Synthesis of Geopolymers from Mechanically Activated Coal Fly Ash and Improvement of Their Mechanical Properties
Abstract
1. Introduction
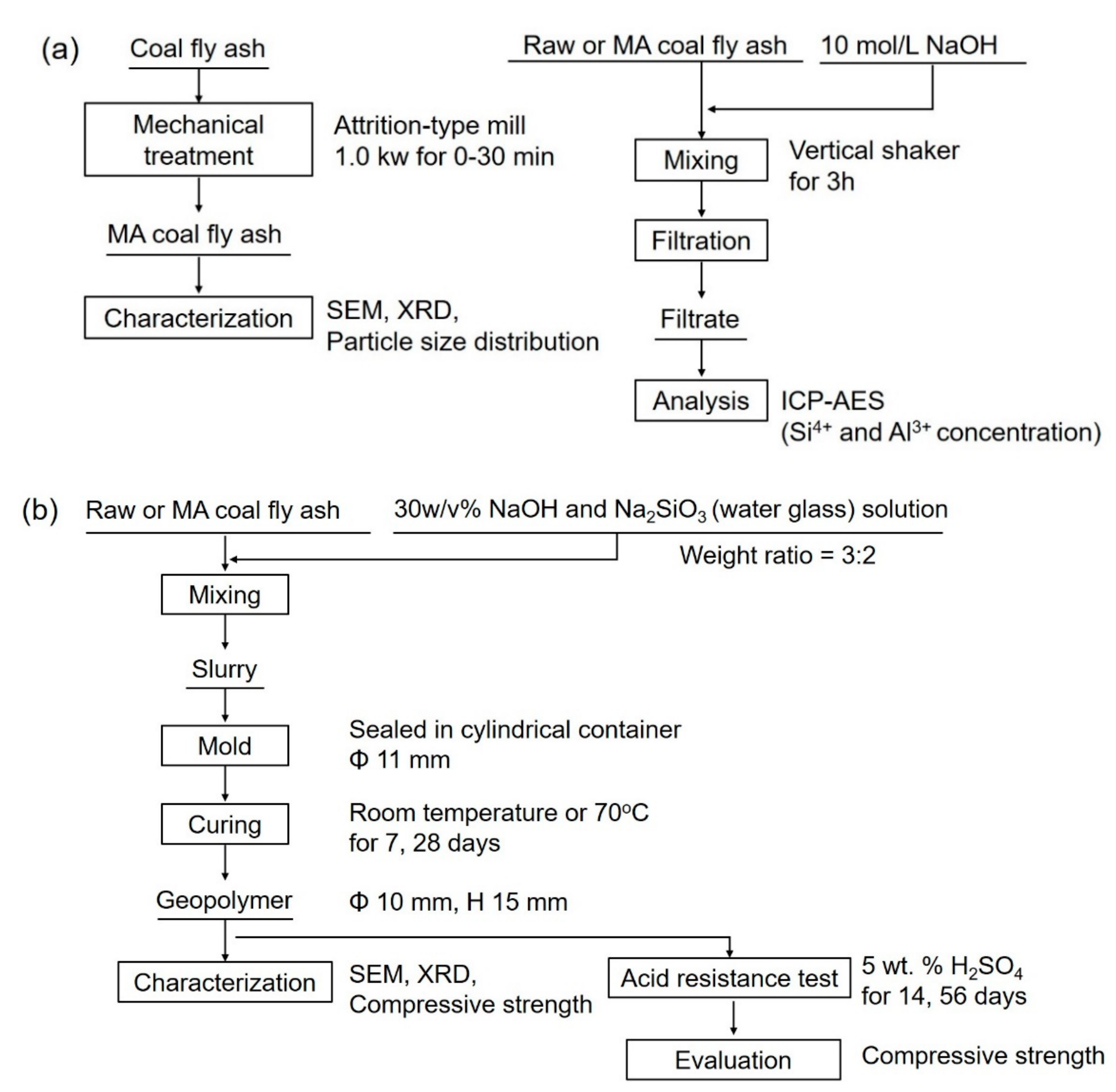
2. Materials and Methods
2.1. Mechanical Activation and Characterization of Coal Fly Ash Powder
2.2. Synthesis and Evaluation of Geopolymers from Mechanically Activated Coal Fly Ash
3. Results and Discussion
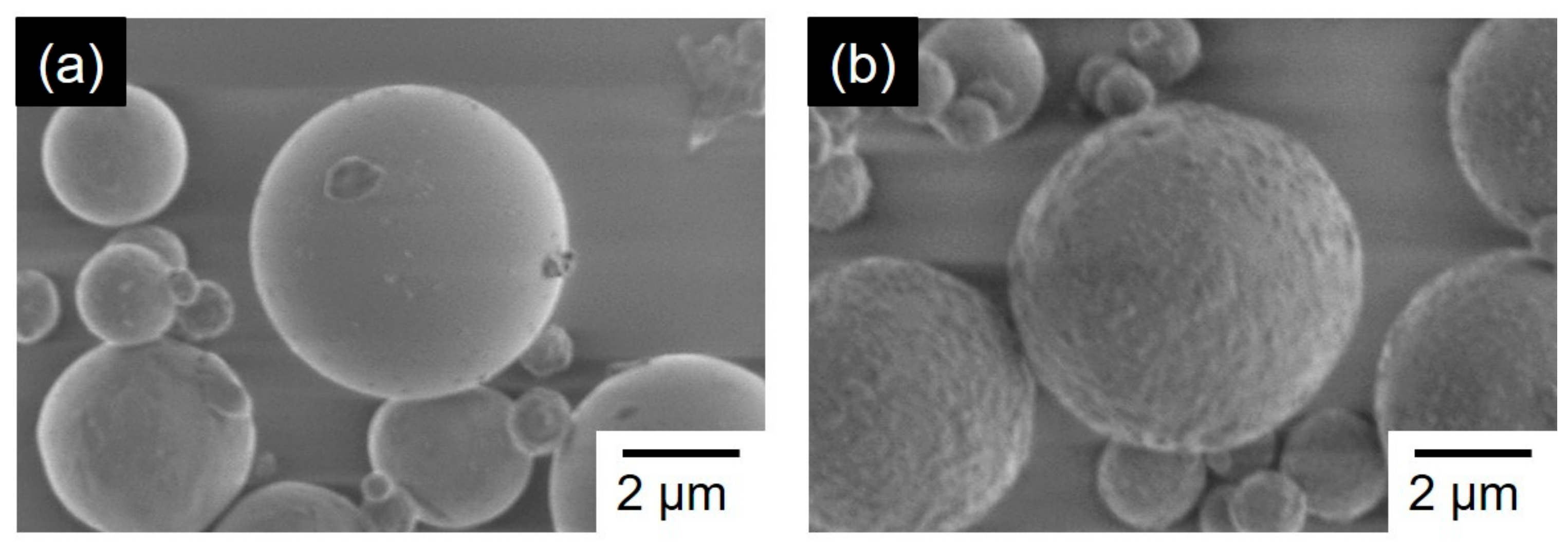
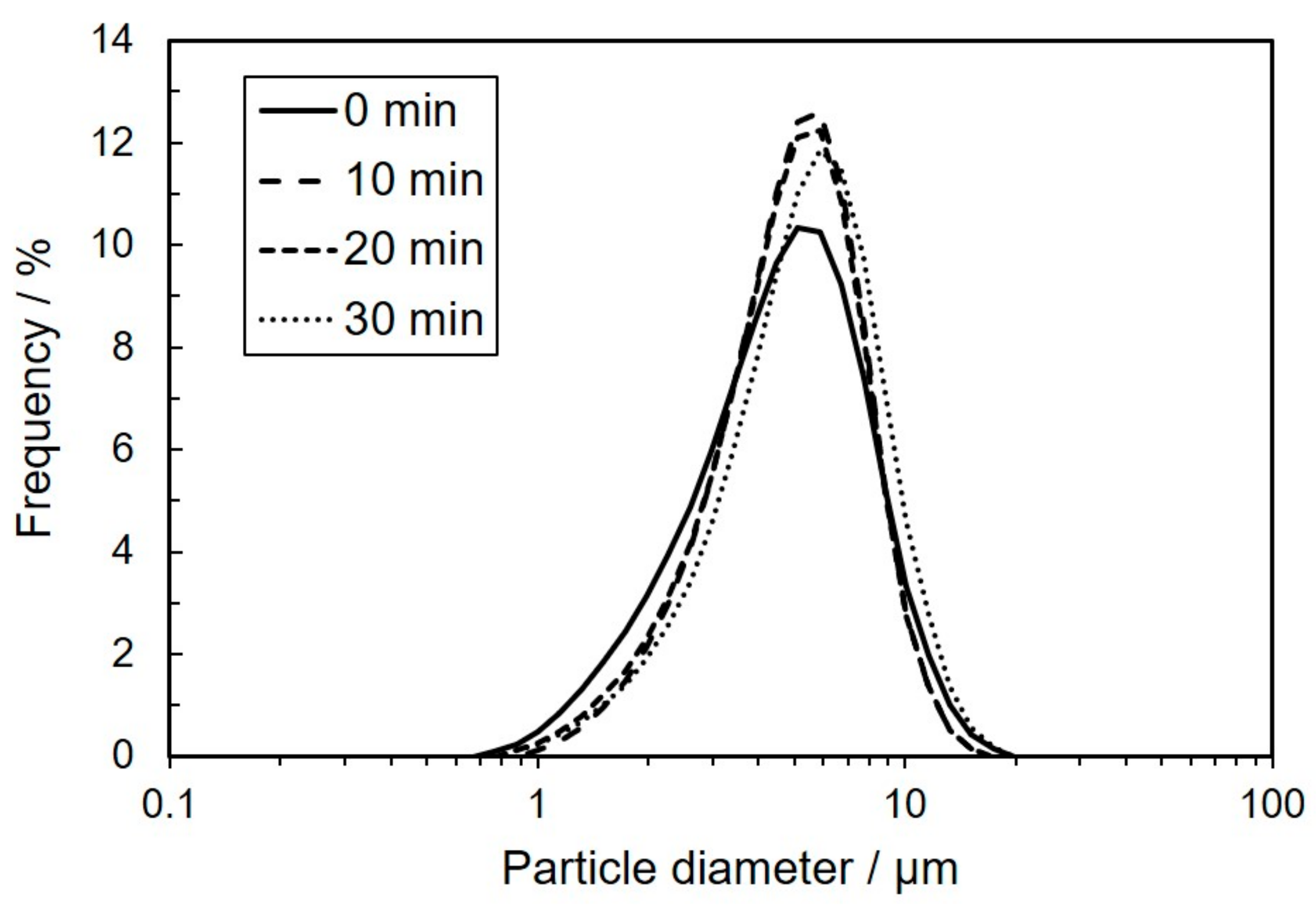
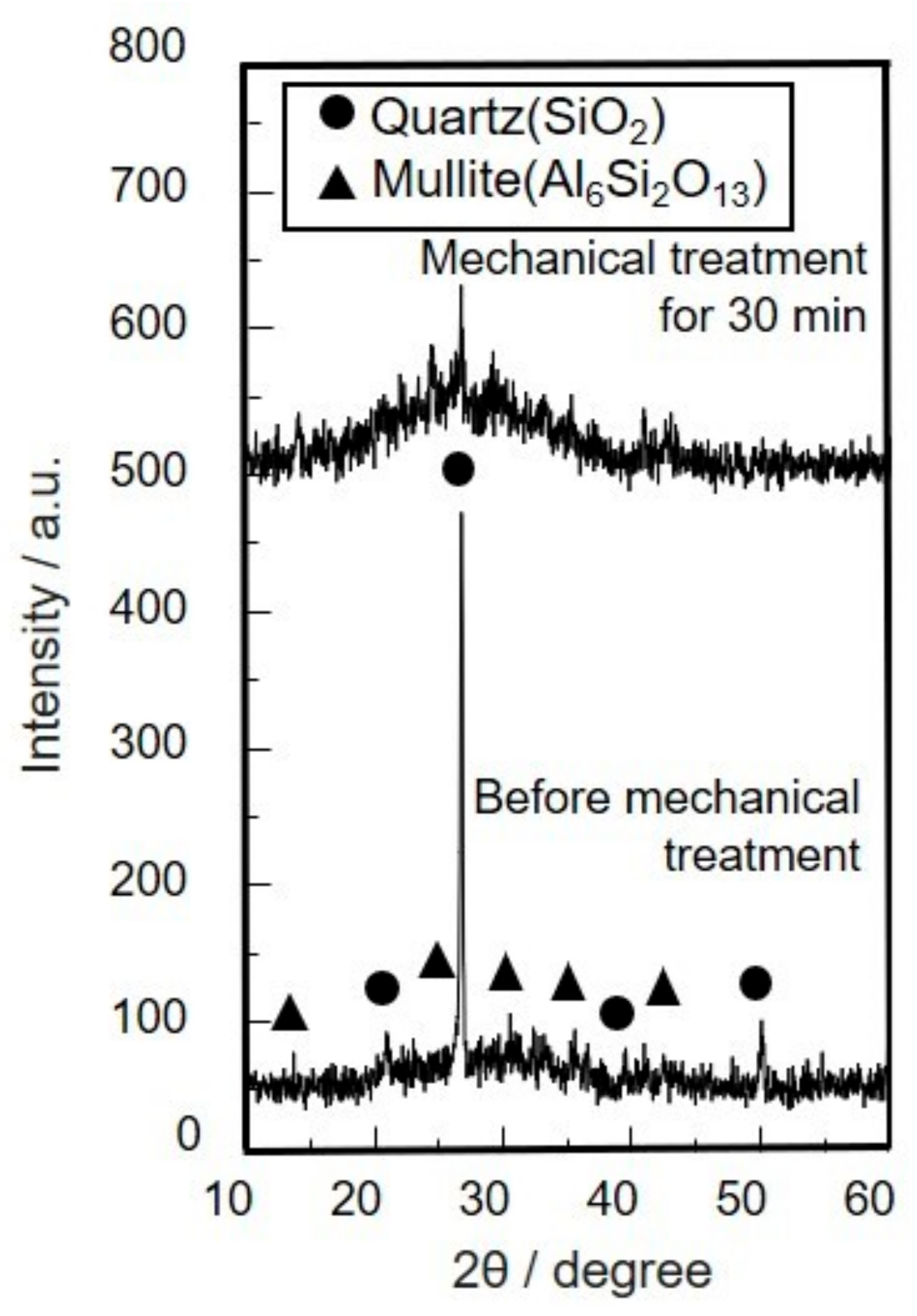
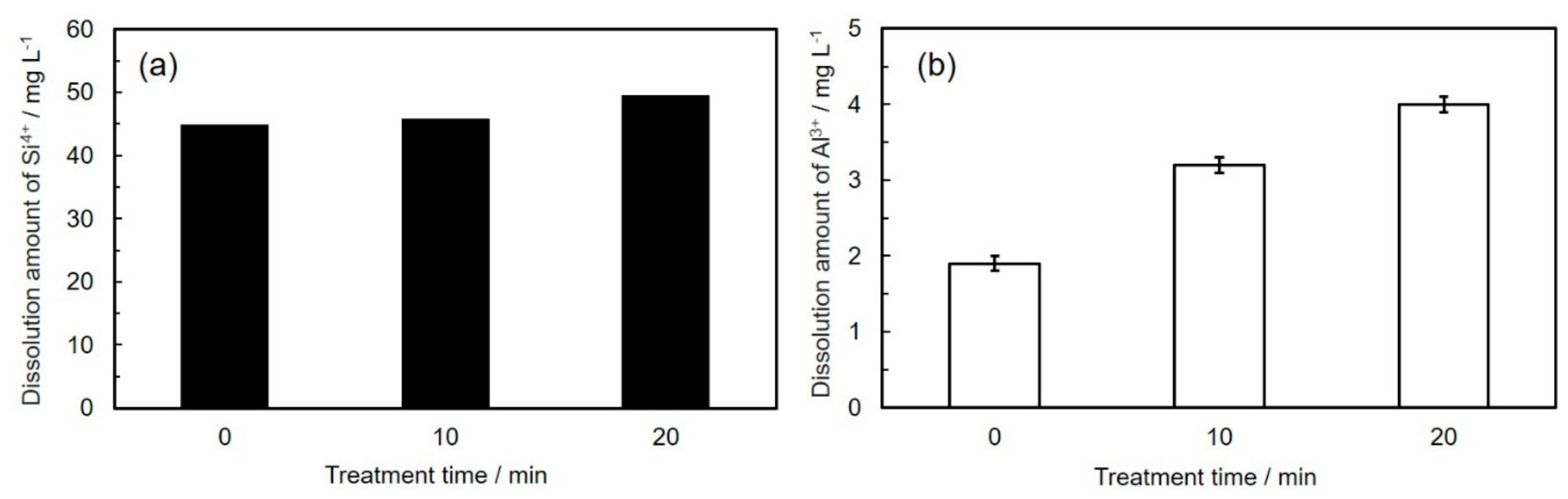
3.1. Characterization for Coal Fly Ash after Mechanical Activation
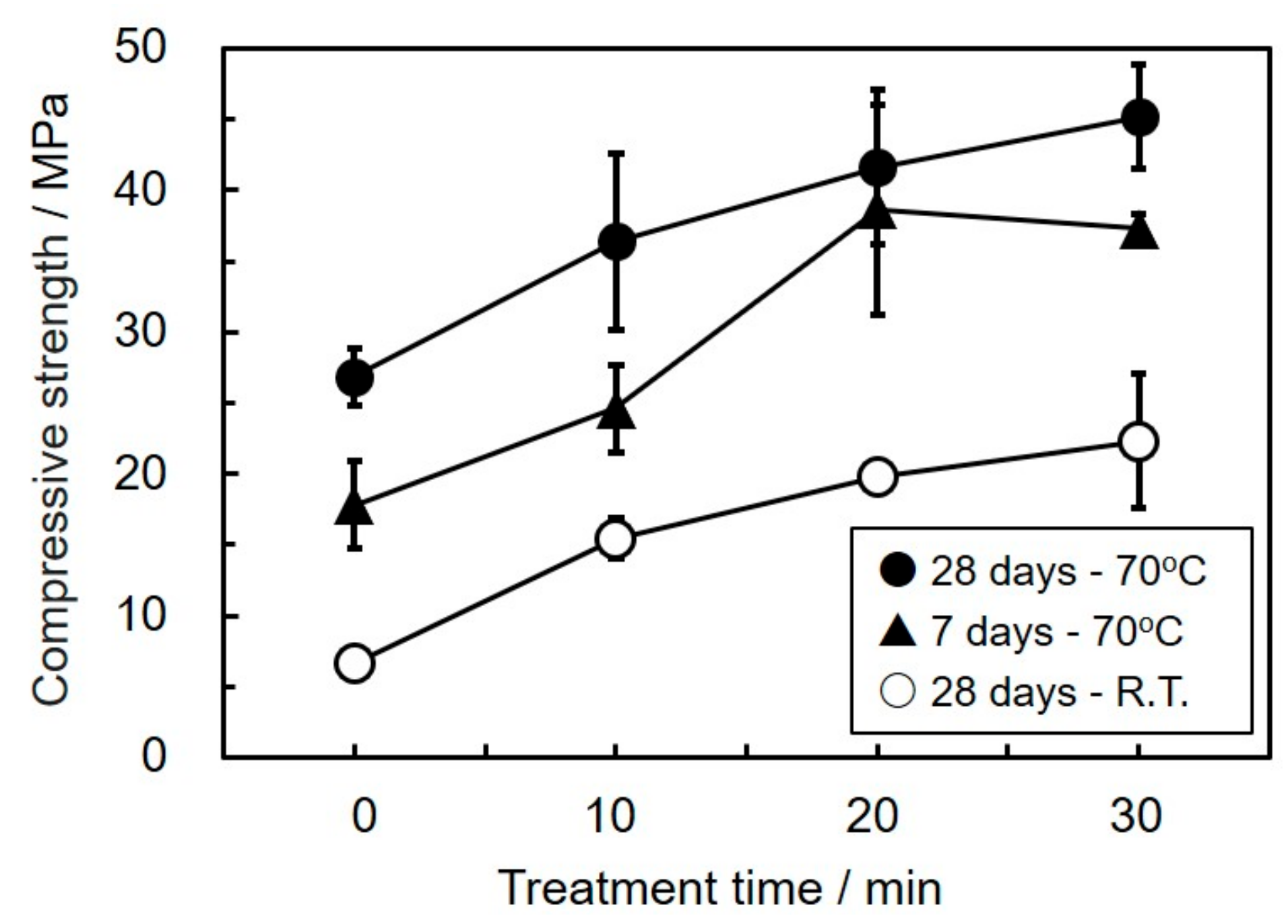
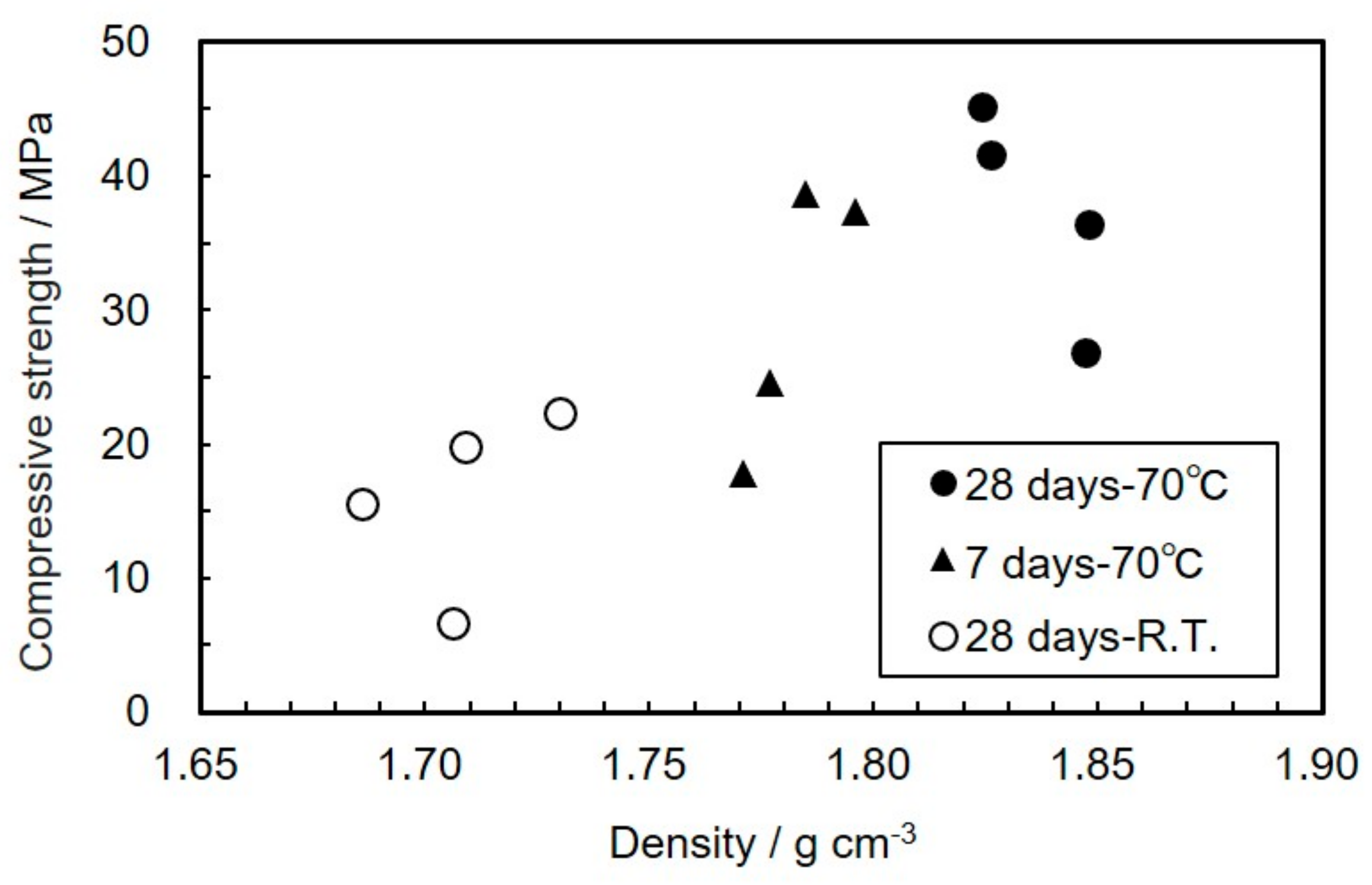
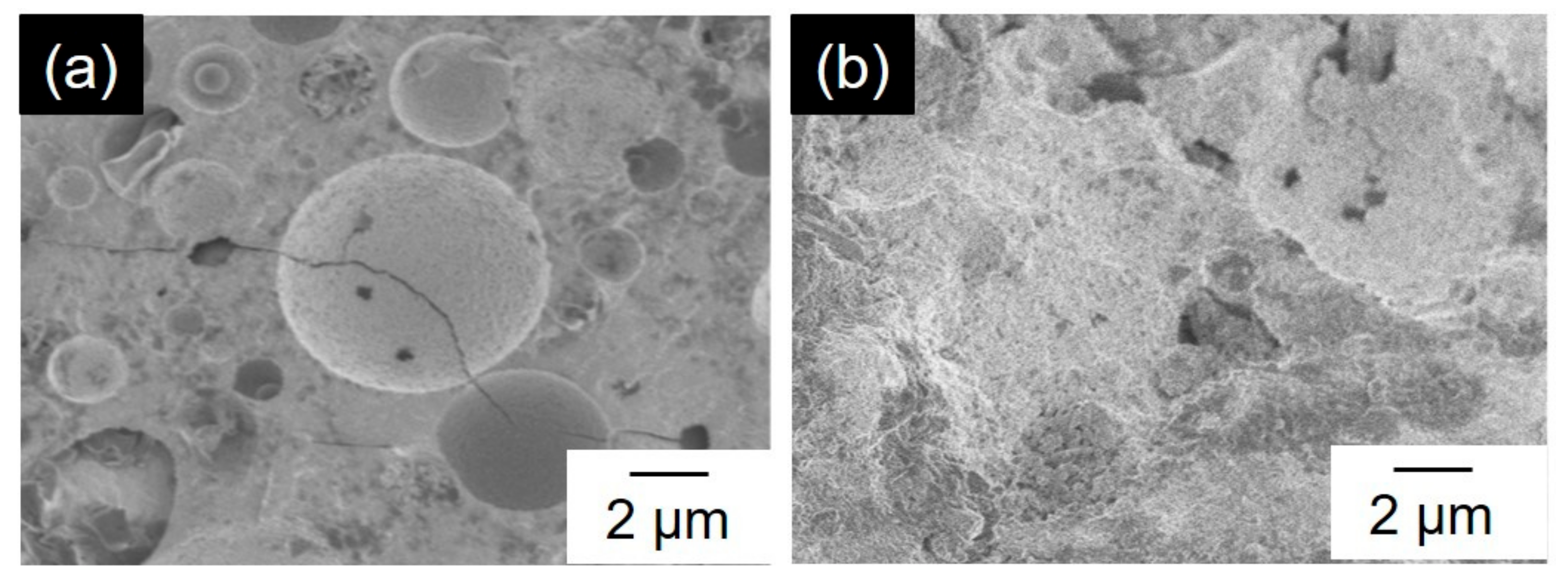
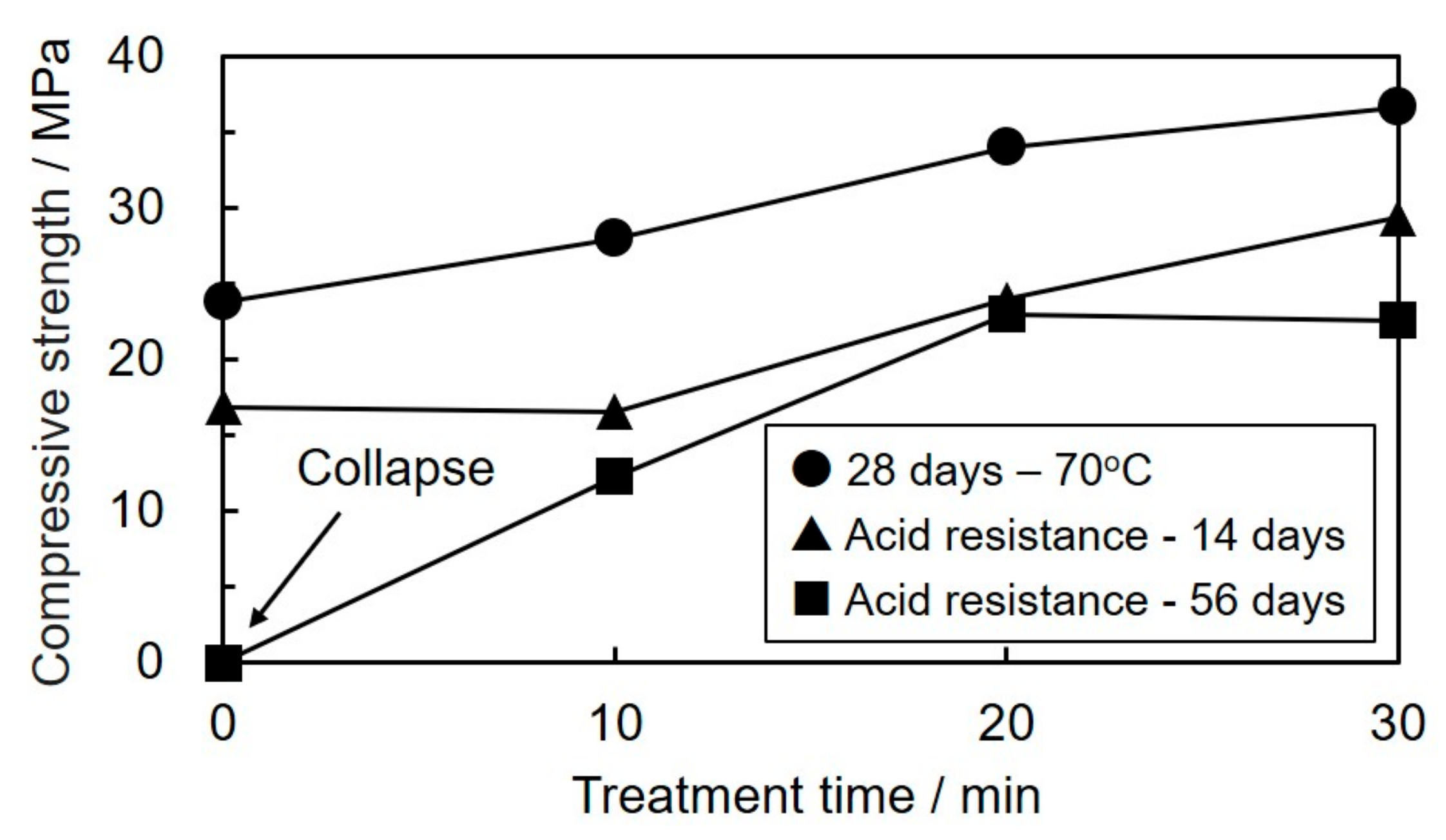
3.2. Effect of Mechanical Activation on Compressive Strength of the Geopolymers
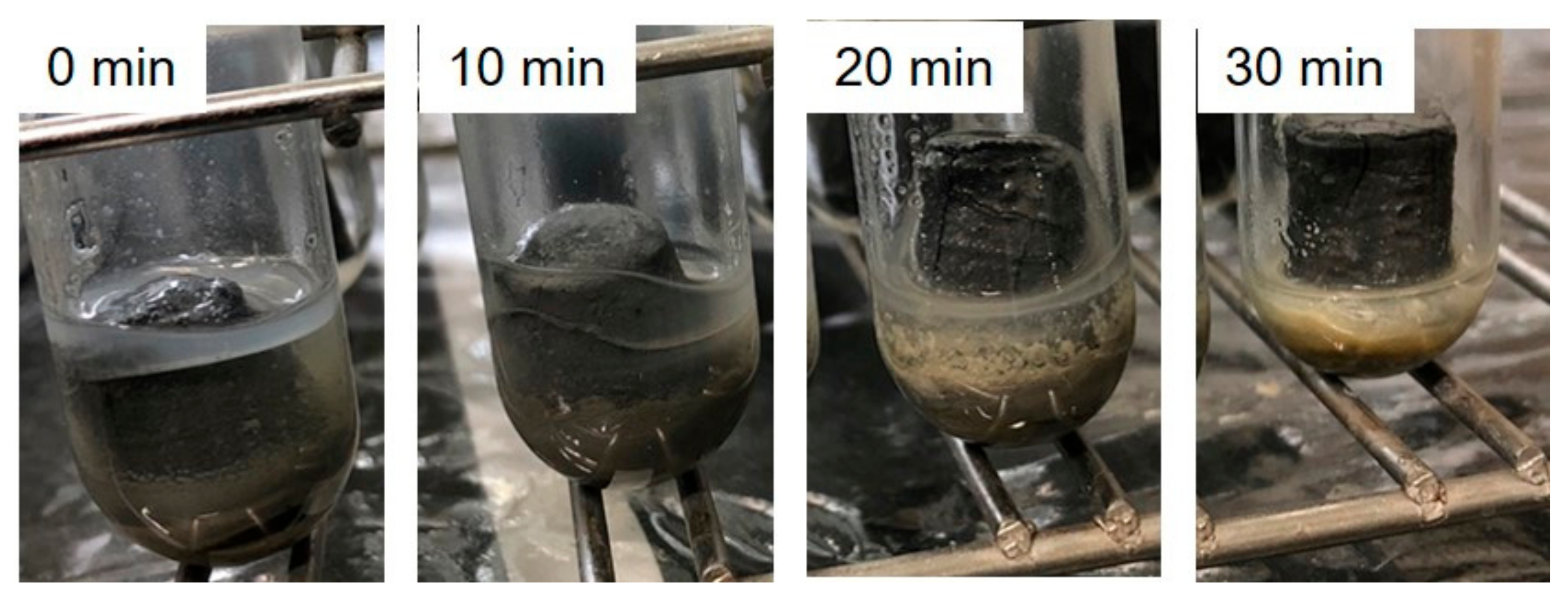
3.3. Acid Resistance Test of Geopolymers Cured from Mechanically Activated Coal Fly Ash
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kumar, S.; Kumar, R.; Mehrotra, S.P. Influence of granulated blast furnace slag on the reaction, structure and properties of fly ash based geopolymer. J. Mater. Sci. 2010, 45, 607–615. [Google Scholar] [CrossRef]
- Nazari, A.; Riahi, S.; Bagheri, A. Designing water resistant lightweight geopolymers produced from waste materials. Mater. Des. 2012, 35, 296–302. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, S. Development of paving blocks from synergistic use of red mud and fly ash using geopolymerization. Constr. Build. Mater. 2013, 38, 865–871. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymers and geopolymeric materials. J. Therm. Anal. Calorim. 1989, 35, 429–441. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymers: Inorganic polymeric new materials. J. Therm. Anal. Calorim. 1991, 37, 1633. [Google Scholar] [CrossRef]
- Takeda, H.; Hashimoto, S.; Yokoyama, H.; Honda, S.; Iwamoto, Y. Characterization of zeolite in zeolite-geopolymer hybrid bulk materials derived from kaolinitic clays. Materials 2013, 6, 1767–1778. [Google Scholar] [CrossRef] [PubMed]
- Ryu, G.S.; Lee, Y.B.; Koh, K.T.; Chung, Y.S. The mechanical properties of fly ash-based geopolymer concrete with alkaline activators. Constr. Build. Mater. 2013, 47, 409–418. [Google Scholar] [CrossRef]
- Van Jaarsveld, J.G.S.; van Deventer, J.S.J.; Lukey, G.C. The effect of composition and temperature on the properties of fly ash- and kaolinite-based geopolymers. Chem. Eng. J. 2002, 89, 63–73. [Google Scholar] [CrossRef]
- Lee, N.K.; Lee, H.K. Setting and mechanical properties of alkali-activated fly ash/slag concrete manufactured at room temperature. Constr. Build. Mater. 2013, 47, 1201–1209. [Google Scholar] [CrossRef]
- Fan, Y.; Yin, S.; Wen, Z.; Zhong, J. Activation of fly ash and its effects on cement properties. Cem. Concr. Res. 1999, 29, 467–472. [Google Scholar] [CrossRef]
- Temuujin, J.; MacKenzie, K.J.D.; Burmaa, G.; Tsend-Ayush, D.; Jadambaa, T.; Riessen, A.V. Mechanical activation of MoS2 + Na2O2 mixtures. Miner. Eng. 2009, 22, 415–418. [Google Scholar] [CrossRef]
- Hamzaoui, R.; Bouchenafa, O.; Guessasma, S.; Leklou, N.; Bouaziz, A. The sequel of modified fly ashes using high energy ball milling on mechanical performance of substituted past cement. Mater. Des. 2016, 90, 29–37. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, R. Mechanical activation of fly ash: Effect on reaction, structure and properties of resulting geopolymer. Ceram. Int. 2011, 37, 533–541. [Google Scholar] [CrossRef]
- Marjanovic, N.; Komljenović, M.; Baščarević, Z.; Nikolić, V. Improving reactivity of fly ash and properties of ensuing geopolymers through mechanical activation. Constr. Build. Mater. 2014, 57, 151–162. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, S.; Mehrotra, S.P. Towards sustainable solutions for fly ash through mechanical activation. Resour. Conserv. Recycl. 2007, 52, 157–179. [Google Scholar] [CrossRef]
- Mucsi, G.; Molnar, Z.; Kumar, S. Geopolymerisation of mechanically activated lignite and brown coal fly ash. Acta Phys. Pol. A 2014, 126, 994–998. [Google Scholar] [CrossRef]
- Boldyrev, V.V. Mechanochemistry and mechanical activation of solids. Bull. Acad. Sci. USSR Div. Chem. Sci. 1990, 39, 2029–2044. [Google Scholar] [CrossRef]
- Baláž, P.; Dutková, E. Fine milling in applied mechanochemistry. Miner. Eng. 2009, 22, 681–694. [Google Scholar] [CrossRef]
- Craig, S.L. Mechanochemistry: A tour of force. Nature 2012, 487, 176–177. [Google Scholar] [CrossRef]
- Kato, K.; Xin, Y.; Hitomi, T.; Shirai, T. Surface modification of fly ash by mechano-chemical treatment. Ceram. Int. 2019, 45, 849–853. [Google Scholar] [CrossRef]
- Kumar, S.; Kristály, F.; Mucsi, G. Geopolymerisation behaviour of size fractioned fly ash. Adv. Powder Technol. 2015, 26, 24–30. [Google Scholar] [CrossRef]
- Petrakis, E.; Karmali, V.; Bartzas, G.; Komnitsas, K. Grinding kinetics of slag and effect of final particle size on the compressive strength of alkali activated materials. Minerals 2019, 9, 714. [Google Scholar] [CrossRef]
- Riahi, S.; Nazari, A. The effects of nanoparticles on early age compressive strength of ash-based geopolymers. Ceram. Int. 2012, 38, 4467–4476. [Google Scholar] [CrossRef]
- Guo, X.; Hu, W.; Shi, H. Microstructure and self-solidification/stabilization (S/S) of heavy metals of nano-modified CFA-MSWIFA composite geopolymers. Constr. Build. Mater. 2014, 56, 81–86. [Google Scholar] [CrossRef]
- Matsuoka, M.; Kondo, A.; Kozawa, T.; Naito, M.; Koga, H.; Saito, T.; Iba, H. Effect of carbon addition on one-step mechanical synthesis of LiCoPO4/C composite granules and their powder characteristics. Ceram. Int. 2017, 43, 938–943. [Google Scholar] [CrossRef]
- Kumar, S.; Mucsi, G.; Kristály, F.; Pekker, P. Mechanical activation of fly ash and its influence on micro and nano-structural behaviour of resulting geopolymers. Adv. Powder Technol. 2017, 28, 805–813. [Google Scholar] [CrossRef]
| Products | Si | Al | Na | K | Ca | Fe | Mg |
|---|---|---|---|---|---|---|---|
| Coal fly ash | 62.7 | 21.6 | 0.9 | 2.0 | 4.6 | 7.1 | 1.1 |
| Characteristic | Mechanical Treatment Time (min) | |||
|---|---|---|---|---|
| 0 | 10 | 20 | 30 | |
| Mean Particle size d50 (μm) | 4.4 | 4.7 | 4.6 | 5.1 |
| Specific surface area (m2 g−1) | 1.8 | 2.2 | 1.9 | 2.2 |
| Curing Conditions | 28 days–70 °C | 7 days–70 °C | 28 days–R.T. |
|---|---|---|---|
| Compressive strength | 26.9 | 17.8 | 6.7 |
| (MPa) | (SD 4.0) | (SD 6.1) | (SD 2.2) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsuoka, M.; Yokoyama, K.; Okura, K.; Murayama, N.; Ueda, M.; Naito, M. Synthesis of Geopolymers from Mechanically Activated Coal Fly Ash and Improvement of Their Mechanical Properties. Minerals 2019, 9, 791. https://doi.org/10.3390/min9120791
Matsuoka M, Yokoyama K, Okura K, Murayama N, Ueda M, Naito M. Synthesis of Geopolymers from Mechanically Activated Coal Fly Ash and Improvement of Their Mechanical Properties. Minerals. 2019; 9(12):791. https://doi.org/10.3390/min9120791
Chicago/Turabian StyleMatsuoka, Mitsuaki, Kaho Yokoyama, Kohei Okura, Norihiro Murayama, Masato Ueda, and Makio Naito. 2019. "Synthesis of Geopolymers from Mechanically Activated Coal Fly Ash and Improvement of Their Mechanical Properties" Minerals 9, no. 12: 791. https://doi.org/10.3390/min9120791
APA StyleMatsuoka, M., Yokoyama, K., Okura, K., Murayama, N., Ueda, M., & Naito, M. (2019). Synthesis of Geopolymers from Mechanically Activated Coal Fly Ash and Improvement of Their Mechanical Properties. Minerals, 9(12), 791. https://doi.org/10.3390/min9120791




