Formation of Au-Bearing Antigorite Serpentinites and Magnetite Ores at the Massif of Ophiolite Ultramafic Rocks: Thermodynamic Modeling
Abstract
1. Introduction
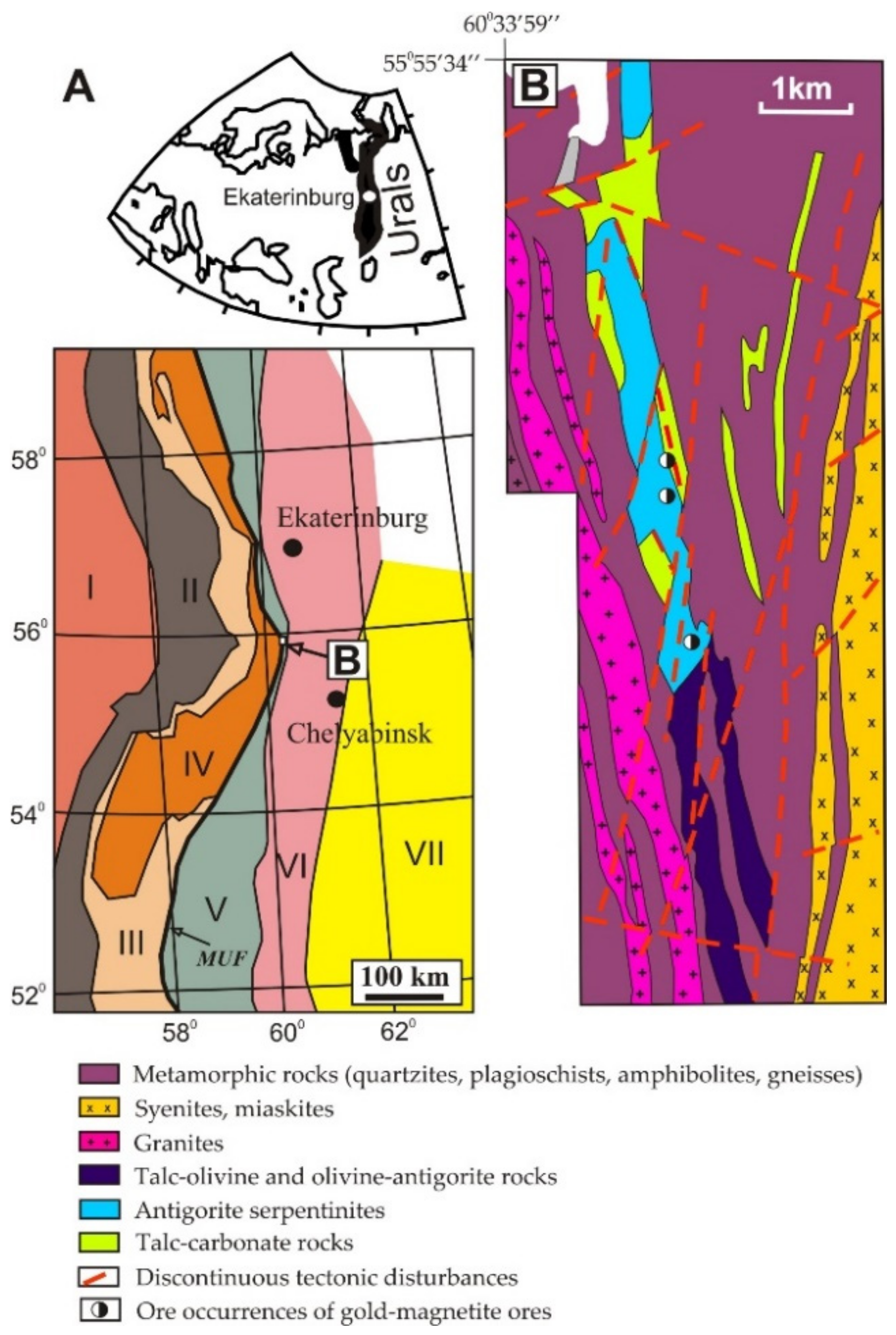
2. Geological and Metallogenic Overview
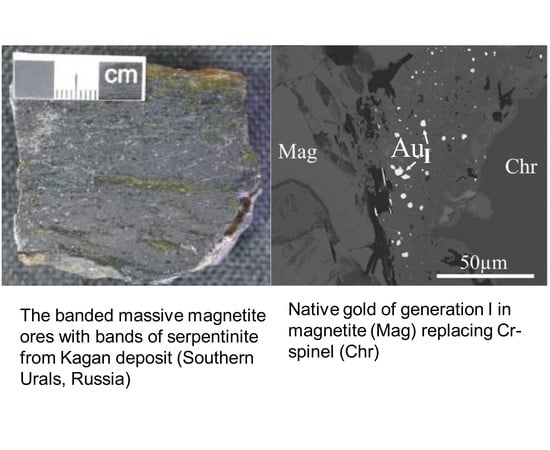
2.1. Geological Setting and Types of Gold Mineralization of the Kagan Massif
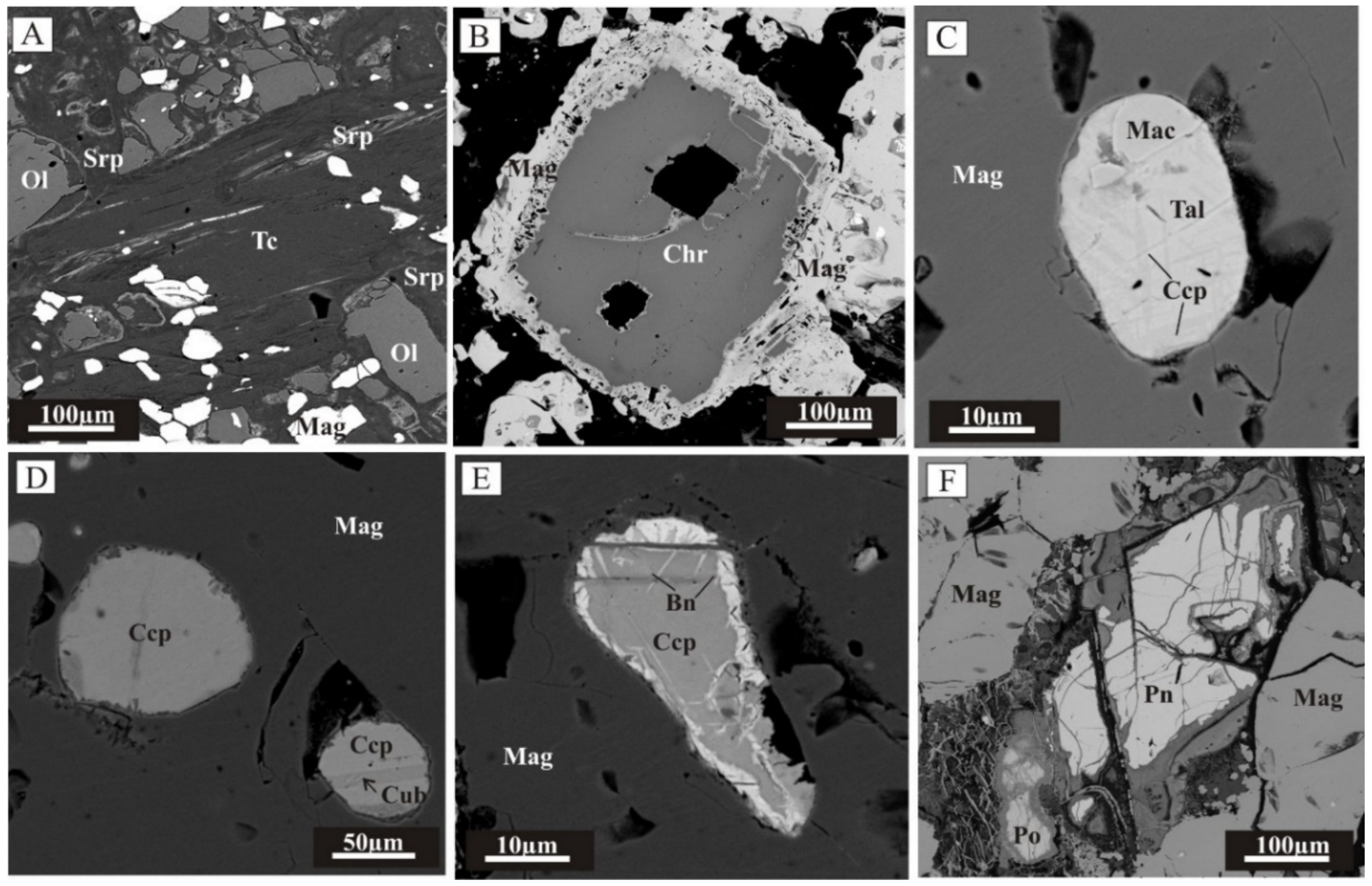
2.2. Mineralogy of Gold-Bearing Antigorite Serpentinites and Gold–Sulfide–Magnetite Ores
2.3. Physico-Chemical Conditions, Sources of Substance and Fluid and the Sequence of Formation of Gold Mineralization
3. Research Methods
3.1. Software and Thermodynamic Dataset for Modeling
3.2. Initial Data for Thermodynamic Modeling
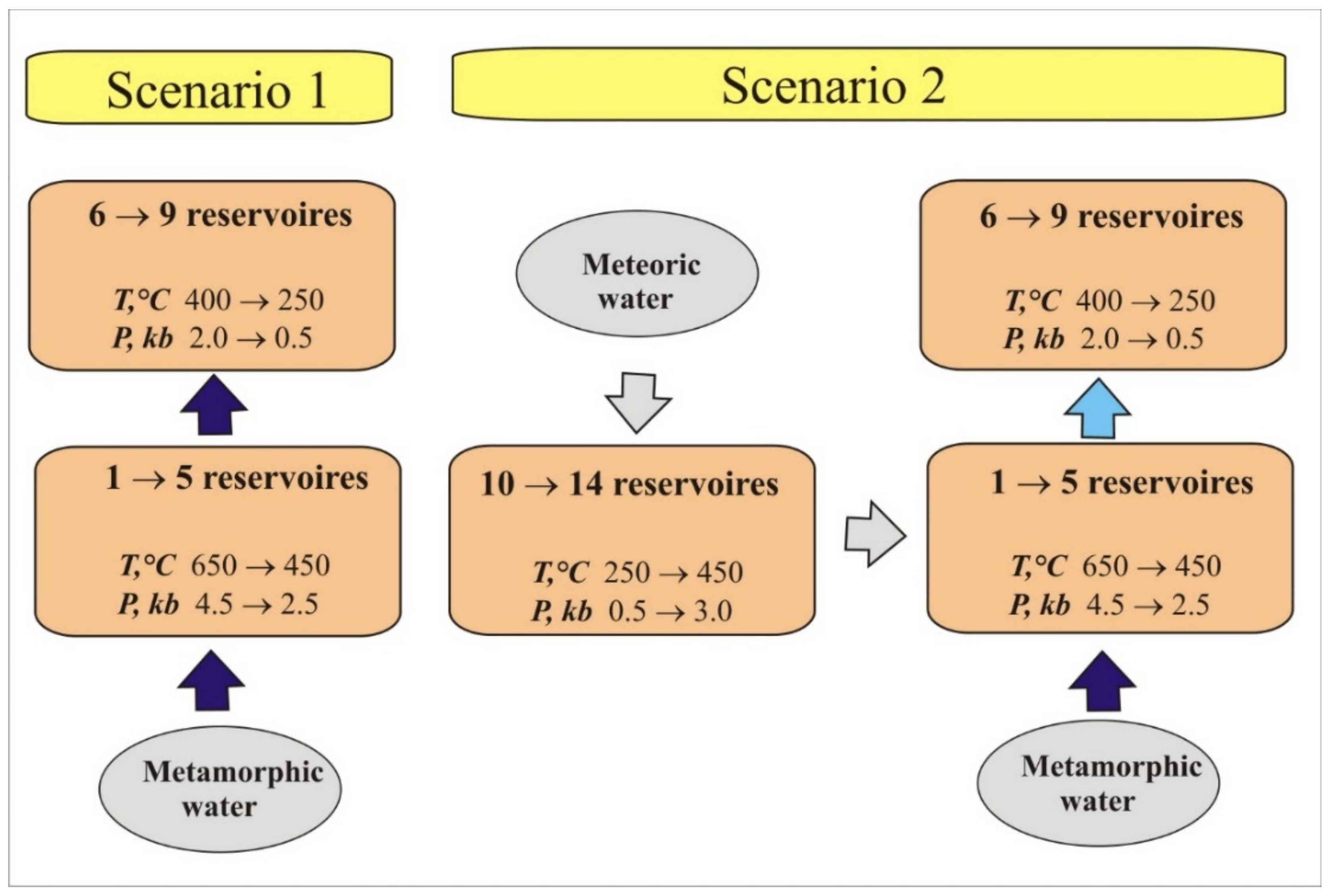
3.3. Scenarios of Thermodynamic Modeling
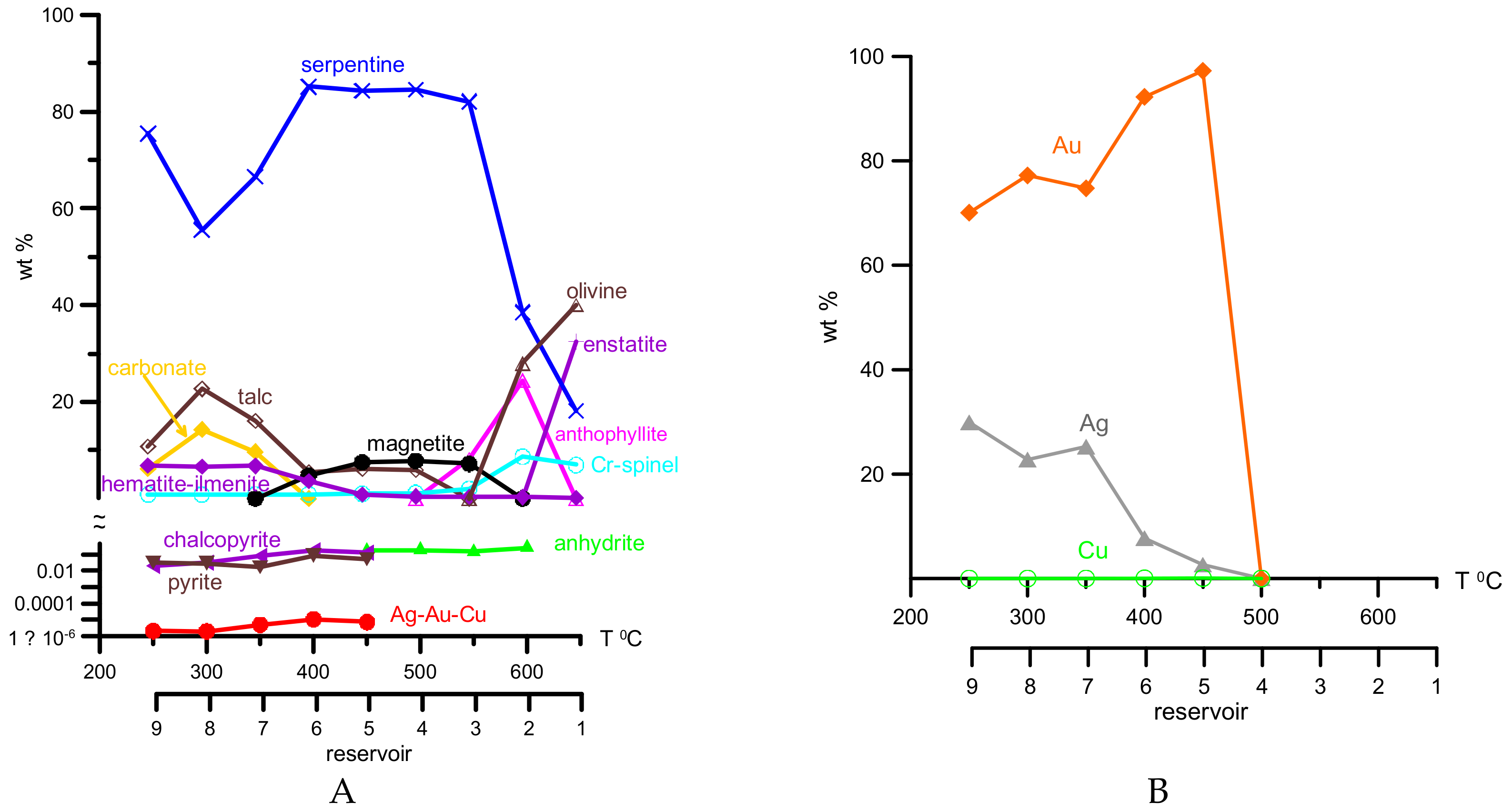
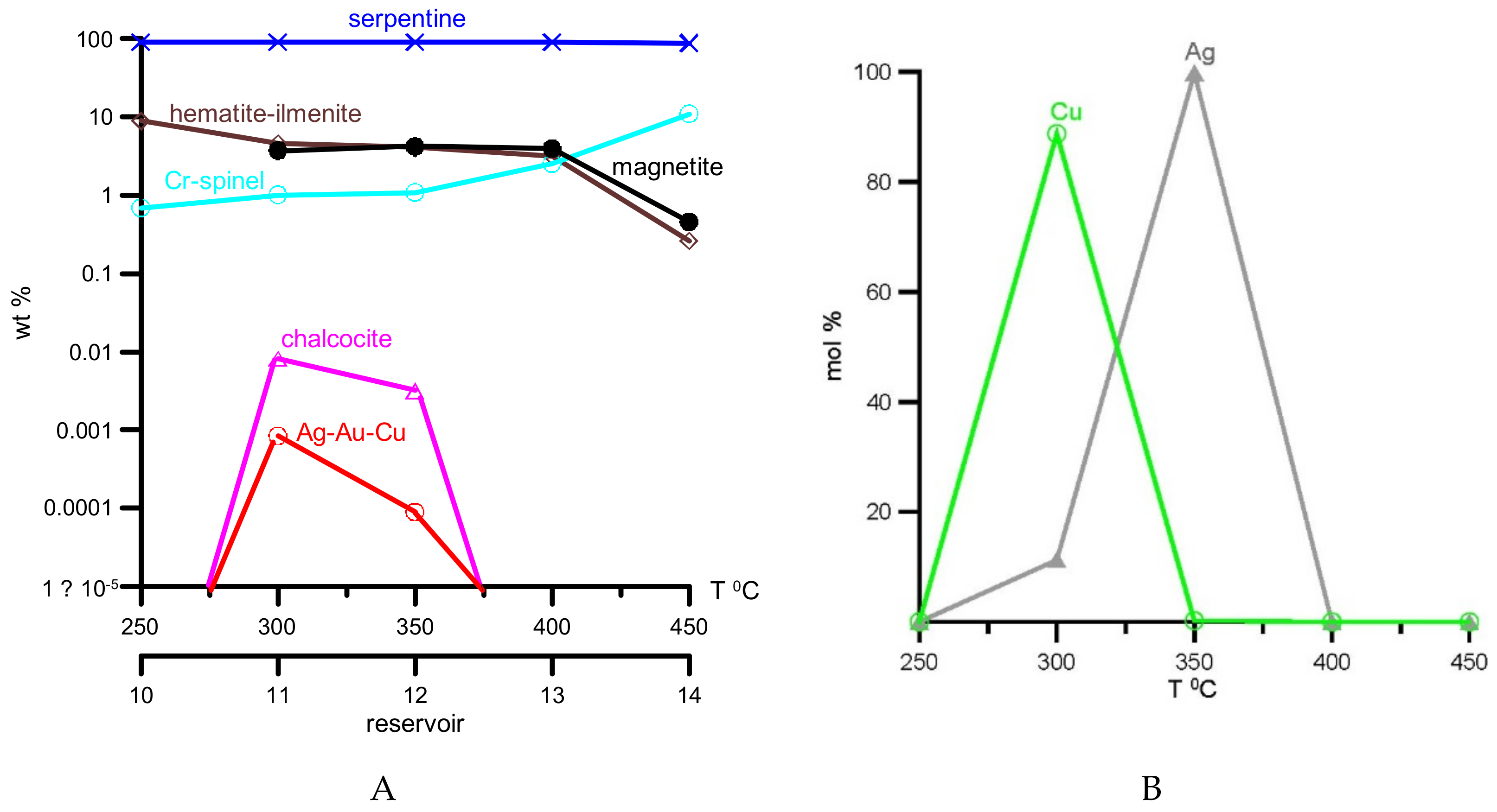
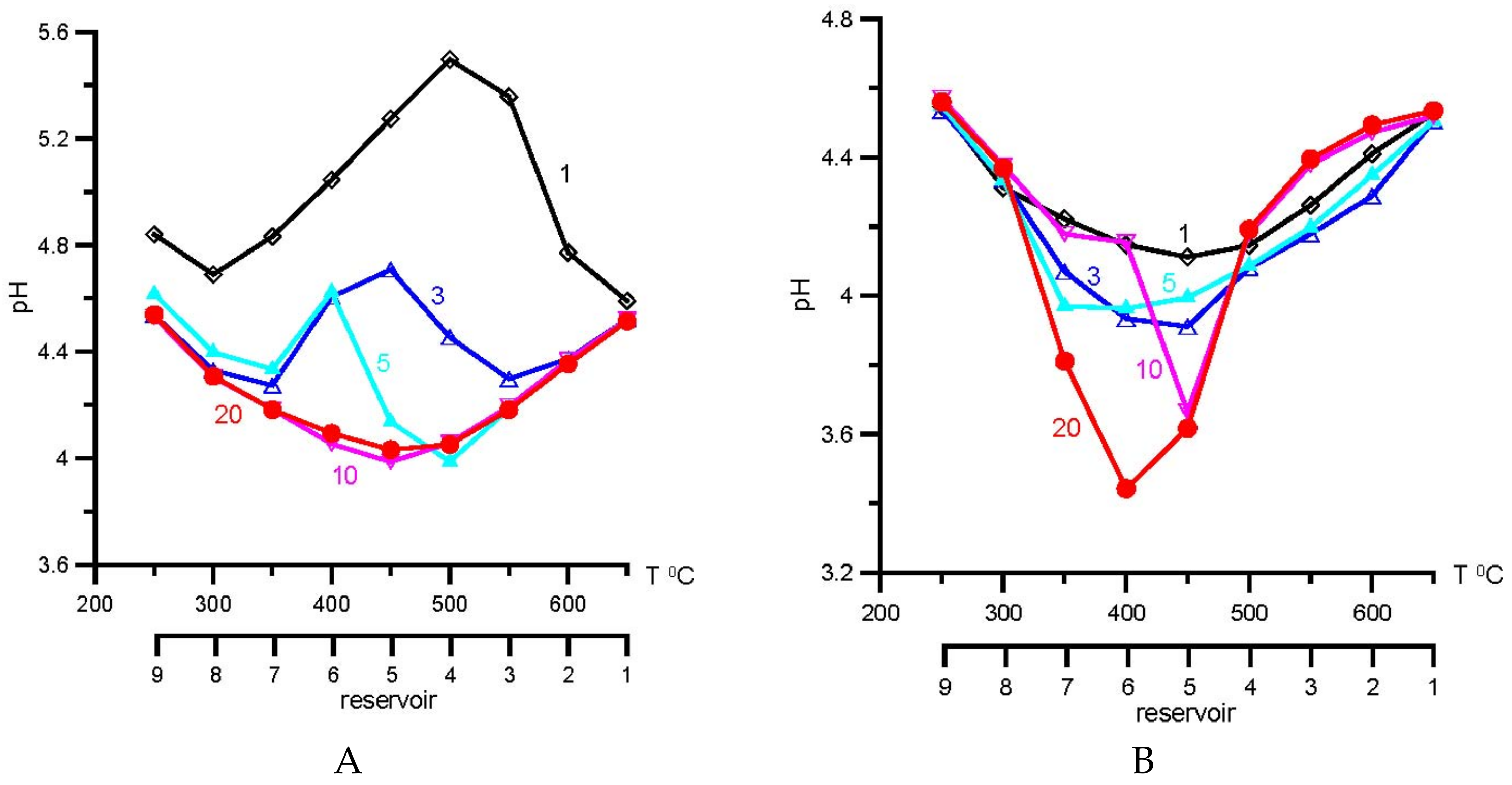
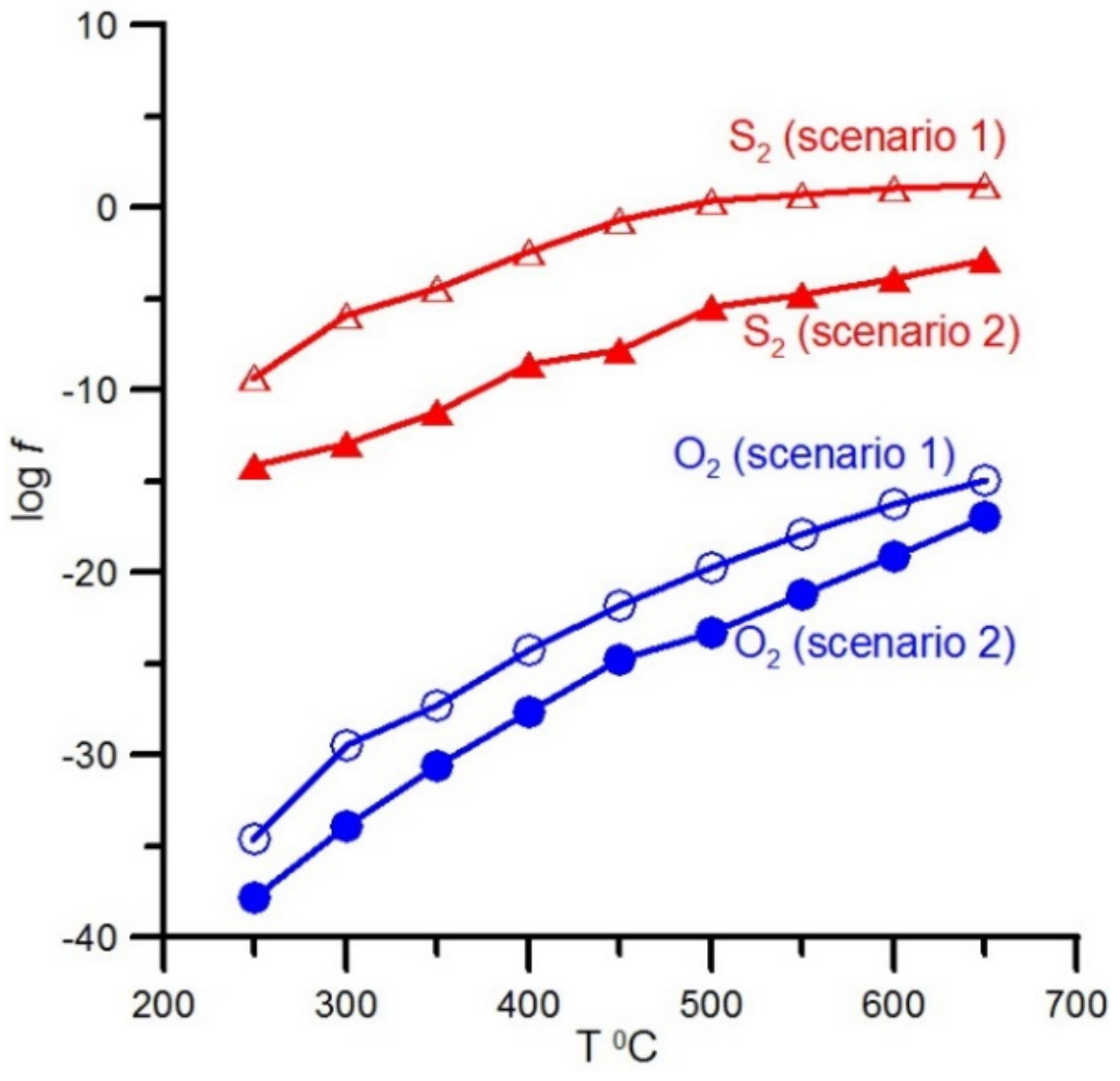
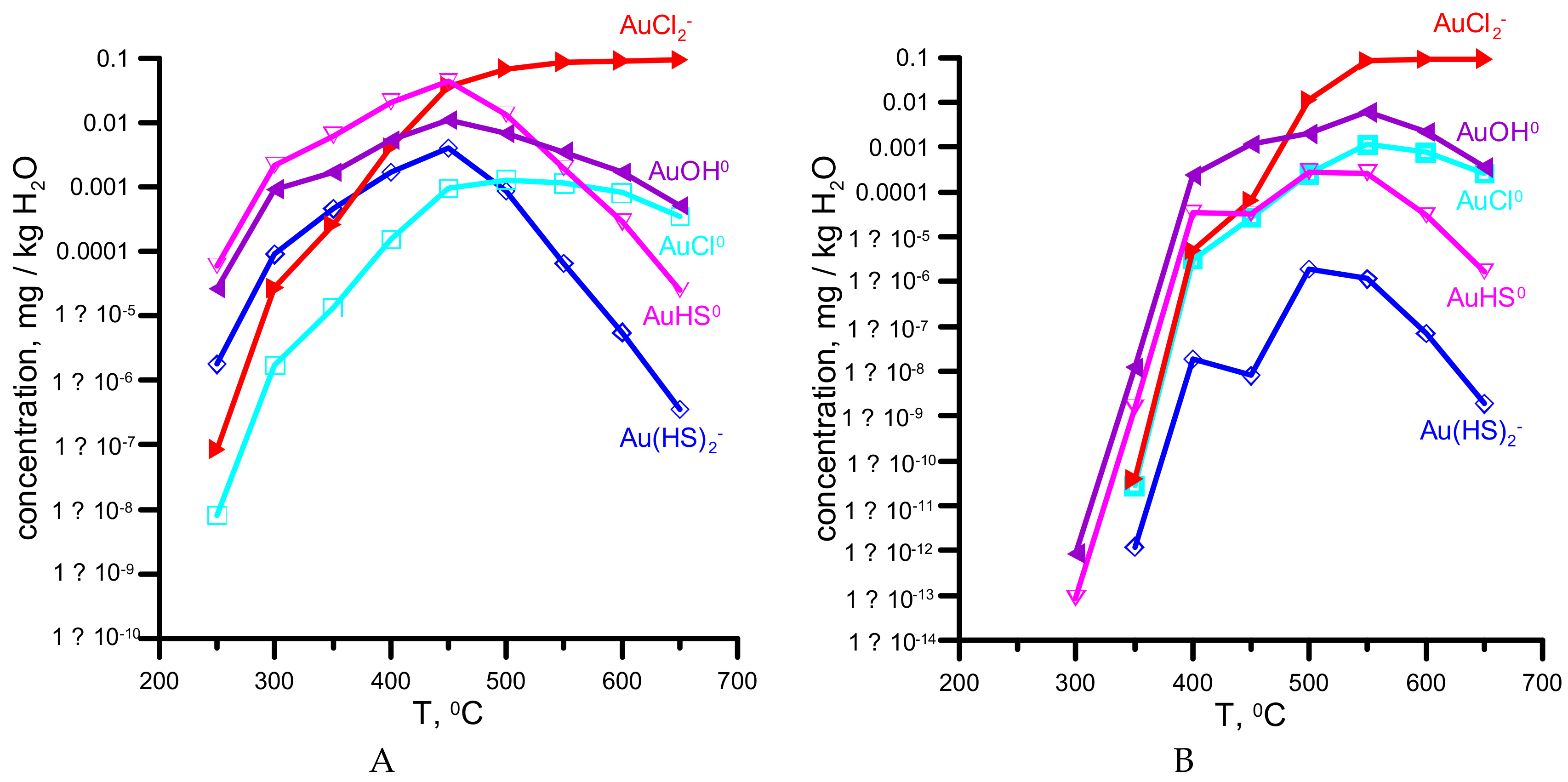
4. Results of Thermodynamic Modeling
5. Discussion
5.1. Comparative Analysis of Agreement of Model and Natural Mineral Associations
5.2. The Possible Sources of Gold, Silver, and Other Metals
5.3. Sulfur Fugacity Evolution During Gold-Ore Mineralization Formation in Ultramafic Rocks and Magnetite Ores
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Benevolsky, B.I. Gold of Russia. Problems of the Use and Reproduction of the Mineral Resource Base; Geoinformmark: Moscow, Russia, 1995; 87p. (In Russian) [Google Scholar]
- Sazonov, V.N.; Ogorodnikov, V.N.; Koroteev, V.A.; Polenov, I.A. Gold Deposits of the Urals, 2nd ed.; Ural Mining and Geological Academy: Yekaterinburg, Russia, 2001; 622p. (In Russian) [Google Scholar]
- Ovchinnikov, L.N. Minerals and Metallogeny of the Urals; Geoinformmark: Moscow, Russia, 1998; 412p. (In Russian) [Google Scholar]
- Puchkov, V.N. General features relating to the occurrence of mineral deposits in the Urals: What, where, when and why. Ore Geol. Rev. 2017, 85, 4–29. [Google Scholar] [CrossRef]
- Borodaevsky, N.I. Types of gold deposits related to ultramafic rocks in the Miass and Uchaly districts in the southern Urals. In 200 Years of Gold Industry of the Urals; Ivanov, A.A., Rozhkov, I.S., Eds.; UFAN SSSR: Sverdlovsk, Russia, 1948; pp. 316–330. (In Russian) [Google Scholar]
- Berzon, R.O. Gold Resource Potential of Ultramafics; VIEMS: Moscow, Russia, 1983; p. 47. (In Russian) [Google Scholar]
- Sazonov, V.N. Gold-Productive Metasomatic Rocks in Mobile Belts: Geodynamic Settings and PTX Parameters of Their Formation and Implications for Forecasting; Ural Mining and Geological Academy: Yekaterinburg, Russia, 1998; 181p. (In Russian) [Google Scholar]
- Spiridonov, E.M.; Pletnev, P.A. Zolotaya Gora Deposit of Cupriferous Gold; Nauchnyi Mir: Moscow, Russia, 2002; 220p. (In Russian) [Google Scholar]
- Murzin, V.V.; Varlamov, D.A.; Shanina, S.N. New Data on the Gold–Antigorite Association of the Urals. Dokl. Earth Sci. 2007, 417, 1436–1439. [Google Scholar] [CrossRef]
- Murzin, V.V.; Varlamov, D.A.; Ronkin, Y.L.; Shanina, S.N. Origin of Au-bearing Rodingite in the Karabash Massif of Alpine-Type Ultramafic Rocks in the Southern Urals. Geol. Ore Depos. 2013, 55, 278–297. [Google Scholar] [CrossRef]
- Murzin, V.; Chudnenko, K.; Palyanova, G.; Kissin, A.; Varlamov, D. Physicochemical model of formation of gold-bearing magnetite–chlorite–carbonate rocks at the Karabash ultramafic massif (Southern Urals, Russia). Minerals 2018, 8, 306. [Google Scholar] [CrossRef]
- Murzin, V.V.; Chudnenko, K.V.; Palyanova, G.A.; Varlamov, D.A.; Naumov, E.A.; Pirajno, F. Physicochemical model of formation of Cu–Ag–Au–Hg solid solutions and intermetallic alloys in the rodingites of the Zolotaya Gora gold deposit (Urals, Russia). Ore Geol. Rev. 2018, 93, 81–97. [Google Scholar] [CrossRef]
- Bazhin, E.A. Gold Content of the Talovskii Gabbro-Ultramafic Massif (Southern Urals). Problems of Mineralogy, Petrography and Metallogeny; Scientific Readings in Memory of P.N.Chirvinskii: Collection of Scientific Articles; Issue 15; Perm State Research University: Perm, Russia, 2012; pp. 294–298. (In Russian) [Google Scholar]
- Varlakov, A.S.; Kuznetsov, G.P.; Korablev, G.G.; Murkin, V.P. The Ultrabasites of Vishnyevogorsk-Ilmenogorsk Metamorphic Complex (South Urals); Edition of Institute of Mineralogy Ural Division of Russian Academy of Sciences: Miass, Russia, 1998; 195p. (In Russian) [Google Scholar]
- Murzin, V.V. Oxygen and Hydrogen Isotopic Composition of the Fluid during Formation of Anthophyllite Metaultramafic Rocks in the Sysert Metamorphic Complex, Central Urals. Dokl. Earth Sci. 2014, 459, 1548–1552. [Google Scholar] [CrossRef]
- Murzin, V.V.; Hiller, V.V.; Varlamov, D.A. The age position of gold–sulfide mineralization in the metahyperbasites of the Sysert’ metamorphic complex in the Middle Urals. Litosfera 2015, 4, 87–92. (In Russian) [Google Scholar]
- Reith, F.; Brugger, J.; Zammit, C.M.; Nies, D.H.; Southam, G. Geobiological cycling of gold: From fundamental process understanding to exploration solutions. Minerals 2013, 3, 367–394. [Google Scholar] [CrossRef]
- Kerr, G.; Craw, D. Mineralogy and geochemistry of biologically-mediated gold mobilisation and redeposition in a semiarid climate, Southern New Zealand. Minerals 2017, 7, 147. [Google Scholar] [CrossRef]
- Kalinin, Y.A.; Palyanova, G.A.; Naumov, E.A.; Kovalev, K.R.; Pirajno, F. Supergene remobilization of Au in Au-bearing regolith related to orogenic deposits: A case study from Kazakhstan. Ore Geol. Rev. 2019, 109, 358–369. [Google Scholar] [CrossRef]
- Kalinin, Y.A.; Palyanova, G.A.; Bortnikov, N.S.; Naumov, E.A.; Kovalev, K.R. Aggregation and Differentiation of Gold and Silver during the Formation of the Gold-Bearing Weathering Crusts (on the Example of Kazakhstan Deposits). Dokl. Earth Sci. 2018, 482, 1193–1198. [Google Scholar] [CrossRef]
- Saunders, J.A.; Burke, M. Formation and aggregation of gold (electrum) nanoparticles in epithermal ores. Minerals 2017, 7, 163. [Google Scholar] [CrossRef]
- Murzin, V.V.; Varlamov, D.A. Noble metals in magnetite ores of the Kagan ultramafic massif in the Southern Urals. In Yearbook-2005/Institute of Geology and Geochemistry; Collection of Scientific Papers; IGG UrB RAS: Yekaterinburg, Russia, 2006; pp. 379–381. (In Russian) [Google Scholar]
- Brown, A.V.; Page, N.J.; Love, A.H. Geology and platinum-group element geochemistry of the Serpentine Hill complex, Dundas Trough, Western Tasmania. Can. Mineral. 1988, 26, 161–175. [Google Scholar]
- Arai, S.; Akizawa, N. Precipitation and dissolution of chromite by hydrothermal solutions in the Oman ophiolite: New behavior of Cr and chromite. Am. Mineral. 2014, 99, 28–34. [Google Scholar] [CrossRef]
- Kapsiotis, A.; Rassios, A.E.; Antonelou, A.; Tzamos, E. Genesis and Multi-Episodic Alteration of Zircon-Bearing Chromitites from the Ayios Stefanos Mine, Othris Massif, Greece: Assessment of an Unconventional Hypothesis on the Origin of Zircon in Ophiolitic Chromitites. Minerals 2016, 6, 124. [Google Scholar] [CrossRef]
- Colás, V.; Padrón-Navarta, J.A.; González-Jiménez, J.M.; Fanlo, I.; Sánchez-Vizcaíno, V.L.; Gervilla, F.; Castroviejo, R. The role of silica in the hydrous metamorphism of chromite. Ore Geol. Rev. 2017, 90, 274–286. [Google Scholar] [CrossRef]
- Dritz, M.; Bochvar, N.R.; Guzei, L.S. Binary and Multicomponent Copper-Based Systems: Handbook; Nauka: Moscow, Russia, 1979; 248p. (In Russian) [Google Scholar]
- Murzin, V.V.; Varlamov, D.A. Chemical composition of native gold in magnetite ore from the Kagan ultramafic massif (S. Urals). In Yearbook-Proceedings of the IGG UrB RAS; IGG UrB RAS: Yekaterinburg, Russia, 2018; Volume 165, pp. 194–199. (In Russian) [Google Scholar]
- Wenner, D.; Taylor, H.P., Jr. O/H and O16/O18 studies of serpentinization of ultramafic rocks. Geochim. Cosmochim. Acta 1974, 38, 1255–1286. [Google Scholar] [CrossRef]
- Fruh-Green, G.L.; Scambelluri, M.; Vallis, F. O–H isotop ratios of high pressure ultramafic rocks: Implications for fluid sources and mobility in the subducted mantle. Contrib. Mineral. Petrol. 2001, 141, 145–159. [Google Scholar] [CrossRef]
- Murzin, V.V.; Shanina, S.N. Fluid regime and origin of gold-bearing rodingites from the Karabash alpine-type ultrabasic massif, Southern Ural. Geochem. Int. 2007, 45, 998–1011. [Google Scholar] [CrossRef]
- Karpov, I.K.; Chudnenko, K.V.; Kulik, D.A. Modeling chemical mass transfer in geochemical processes: Thermodynamic relations, conditions of equilibria, and numerical algorithms. Am. J. Sci. 1997, 297, 767–806. [Google Scholar] [CrossRef]
- Chudnenko, K.V. Thermodynamic Modeling in Geochemistry: Theory, Algorithms, Software, Applications; Academic Publishing House Geo: Novosibirsk, Russia, 2010; 287p, ISBN 978-5-904682-18-7. (In Russian) [Google Scholar]
- Kulik, D.A.; Wagner, T.; Dmytrieva, S.V.; Kosakowski, G.; Hingerl, F.F.; Chudnenko, K.V.; Berner, U.R. GEM-Selektor geochemical modeling package: Revised algorithm and GEMS3K numerical kernel for coupled simulation codes. Comput. Geosci. 2013, 17, 1–24. [Google Scholar] [CrossRef]
- Chudnenko, K.; Palyanova, G. Thermodynamic modeling of native formation Cu–Ag–Au–Hg solid solutions. Appl. Geochem. 2016, 66, 88–100. [Google Scholar] [CrossRef]
- Holland, T.J.B.; Powell, R. An internally consistent thermodynamic data set for phases of petrological interest. J. Metamorph. Geol. 1998, 16, 309–343. [Google Scholar] [CrossRef]
- Helgeson, H.C.; Delany, J.M.; Nesbitt, H.W.; Bird, D.K. Summary and critique of the thermodynamic properties of rock-forming minerals. Am. J. Sci. 1978, 278, 1–229. [Google Scholar]
- Robie, R.A.; Hemingway, B.S. Thermodynamic Properties of Minerals and Related Substances at 298.15 K and 1 Bar (105 Pascals) Pressure and at Higher Temperatures; United States Geological Survey: Washington, DC, USA, 1995; 458p. [Google Scholar]
- Tagirov, B.R.; Baranova, N.N.; Zotov, A.V.; Schott, J.; Bannykh, L.N. Experimental determination of the stabilities of Au2S(cr) at 25 °C and Au(HS)2- at 25–250 °C. Geochim. Cosmochim. Acta 2006, 70, 3689–3701. [Google Scholar] [CrossRef]
- Shock, E.L.; Sassani, D.C.; Willis, M.; Sverjensky, D.A. Inorganic species in geologic fluids: Correlation among standard molal thermodynamic properties of aqueous ions and hydroxide complexes. Geochim. Cosmochim. Acta 1997, 61, 907–950. [Google Scholar] [CrossRef]
- Stefansson, A. Dissolution of primary minerals of basalt in natural waters. I. Calculation of mineral solubilities from 0 °C to 350 °C. Chem. Geol. 2001, 172, 225–250. [Google Scholar] [CrossRef]
- Dorogokupets, P.I.; Karpov, I.K. Thermodynamics of Minerals and Mineral Equilibria; Nauka: Novosibirsk, Russia, 1984; 186p. (In Russian) [Google Scholar]
- Reid, R.C.; Prausnitz, J.M.; Sherwood, T.K. The Properties of Gases and Liquids; McGraw-Hill Book Company: New York, NY, USA, 1977; 688p. [Google Scholar]
- Sverjensky, D.A.; Shock, E.L.; Helgeson, H.C. Prediction of the thermodynamic properties of aqueous metal complexes to 1000 °C and 5 kb. Geochim. Cosmochim. Acta 1997, 61, 1359–1412. [Google Scholar] [CrossRef]
- Shock, E.L.; Helgeson, H.C.; Sverjensky, D.A. Calculation of the thermodynamic and transport properties of aqueous species at high pressures and temperatures: Standard partial molal properties of inorganic neutral species. Geochim. Cosmochim. Acta 1989, 53, 2157–2183. [Google Scholar] [CrossRef]
- Akinfiev, N.N.; Zotov, A.V. Thermodynamic description of chloride, hydrosulfide, and hydroxo complexes of Ag(I), Cu(I), and Au(I) at temperatures of 25–500 °C and pressures of 1–2000 bars. Geochem. Int. 2001, 39, 990–1006. [Google Scholar]
- Akinfiev, N.N.; Zotov, A.V. Thermodynamic description of aqueous species in the system Cu–Ag–Au–S–O–H at temperatures of 0–600 °C and pressures of 1–3000 bar. Geochem. Int. 2010, 48, 714–720. [Google Scholar] [CrossRef]
- Johnson, J.W.; Oelkers, E.H.; Helgeson, H.C. SUPCRT92: Software package for calculating the standard molal thermodynamic properties of mineral, gases, aqueous species, and reactions from 1 to 5000 bars and 0 to 1000 °C. Comput. Geosci. 1992, 18, 899–947. [Google Scholar] [CrossRef]
- Pokrovskii, V.A.; Helgeson, H.C. Thermodynamic properties of aqueous species and the solubilities of minerals at high pressures and temperatures: The system Al2O3–H2O–NaCl. Am. J. Sci. 1995, 295, 1255–1342. [Google Scholar] [CrossRef]
- Diakonov, I.; Pokrovski, G.; Schott, J.; Castet, S.; Gout, R. An experimental and computational study of sodium–aluminum complexing in crustal fluids. Geochim. Cosmochim. Acta 1996, 60, 197–211. [Google Scholar] [CrossRef]
- Bessinger, B.; Apps, J.A. The Hydrothermal Chemistry of Gold, Arsenic, Antimony, Mercury and Silver. 2003. Available online: http://repositories.cdlib.org/lbnl/LBNL-57395 (accessed on 4 October 2019).
- Shock, E.L.; Helgeson, H.C. Calculation of the thermodynamic and transport properties of aqueous species at high pressures and temperatures: Correlation algorithms for ionic species and equation of state predictions to 5 kb and 1000 °C. Geochim. Cosmochim. Acta 1988, 52, 2009–2036. [Google Scholar] [CrossRef]
- Chudnenko, K.V.; Pal’yanova, G.A.; Anisimova, G.S.; Moskvitin, S.G. Physicochemical modeling of formation of Ag–Au–Hg solid solutions: Kyuchyus deposit (Yakutia, Russia) as an example. Appl. Geochem. 2015, 55, 138–151. [Google Scholar] [CrossRef]
- Palyanova, G. Physicochemical modeling of the coupled behavior of gold and silver in hydrothermal processes: Gold fineness, Au/Ag ratios and their possible implications. Chem. Geol. 2008, 255, 399–413. [Google Scholar] [CrossRef]
- Zhuravkova, T.V.; Palyanova, G.A.; Chudnenko, K.V.; Kravtsova, R.G.; Prokopyev, I.R.; Makshakov, A.S.; Borisenko, A.S. Physicochemical models of formation of gold–silver ore mineralization at the Rogovik deposit (Northeastern Russia). Ore Geol. Rev. 2017, 91, 1–20. [Google Scholar] [CrossRef]
- Chudnenko, K.V.; Pal’yanova, G.A. Thermodynamic properties of solid solutions in the Ag–Au–Cu system. Russ. Geol. Geophys. 2014, 55, 349–360. [Google Scholar] [CrossRef]
- Yokokawa, H. Tables of thermodynamic properties of inorganic compounds. J. Natl. Chem. Lab. Ind. 1988, 83, 27–118. [Google Scholar]
- Likhachev, A.P. The conditions of magnetite and its ore clusters formation. Natl. Geol. 2017, 4, 44–53. (In Russian) [Google Scholar]
- Zotov, A.; Kuzmin, N.; Reukov, V.; Tagirov, B. Stability of AuCl2− from 25 to 1000 °C at pressures to 5000 bar, and consequences for hydrothermal gold mobilization. Minerals 2018, 8, 286. [Google Scholar] [CrossRef]
- Seward, T.M.; Williams-Jones, A.E.; Migdisov, A.A. The chemistry of metal transport and deposition by ore-forming hydrothermal fluids. In Treatise on Geochemistry, 2nd ed.; Turekian, K., Holland, H., Eds.; Elsevier: New York, NY, USA, 2014; pp. 29–57. [Google Scholar]
- Murzin, V.V. Gold Mineralization in the Ultramafites of the Urals. Mafic-Ultramafic Complexes of Folded Regions and Related Deposits; Abstract of the 3D International Conference; Institute of Geology and Geochemistry UB of RAS: Ekaterinburg, Russia, 2009; Volume 2, pp. 61–64. [Google Scholar]
- Murzin, V.V.; Sustavov, S.G.; Mamin, N.A. Gold and Platinum-Group Element Mineralization of Placer Deposits of the Verkh-Neivinsky Massif of Alpine-Type Ultrabasites (the Middle Urals); Ural Mining and Geological Academy: Yekaterinburg, Russia, 1999; 93p. [Google Scholar]
- Sazonov, V.N.; Murzin, V.V.; Ogorodnikov, V.N.; Volchenko, Y.A. Gold mineralization associated with alpinotype ultrabasites (on the example of the Urals). Litosfera 2002, 4, 63–77. (In Russian) [Google Scholar]
- Zhmodik, S.M.; Mironov, A.G.; Zhmodik, A.S. Gold-Concentrating Systems of Ophiolite Belts (by the Example of the Sayan-Baikal-Muya Belt); Academic Publishing House Geo: Novosibirsk, Russia, 2008; 304p. [Google Scholar]
- Paraskevopoulos, G.M.; Economou, M.I. Genesis of magnetite ore occurrences by metasomatism of chromite ores in Greece. Neues Jahrb. Mineral. Abh. 1980, 140, 29–53. [Google Scholar]
- Bakirov, A.G. The relationship between pyrite mineralization and magnetite and sulfide occurrences in ultramafic rocks of the Southern Urals. In Minerals of Ore Deposits and Pegmatites of the Urals; UBAS USSR: Sverdlovsk, Russia, 1965; Volume 6, pp. 185–192. (In Russian) [Google Scholar]
- Singh, R.N.; Srivastava, S.N.P. Ophiolites and associated mineralization in the Naga Hills, north-eastern India. In Ophiolites, Proceedings of the International Ophiolite Symposium, Cyprus, Nicosia, 1979; Panayiotou, A., Ed.; Cyprus Geological Survey: Nicosia, Cyprus, 1980; pp. 758–764. [Google Scholar]
- Zucchetti, S.; Mastrangelo, F.; Rossetti, P.; Sandrone, R. Serpentinization and metamorphism: Their relationships with metallogeny in some ophiolitic ultramafics from the Alps. In Zuffar’ Days Symposium in Honor of Piero Zuffardi; University of Cagliari: Cagliari, Italy, 1988; pp. 137–159. [Google Scholar]
- Diella, V.; Ferrario, A.; Rossetti, P. The magnetite ore deposits of the southern Aosta Valley: Chromitite transformed during an alpine metamorphic event. Ofioliti 1994, 19, 247–256. [Google Scholar]
- Agafonov, L.V.; Lkhamsuren, J.; Kuzhuget, K.S.; Oidup, C.K. Platinum-Group Element Mineralization of Ultramafic-Mafic Rocks in Mongolia and Tuva; Mongolian State University of Science and Technology: Ulaanbaatar, Mongolia, 2015; 224p. (In Russian) [Google Scholar]
- Gahlan, H.A.; Arai, S.; Ahmed, A.H.; Ishida, Y.; Abdel-Aziz, Y.M.; Rahimi, A. Origin of magnetite veins in serpentinite from the Late Proterozoic Bou-Azzer ophiolite, Anti-Atlas, Morocco: An implication for mobility of iron during serpentinization. J. Afr. Earth Sci. 2006, 46, 318–330. [Google Scholar] [CrossRef]
- Einaudi, M.T.; Hedenquist, J.W.; Inan, E.E. Sulfidation state of fluids in active and extinct hydrothermal systems: Transitions from porphyry to epithermal environments. In Volcanic, Geothermal and Ore-Forming Fluids: Rulers and Witnesses of Processes within the Earth; Simmons, S.F., Graham, I., Eds.; Society of Economic Geologists Special Publication: Littleton, CO, USA, 2003; pp. 285–314. [Google Scholar]
- Barton, P.; Toulmin, J.P. The electrum-tarnish method for the determination of the fugacity of sulfur in laboratory sulfide systems. Geochim. Cosmochim. Acta 1984, 28, 619–640. [Google Scholar] [CrossRef]

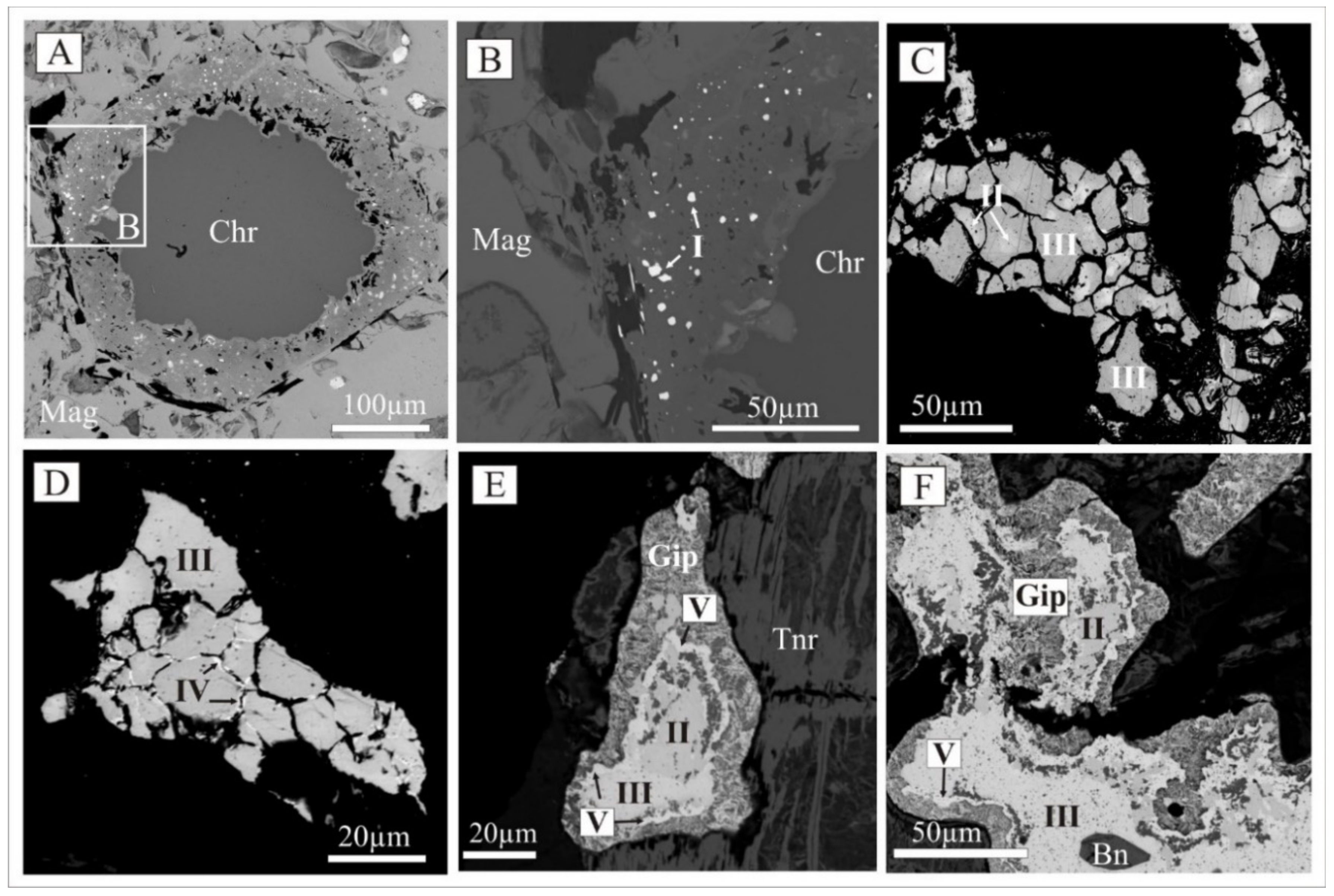




| Stages of Metamorphism and Metasomatism, Age | Composition of Metamorphic and Metasomatic Rocks (From High to Low Temperature) | Type of Mineralization |
|---|---|---|
| Regional zonal dynamothermal metamorphism, Late Precambrian | Enstatite–olivine | |
| Talc–olivine | ||
| Antigorite–olivine | ||
| Antigorite | Gold–sulfide–magnetite, Gold–antigorite | |
| Tremolite–chlorite | ||
| Regional silicic acid metasomatism, O2–S1 | Enstatite | |
| Anthophyllite, tremolite–anthophyllite | Gold–sulfide | |
| Local silicic acid metasomatism, P3–T | enstatite–anthophyllite, talc–carbonate–anthophyllite | Anthophyllite–asbest |
| chlorite-biotite, chlorite–actinolite |
| No. Sample/ Grain | Au wt % | Ag wt % | Cu wt % | Hg wt % | Total, wt % | Fineness, ‰ * |
|---|---|---|---|---|---|---|
| K-10/1 | 99.06 | 0.04 | 0.63 | 0.0 | 99.73 | 993 |
| K-10/2 | 98.98 | 0.09 | 0.59 | 0.0 | 99.66 | 993 |
| K-10/10 | 92.21 | 0.43 | 6.52 | 0.0 | 99.16 | 930 |
| K-5/11 | 85.86 | 13.93 | 0.34 | 0.0 | 100.13 | 857 |
| K-6/12 | 77.41 | 22.23 | 0.60 | 0.0 | 100.24 | 772 |
| C-437/13 | 92.55 | 5.44 | 0.21 | 1.72 | 99.92 | 926 |
| Sp-207/14 | 92.17 | 8.13 | 0.26 | 0.11 | 100.67 | 916 |
| Sp-207/15 | 95.10 | 4.23 | 0.36 | 0.03 | 99.72 | 954 |
| Sp-207/16 | 99.13 | 0.92 | 0.31 | 0.0 | 100.36 | 988 |
| Sp-207/17 | 97.70 | 2.43 | 0.78 | 0.0 | 100.91 | 968 |
| Sp-201/18 | 90.74 | 9.66 | 0.29 | 0.0 | 100.69 | 901 |
| Sp-212/19 | 99.90 | 0.16 | 0.47 | 0.0 | 100.53 | 994 |
| Sp-212/20 | 89.03 | 9.17 | 0.33 | 0.53 | 99.06 | 899 |
| Sp-212/21 | 99.14 | 0.0 | 0.41 | 0.0 | 99.55 | 996 |
| Sp-212/22 | 91.96 | 8.18 | 0.29 | 0.0 | 100.43 | 916 |
| Sp-212/23 | 97.72 | 1.74 | 0.78 | 0.0 | 100.24 | 975 |
| Sp-328/1 | 94.05 | 5.52 | 0.42 | 0.0 | 99.99 | 941 |
| Sp-328/25 | 92.87 | 6.79 | 0.41 | 0.0 | 100.07 | 928 |
| Sp-328/26 | 95.70 | 4.11 | 0.52 | 0.0 | 100.33 | 954 |
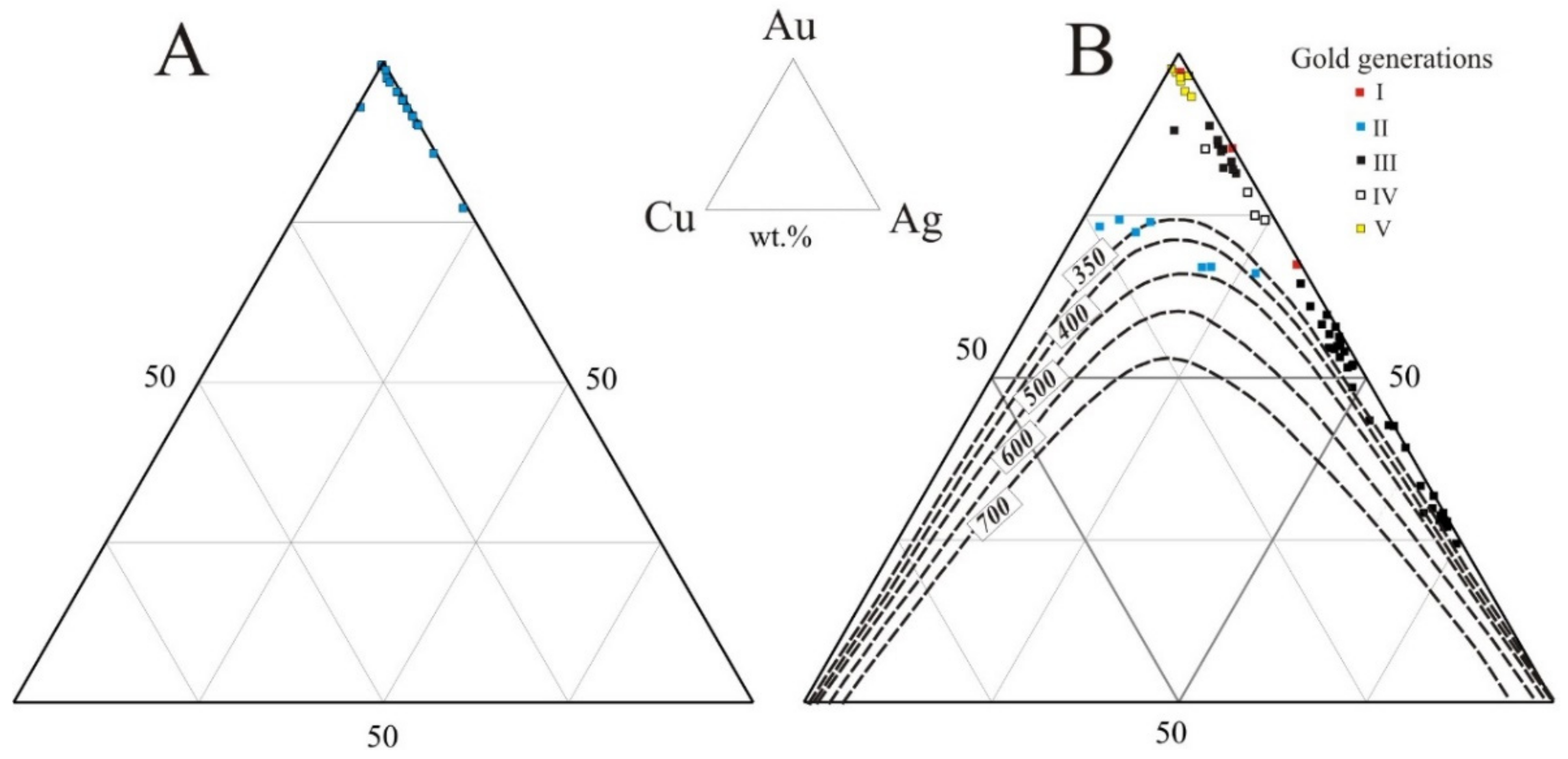
| No. Sample/Grain | Generation | Au wt % | Ag wt % | Cu wt % | Hg wt % | Pd wt % | Total wt % | Fineness ‰ |
|---|---|---|---|---|---|---|---|---|
| 888/23 | I | 93.99 | 2.00 | 1.33 | 0.00 | 0.44 | 97.76 | 961 |
| 888/24 | 81.58 | 13.79 | 0.20 | 0.00 | 0.75 | 96.33 | 847 | |
| 888/25 | 55.24 | 40.11 | 0.12 | 0.00 | 0.97 | 95.44 | 579 | |
| KAG-3/16 | II | 67.51 | 19.70 | 13.48 | 0.26 | 0.00 | 100.95 | 669 |
| KAG-3/20 | 74.18 | 2.82 | 24.19 | 0.04 | 0.00 | 101.23 | 733 | |
| KAG-3/31 | 73.81 | 4.84 | 20.57 | 0.00 | 0.00 | 99.22 | 744 | |
| KAG-3/33 | 73.51 | 9.10 | 16.68 | 0.32 | 0.00 | 99.61 | 738 | |
| KAG-3/35 | 71.94 | 8.02 | 19.41 | 0.00 | 0.00 | 99.37 | 724 | |
| 890b/42 | 64.86 | 26.70 | 6.58 | 2.00 | 0.00 | 100.14 | 648 | |
| KAG-3/5 | 65.82 | 20.36 | 11.91 | 0.00 | 0.00 | 98.09 | 671 | |
| 890s/1 | III | 31.73 | 67.93 | 0.08 | 0.49 | 0.00 | 100.23 | 317 |
| 890s/6 | 28.22 | 72.28 | 0.56 | 0.00 | 0.00 | 101.06 | 279 | |
| 890s/7 | 43.51 | 53.86 | 2.89 | 0.32 | 0.00 | 100.58 | 433 | |
| KAG-3/27 | 84.66 | 13.19 | 1.94 | 0.00 | 0.00 | 99.79 | 848 | |
| KAG-3/38 | 51.45 | 46.82 | 0.98 | 0.82 | 0.00 | 100.07 | 514 | |
| 890/13 | 33.35 | 65.60 | 1.08 | 0.00 | 0.00 | 100.03 | 333 | |
| 890s/9 | IV | 78.43 | 19.88 | 1.51 | 1.38 | 0.00 | 101.20 | 775 |
| 890s/4 | 74.11 | 22.35 | 2.31 | 1.43 | 0.00 | 100.20 | 740 | |
| 890/7 | 84.88 | 10.85 | 3.79 | 0.00 | 0.00 | 99.52 | 853 | |
| 890/14 | 74.09 | 24.26 | 1.38 | 0.00 | 0.00 | 99.73 | 743 | |
| KAG-3/29 | V | 95.60 | 2.99 | 0.45 | 0.00 | 0.00 | 99.04 | 965 |
| KAG-3/45 | 96.46 | 0.20 | 2.21 | 0.00 | 0.00 | 98.87 | 976 | |
| KAG-3/47 | 92.24 | 4.99 | 1.62 | 0.00 | 0.00 | 98.85 | 933 |
| Components | 1 * | 2 ** |
|---|---|---|
| SiO2 | 38.93 | 39.0 |
| TiO2 | 0.01 | 0.025 |
| Al2O3 | 1.39 | 1.49 |
| Cr2O3 | 0.38 | 0.197 |
| Fe2O3 | 5.33 | 5.90 |
| FeO | 2.2 | 2.4 |
| MnO | 0.07 | 0.07 |
| MgO | 36.98 | 38.0 |
| CaO | 0.82 | 0.04 |
| K2O | - | 0.01 |
| H2O | 1.15 | - |
| LOI | 13.23 | 12.5 |
| CO2 | 1.15 | - |
| CuO | - | 0.03 |
| S | - | 0.02 |
| Au (ppm) | 0.010 | |
| Ag (ppm) | 0.888 |
| Components | Composition, mol/kg H2O |
|---|---|
| C | 1.811 |
| Mg | 3.980 × 10−3 |
| Mn | 6.682 × 10−2 |
| Al | 6.552 × 10−8 |
| Si | 1.947 × 10−1 |
| S | 4.323 × 10−2 |
| Cl | 6.932 × 10−1 |
| Ca | 2.404 × 10−1 |
| Fe | 3.326 × 10−2 |
| Cu | 7.608 × 10−3 |
| Ag | 5.705 × 10−5 |
| Au | 3.519 × 10−7 |
| Cr | 6.904 × 10−10 |
| H | 4.199 × 10−1 |
| O | 4.092 |
| pH | 4.357 |
| log fO2 | –15.05 |
| log fS2 | 0.829 |
| Reservoir | T, °C | P, kbar |
|---|---|---|
| 1 | 650 | 4.5 |
| 2 | 600 | 4 |
| 3 | 550 | 3.5 |
| 4 | 500 | 3 |
| 5 | 450 | 2.5 |
| 6 | 400 | 2 |
| 7 | 350 | 1.5 |
| 8 | 300 | 1 |
| 9 | 250 | 0.5 |
| 10 | 250 | 0.5 |
| 11 | 300 | 1 |
| 12 | 350 | 1.5 |
| 13 | 400 | 2 |
| 14 | 450 | 3 |
| Characteristics | Kagan Massif | Calculated Data | ||
|---|---|---|---|---|
| 1 Type of Gold Mineralization | 2 Type of Gold Mineralization | Scenario 1 | Scenario 2 | |
| Quantitative ratios of main minerals * | Atg > Ctl, Tc > Chl > Tr, Mag | Mag >> Atg > Tc > Tr, Chl, Ctl, Dol | Srp >> Tc, Mag > Ilm–Hem | Mag >> Srp > Tc > Tr, Chl |
| Quantitative ratios of accessory minerals | Pn > Au(ss) | Ccp > Po, Pn > Cub, Bn, Tal, Sp > Au(ss) | Ccp, Py >> Au(ss) | Ilm–Hem > Cct > Bn, Au(ss) |
| Sequence of mineral deposition ** | Atg, Tr, Mag, Pn, Au(ss) → Tc, Chl, Ctl | Mag, Atg, Ccp, Po, Cub, Tal, Pn, Au(ss) → Tr, Tc, Ctl → Ccp, Bn, Po, Sp, Au(ss) → Chl, Srp, Dol | Srp, Mag, Tc, Ccp, Py, Au(ss) → Srp, Tc, Cb, Ilm–Hem, Au(ss) | Ol, Srp, Ath, Tr → Mag, Chl, Tc, Ilm–Hem, Au(ss) → Mag, Chl, Cct, Au(ss) → Srp, Tc, Cb, Bn, Au(ss) |
| T °C of gold deposition | 500–300 | 450–250 | 500–350 | |
| Composition of solid solutions gold/fineness ‰ | ss(Au,Ag)/772–996 | ss(Au,Ag)/580–960, ss (Au,Ag,Cu)/648–744 ss (Au,Ag,Cu)/280–853 | ss(Au,Ag)/900–950 ss(Au,Ag)/700–800 | ss(Au,Ag)/600–900 ss(Au,Ag)/600 ss(Ag,Au)/0–600 |
| Gold content in rock/ore, ppm | To 0.1 | 0.2–1.2 and more | To 0.1 | To 10 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murzin, V.; Chudnenko, K.; Palyanova, G.; Varlamov, D. Formation of Au-Bearing Antigorite Serpentinites and Magnetite Ores at the Massif of Ophiolite Ultramafic Rocks: Thermodynamic Modeling. Minerals 2019, 9, 758. https://doi.org/10.3390/min9120758
Murzin V, Chudnenko K, Palyanova G, Varlamov D. Formation of Au-Bearing Antigorite Serpentinites and Magnetite Ores at the Massif of Ophiolite Ultramafic Rocks: Thermodynamic Modeling. Minerals. 2019; 9(12):758. https://doi.org/10.3390/min9120758
Chicago/Turabian StyleMurzin, Valery, Konstantin Chudnenko, Galina Palyanova, and Dmitry Varlamov. 2019. "Formation of Au-Bearing Antigorite Serpentinites and Magnetite Ores at the Massif of Ophiolite Ultramafic Rocks: Thermodynamic Modeling" Minerals 9, no. 12: 758. https://doi.org/10.3390/min9120758
APA StyleMurzin, V., Chudnenko, K., Palyanova, G., & Varlamov, D. (2019). Formation of Au-Bearing Antigorite Serpentinites and Magnetite Ores at the Massif of Ophiolite Ultramafic Rocks: Thermodynamic Modeling. Minerals, 9(12), 758. https://doi.org/10.3390/min9120758