Composition and Ligand Microstructure of Arsenopyrite from Gold Ore Deposits of the Yenisei Ridge (Eastern Siberia, Russia)
Abstract
1. Introduction
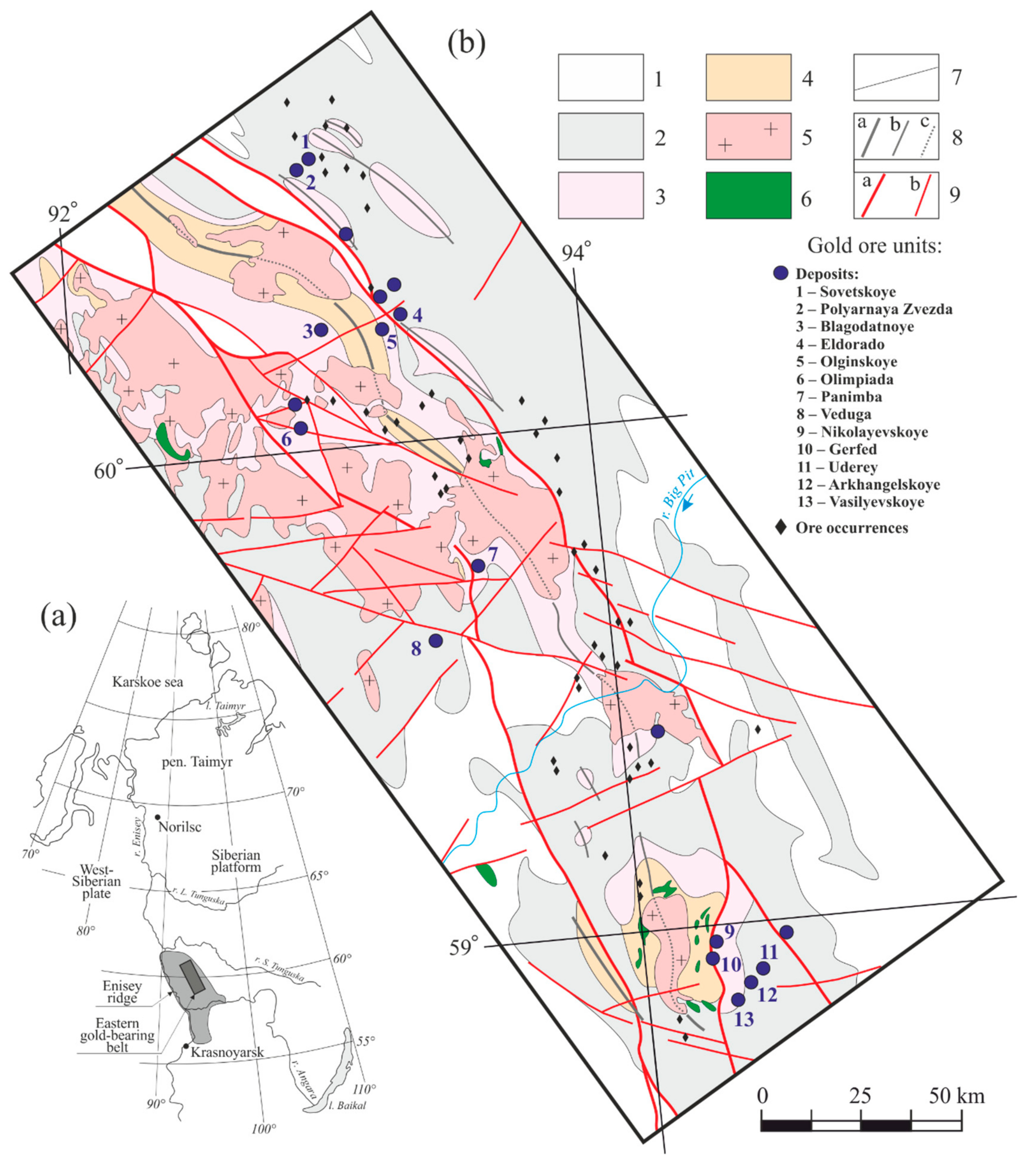
2. General Data on Geology of Gold-Arsenopyrite Mineralization of the Yenisei Ridge
3. Preparations and Research Methods
4. Results and Discussion
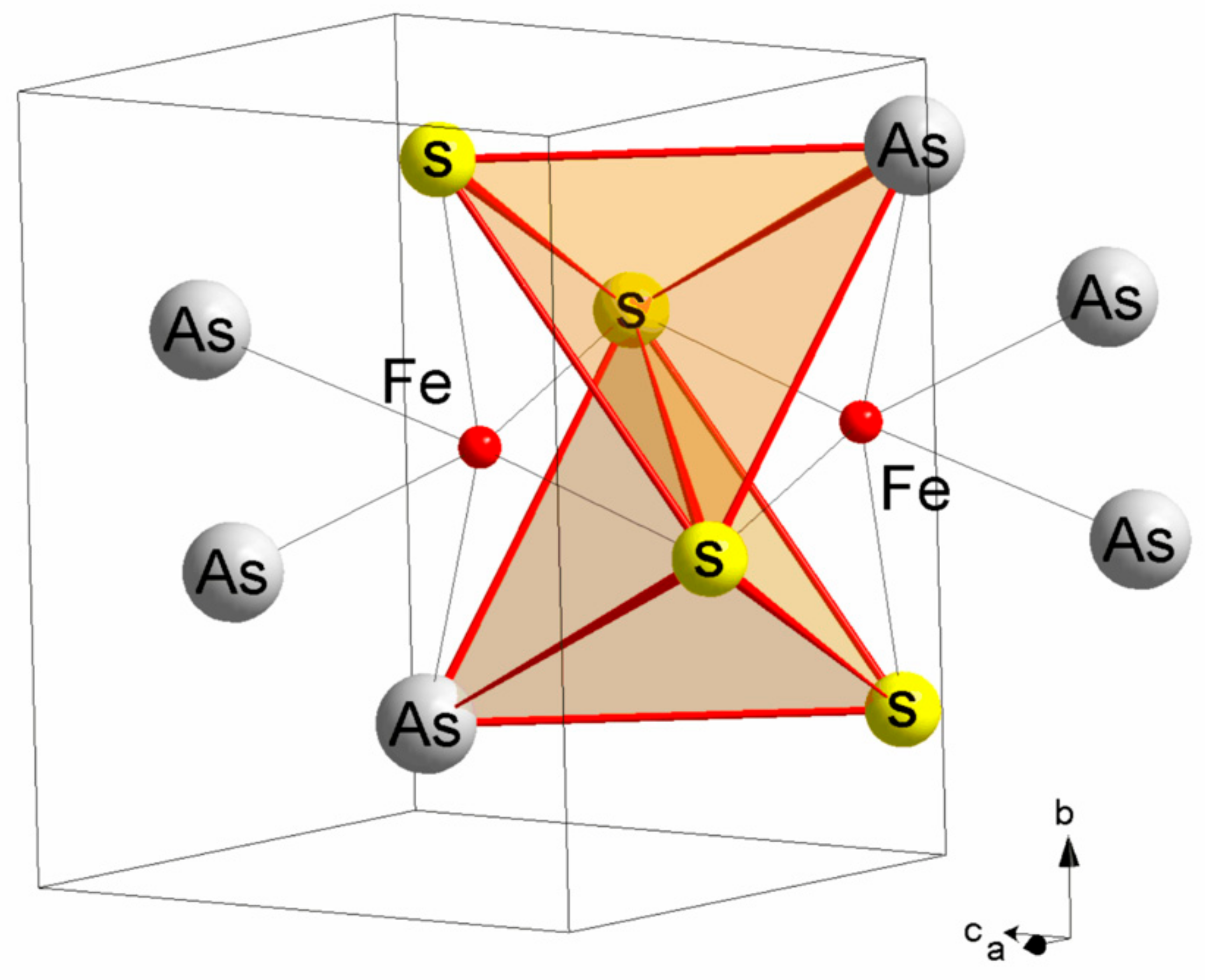
4.1. Mössbauer Spectroscopy
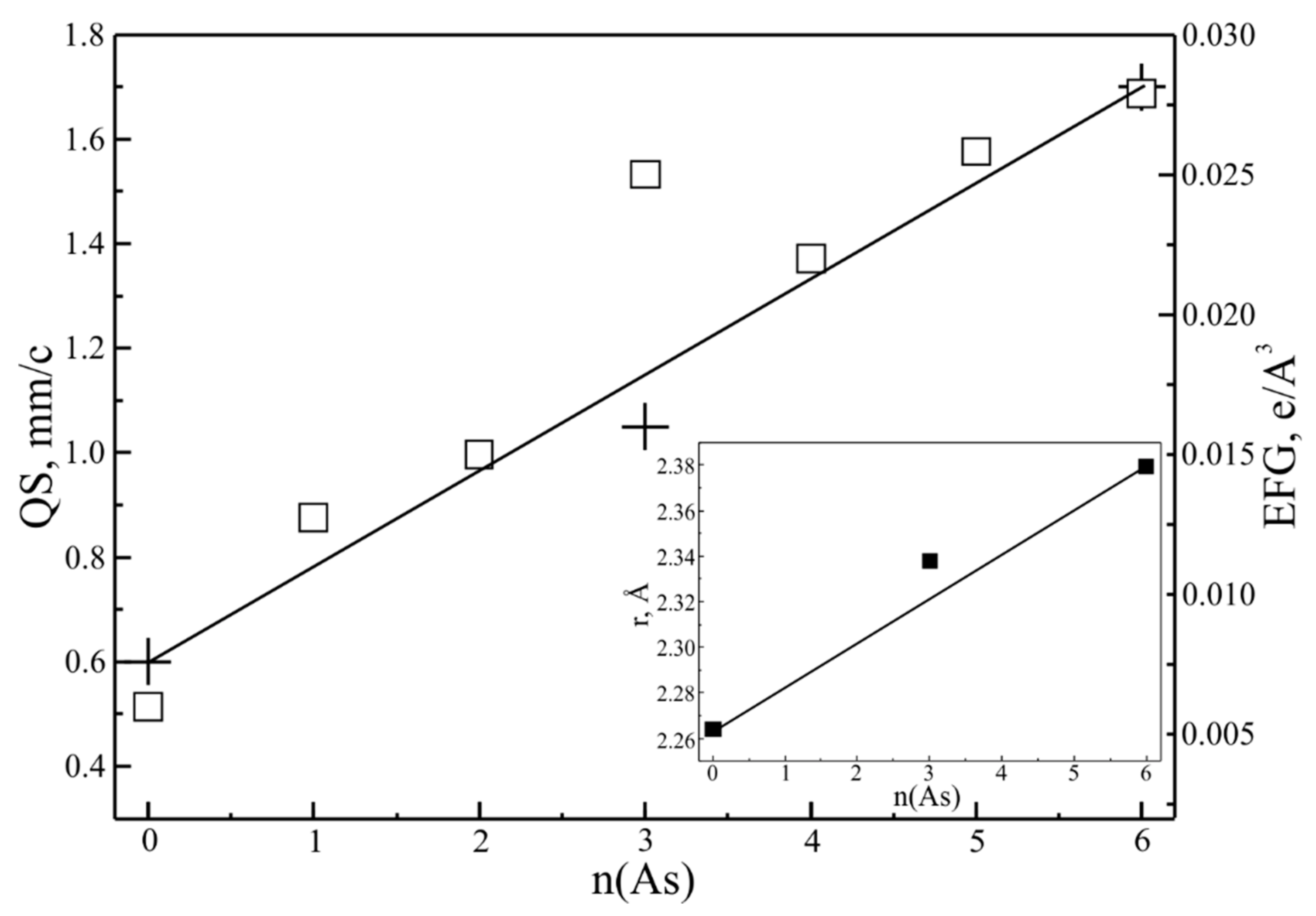
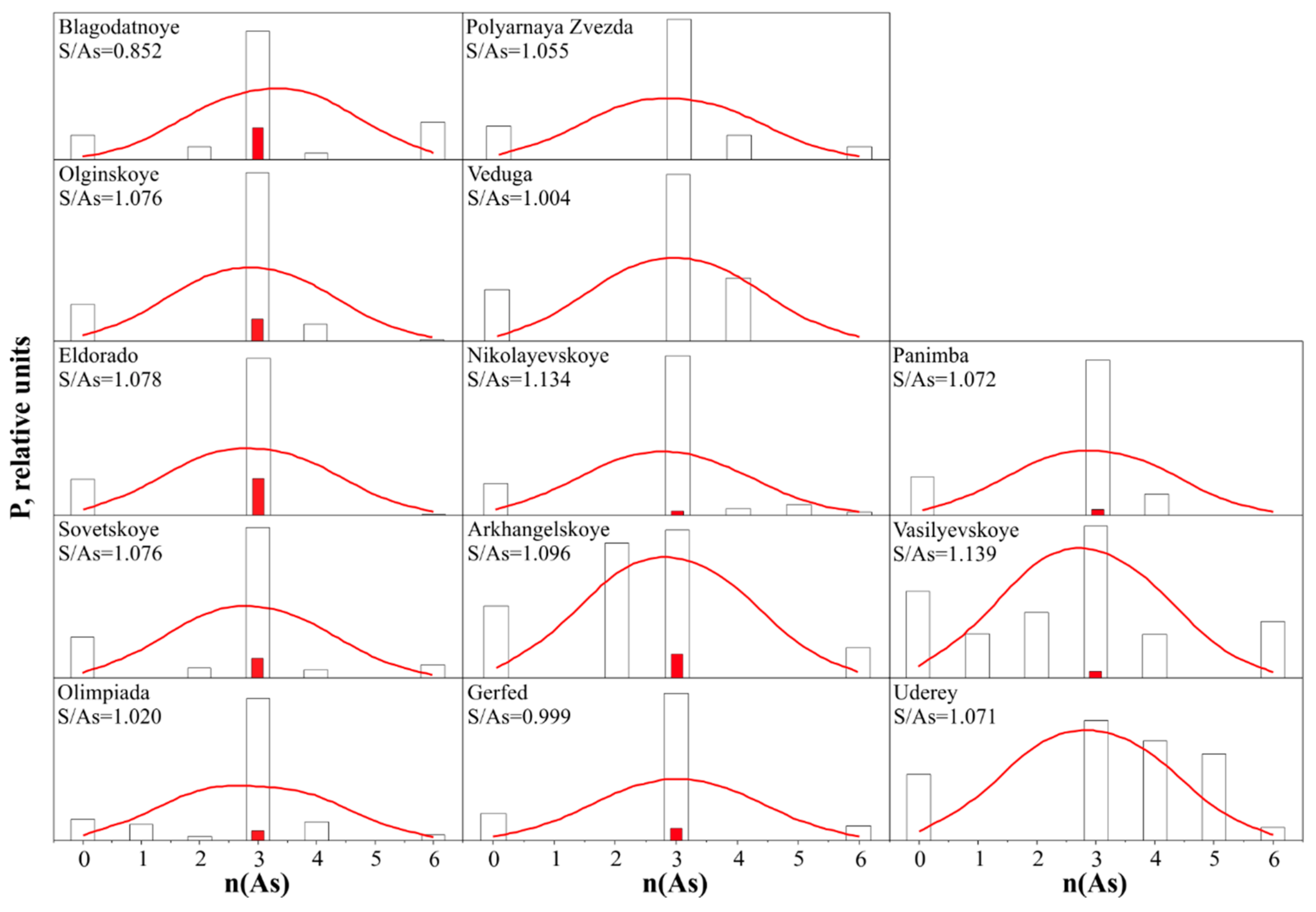
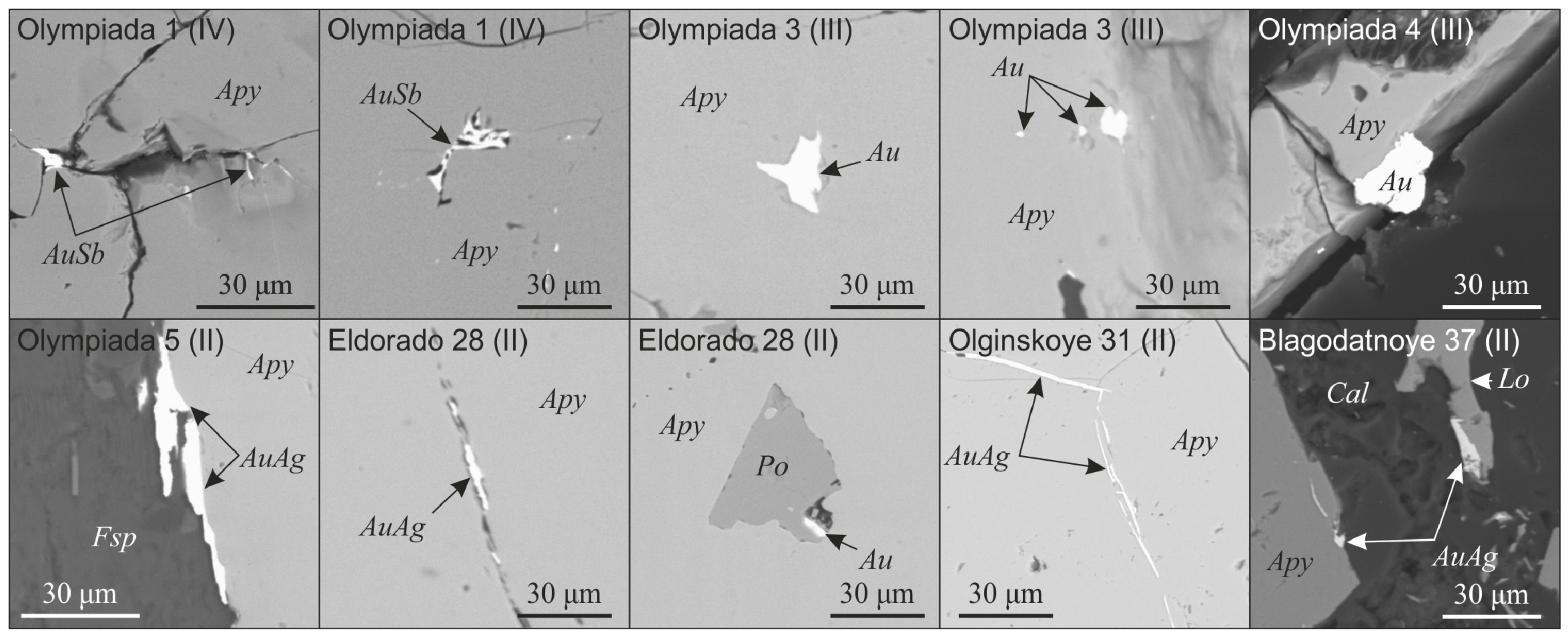
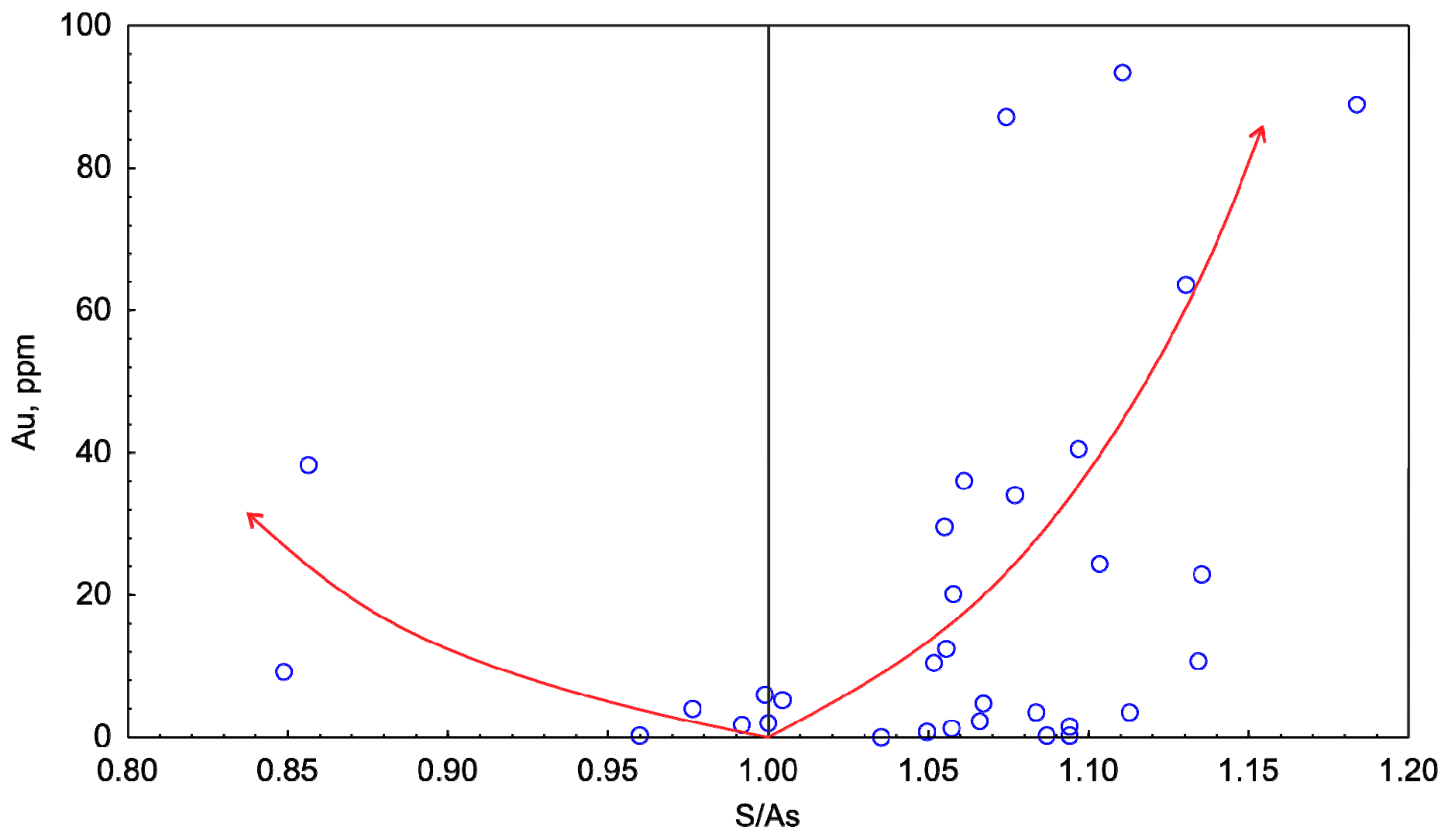
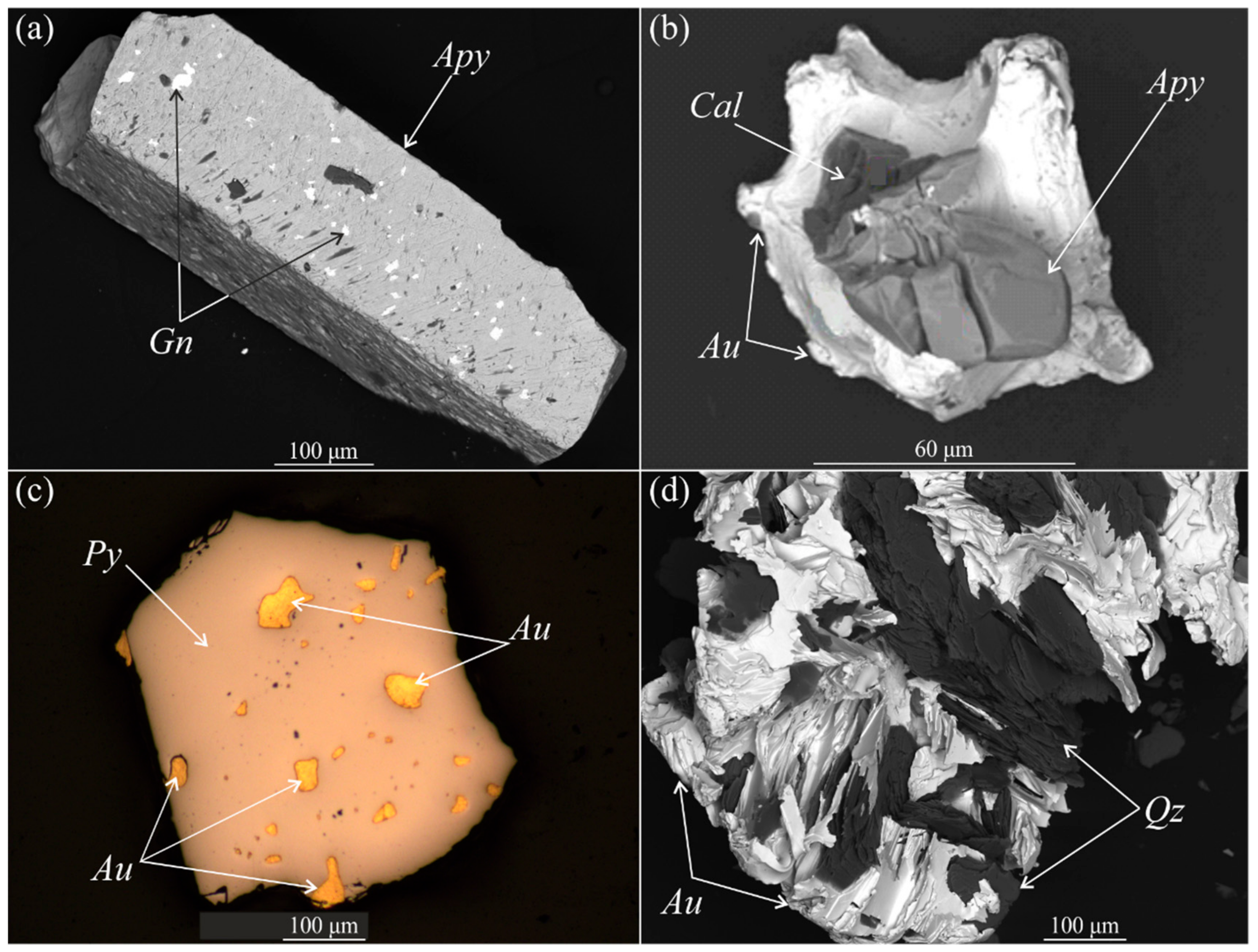
4.2. Arsenopyrite Composition and Distribution of Impurities of Precious Metals
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kretschmar, U.; Scott, S.D. Phase relations involving arsenopyrite in the system Fe–As–S and their application. Can. Mineral. 1976, 14, 364–386. [Google Scholar]
- Sharp, Z.D.; Essene, E.J.; Kelly, W.C. A reexamination of the arsenopyrite geothermometer: Pressure considerations and applications to natural assemblages. Can. Mineral. 1985, 23, 517–534. [Google Scholar]
- Sustavov, S.G.; Murzin, V.V.; Ivanov, O.K.; Litoshko, D.N. Arsenopyrite. In Ural Mineralogy: Arsenides and stibnides. Tellurides. Selenides. Fluorides. Chlorides and Bromides; Ural Branch of the Russian Academy of Sciences: Sverdlovsk, Russia, 1991; pp. 4–12. (In Russian) [Google Scholar]
- Murzin, V.V.; Semenkin, V.A.; Pikulev, A.I.; Mil’der, O.B.; Sustavov, S.G.; Krinov, D.I. Mössbauer spectroscopic evidence for nonequivalent sites of iron atoms in gold-bearing arsenopyrite. Geochem. Int. 2003, 41, 812–820. [Google Scholar]
- Tyukova Ye, E.; Voroshin, S.V. Composition and Parageneses of Arsenopyrite in Deposits and Hosting Rocks of the Upper Kolyma Region (for Interpretation of Sulfide Associations Genesis); SVKNII of the Far-Eastern Branch of the Russian Academy of Sciences: Moscow, Russia, 2007; p. 107. (In Russian) [Google Scholar]
- Sazonov, A.M.; Kirik, S.D.; Silyanov, S.A.; Bayukov, O.A.; Tushin, P.A. Typomorphism of arsenopyrite from Blagodatnoye and Olimpiada gold ore deposits (Yenisei Ridge). Mineralogy 2016, 3, 52–70. (In Russian) [Google Scholar]
- Stankovič, J.; Jančula, D. K typochemizmu arzenopyritu z niektorých ložísk na južných svahoch Nízkych Tatier. Miner. Slovaca 1981, 13, 373–377. [Google Scholar]
- Boiron, M.C.; Cathelineau, M.; Trescases, J.J. Conditions of gold-bearing arsenopyrite crystallization in the Villeranges Basin, Marche-Combrailles shear zone, France: A mineralogical and fluid inclusion study. Econ. Geol. 1989, 84, 1340–1362. [Google Scholar] [CrossRef]
- Genkin, A.D. Gold-bearing arsenopyrite from gold ore deposits: Internal structure of grains, composition, growth mechanism and state of gold. Geol. ore Depos. 1998, 40, 551–557. (In Russian) [Google Scholar]
- Kovalev, K.R.; Kalinin, Y.A.; Naumov, Y.A.; Kolesnikova, M.K.; Korolyuk, V.N. Gold-bearing arsenopyrite in eastern Kazakhstan gold-sulfide deposits. Russ. Geol. Geophys. 2011, 52, 178–192. [Google Scholar] [CrossRef]
- Fuess, H.; Kratz, T.; Töpel-Schadt, J.; Miehe, G. Crystal structure refinement and electron microscopy of arsenopyrite. Z. Krist. 1987, 179, 335–346. [Google Scholar] [CrossRef]
- Wagner, F.E.; Marion, P.; Regnard, J.-R. 197Au Mössbauer study of gold ores, mattes, roaster products, and gold minerals. Hyperfine Interact. 1988, 41, 851–854. [Google Scholar] [CrossRef]
- Johan, Z.; Marcoux, E.; Bonnemaison, M. Arsenopyrite aurifere: Mode de substitution de Au dans la structure de FeAsS. Comptes Rendus Acad. Sci. 1989, 308, 185–191. [Google Scholar]
- Friedl, J.; Wagner, F.E.; Sawicki, J.A.; Harris, D.C.; Mandarino, J.A.; Marion, P. 197Au, 57Fe and 121Sb Mössbauer study of gold minerals and ores. Hyperfine Interact. 1992, 70, 945–948. [Google Scholar] [CrossRef]
- Mumin, A.H.; Fleet, M.E.; Chryssoulis, S.L. Gold mineralization in As-rich mesothermal gold ores of the Bogosu–Prestea mining district of the Ashanti Gold Belt, Ghana: Remobilization of “invisible” gold. Miner. Depos. 1994, 29, 445–460. [Google Scholar] [CrossRef]
- Jiuling, L.; Daming, F.; Jeng, Q.; Guilan, Z. The existence of the negative valence state of gold in sulfide minerals and its formation mechanism. Acta Geol. Sin. 1995, 69, 67–77. [Google Scholar]
- Genkin, A.D.; Bortnikov, N.S.; Cabri, L.J.; Wagner, F.E.; Stanley, C.J.; Safonov, Y.G.; McMahon, G.; Friedl, J.; Kerzin, A.L.; Gamyanin, G.N. A multidisciplinary study of invisible gold in arsenopyrite from four mesothermal gold deposits in Siberia, Russian Federation. Econ. Geol. 1998, 93, 463–487. [Google Scholar] [CrossRef]
- Cabri, L.J.; Newville, M.; Gordon, R.A.; Crozier, E.D.; Sutton, S.R.; McMahon, G.; Jiang, D.-T. Chemical speciation of gold in arsenopyrite. Can. Mineral. 2000, 38, 1265–1281. [Google Scholar] [CrossRef]
- Sung, Y.-H.; Brugger, J.; Ciobanu, C.L.; Pring, A.; Skinner, W.; Nugus, M. Invisible gold in arsenian pyrite and arsenopyrite from a multistage Archaean gold deposit: Sunrise Dam, Eastern Goldfields Province, Western Australia. Miner. Depos. 2009, 44, 765–775. [Google Scholar] [CrossRef]
- Zhu, Y.; Fang, A.; Tan, J. Geochemistry of hydrothermal gold deposits: A review. Geosci. Front. 2011, 2, 367–374. [Google Scholar] [CrossRef]
- Silva, J.C.M.; De Abreu, H.A.; Duarte, H.A. Electronic and structural properties of bulk arsenopyrite and its cleavage surfaces—A DFT study. RSC Adv. 2015, 5, 2013–2023. [Google Scholar] [CrossRef]
- Kravtsova, R.G.; Tauson, V.L.; Nikitenko, E.M. Modes of Au, Pt, and Pd occurrence in arsenopyrite from the Natalkinskoe deposit, NE Russia. Geochem. Int. 2015, 53, 964–972. [Google Scholar] [CrossRef]
- Buerger, M.J. The symmetry and crystal structure of the minerals of the arsenopyrite group. Z. Krist. 1936, 95, 83–113. [Google Scholar] [CrossRef]
- Morimoto, N.; Clark, L.A. Arsenopyrite crystal-chemical relations. Am. Mineral. 1961, 46, 1448–1469. [Google Scholar]
- Kirik, S.D.; Sazonov, A.M.; Silyanov, S.A.; Bayukov, O.A. Investigation of disordering in natural arsenopyrite by X-ray powder crystal structure analysis and nuclear gamma resonance. J. Sib. Fed. Univ. Eng. Technol. Ser. 2017, 10, 578–592. (In Russian) [Google Scholar] [CrossRef]
- Nickel, E.H. Structural stability of minerals with the pyrite, marcasite, arsenopyrite and loellingite structures. Can. Mineral. 1968, 9, 311–323. [Google Scholar]
- Bindi, L.; Moëlo, Y.; Léone, P.; Suchaud, M. Stoichiometric arsenopyrite, FeAsS, from La Roche-Balue Quarry Loire-Atlantique, France: Crystal structure and Mössbauer study. Can. Mineral. 2012, 50, 471–479. [Google Scholar] [CrossRef]
- Kerler, W.; Neuwirth, W.; Fluck, E.Z. Isomerieverschiebung und Quadrupolaufspaltung beim Mößbauer-Effekt von Fe57 in Eisenverbindungen. Z. Phys. 1963, 175, 200–220. [Google Scholar] [CrossRef]
- Evans, B.J.; Johnson, R.G.; Senftle, F.E.; Blaine Cecil, C.; Dulong, F. The 57Fe Mössbauer parameters of pyrite and marcasite with different provenances. Geochim. Cosmochim. Acta 1982, 46, 761–775. [Google Scholar] [CrossRef]
- Imbert, P.; Gerard, A.; Wintenberger, M. Study of naturally occurring sulfides, sulfarsenides, and arsenides of iron by Mössbauer effect. Comptes Rendus 1963, 256, 4391–4393. [Google Scholar]
- Kjekshus, A.; Nicholson, D.G. The significance of π Back-Bonding in compounds with pyrite, marcasite and arsenopyrite Type structures. Acta Chem. Scand. 1971, 25, 866–876. [Google Scholar] [CrossRef]
- Sazonov, A.M.; Zvyagina Ye, A.; Silyanov, S.A.; Lobanov, K.V.; Leontyev, S.I.; Kalinin Yu, A.; Savichev, A.A.; Tishin, P.A. Ore genesis of the Olimpiada gold deposit (Yenisei Ridge, Russia). Geosph. Res. 2019, 17–43. (In Russian) [Google Scholar] [CrossRef]
- Sazonov, A.M.; Bernatonis, V.K. Features of formation of gold-bearing quartz-vein zones in crystalline slates. In Geological and Geochemical Criteria of Gold Mineralization; Nauka: Novosibirsk, Russia, 1990; pp. 44–57. (In Russian) [Google Scholar]
- Sazonov, A.M.; Ananiev, A.A.; Vlasov, V.S. On the conditions of spatial combination of gold ore and antimony mineralization in slate strata of one of the Siberian regions. In Geological and Geochemical Criteria of Gold Mineralization; Nauka: Novosibirsk, Russia, 1990; pp. 84–96. (In Russian) [Google Scholar]
- Sazonov, A.M.; Sarayev, V.A.; Ananiev, A.A. Sulfide and quartz gold deposits in metamorphic strata of the Yenisei Ridge. Geol. Geophys. 1991, 5, 28–37. (In Russian) [Google Scholar]
- Sazonov, A.M.; Zvyagina, E.A.; Romanovsky, A.E.; Shvedov, G.I.; Leontiev, S.I. Aposlate gold-bearing metasomatites of beresite formation (Yenisei Ridge). Geol. Geophys. 1995, 36, 53–61. (In Russian) [Google Scholar]
- Sazonov, A.M.; Ananyev, A.A.; Poleva, T.V.; Khokhlov, A.N.; Vlasov, V.S.; Zvyagina, E.A.; Fedorova, A.V.; Tishin, P.A.; Leontyev, S.I. Gold metallogeny of the Yenisei Ridge: Geological and structural position, structural types of ore fields. J. Sib. Fed. Univ. Ser. Eng. Technol. 2010, 3, 371–395. (In Russian) [Google Scholar]
- Gibsher, N.A.; Sazonov, A.M.; Travin, A.V.; Tomilenko, A.A.; Ponomarchuk, A.V.; Sil’yanov, S.A.; Nekrasova, N.A.; Shaparenko, E.O.; Ryabukha, M.A.; Khomenko, M.O. Age and duration of the formation of the Olimpiadinski gold deposit (Yenisei ridge, Russia). Geochem. Int. 2019, 57, 593–599. [Google Scholar] [CrossRef]
- Sazonov, A.M. Geochemistry of Gold in Metamorphic Strata; Publishing House of TPU: Tomsk, Russia, 1998; p. 168. (In Russian) [Google Scholar]
- Brostigen, G.; Kjekshus, A. Redetermined Crystal Structure of FeS2 (Pyrite). Acta Chem. Scand. 1969, 23, 2186–2188. [Google Scholar] [CrossRef]
- Lutz, H.D.; Jung, M.; Waschenbach, G. Kristallstrukturen des lollingits FeAs2 und des pyrits RuTe2. Z. Anorg. Allg. Chem. 1987, 554, 87–91. [Google Scholar] [CrossRef]
- Goldansky, V.I. Chemical Applications of Moessbauer Spectroscopy; Mir: Moscow, Russia, 1970; p. 504. (In Russian) [Google Scholar]
- Scott, S.D. Chemical behaviour of sphalerite and arsenopyrite in hydrothermal and metamorphic environments. Mineral. Mag. 1983, 47, 427–435. [Google Scholar] [CrossRef]
- Pavlov, A.L.; Pavlova, L.K. Elements of thermodynamics of gold behavior in ore formation sample. In Physics and Physicochemistry of Ore-Forming Processes; Nauka: Novosibirsk, Russia, 1971; pp. 121–147. (In Russian) [Google Scholar]
- Kolonin, G.R.; Palyanova, G.A.; Shironosova, G.P. Stability and solubility of arsenopyrite in hydrothermal solutions. Geochemistry 1988, 6, 843–855. (In Russian) [Google Scholar]
- Palyanova, G.A.; Kolonin, G.R. Arsenopyrite-containing mineral associations as indicators of physical and chemical conditions of hydrothermal ore formation. Geochemistry 1991, 10, 1481–1492. (In Russian) [Google Scholar]
- Reich, M.; Kesler, S.; Utsunomiya, S.; Palenik, C.S.; Chryssoulis, S.L.; Ewing, R.C. Solubility of gold in arsenian pyrite. Geochim. Cosmochim. Acta 2005, 69, 2781–2796. [Google Scholar] [CrossRef]
- Pokrovski, G.S.; Roux, J.; Ferlat, G.; Jonchiere, R.; Seitsonen, A.P.; Vuilleumier, R.; Hazemann, J.-L. Silver in geological fluids from in situ X-ray absorption spectroscopy and first-principles molecular dynamics. Geochim. Cosmochim. Acta 2013, 106, 501–523. [Google Scholar] [CrossRef]
- Pokrovski, G.S.; Tagirov, B.R.; Schott, J.; Hazemann, J.-L.; Proux, O. A new view on gold speciation in sulfurbearing hydrothermal fluids from in situ X-ray absorption spectroscopy and quantum-chemical modeling. Geochim. Cosmochim. Acta 2009, 73, 5406–5427. [Google Scholar] [CrossRef]
- Pokrovski, G.S.; Tagirov, B.R.; Schott, J.; Bazarkina, E.F.; Hazemann, J.-L.; Proux, O. An in situ X-ray absorption spectroscopy study of gold–chloride complexing in hydrothermal fluids. Chem. Geol. 2009, 259, 17–29. [Google Scholar] [CrossRef]
- Mikhlin, Y.; Likhatski, M.; Tomashevich, Y.; Romanchenko, A.; Erenburg, S.; Trubina, S. XAS and XPS examination of the Au–S nanostructures produced via the reduction of aqueous gold(III) by sulfide ions. J. Electron Spectrosc. Relat. Phenom. 2010, 177, 24–29. [Google Scholar] [CrossRef]
- Pokrovski, G.S.; Kara, S.; Roux, J. Stability and solubility of arsenopyrite, FeAsS, in crustal fluids. Geochim. Cosmochim. Acta 2002, 66, 2361–2378. [Google Scholar] [CrossRef]
- Morey, A.A.; Tomkins, A.G.; Bierlein, F.P.; Weinberg, R.F.; Davidson, G.J. Bimodal Distribution of Gold in Pyrite and Arsenopyrite: Examples from the Archean Boorara and Bardoc Shear Systems, Yilgarn Craton, Western Australia. Econ. Geol. 2008, 103, 599–614. [Google Scholar] [CrossRef]
- Xing, Y.; Brugger, J.; Tomkins, A.; Shvarov, Y. Arsenic evolution as a tool for understanding formation of pyritic gold ores. Geology 2019, 47, 335–338. [Google Scholar] [CrossRef]
- Vilor, N.V.; Pavlova, L.A.; Kaz’min, L.A. Aresenopyrite–pyrite paragenesis in gold deposits (thermodynamic modeling). Russ. Geol. Geophys. 2014, 55, 824–841. [Google Scholar] [CrossRef]
- Trigub, A.L.; Tagirov, B.R.; Kvashnina, K.O.; Lafuerza, S.; Filimonova, O.N.; Nickolsky, M.S. Experimental determination of gold speciation in sulfide-rich hydrothermal fluids under a wide range of redox conditions. Chem. Geol. 2017, 471, 52–64. [Google Scholar] [CrossRef]
- Trigub, A.L.; Tagirov, B.R.; Kvashnina, K.O.; Chareev, D.A.; Nickolsky, M.S.; Shiryaev, A.A.; Baranova, N.N.; Kovalchuk, E.V.; Mokhov, A.V. X-ray spectroscopy study of the chemical state of “invisible” Au in synthetic minerals in the Fe-As-S system. Am. Mineral. 2017, 102, 1057–1065. [Google Scholar] [CrossRef]
- Sazonov, A.M.; Ananyev, A.A.; Ananyeva, T.A.; Leontiev, S.I. Reflection of gold-bearing content of low-sulphide quartz veins in typomorphism of constituent minerals. ZVMO 1991, 2, 17–24. (In Russian) [Google Scholar]
- Sazonov, A.M.; Zvyagina, E.A.; Krivoputskaya, L.M. Structural and chemical heterogeneity of pyrite of Sarala deposit (Kuznetsky Alatau). Geol. Geophys. 1992, 8, 87–95. (In Russian) [Google Scholar]
- Fougerouse, D.; Micklethwaite, S.; Tomkins, A.G.; Mei, Y.; Kilburn, M.; Guagliardo, P.; Fisher, L.A.; Halfpenny, A.; Gee, M.; Paterson, D.; et al. Gold remobilisation and formation of high grade ore shoots driven by dissolution–reprecipitation replacement and Ni substitution into auriferous arsenopyrite. Geochim. Cosmochim. Acta 2016, 178, 143–159. [Google Scholar] [CrossRef]
- Sazonov, A.M.; Onufrienok, V.V.; Kolmakov, Y.V.; Nekrasova, N.A. Pyrrhotite of gold-bearing ores: Composition, spot defects, magnetic properties, gold distribution. J. Sib. Fed. Univ. Eng. Technol. 2014, 7, 717–737. (In Russian) [Google Scholar]
- Palyanova, G.A.; Sazonov, A.M.; Zhuravkova, T.V.; Silyanov, S.A. Composition of pyrrhotite as an indicator of mineralization conditions at the Sovetskoye gold deposit. Russ. Geol. Geophys. 2019, 60, 735–751. [Google Scholar] [CrossRef]
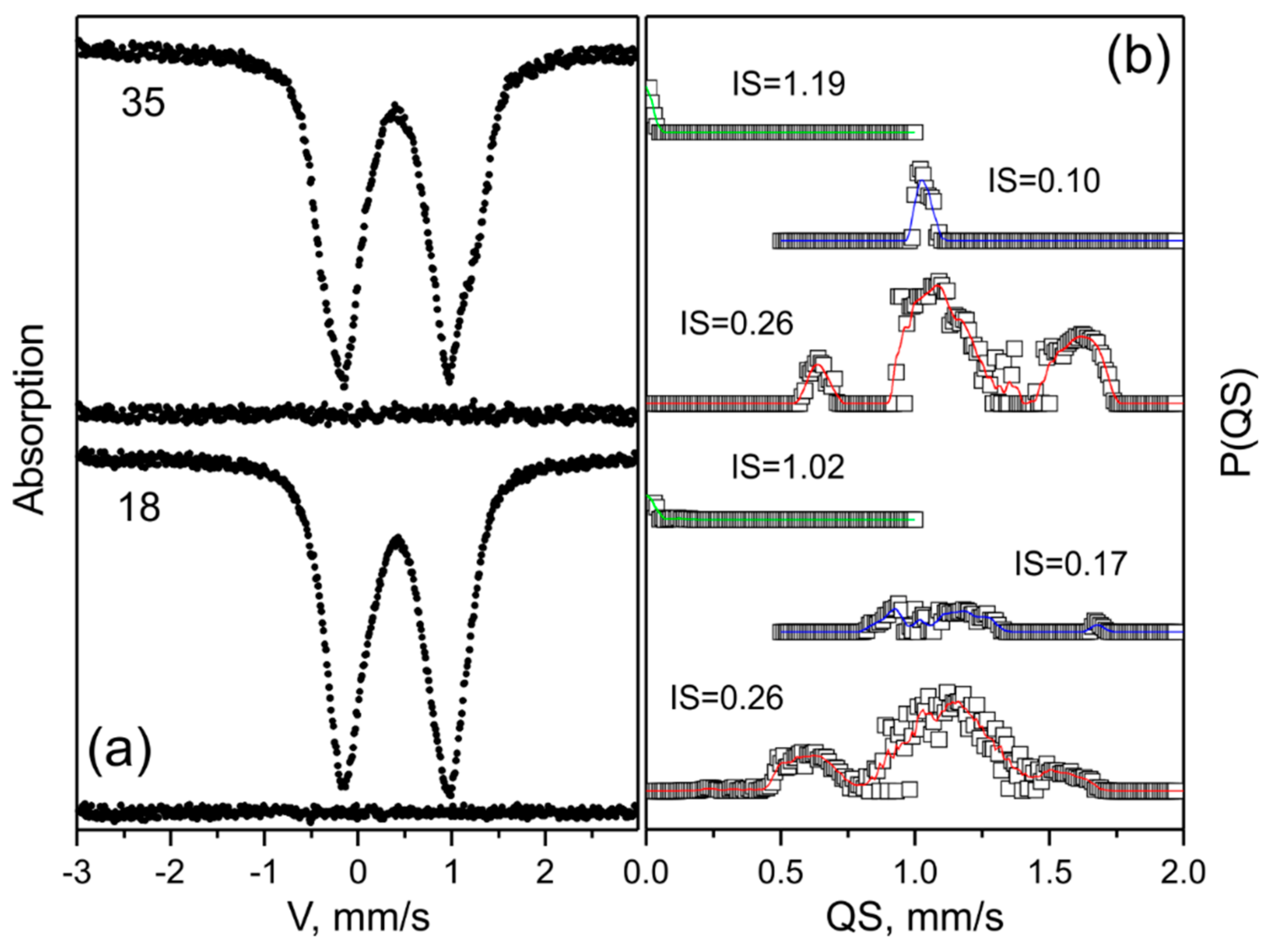
| Specimen Number, Deposit, Morphology of Arsenopyrite Grains (Mineralization Stage) | IS, mm/s (±0.005) | QS, mm/s (±0.02) | W, mm/s (±0.02) | A, % (±3) | Configuration of Ligands |
|---|---|---|---|---|---|
| 1. Olimpiada, isometric pseudopyramidal grains (IV) | 0.26 | 0.58 | 0.33 | 8 | {6S} |
| 0.25 | 1.11 | 0.33 | 68 | {3S3As} | |
| 0.25 | 1.20 | 0.27 | 15 | {2S4As} | |
| 0.11 | 0.97 | 0.23 | 3 | Fe-LC-1 | |
| 0.21 | 1.56 | 0.28 | 4 | Fe-LC-2 | |
| 1.00 | 0.11 | 0.24 | 2 | Fe-HS | |
| 2. Olimpiada, isometric pseudopyramidal grains (IV) | 0.29 | 0.58 | 0.28 | 8 | {6S} |
| 0.25 | 1.14 | 0.35 | 69 | {3S3As} | |
| 0.28 | 1.28 | 0.36 | 18 | {2S4As} | |
| 0.13 | 0.94 | 0.26 | 2 | Fe-LC-1 | |
| 0.15 | 1.28 | 0.22 | 3 | Fe-LC-2 | |
| 3. Olimpiada, prismatic grains (IV) | 0.26 | 0.64 | 0.36 | 13 | {6S} |
| 0.24 | 1.11 | 0.33 | 66 | {3S3As} | |
| 0.26 | 1.30 | 0.26 | 8 | {2S4As} | |
| 0.26 | 1.66 | 0.40 | 11 | {6As} | |
| 0.90 | 0.11 | 0.22 | 2 | Fe-HS | |
| 4. Olimpiada, needle-like grains (IV) | 0.28 | 0.53 | 0.32 | 14 | {6S} |
| 0.25 | 0.75 | 0.24 | 6 | {5S1As} | |
| 0.25 | 1.10 | 0.35 | 65 | {3S3As} | |
| 0.26 | 1.27 | 0.23 | 5 | {2S4As} | |
| 0.24 | 1.58 | 0.24 | 3 | {6As} | |
| 0.15 | 1.14 | 0.28 | 5 | Fe-LC-1 | |
| 1.00 | 0.14 | 0.22 | 2 | Fe-HS | |
| 5. Olimpiada, prismatic grains (III) | 0.26 | 0.62 | 0.38 | 18 | {6S} |
| 0.26 | 1.10 | 0.29 | 54 | {3S3As} | |
| 0.25 | 1.22 | 0.26 | 11 | {2S4As} | |
| 0.24 | 1.55 | 0.29 | 7 | {6As} | |
| 0.16 | 1.04 | 0.29 | 9 | Fe-LC-1 | |
| 0.92 | 0.15 | 0.28 | 2 | Fe-HS | |
| 6. Olimpiada, isometric pseudopyramidal grains (V) | 0.25 | 0.58 | 0.33 | 10 | {6S} |
| 0.26 | 1.10 | 0.27 | 63 | {3S3As} | |
| 0.25 | 1.32 | 0.23 | 8 | {2S4As} | |
| 0.25 | 1.64 | 0.36 | 6 | {6As} | |
| 0.15 | 1.06 | 0.22 | 8 | Fe-LC-1 | |
| 0.20 | 1.61 | 0.23 | 3 | Fe-LC-2 | |
| 0.92 | 0.16 | 0.22 | 2 | Fe-HS | |
| 7. Olimpiada, prismatic grains (II) | 0.27 | 0.61 | 0.32 | 8 | {6S} |
| 0.25 | 1.10 | 0.29 | 70 | {3S3As} | |
| 0.23 | 1.21 | 0.24 | 12 | {2S4As} | |
| 0.20 | 1.58 | 0.23 | 2 | {6As} | |
| 0.18 | 0.95 | 0.22 | 4 | Fe-LC-1 | |
| 0.18 | 1.36 | 0.21 | 2 | Fe-LC-2 | |
| 1.04 | 0.10 | 0.29 | 2 | Fe-HS | |
| 18. Sovetskoye, aggregates of coalescent pseudopyramidal grains (III) | 0.31 | 0.60 | 0.31 | 12 | {6S} |
| 0.26 | 0.81 | 0.29 | 10 | {4S2As} | |
| 0.26 | 1.13 | 0.33 | 59 | {3S3As} | |
| 0.23 | 1.56 | 0.22 | 8 | {6As} | |
| 0.12 | 0.80 | 0.23 | 5 | Fe-LC-1 | |
| 0.15 | 1.40 | 0.28 | 4 | Fe-LC-2 | |
| 1.02 | 0.09 | 0.22 | 2 | Fe-HS | |
| 19. Sovetskoye, aggregates of coalescent pseudopyramidal grains (II) | 0.29 | 0.58 | 0.33 | 14 | {6S} |
| 0.27 | 1.11 | 0.28 | 58 | {3S3As} | |
| 0.26 | 1.15 | 0.22 | 8 | {2S4As} | |
| 0.29 | 1.58 | 0.22 | 6 | {6As} | |
| 0.14 | 1.05 | 0.26 | 13 | Fe-LC-1 | |
| 0.97 | 0.20 | 0.22 | 1 | Fe-HS | |
| 28. Eldorado, aggregates of coalescent pseudopyramidal grains (II) | 0.28 | 0.61 | 0.33 | 13 | {6S} |
| 0.25 | 1.12 | 0.34 | 70 | {3S3As} | |
| 0.17 | 0.99 | 0.36 | 14 | Fe-LC-1 | |
| 0.92 | 0.12 | 0.24 | 2 | Fe-HS | |
| 29. Eldorado, aggregates of coalescent pseudopyramidal grains (III) | 0.27 | 0.62 | 0.37 | 15 | {6S} |
| 0.30 | 1.11 | 0.32 | 66 | {3S3As} | |
| 0.12 | 1.06 | 0.28 | 7 | Fe-LC-3 | |
| 0.19 | 1.11 | 0.22 | 8 | Fe-LC-1 | |
| 0.19 | 1.70 | 0.24 | 4 | Fe-LC-2 | |
| 30. Eldorado, aggregates of coalescent pseudopyramidal grains (II) | 0.29 | 0.62 | 0.30 | 12 | {6S} |
| 0.25 | 1.14 | 0.30 | 68 | {3S3As} | |
| 0.27 | 1.58 | 0.27 | 6 | {6As} | |
| 0.18 | 0.78 | 0.34 | 12 | Fe-LC-1 | |
| 0.87 | 0.25 | 0.23 | 2 | Fe-HS | |
| 31. Olginskoye, aggregates of coalescent pseudopyramidal grains (III) | 0.28 | 0.58 | 0.32 | 12 | {6S} |
| 0.25 | 1.08 | 0.33 | 71 | {3S3As} | |
| 0.26 | 1.25 | 0.22 | 6 | {2S4As} | |
| 0.17 | 1.03 | 0.31 | 8 | Fe-LC-1 | |
| 0.92 | 0.18 | 0.28 | 2 | Fe-HS | |
| 32. Olginskoye, aggregates of coalescent pseudopyramidal grains (III) | 0.27 | 0.58 | 0.32 | 12 | {6S} |
| 0.25 | 1.09 | 0.34 | 70 | {3S3As} | |
| 0.28 | 1.27 | 0.25 | 10 | {2S4As} | |
| 0.18 | 1.61 | 0.26 | 5 | {6As} | |
| 0.85 | 0.41 | 0.22 | 2 | Fe-HS | |
| 35. Blagodatnoye, disseminated aggregates of pseudopyramidal grains (III) | 0.27 | 0.63 | 0.26 | 8 | {6S} |
| 0.28 | 1.12 | 0.30 | 54 | {3S3As} | |
| 0.26 | 1.56 | 0.27 | 23 | {6As} | |
| 0.17 | 1.14 | 0.26 | 12 | Fe-LC-1 | |
| 1.03 | 0.07 | 0.23 | 3 | Fe-HS | |
| 37. Blagodatnoye, disseminated aggregates of pseudopyramidal grains (III) | 0.29 | 0.64 | 0.33 | 13 | {6S} |
| 0.28 | 0.96 | 0.25 | 8 | {4S2As} | |
| 0.27 | 1.12 | 0.29 | 50 | {3S3As} | |
| 0.28 | 1.40 | 0.22 | 8 | {2S4As} | |
| 0.26 | 1.67 | 0.28 | 9 | {6As} | |
| 0.18 | 0.80 | 0.25 | 7 | Fe-LC-1 | |
| 0.17 | 1.33 | 0.26 | 5 | Fe-LC-2 | |
| 40. Gerfed, aggregates of coalescent pseudopyramidal grains (III) | 0.26 | 0.61 | 0.32 | 13 | {6S} |
| 0.27 | 1.12 | 0.32 | 69 | {3S3As} | |
| 0.26 | 1.59 | 0.26 | 8 | {6As} | |
| 0.14 | 1.07 | 0.22 | 8 | Fe-LC-1 | |
| 0.97 | 0.09 | 0.22 | 2 | Fe-HS | |
| 41. Arkhangelskoye, aggregates of coalescent pseudopyramidal grains (III) | 0.29 | 0.56 | 0.33 | 18 | {6S} |
| 0.27 | 0.97 | 0.33 | 33 | {4S2As} | |
| 0.27 | 1.23 | 0.31 | 36 | {3S3As} | |
| 0.26 | 1.66 | 0.29 | 7 | {6As} | |
| 0.10 | 1.08 | 0.21 | 6 | Fe-LC-1 | |
| 42. Nikolayevskoye, flattened pseudopyramidal grains (III) | 0.28 | 0.57 | 0.31 | 10 | {6S} |
| 0.27 | 1.12 | 0.32 | 76 | {3S3As} | |
| 0.25 | 1.52 | 0.27 | 6 | {1S5As} | |
| 0.16 | 1.08 | 0.24 | 8 | Fe-LC-1 | |
| 43. Nikolayevskoye, flattened pseudopyramidal grains (III) | 0.27 | 0.65 | 0.34 | 15 | {6S} |
| 0.24 | 1.11 | 0.28 | 70 | {3S3As} | |
| 0.26 | 1.33 | 0.23 | 9 | {2S4As} | |
| 0.24 | 1.56 | 0.24 | 6 | {6As} | |
| 44. Veduga, aggregates of coalescent pseudopyramidal grains (III) | 0.26 | 0.66 | 0.34 | 18 | {6S] |
| 0.25 | 1.10 | 0.34 | 59 | {3S3As} | |
| 0.28 | 1.44 | 0.35 | 22 | {2S4As} | |
| 45 Polyarnaya Zvezda, aggregates of coalescent pseudopyramidal grains (II) | 0.27 | 0.62 | 0.31 | 16 | {6S} |
| 0.25 | 1.09 | 0.37 | 66 | {3S3As} | |
| 0.28 | 1.31 | 0.26 | 11 | {2S4As} | |
| 0.28 | 1.57 | 0.24 | 6 | {6As} | |
| 46. Uderey, needle-like grains (III) | 0.29 | 0.57 | 0.31 | 19 | {6S} |
| 0.25 | 0.98 | 0.31 | 36 | {4S2As} | |
| 0.26 | 1.20 | 0.26 | 30 | {3S3As} | |
| 0.26 | 1.52 | 0.30 | 15 | {1S5As} | |
| 47. Uderey, needle-like grains (II) | 0.29 | 0.54 | 0.28 | 11 | {6S} |
| 0.26 | 0.95 | 0.36 | 45 | {4S2As} | |
| 0.25 | 1.18 | 0.23 | 22 | {3S3As} | |
| 0.27 | 1.37 | 0.22 | 14 | {2S4As} | |
| 0.25 | 1.64 | 0.32 | 8 | {6As} | |
| 49. Uderey, needle-like grains (V) | 0.29 | 0.59 | 0.32 | 22 | {6S} |
| 0.24 | 0.93 | 0.31 | 26 | {4S2As} | |
| 0.25 | 1.13 | 0.23 | 22 | {3S3As} | |
| 0.26 | 1.33 | 0.29 | 26 | {2S4As} | |
| 0.26 | 1.66 | 0.24 | 5 | {6As} | |
| 56. Vasilyevskoye, prismatic grains (III) | 0.29 | 0.56 | 0.34 | 21 | {6S} |
| 0.25 | 0.90 | 0.30 | 23 | {4S2As} | |
| 0.25 | 1.16 | 0.28 | 32 | {3S3As} | |
| 0.27 | 1.42 | 0.31 | 20 | {2S4As} | |
| 0.22 | 1.71 | 0.38 | 4 | {6As} | |
| 57. Vasilyevskoye, prismatic grains (III) | 0.29 | 0.50 | 0.34 | 19 | {6S} |
| 0.28 | 0.79 | 0.24 | 13 | {5S1As} | |
| 0.28 | 1.00 | 0.22 | 12 | {4S2As} | |
| 0.28 | 1.21 | 0.28 | 34 | {3S3As} | |
| 0.26 | 1.57 | 0.31 | 16 | {6As} | |
| 0.12 | 1.01 | 0.24 | 6 | Fe-LC-1 | |
| 58. Vasilyevskoye, prismatic grains (III) | 0.28 | 0.51 | 0.35 | 18 | {6S} |
| 0.28 | 0.80 | 0.28 | 14 | {5S1As} | |
| 0.28 | 0.99 | 0.23 | 14 | {4S2As} | |
| 0.28 | 1.21 | 0.29 | 32 | {3S3As} | |
| 0.26 | 1.59 | 0.36 | 16 | {6As} | |
| 0.11 | 1.01 | 0.22 | 6 | Fe-LC-1 | |
| 61. Panimba, isometric pseudopyramidal grains (III) | 0.28 | 0.58 | 0.33 | 16 | {6S} |
| 0.25 | 1.1 | 0.33 | 74 | {3S3As} | |
| 0.27 | 1.43 | 0.27 | 10 | {2S4As} | |
| 62. Panimba, isometric pseudopyramidal grains (III) | 0.32 | 0.67 | 0.39 | 21 | {6S} |
| 0.26 | 1.13 | 0.32 | 66 | {3S3As} | |
| 0.33 | 1.35 | 0.22 | 5 | {2S4As} | |
| 0.06 | 1.08 | 0.28 | 8 | Fe-LC-3 | |
| 63. Panimba, isometric pseudopyramidal grains (II) | 0.28 | 0.60 | 0.32 | 14 | {6S} |
| 0.25 | 1.09 | 0.33 | 70 | {3S3As} | |
| 0.27 | 1.32 | 0.31 | 16 | {2S4As} |
| No. | Fe | As | S | T °C | log fS2 | Au | Ag | Ru | Pd | Pt | ∑ | S/As | (S + As)/Fe |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 33.9 | 46.4 | 19.7 | 300 | –14.6 | 1.94 | 0.11 | 0.001 | 0.03 | 0.01 | 2.09 | 0.99 | 2.03 |
| 2 | 34.1 | 45.5 | 20.4 | 460 | –5.9 | 0.88 | 3.21 | 0.001 | 0.09 | 0.01 | 4.19 | 1.05 | 2.03 |
| 3 | 33.6 | 47.1 | 19.3 | 340 | –12.8 | 0.36 | 2.18 | 0.001 | 0.07 | 0.00 | 2.61 | 0.96 | 2.05 |
| 4 | 34.0 | 44.7 | 21.2 | 430 | –6.5 | 93.67 | 1.13 | 0.001 | 0.08 | 0.02 | 94.90 | 1.11 | 2.07 |
| 5 | 34.1 | 45.4 | 20.5 | 450 | –5.9 | 12.52 | 1.05 | 0.002 | 0.14 | 0.02 | 13.73 | 1.06 | 2.04 |
| 6 | 33.7 | 46.7 | 19.5 | 320 | –13.4 | 4.13 | 6.35 | 0.002 | 0.09 | 0.00 | 10.57 | 0.98 | 2.04 |
| 7 | 33.7 | 46.4 | 19.9 | 300 | –14.6 | 2.22 | 0.55 | 0.001 | 0.09 | 0.02 | 2.87 | 1.00 | 2.05 |
| 18 | 33.9 | 45.5 | 20.6 | 460 | –5.9 | 20.26 | 46.69 | 0.003 | 0.22 | 0.05 | 67.23 | 1.06 | 2.06 |
| 19 | 34.0 | 44.9 | 21.0 | 440 | –6.4 | 0.38 | 0.85 | 0.001 | 0.02 | 0.00 | 1.25 | 1.09 | 2.06 |
| 28 | 34.4 | 44.5 | 21.2 | 420 | –6.7 | 3.63 | 14.16 | 0.003 | 0.10 | 0.57 | 18.46 | 1.11 | 2.04 |
| 29 | 34.4 | 44.8 | 20.8 | 440 | –6.4 | 0.32 | 22.98 | 0.003 | 0.10 | 0.49 | 23.90 | 1.09 | 2.02 |
| 30 | 34.2 | 45.6 | 20.2 | 460 | –5.9 | 0.15 | 0.84 | 0.001 | 0.02 | 0.01 | 1.01 | 1.03 | 2.02 |
| 31 | 34.0 | 45.4 | 20.5 | 460 | –5.9 | 1.40 | 3.44 | 0.002 | 0.32 | 0.05 | 5.22 | 1.06 | 2.05 |
| 32 | 34.2 | 44.9 | 21.0 | 440 | –6.4 | 1.56 | 2.72 | 0.001 | 0.15 | 0.03 | 4.46 | 1.09 | 2.05 |
| 35 | 32.8 | 49.1 | 17.8 | 530 | –7.4 | 9.44 | 6.03 | 0.013 | 0.17 | 0.04 | 15.69 | 0.85 | 2.06 |
| 37 | 32.9 | 49.1 | 18.0 | 510 | –7.5 | 38.45 | 63.66 | 0.001 | 0.06 | 0.65 | 102.82 | 0.86 | 2.06 |
| 40 | 33.9 | 46.2 | 19.8 | 300 | –14.6 | 6.14 | 49.92 | 0.002 | 0.16 | 0.73 | 56.96 | 1.00 | 2.03 |
| 41 | 34.3 | 44.7 | 21.0 | 430 | –6.5 | 40.56 | 15.27 | 0.001 | 0.07 | 0.12 | 56.02 | 1.10 | 2.04 |
| 42 | 34.4 | 44.1 | 21.4 | 410 | –6.9 | 22.98 | 28.53 | 0.002 | 0.12 | 0.69 | 52.33 | 1.14 | 2.04 |
| 43 | 34.3 | 44.2 | 21.4 | 415 | –6.8 | 10.90 | 25.81 | 0.003 | 0.11 | 0.00 | 36.83 | 1.13 | 2.05 |
| 44 | 33.8 | 46.2 | 19.8 | 300 | –14.6 | 5.40 | 23.52 | 0.002 | 0.03 | 0.00 | 28.96 | 1.00 | 2.04 |
| 45 | 34.2 | 45.3 | 20.4 | 455 | –6.0 | 29.70 | 26.00 | 0.003 | 0.08 | 0.00 | 55.79 | 1.05 | 2.03 |
| 46 | 34.3 | 45.2 | 20.5 | 450 | –6.2 | 36.18 | 18.54 | 0.002 | 0.26 | 0.02 | 55.00 | 1.06 | 2.02 |
| 47 | 34.4 | 45.0 | 20.7 | 445 | –6.4 | 87.41 | 378.71 | 0.001 | 0.09 | 0.02 | 466.22 | 1.07 | 2.02 |
| 49 | 34.3 | 44.9 | 20.7 | 445 | –6.4 | 34.11 | 21.61 | 0.000 | 0.27 | 0.02 | 56.02 | 1.08 | 2.03 |
| 56 | 34.2 | 44.6 | 21.1 | 430 | –6.5 | 24.46 | 10.20 | 0.002 | 0.15 | 0.02 | 34.84 | 1.10 | 2.04 |
| 57 | 34.7 | 43.3 | 21.9 | 390 | –7.4 | 89.13 | 3.64 | 0.001 | 0.05 | 0.01 | 92.83 | 1.18 | 2.03 |
| 58 | 34.5 | 44.2 | 21.4 | 415 | –6.8 | 63.78 | 9.66 | 0.002 | 0.02 | 0.00 | 73.46 | 1.13 | 2.03 |
| 61 | 34.4 | 45.0 | 20.5 | 445 | –6.4 | 4.92 | 18.08 | 0.004 | 0.14 | 0.04 | 23.19 | 1.07 | 2.01 |
| 62 | 34.2 | 44.9 | 20.8 | 445 | –6.4 | 3.61 | 11.49 | 0.003 | 0.23 | 0.04 | 15.38 | 1.08 | 2.04 |
| 63 | 34.1 | 45.2 | 20.6 | 450 | –6.1 | 2.40 | 10.59 | 0.002 | 0.39 | 0.04 | 13.42 | 1.07 | 2.04 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sazonov, A.M.; Silyanov, S.A.; Bayukov, O.A.; Knyazev, Y.V.; Zvyagina, Y.A.; Tishin, P.A. Composition and Ligand Microstructure of Arsenopyrite from Gold Ore Deposits of the Yenisei Ridge (Eastern Siberia, Russia). Minerals 2019, 9, 737. https://doi.org/10.3390/min9120737
Sazonov AM, Silyanov SA, Bayukov OA, Knyazev YV, Zvyagina YA, Tishin PA. Composition and Ligand Microstructure of Arsenopyrite from Gold Ore Deposits of the Yenisei Ridge (Eastern Siberia, Russia). Minerals. 2019; 9(12):737. https://doi.org/10.3390/min9120737
Chicago/Turabian StyleSazonov, Anatoly M., Sergey A. Silyanov, Oleg A. Bayukov, Yuriy V. Knyazev, Yelena A. Zvyagina, and Platon A. Tishin. 2019. "Composition and Ligand Microstructure of Arsenopyrite from Gold Ore Deposits of the Yenisei Ridge (Eastern Siberia, Russia)" Minerals 9, no. 12: 737. https://doi.org/10.3390/min9120737
APA StyleSazonov, A. M., Silyanov, S. A., Bayukov, O. A., Knyazev, Y. V., Zvyagina, Y. A., & Tishin, P. A. (2019). Composition and Ligand Microstructure of Arsenopyrite from Gold Ore Deposits of the Yenisei Ridge (Eastern Siberia, Russia). Minerals, 9(12), 737. https://doi.org/10.3390/min9120737





