C- and N-Bearing Species in Reduced Fluids in the Simplified C–O–H–N System and in Natural Pelite at Upper Mantle P–T Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.3. Analytical Techniques
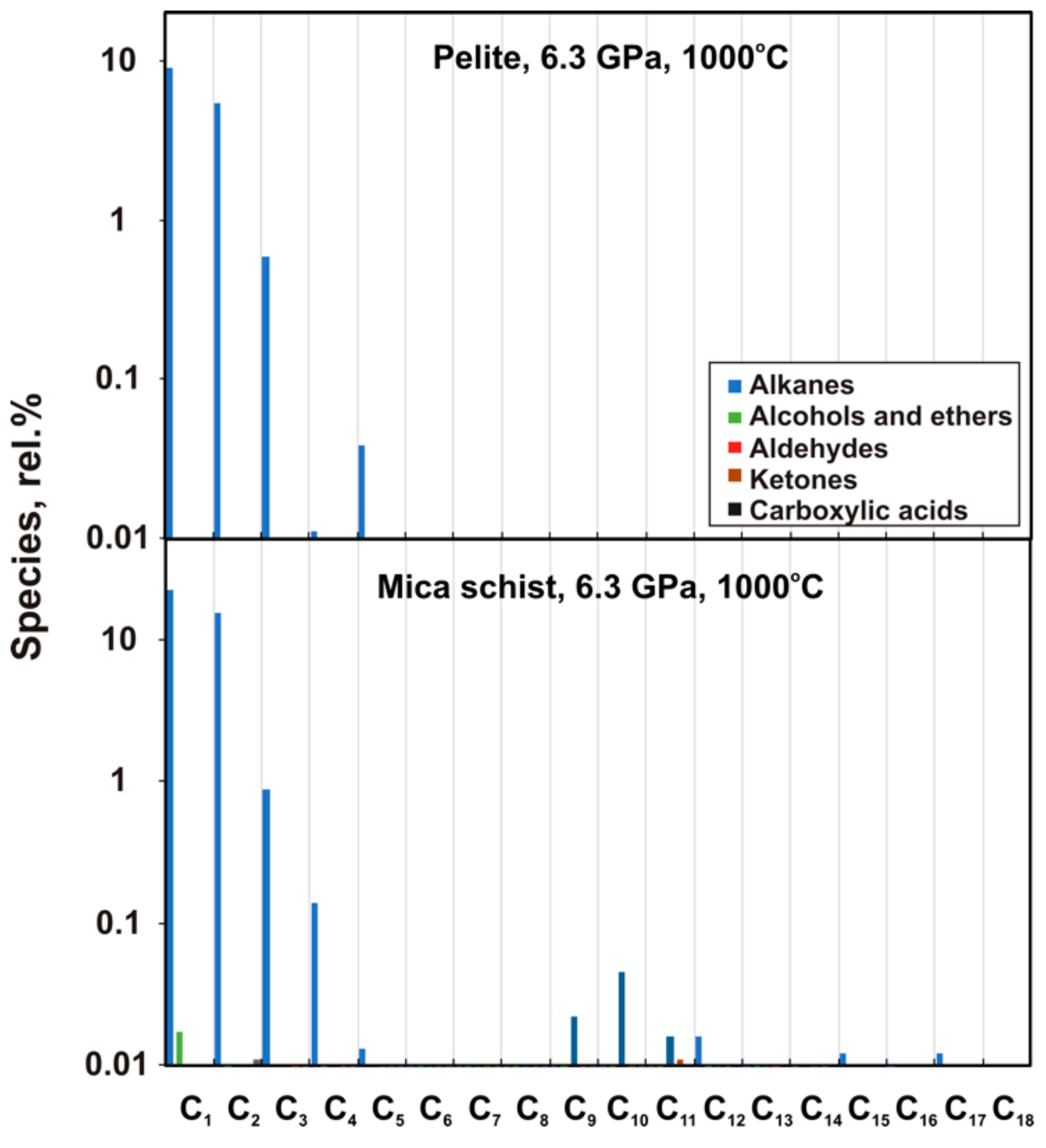
3. Results
4. Discussion
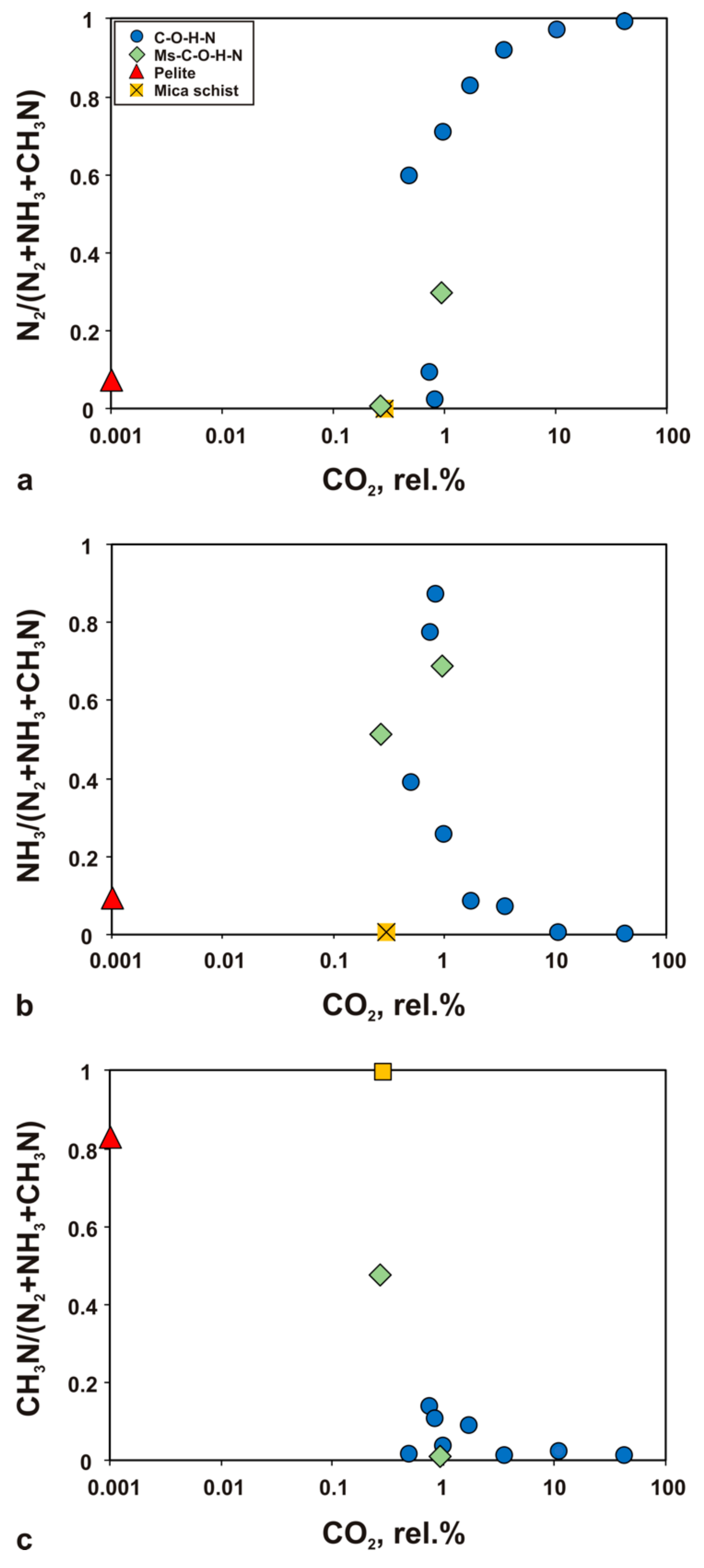
4.1. Carbon Species
4.2. N-Bearing Species
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Etiope, G.; Sherwood Lollar, B. Abiotic methane on Earth. Rev. Geophys. 2013, 51, 276–299. [Google Scholar] [CrossRef]
- Palyanov, Y.N.; Bataleva, Y.V.; Sokol, A.G.; Borzdov, Y.M.; Kupriyanov, I.N.; Reutsky, V.N.; Sobolev, N.V. Mantle-slab interaction and redox mechanism of diamond formation. Proc. Natl. Acad. Sci. USA 2013, 110, 20408–20413. [Google Scholar] [CrossRef] [PubMed]
- Luth, R.W. Volatiles in Earth’s mantle. In Treatise on Geochemistry, 2nd ed.; Elsevier: Oxford, UK, 2014; Volume 3, pp. 355–391. [Google Scholar]
- Sverjensky, D.A.; Stagno, V.; Huang, F. Important role for organic carbon in subduction-zone fluids in the deep carbon cycle. Nat. Geosci. 2014, 7, 909. [Google Scholar] [CrossRef]
- Smith, E.M.; Shirey, S.B.; Nestola, F.; Bullock, E.S.; Wang, J.; Richardson, S.H.; Wang, W. Large gem diamonds from metallic liquid in Earth’s deep mantle. Science 2016, 354, 1403–1405. [Google Scholar] [CrossRef]
- Bebout, G.E.; Lazzeri, K.E.; Geiger, C.A. Pathways for nitrogen cycling in Earth’s crust and upper mantle: A review and new results for microporous beryl and cordierite. Am. Mineral. 2016, 101, 7–24. [Google Scholar] [CrossRef]
- Kolesnikov, A.Y.; Saul, J.M.; Kutcherov, V.G. Chemistry of hydrocarbons under extreme thermobaric conditions. ChemistrySelect 2017, 2, 1336–1352. [Google Scholar] [CrossRef]
- Matveev, S.; Ballhaus, C.; Fricke, K.; Truckenbrodt, J.; Ziegenben, D. Volatiles in the Earth’s mantle: I. Synthesis of CHO fluids at 1273 K and 2.4 GPa. Geochim. Cosmochim. Acta 1997, 61, 3081–3088. [Google Scholar] [CrossRef]
- Sokol, A.G.; Palyanova, G.A.; Palyanov, Y.N.; Tomilenko, A.A.; Melenevsky, V.N. Fluid regime and diamond formation in the reduced mantle: Experimental constraints. Geochim. Cosmochim. Acta 2009, 73, 5820–5834. [Google Scholar] [CrossRef]
- Sokol, A.G.; Tomilenko, A.A.; Bul’bak, T.A.; Palyanova, G.A.; Sokol, I.A.; Palyanov, Y.N. Carbon and Nitrogen Speciation in N-poor C–O–H–N Fluids at 6.3 GPa and 1100–1400 °C. Sci. Rep. 2017, 7, 706. [Google Scholar] [CrossRef]
- Matjuschkin, V.; Woodland, A.B.; Yaxley, G.M. Methane-bearing fluids in the upper mantle: An experimental approach. Contrib. Mineral. Petrol. 2019, 174, 1. [Google Scholar] [CrossRef]
- Kenney, J.F.; Kutcherov, V.A.; Bendeliani, N.A.; Alekseev, V.A. The evolution of multicomponent systems at high pressures: The thermodynamic stability of the hydrogen-carbon system: The genesis of hydrocarbons and the origin of petroleum. Proc. Nat. Acad. Sci. USA 2002, 99, 10976–10981. [Google Scholar] [CrossRef] [PubMed]
- Scott, H.P.; Hemley, R.J.; Mao, H.; Herschbach, D.R.; Fried, L.E.; Howard, W.M.; Bastea, S. Generation of methane in the Earth’s mantle: In situ high pressure–temperature measurements of carbonate reduction. Proc. Nat. Acad. Sci. USA 2004, 101, 14023–14026. [Google Scholar] [CrossRef] [PubMed]
- Kutcherov, V.G.; Kolesnikov, A.Y.; Dyuzheva, T.I.; Kulikova, L.F.; Nikolaev, N.N.; Sazanova, O.A.; Braghkin, V.V. Synthesis of Complex Hydrocarbon Systems at Temperatures and Pressures Corresponding to the Earth’s Upper Mantle Conditions. Dokl. Phys. Chem. 2010, 433, 132–135. [Google Scholar] [CrossRef]
- Palyanov, Y.N.; Borzdov, Y.M.; Kupriyanov, I.N.; Khokhryakov, A.F. Effect of H2O on diamond crystal growth in metal–carbon systems. Cryst. Growth Des. 2010, 12, 5571–5578. [Google Scholar] [CrossRef]
- Mukhina, E.; Kolesnikov, A.; Kutcherov, V. The lower pT limit of deep hydrocarbon synthesis by CaCO3 aqueous reduction. Sci. Rep. 2017, 7, 5749. [Google Scholar] [CrossRef] [PubMed]
- Tao, R.; Zhang, L.; Tian, M.; Zhu, J.; Liu, X.; Liu, J.; Höfer, H.E.; Stagno, V.; Fei, Y. Formation of abiotic hydrocarbon from reduction of carbonate in subduction zones: Constraints from petrological observation and experimental simulation. Geochim. Cosmochim. Acta 2018, 239, 390–408. [Google Scholar] [CrossRef]
- Sokol, A.G.; Tomilenko, A.A.; Bul’bak, T.A.; Sokol, I.A.; Persikov, E.S.; Bukhtiyarov, P.G.; Palyanov, Y.N. Distribution of light alkanes in the reaction of graphite hydrogenation at pressure of 0.1–7.8 GPa and temperatures of 1000–1350 °C. High Press. Res. 2018, 38, 468–481. [Google Scholar] [CrossRef]
- Sokol, A.G.; Tomilenko, A.A.; Bul’bak, T.A.; Sokol, I.A.; Zaikin, P.A.; Palyanova, G.A.; Palyanov, Y.N. Hydrogenation of carbon at 5.5–7.8 GPa and 1100–1400 °C: Implications to formation of hydrocarbons in reduced mantles of terrestrial planets. Phys. Earth Planet. Inter. 2019, 291, 12–23. [Google Scholar] [CrossRef]
- Sokol, A.; Tomilenko, A.; Sokol, I.; Zaikin, P.; Bul’bak, T. Formation of Hydrocarbons in the Presence of Native Iron at Upper Mantle Conditions: Experimental Constraints. Minerals 2019, in press. [Google Scholar]
- Mikhail, S.; Sverjensky, D.A. Nitrogen speciation in upper mantle fluids and the origin of Earth’s nitrogen-rich atmosphere. Nat. Geosci. 2014, 7, 816–819. [Google Scholar] [CrossRef]
- Sokol, A.G.; Tomilenko, A.A.; Bul’bak, T.A.; Kruk, A.N.; Sokol, I.A.; Palyanov, Y.N. Fate of fluids at the base of subcratonic lithosphere: Experimental constraints at 5.5–7.8 GPa and 1150–1350 deg C. Lithos 2018, 318, 419–433. [Google Scholar] [CrossRef]
- Chukanov, N.V.; Pekov, I.V.; Sokolov, S.V.; Nekrasov, A.N.; Chukanova, V.N.; Naumova, I.S. On the problem of the formation and geochemical role of bituminous matter in pegmatites of the Khibiny and Lovozero alkaline massifs, Kola Peninsula, Russia. Geochem. Int. 2006, 44, 715–728. [Google Scholar] [CrossRef]
- Smith, E.M.; Shirey, S.B.; Richardson, S.H.; Nestola, F.; Bullock, E.S.; Wang, J.; Wang, W. Blue boron-bearing diamonds from Earth’s lower mantle. Nature 2018, 560, 84. [Google Scholar] [CrossRef] [PubMed]
- Sobolev, N.V.; Sobolev, A.V.; Tomilenko, A.A.; Kuz’min, D.V.; Grakhanov, S.A.; Batanova, V.G.; Logvinova, A.M.; Bul’bak, T.A.; Kostrovitskii, S.I.; Yakovlev, D.A.; et al. Prospects of search for diamondiferous kimberlites in the northeastern Siberian. Platform. Russ. Geol. Geophys. 2018, 59, 1365–1379. [Google Scholar] [CrossRef]
- Sobolev, N.V.; Tomilenko, A.A.; Bul’bak, T.A.; Logvinova, A.M. Composition of volatile components in the diamonds, associated garnet and olivine from diamondiferous peridotites from the Udachnaya pipe, Yakutia, Russia (by coupled gas chromatographic-mass spectrometric analysis). Engineering 2019, 5, 471–478. [Google Scholar] [CrossRef]
- Sobolev, N.V.; Logvinova, A.M.; Tomilenko, A.A.; Wirth, R.; Bul’bak, T.A.; Luk’yanova, L.I.; Fedorova, E.N.; Reutsky, V.N.; Efimova, E.S. Mineral and fluid inclusions in diamonds from the Urals placers, Russia: Evidence for solid molecular N2 and hydrocarbons in fluid inclusions. Geochim. Cosmochim. Acta 2019, 266, 197–219. [Google Scholar] [CrossRef]
- Plank, T.; Langmuir, C.H. The chemical composition of subducting sediment and its consequences for the crust and mantle. Chem. Geol. 1998, 145, 325–394. [Google Scholar] [CrossRef]
- Schmidt, M.; Poli, S. Devolatilization during subduction. In Treatise on Geochemistry, 2nd ed.; Elsevier: Oxford, UK, 2014; pp. 669–701. [Google Scholar]
- Watenphul, A.; Wunder, B.; Heinrich, W. High-pressure ammonium-bearing silicates: Implications for nitrogen and hydrogen storage in the Earth’s mantle. Am. Mineral. 2009, 94, 283–292. [Google Scholar] [CrossRef]
- Watenphul, A.; Wunder, B.; Wirth, R.; Heinrich, W. Ammonium-bearing clinopyroxene: A potential nitrogen reservoir in the Earth’s mantle. Chem. Geol. 2010, 270, 240–248. [Google Scholar] [CrossRef]
- Domanik, K.J.; Holloway, J.R. The stability and composition of phengitic muscovite and associated phases from 5.5 to 11 GPa: Implications for deeply subducted sediments. Geochim. Cosmochim. Acta 1996, 60, 4133–4150. [Google Scholar] [CrossRef]
- Schmidt, M.W.; Vielzeuf, D.; Auzanneau, E. Melting and dissolution of subducting crust at high pressurfufes: The key role of white mica. Earth Planet. Sci. Lett. 2004, 228, 65–84. [Google Scholar] [CrossRef]
- Busigny, V.; Cartigny, P.; Philippot, P.; Ader, M.; Javoy, M. Massive recycling of nitrogen and other fluid-mobile elements (K, Rb, Cs, H) in a cold slab environment: Evidence from HP to UHP oceanic metasediments of the Schistes Lustrés nappe (western Alps, Europe). Earth Planet. Sci. Lett. 2003, 215, 27–42. [Google Scholar] [CrossRef]
- Cartigny, P.; Harris, J.W.; Javoy, M. Diamond genesis, mantle fractionations and mantle nitrogen content: A study of δ13C–N concentrations in diamonds. Earth Planet. Sci. Lett. 2001, 185, 85–98. [Google Scholar] [CrossRef]
- Li, Y.; Keppler, H. Nitrogen speciation in mantle and crustal fluids. Geochim. Cosmochim. Acta 2014, 129, 13–32. [Google Scholar] [CrossRef]
- Sokol, A.G.; Tomilenko, A.A.; Bul’bak, T.A.; Kruk, A.N.; Zaikin, P.A.; Sokol, I.A.; Seryotkin, Y.V.; Palyanov, Y.N. The Fe–C–O–H–N system at 6.3–7.8 GPa and 1200–1400 °C: Implications for deep carbon and nitrogen cycles. Contrib. Mineral. Petrol. 2018, 173, 47. [Google Scholar] [CrossRef]
- Sokol, A.G.; Palyanov, Y.N.; Tomilenko, A.A.; Bul’bak, T.A.; Palyanova, G.A. Carbon and nitrogen speciation in nitrogen-rich C–O–H–N fluids at 5.5–7.8 GPa. Earth Planet. Sci. Lett. 2017, 460, 234–243. [Google Scholar] [CrossRef]
- Sokol, E.; Kokh, S.; Kozmenko, O.; Novikova, S.; Khvorov, P.; Nigmatulina, E.; Belogub, E.; Kirillov, M. Mineralogy and Geochemistry of Mud Volcanic Ejecta: A New Look at Old Issues (A Case Study from the Bulganak Field, Northern Black Sea). Minerals 2018, 8, 344. [Google Scholar] [CrossRef]
- Karpenko, V.Y.; Pautov, L.A.; Agakhanov, A.A.; Khvorov, P.V. On Nitrogen Content in the Schist of the Mun’-Khambo Ridge (N. Ural); The Ural Mineralogical Collected Papers #11. Scientific Edition; Institute of Mineralogy, Ural Branch of Russian Academy of Sciences: Miass, Russia, 2001; p. 330. [Google Scholar]
- Li, L.; Bebout, G.E. Carbon and nitrogen geochemistry of sediments in the Central American convergent margin: Insights regarding subduction input fluxes, diagenesis, and paleoproductivity. J. Geophys. Res. Solid Earth 2005, 110, B11202. [Google Scholar] [CrossRef]
- Busigny, V.; Cartigny, P.; Philippot, P. Nitrogen isotopes in ophiolitic metagabbros: A re-evaluation of modern nitrogen fluxes in subduction zones and implication for the early Earth atmosphere. Geochim. Cosmochim. Acta 2011, 75, 7502–7521. [Google Scholar] [CrossRef]
- Bebout, G.E.; Agard, P.; Kobayashi, K.; Moriguti, T.; Nakamura, E. Devolatilization history and trace element mobility in deeply subducted sedimentary rocks: Evidence from Western Alps HP/UHP suites. Chem. Geol. 2013, 342, 1–20. [Google Scholar] [CrossRef]
- Busigny, V.; Bebout, G.E. Nitrogen in the silicate Earth: Speciation and isotopic behavior during mineral–fluid interactions. Elements 2013, 9, 353–358. [Google Scholar] [CrossRef]
- Palyanov, Y.N.; Borzdov, Y.M.; Khokhryakov, A.F.; Kupriyanov, I.N.; Sokol, A.G. Effect of nitrogen impurity on diamond crystal growth processes. Cryst. Growth Des. 2010, 10, 3169–3175. [Google Scholar] [CrossRef]
- Sokol, A.G.; Borzdov, Y.M.; Palyanov, Y.N.; Khokhryakov, A.F. High-temperature calibration of a multi-anvil high-pressure apparatus. High Press. Res. 2015, 35, 139–147. [Google Scholar] [CrossRef]
- Luth, R.W. Natural versus experimental control of oxidation state: Effects on the composition and speciation of C–O–H fluids. Am. Mineral. 1989, 74, 50–57. [Google Scholar]
- Dementyev, S.N.; Drebushchak, V.A. Zeolites’ dehydration under dynamic regime. Geochem. Int. 1992, 9, 1361–1367. [Google Scholar]
- Theule, P.; Borget, F.; Mispelaer, F.; Danger, G.; Duvernay, F.; Guillemin, J.C.; Chiavassa, T. Hydrogenation of solid hydrogen cyanide HCN and methanimine CH2NH at low temperature. Astron. Astrophys. 2011, 534, A64. [Google Scholar] [CrossRef]
- Foley, S. A reappraisal of redox melting in the Earth’s mantle as a function of tectonic setting and time. J. Petrol. 2011, 52, 1363–1391. [Google Scholar] [CrossRef]
- Stagno, V.; Ojwang, D.O.; McCammon, C.A.; Frost, D.J. The oxidation state of the mantle and the extraction of carbon from Earth’s interior. Nature 2013, 493, 84. [Google Scholar] [CrossRef]
- Robertson, A.J.B. The Pyrolysis of Methane, Ethane and n-butane on a Platinum Filament. Proc. R. Soc. Lond. A Math. Phys. Eng. Sci. 1949, 199, 394. [Google Scholar]
- Belgued, M.; Amariglio, A.; Paréja, P.; Amariglio, H. Oxygen-Free conversion of methane to higher alkanes through an isothermal two-step reaction on platinum (EUROPT-1): II. hydrogenation of the adspecies resulting from the chemisorption of methane. J. Catal. 1996, 159, 449–457. [Google Scholar] [CrossRef]
- Hammer, B.; Nørskov, J. Why gold is the noblest of all the metals. Nature 1995, 376, 238. [Google Scholar] [CrossRef]
- McEwan, L.; Julius, M.; Roberts, S.; Fletcher, J.C.Q. A review of the use of gold catalysts in selective hydrogenation reactions. Gold Bull. 2010, 43, 298. [Google Scholar]
- Mowbray, D.J.; Migani, A.; Walther, G.; Cardamone, D.M.; Rubio, A. Gold and methane: A noble combination for delicate oxidation. J. Phys. Chem. Lett. 2013, 4, 3006–3012. [Google Scholar] [CrossRef]
- Sharma, A.; Cody, G.D.; Hemley, R.J. In situ diamond-anvil cell observations of methanogenesis at high pressures and temperatures. Energy Fuels 2009, 23, 5571–5579. [Google Scholar] [CrossRef]
- Stachel, T.; Luth, R.W. Diamond formation—Where, when and how? Lithos 2015, 220, 200–220. [Google Scholar] [CrossRef]



| Run# | P (GPa) | T (°C) | Buffer | Capsule | τ (h) | Compositions of Samples, mg | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gr | Ms | Pelite | Mica Schist | C3H6N6 | H2O | C22H46 | Ag2C2O4 | Dm | ||||||
| 1668_2_1 | 5.5 | 1150 | - | Pt | 10 | 10.1 | - | - | - | - | - | - | - | - |
| 1906_2_1 | 6.3 | 1400 | - | Pt | 0.33 | 8.4 | - | - | - | - | - | - | - | - |
| 1969_2_1 | 5.5 | 1150 | MMO | Pt | 40 | 8.5 | - | - | - | - | 0.8 | - | - | |
| 996_5_6 | 5.5 | 1200 | MMO | Pt | 10 | 9.8 | - | - | - | - | 0.5 | - | ||
| 2107_2_3 * | 6.3 | 1200 | MMO | Au | 10 | 9.5 | - | - | - | - | 0.5 | - | - | |
| 2107_2_4 * | 6.3 | 1200 | MMO | Au | 10 | 10.8 | - | - | - | - | - | - | - | - |
| 1942_2_2 | 7.8 | 1350 | MMO | Pt | 10 | 9.7 | - | - | - | - | - | - | - | - |
| 1975_2_1 | 7.8 | 1350 | IW | Pt | 10 | 16.4 | - | - | - | - | - | - | - | - |
| 1670_2_1 | 7.8 | 1350 | IW | Pt | 10 | 11.4 | - | - | - | - | - | - | - | - |
| 1670_2_3 | 7.8 | 1350 | IW | Pt | 10 | - | - | - | - | - | - | - | 8.1 | |
| 1695_1_4 | 3.8 | 800 | MMO | Au | 40 | - | 8.5 | - | - | 0.5 | - | - | - | |
| 1981_2_6 | 5.5 | 1000 | MMO | Au | 40 | 2.1 | 8.3 | - | - | - | 0.9 | - | - | - |
| 2093_2_1 | 6.3 | 1000 | MMO | Au | 60 | - | 9.8 | - | - | - | - | - | - | |
| 2093_2_3 | 6.3 | 1000 | MMO | Au | 60 | - | - | 10.9 | - | - | - | - | - | |
| Muscovite | Pelite * (Maykop Fm. Russia) | Mica Schist ** (Polar Ural, Russia) | |
|---|---|---|---|
| SiO2 | 46.3 | 53.9 | 48.4 |
| TiO2 | 0.1 | 0.8 | 1.4 |
| Al2O3 | 34.6 | 16.3 | 22.4 |
| FeO | 1.5 | 7.3 | 10.8 |
| MnO | 0.1 | 0.1 | 0.2 |
| MgO | 0.8 | 3.2 | 4.3 |
| CaO | - | 1.8 | 0.6 |
| Na2O | 0.5 | 1.3 | 3.1 |
| K2O | 10.8 | 2.9 | 3.3 |
| P2O5 | - | 0.1 | 0.3 |
| BaO | 0.4 | - | 0.1 |
| LOI | - | 11.1 | 4.1 |
| Total | 95.1 | 99.0 | 99.2 |
| Run# | Phase | SiO2 | TiO2 | Al2O3 | FeO | MnO | MgO | CaO | Na2O | K2O | BaO | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1695_1_4 | Ms | 45.5 | 0.6 | 31.7 | 2.9 | – | 1.6 | - | 0.5 | 10.7 | 0.4 | 93.9 |
| 1981_2_6 | Ms | 47.6 | 0.2 | 29.5 | 3.1 | - | 1.7 | - | - | 11.3 | 0.5 | 93.8 |
| Ky | 36.6 | 0.0 | 63.5 | 0.3 | - | - | - | - | 100.4 | |||
| 2093_2_1 | Ms | 52.5 | 1.1 | 23.5 | 1.6 | - | 4.6 | - | - | 11.5 | - | 94.9 |
| Grt | 40.5 | 0.6 | 21.9 | 20.9 | 0.7 | 8.3 | 6.0 | - | - | - | 99.7 | |
| Omp | 57.6 | 0.3 | 21.1 | 1.4 | - | 3.1 | 3.5 | 11.7 | - | - | 98.5 | |
| Coe | 98.9 | - | - | - | - | - | - | - | - | - | 98.9 | |
| Ky | 38.2 | - | 59.5 | 1.2 | - | - | - | - | - | - | 98.8 | |
| Ru | 0.5 | 95.7 | 1.6 | 0.6 | - | - | - | - | - | - | 98.3 | |
| 2093_2_3 | Ms | 52.3 | 1.1 | 24.5 | 2.5 | - | 4.2 | - | - | 10.7 | - | 95.3 |
| Grt | 38.1 | 0.2 | 21.2 | 29.5 | 0.5 | 9.4 | 0.6 | - | - | 99.6 | ||
| Omp | 58.7 | 0.4 | 23.4 | 1.7 | 0.0 | 1.2 | 1.2 | 13.4 | 0.0 | - | 100.0 | |
| Ky | 36.8 | 0.0 | 62.8 | 0.4 | - | - | - | - | - | - | 100.0 | |
| Ru | 0.5 | 96.1 | 1.8 | 0.9 | - | - | - | - | - | - | 99.3 |
| Run # | Alkanes | Alcohols, Ethers | Aldehydes | Ketones | Carb. Acids | H2O | CO2 | N2 | CH3N | H3N |
|---|---|---|---|---|---|---|---|---|---|---|
| 1668_2_1 | 3.9 | 3.3 | 15.2 | 5.3 | 1.6 | 4.1 | 42.9 | 17.7 | 0.2 | 0.0 |
| 1906_2_1 | 26.6 | 0.4 | 1.7 | 0.6 | 16.9 | 37.5 | 10.7 | 4.3 | 0.1 | 0.0 |
| 1969_2_1 | 69.9 | 1.2 | 2.5 | 2.2 | 1.9 | 11.7 | 0.8 | 0.6 | 0.9 | 5.1 |
| 996_5_6 | 25.3 | 0.8 | 0.9 | 0.4 | 1.7 | 62.0 | 0.9 | 0.2 | 0.7 | 6.0 |
| 1942_2_ 2 * | 30.4 | 3.6 | 15.1 | 9.2 | 5.7 | 13.1 | 3.6 | 7.2 | 0.1 | 0.5 |
| 1975_2_1 | 57.3 | 1.0 | 1.2 | 1.3 | 2.4 | 16.9 | 0.5 | 10.6 | 0.3 | 6.9 |
| 1670_2_1 | 53.1 | 2.2 | 3.6 | 2.3 | 17.3 | 9.8 | 1.7 | 4.3 | 0.5 | 0.4 |
| 1670_2_3 | 52.2 | 3.6 | 2.2 | 2.2 | 2.2 | 12.4 | 1.0 | 15.2 | 0.8 | 5.5 |
| 1695_1_4 | 27.0 | 0.1 | 0.2 | 0.1 | 0.3 | 26.4 | 0.9 | 13.3 | 0.5 | 30.8 |
| 1981_2_6 | 14.7 | 0.2 | 0.2 | 0.0 | 0.2 | 81.4 | 0.3 | 0.0 | 1.1 | 1.2 |
| 2093_2_1 | 15.3 | 0.0 | 0.0 | 0.0 | 0.0 | 80.8 | 0.0 | 0.3 | 3.2 | 0.4 |
| 2093_2_3 | 39.0 | 0.1 | 0.1 | 0.1 | 0.0 | 48.0 | 0.3 | 0.0 | 12.4 | 0.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sokol, I.; Sokol, A.; Bul’bak, T.; Nefyodov, A.; Zaikin, P.; Tomilenko, A. C- and N-Bearing Species in Reduced Fluids in the Simplified C–O–H–N System and in Natural Pelite at Upper Mantle P–T Conditions. Minerals 2019, 9, 712. https://doi.org/10.3390/min9110712
Sokol I, Sokol A, Bul’bak T, Nefyodov A, Zaikin P, Tomilenko A. C- and N-Bearing Species in Reduced Fluids in the Simplified C–O–H–N System and in Natural Pelite at Upper Mantle P–T Conditions. Minerals. 2019; 9(11):712. https://doi.org/10.3390/min9110712
Chicago/Turabian StyleSokol, Ivan, Alexander Sokol, Taras Bul’bak, Andrey Nefyodov, Pavel Zaikin, and Anatoly Tomilenko. 2019. "C- and N-Bearing Species in Reduced Fluids in the Simplified C–O–H–N System and in Natural Pelite at Upper Mantle P–T Conditions" Minerals 9, no. 11: 712. https://doi.org/10.3390/min9110712
APA StyleSokol, I., Sokol, A., Bul’bak, T., Nefyodov, A., Zaikin, P., & Tomilenko, A. (2019). C- and N-Bearing Species in Reduced Fluids in the Simplified C–O–H–N System and in Natural Pelite at Upper Mantle P–T Conditions. Minerals, 9(11), 712. https://doi.org/10.3390/min9110712






