wood by multivariate analysis of LIBS spectra. Spectrochim. Acta Part B Atomic Spectrosc. 2005, 60, 1179–1185. [Google Scholar] [CrossRef]
- Boumans, P.W.J.M. (Ed.) Basic concepts and characteristics of ICP-AES. In Inductively Coupled Plasma Emission Spectrosc.opy: Part 1, Methodology, Instrumentation, and Performance; Wiley: New York, NY, USA, 1987; Chapter 4; p. 100. [Google Scholar]
- Boumans, P.W.J.M.; Tielrooy, J.A.; Maessen, F.J.M.J. Mutual spectral interferences of rare earth elements in inductively coupled plasma atomic emission spectrometry-I. Rational line selection and correction procedure. Spectrochim. Acta Part B Atomic Spectrosc. 1988, 43, 173–199. [Google Scholar] [CrossRef]
- Boumans, P.W.J.M.; Vrakking, J.J.A.M.; Heijms, A.H.M. Mutual spectral interferences of rare earth elements in inductively coupled plasma atomic emission spectrometry-II: Approach to the compilation and use of pseudo physically resolved spectral data. Spectrochim. Acta Part B Atomic Spectrosc. 1988, 43, 1365–1488. [Google Scholar] [CrossRef]
- Boumans, P.W.J.M.; Zhuang, H.Z.; Vrakking, J.J.A.M.; Tielrooy, J.A.; Maessen, F.J.M.J. Mutual spectral interferences of rare earth elements in inductively coupled plasma atomic emission spectrometry-III. Pseudo physically resolved spectral data: Complete results and evaluation. Spectrochim. Acta Part B Atomic Spectrosc. 1989, 44, 31–93. [Google Scholar] [CrossRef]
- Huang, B.; Ying, H.; Yang, P.; Wang, X.; Gu, S.; Zhang, Z.; Zhuang, Z.; Sun, Z.; Li, B. An Atlas of High Resolution Spectra of Rare Earth Elements for ICP-AES; Royal Society of Chemistry: London, UK, 2000. [Google Scholar]
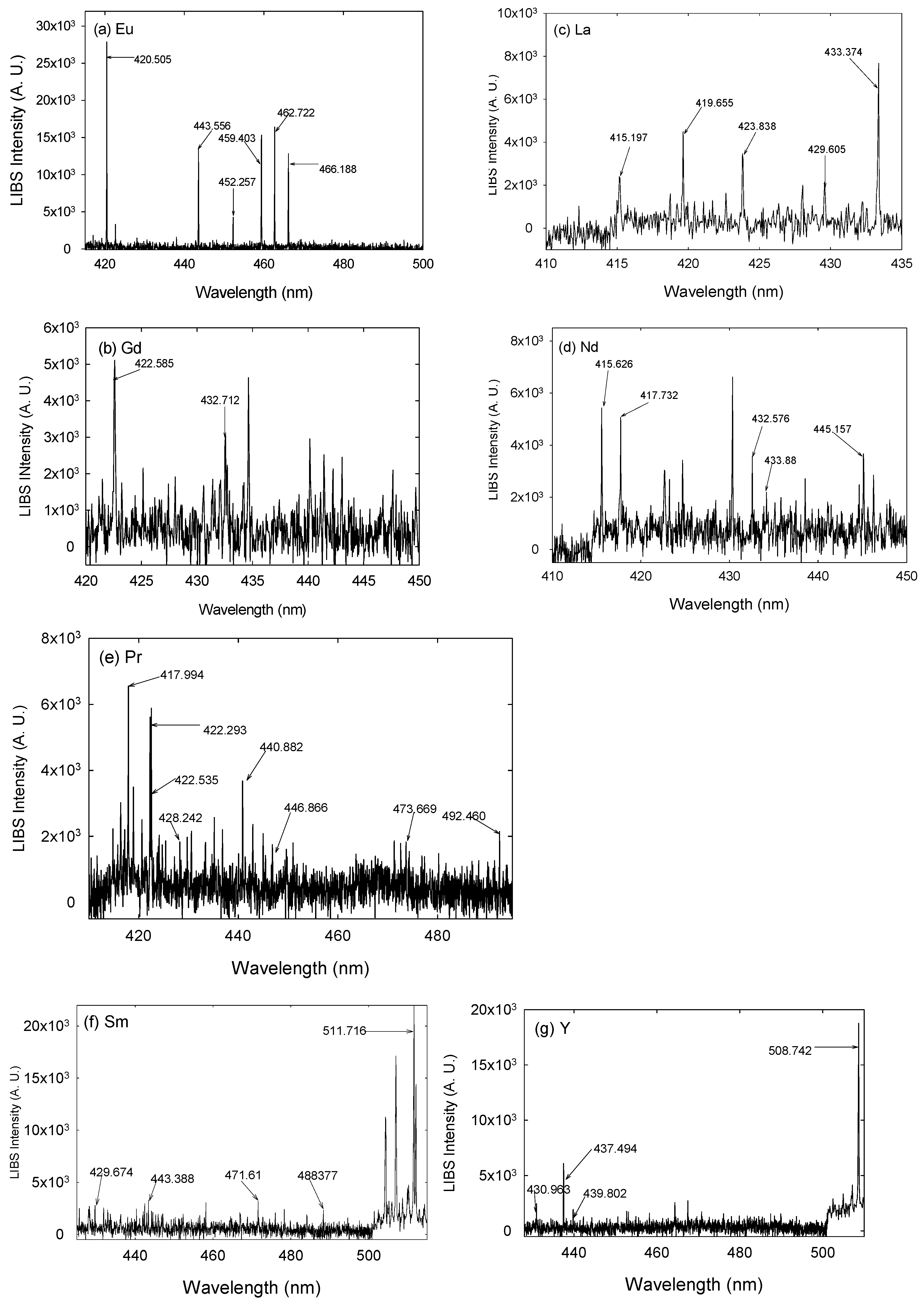
| Element | Wavelength (nm) | Element | Wavelength (nm) |
|---|---|---|---|
| Eu II | 381.967 | La II | 415.197 |
| Eu II | 393.048 | La I | 418.732 |
| Eu II | 397.196 | La II | 419.655 |
| Eu II | 420.505 | La II | 423.838 |
| Eu II | 443.556 | La II | 429.605 |
| Eu II | 452.257 | La II | 433.374 |
| Eu I | 459.403 | La II | 452.237 |
| Eu I | 462.722 | La II | 452.612 |
| Eu I | 466.188 | La II | 455.846 |
| Eu I | 490.086 | La I | 521.186 |
| Eu I | 535.761 | La I | 545. 515 |
| Eu I | 548.865 | La I | 639.423 |
| Eu I | 576.520 | Pr II | 417.939 |
| Eu I | 583.098 | Pr II | 422.293 |
| Eu I | 596.710 | Pr II | 422.535 |
| Nd II | 415.626 | Pr II | 428.242 |
| Nd II | 417.732 | Pr II | 440.882 |
| Nd II | 432.576 | Pr II | 446.866 |
| Nd II | 433.88 | Pr I | 473.669 |
| Nd II | 445.157 | Pr I | 492.460 |
| Nd II | 527.343 | Pr II | 511.038 |
| Nd II | 543.153 | Pr I | 513.344 |
| Nd II | 582.587 | Pr II | 522.011 |
| Gd I | 422.585 | Pr II | 532.276 |
| Gd I | 432.712 | Pr II | 535.240 |
| Gd I | 501.504 | Pr I | 552.415 |
| Gd I | 510.345 | Pr I | 553.837 |
| Gd I | 515.584 | Pr II | 562.305 |
| Gd I | 537.063 | Pr II | 581.533. |
| Gd I | 545.346 | Sm I | 429.674 |
| Gd I | 561.791 | Sm II | 443. 388 |
| Gd I | 570.135 | Sm I | 471.610 |
| Gd I | 574.636 | Sm I | 488.377 |
| Gd I | 577.602 | Sm I | 511.716 |
| Gd I | 579.138 | Y II | 430.963 |
| Gd I | 585.163 | Y II | 437.494 |
| Gd II | 585.524 | Y II | 439.802 |
| Gd I | 585.622 | Y II | 508.742 |
| Y I | 570.671 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martin, M.; Hamm, D.; Martin, S.; Allman, S.; Bell, G.; Martin, R. Micro-Laser-Induced Breakdown Spectroscopy: A Novel Approach Used in the Detection of Six Rare Earths and One Transition Metal. Minerals 2019, 9, 103. https://doi.org/10.3390/min9020103
Martin M, Hamm D, Martin S, Allman S, Bell G, Martin R. Micro-Laser-Induced Breakdown Spectroscopy: A Novel Approach Used in the Detection of Six Rare Earths and One Transition Metal. Minerals. 2019; 9(2):103. https://doi.org/10.3390/min9020103
Chicago/Turabian StyleMartin, Madhavi, Daniel Hamm, Samir Martin, Steve Allman, Gary Bell, and Rodger Martin. 2019. "Micro-Laser-Induced Breakdown Spectroscopy: A Novel Approach Used in the Detection of Six Rare Earths and One Transition Metal" Minerals 9, no. 2: 103. https://doi.org/10.3390/min9020103
APA StyleMartin, M., Hamm, D., Martin, S., Allman, S., Bell, G., & Martin, R. (2019). Micro-Laser-Induced Breakdown Spectroscopy: A Novel Approach Used in the Detection of Six Rare Earths and One Transition Metal. Minerals, 9(2), 103. https://doi.org/10.3390/min9020103




