Abstract
Determining the V-x parameters of H2O–NaCl–CO2 fluid inclusions (total density of inclusions, gas content, homogenization pressure, etc.) is of great value for the exploration of hydrothermal deposits. However, previous accurate calculation methods are only applicable to H2O–NaCl–CO2 fluid inclusions with homogenization temperature above 300 °C and CO2 phase homogenization temperature above the CO2 clathrate melting temperature. In this paper, a new calculation method is proposed to accurately solve the V-x parameters of H2O–NaCl–CO2 fluid inclusions with complete homogenization temperature lower than 300 °C. The algorithm first determines the salinity of inclusions with respect to the melting temperature of CO2 clathrate and the partial homogenization temperature of the CO2 phase and then determines the internal pressure of inclusions when CO2 clathrate is completely melted. The V-x parameters of the inclusions are then iteratively solved. The new algorithm does not require a visual estimation of the volume fraction of the CO2 phase as an input parameter. It is possible to avoid the significant error brought about by traditional method of calculating the inclusion V-x parameters involving visual estimation of the CO2 phase volume fraction. A computer program is developed on the basis of the new method and is applied to the analysis of fluid inclusions in medium and low temperature hydrothermal gold ore.
1. Introduction
Geological fluid, which is involved in and has a crucial effect on numerous geological processes, is among frontiers and hotspots in geoscience [1,2,3,4]. NaCl and CO2 are the most common solute components in various geological fluids. Many geological fluids can be approximated as H2O–CO2 or H2O–NaCl–CO2 systems, such as hydrothermal gold ore-forming fluids, intermediate-grade metamorphic fluids, etc. Therefore, determining the physical and chemical parameters, e.g., density, salinity, composition, and homogenization pressure of H2O–NaCl–CO2 inclusions, is one of the important foundations for studying the source and evolution of ore-forming fluids and exploring the formation mechanism of hydrothermal deposits [5,6,7,8,9].
Previous studies have shown that most hydrothermal gold ore-forming fluids have medium to low salinity (NaCl less than 6–10 wt %) and relatively high CO2 content, and the complete homogenization temperature of the inclusions is between 200–400 °C [9,10,11]. At room temperature, the inclusions are either gas-liquid-liquid or gas-liquid, and lack halite daughter minerals. H2S and N2 are also very low. Therefore, a V-x parameter calculation program for the H2O–NaCl–CO2 inclusions is adequate for the research of hydrothermal gold ore fluid inclusion.
The thermodynamic model and calculation method are both necessary for the development of the calculation program. The determination of V-x parameters of the inclusions requires accurate calculations of the P-V-T-x and gas-liquid equilibrium composition of the H2O–NaCl–CO2 fluid system at 0–400 °C, 0–3000 bar. Previous state equations for H2O–NaCl–CO2–CH4 system at medium-high temperature (T > 300 °C) or medium-low temperature equations [12,13,14], as well as the CO2 solubility model could basically meet the calculation requirement [14,15,16]. For the latter, Song’s [17] method is adequate for the calculation of H2O–NaCl–CO2 inclusions where the CO2 phase homogenization temperature is higher than the CO2 clathrate melting temperature. Mao et al. [18] proposed a method for the calculation of H2O–NaCl–CO2 inclusions with complete homogenization to liquid phase and homogenization pressure not higher than 1500 bar, which is based on the model of Song [17]. For the calculation of the V-x parameter of the H2O–NaCl–CO2 inclusions whose CO2 phase homogenization temperature is lower than the melting temperature of the CO2 clathrate, a valid algorithm that does not require a visual estimation of gas-liquid ratiois still absent.
This study aims to solve the above problems by developing a computer program that can accurately calculate the V-x parameters of H2O–NaCl–CO2 inclusions. Two major contributions are included in this study, as described below:
1. A new iterative algorithm is proposed to solve the V-x parameters of H2O–NaCl–CO2 inclusions with a CO2 phase homogenization temperature lower than the CO2 clathrate melting temperature and to develop a calculation program. This new algorithm only takes the temperature measurement data as the input parameter and does not need to visually measure the volume fraction of the CO2 phase.
2. A new application is developed that can accurately calculate the V-x parameters of H2O–NaCl–CO2 inclusions, which is based on the algorithms of Song et al. [17] and this paper. The thermodynamic properties of the H2O–NaCl–CO2 system with temperatures above 300 °C and temperatures below 300 °C were calculated using the equations established by Duan et al. [12], Sun and Dubessy [13]. The application is valid at a full homogenization temperature range of 200–450 °C, and is suitable for both situations in which CO2 phase homogenization temperature is either higher or lower than CO2 clathrate melting temperature, with no halite daughter minerals present.
2. Calculation Method
2.1. Salinity Calculation of H2O–NaCl–CO2 Fluid Inclusions
For H2O–NaCl–CO2 inclusions, salinity is generally expressed in mass fraction or mass percentage S, representing the mass fraction of NaCl in H2O–NaCl solution. At room temperature, the H2O–NaCl–CO2 inclusions generally appear in two or three phases. The corresponding salinity calculation method will be introduced below with respect to these two different situations.
2.1.1. Salinity Calculation of Three-Phase H2O–NaCl–CO2Inclusions at Room Temperature
At room temperature, three-phase H2O–NaCl–CO2 inclusions (aqueous phase + CO2 liquid phase + CO2 gas phase) cool down to form CO2 clathrate. After re-heating, the melting temperature of the CO2 clathrate will be lower than the homogenization temperature of the CO2 phase. Therefore, CO2clathrate in the inclusion disappeared by heating so that the salinity of the inclusion could be determined with respect to the temperature. During the heating, the CO2 clathrate disappears and the system is in four-phase equilibrium. According to the Gibbs phase rule:
the number of components C = 3, the number of phases φ = 4, the four-phase degree of freedom F = 1, the salinity then has a one-to-one correspondence to the melting temperature of the clathrate, and the NaCl content of the aqueous phase is also determined when melting temperature is determined. In addition, in the case of lower temperature, the content of NaCl and water vapor in the CO2 gas phase (bubble) and CO2 liquid phase is negligible, and the NaCl content in the aqueous phase could be used to calculate the salinity of the entire inclusion. Salinity could be calculated by the equation below [19,20]:
where T is the melting temperature of CO2 hydrate (−10–10 °C). This equation is valid in the NaCl salinity range of 0–24.2 wt %.
F = C − φ + 2
S (wt %) = 0.00098241 × (10 − T) × (T2 + 45.385T + 1588.75)
2.1.2. Salinity Calculation of Two-Phase H2O–NaCl–CO2Inclusions at Room Temperature
The method above is not valid for the determination of salinity for H2O–NaCl–CO2 fluid inclusions exhibiting two phases at room temperature (aqueous phase + CO2 liquid phase or CO2 gas phase). When the inclusions cool down, CO2 hydrate will also form. When the system is heated, the hydrate will disappear at a certain temperature. At this time, the inclusion system is on the three-phase equilibrium interface. Since the number of components is C = 3, the number of phases is φ = 4, and the degree of freedom of the system is F = 2, which means that the salinity changes not only with temperature but also with pressure. Then the aforementioned method for determining the salinity of three-phase H2O–NaCl–CO2 inclusions is no longer applicable.
The following two calculation methods are mainly used for this occasion. The first method was proposed by Diamond [21], which determines the salinity in combination with the hydrate melting temperature and the partial homogenization temperature of the CO2 measured in the metastable state. When the CO2 gas phase and the liquid phase are homogeneous to the gas phase, the salinity is calculated as follow:
x is the partial homogenization temperature of the CO2 phase, and the range of application is between −20 °C to 10 °C. y is the melting temperature of the hydrate and is valid between −5 °C to 13 °C. The range of salinity S is 0–21 wt % NaCl (relative to the binary H2O–NaCl subsystem without CO2). When the CO2 gas phase and the liquid phase are homogeneous to the liquid phase, the salinity is calculated as follow:
S (wt %) = 15.6151 − 0.03627x + 0.00164x2 − 0.949y − 0.00287xy − 0.02464y2 − 0.00107xy2 − 0.00222y3
S (wt %) = 15.6151 − 0.065705x + 0.00778x2 − 1.05135y − 0.02687xy − 0.04717y2 − 0.00138xy2 − 0.00411y3
The valid range of x and S is the same as the previous equation while y is valid between −8 °C to 10 °C.
The second method, which is proposed by Fall et al. [22], determines the CO2 phase pressure according to the carbon dioxide Fermi peak displacement method, and also determines the NaCl content according to the gas hydrate three-phase equilibrium model by Bakker [23], Duan and Sun [15]. It brings smaller salinity error for H2O–NaCl–CO2 two-phase inclusion in which CO2is a liquid phase (0.3 wt %) and much more significant error for those in which CO2 exhibits gas phase (~3.2 wt %)
2.2. Calculation Method of V-x Parameters of H2O–NaCl–CO2 Inclusions
For single-component fluid inclusions, the microthermometry measurement data can be substituted into the formula to calculate the total density of inclusions and homogenization pressure. For ternary system fluid inclusions, the total density, composition, homogenization pressure and other parameters need to be determined by means of more complex thermodynamic calculation models or diagrams.
The V-x parameter depends on the thermodynamic properties of the fluid system under different temperature and pressure conditions. A few of them can be calculated by simple formulas, while for the rest, an accurate solution can only be provided by thermodynamic models with sound theoretical basis. Due to the non-ideality of the system, there are few equations that can accurately describe the thermodynamic properties of H2O–NaCl–CO2 systems. Parameters and the corresponding models include (1) Gas-liquid phase equilibrium composition and density of H2O–NaCl–CO2 ternary system at the homogenization temperature are calculated by equation established by Duan et al. [12,14], Sun and Dubessy [13]. (2) Solubility of CO2 in aqueous sodium chloride solution at room temperature is described by the concentration of CO2 in aqueous NaCl solution, which could be provided by the CO2 solubility model established and improved by Duan and collaborators [14,15,16,24], and the density of aqueous solutions described by the Pitzer’s [25] activity coefficient model. (3) Triple-phase equilibrium pressure of CO2 clathrate is determined by the equilibrium model established by Duan and Sun [15].
2.2.1. Traditional Algorithm for V-x Parameters of H2O–NaCl–CO2 Fluid Inclusions
As mentioned above, the salinity of the H2O–NaCl–CO2 fluid inclusions can be determined by the melting temperature of the CO2 clathrate, but there is no simple calculation method for the CO2 content of the inclusions. It is generally necessary to visually estimate the volume ratio of the CO2 phase to the aqueous phase, and then the homogenization pressure and isometrics of H2O–NaCl–CO2 inclusions can be obtained by using the appropriate equation of state on the basis of density, composition and the estimated homogenization temperature. Nonetheless, the visual volume fraction inevitably produces large errors, giving rise to inaccurate calculation results of parameters [26,27].
Combined with theoretical calculations, Schwarz [28] plotted the V-x diagram of H2O–CO2 inclusions without NaCl and H2O–NaCl–CO2 inclusions with a salinity of 6 wt %. Bakker and Diamond [29,30] also mapped the V-x of H2O–CO2 inclusions based on experimental data published by Sterner and Bodnar [31]. Although the using of these diagrams does not need a visual estimation of the volume fraction of the CO2 phase, the accuracy is poor in the low temperature region, and the applicable range of salinity is small.
2.2.2. Iterative Calculation Method for V-x Parameters of H2O–NaCl–CO2Fluid Inclusions at Room Temperature
The phase volume of a H2O–NaCl–CO2 fluid inclusion is limited by the density, complete homogenization temperature and homogenization of the CO2 phase due to that the inclusion is a closed system with constant volume. Parry [32] proposed an iterative calculation method to solve the CO2 content and total density of the three-phase inclusions of H2O–NaCl–CO2 system. This method used the Bowers-Helgeson [33] equation to calculate the fluid thermodynamic parameters (gas-liquid phase equilibrium composition, gas phase, and aqueous phase density, etc.) required during the iterative process. Parry’s method does not need to visually measure the volume or phase proportion of the inclusions, and density and XCO2 could be calculated using iterative method with the measurement of Th,CO2, S, and Th. Due to the Bowers-Helgeson equation, Parry’s iterative algorithm can be used to calculate the V-x parameters of H2O–NaCl–CO2 fluid inclusions with a homogenization temperature above 300 °C. However, Parry’s method ignores the solubility of CO2 in NaCl aqueous solution, so the mathematical function of XCO2 constructed and the calculated XCO2 is not sufficiently accurate, and then the pressure error calculated by iterative calculation is ineligible. Furthermore, Parry’s method cannot be applied for the H2O–NaCl–CO2system at 0–300 °C, due to the lack of validated fluid thermodynamic functions over this temperature range.
Liu and Shen [6], and Song [17] take into account the solubility of CO2 in aqueous sodium chloride solution at room temperature and improved the calculation accuracy of the Parry method. Song et al. [17] also used the more accurate H2O–NaCl–CO2 system state equation (DMW95 equation) established by Duan et al. [12] instead of the Bowers-Helgeson [17] equation. DMW95 is a state equation capable of accurately predicating P-V-T-X, phase equilibria, solubility, and activity of the H2O–NaCl–CO2 system. Since the DMW95 equation is only applicable to high temperature (T > 300 °C) H2O–NaCl–CO2 system, this improved calculation method is still not valid at complete homogenization temperature below 300 °C.
On the basis of Song’s method, Xi et al. [34]. proposed that the dissolved amount of CO2 in aqueous solution MCO2 can be calculated by the solubility model proposed by Duan and Sun [14,35]. This method can only be applied to H2O–NaCl–CO2 inclusions in which the CO2 partial homogenization temperature is higher than the melting temperature of the clathrate, and the halite daughter minerals are absent. The inclusion should also be completely homogeneous to aqueous solutions. Xi et al. [34] also suggested that for H2O–NaCl–CO2 inclusions with complete homogenization temperature below 300 °C and salinity below 4%, the ternary system can be approximated as a H2O–CO2 binary system, which expands the applicable range of temperature and pressure of the method.
Xu et al. [36] combined the improved algorithms of Song with the state equation established by Mao et al. [37] and developed an application capable of calculating V-x parameters of CO2–H2O fluid inclusions with a complete homogenization temperature below 350 °C, homogenization pressure below 100 MPa, which are completely homogenized to liquid phase.
Mao et al. [18] proposed a new algorithm for calculating the V-x parameters of H2O–NaCl–CO2 inclusions. The method uses the improved CO2 solubility model of them in combination with the previously established aqueous solution density model to calculate the relevant thermodynamic properties of the H2O–NaCl–CO2 system. A calculation program is also developed based on this new algorithm, which applies to a maximum homogenization temperature of 723 K, but only for H2O–NaCl–CO2 inclusions that are completely homogeneous to the liquid phase [18].
3. Calculation Program Description
3.1. A New Algorithm for V-x Parameters of H2O–NaCl–CO2 Two-Phase Inclusions at Room Temperature
As mentioned above, the iterative method initiated by Parry [32] and further improved by Song et al. [17] is only applicable to H2O–NaCl–CO2 three-phase inclusions without halite daughter minerals, and is not suitable for the two-phase inclusion of H2O–NaCl–CO2, in which the homogenization temperature of the CO2 phase is lower than the melting temperature of the CO2 clathrate.
This study proposes a new iterative algorithm to accurately calculate the V-x parameters of the H2O–NaCl–CO2 two-phase inclusion. The algorithm first uses the method of Diamond [21] to determine the salinity of H2O–NaCl–CO2 two-phase inclusions with respect to the melting temperature of CO2 clathrate and the partial homogenization temperature of CO2 phase measured under metastable state, and uses the three-phase equilibrium calculation model of CO2 hydrate established by Duan and Sun [15] to determine the internal pressure of inclusions when CO2 clathrate is completely melted. Then, the V-x parameters of the inclusions are iteratively solved by the relationship between the phase change and the volume change when the CO2 clathrate is melted and the inclusions are completely homogenized. The new algorithm also does not require a visual estimation of the volume fraction of the CO2 phase as an input parameter. Table 1 and Table 2 provide a brief description of the input and output parameters of the method and program

Table 1.
Input parameter of the program for H2O–NaC–CO2 inclusion.

Table 2.
Output parameters of the program for H2O–NaCl–CO2 inclusions.
The specific iterative steps of the algorithm, as shown in Figure 1, are described as follows:
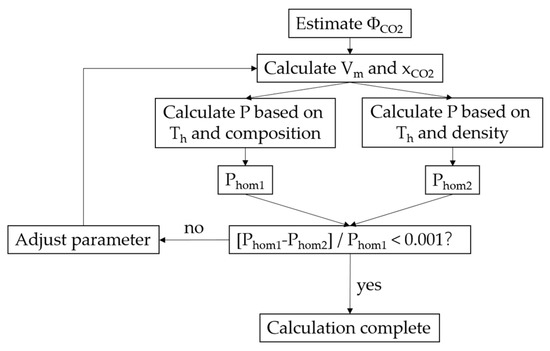
Figure 1.
Flowchart for the calculation of V-x properties of two-phase CO2–H2O–NaCl fluid inclusions.
1. After determining the salinity of the inclusions, the internal pressure of the inclusions after the carbon dioxide hydrate is completely melted is determined according to the gas hydrate three-phase equilibrium model, and then the density of the CO2 phase and the aqueous phase at this temperature are calculated using a proper thermodynamic model.
2. Calculating the total density (or molar volume) and CO2 concentration XCO2 of the inclusion based on an initial estimation of ΦCO2.
3. Determining the pressure Phoml at which the inclusions are at homogenization temperature Th, which refers to the minimum pressure at which the solution or gas phase is saturated at Th.
4. Calculating the pressure Phoml2 at which the inclusions with certain composition and total density exhibit a single phase at the homogenization temperature.
5. If Phoml and Phoml2 are significantly different, change ΦCO2 and re-calculate the total density (or molar volume) and XCO2 until the calculated relative difference of Phoml and Phoml2 is less than 0.1%. At this time, the final total density, XCO2, and ΦCO2 are obtained.
The above step 3 needs to calculate the gas-liquid equilibrium composition of the H2O–NaCl–CO2 system on the basis of a suitable equation of state, and step 4 requires the calculation of the P-V-T-x of the H2O–NaCl–CO2 system. The DMW95 equation is used when Th > 573 K, while the equation of state for the medium-low temperature H2O–NaCl–CO2 system established by Sun and Dubessy [13] is applicable when Th ≤ 573 K.
3.2. H2O–NaCl–CO2 Inclusion Parameter Calculation Program Description
Combining the iterative algorithm for calculating the V-x parameters of H2O–NaCl–CO2 inclusions with the related thermodynamic model introduced above, a computer program for the accurate calculation of V-x parameters of H2O–NaCl–CO2 inclusions is written in FORTRAN90 language, which only takes microthermometry measurement data as input parameters with no gas phase volume fraction required. Some calculation examples were also given below.
3.2.1. Program Function
An important feature of the calculation program developed in this study is that the input parameters are all microthermometry measurement data without visually measuring the CO2 phase volume fraction. The Raman peak displacement of CO2 is also not required as an input parameter to determine the internal pressure and composition of the inclusion. In this study, the improved algorithm of Song et al. [17] was used to calculate the V-x parameters of H2O–NaCl–CO2 inclusions in which the partial homogenization temperature of the CO2 phase is higher than the melting temperature of CO2 clathrate. For the V-x parameters of the H2O–NaCl–CO2 inclusions where the CO2 phase partial homogenization temperature is lower than the CO2 clathrate melting temperature, the new algorithm proposed in this study is applied, which is not only suitable for three-phase H2O–NaCl–CO2 inclusions but also for two-phase H2O–NaCl–CO2 inclusions at room temperature.
The calculation program developed in this paper is based on the state equation of a wide temperature and pressure range and high precision, thus it is more applicable than any existing calculation procedures in terms of temperature and pressure condition. The program is suitable for inclusions that are either completely homogeneous to the liquid phase or completely homogeneous to gas phase. Due to the inherent defects of the thermodynamic model, the program has a large V-x parameters calculation error for critically homogeneous and near-critically homogeneous inclusions, which requires further improvement.
3.2.2. Steps for Usage
The program is provided as Supplementary File S1. The procedure for calculating the V-x value of H2O–NaCl–CO2 inclusions is as follows:
1. Enter the microthermometry measurement parameters required for the calculation, including the partial homogenization temperature of the CO2 phase, the melting temperature of the CO2clathrates, and the complete homogenization temperature of the inclusions.
2. Select the CO2 phase partial homogenization mode and the inclusion completely homogenization mode, and run the Fortran executive program.
3. The calculation outputs all the seven parameters as shown in Table 2.
3.3. Calculation Example

Table 3.
Samples for V-x parameters of H2O–NaCl–CO2 inclusions (liquid CO2 + vapor CO2 homogenized to vapor phase).

Table 4.
Samples for V-x parameters of H2O–NaCl–CO2 inclusions (liquid CO2 + vapor CO2 homogenized to liquid phase).
4. Conclusions
The ore-forming fluid of hydrothermal gold ore can be approximated as H2O–NaCl–CO2 system. Therefore, it is important to determine the V-x parameters of H2O–NaCl–CO2 fluid inclusions.
A new algorithm is proposed in this study for the accurate calculation of the V-x parameters of H2O–NaCl–CO2 two-phase inclusions, i.e., inclusions with a CO2 phase homogenization temperature lower than the melting temperature of CO2 clathrate based on microthermometry measurement data.
A calculation program is also developed for the calculation of the V-x parameters of the H2O–NaCl–CO2 inclusions. The program uses the new algorithm proposed in this study to calculate the V-x parameters of H2O–NaCl–CO2 two-phase inclusions and uses the improved Parry algorithm to calculate the V-x parameters of H2O–NaCl–CO2 three-phase inclusions, i.e., the CO2 phase homogenization temperature is higher than the CO2 clathrate.
Supplementary Materials
The following are available online at https://www.mdpi.com/2075-163X/9/11/673/s1, File S1: calculation program.exe.
Author Contributions
Conceptualization, R.S.; methodology, R.S.; software, X.L.; resources, Z.L.; data curation, J.W.; visualization, J.W.; writing—original draft preparation, S.Y.; writing—review and editing, R.S.; supervision, R.S.; project administration, R.S.; funding acquisition, R.S.
Funding
This research was funded by the National Natural Science Foundation of China (No.41073049).
Acknowledgments
This article is an extension of my project when I was studying in Northwest University, and it under the guidance of Rui Sun. The authors thank Mengjue Wang from Peking University and Jie Zeng from China University of Geosciences (Beijing) for language polishing.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lu, H.Z.; Wang, Z.G.; Li, Y.S. Magma-Fluid Transition and the Genesis of Pegmatite Dike No.3, Altay, Xinjiang, Northwest China. Chin. J. Geochem. 1997, 16, 43–52. [Google Scholar] [CrossRef]
- Fyfe, W.S.; Price, N.J.; Thompson, A.B. Fluids in the Earth’s Crust; Elsevier: New York, NY, USA, 1979. [Google Scholar]
- Liu, J.M.; Chu, X.L.; Liu, W.; Sun, S.H.; Xu, C. Ore-Forming Fluid Systems in Crust. Prog. Geophys. 1997, 12, 31–40. (In Chinese) [Google Scholar]
- Liu, C.Q.; Zhang, B.R.; Fu, J.M. Recent Advances in Geochemistry of Geological Fluids/Progress in Geochemistry; Chemical Industry Press: Beijing, China, 2005. (In Chinese) [Google Scholar]
- Labotka, T.C. Chemical and Physical Properties of Fluids. Rev. Mineral. 1991, 26, 43–104. [Google Scholar]
- Bin, L.; Kun, S. Thermodynamics of Fluid Inclusions; Geological Publishing House: Beijing, China, 1999; Volume 2, pp. 44–72. (In Chinese) [Google Scholar]
- Wilkinson, J.J. Fluid Inclusions in Hydrothermal Ore Deposits. Lithos 2001, 55, 229–272. [Google Scholar] [CrossRef]
- Chi, G. An Overview on Current Fluid-Inclusion Research and Applications. Acta Petrol. Sin. 2003, 19, 201–212. [Google Scholar]
- Lu, H.Z.; Fan, H.R.; Ni, P.; Ou, G.X.; Shen, K.; Zhang, W.R. Fluid Inclusions; Science Press: Beijing, China, 2004. (In Chinese) [Google Scholar]
- Hu, W.X.; Sun, R.; Zhang, W.L.; Sun, G.X. Characteristics of Gold Ore-Forming Fluids and Metallogenic Process by Mutual Mixing and Acting of Deep-Derived Fleids and Shallow-Seeped Ones. Earth Sci. Front. 2001, 8, 281–288. (In Chinese) [Google Scholar]
- Chen, Y.J.; Ni, P.; Fan, H.R.; Pirajno, F.; Lai, Y.; Su, W.C.; Zhang, R. Diagnostic Fluid Inclusion of Different Types Hydrothermal Gold Deposits. Acta Petrol. Sin. 2007, 23, 2085–2108. (In Chinese) [Google Scholar]
- Duan, Z.; Møller, N.; Weare, J.H. Equation of State for the NaCl–H2O–CO2 System: Prediction of Phase Equilibria and Volumetric Properties. Geochim. Cosmochim. Acta 1995, 59, 2869–2882. [Google Scholar] [CrossRef]
- Rui, S.; Dubessy, J. Prediction of Vapor–Liquid Equilibrium and Pvtx Properties of Geological Fluid System with Saft-Lj Eos Including Multi-Polar Contribution. Part II: Application to H2O–NaCl and CO2–H2O–NaCl System. Geochim. Cosmochim. Acta 2012, 88, 130–145. [Google Scholar]
- Duan, Z.; Sun, R. An Improved Model Calculating CO2 Solubility in Pure Water and Aqueous Nacl Solutions from 273 to 533 K and from 0 to 2000 Bar. Chem. Geol. 2003, 193, 257–271. [Google Scholar] [CrossRef]
- Duan, Z.; Sun, R. A Model to Predict Phase Equilibrium of Ch4 and CO2 Clathrate Hydrate in Aqueous Electrolyte Solutions. Am. Mineral. 2006, 91, 1346–1354. [Google Scholar] [CrossRef]
- Duan, Z.; Mao, S. A Thermodynamic Model for Calculating Methane Solubility, Density and Gas Phase Composition of Methane-Bearing Aqueous Fluids from 273 to 523 K and from 1 to 2000 Bar. Geochim. Cosmochim. Acta 2006, 70, 3369–3386. [Google Scholar] [CrossRef]
- Song, Y.; Hu, W.; Ni, P.; Duan, Z.; Zhang, X. Improved Method to Determine the Molar Volume and Compositions of the NaCl–H2O–CO2 System Inclusion. Sci. China Ser. D Earth Sci. 2007, 50, 385–391. [Google Scholar] [CrossRef]
- Mao, S.; Zhang, D.; Li, Y.; Liu, N. An Improved Model for Calculating CO2 Solubility in Aqueous NaCl Solutions and the Application to CO2–H2O–NaCl Fluid Inclusions. Chem. Geol. 2013, 347, 43–58. [Google Scholar] [CrossRef]
- Bozzo, A.T.; Chen, H.S.; Kass, J.R.; Barduhn, A.J. The Properties of the Hydrates of Chlorine and Carbon Dioxide. Desalination 1975, 16, 303–320. [Google Scholar] [CrossRef]
- Chen, H.S. The thermodynamics and composition of carbon dioxide hydrate. Ph.D. Thesis, Syracuse University, Syracuse, NY, USA, 1972. [Google Scholar]
- Diamond, L.W. Stability of CO2 Clathrate Hydrate + CO2 Liquid + CO2 Vapour + Aqueous KCl-NaCl Solutions: Experimental Determination and Application to Salinity Estimates of Fluid Inclusions. Geochim. Cosmochim. Acta 1992, 56, 273–280. [Google Scholar] [CrossRef]
- Fall, A.; Tattitch, B.; Bodnar, R.J. Combined Microthermometric and Raman Spectroscopic Technique to Determine the Salinity of H2O–CO2–NaCl Fluid Inclusions Based on Clathrate Melting. Geochim. Cosmochim. Acta 2011, 75, 951–964. [Google Scholar] [CrossRef]
- Bakker, R.J.; Dubessy, J.; Cathelineau, M. Improvements in Clathrate Modelling: I. The H2O–CO2 System with Various Salts. Geochim. Cosmochim. Acta 1996, 60, 1657–1681. [Google Scholar] [CrossRef]
- Duan, Z.; Møller, N.; Weare, J.H. An Equation of State for the CH4–CO2–H2O System: I. Pure Systems from 0 to 1000 °C and 0 to 8000 Bar. Geochim. Cosmochim. Acta 1992, 56, 2605–2617. [Google Scholar] [CrossRef]
- Pitzer, K.S. Thermodynamics of Electrolytes. I. Theoretical Basis and General Equations. J. Phys. Chem. 1973, 77, 268–277. [Google Scholar] [CrossRef]
- Brown, P.E.; Hagemann, S.G. Macflincor and Its Application to Fluids in Archean Lode-Gold Deposits. Geochim. Cosmochim. Acta 1995, 59, 3943–3952. [Google Scholar] [CrossRef]
- Bakker, R.J. Package Fluids 1. Computer Programs for Analysis of Fluid Inclusion Data and for Modelling Bulk Fluid Properties. Chem. Geol. 2003, 194, 3–23. [Google Scholar] [CrossRef]
- Schwartz, M.O. Determining Phase Volumes of Mixed CO2–H2O Inclusions Using Microthermometric Measurements. Miner. Depos. 1989, 24, 43–47. [Google Scholar] [CrossRef]
- Bakker, R.J.; Diamond, L.W. Determination of the Composition and Molar Volume of H2O–CO2 Fluid Inclusions by Microthermometry. Geochim. Cosmochim. Acta 2000, 64, 1753–1764. [Google Scholar] [CrossRef]
- Diamond, L.W. Review of the Systematics of CO2–H2O Fluid Inclusions. Lithos 2001, 55, 69–99. [Google Scholar] [CrossRef]
- Sterner, S.M.; Bodnar, R.J. Synthetic Fluid Inclusions; X, Experimental Determination of P-V-T-X Properties in the CO2–H2O System to 6 Kb and 700 Degrees C. Am. J. Sci. 1991, 291, 1–54. [Google Scholar] [CrossRef]
- Parry, W.T. Estimation of Xco2, P and Fluid Inclusion Volume from Fluid Inclusion Temperature Measurements in the System Nacl-H2O-CO2. Econ. Geol. 1986, 81, 1009–1013. [Google Scholar] [CrossRef]
- Bowers, T.S.; Helgeson, H.C. Calculation of the Thermodynamic and Geochemical Consequences of Nonideal Mixing in the System H2O–CO2–NaCl on Phase Relations in Geologic Systems: Equation of State for H2O–CO2–NaCl Fluids at High Pressures and Temperatures. Geochim. Cosmochim. Acta 1983, 47, 1247–1275. [Google Scholar] [CrossRef]
- Xi, B.B.; Shi, W.J.; Zhang, D.H.; Xu, W.G.; Jiang, H.; Wang, C. Improvements and application of iterative Method for Calculating Homogenization Pressure of H2O–CO2–NaCl Inclusion System. Miner. Depos. 2010, 29, 1138–1144. (In Chinese) [Google Scholar]
- Duan, Z.; Sun, R.; Zhu, C.; Chou; Ming, I. An Improved Model for the Calculation of CO2 Solubility in Aqueous Solutions Containing Na+, K+, Ca2+, Mg2+, Cl−, and SO24−. Mar. Chem. 2006, 98, 131–139. [Google Scholar] [CrossRef]
- Xu, W.G.; Zhang, D.H.; Huang, Z.F.; Xi, B.B.; Fan, H.R. A New Method Used to Calculate the Homogenization Pressure and Related Thermodynamic Parameters of CO2–H2O System at T < 623.15 K and P < 100 MPA. Geol. Rev. 2012, 58, 175–182. (In Chinese) [Google Scholar]
- Shide, M.; Zhenhao, D.; Wenxuan, H. A Vapor—Liquid Phase Equilibrium Model for Binary CO2–H2O and CH4–H2O Systems above 523K for Application to Fluid Inclusions. J. Supercrit. Fluids 2009, 50, 13–21. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).