Trace Elements in Magnetite from the Pagoni Rachi Porphyry Prospect, NE Greece: Implications for Ore Genesis and Exploration
Abstract
1. Introduction
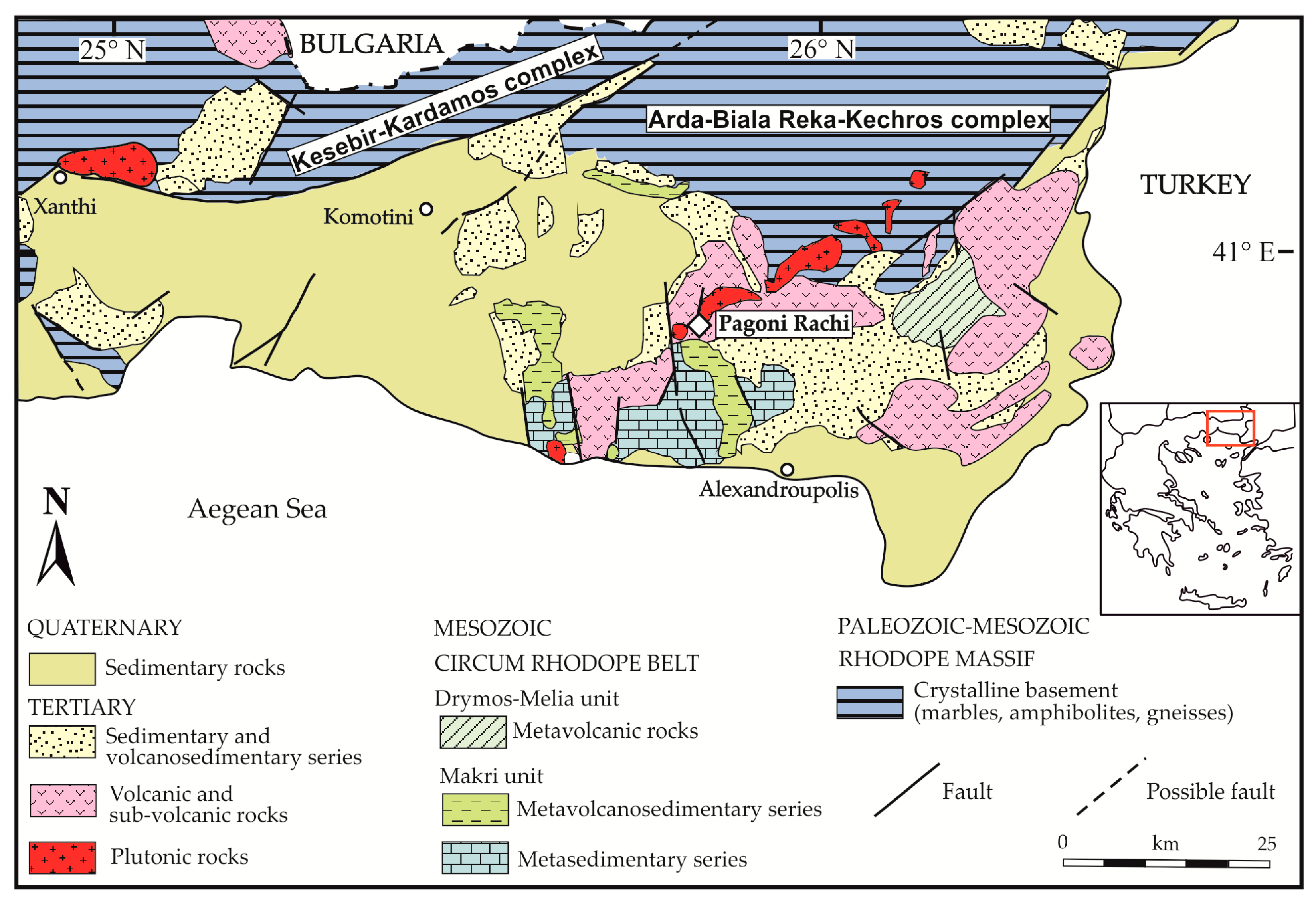
2. Geological Setting
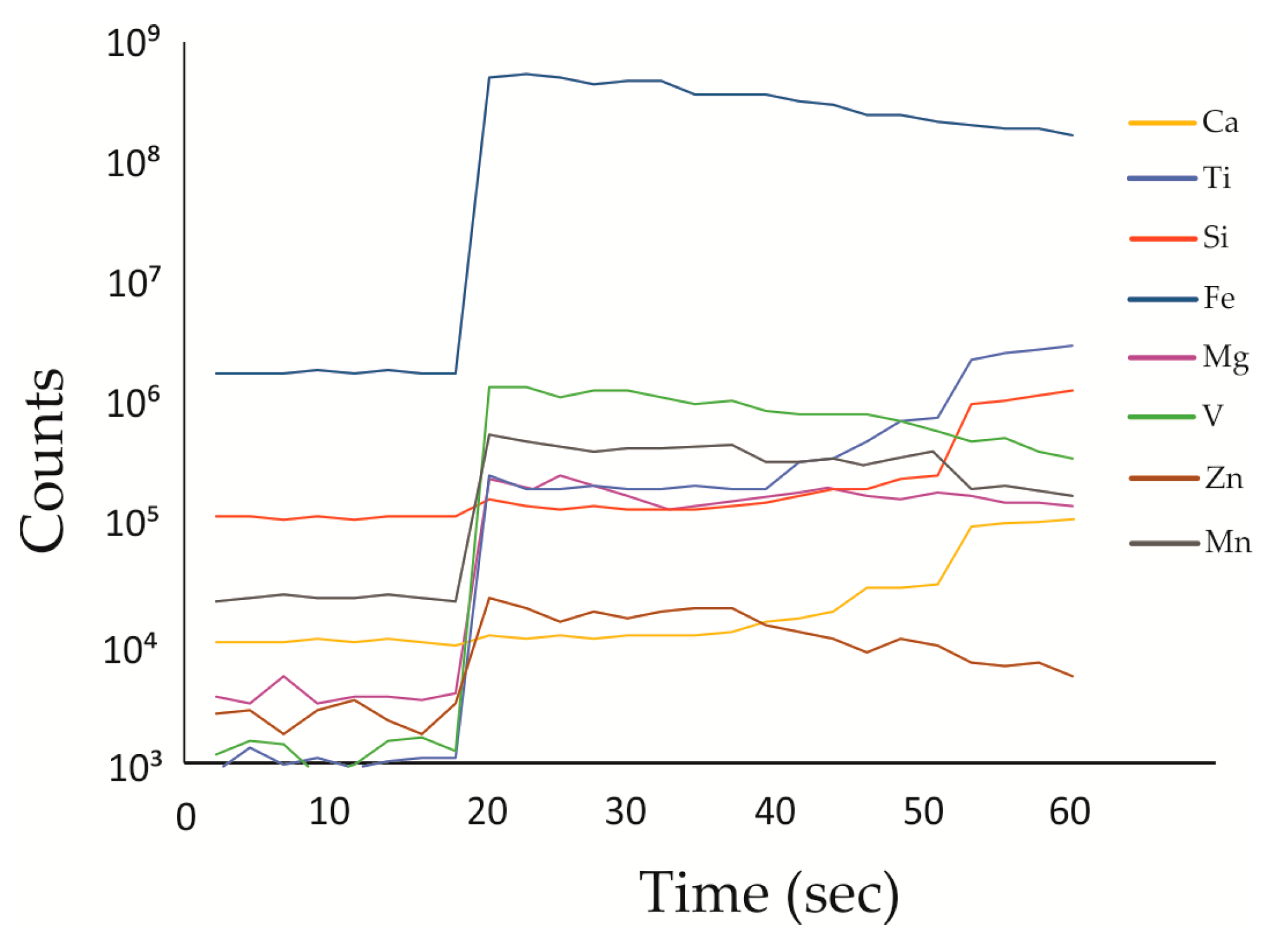
3. Materials and Methods
4. Results
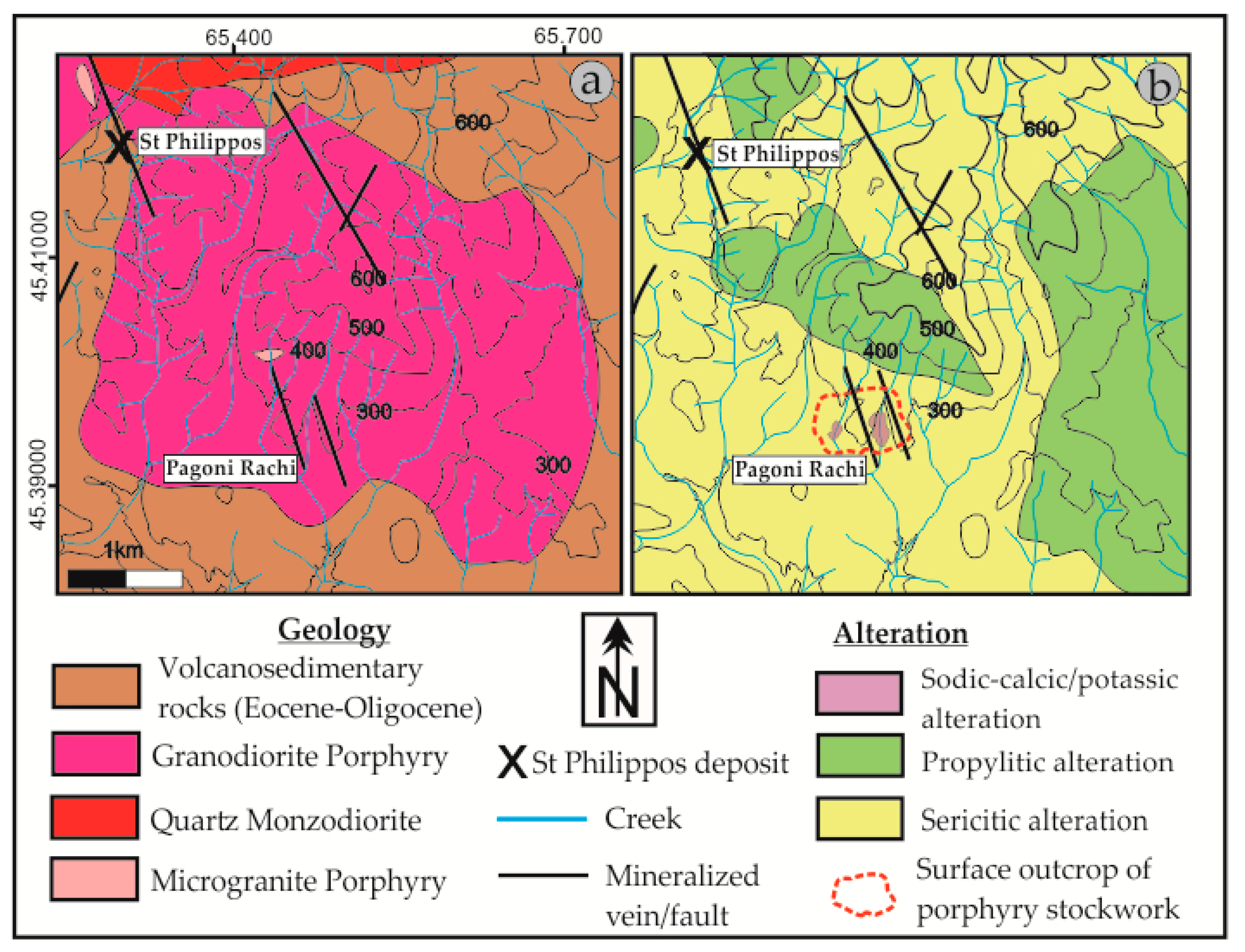
4.1. Geology and Description of the Pagoni Rachi Prospect
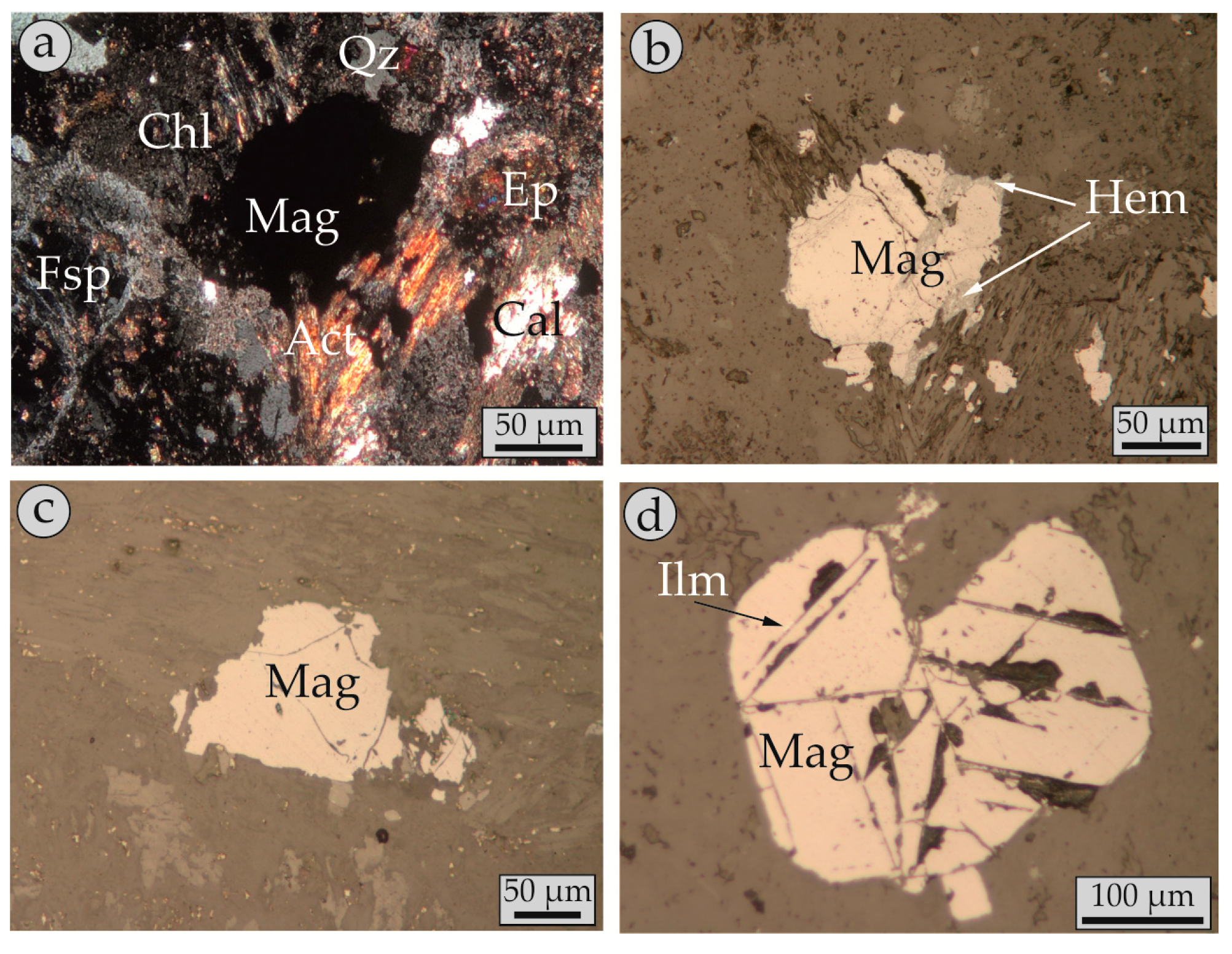
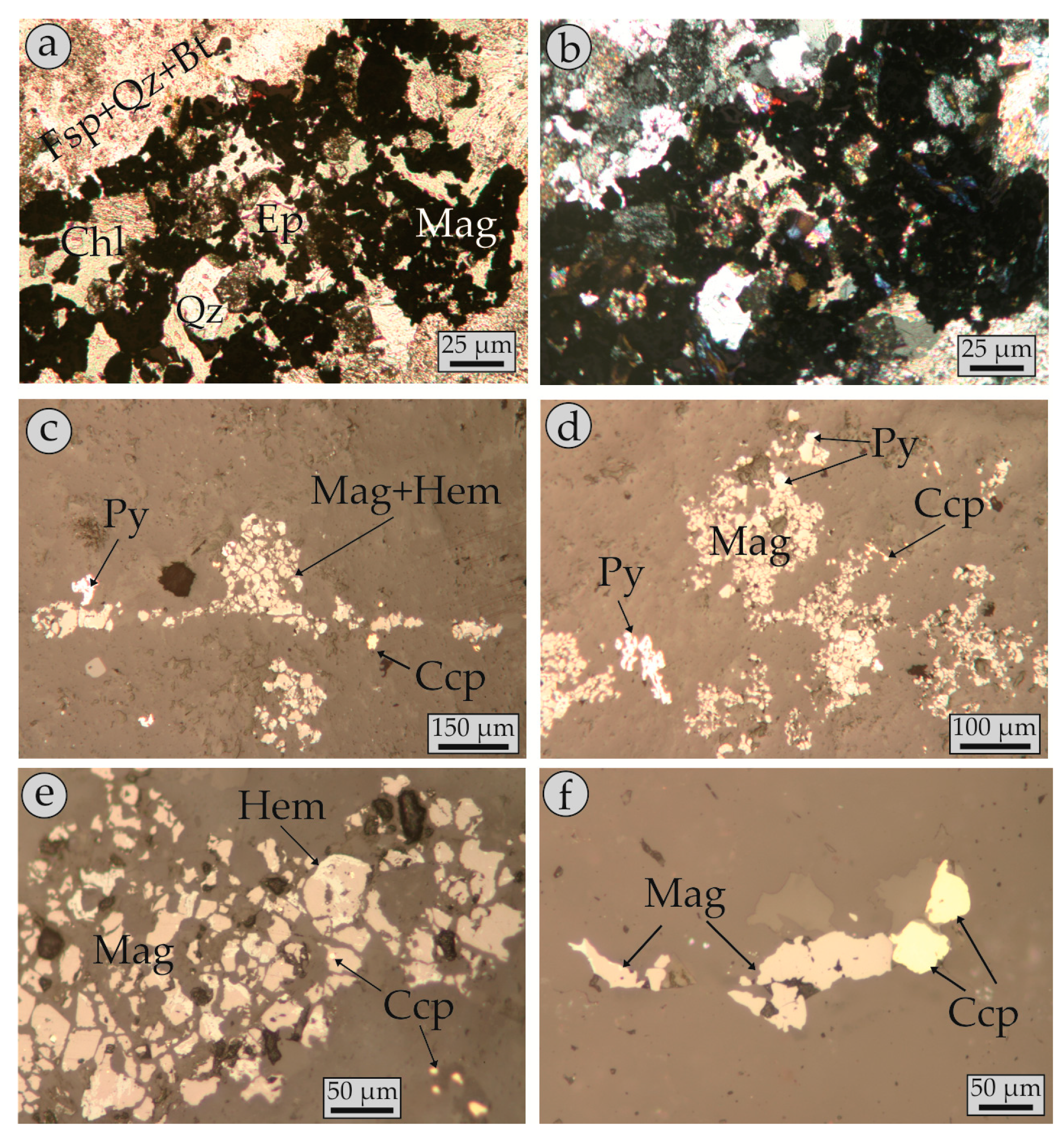
4.2. Modes of Magnetite Occurrence (Petrography)
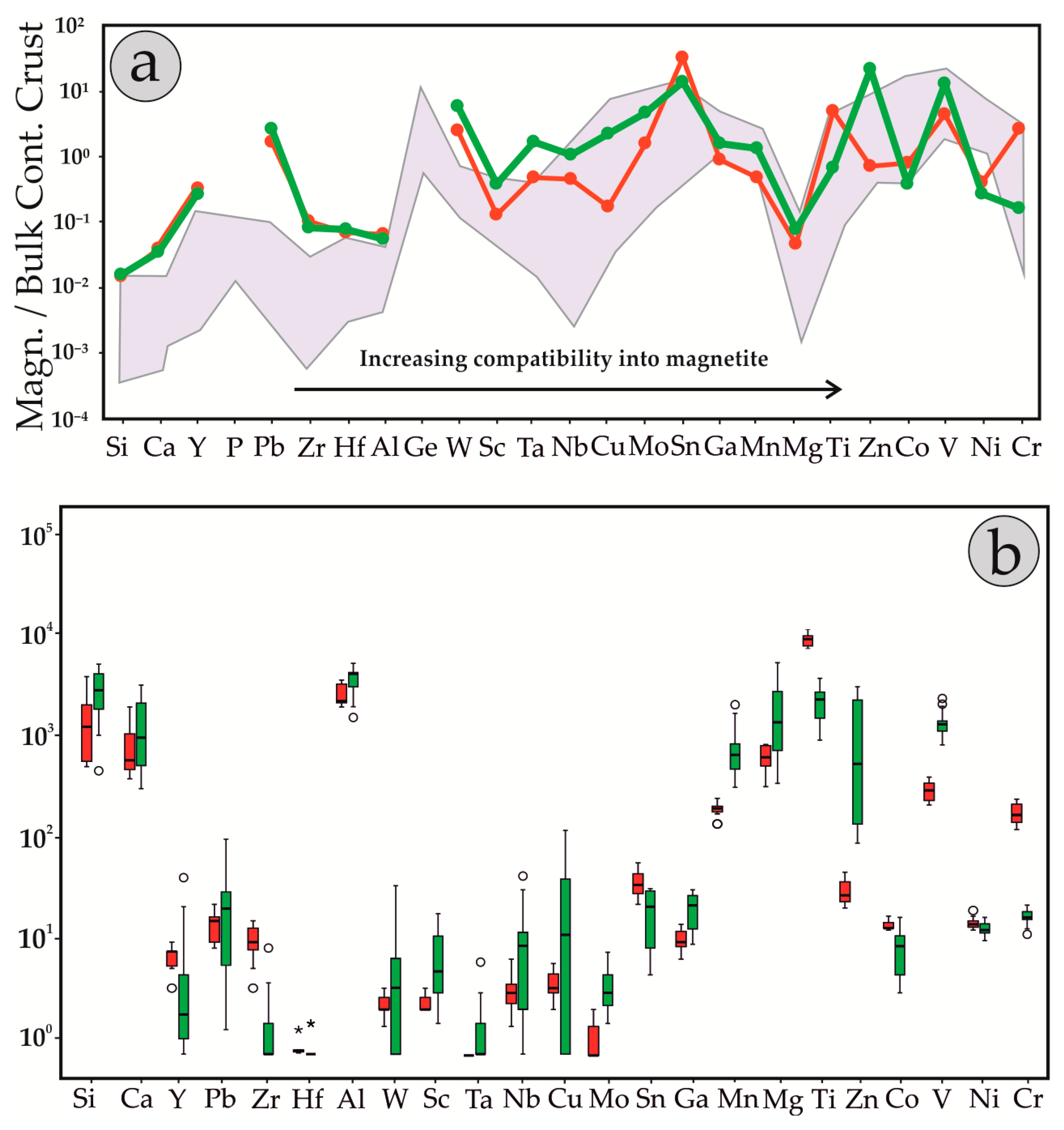
4.3. Chemical Composition of Magnetite
5. Discussion
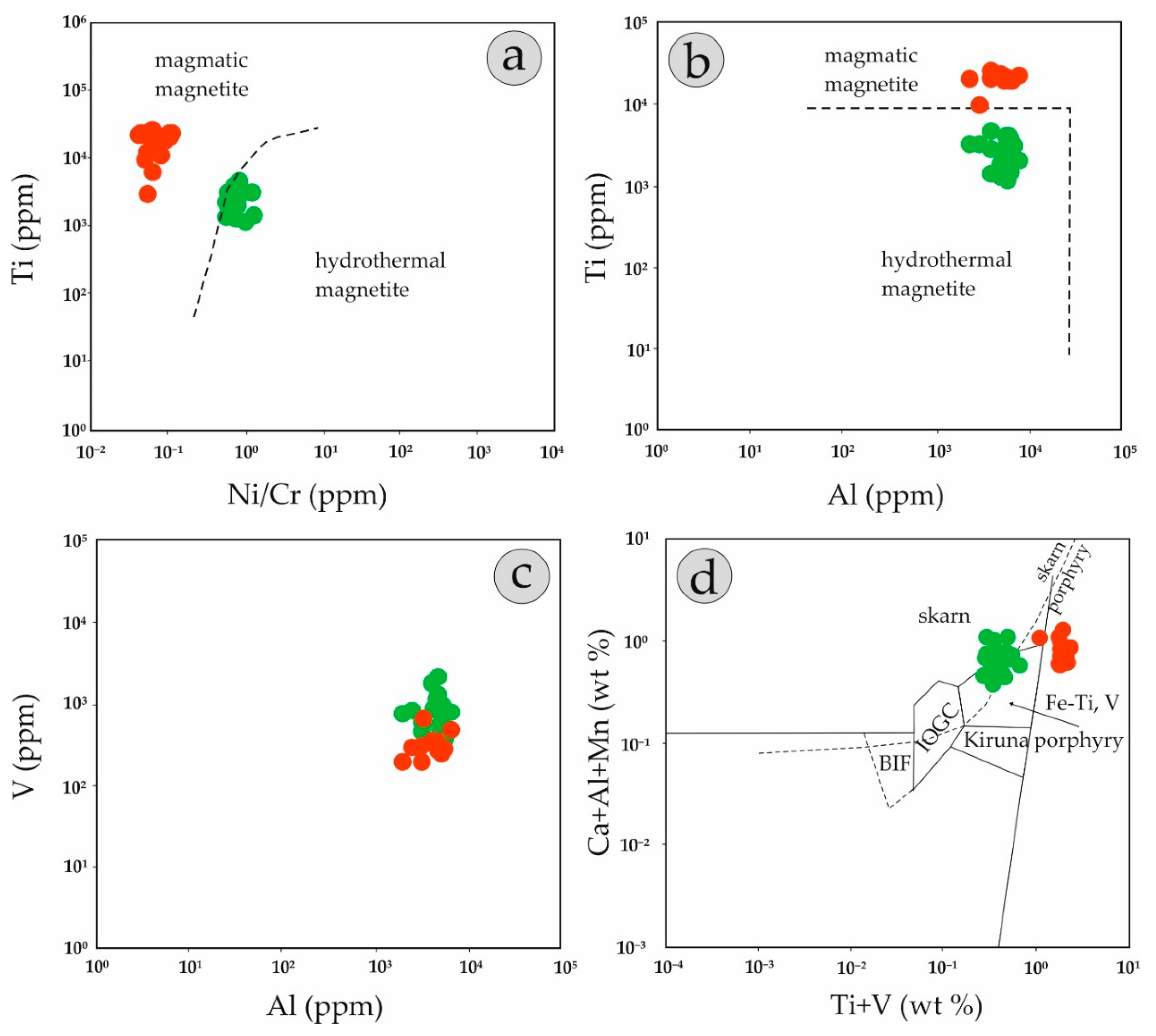
5.1. Fingerprinting Magmatic and Hydrothermal Processes
5.2. Genetic Considerations—Implications for Exploration
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dare, S.A.S.; Barnes, S.-J.; Beaudoin, G.; Méric, J.; Boutroy, E.; Potvin-Doucet, C. Trace elements in magnetite as petrogenetic indicators. Minim. Depos. 2014, 49, 785–796. [Google Scholar] [CrossRef]
- Sack, R.O.; Ghiorso, M.S. Chromian spinels as petrogenetic indicators: Thermodynamics and petrological applications. Am. Mineral. 1991, 76, 827–847. [Google Scholar]
- Roeder, P.L. Chromite: From the fiery rain of chondrules to the Kilauea Iki lava lake. Can. Mineral. 1994, 32, 729–746. [Google Scholar]
- Barnes, S.J.; Roeder, P.L. The range of spinel compositions in terrestrial mafic and ultramafic rocks. J. Pet. 2001, 42, 2279–2302. [Google Scholar] [CrossRef]
- Nadoll, P.; Mauk, J.L.; Hayes, T.S.; Koenig, A.E.; Box, S.E. Geochemistry of magnetite from hydrothermal ore deposits and host rocks of the Mesoproterozoic Belt Supergroup, United States. Econ. Geol. 2012, 107, 1275–1292. [Google Scholar] [CrossRef]
- Chen, W.; Ying, Y.C.; Bai, T.; Zhang, J.J.; Jiang, S.Y.; Zhao, K.D.; Shin, D.; Kynicky, J. In situ major and trace element analysis of magnetite from carbonatite-related complexes: Implications for petrogenesis and ore genesis. Ore Geol. Rev. 2019, 107, 30–40. [Google Scholar] [CrossRef]
- Dupuis, C.; Beaudoin, G. Discriminant diagrams for iron oxide trace element fingerprinting of mineral deposit types. Minim. Depos. 2011, 46, 319–335. [Google Scholar] [CrossRef]
- Liu, Y.S.; Hu, Z.C.; Gao, S.; Günther, D.; Xu, J.; Gao, C.G. In situ analysis of major and trace elements of anhydrous minerals by LA-ICP-MS without applying an internal standard. Chem. Geol. 2008, 257, 34–43. [Google Scholar] [CrossRef]
- Wijbrans, C.H.; Klemme, S.; Berndt, J.; Vollmer, C. Experimental determination of trace element partition coefficients between spinel and silicate melt: The influence of chemical composition and oxygen fugacity. Contrib Mineral. Petrol. 2015, 69, 45–77. [Google Scholar] [CrossRef]
- Müller, B.; Axelsson, M.D.; Ohlander, B. Trace elements in magnetite from Kiruna, northern Sweden, as determined by LA-ICP-MS. GFF 2003, 125, 1–5. [Google Scholar] [CrossRef]
- Carew, M.J. Controls on Cu–Au Mineralisation and Fe-Oxide Metasomatism in the Eastern Fold Belt, NW Queensland, Australia. Ph.D. Thesis, James Cook University, Townsville, Australia, 2004. [Google Scholar]
- Dare, S.A.S.; Barnes, S.-J.; Beaudoin, G. Variation in trace element content of magnetite crystallized from a fractionating sulfide liquid, Sudbury, Canada: Implications for provenance discrimination. Geochim. Cosmochim. Acta 2012, 88, 27–50. [Google Scholar] [CrossRef]
- Nadoll, P.; Angerer, T.; Mauk, J.L.; French, D.; Walshe, J. The chemistry of hydrothermal magnetite: A review. Ore Geol. Rev. 2014, 61, 1–32. [Google Scholar] [CrossRef]
- Nadoll, P.; Mauk, J.L.; Leveille, R.A.; Koenig, A.E. Geochemistry of magnetite from porphyry Cu and skarn deposits in the southwestern United States. Miner. Depos. 2015, 50, 493–515. [Google Scholar] [CrossRef]
- Canil, D.; Grondahl, C.; Lacourse, T.; Pisiak, L.K. Trace elements in magnetitefrom porphyry Cu-Mo-Au deposits in British Columbia, Canada. Ore Geol. Rev. 2016, 72, 1116–1128. [Google Scholar] [CrossRef]
- Huang, X.W.; Sappin, A.A.; Boutroy, E.; Beaudoin, G.; Makvandi, S. Trace Element Composition of Igneous and Hydrothermal Magnetite from Porphyry Deposits: Relationship to Deposit Subtypes and Magmatic Affinity. Econ. Geol. 2019, 114, 917–952. [Google Scholar] [CrossRef]
- Wen, G.; Li, J.W.; Hofstra, A.H.; Koenig, A.E.; Lowers, H.A.; Adams, D. Hydrothermal reequilibration of igneous magnetite in altered granitic plutons and its implications for magnetite classification schemes: Insights from the Handan-Xingtai iron district, North China Craton Guang. Geochim. Cosmochim. Acta 2017, 213, 255–270. [Google Scholar] [CrossRef]
- Pisiak, L.K.; Canil, D.; Lacourse, T.; Plouffe, A.; Ferbey, T. Magnetite as an indicator mineral in the exploration of porphyry deposits: A case study in till near the Mount Polley Cu-Au deposit, British Columbia, Canada. Econ. Geol. 2017, 112, 919–940. [Google Scholar] [CrossRef]
- Averill, S.A. The application of heavy indicator mineralogy in mineral exploration with emphasis on base metal indicators in glaciated metamorphic and plutonic terrains. In Drift Exploration in Glaciated Terrane; McClenaghan, M.B., Bobrowsky, P.T., Hall, G.E.M., Cook, S.J., Eds.; Geological Society of London Special Publications, Burlington House: Picadilly, Londn, UK, 2001; Volume 185, pp. 69–81. [Google Scholar] [CrossRef]
- Kelley, K.D.; Eppinger, R.G.; Lang, J.; Smith, S.M.; Fey, D.L. Porphyry copper indicator minerals (PCIMs) in glacial till samples as an exploration tool: Example from the giant Pebble porphyry Cu-Au-Mo deposit. Geochem. Explor. Environ. Anal. 2011, 11, 321–334. [Google Scholar] [CrossRef]
- Celis, M.A.; Bouzari, F.; Bissig, T.; Hart, C.J.R.; Ferbey, T. Petrographic Characteristics of Porphyry Indicator Minerals from Alkalic Porphyry Copper-Gold Deposits in South-Central British Columbia (NTS 092, 093): Geoscience BC, Summary of Activities 2013; Report 2014-1; Geoscience BC: Vancouver, BC, Canada; pp. 53–62.
- Hashmi, S.; Ward, B.C.; Plouffe, A.; Leybourne, M.I.; Ferbey, T. Geochemical and mineralogical dispersal in till from the Mount Polley Cu-Au porphyry deposit, central British Columbia, Canada. Geochem. Explor. Environ. Anal. 2015, 15, 234–249. [Google Scholar] [CrossRef]
- Ricou, L.E.; Burg, J.P.; Godfriaux, I.; Ivanov, Z. Rhodope and Vardari the metamorphic and the olistostromic paired belts related to the Cretaceous subduction under Europe. Geod. Acta 1998, 11, 285–309. [Google Scholar] [CrossRef]
- Mountrakis, D. Tertiary and Quaternary tectonics of Greece. In Postcollisional Tectonics and Magmatism in the Mediterranean Region and Asia: Geological Society of America Special Paper; Dilek, Y., Pavlides, S., Eds.; Geological Society of America: Boulder, CO, USA, 2006; Volume 409, pp. 125–136. [Google Scholar] [CrossRef]
- Jolivet, L.; Brunn, J.P. Cenozoic geodynamic evolution of the Aegean Region. Int. J. Earth. Sci. 2010, 99, 109–138. [Google Scholar] [CrossRef]
- Ring, U.; Glodny, J.; Will, T.; Thomson, S. The Hellenic subduction system: High pressure metamorphism, exhumation, normal faulting, and large-scale extension. Ann. Rev. Earth Planet. Sci. 2010, 38, 45–76. [Google Scholar] [CrossRef]
- Jolivet, L.; Faccenna, C.; Huet, B.; Labrousse, L.; Le Pourhiet, L.; Lacombe, O.; Lecomte, E.; Burov, E.; Denèle, Y.; Brun, J.-P.; et al. Aegean tectonics: Strain localization, slab tearing and trench retreat. Tectonophysics 2013, 597, 1–33. [Google Scholar] [CrossRef]
- Kydonakis, K.; Brunn, J.-P.; Sokoutis, D. North Aegean core complexes, the gravity spreading of a thrust wedge. J. Geophys. Res. Solid Earth 2015, 120, 1601. [Google Scholar] [CrossRef]
- Kydonakis, K.; Moulas, E.; Chatzitheodoridis, E.; Brunn, J.-P.; Kostopoulos, D. First-report on Mesozoic eclogite-facies metamorphism preceding Barrovian overprint from the western Rhodope (Chalkidiki, northern Greece). Lithos 2015, 220–223, 147–163. [Google Scholar] [CrossRef]
- Voudouris, P.; Tarkian, M.; Arikas, K. Mineralogy of telluride-bearing epithermal ores in Kassiteres-Sappes area, western Thrace, Greece. Minim. Petrol 2006, 87, 31–52. [Google Scholar] [CrossRef]
- Mavrogonatos, C.; Voudouris, P.; Spry, P.G.; Melfos, V.; Klemme, S.; Berndt, J.; Baker, T.; Moritz, R.; Bissig, T.; Monecke, T.; et al. Mineralogical Study of the Advanced Argillic Alteration Zone at the Konos Hill Mo–Cu–Re–Au Porphyry Prospect, NE Greece. Minerals 2018, 8, 479. [Google Scholar] [CrossRef]
- Brun, J.P.; Sokoutis, D. Core complex segmentation in North Aegean, a dynamic view. Tectonics 2018, 37, 1797–1830. [Google Scholar] [CrossRef]
- Turpaud, P.; Reischmann, T. Characterisation of igneous terranes by zircon dating: Implications for UHP occurrences and suture identification in the Central Rhodope, northern Greece. Int. J. Earth Sci. 2010, 99, 567–591. [Google Scholar] [CrossRef]
- Meinhold, G.; Kostopoulos, D.K. The Circum-Rhodope Belt, northern Greece: Age, provenance, and tectonic setting. Tectonophysics 2013, 595–596, 55–68. [Google Scholar] [CrossRef]
- Bonev, N.; Marchev, P.; Moritz, R.; Collings, D. Jurassic subduction zone tectonics of the Rhodope Massif in the Thrace region (NE Greece) as revealed by new U-Pb and 40Ar/39Ar geochronology of the Evros ophiolite and high-grade basement rocks. Gondwana Res. 2015, 27, 760–775. [Google Scholar] [CrossRef]
- Wuthrich, E. Low Temperature Thermochronology of the North Aegean Rhodope Massif. Ph.D. Thesis, Swiss Federal Institute of Technology, Zurich, Switzerland, 2009. [Google Scholar]
- Kilias, A.; Falalakis, G.; Sfeikos, A.; Papadimitriou, E.; Vamvaka, A.; Gkarlaouni, C. The Thrace basin in the Rhodope province of NE Greece—A Tertiary supra-detachment basin and its geodynamic implications. Tectonophysics 2013, 595–596, 90–105. [Google Scholar] [CrossRef]
- Del Moro, A.; Innocenti, F.; Kyriakopoulos, C.; Manetti, P.; Papadopoulos, P. Tertiary granitoids from Thrace (Northern Greece): Sr isotopic and petrochemical data. Neues Jahrb. Mineral. Abh. 1988, 159, 113–115. [Google Scholar]
- Marchev, P.; Kaiser-Rohrmeier, B.; Heinrich, C.; Ovtcharova, M.; von Quadt, A.; Raicheva, R. Hydrothermal ore deposits related to post-orogenic extensional magmatism and core complex formation: The Rhodope Massif of Bulgaria and Greece. Ore Geol. Rev. 2005, 27, 53–89. [Google Scholar] [CrossRef]
- Ersoy, E.Y.; Palmer, M.R. Eocene-Quaternary magmatic activity in the Aegean: Implications for mantle metasomatism and magma genesis in an evolving orogeny. Lithos 2013, 180–181, 5–24. [Google Scholar] [CrossRef]
- Pe-Piper, G.; Piper, D.J.W. The igneous Rocks of Greece: The anatomy of an orogen. In Beiträge der Regionalen Geologie der Erde; Gebrüder Borntraeger: Berlin, Germany, 2002; p. 573. [Google Scholar]
- Moritz, R.; Márton, I.; Ortelli, M.; Marchev, P.; Voudouris, P.; Bonev, N.; Spikings, R.; Cosca, M. A review of age constraints of epithermal precious and base metal deposits of the Tertiary Eastern Rhodopes: Coincidence with Late Eocene-Early Oligocene tectonic plate reorganization along the Tethys. In XIX Congress of the Carpathian Balkan Geological Association; Christofides, G., Kantiranis, D., Kostopoulos, D.S., Chatzipetros, A., Eds.; Scientific Annals of the School of Geology A.U.Th. 100: Thessaloniki, Greece, 2010; pp. 351–358. [Google Scholar]
- Menant, A.; Jolivet, L.; Vrielynck, B. Kinematic reconstruction and magmatic evolution illuminating crystal and mantle dynamics of the eastern Mediterranean region since the late Cretaceous. Tectonophysics 2016, 675, 103–140. [Google Scholar] [CrossRef]
- Melfos, V.; Voudouris, P. Cenozoic metallogeny of Greece and potential for precious, critical and rare metals exploration. Ore Geol. Rev. 2017, 59, 1030–1057. [Google Scholar] [CrossRef]
- Voudouris, P.; Mavrogonatos, C.; Spry, P.G.; Baker, T.; Melfos, V.; Klemd, R.; Haase, K.; Repstock, A.; Djiba, A.; Bismayer, U.; et al. Porphyry and epithermal deposits in Greece: An overview, new discoveries, and mineralogical constraints on their genesis. Ore Geol. Rev. 2019, 107, 654–691. [Google Scholar] [CrossRef]
- Voudouris, P. Mineralogical, Geochemical and Fluid Inclusion Studies on Epithermal Vein Type Gold/Silver Mineralizations at Kassiteres/Sapes, (NE- Greece). Ph.D. Thesis, University of Hamburg, Hamburg, Germany, 1993. [Google Scholar]
- Ortelli, M.; Moritz, R.; Voudouris, P.; Spangenberg, J. Tertiary porphyry and epithermal association of the Sapes-Kassiteres district, Eastern Rhodopes, Greece. In Proceedings of the 10th Biennial SGA Meeting, Townsville, Australia, 17–20 August 2009; pp. 536–538. [Google Scholar]
- Voudouris, P. Hydrothermal corundum, topaz, diaspore and alunite supergroup minerals in the advanced argillic alteration lithocap of the Kassiteres-Sapes porphyry-epithermal system, western Thrace, Greece. Neues Jahrb. Mineral. 2014, 191, 117–136. [Google Scholar] [CrossRef]
- Mavrogonatos, C.; Voudouris, P.; Spry, P.G.; Melfos, V.; Klemme, S.; Berndt, J.; Moritz, R.; Kanellopoulos, C. First zunyite-bearing lithocap in Greece: The case of Konos Hill Mo-Re-Cu-Au porphyry system. In Proceedings of the 1st International Electronic Conference on Mineral Science, At sciforum, Basel, Switzerland, 16–31 July 2018; Volume 1. [Google Scholar] [CrossRef]
- Voudouris, P.; Melfos, V.; Spry, P.G.; Bindi, L.; Kartal, T.; Arikas, K.; Moritz, R.; Ortelli, M. Rhenium-rich molybdenite and rheniite (ReS2) in the Pagoni Rachi-Kirki Mo-Cu-Te-Ag-Au deposit, Northern Greece. implications for the rhenium geochemistry of porphyry style Cu-Mo and Mo mineralization. Can. Miner. 2009, 47, 1013–1036. [Google Scholar] [CrossRef]
- Voudouris, P.; Melfos, V.; Spry, P.G.; Kartal, T.; Schleicher, H.; Moritz, R.; Ortelli, M. The Pagoni Rachi/Kirki Cu-Mo-Re-Au-Ag-Te deposit, northern Greece: Mineralogical and fluid inclusion constraints on the evolution of a telescoped porphyry-epithermal system. Can. Miner. 2013, 51, 411–442. [Google Scholar] [CrossRef]
- Mavrogonatos, C.; Voudouris, P.; Spry, P.G.; Melfos, V.; Klemme, S.; Berndt, J.; Periferakis, A. Biotite Chemistry from Porphyry-Style Mineralization in Western Thrace, Greece. In Proceedings of the 8th Geochemistry symposium, Antalya, Turkey, 2–6 May 2018; Volume 193. [Google Scholar]
- Melfos, V.; Vavelidis, M.; Christofides, G.; Seidel, E. Origin and evolution of the Tertiary Maronia porphyry copper-molybdenum deposit, Thrace, Greece. Min. Depos. 2002, 37, 648–668. [Google Scholar] [CrossRef]
- Galanopoulos, E.; Voudouris, P.; Mavrogonatos, C.; Spry, P.G.; Hart, C.; Melfos, V.; Zaccarini, F.; Alfieris, D.A. New porphyry Mo mineralization at Aisymi-Leptokarya, South-Eastern Rhodope, North-East Greece: Geological and mineralogical constraints. Geosciences 2018, 8, 435. [Google Scholar] [CrossRef]
- Kilias, S.P.; Naden, J.; Paktsevanoglou, M.; Giampouras, M.; Stavropoulou, A.; Apeiranthiti, D.; Mitsis, I.; Koutles, T.; Michael, K.; Christidis, C. Multistage alteration, mineralization and ore–forming fluid properties at the Viper (Sappes) Au–Cu–Ag–Te ore body, W. Thrace, Greece. Bull. Geol. Soc. Greece 2013, 47, 1635–1644. [Google Scholar] [CrossRef]
- Voudouris, P.; Melfos, V.; Spry, P.G.; Moritz, R.; Papavasiliou, C.; Falalakis, G. Mineralogy and geochemical environment of formation of the Perama Hill high sulfidation epithermal Au-Ag-Te-Se deposit, Petrota graben, NE Greece. Min. Pet. 2011, 103, 79–100. [Google Scholar] [CrossRef]
- Dimou, Ε. A correlative mineralogical study of Achla Tarla and St. Philippos mineralization, Kirki area (NE Greece). Bull. Geol. Soc. Greece 1993, 28, 37–54. (In Greek) [Google Scholar]
- Repstock, A.; Voudouris, P.; Kolitsch, U. New occurrences of watanabeite, colusite, “arsenosulvanite” and Cu-excess tetrahedrite-tennantite at the Pefka high-sulfidation epithermal deposit, northeastern Greece. N. Jahrb. Min. 2015, 192, 135–149. [Google Scholar] [CrossRef]
- Pouchou, J.L.; Pichoir, F. Quantitative analysis of homogeneous or stratified microvolumes applying the model “PAP”. In Electron Probe Quantitation; Heinrich, K.F.J., Newbury, D.E., Eds.; Plenum Press: New York, NY, USA, 1991; pp. 31–75. [Google Scholar]
- Milani, L.; Bolhar, R.; Cawthorn, R.G.; Frei, D. In situ LA-ICP-MS and EPMA trace element characterization of Fe-Ti oxides from the phoscorite-carbonatite association at Phalaborwa, South Africa. Min. Depos. 2017, 52, 747–768. [Google Scholar] [CrossRef]
- van Achterbergh, E.; Ryan, C.G.; Jackson, S.E.; Griffin, W.L. Data reduction software for LA-ICP-MS: Appendix. In Laser Ablation-ICP Mass Spectrometry in the Earth Sciences: Principles and Applications; Sylvester, P.J., Ed.; Mineralogical Association Canada (MAC) Short Course Series; MAC: Ottawa, ON, Canada, 2001; Volume 29, pp. 239–243. [Google Scholar]
- Griffin, W.L.; Powell, W.J.; Pearson, N.J.; O’Reilly, S.Y. GLITTER: Data reduction software for laser ablation ICP-MS. In Laser Ablation ICP-MS in the Earth Sciences: Current Practices and Outstanding Issues; Sylvester, P., Ed.; Short Course Series 40; Mineralogical Association of Canada: Quebec City, QC, Canada, 2008; pp. 308–311. [Google Scholar]
- Jochum, K.P.; Weis, U.; Stoll, B.; Kuzmin, D.; Yang, Q.; Raczek, I.; Jacob, D.E.; Stracke, A.; Birbaum, K.; Frick, D.A.; et al. Determinationof reference values for NIST SRM 610–617 glasses following ISO guidelines. Geostand. Geoanal. Res. 2011, 35, 397–429. [Google Scholar] [CrossRef]
- Arikas, K. The porphyry Mo occurrence at Panoni Rachi (NE Kirki) and the mineralogy of hydrothermally altered subvolcanic rocks at Kirki–Esymi area. Bull. Geol. Soc. Greece 1991, 25, 259–274. (In Greek) [Google Scholar]
- Perkins, R.; Copper, F.J.; Condon, D.J.; Tattitsch, B.; Naden, J. Post-collisional Cenozoic extension in the northern Aegean: The high-K to shoshonitic intrusive rocks of the Maronia Magmatic Corridor, northeastern Greece. Lithosphere 2018, 10, 582–601. [Google Scholar] [CrossRef]
- McQueen, K.G.; Cross, A.J. Magnetite as a geochemical sampling medium: Application to skarn deposits. In The State of the Regolith; Eggleton, R.A., Ed.; Geological Society of Australia: Brisbane, Australia, 1998; pp. 194–199. [Google Scholar]
- Deditius, A.P.; Reich, M.; Simon, A.C.; Suvorova, A.; Knipping, I.; Roberts, M.P.; Rubanov, S.; Dodd, A.; Saunders, M. Nanogeochemistry of hydrothermal magnetite. Contr. Miner. Pet. 2018, 173. [Google Scholar] [CrossRef]
- Frost, B.R.; Lindsley, D.H. Equilibria among Fe-Ti oxides, pyroxenes, olivine, and quartz: Part II. Application. Am. Miner. 1992, 77, 1004–1020. [Google Scholar]
- Toplis, M.J.; Corgne, A. An experimental study of element partitioning between magnetite, clinopyroxene and iron-bearing silicate liquids with particular emphasis on vanadium. Contrib. Min. Pet. 2002, 144, 22–37. [Google Scholar] [CrossRef]
- Sievwright, R.H.; Wilkinson, J.J.; O’Neill, H.S.C.; Berry, A.J. Thermodynamic controls on element partitioning between titanomagnetite and andesitic-dacitic silicate melts. Contrib. Min. Pet. 2017, 172, 1–33. [Google Scholar] [CrossRef]
- Sossi, P.A.; Prytulak, J.; O’Neill, H.S.C. Experimental calibration of vanadium partitioning and stable isotope fractionation between hydrous granitic melt and magnetite at 800 °C and 0.5 GPa. Contrib. Miner. Pet. 2018, 173, 27. [Google Scholar] [CrossRef]
- Sillitoe, R.H. Characteristics and controls of the largest porphyry copper-gold and epithermal gold deposits in the circum-Pacific region. Aust. J. Earth Sci. 1997, 44, 373–388. [Google Scholar] [CrossRef]
- Sinclair, W.D. Porphyry Deposits; Special Publication 5; Geological Association of Canada, Mineral Deposits Division: St. John’s, NL, Canada, 2007; pp. 223–243. [Google Scholar]
- Simon, A.C.; Pettke, T.; Candela, P.A.; Piccoli, P.M.; Heinrich, C.A. Magnetite solubility and iron transport in magmatic-hydrothermal environments. Geochim. Cosmochim. Acta 2004, 68, 4905–4914. [Google Scholar] [CrossRef]
- Oliver, N.H.; Cleverley, J.S.; Mark, G.; Pollard, P.J.; Fu, B.; Marshall, L.J.; Rubenach, M.J.; Williams, P.J.; Baker, T. Modeling the role of sodic alteration in the genesis of iron oxide-copper-gold deposits, Eastern Mount Isa block, Australia. Econ. Geol. 2004, 99, 1145–1176. [Google Scholar] [CrossRef]
- Nadoll, P.; Koenig, A.E. LA-ICP-MS of magnetite: Methods and reference materials. J. Anal. At. Spectrom. 2011, 26, 1872–1877. [Google Scholar] [CrossRef]
- Rudnick, R.L.; Gao, S. Composition of the continental crust. In Treatise on Geochemistry; Holland, H.D., Turekian, K.K., Eds.; The Crust Elsevier-Pergamon: Oxford, UK, 2003; Volume 3, pp. 1–64. [Google Scholar]
- Ciobanu, C.L.; Cook, N.J. Skarn textures and a case study: The Ocna de Fier-Dognecea orefield, Banat, Romania. Ore. Geol. Rev. 2004, 24, 315–370. [Google Scholar] [CrossRef]
- Einaudi, M.T.; Meinert, L.D.; Newberry, R.J. Skarn Deposits: 75th Anniversary Volume; Society of Economic Geologists: Littleton, CO, USA, 1981; pp. 317–391. [Google Scholar]
- Meinert, L.D.; Dipple, G.M.; Nicolescu, S. World Skarn Deposits: Economic Geology 100th Anniversary Volume; Society of Economic Geologists: Littleton, CO, USA, 2005; pp. 299–336. [Google Scholar]
- Pearce, J.A.; Cann, J. Tectonic setting of basic volcanic rocks determined using trace element analyses. Earth Plan. Sci. Lett. 1973, 19, 290–300. [Google Scholar] [CrossRef]
- Floyd, P.A.; Winchester, J.A. Identification and discrimination of altered and metamorphosed volcanic rocks using immobile elements. Chem. Geol. 1978, 21, 291–306. [Google Scholar] [CrossRef]
- Middelburg, J.J.; van der Weijden, C.H.; Woittiez, J.R.W. Chemical processes affecting the mobility of major, minor and trace elements during weathering of granitic rocks. Chem. Geol. 1988, 68, 253–273. [Google Scholar] [CrossRef]
- Hough, R.M.; Noble, R.R.P.; Hitchen, G.J.; Hart, R.; Reddy, S.M.; Saunders, M.; Clode, P.; Vaughan, D.; Lowe, J.; Anand, R.R.; et al. Naturally occurring gold nanoparticles and nanoplates. Geology 2008, 36, 571–574. [Google Scholar] [CrossRef]
- Whalen, J.B.; Anderson, R.G.; Struik, L.C.; Villeneuve, M.E. Gochemistry and Nd isotopes of the François Lake plutonic suite, Endako batholith: Host and progenitor to the Endako molybdenum camp, central British Columbia. Can. J. Earth Sci. 2001, 38, 603–618. [Google Scholar] [CrossRef]
- Yang, X.-M.; Lentz, D.R.; McCutcheon, S.R. Petrochemical evolution of subvolcanic granitoid intrusions within the Late Devonian Mount Pleasant caldera, southwestern New Brunswick, Canada: Comparison of Au versus Sn-W-Mo-polymetallic mineralization systems. Atl. Geol. 2003, 39, 97–121. [Google Scholar] [CrossRef]

| Sample | KM81 | KM68 | KM74 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Type | Magmatic (n = 15) | Hydrothermal (n = 29) | Hydrothermal (n = 30) | ||||||
| (wt %) | avg | min | max | avg | min | max | avg | min | max |
| MgO | 0.01 | bdl | 0.03 | 0.01 | bdl | 0.24 | 0.01 | bdl | 0.88 |
| CaO | 0.05 | 0.02 | 0.34 | 0.04 | bdl | 0.07 | 0.03 | bdl | 0.23 |
| MnO | 0.01 | bdl | 0.18 | 0.01 | bdl | 0.10 | 0.01 | bdl | 0.32 |
| FeOtot | 97.10 | 95.44 | 98.69 | 97.94 | 96.88 | 98.40 | 98.11 | 97.03 | 99.19 |
| TiO2 | 1.41 | 1.05 | 2.77 | 0.39 | 0.20 | 0.78 | 0.47 | 0.11 | 1.13 |
| Al2O3 | 0.04 | bdl | 0.58 | 0.16 | 0.22 | 0.46 | 0.05 | bdl | 0.17 |
| SiO2 | 0.06 | bdl | 0.52 | 0.07 | bdl | 0.30 | 0.05 | bdl | 0.18 |
| Cr2O | 0.02 | 0.01 | 0.08 | 0.02 | bdl | 0.04 | 0.01 | bdl | 0.06 |
| NiO | 0.01 | bdl | 0.04 | 0.01 | bdl | 0.06 | 0.02 | bdl | 0.07 |
| ZnO | 0.01 | bdl | 0.05 | 0.04 | 0.03 | 0.18 | 0.01 | bdl | 0.03 |
| V2O | 0.16 | bdl | 0.28 | 0.21 | 0.05 | 0.32 | 0.22 | 0.09 | 0.33 |
| Total | 97.70 | 98.04 | 99.13 | 98.87 | 97.01 | 99.41 | 98.85 | 94.71 | 99.28 |
| Sample | KM81 | KM68 | KM74 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Type | Magmatic (n = 12) | Hydrothermal (n = 15) | Hydrothermal (n = 15) | ||||||
| (ppm) | avg | min | max | avg | min | max | avg | min | max |
| Mg | 1271 | 612 | 2678 | 2643 | 550 | 6896 | 2043 | 451 | 3613 |
| Al | 5679 | 4112 | 7845 | 5131 | 3354 | 5977 | 4676 | 2002 | 6808 |
| Si | 3120 | 991 | 8521 | 3376 | 1338 | 6247 | 4481 | 1982 | 6679 |
| Ca | 1755 | 740 | 4125 | 1386 | 980 | 2065 | 2749 | 910 | 4158 |
| Sc | 3 | 2 | 4 | 4 | 1 | 18 | 12 | 4 | 23 |
| Ti | 20,179 | 9915 | 22,545 | 3202 | 1200 | 4852 | 2419 | 1305 | 11,517 |
| V | 561 | 395 | 770 | 1777 | 1622 | 1968 | 1784 | 1077 | 3079 |
| Cr | 329 | 221 | 454 | 20 | 16 | 25 | 23 | 14 | 28 |
| Mn | 316 | 224 | 412 | 1047 | 637 | 2199 | 954 | 412 | 2672 |
| Co | 22 | 19 | 27 | 12 | 3 | 21 | 7 | 4 | 14 |
| Ni | 23 | 19 | 31 | 16 | 12 | 21 | 16 | 14 | 19 |
| Cu | 5 | 2 | 8 | 56 | bdl | 156 | 21 | bdl | 152 |
| Zn | 51 | 33 | 79 | 189 | 1417 | 2163 | 2926 | 1860 | 4004 |
| Ga | 15 | 9 | 22 | 17 | 11 | 35 | 34 | 31 | 40 |
| Rb | 3 | 2 | 5 | 2 | bdl | 7 | 4 | bdl | 19 |
| Sr | 6 | 4 | 8 | 11 | 2 | 30 | 18 | bdl | 72 |
| Y | 10 | 4 | 14 | 11 | 1 | 53 | 1 | bdl | 10 |
| Zr | 15 | 4 | 24 | 1 | bdl | 10 | 4 | bdl | 23 |
| Nb | 4 | 1 | 9 | 8 | bdl | 55 | 10 | bdl | 40 |
| Mo | 1 | bdl | 2 | 4 | 1 | 9 | 3 | 1 | 6 |
| Sn | 64 | 36 | 99 | 13 | 5 | 39 | 35 | 26 | 41 |
| Ba | 6 | 2 | 11 | 5 | 3 | 8 | 4 | bdl | 11 |
| Hf | 0.3 | bdl | 1.2 | 0.2 | bdl | 1 | 0.4 | bdl | 2 |
| Ta | bdl | bdl | 1 | 1 | bdl | 7 | 1 | bdl | 3 |
| W | 2 | 1 | 4 | 6 | bdl | 44 | 3 | bdl | 9 |
| Pb | 21 | 12 | 36 | 49 | 10 | 128 | 6 | bdl | 20 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mavrogonatos, C.; Voudouris, P.; Berndt, J.; Klemme, S.; Zaccarini, F.; Spry, P.G.; Melfos, V.; Tarantola, Α.; Keith, M.; Klemd, R.; et al. Trace Elements in Magnetite from the Pagoni Rachi Porphyry Prospect, NE Greece: Implications for Ore Genesis and Exploration. Minerals 2019, 9, 725. https://doi.org/10.3390/min9120725
Mavrogonatos C, Voudouris P, Berndt J, Klemme S, Zaccarini F, Spry PG, Melfos V, Tarantola Α, Keith M, Klemd R, et al. Trace Elements in Magnetite from the Pagoni Rachi Porphyry Prospect, NE Greece: Implications for Ore Genesis and Exploration. Minerals. 2019; 9(12):725. https://doi.org/10.3390/min9120725
Chicago/Turabian StyleMavrogonatos, Constantinos, Panagiotis Voudouris, Jasper Berndt, Stephan Klemme, Federica Zaccarini, Paul G. Spry, Vasilios Melfos, Αlexandre Tarantola, Manuel Keith, Reiner Klemd, and et al. 2019. "Trace Elements in Magnetite from the Pagoni Rachi Porphyry Prospect, NE Greece: Implications for Ore Genesis and Exploration" Minerals 9, no. 12: 725. https://doi.org/10.3390/min9120725
APA StyleMavrogonatos, C., Voudouris, P., Berndt, J., Klemme, S., Zaccarini, F., Spry, P. G., Melfos, V., Tarantola, Α., Keith, M., Klemd, R., & Haase, K. (2019). Trace Elements in Magnetite from the Pagoni Rachi Porphyry Prospect, NE Greece: Implications for Ore Genesis and Exploration. Minerals, 9(12), 725. https://doi.org/10.3390/min9120725








