Britholite Group Minerals from REE-Rich Lithologies of Keivy Alkali Granite—Nepheline Syenite Complex, Kola Peninsula, NW Russia
Abstract
1. Introduction
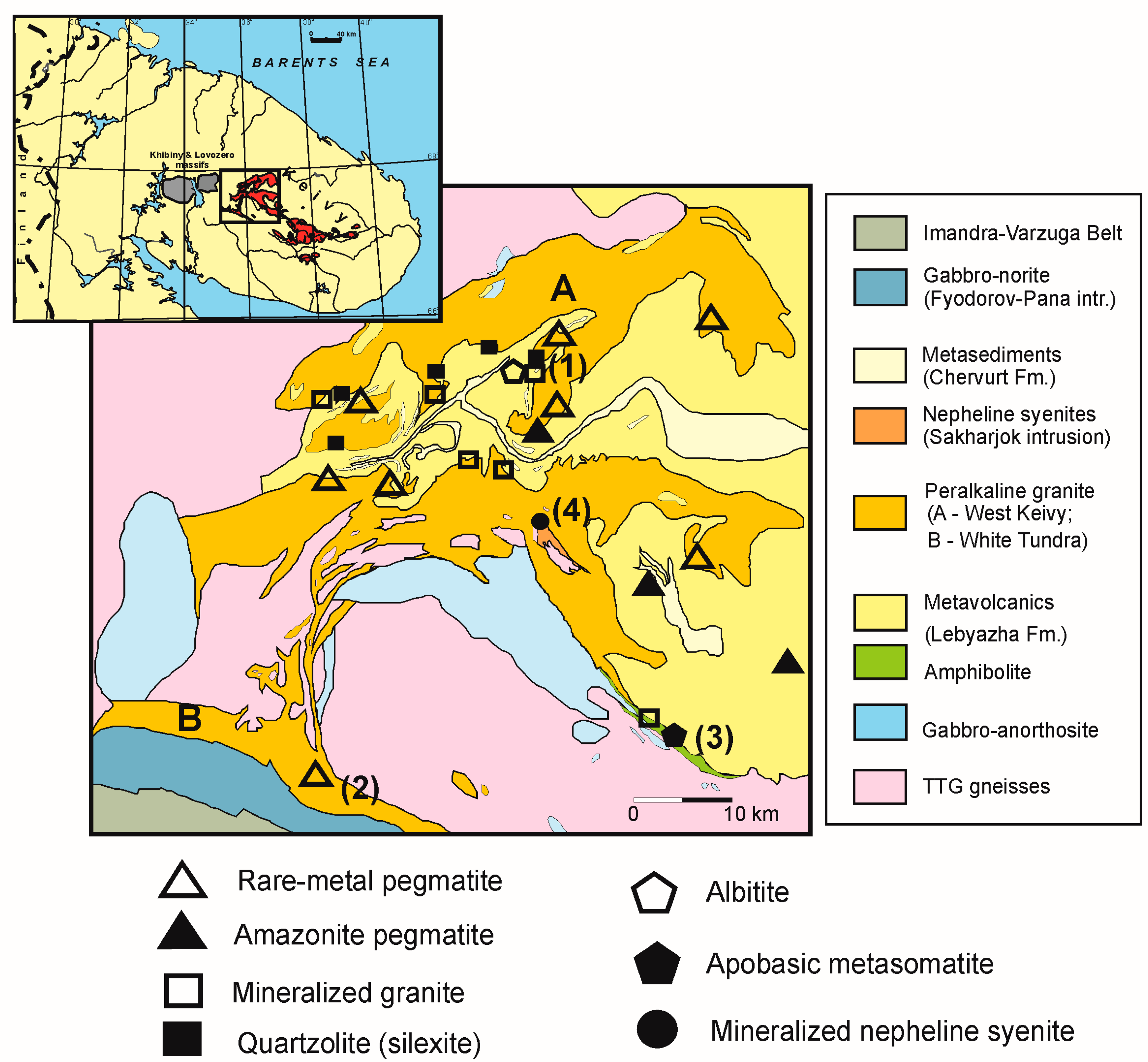
2. Geological Background and REE-Rich Occurrences
2.1. Occurrences Related to Keivy Alkali Granite
2.2. Occurrences Related to Sakharjok Nepheline Syenite
3. Analytical Methods
4. Results
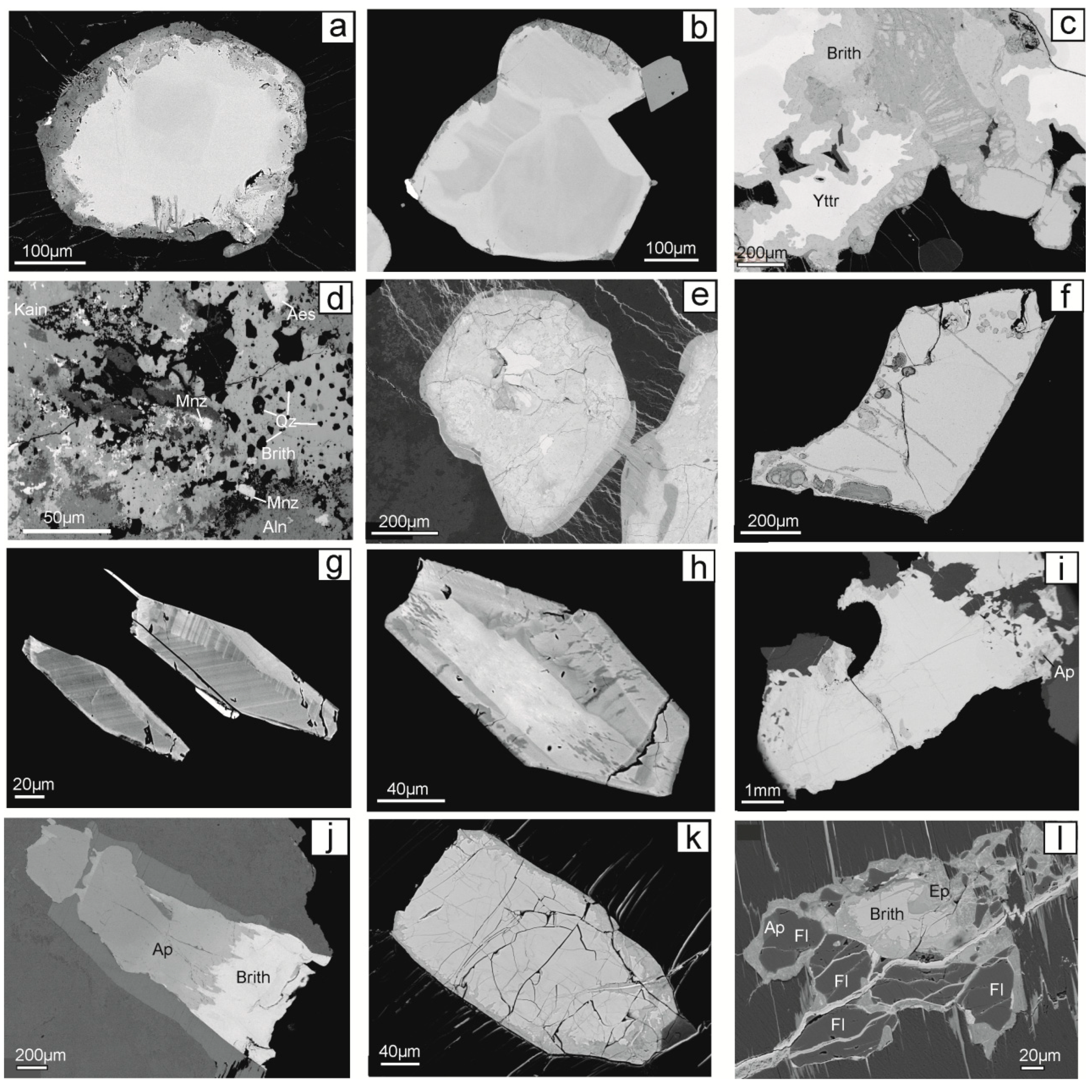
4.1. Morphology, Internal Textures, Associated Minerals, and Alteration
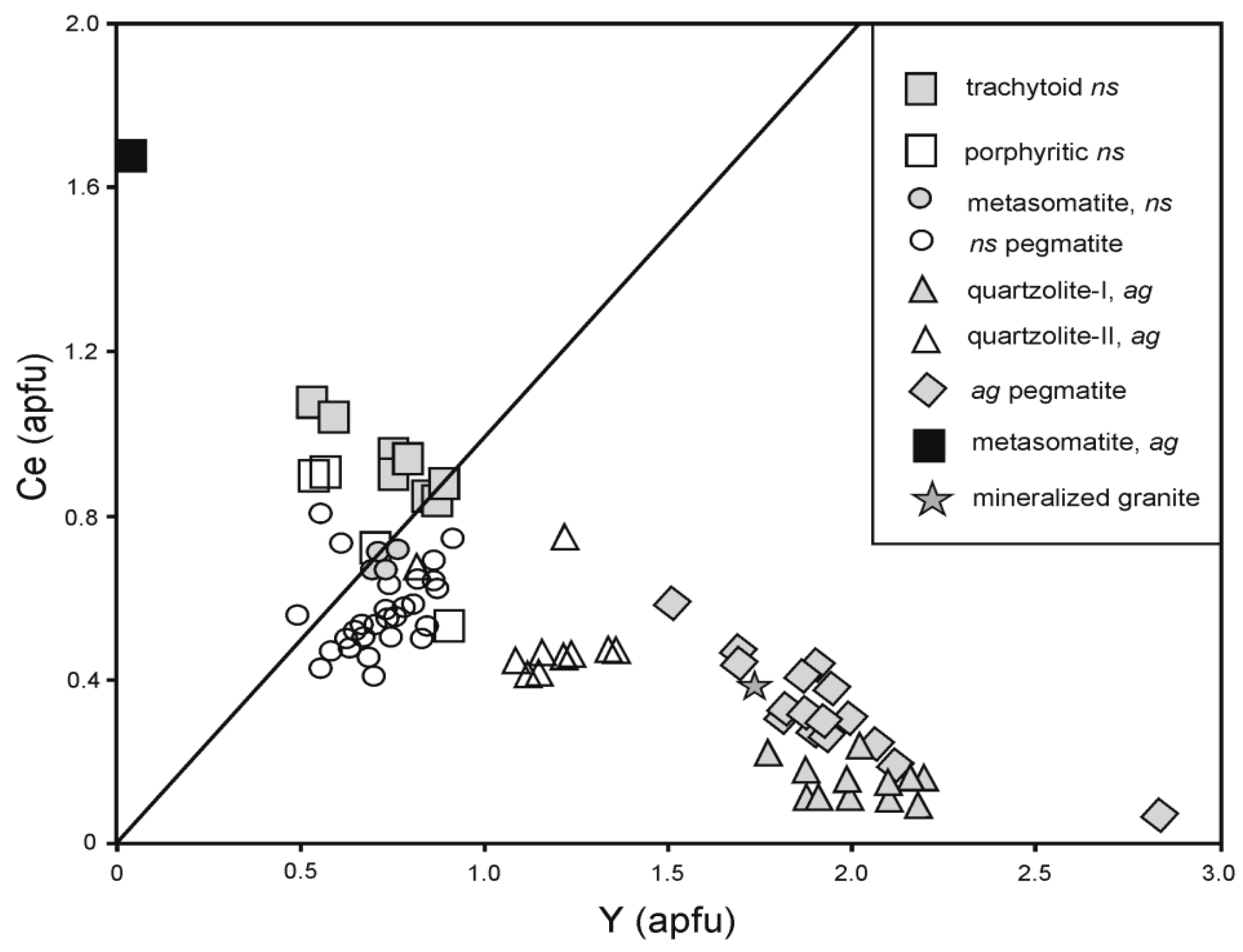
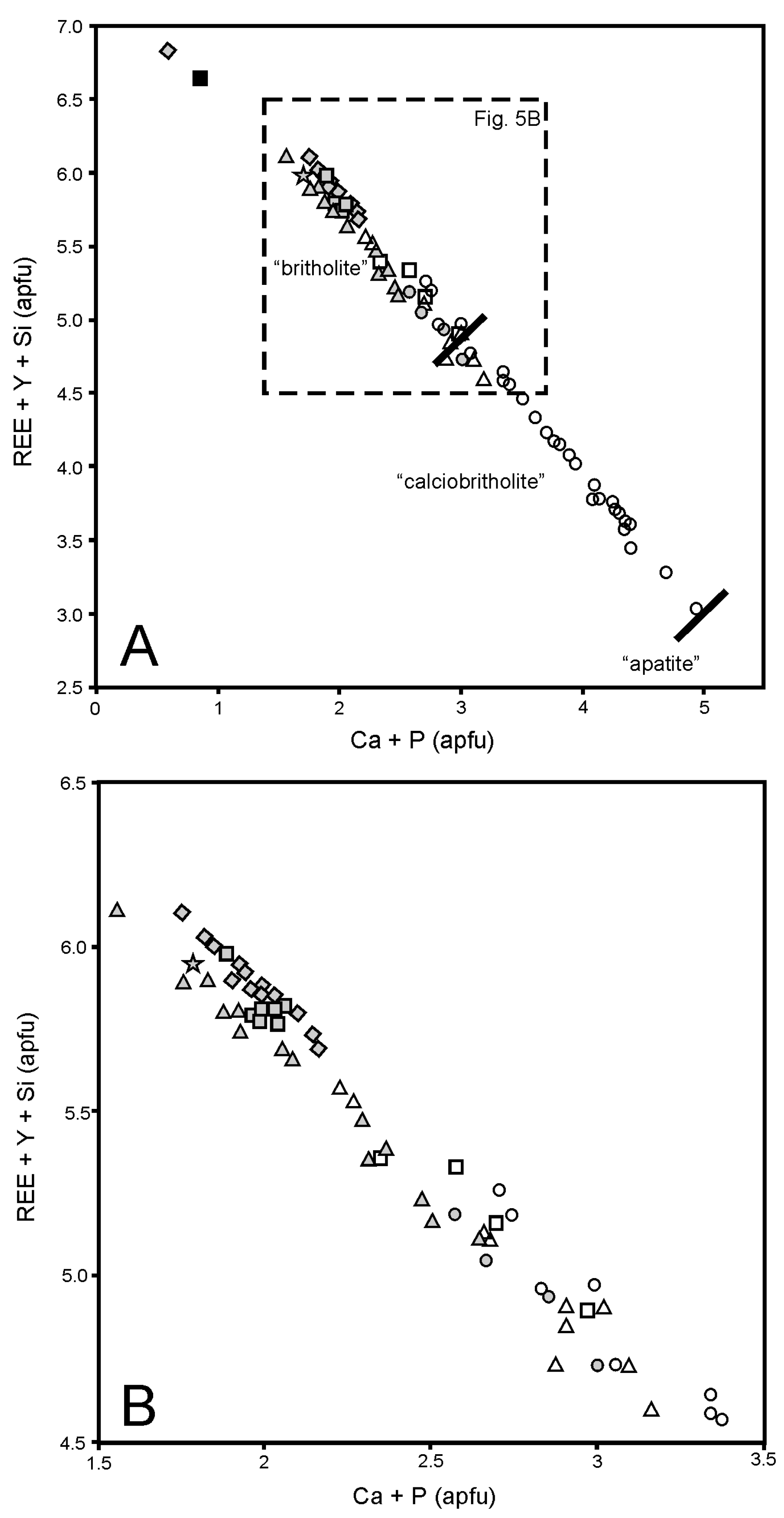
4.2. BGM Composition and Mineral Species
4.3. BGM Substitution Schemes and the ‘Calciobritholite’-‘Apatite’ Series
5. Discussion
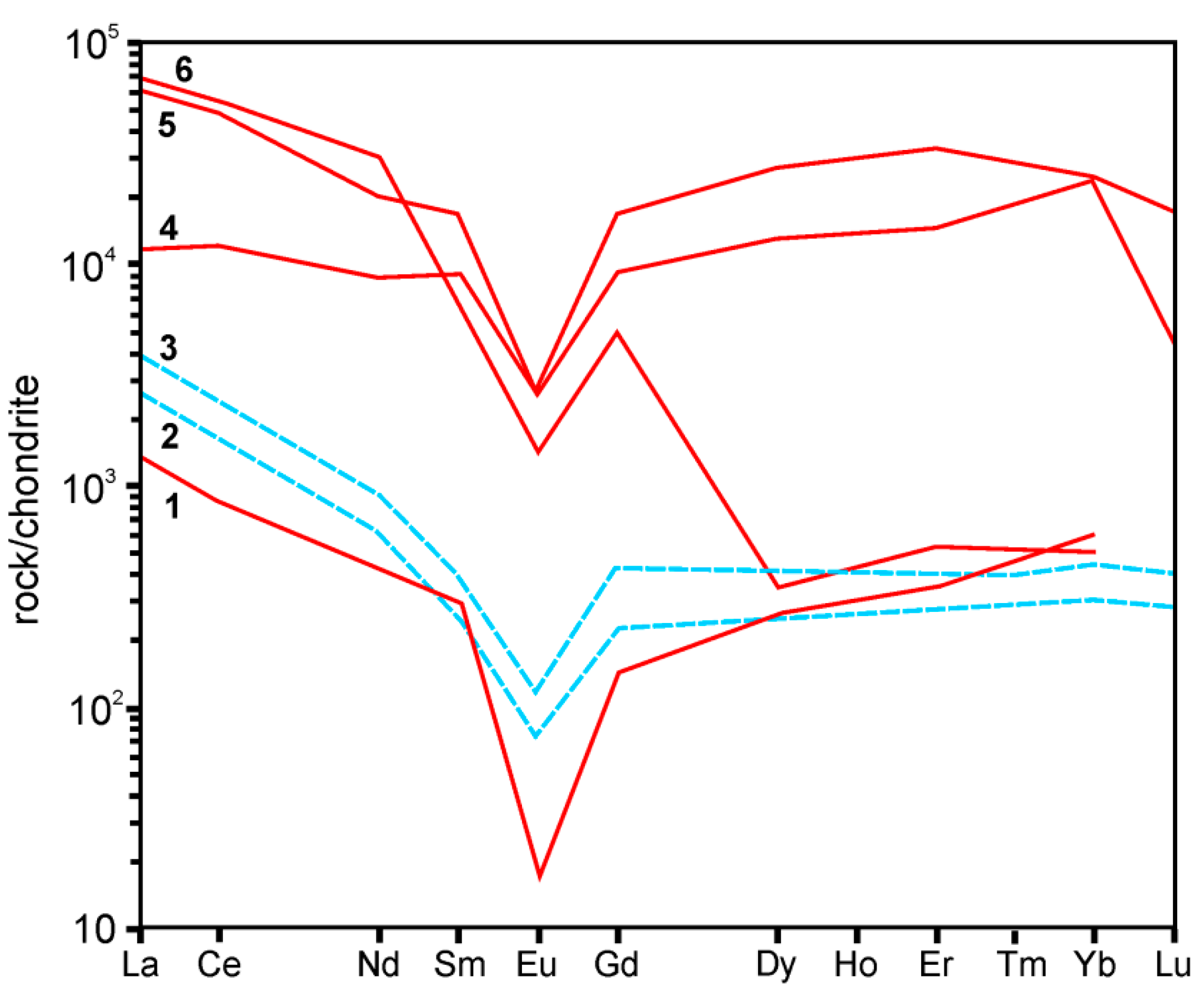
5.1. REE Enrichment Processes in Keivy Alkali Granite-Nepheline Syenite Complex
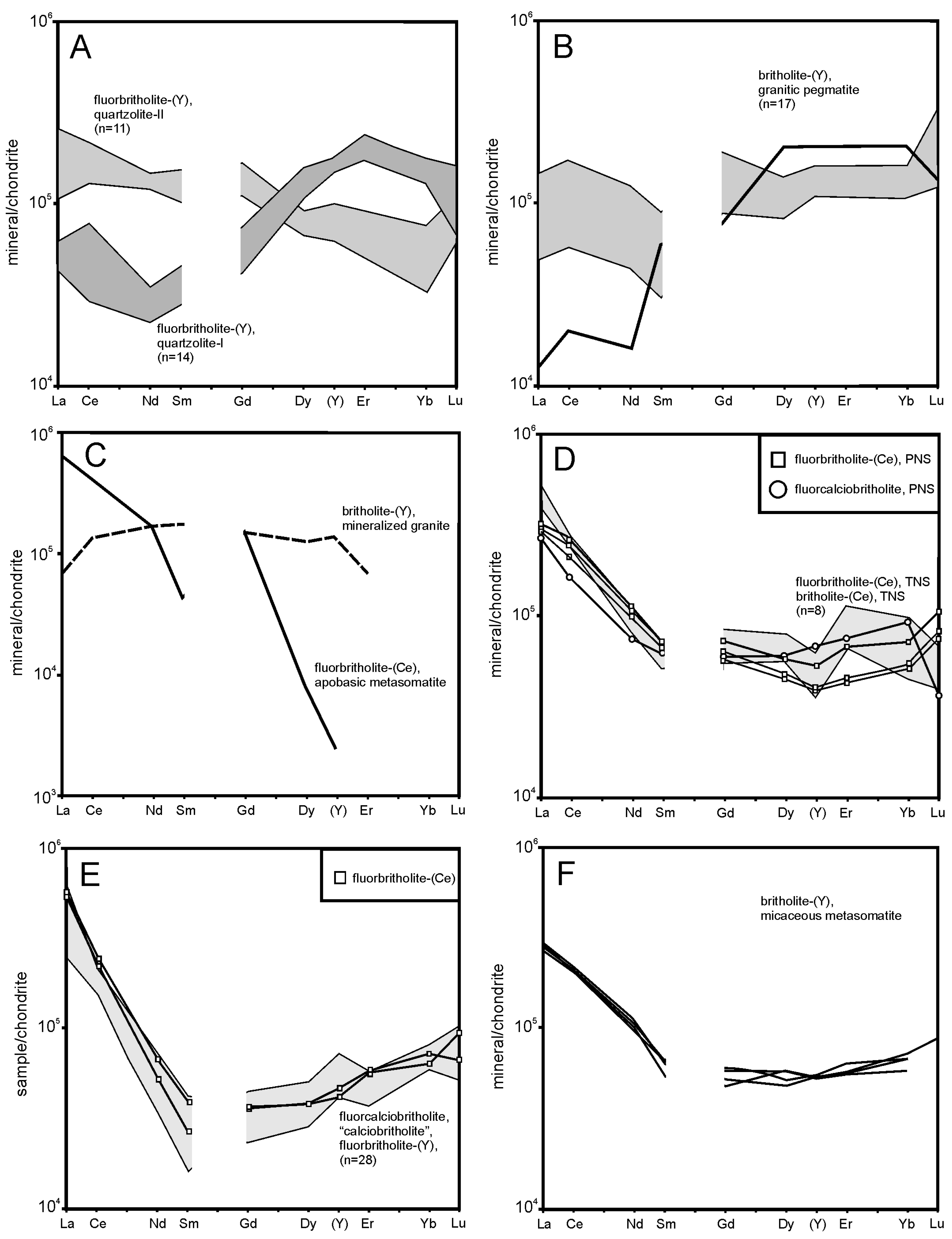
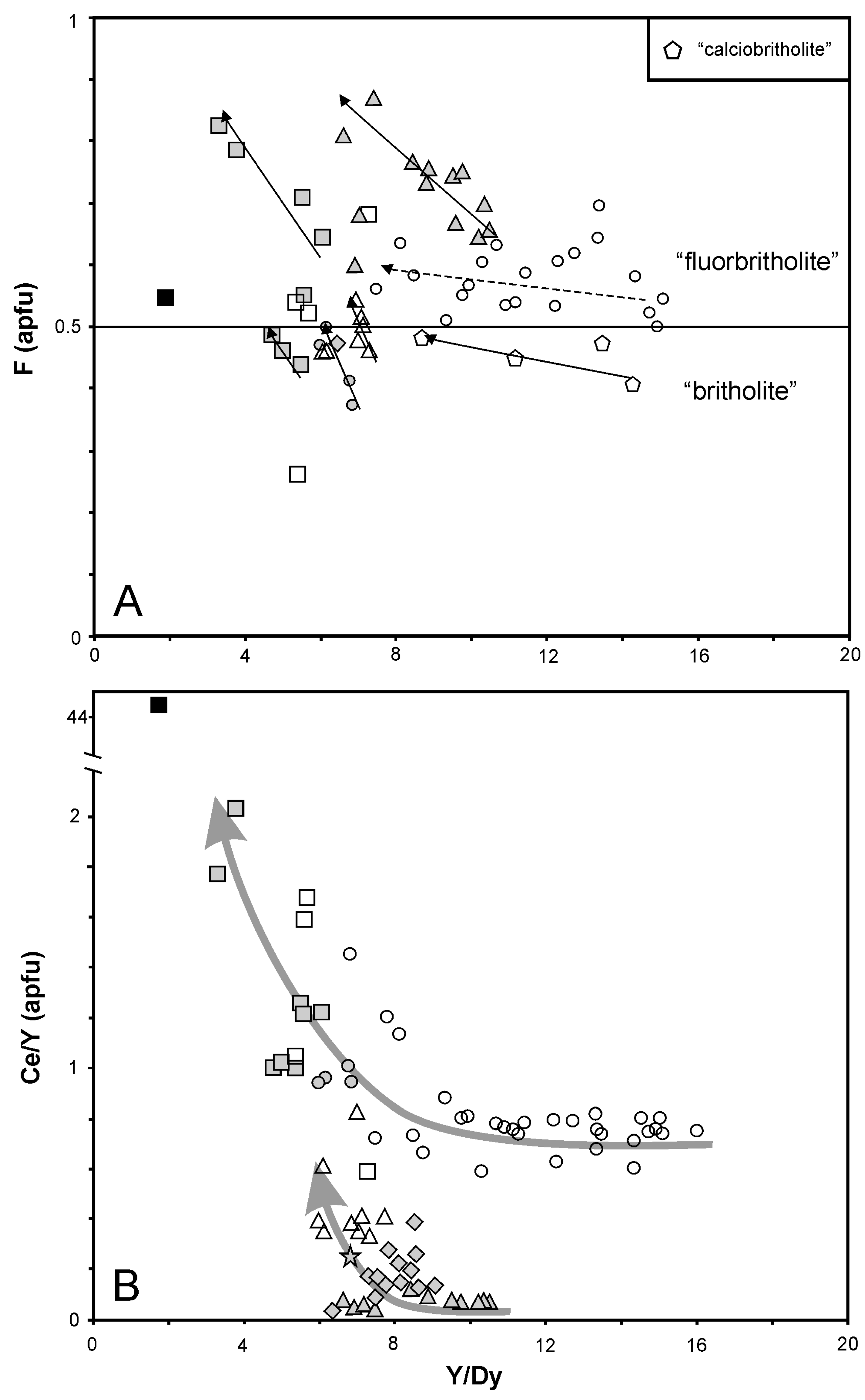
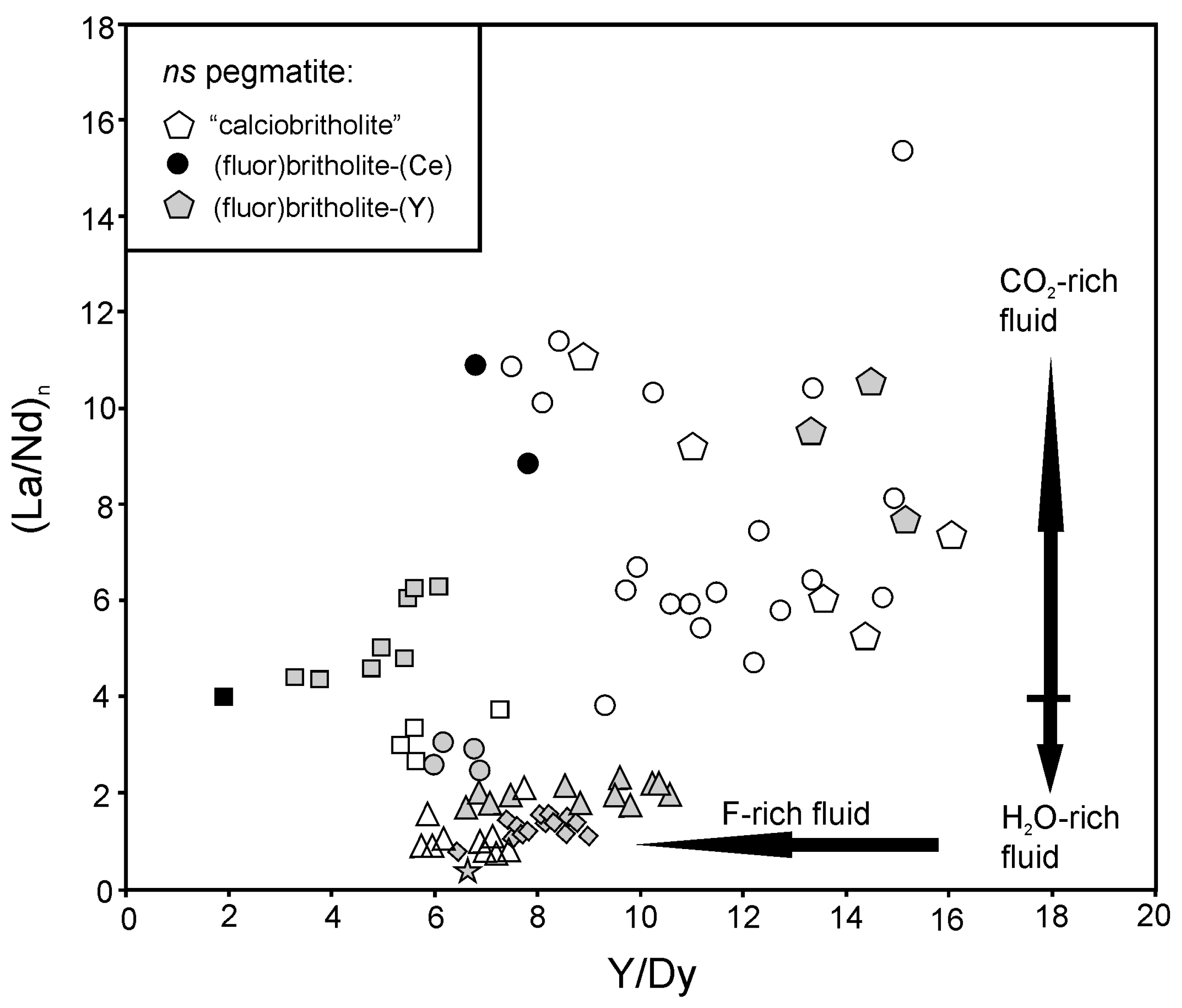
5.2. Fluid-Driven Diversity of BGM and Their Origin
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ahijado, A.; Casillas, R.; Nagy, G.; Fernandez, C. Sr-rich minerals in a carbonatite skarn, Fuerteventura, Canary Islands (Spain). Miner. Pet. 2005, 84, 107–127. [Google Scholar] [CrossRef]
- Arden, K.M.; Halden, N.M. Crystallization and alteration history of britholite in rare-earth-element-enriched pegmatitic segregations associated with the Eden Lake Complex, Manitoba, Canada. Can. Miner. 1999, 37, 1239–1253. [Google Scholar]
- Della Ventura, G.; Williams, C.T.; Cabella, R.; Oberti, R.; Caprilli, E.; Bellatreccia, F. Britholite-hellandite intergrowths and associated REE-minerals from the alkali-syenitic ejecta of the Vico volcanic complex (Latium, Italy): Petrological implications bearing on REE mobility in volcanic systems. Eur. J. Miner. 1999, 11, 843–854. [Google Scholar] [CrossRef]
- Doroshkevich, A.G.; Viladkar, S.G.; Ripp, G.S.; Burtseva, M.V. Hydrothermal REE mineralization in the Amba Dongar carbonatite complex, Gujarat, India. Can. Miner. 2009, 47, 1105–1116. [Google Scholar] [CrossRef]
- Griffin, W.L.; Nilssen, B.; Jensen, B.B. Britholite-(Y) and its alteration—Reiarsdal, Vest-Agder, South-Norway—contributions to the mineralogy of Norway, NO-64. Nor. Geol. Tidsskr. 1979, 59, 265–271. [Google Scholar]
- Gu, J.; Chao, I.Y.; Tang, S. A new mineral—Fluorbritholite-(Ce). J. Wuhan Univ. Technol. 1994, 9, 9–14. [Google Scholar]
- Kolyago, E.K.; Lapin, A.V. Britholite from metasomatites of the Kii alkali massif, Yenisei Range. Zap. Vsesoyuznogo Mineral. Obs. 1990, 119, 107–110. (In Russian) [Google Scholar]
- Macdonald, R.; Bagiński, B.; Dzierzanowski, P.; Jokubauskas, P. Apatite-supergroup minerals in UK Palaeogene granites: Composition and relationship to host-rock composition. Eur. J. Miner. 2013, 25, 461–471. [Google Scholar] [CrossRef]
- Melluso, L.; Morra, V.; de’ Gennaro, R. Coexisting Ba-feldspar and melilite in a melafoidite lava at Mt. Vulture, Italy: Role of volatiles and alkaline earths in bridging a petrological incompatibility. Can. Miner. 2011, 49, 983–1000. [Google Scholar] [CrossRef]
- Mel’nikov, V.S.; Grechanovskyaya, E.E. Pseudomorphic substitution of britholite of the Azov zirconium–rare-earth deposit: Role of metamict transformations and metasomatism. Miner. Zhurnal 2010, 32, 11–21. (In Russian) [Google Scholar]
- Orlandi, P.; Perchiazzi, N.; Mannucci, G. First occurrence of britholite-(Ce) in Italy (Monte Somma, Vesuvius). Eur. J. Miner. 1989, 1, 723–725. [Google Scholar] [CrossRef]
- Pekov, I.V.; Pasero, M.; Yaskovskaya, A.N.; Chukanov, N.V.; Pushcharovsky, D.Y.; Merlino, S.; Zubkova, N.V.; Kononkova, N.N.; Men’shikov, Y.P.; Zadov, A.E. Fluorcalciobritholite, (Ca,REE)(5) (Si,P)O-4 (3)F, a new mineral: Description and crystal chemistry. Eur. J. Miner. 2007, 19, 95–103. [Google Scholar] [CrossRef]
- Pekov, I.V.; Zubkova, N.V.; Chukanov, N.V.; Husdal, T.A.; Zadov, A.E.; Pushcharovsky, D.Y. Fluorbritholite-(Y), (Y,Ca,Ln)(5) (Si,P)O-4 (3)F, a new mineral of the britholite group. Neus. Jahrb. Miner. Abh. 2011, 188, 191–197. [Google Scholar] [CrossRef]
- Ripp, G.S.; Karmanov, N.S.; Kanakin, S.V.; Doroshkevich, A.G.; Andreev, G.V. Cerium britholite of the Mushugui deposit, Mongolia. Zap. Ross. Miner. Obs. 2005, 134, 91–103. (In Russian) [Google Scholar]
- Torró, L.; Villanova, C.; Castillo, M.; Campeny, M.; Gonçalves, A.O.; Melgarejo, J.C. Niobium and rare earth minerals from the Virulundo carbonatite, Namibe, Angola. Miner. Mag. 2012, 76, 393–409. [Google Scholar] [CrossRef]
- Uher, P.; Ondrejka, M.; Bačik, P.; Broska, I.; Konečný, P. Britholite, monazite, REE carbonates, and calcite: Products of hydrothermal alteration of allanite and apatite in A-type granite from Stupne, Western Carpathians, Slovakia. Lithos 2015, 236, 212–225. [Google Scholar] [CrossRef]
- Lyalina, L.M.; Zozulya, D.R.; Savchenko, Y.E.; Tarasov, M.P.; Selivanova, E.A.; Tarasova, E. Fluorbritholite-(Y) and yttrialite-(Y) from silexites of the Keivy alkali granites, Kola Peninsula. Geol. Ore Depos. 2014, 56, 589–602. [Google Scholar] [CrossRef]
- Mel’nikov, V.S.; Vozhyak, D.K.; Grechanovskyaya, E.E.; Gurskii, D.S.; Kulchitskaya, A.A.; Strekozov, S.N. Azov zirconium–rare-earth deposit: Mineralogical and genetic features. Miner. Zhurnal 2000, 22, 42–61. (In Russian) [Google Scholar]
- Lira, R.; Ripley, E.M. Hydrothermal alteration and REE-Th mineralization at the Rodeo-De-Los-Molles deposit, Las-Chacras batholith, Central Argentina. Contrib. Miner. Pet. 1992, 110, 370–386. [Google Scholar] [CrossRef]
- Lorenz, M.; Altenberger, U.; Trumbull, R.B.; Lira, R.; López de Luchi, M.; Günter, C.; Eidner, S. Chemical and textural relations of britholite- and apatite-group minerals from hydrothermal REE mineralization at the Rodeo de los Molles deposit, Central Argentina. Miner. Mag. 2019, in press. [Google Scholar] [CrossRef]
- Zozulya, D.R.; Lyalina, L.M.; Savchenko, Y.E. Britholite Ores of the Sakharjok Zr-Y-REE Deposit, Kola Peninsula: Geochemistry, Mineralogy, and Formation Stages. Geochem. Int. 2015, 53, 892–902. [Google Scholar] [CrossRef]
- Petrella, L.; Williams-Jones, A.E.; Goutier, J.; Walsh, J. The Nature and Origin of the Rare Earth Element Mineralization in the Misery Syenitic Intrusion, Northern Quebec, Canada. Econ. Geol. 2014, 109, 1643–1666. [Google Scholar] [CrossRef]
- Wu, C.; Yuan, Z.; Bai, G. Rare earth deposits in China. In Rare Earth Minerals. Chemistry, Origin and Ore Deposits; Jones, A.P., Wall, F., Williams, C.T., Eds.; Chapman & Hall: London, UK, 1996; pp. 281–310. [Google Scholar]
- Mariano, A.N. Economic geology of rare-earth elements. Rev. Miner. 1989, 21, 309–337. [Google Scholar]
- Pasero, M.; Kampf, A.R.; Ferraris, C.; Pekov, I.V.; Rakovan, J.; White, T.J. Nomenclature of the apatite supergroup minerals. Eur. J. Miner. 2010, 22, 163–179. [Google Scholar] [CrossRef]
- Zozulya, D.R.; Lyalina, L.M.; Savchenko, Y.E. Britholite-group minerals as sensitive indicators of changing fluid composition during pegmatite formation: Evidence from the Keivy alkaline province, Kola Peninsula, NW Russia. Miner. Pet. 2017, 111, 511–522. [Google Scholar] [CrossRef]
- Zozulya, D.R.; Lyalina, L.M.; Eby, N.; Savchenko, Y.E. Ore geochemistry, zircon mineralogy, and genesis of the Sakharjok Y-Zr deposit, Kola Peninsula, Russia. Geol. Ore Depos. 2012, 54, 81–98. [Google Scholar] [CrossRef]
- Vinogradov, A.N.; Batieva, I.D.; Zozulya, D.R.; Kalinnikov, V.T.; Lebedev, V.N.; Masloboyev, V.A.; Rakaev, A.I.; Gritzay, Z.D. Complex rare‐earths—zirconium mineralization of Sakharjok alkaline massif. Miner. Syr’yo 2000, 7, 25–34. (In Russian) [Google Scholar]
- Mitrofanov, F.P.; Zozulya, D.R.; Bayanova, T.B.; Levkovich, N.V. The world’s oldest anorogenic alkali granitic magmatism in the Keivy structure on the Baltic Shield. Dokl. Earth Sci. 2000, 374, 1145–1148. [Google Scholar]
- Zozulya, D.R.; Bayanova, T.B.; Eby, G.N. Geology and age of the Late Archean Keivy alkaline province, Northeastern Baltic Shield. J. Geol. 2005, 113, 601–608. [Google Scholar] [CrossRef]
- Ćerny, P.; Ercit, T.S. The classification of granitic pegmatites revisited. Can. Miner. 2005, 43, 2005–2026. [Google Scholar] [CrossRef]
- Bagiński, B.; Zozulya, D.; Macdonald, R.; Kartashov, P.M.; Dzierzanowski, P. Low-temperature hydrothermal alteration of a rare-metal rich quartz-epidote metasomatite from the El’ozero deposit, Kola Peninsula, Russia. Eur. J. Miner. 2016, 28, 789–810. [Google Scholar] [CrossRef]
- Batieva, I.D.; Bel’kov, I.V. Sakharjok Alkaline Massif, Constituent Rocks, and Minerals; Kola Branch, USSR Academy of Sciences: Apatity, Russia, 1984; p. 133. (In Russian) [Google Scholar]
- Lyalina, L.M.; Zozulya, D.R.; Savchenko, E.E. Multiple crystallization of zircon in the Sakharjok rare-earth element–zirconium deposit, Kola Peninsula. Dokl. Earth Sci. 2010, 430, 120–124. [Google Scholar] [CrossRef]
- Lyalina, L.; Zolotarev, A., Jr.; Selivanova, E.; Savchenko, Y.; Zozulya, D.; Krivovichev, S.; Mikhailova, Y. Structural characterization and composition of Y-rich hainite from Sakharjok nepheline syenite pegmatite (Kola Peninsula, Russia). Miner. Pet. 2015, 109, 443–451. [Google Scholar] [CrossRef]
- Pouchou, J.L.; Pichoir, J.F. Quantitative analysis of homogeneous or stratified microvolumes applying the model ‘PAP’. In Electron Probe Quantitation; Newbury, H., Ed.; Plenum Press: New York, NY, USA, 1991; pp. 31–75. [Google Scholar]
- Merlet, C. An accurate computer correction program for quantitative electron probe microanalysis. Microchim. Acta 1994, 114, 363–376. [Google Scholar] [CrossRef]
- Mikhailova, J.A.; Pakhomovsky, Y.A.; Ivanyuk, G.Y.; Bazai, A.V.; Yakovenchuk, V.N.; Elizarova, I.R.; Kalashnikov, A.O. REE mineralogy and geochemistry of the Western Keivy peralkaline granite massif, Kola Peninsula, Russia. Ore Geol. Rev. 2017, 82, 181–197. [Google Scholar] [CrossRef]
- Sun, S.S.; McDonough, W.F. Chemical and isotopic systematics of oceanic basalts: Implications for mantle composition and processes. Geol. Soc. Lond. Spec. Publ. 1989, 42, 313–345. [Google Scholar] [CrossRef]
- Rønsbo, J.G. Coupled substitutions involving REEs and Na and Si in apatites in alkaline rocks from the Ilimaussaq intrusion, South Greenland, and the petrological implications. Am. Miner. 1989, 74, 896–901. [Google Scholar]
- Boily, M.; Williams-Jones, A.E. The role of magmatic and hydrothermal processes in the chemical evolution of the Strange Lake plutonic complex, Québec-Labrador. Contrib. Miner. Pet. 1994, 118, 33–47. [Google Scholar] [CrossRef]
- Linnen, R.L.; Samson, I.M.; Williams-Jones, A.E.; Chakhmouradian, A.R. Geochemistry of the Rare-Earth Elements, Nb, Ta, Hf, and Zr Deposits. In Treatise on Geochemistry, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 543–568. [Google Scholar]
- Schmitt, A.K.; Trumbull, R.B.; Dulski, P.; Emmermann, R. Zr-Nb-REE mineralization in peralkaline granites from the Amis Complex, Brandberg (Namibia): Evidence for magmatic pre-enrichment from melt inclusions. Econ. Geol. 2002, 97, 399–413. [Google Scholar] [CrossRef]
- Salvi, S.; Williams-Jones, A.E. Alkaline granite-syenite hosted deposits. In Rare-Element Geochemistry and Mineral Deposits; Linnen, R.L., Samson, I.M., Eds.; Short Course Notes; Geological Association of Canada: Ottawa, ON, Canada, 2005; Volume 7, pp. 315–341. [Google Scholar]
- Salvi, S.; Williams-Jones, A.E. Alteration, HFSE mineralisation and hydrocarbon formation in peralkaline igneous systems: Insights from the Strange Lake Pluton, Canada. Lithos 2006, 91, 19–34. [Google Scholar] [CrossRef]
- Sheard, E.R.; Williams-Jones, A.E.; Heiligmann, M.; Pederson, C.; Trueman, D.L. Controls of the concentration of zirconium, niobium, and the rarer earth elements in the Thor Lake rare metal deposit, Northwest Territories, Canada. Econ. Geol. 2012, 107, 81–104. [Google Scholar] [CrossRef]
- Dostal, J.; Kontak, D.J.; Karl, S.M. The Early Jurassic Bokan Mountain peralkaline granitic complex (southeastern Alaska): Geochemistry, petrogenesis and rare-metal mineralization. Lithos 2014, 202–203, 395–412. [Google Scholar]
- Zozulya, D.R.; Bayanova, T.B.; Serov, P.N. Age and Isotopic Geochemical Characteristics of Archean Carbonatites and Alkaline Rocks of the Baltic Shield. Dokl. Earth Sci. 2007, 415A, 874–879. [Google Scholar] [CrossRef][Green Version]
- Kosterin, A.V. On possible forms of transfer of rare-earth elements by hydrothermal solutions. Geokhimiya 1959, 4, 310–315. (In Russian) [Google Scholar]
- Mineev, D.A. Geochemical differentiation of rare-earth elements. Geokhimiya 1963, 12, 1082–1100. (In Russian) [Google Scholar]
- Mineev, D.A. Geochemistry of Apogranites and Rare-Metal Metasomatites of Northwestern Tarbagatai; Nauka: Moscow, Russia, 1968; p. 185. (In Russian) [Google Scholar]
- Taylor, R.P.; Strong, D.F.; Fryer, B.J. Volatile control of contrasting trace-element distributions in peralkaline granitic and volcanic-rocks. Contrib. Miner. Pet. 1981, 77, 267–271. [Google Scholar] [CrossRef]
- Charoy, B.; Raimbault, L. Zr-rich, Th-rich, and REE-rich biotite differentiates in the A-type granite pluton of Suzhou (Eastern China)—the key role of fluorine. J. Pet. 1994, 35, 919–962. [Google Scholar] [CrossRef]
- Keppler, H.; Wyllie, P.J. Role of fluids in transport and fractionation of uranium and thorium in magmatic processes. Nature 1990, 348, 531–533. [Google Scholar] [CrossRef]
- Trueman, D.L.; Pedersen, J.C.; de St Jorre, L.; Smith, D.G.W. The Thor Lake rare-metal deposits, North-West Territories. In Recent Advances in the Geology of Granite-Related Mineral Deposits; Taylor, R.P., Strong, D.F., Eds.; Canadian Institute of Mining and Metallurgy: Montreal, QC, Canada, 1988; Volume 39, pp. 280–290. [Google Scholar]
- Macdonald, R.; Bagiński, B.; Zozulya, D. Differing responses of zircon, chevkinite-(Ce), monazite-(Ce) and fergusonite-(Y) to hydroth;rmal alteration: Evidence from the Keivy alkaline province, Kola Peninsula, Russia. Miner. Pet. 2017, 111, 523–545. [Google Scholar] [CrossRef]
- Keppler, H. Influence of fluorine on the enrichment of high-field strength trace-elements in granitic rocks. Contrib. Miner. Pet. 1993, 114, 479–488. [Google Scholar] [CrossRef]
- Migdisov, A.A.; Williams-Jones, A.E.; Wagner, T. An experimental study of the solubility and speciation of the Rare Earth Elements (III) in fluoride- and chloride-bearing aqueous solutions at temperatures up to 300 degrees C. Geochim. Cosmochim. Acta 2009, 73, 7087–7109. [Google Scholar] [CrossRef]
- London, D.; Hervig, R.L.; Morgan, G.B. Melt-vapor solubilities and elemental partitioning in peraluminous granite-pegmatite systems-experimental results with Macusani glass at 200 Mpa. Contrib. Miner. Pet. 1988, 99, 360–373. [Google Scholar] [CrossRef]
- Agangi, A.; Kamenetsky, V.S.; McPhie, J. The role of fluorine in the concentration and transport of lithophile trace elements in felsic magmas: Insights from the Gawler Range Volcanics, South Australia. Chem. Geol. 2010, 273, 314–325. [Google Scholar] [CrossRef]
- Ekambaram, V.; Brookins, D.G.; Rosenberg, P.E.; Emanuel, K.M. Rare-earth element geochemistry of fluorite-carbonate deposits in Western Montana, USA. Chem. Geol. 1986, 54, 319–331. [Google Scholar] [CrossRef]
- McLennan, S.M.; Taylor, S.R. Rare-earth element mobility associated with uranium mineralization. Nature 1979, 282, 247–250. [Google Scholar] [CrossRef]
- Salvi, S.; Williams-Jones, A.E. The role of hydrothermal processes in concentrating high-field strength elements in the Strange Lake peralkaline complex, northeastern Canada. Geochim. Cosmochim. Acta 1996, 60, 1917–1932. [Google Scholar] [CrossRef]
- Williams-Jones, A.E.; Samson, I.M.; Olivo, G.R. The genesis of hydrothermal fluorite-REE deposits in the Gallinas Mountains, New Mexico. Econ. Geol. Bull. Soc. Econ. Geol. 2000, 95, 327–341. [Google Scholar] [CrossRef]
- Gramaccioli, C.M.; Diella, V.; Demartin, F. The role of fluoride complexes in REE geochemistry and the importance of 4f electrons: Some examples in minerals. Eur. J. Miner. 1999, 11, 983–992. [Google Scholar] [CrossRef]
- Brookins, D.G. Aqueous geochemistry of rare earth elements. Rev. Miner. Geochem. 1989, 21, 201–225. [Google Scholar]
- Smith, M.P.; Henderson, P.; Campbell, L.S. Fractionation of the REE during hydrothermal processes: Constraints from the Bayan Obo Fe-REE-Nb deposit, Inner Mongolia, China. Geochim. Cosmochim. Acta 2000, 64, 3141–3160. [Google Scholar] [CrossRef]
| Mineralized Granite | Alkali Granite Pegmatite | Quartzolite | Apobasic Quartz-Epidote Metasomatite | Mineralized Nepheline Syenite | Nepheline Syenite Pegmatite |
|---|---|---|---|---|---|
| zircon, fergusonite-(Y), chevkinite-(Ce), bastnäsite-(Ce), monazite-(Ce), britholite-(Y), allanite-(Ce), thorite, xenotime-(Y), REE-rich fluorapatite, pyrochlore group minerals Zr (700–21000 ppm, average 4700 ppm), Y (100–3900 ppm, average 550 ppm), Nb (40–400 ppm, average 180 ppm), REE (1000–1800 ppm, average 1300 ppm), Th (20–150 ppm), U (5–23 ppm) | zircon, allanite-(Ce), kainosite-(Y), fergusonite-(Y), gadolinite-(Y), thorite, monazite-(Ce), REE-bearing titanite, britholite group minerals, tengerite-(Y), bastnäsite-(Y) | zircon, fergusonite-(Y), britholite group minerals, aeschynite-(Y), chevkinite-(Ce), yttrialite-(Y), thorite, monazite-(Ce), xenotime-(Y), bastnäsite-(Ce) ZrO2 6.5 wt %, Y2O3 2.5 wt % REE (30000– 40000 ppm), Nb 14000 ppm, | thorite, zircon, allanite-(Ce), fergusonite-(Y), chevkinite-(Ce), monazite-(Ce), ferriallanite-(Ce), samarskite-(Y), aeschynite-(Y), Nb-bearing titanite, uraninite, fluorbritholite-(Ce), pyrochlore group minerals, gadolinite group minerals, REE-carbonates REE 85000 ppm, Y 42000 ppm, Nb 62000 ppm, Zr 67000 ppm, Th 17000 ppm, U 7700 ppm | zircon, britholite group minerals, pyrochlore group minerals Zr (10000–16000 ppm), Y 900 ppm, REE2O3 (0.1–0.3 wt %), Nb (up to 1200 ppm) | britholite group minerals, REE-rich fluorapatite, hainite-(Y), batievaite-(Y), meliphanite, leucophanite, zircon, pyrochlore group minerals, gadolinite group minerals, zirkelite, cerianite-(Ce), behoite |
| Sample | 172 | 9 | 2 | 9/1 | 162/60 | 302 | 300 | 529 | 532 | 633 |
|---|---|---|---|---|---|---|---|---|---|---|
| wt % | ||||||||||
| P2O5 | 4.88 | 5.15 | 0.00 | 2.23 | b.d. | 3.38 | 1.60 | 11.86 | 10.57 | 4.84 |
| SiO2 | 21.92 | 19.75 | 23.17 | 22.74 | 21.09 | 21.52 | 20.91 | 16.16 | 18.14 | 19.80 |
| ThO2 | 0.78 | 0.75 | b.d. | b.d. | b.d. | 2.85 | 0.85 | 0.27 | b.d. | 2.44 |
| UO2 | b.d. | 0.28 | b.d. | 0.12 | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. |
| SO2 | n.a. | n.a. | n.a. | b.d. | n.a. | n.a. | n.a. | n.a. | n.a. | 0.46 |
| TiO2 | n.a. | n.a. | n.a. | b.d. | 0.76 | n.a. | n.a. | n.a. | n.a. | n.a. |
| ZrO2 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 0.88 |
| Al2O3 | b.d. | b.d. | b.d. | b.d. | b.d. | 0.28 | 0.05 | 0.07 | 0.15 | 0.17 |
| Y2O3 | 30.64 | 18.11 | 41.11 | 28.11 | 0.48 | 10.53 | 10.34 | 10.36 | 12.40 | 10.61 |
| La2O3 | 1.61 | 3.32 | 0.34 | 2.57 | 18.82 | 8.52 | 13.88 | 8.73 | 9.10 | 7.87 |
| Ce2O3 | 4.25 | 9.94 | 1.46 | 7.01 | 31.26 | 15.66 | 18.91 | 11.93 | 13.37 | 14.85 |
| Pr2O3 | 0.45 | 1.83 | 0.13 | 0.58 | 2.40 | 1.52 | 1.25 | 0.78 | 0.98 | 1.43 |
| Nd2O3 | 1.80 | 7.36 | 0.85 | 3.68 | 9.08 | 6.08 | 4.39 | 2.80 | 3.04 | 5.09 |
| Sm2O3 | 0.51 | 2.23 | 1.00 | 1.41 | 0.75 | 1.24 | 0.89 | 0.59 | 0.67 | 1.12 |
| Gd2O3 | 1.07 | 3.30 | 1.77 | 3.74 | 3.49 | 1.33 | 1.25 | 0.70 | 0.62 | 1.37 |
| Tb2O3 | 0.28 | 0.32 | 0.55 | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. |
| Dy2O3 | 3.12 | 2.28 | 5.78 | 3.37 | 0.23 | 1.68 | 1.70 | 0.74 | 1.00 | 1.56 |
| Ho2O3 | 0.70 | 0.65 | 1.39 | 0.11 | b.d. | 0.20 | 0.28 | b.d. | 0.48 | b.d. |
| Er2O3 | 3.26 | b.d. | 5.33 | b.d. | b.d. | 1.23 | 1.48 | 0.80 | 0.91 | 1.07 |
| Tm2O3 | 0.57 | b.d. | 0.78 | b.d. | b.d. | 0.11 | 0.14 | 0.19 | 0.22 | 0.24 |
| Yb2O3 | 2.35 | 1,17 | 3.79 | 2.35 | b.d. | 1.33 | 1.57 | 1.22 | 1.21 | 1.24 |
| Lu2O3 | 0.25 | b.d. | 0.38 | 0.56 | b.d. | 0.23 | 0.14 | b.d. | 0.08 | 0.25 |
| FeO | 0.71 | 0.36 | 0.88 | 0.50 | 3.36 | 0.05 | 0.17 | b.d. | 0.05 | 0.07 |
| MnO | 1.82 | 1.15 | 0.58 | 0.33 | 0.10 | 0.06 | 0.67 | 0.05 | b.d. | 0.17 |
| CaO | 16.48 | 15.72 | 4.21 | 14.61 | 5.36 | 17.35 | 12.45 | 26.08 | 24.63 | 18.86 |
| SrO | 0.25 | 0.04 | b.d. | b.d. | n.a. | n.a. | n.a. | b.d. | b.d. | b.d. |
| BaO | n.a. | 0.00 | n.a. | 0.11 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. |
| PbO | n.a. | n.a. | n.a. | 0.37 | n.a. | 0.30 | b.d. | b.d. | b.d. | b.d. |
| Na2O | 0.19 | b.d. | 1.70 | 0.16 | b.d. | b.d. | 0.18 | 0.09 | 0.08 | 0.18 |
| K2O | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | 0.04 | b.d. | b.d. | b.d. |
| F | 2.08 | 1.29 | 1.16 | n.a | 1.18 | 1.36 | 1.65 | 1.72 | 1.26 | 1.28 |
| Cl | b.d. | b.d. | b.d. | 0.04 | b.d. | b.d. | b.d. | b.d. | b.d. | 0.05 |
| Sum | 99.98 | 95.00 | 96.37 | 98.58 | 98.48 | 96.80 | 94.77 | 95.14 | 98.98 | 95.90 |
| O = -F | 0.88 | 0.54 | 0.49 | 1.60 | 0.50 | 0.57 | 0.69 | 0.72 | 0.53 | 0.54 |
| O = -Cl | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 |
| Total | 99.10 | 94.46 | 95.88 | 96.98 | 97.98 | 96.22 | 94.08 | 94.41 | 98.45 | 95.35 |
| Formulae on the basis of 8 total (M + T) cations | ||||||||||
| P | 0.47 | 0.55 | 0.00 | 0.23 | 0.00 | 0.36 | 0.18 | 1.14 | 1.00 | 0.51 |
| Si | 2.52 | 2.49 | 3.00 | 2.79 | 3.09 | 2.70 | 2.84 | 1.84 | 2.02 | 2.44 |
| Sum T | 2.99 | 3.04 | 3.00 | 3.02 | 3.09 | 3.06 | 3.02 | 2.98 | 3.02 | 3.00 |
| Th | 0.02 | 0.02 | 0.00 | 0.00 | 0.00 | 0.08 | 0.03 | 0.01 | 0.00 | 0.07 |
| U | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| S | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.05 |
| Ti | 0.00 | 0.00 | 0.00 | 0.00 | 0.08 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Zr | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.05 |
| Al | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.04 | 0.01 | 0.01 | 0.02 | 0.03 |
| Y | 1.87 | 1.21 | 2.84 | 1.84 | 0.04 | 0.70 | 0.75 | 0.63 | 0.74 | 0.70 |
| La | 0.07 | 0.15 | 0.02 | 0.12 | 1.02 | 0.39 | 0.70 | 0.37 | 0.37 | 0.36 |
| Ce3+ | 0.18 | 0.46 | 0.07 | 0.31 | 1.68 | 0.72 | 0.94 | 0.50 | 0.55 | 0.67 |
| Pr | 0.02 | 0.08 | 0.01 | 0.03 | 0.13 | 0.07 | 0.06 | 0.03 | 0.04 | 0.06 |
| Nd | 0.07 | 0.33 | 0.04 | 0.16 | 0.48 | 0.27 | 0.21 | 0.11 | 0.12 | 0.22 |
| Sm | 0.02 | 0.10 | 0.04 | 0.06 | 0.04 | 0.05 | 0.04 | 0.02 | 0.03 | 0.05 |
| Gd | 0.04 | 0.14 | 0.08 | 0.15 | 0.17 | 0.06 | 0.06 | 0.03 | 0.02 | 0.06 |
| Tb | 0.01 | 0.01 | 0.02 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Dy | 0.12 | 0.09 | 0.24 | 0.13 | 0.01 | 0.07 | 0.07 | 0.03 | 0.04 | 0.06 |
| Ho | 0.03 | 0.03 | 0.06 | 0.00 | 0.00 | 0.01 | 0.01 | 0.00 | 0.02 | 0.00 |
| Er | 0.12 | 0.00 | 0.22 | 0.00 | 0.00 | 0.05 | 0.06 | 0.03 | 0.03 | 0.04 |
| Tm | 0.02 | 0.00 | 0.03 | 0.00 | 0.00 | 0.00 | 0.01 | 0.01 | 0.01 | 0.01 |
| Yb | 0.08 | 0.04 | 0.15 | 0.09 | 0.00 | 0.05 | 0.07 | 0.04 | 0.04 | 0.05 |
| Lu | 0.01 | 0.00 | 0.01 | 0.02 | 0.00 | 0.01 | 0.01 | 0.00 | 0.00 | 0.01 |
| Fe2+ | 0.07 | 0.04 | 0.09 | 0.05 | 0.41 | 0.00 | 0.02 | 0.00 | 0.01 | 0.01 |
| Mn2+ | 0.18 | 0.12 | 0.06 | 0.03 | 0.01 | 0.01 | 0.08 | 0.00 | 0.00 | 0.02 |
| Ca | 2.03 | 2.12 | 0.58 | 1.92 | 0.84 | 2.33 | 1.81 | 3.18 | 2.94 | 2.49 |
| Sr | 0.02 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Ba | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Pb | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 |
| Na | 0.04 | 0.00 | 0.43 | 0.04 | 0.00 | 0.00 | 0.05 | 0.02 | 0.02 | 0.04 |
| K | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 |
| Sum M | 5.01 | 4.96 | 5.00 | 4.98 | 4.90 | 4.94 | 4.98 | 5.02 | 4.98 | 5.00 |
| F | 0.76 | 0.51 | 0.47 | 0.55 | 0.54 | 0.71 | 0.62 | 0.44 | 0.50 | |
| Cl | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | |
| OH | 0.13 | 0.16 | 0.12 | 0.31 | 0.07 | 0.38 | 0.52 | 0.49 | ||
| O | 0.11 | 0.33 | 0.41 | 0.45 | 0.15 | 0.22 | 0.04 | |||
| Sum X | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zozulya, D.; Lyalina, L.; Macdonald, R.; Bagiński, B.; Savchenko, Y.; Jokubauskas, P. Britholite Group Minerals from REE-Rich Lithologies of Keivy Alkali Granite—Nepheline Syenite Complex, Kola Peninsula, NW Russia. Minerals 2019, 9, 732. https://doi.org/10.3390/min9120732
Zozulya D, Lyalina L, Macdonald R, Bagiński B, Savchenko Y, Jokubauskas P. Britholite Group Minerals from REE-Rich Lithologies of Keivy Alkali Granite—Nepheline Syenite Complex, Kola Peninsula, NW Russia. Minerals. 2019; 9(12):732. https://doi.org/10.3390/min9120732
Chicago/Turabian StyleZozulya, Dmitry, Lyudmila Lyalina, Ray Macdonald, Bogusław Bagiński, Yevgeny Savchenko, and Petras Jokubauskas. 2019. "Britholite Group Minerals from REE-Rich Lithologies of Keivy Alkali Granite—Nepheline Syenite Complex, Kola Peninsula, NW Russia" Minerals 9, no. 12: 732. https://doi.org/10.3390/min9120732
APA StyleZozulya, D., Lyalina, L., Macdonald, R., Bagiński, B., Savchenko, Y., & Jokubauskas, P. (2019). Britholite Group Minerals from REE-Rich Lithologies of Keivy Alkali Granite—Nepheline Syenite Complex, Kola Peninsula, NW Russia. Minerals, 9(12), 732. https://doi.org/10.3390/min9120732






