Distribution of “Invisible” Noble Metals between Pyrite and Arsenopyrite Exemplified by Minerals Coexisting in Orogenic Au Deposits of North-Eastern Russia
Abstract
1. Introduction
2. Previous Studies and Problem Statement
3. Objects
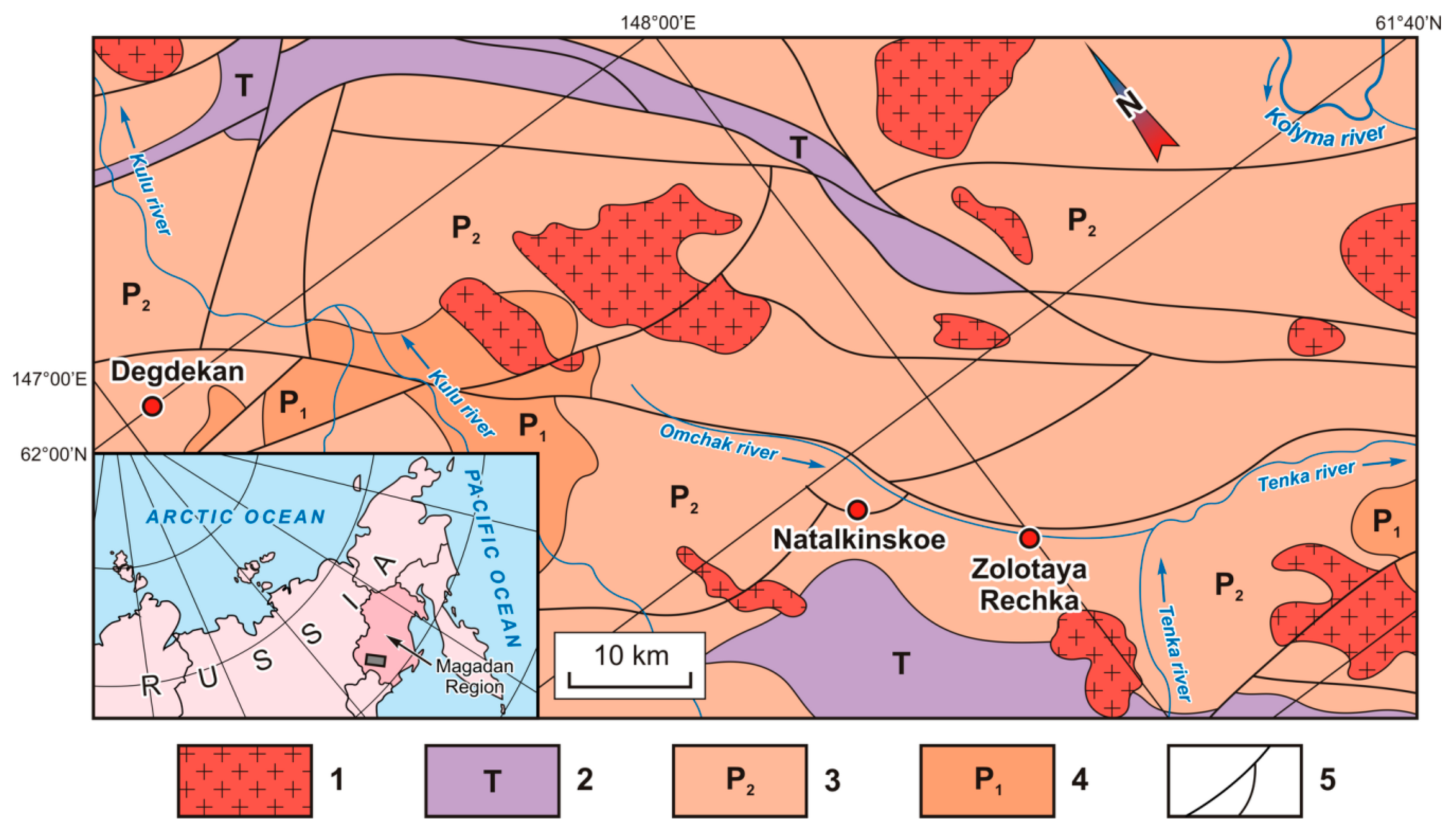
3.1. The Natalkinskoe Deposit
3.2. The Degdekan Deposit
3.3. The Zolotaya Rechka Deposit
4. Methods
4.1. Electron Probe Microanalysis (EPMA)
4.2. X-Ray Diffraction (XRD)
4.3. Atomic Absorption Spectrometry with ADSSC Data Processing (AAS-ADSSC)
4.4. Laser Ablation Inductively Coupled Plasma Mass Spectrometry (LA-ICP-MS)
5. Results
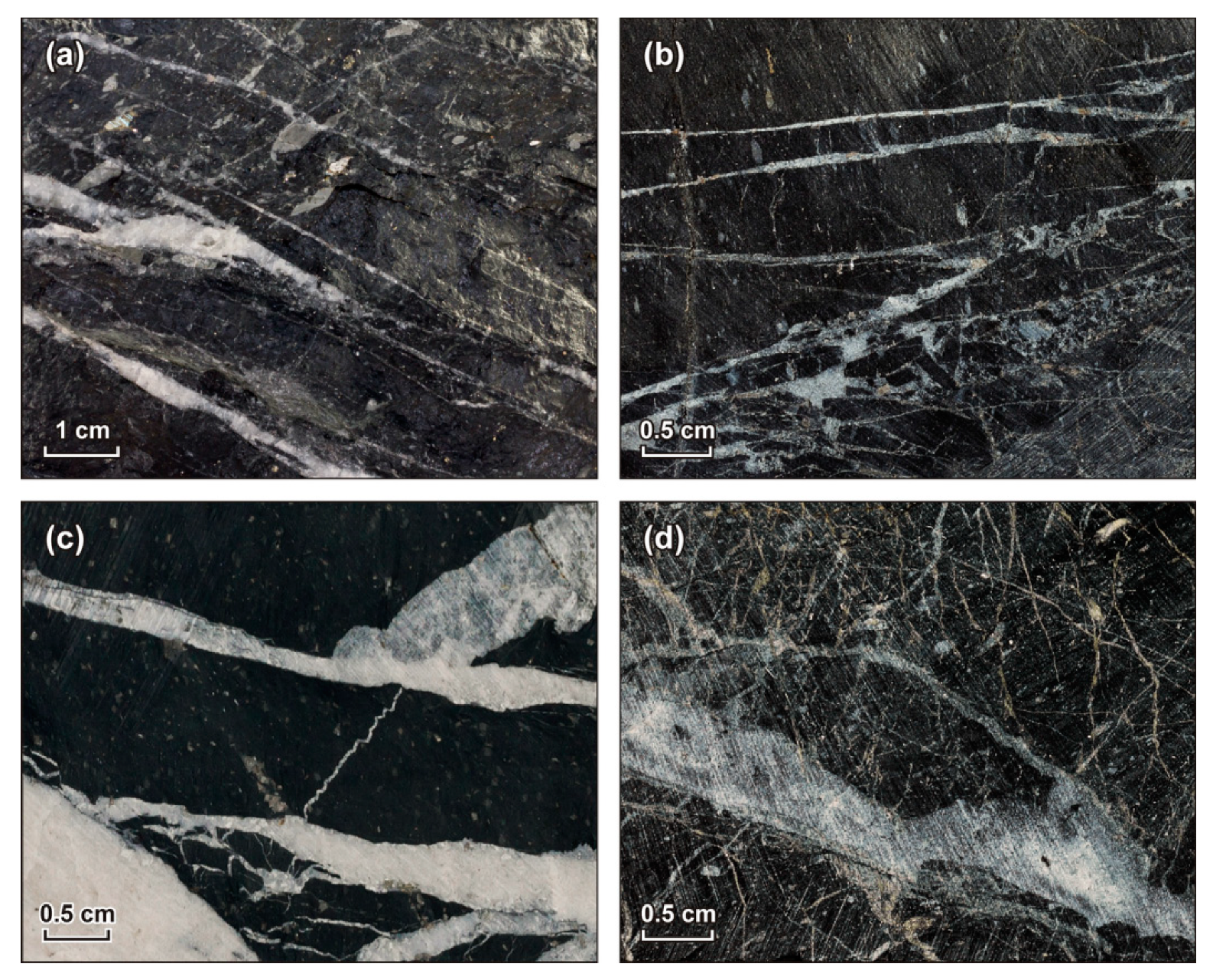
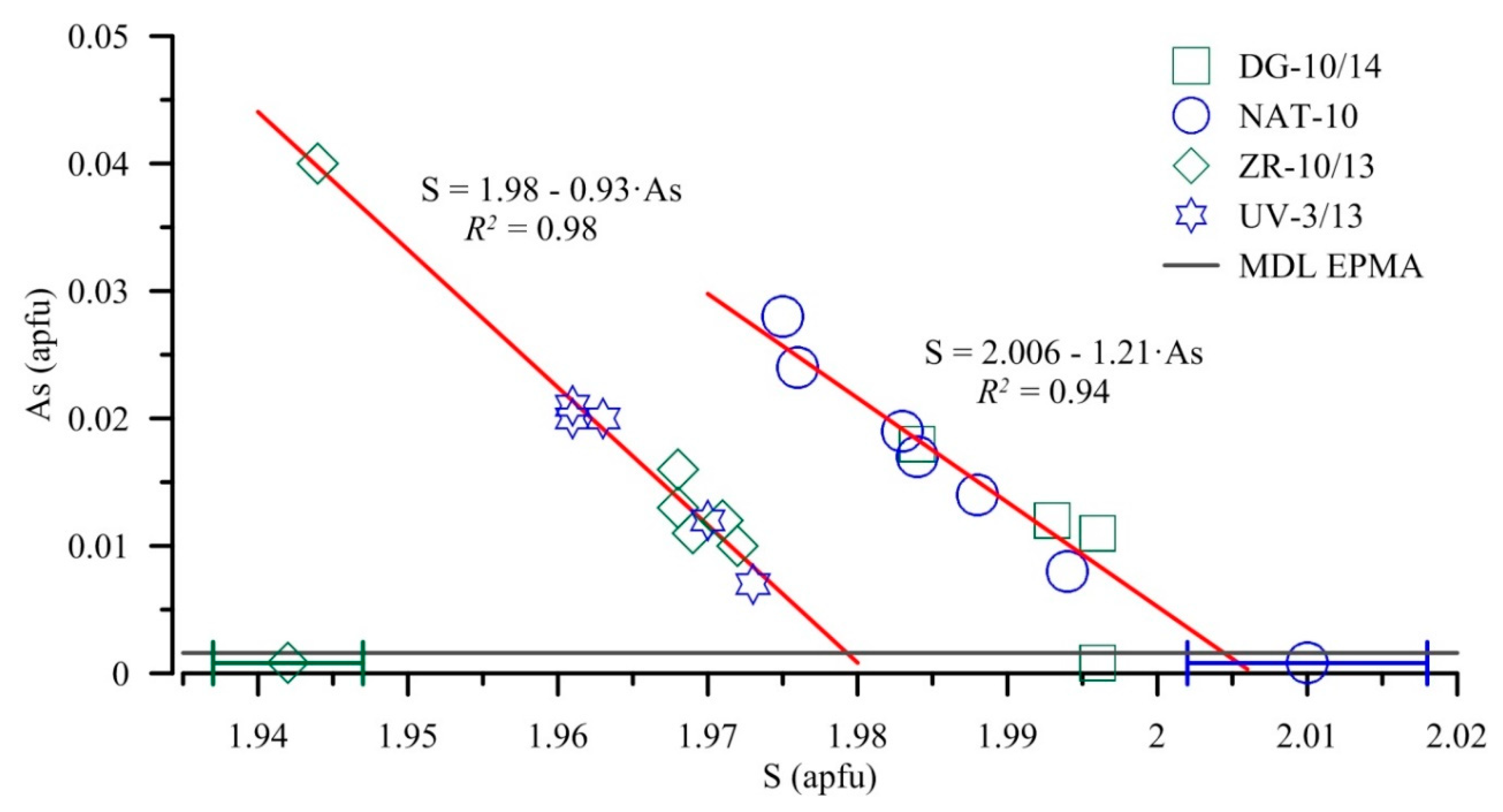
5.1. EPMA
5.2. XRD
5.3. AAS-ADSSC
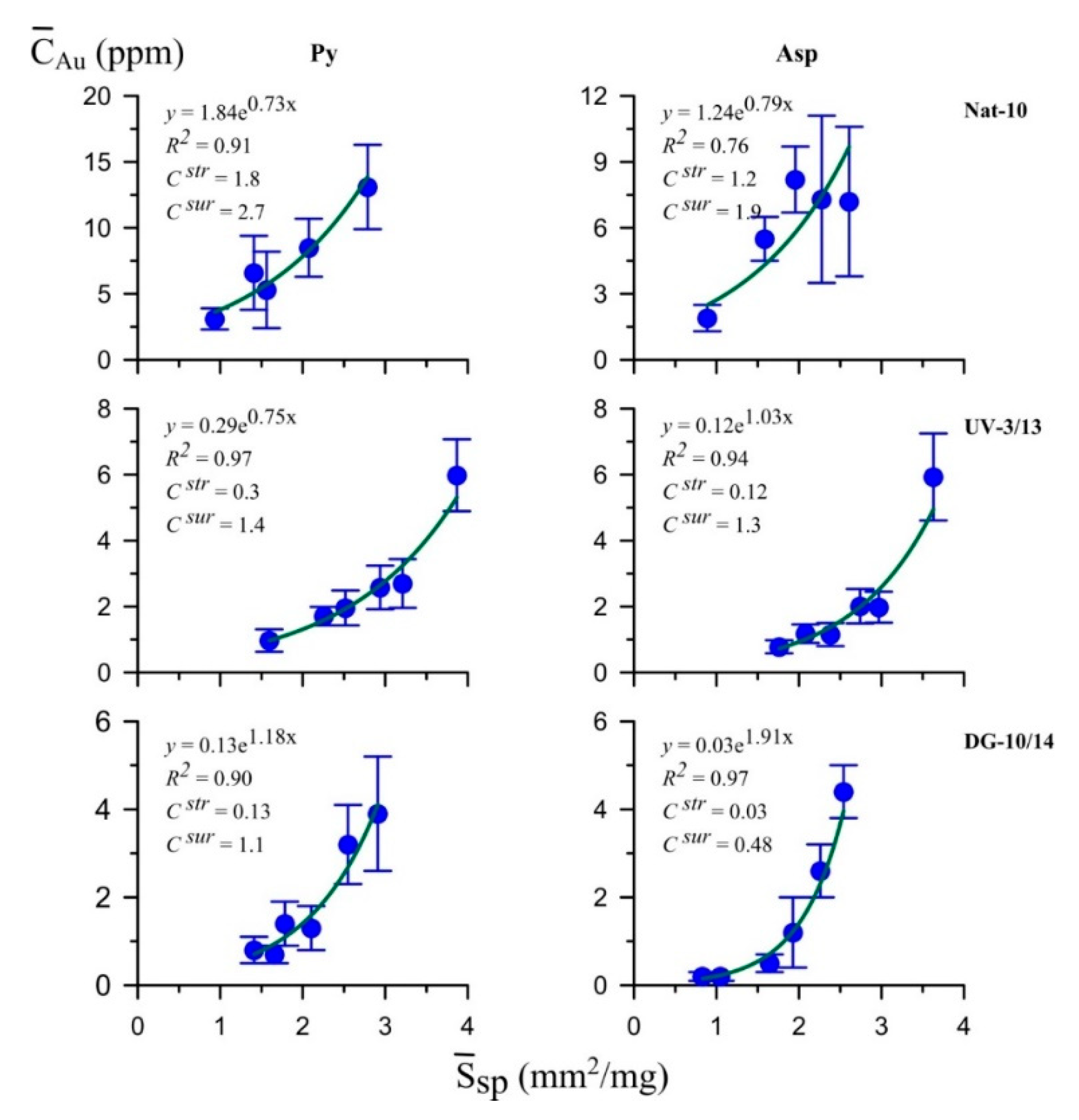
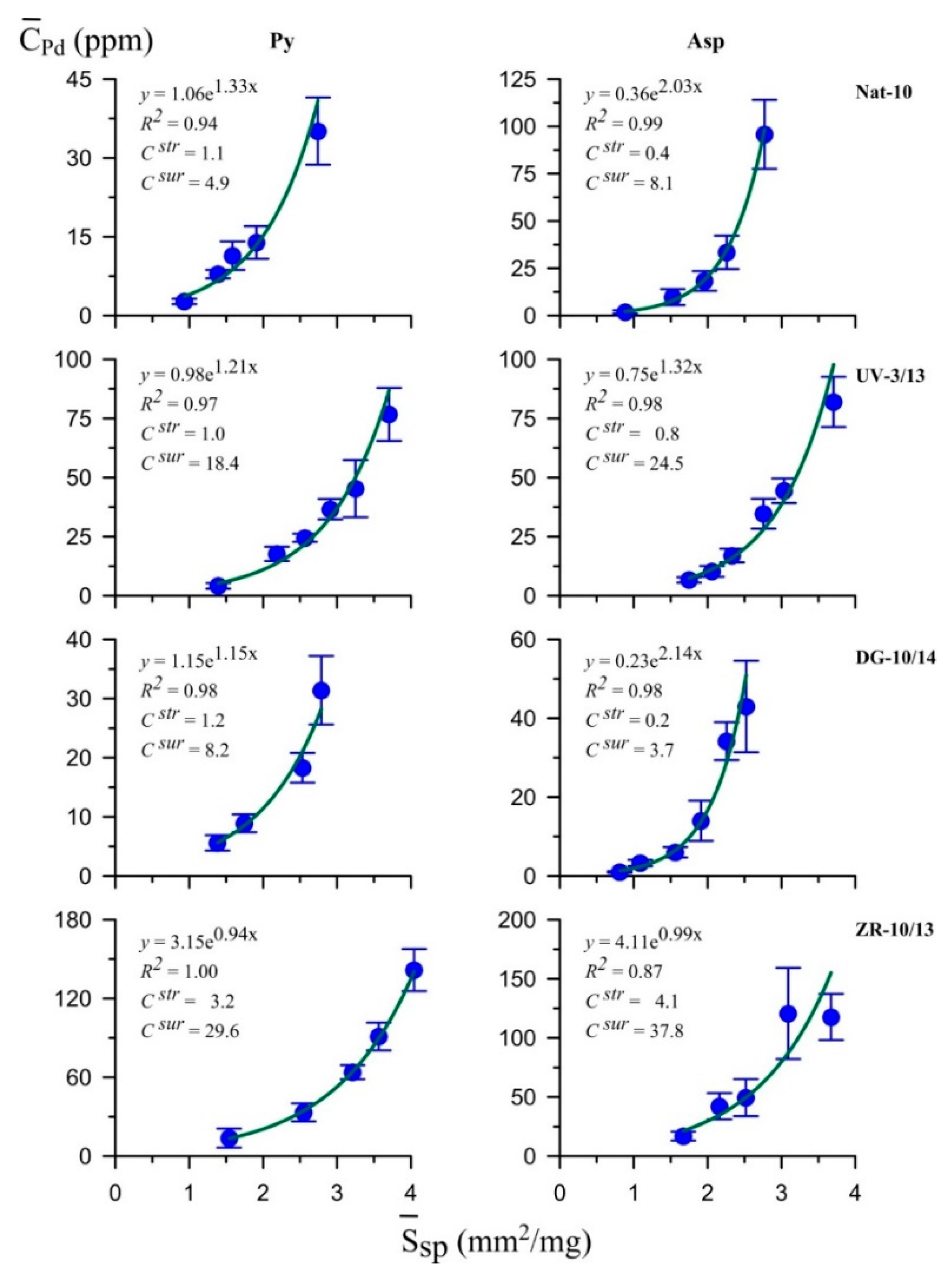
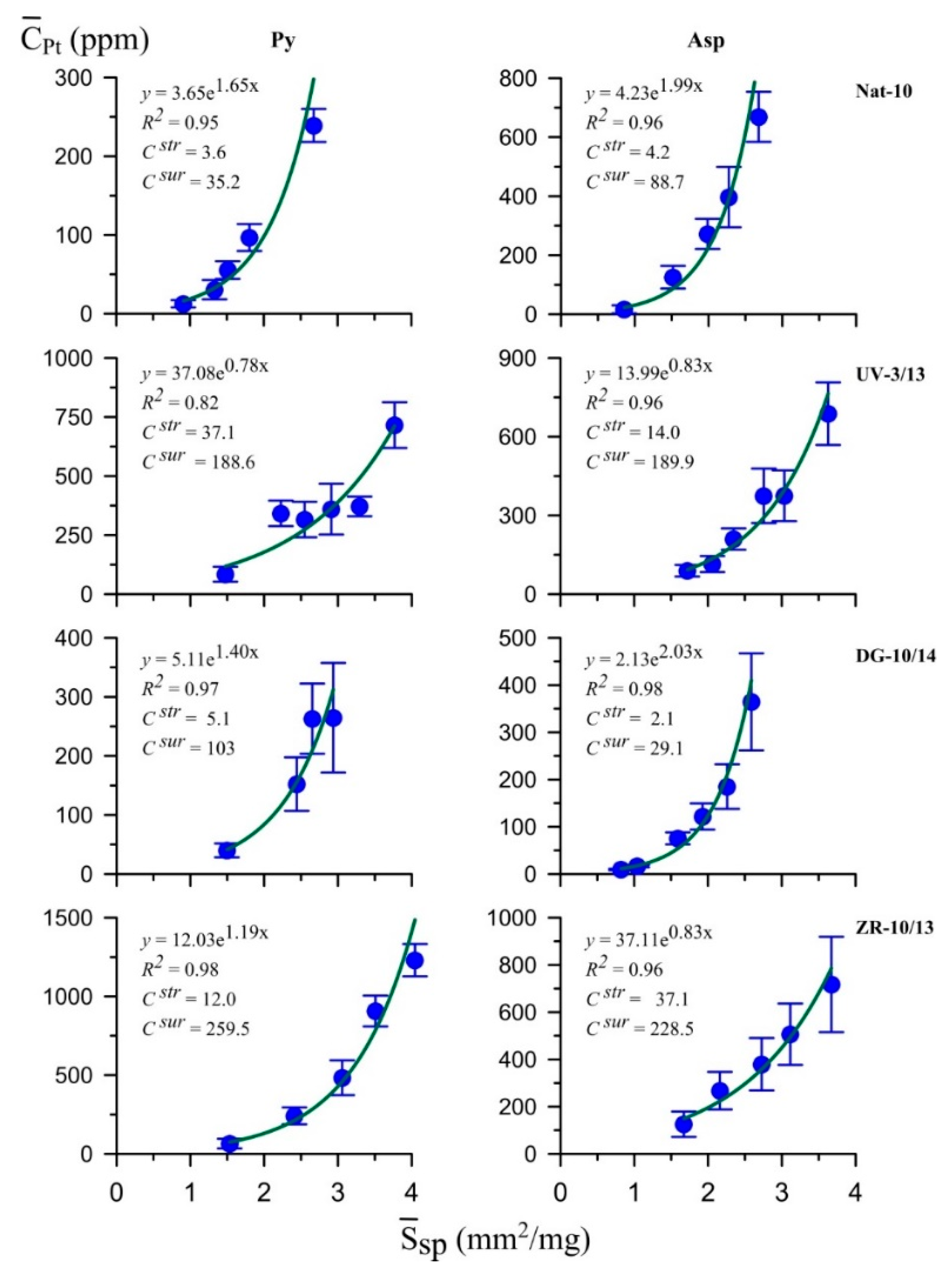
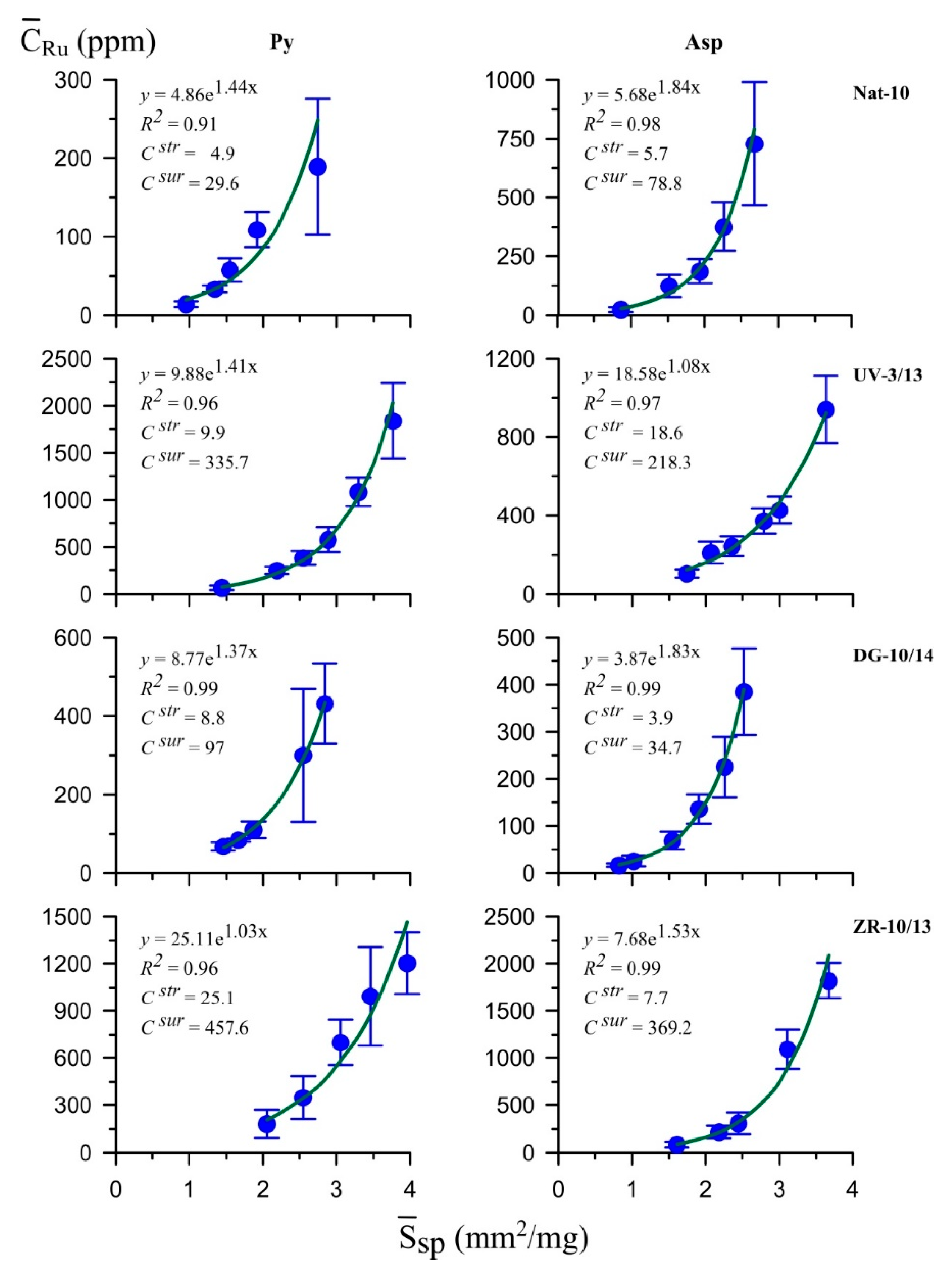
5.4. LA-ICP-MS
6. Discussion
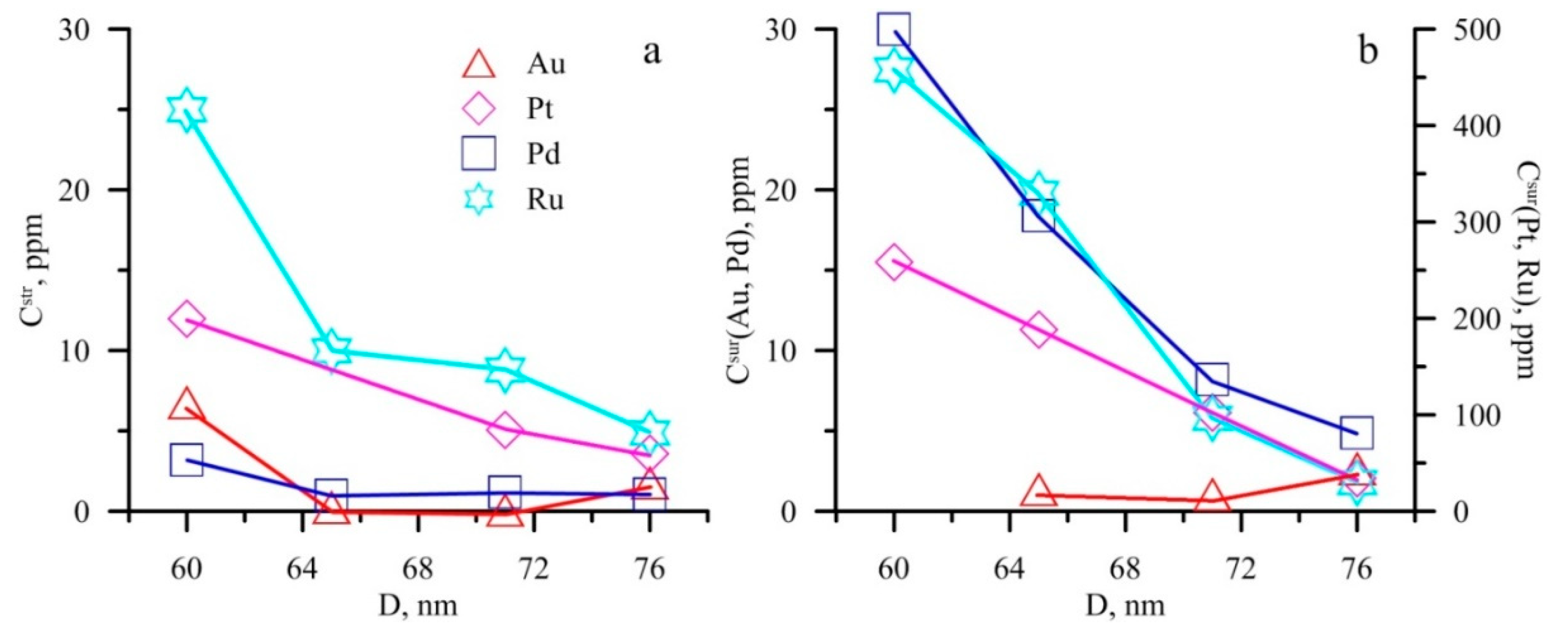
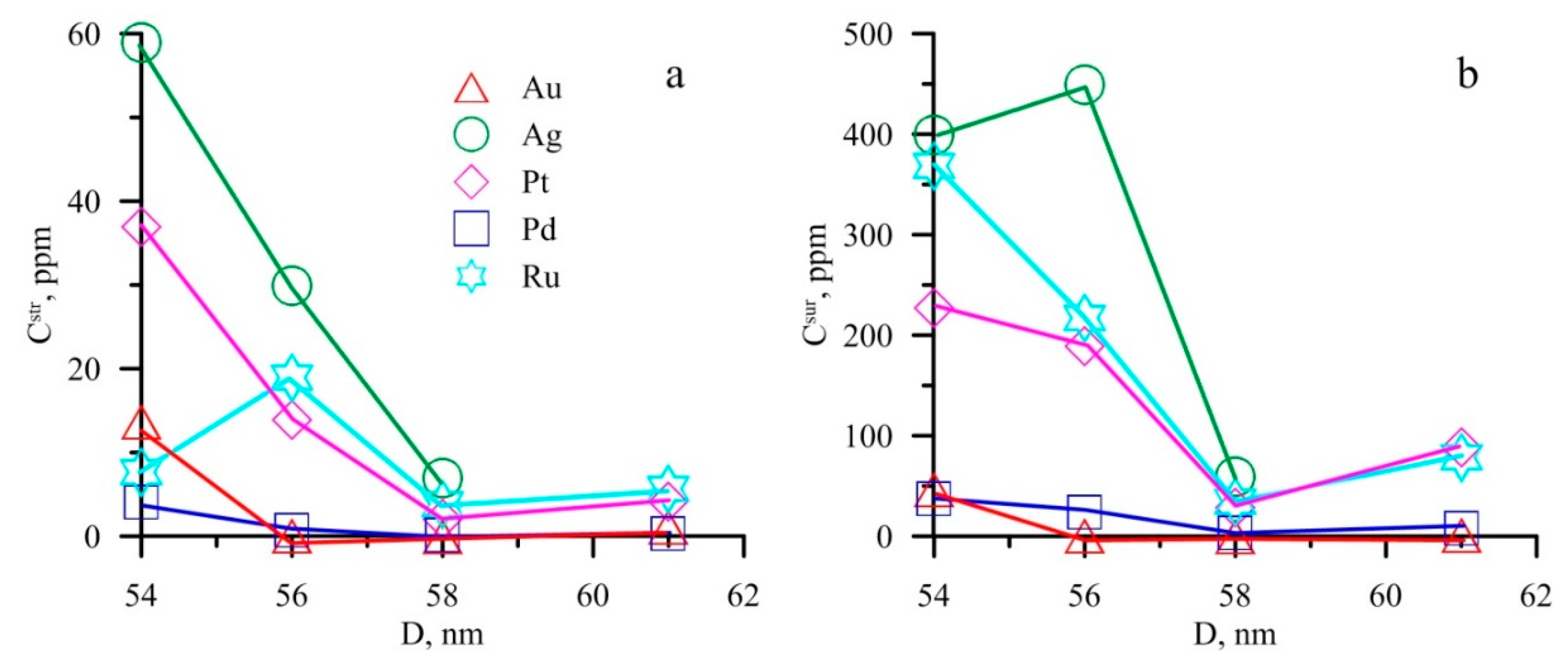
6.1. Surficial NM Accumulation
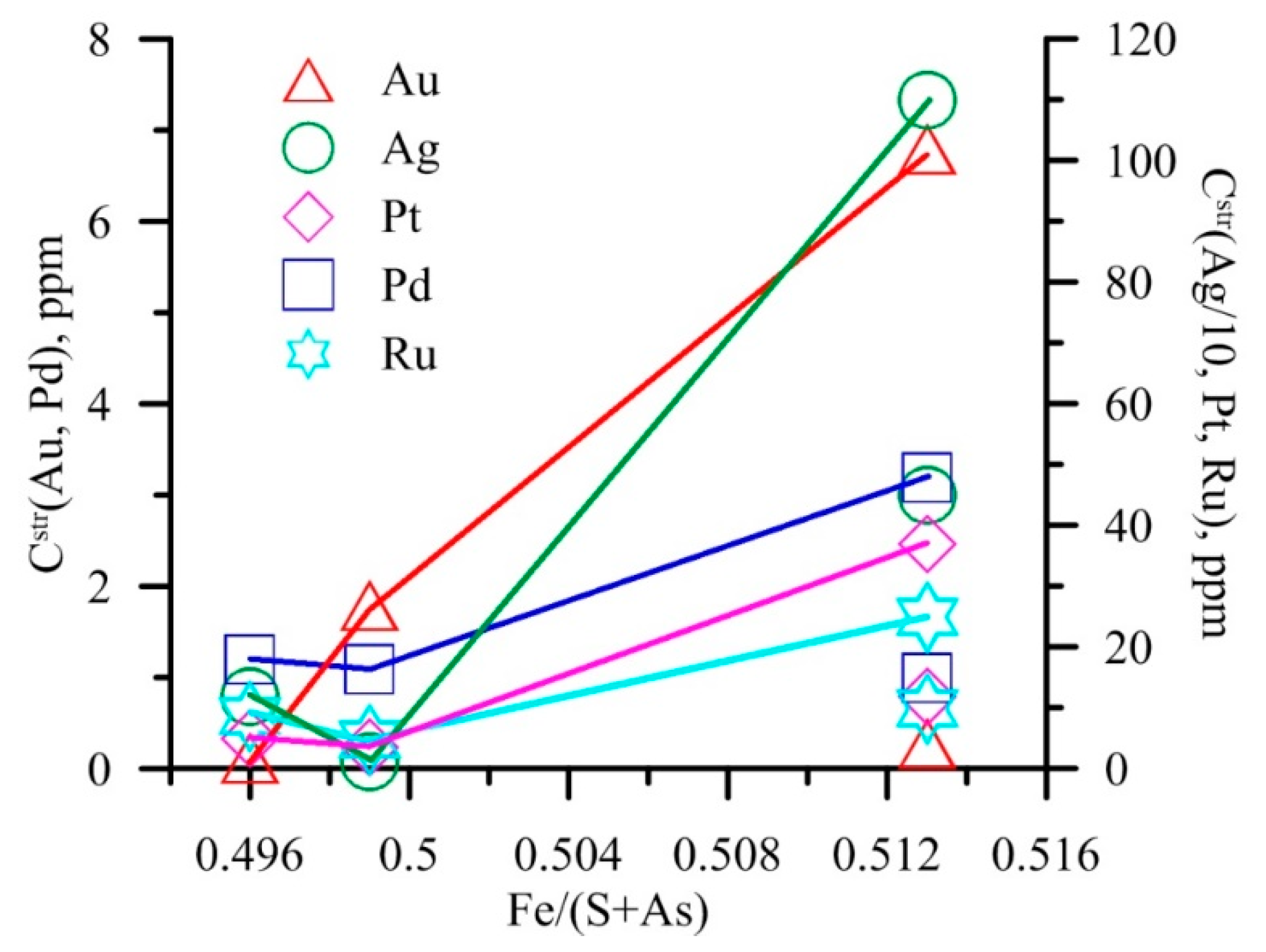
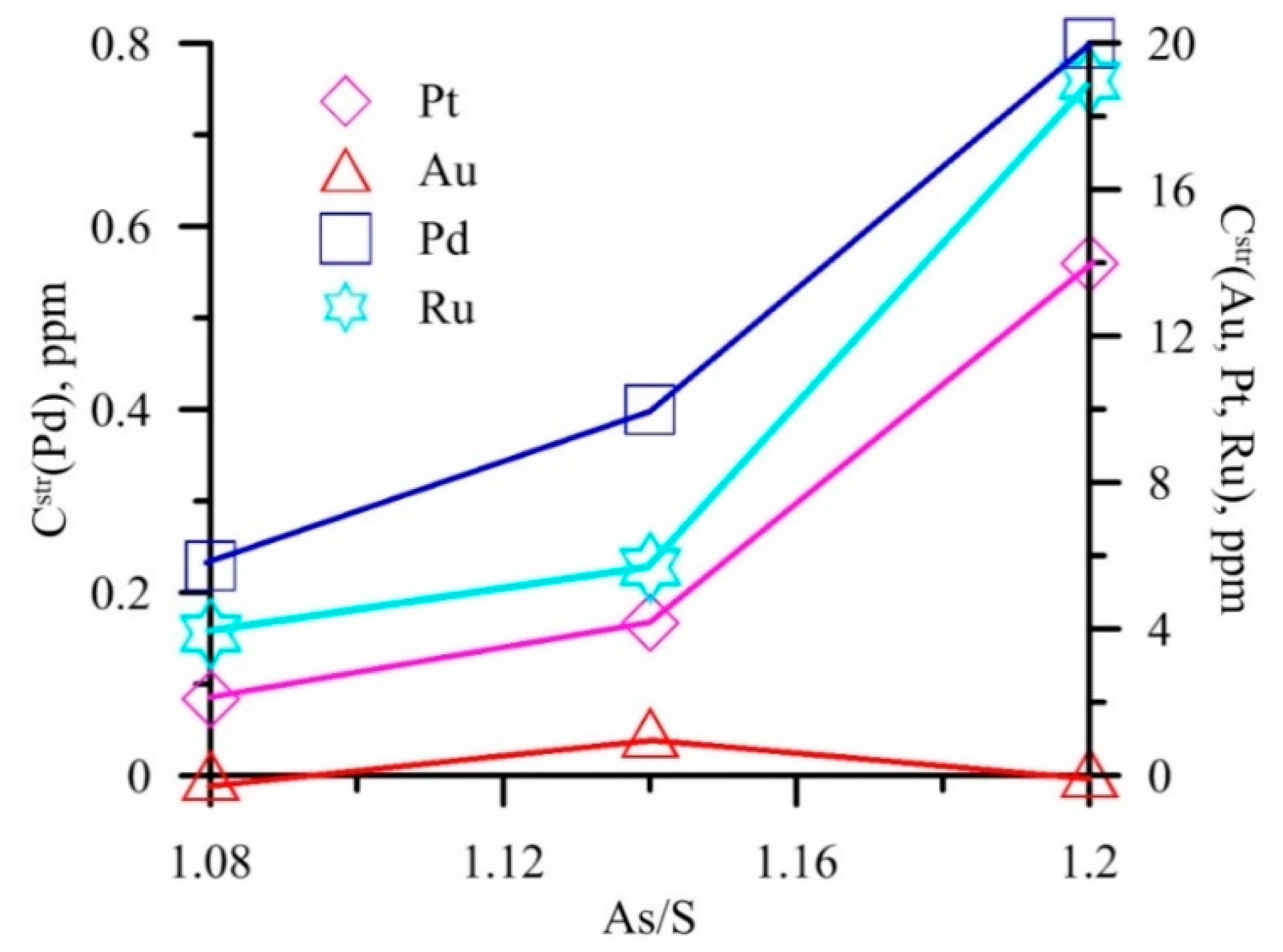
6.2. Arsenic in Pyrite: Assistance in NM Incorporation
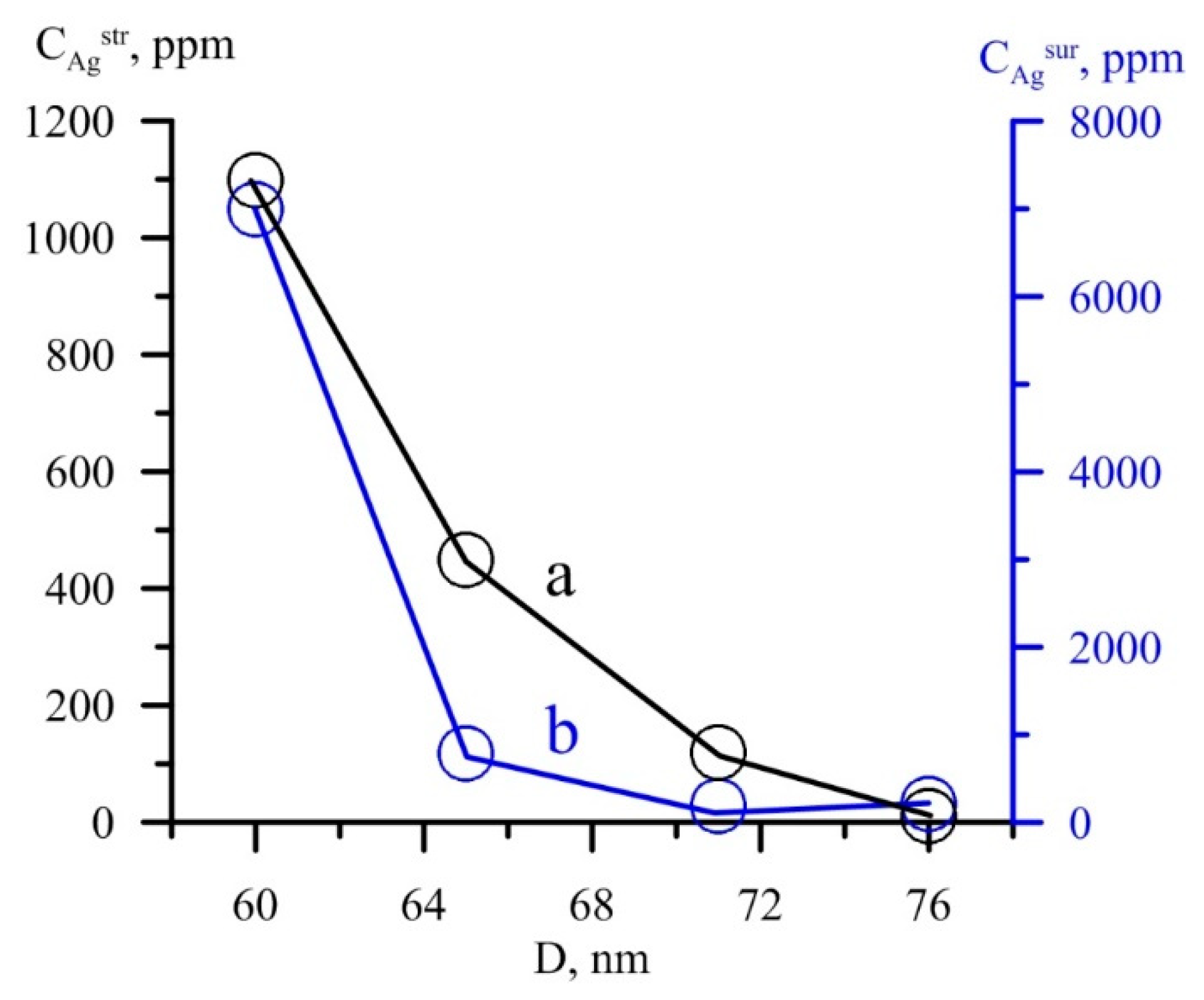
6.3. Crystallite Sizes
6.4. Non-Stoichiometry
6.5. Non-Precise Metal Impurities
6.6. Effect of Crystal Inhomogeneity
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gervilla, F.; Papunen, H.; Kojonen, K.; Johanson, B. Platinum-, palladium- and gold-rich arsenide ores from the Kylmäkoski Ni-Cu deposit (Vammala Nickel Belt, SW Finland). Mineral. Petrol. 1998, 64, 163–185. [Google Scholar] [CrossRef]
- Dare, S.A.S.; Barnes, S.-J.; Prichard, H.M. The distribution of platinum group elements and other chalcophile elements among sulfides from the Creighton Ni-Cu-PGE sulfide deposit, Sudbury, Canada, and the origin of Pd in pentlandite. Miner. Depos. 2010, 45, 765–793. [Google Scholar] [CrossRef]
- Cafagna, F.; Jugo, P.J. An experimental study on the geochemical behavior of highly siderophile elements (HSE) and metalloids (As, Se, Sb, Te, Bi) in a mss-iss-pyrite system at 650 °C: A possible magmatic origin for Co-HSE-bearing pyrite and the role of metalloid-rich phases in the fractionation of HSE. Geochim. Cosmochim. Acta 2016, 178, 233–258. [Google Scholar] [CrossRef]
- Duran, C.J.; Barnes, S.-J.; Corkery, J.T. Chalcophile and platinum-group element distribution in pyrites from the sulfide-rich pods of the Lac des Iles Pd deposit, Western Ontario, Canada: Implications for post-cumulus re-equilibration of the ore and the use of pyrite composition in exploration. J. Geochem. Explor. 2015, 158, 223–242. [Google Scholar] [CrossRef]
- Pašava, J.; Zaccarini, F.; Aiglsperger, T.; Vymazalová, A. Platinum-group elements (PGE) and their principal carriers in metal-rich black shales: An overview with a new data from Mo-Ni-PGE black shales (Zunyi region, Guizhou Province, south China). J. Geosci. 2013, 58, 209–216. [Google Scholar] [CrossRef]
- Reich, M.; Kesler, S.E.; Utsunomiya, S.; Palenik, C.S.; Chryssoulis, S.L.; Ewing, R.C. Solubility of gold in arsenian pyrite. Geochim. Cosmochim. Acta 2005, 69, 2781–2796. [Google Scholar] [CrossRef]
- Deditius, A.P.; Reich, M.; Kesler, S.E.; Utsunomiya, S.; Chryssoulis, S.L.; Walshe, J.; Ewing, R.C. The coupled geochemistry of Au and As in pyrite from hydrothermal ore deposit. Geochim. Cosmochim. Acta 2014, 140, 644–670. [Google Scholar] [CrossRef]
- Dare, S.A.S.; Barnes, S.-J.; Prichard, H.M.; Fisher, P.C. Chalcophile and platinum-group element (PGE) concentrations in the sulfide minerals from the McCreedy East deposit, Sudbury, Canada, and the origin of PGE in pyrite. Miner. Depos. 2011, 46, 381–407. [Google Scholar] [CrossRef]
- Filimonova, O.N.; Nickolsky, M.S.; Trigub, A.L.; Chareev, D.A.; Kvashnina, K.O.; Kovalchuk, E.V.; Vikentyev, I.V.; Tagirov, B.R. The state of platinum in pyrite studied by X-ray absorption spectroscopy of synthetic crystals. Econ. Geol. in press.
- Gervilla, F.; Cabri, L.J.; Kojonen, K.; Oberthür, T.; Weiser, T.W.; Johanson, B.; Sie, S.H.; Campbell, J.L.; Teesdale, W.J.; Laflamme, J.H.G. Platinum-group element distribution in some ore deposits: Results of EPMA and Micro-PIXE analyses. Microchim. Acta 2004, 147, 167–173. [Google Scholar] [CrossRef]
- Parviainen, A.; Gervilla, F.; Melgarejo, J.-C.; Johanson, B. Low-temperature, platinum-group elements-bearing Ni arsenide assemblage from the Atrevida mine (Catalonian Coastal Ranges, NE Spain). Neues Jahrb. Mineral. Abh. 2008, 185, 33–49. [Google Scholar] [CrossRef]
- Tauson, V.L.; Parkhomenko, I.Y.; Babkin, D.N.; Men’shikov, V.I.; Lustenberg, E.E. Cadmium and mercury uptake by galena crystals under hydrothermal growth: A spectroscopic and element thermo-release atomic absorption study. Eur. J. Mineral. 2005, 17, 599–610. [Google Scholar] [CrossRef]
- Tauson, V.L.; Lipko, S.V.; Smagunov, N.V.; Kravtsova, R.G. Trace element partitioning dualism under mineral-fluid interaction: Origin and geochemical significance. Minerals 2018, 8, 282. [Google Scholar] [CrossRef]
- Tauson, V.L.; Lipko, S.V.; Arsent’ev, K.Y.; Mikhlin, Y.L.; Babkin, D.N.; Smagunov, N.V.; Pastushkova, T.M.; Voronova, I.Y.; Belozerova, O.Y. Dualistic distribution coefficients of trace elements in the system mineral-hydrothermal solution. IV. Platinum and silver in pyrite. Geochem. Int. 2017, 55, 753–774. [Google Scholar] [CrossRef]
- Ballhaus, C.; Sylvester, P. Noble metal enrichment processes in the Merensky Reef, Bushveld Complex. J. Petrol. 2000, 41, 545–561. [Google Scholar] [CrossRef]
- Morishita, Y.; Shimada, N.; Shimada, K. Invisible gold in arsenian pyrite from high-grade Hishikari gold deposit, Japan: Significance of variation and distribution of Au/As ratio in pyrite. Ore Geol. Rev. 2018, 95, 79–93. [Google Scholar] [CrossRef]
- Akimov, V.V.; Gerasimov, I.N.; Rigin, A.V. Behavior of gold nanoparticles in formation of surficial sulfide micro- and nanophases on substrates of single crystals of arsenopyrite and chalcopyrite. AIP Conf. Proc. 2019, 2064, 030001. [Google Scholar] [CrossRef]
- Sazonov, A.M.; Kirik, S.D.; Silyanov, S.A.; Bayukov, O.A.; Tishin, P.A. Typomorphism of arsenopyrite from the Blagodatnoe and Olimpiada gold deposits (Yenisei Ridge). Mineralogiya 2016, 3, 53–70. (In Russian) [Google Scholar]
- Tauson, V.L.; Mironov, A.G.; Smagunov, N.V.; Bugaeva, N.G.; Akimov, V.V. Gold in sulfides: State of art of occurrence and perspectives for experimental studies. Geol. Geofiz. 1996, 37, 3–14. (In Russian) [Google Scholar]
- Pina, R.; Gervilla, F.; Barnes, S.-J.; Ortega, L.; Lunar, R. Partition coefficients of platinum group and chalcophile elements between arsenide and sulfide phases as determined in the Beni Bousera Cr-Ni mineralization. Econ. Geol. 2013, 108, 935–951. [Google Scholar] [CrossRef]
- Kravtsova, R.G.; Tauson, V.L.; Nikitenko, E.M. Modes of Au, Pt, and Pd occurrence in arsenopyrite from the Natalkinskoe deposit, NE Russia. Geochem. Int. 2015, 53, 964–972. [Google Scholar] [CrossRef]
- Cook, N.J.; Ciobanu, C.L.; Meria, D.; Silcock, D.; Wade, B. Arsenopyrite-pyrite association in an orogenic gold ore: Tracing mineralization history from textures and trace elements. Econ. Geol. 2013, 108, 1273–1283. [Google Scholar] [CrossRef]
- Su, W.; Zhang, H.; Hu, R.; Ge, X.; Xia, B.; Chen, Y.; Zhu, C. Mineralogy and geochemistry of gold-bearing arsenian pyrite from the Shuiyindong Carlin-type gold deposit, Guizhou, China: Implications for gold depositional processes. Mineral. Depos. 2012, 47, 653–662. [Google Scholar] [CrossRef]
- Deol, S.; Deb, M.; Large, R.R.; Gilbert, S. LA-ICPMS and EPMA studies of pyrite, arsenopyrite and loellingite from the Bhukia-Jagpura gold prospect, southern Rajasthan, India: Implications for ore genesis and gold remobilization. Chem. Geol. 2012, 326–327, 72–87. [Google Scholar] [CrossRef]
- Benzaazoua, M.; Marion, P.; Robaut, F.; Pinto, A. Gold-bearing arsenopyrite and pyrite in refractory ores: Analytical refinements and new understanding of gold mineralogy. Mineral. Mag. 2007, 71, 123–142. [Google Scholar] [CrossRef]
- Hazarika, P.; Mishra, B.; Pruseth, K.L. Trace-element geochemistry of pyrite and arsenopyrite: Ore genetic implications for late Archean orogenic gold deposits in southern India. Mineral. Mag. 2017, 81, 661–678. [Google Scholar] [CrossRef]
- Kojonen, K.; Johanson, B. Determination of refractory gold distribution by microanalysis, diagnostic leaching and image analysis. Mineral. Petrol. 1999, 67, 1–19. [Google Scholar] [CrossRef]
- Hinchey, J.G.; Wilton, D.H.C.; Tubrett, M.N. A LAM-ICP-MS study of the distribution of gold in arsenopyrite from the Lodestar prospect, Newfoundland, Canada. Can. Mineral. 2003, 41, 353–364. [Google Scholar] [CrossRef]
- Wagner, T.; Klemd, R.; Wenzel, T.; Mattsson, B. Gold upgrading in metamorphosed massive sulfide ore deposits: Direct evidence from laser-ablation-inductively coupled plasma-mass spectrometry analysis of invisible gold. Geology 2007, 35, 775–778. [Google Scholar] [CrossRef]
- Sahoo, P.R.; Venkatesh, A.S. Constrains of mineralogical characterization of gold ore: Implication for genesis, controls and evolution of gold from Kundarkocha gold deposit, eastern India. J. Asian Earth Sci. 2015, 96, 136–149. [Google Scholar] [CrossRef]
- Zachariáš, J.; Frýda, J.; Paterová, B.; Mihaljevič, M. Arsenopyrite and As-pyrite from the Roudný deposit, Bohemian Massif. Mineral. Mag. 2004, 68, 31–46. [Google Scholar] [CrossRef]
- Tarnocai, C.A.; Hattori, K.; Cabri, L.J. “Invisible” gold in sulfides from Campbell mine, Red Lake greenstone belt, Ontario: Evidence for mineralization during the peak of metamorphism. Can. Mineral. 1997, 35, 805–815. [Google Scholar]
- McClenaghan, S.H.; Lentz, D.R.; Cabri, L.J. Abundance and speciation of gold in massive sulfides of the Bathurst Mining Camp, New Brunswick, Canada. Can. Mineral. 2004, 42, 851–871. [Google Scholar] [CrossRef]
- Liang, J.; Sun, W.; Zhu, S.; Li, H.; Liu, Y.; Zhai, W. Mineralogical study of sediment-hosted gold deposit in the Yangshan ore field, Western Qinling Orogen, Central China. J. Asian Earth Sci. 2014, 85, 40–52. [Google Scholar] [CrossRef]
- Zhang, J.; Li, L.; Gilbert, S.; Liu, J.-J.; Shi, W.-S. LA-ICP-MS and EPMA studies on the Fe-S-As minerals from the Jinlongshan gold deposit, Qinling Orogen, China: Implications for ore-forming processes. Geol. J. 2014, 49, 482–500. [Google Scholar] [CrossRef]
- Yang, S.; Blum, N.; Rahders, E.; Zhang, Z. The nature of invisible gold in sulfides from the Xiangxi Au-Sb-W ore deposit in northwestern Hunan, People’s Republic of China. Can. Mineral. 1998, 36, 1361–1372. [Google Scholar]
- Morey, A.A.; Tomkins, A.G.; Bierlein, F.P.; Weinberg, R.F.; Davidson, G.J. Bimodal distribution of gold in pyrite and arsenopyrite: Examples from the Archean Boorara and Bardoc shear systems, Yilgarn Craton, Western Australia. Econ. Geol. 2008, 103, 599–614. [Google Scholar] [CrossRef]
- Tauson, V.L. Gold solubility in the common gold-bearing minerals: Experimental evaluation and application to pyrite. Eur. J. Mineral. 1999, 11, 937–947. [Google Scholar] [CrossRef]
- Tauson, V.L.; Babkin, D.N.; Akimov, V.V.; Lipko, S.V.; Smagunov, N.V.; Parkhomenko, I.Y. Trace elements as indicators of the physicochemical conditions of mineral formation in hydrothermal sulfide systems. Rus. Geol. Geophys. 2013, 54, 526–543. [Google Scholar] [CrossRef]
- Goncharov, V.I.; Voroshin, S.V.; Sidorov, V.A. Natalkinskoe Gold-Ore Deposit; North-East Complex Research Institute, Far-East Branch of RAS: Magadan, Russia, 2002. (In Russian) [Google Scholar]
- Goryachev, N.A.; Pirajno, F. Gold deposits and gold metallogeny of Far East Russia. Ore Geol. Rev. 2014, 59, 123–151. [Google Scholar] [CrossRef]
- Goryachev, N.A.; Vikent’eva, O.V.; Bortnikov, N.S.; Prokof’ev, V.Y.; Alpatov, V.A.; Golub, V.V. The world-class Natalka gold deposit, Northeast Russia: REE patterns, fluid inclusions, stable oxygen isotopes, and formation conditions of ore. Geol. Ore Depos. 2008, 50, 362–390. [Google Scholar] [CrossRef]
- Mikhailov, B.K.; Struzhkov, S.F.; Natalenko, M.V.; Cimbalyuk, N.V. Multifactor model of large-volume gold-ore deposit Degdekan (Magadan region). Otechestvennaya Geol. 2010, 2, 20–31. [Google Scholar]
- Khanchuk, A.I.; Plyusnina, L.P.; Nikitenko, E.M.; Kuzmina, T.V.; Barinov, N.N. The noble metal distribution in the black shales of the Degdekan gold deposit in Northeast Russia. Russ. J. Pac. Geol. 2011, 5, 89–96. [Google Scholar] [CrossRef]
- Akinin, V.V.; Voroshin, S.V.; Gelman, M.L.; Leonova, V.V.; Miller, E.L. Shrimp-dating of metamorphic xenolyths of lamprophyre at gold deposit Degdekan: To the history of continental earth crust transformations at Ayan-Uriakh anticlinorium (Yano-Kolyma fold system). In Geodynamics, Magmatism and Minerageny of Continental Margins of North Pacific; North-East Complex Research Institute, Far-East Branch of RAS: Magadan, Russia, 2003; Volume 2, pp. 142–146. (In Russian) [Google Scholar]
- Tauson, V.L.; Lustenberg, E.E. Quantitative determination of modes of gold occurrence in minerals by the statistical analysis of analytical data sampling. Geochem. Int. 2008, 46, 423–428. [Google Scholar] [CrossRef]
- Deditius, A.P.; Utsunomiya, S.; Renock, D.; Ewing, R.C.; Ramana, C.V.; Becker, U.; Kesler, S.E. A proposed new type of arsenian pyrite: Composition, nanostructure and geological significance. Geochim. Cosmochim. Acta 2008, 72, 2919–2933. [Google Scholar] [CrossRef]
- Tauson, V.L.; Lipko, S.V.; Smagunov, N.V.; Kravtsova, R.G.; Arsent’ev, K.Y. Distribution and segregation of trace elements during the growth of ore mineral crystals in hydrothermal systems: Geochemical and mineralogical implications. Rus. Geol. Geophys. 2018, 59, 1718–1732. [Google Scholar] [CrossRef]
- Tauson, V.L.; Lipko, S.V.; Arsent’ev, K.Y.; Smagunov, N.V. Crystal growth through the medium of nonautonomous phase: Implications for element partitioning in ore systems. Cryst. Rep. 2019, 64, 496–507. [Google Scholar] [CrossRef]
- Xing, Y.; Brugger, J.; Tomkins, A.; Shvarov, Y. Arsenic evolution as a tool for understanding formation of pyritic gold ores. Geology 2019, 47, 335–338. [Google Scholar] [CrossRef]
- Kusebauch, C.; Gleeson, S.A.; Oelze, M. Coupled partitioning of Au and As into pyrite controls formation of giant Au deposits. Sci. Adv. 2019, 5, eaav5891. [Google Scholar] [CrossRef]
- Morishita, Y.; Hammond, N.Q.; Momii, K.; Konagaya, R.; Sano, Y.; Takahata, N.; Ueno, H. Invisible gold in pyrite from epithermal, banded-iron-formation-hosted, and sedimentary gold deposits: Evidence of hydrothermal influence. Minerals 2019, 9, 447. [Google Scholar] [CrossRef]
- Clark, L.A. The Fe-As-S system: Phase relations and applications. Part 1. Econ. Geol. 1960, 55, 1345–1381. [Google Scholar] [CrossRef]
- Fleet, M.E.; Mumin, A.H. Gold-bearing arsenian pyrite and marcasite and arsenopyrite from Carlin Trend gold deposits and laboratory synthesis. Am. Mineral. 1997, 82, 182–193. [Google Scholar] [CrossRef]
- Le Pape, P.; Blanchard, M.; Brest, J.; Boulliard, J.-C.; Ikogou, M.; Stetten, L.; Wang, S.; Landrot, G.; Morin, G. Arsenic incorporation in pyrite at ambient temperature at both tetrahedral S−1 and octahedral FeII sites: Evidence from EXAFS-DFT analysis. Environ. Sci. Technol. 2017, 51, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Tauson, V.L.; Akimov, V.V. Effect of crystallite size on solid state miscibility: Applications to the pyrite-cattierite system. Geochim. Cosmochim. Acta 1991, 55, 2851–2859. [Google Scholar] [CrossRef]
- Tauson, V.L.; Akimov, V.V. Further experimental evidence for a crystallite size effect in the FeS2-CoS2 system. Chem. Geol. 1993, 109, 113–118. [Google Scholar] [CrossRef]
- Abramovich, M.G.; Shmakin, B.M.; Tauson, V.L.; Akimov, V.V. Mineral typochemistry: Anomalous trace-element concentrations in solid solutions with defect structures. Int. Geol. Rev. 1990, 32, 608–615. [Google Scholar] [CrossRef]
- Urusov, V.S.; Tauson, V.L.; Akimov, V.V. Geochemistry of Solids; GEOS: Moscow, Russia, 1997. (In Russian) [Google Scholar]
- Ermolaev, N.P.; Sozinov, N.A.; Fliciyan, E.S.; Chinenov, V.A.; Khoroshilov, V.L. New Types of Ores of Noble and Rare Elements in Carbonaceous Shales; Beus, A.A., Ed.; Nauka: Moscow, Russia, 1992. (In Russian) [Google Scholar]
- Urusov, V.S.; Dudnikova, V.B. The trace-component trapping effect: Experimental evidence, theoretical interpretation, and geochemical applications. Geochim. Cosmochim. Acta 1998, 62, 1233–1240. [Google Scholar] [CrossRef]
- Tauson, V.L.; Akimov, V.V.; Parkhomenko, I.Y.; Nepomnyashchikh, K.V.; Men’shikov, V.I. A study of cadmium incorporation into pyrrhotites of different stoichiometry. Geochem. Int. 2004, 42, 115–121. [Google Scholar]
- Lehner, S.W.; Savage, K.S.; Ayers, J.C. Vapor growth and characterization of pyrite (FeS2) doped with Co, Ni, and As: Variations in semiconducting properties. J. Cryst. Growth 2006, 286, 306–317. [Google Scholar] [CrossRef]
- Fiechter, S.; Mai, J.; Ennaoui, A.; Szacki, W. Chemical vapor transport of pyrite (FeS2) with halogen (Cl, Br, I). J. Cryst. Growth 1986, 78, 438–444. [Google Scholar] [CrossRef]
- Schieck, R.; Hartmann, A.; Fiechter, S. Electrical properties of natural and synthetic pyrite (FeS2) crystals. J. Mater. Res. 1990, 5, 1567–1572. [Google Scholar] [CrossRef]
- Tomm, Y.; Schieck, R.; Ellmer, K.; Fiechter, S. Growth mechanism and electronic properties of doped pyrite (FeS2) crystals. J. Cryst. Growth 1995, 146, 271–276. [Google Scholar] [CrossRef]


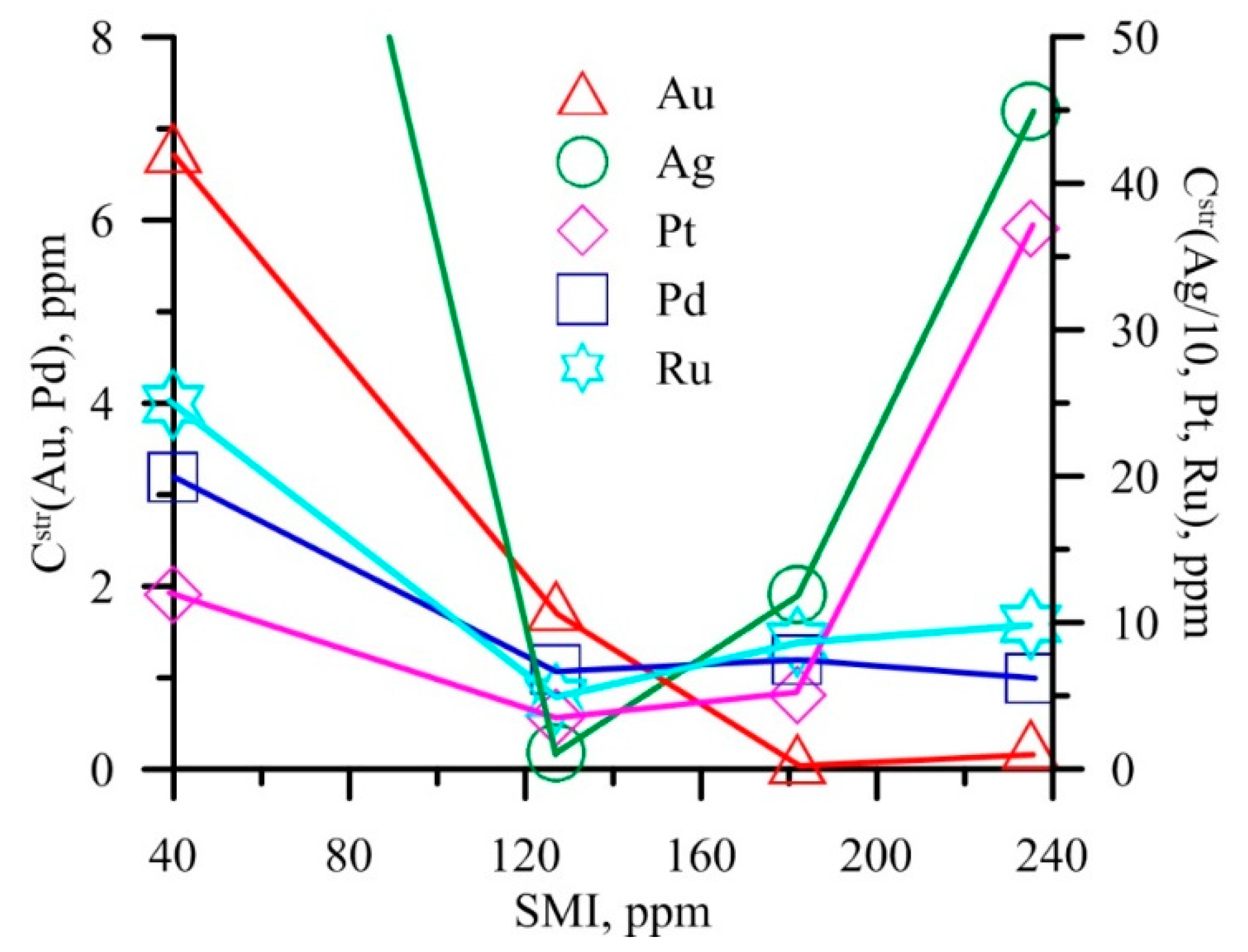
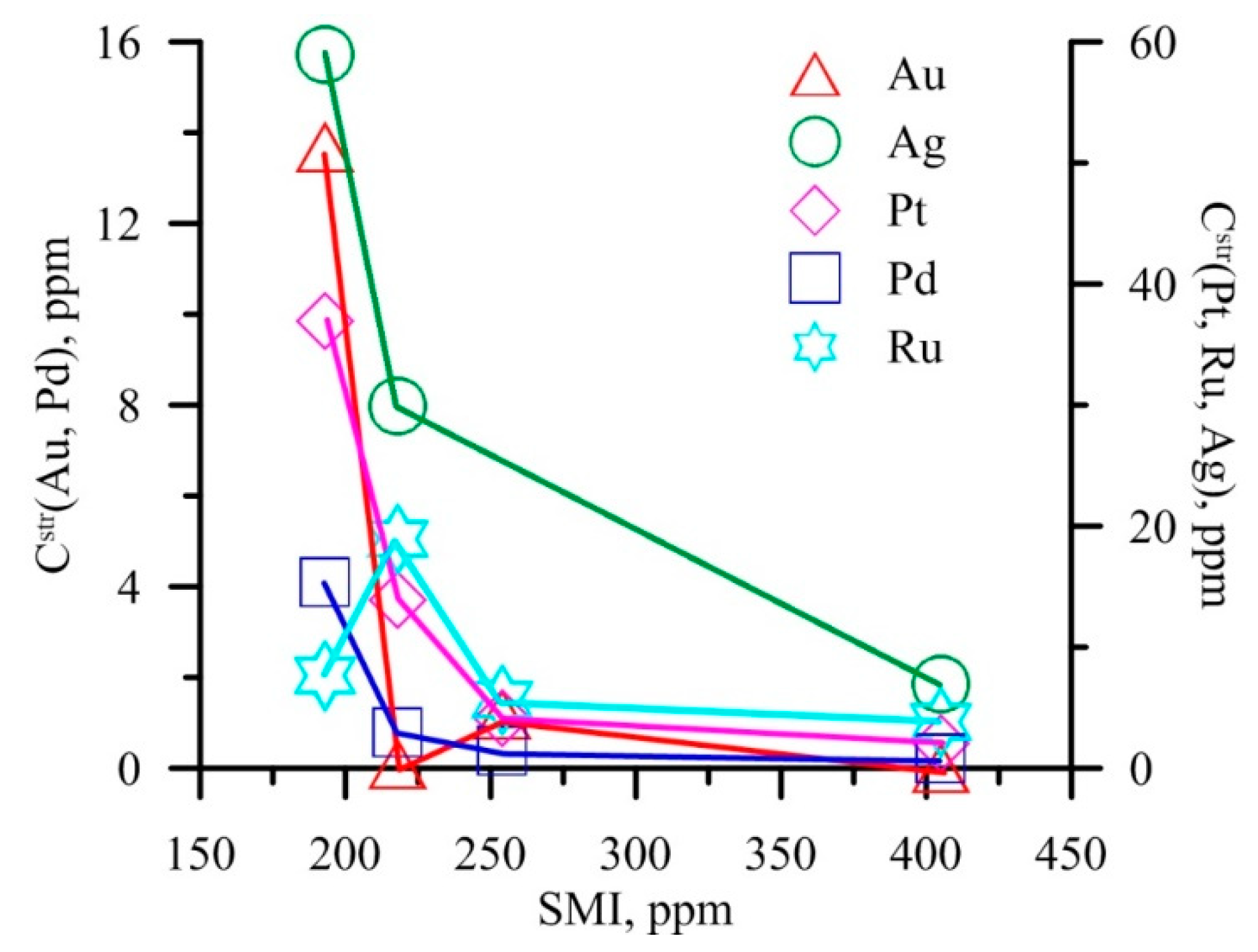
| No | Deposit, Location | Type * | Formation Conditions | Ore Mineral Association ** | Au Content (ppm) | As in Py (ppm) | |
|---|---|---|---|---|---|---|---|
| Py | Asp | ||||||
| 1 | Shuiyindong, Guizhou, China | Carlin | 220 ± 20 °C, 2–4 mol.% CO2, low salinity | Py, Asp, Au | 300–600 | 300–1500 | 7000–12,400 |
| 2 | Bhukia-Jagpura Rajasthan, India | Magmatic-hydrothermal | 460–474 °C, −logfS2 = 4.6–5.8 bar, 24–26 wt % NaCl-eq. | Py, Asp, Po | 0.3–1.6 | 0.28–10 | 143–215 |
| 3 | Neves Norte, Alentejo, Portugal | VHMS | n/a | Py, Asp, Cpy, Gn, St, Tet | 1.7 | 16–35 | <2000 |
| 4 | Hutti, Dharwar Craton, India | Orogenic | 377–450 °C, ~2 kbar | Py, Asp, Po, Sph, Cpy, Au | 0.05–0.45 | 0.43–14.18 | 400–7500 |
| 5 | Suurikuusikko, Kittilä, Finland | VHMS | n/a | Py, Asp, Tet, Bour, Gn, Cpy, Sph, Rut, Au | <22–585 | <22–964 | ~200–40,000 |
| 6 | Lodestar, Newfoundland, Canada | Magmatic-hydrothermal | ~425 °C | Py, Asp, Cpy | 0.03–0.73 0.03–1.33 | 0.12–154 1.31–152 | n/a |
| 7 | Boliden, Skellefte, Sweden | VHMSMetamorphosed | ~400–450 °C, 5 kbar | Py, Asp, Po, Cpy, Gn, Au | ≤0.22 ≤0.19 | 3.8–108 ≤2.7 | n/a |
| 8 | Kundarkocha, Singhbhum, India | Orogenic | 375–390 °C, −logfS2 = 7.5–9.7 | Py, Asp, Po, Cpy, Sph, Pn, Gsd, Gn, Lö, Au | 2100–2700 1300–1500 700–900 | 1600–3600 1200–1300 900–1200 | 1000–2000 |
| 9 | Roudný, Bohemia, Czech Republic | Mesothermal | 320–330 °C | Py, Asp, Mrc, Au | 3–18 | 39–152 | 4000–25,000 |
| 10 | Campbell, Ontario, Canada | Metamorphic-hydrothermal | 450–500 °C, 2–3 kbar | Py, Asp, Gh, Po, Mt, Sph, Auo | 0.1–0.3 0.2–8.5 | 103–5610 118–4522 | n/a |
| 11 | Oberon, Tanami, Australia | Orogenic | 200–430 °C, low-to moderate salinity | Asp, Py, Po, Cpy, Sph, Gn, Au, Cb, Gsd | <1–4.6 | 2–4500 | 2000–4000 |
| 12 | Orvan Brook, Rocky Turn, Murray Brook, Bathurst Mining Camp, New Brunswick, Canada | Massive sulphide Zn–Pb–Cu–Ag | 325–425 °C, 4–6 kbar | Py, Sph, Gn, Cpy, Po, Asp, Mrc, Tet | <0.53–5.6 16.22–31.95 0.56–32.04 | 2.24–9.42 0.11–0.26 1.4–10.91 | bdl EPMA- 9800 |
| 13 | Anba, Qinling Orogen, China | Carlin or Orogenic | n/a | Py, Asp, Ant, Mrc, Sph | 9.7–135.9 | 17.4–461.8 | 11,962–101,332 |
| 14 | Jinlongshan, Shaanxi, China | Carlin | n/a | Py, Asp | 1.9–9.4 21.1–113 | 4.2–429 64.6–576 | bdl EPMA- 62,100 |
| Sample | Grain No. | Average Grain Composition (Atomic Content) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Arsenopyrite | As–Pyrite | Pyrite | |||||||||
| n * | Formula | As/S | n * | Formula | Fe/ (S + As) | As/S | n * | Formula | Fe/S | ||
| Nat- 10 | 1 | 3 | Fe0.999As1.079S0.922 | 1.17 | - | - | - | - | - | - | |
| 2 | 4 | Fe0.993As1.077S0.930 | 1.16 | - | - | - | - | - | - | ||
| 3 | 4 | Fe0.987As1.069S0.944 | 1.13 | - | - | - | - | - | - | ||
| 4 | 4 | Fe0.989As1.065S0.946 | 1.13 | - | - | - | - | - | - | ||
| 5 | 8 | Fe0.993As1.066S0.941 | 1.13 | - | - | - | - | - | - | ||
| 6 | 7 | Fe0.997As1.069S0.934 | 1.14 | - | - | - | - | - | - | ||
| 7 | 5 | Fe0.987As1.059S0.954 | 1.11 | - | - | - | - | - | - | ||
| 8 | 3 | Fe0.994As1.060S0.946 | 1.12 | - | - | - | - | - | - | ||
| 9 | 5 | Fe0.987As1.077S0.936 | 1.15 | - | - | - | - | - | - | ||
| 10 | 5 | Fe0.994As1.069S0.937 | 1.14 | - | - | - | - | - | - | ||
| 11 | 4 | Fe0.992As1.088S0.920 | 1.18 | - | - | - | - | - | - | ||
| 12 | 2 | Fe0.981As1.098S0.921 | 1.19 | 4 | Fe0.998S1.994As0.008 | 0.499 | 0.004 | 2 | FeS2.006 | 0.499 | |
| 13 | 2 | Fe0.973As1.085S0.942 | 1.15 | 6 | Fe0.998S1.988As0.014 | 0.499 | 0.007 | 1 | FeS2.04 | 0.490 | |
| 14 | 1 | n/d | - | 3 | Fe0.997S1.975As0.028 | 0.498 | 0.014 | 3 | FeS2.021 | 0.495 | |
| 15 | 2 | Fe0.991As1.084S0.925 | 1.17 | 5 | Fe0.999S1.984As0.017 | 0.499 | 0.009 | - | - | - | |
| 16 | 1 | Fe0.979As1.076S0.945 | 1.14 | 3 | Fe0.998S1.983As0.019 | 0.499 | 0.01 | 3 | FeS2.009 | 0.498 | |
| 17 | 4 | Fe0.976As1.076S0.948 | 1.14 | 5 | Fe1.000S1.976As0.024 | 0.500 | 0.012 | 3 | FeS2.006 | 0.499 | |
| UV- 3/13 | 1 | 8 | Fe0.982As1.101S0.917 | 1.20 | - | - | - | - | - | - | - |
| 2 | 8 | Fe0.985As1.093S0.922 | 1.19 | - | - | - | - | - | - | - | |
| 3 | 8 | Fe0.986As1.110S0.904 | 1.23 | - | - | - | - | - | - | - | |
| 4 | 7 | Fe0.987As1.087S0.926 | 1.17 | - | - | - | - | - | - | - | |
| 5 | 5 | Fe0.991As1.094S0.915 | 1.20 | - | - | - | - | - | - | - | |
| 6 | 7 | Fe0.990As1.097S0.913 | 1.20 | - | - | - | - | - | - | - | |
| 7 | - | - | - | 9 | Fe1.019S1.961As0.020 | 0.514 | 0.01 | - | - | - | |
| 8 | - | - | - | 13 | Fe1.015S1.964As0.021 | 0.511 | 0.01 | - | - | - | |
| 9 | - | - | - | 12 | Fe1.019S1.973As0.007 | 0.515 | 0.004 | - | - | - | |
| 10 | - | - | - | 16 | Fe1.018S1.970As0.012 | 0.514 | 0.006 | - | - | - | |
| 11 | - | - | - | 9 | Fe1.017S1.963As0.020 | 0.513 | 0.01 | - | - | - | |
| DG- 10/14 | 1 | 7 | Fe0.992As1.054S0.954 | 1.10 | - | - | - | - | - | - | - |
| 2 | 5 | Fe0.999As1.016S0.985 | 1.03 | - | - | - | - | - | - | - | |
| 3 | 5 | Fe0.989As1.056S0.955 | 1.11 | - | - | - | - | - | - | - | |
| 4 | 5 | Fe0.989As1.037S0.974 | 1.06 | - | - | - | - | - | - | - | |
| 5 | 4 | Fe0.999As1.044S0.957 | 1.09 | - | - | - | - | - | - | - | |
| 6 | 3 | Fe0.988As1.050S0.962 | 1.09 | - | - | - | - | - | - | - | |
| 7 | 4 | Fe0.988As1.054S0.958 | 1.10 | - | - | - | - | - | - | - | |
| 8 | 7 | Fe0.992As1.034S0.974 | 1.06 | - | - | - | - | - | - | - | |
| 9 | 7 | Fe0.991As1.043S0.966 | 1.08 | - | - | - | - | - | - | - | |
| 10 | 6 | Fe0.991As1.041S0.968 | 1.08 | - | - | - | - | - | - | - | |
| 11 | 5 | Fe0.988As1.046S0.966 | 1.08 | - | - | - | - | - | - | - | |
| 12 | 6 | Fe0.998As1.049S0.953 | 1.10 | - | - | - | - | - | - | - | |
| 13 | 6 | Fe0.997As1.027S0.976 | 1.05 | - | - | - | - | - | - | - | |
| 14 | 2 | Fe0.980As1.050S0.970 | 1.08 | 6 | Fe0.992S1.996As0.011 | 0.494 | 0.006 | 5 | FeS2.003 | 0.499 | |
| 15 | - | - | - | 6 | Fe0.999S1.984As0.018 | 0.499 | 0.009 | - | - | - | |
| 16 | - | - | - | 5 | Fe0.995S1.993As0.012 | 0.496 | 0.006 | 2 | FeS1.988 | 0.503 | |
| ZR- 10/13 | 1 | 7 | Fe0.997As1.061S0.942 | 1.13 | - | - | - | - | - | - | - |
| 2 | 8 | Fe0.990As1.067S0.943 | 1.13 | - | - | - | - | - | - | - | |
| 3 | 15 | Fe0.994As1.083S0.923 | 1.17 | - | - | - | - | - | - | - | |
| 4 | 14 | Fe0.996As1.075S0.929 | 1.16 | - | - | - | - | - | - | - | |
| 5 | 15 | Fe0.992As1.075S0.933 | 1.15 | - | - | - | - | - | - | - | |
| 6 | 14 | Fe0.987As1.064S0.949 | 1.12 | - | - | - | - | - | - | - | |
| 7 | - | - | - | 11 | Fe1.016S1.968As0.016 | 0.512 | 0.008 | 4 | FeS1.944 | 0.514 | |
| 8 | - | - | - | 11 | Fe1.018S1.972As0.010 | 0.514 | 0.005 | 5 | FeS1.944 | 0.514 | |
| 9 | - | - | - | 11 | Fe1.017S1.971As0.012 | 0.513 | 0.006 | - | - | - | |
| 10 | - | - | - | 8 | Fe1.019S1.968As0.013 | 0.514 | 0.007 | 8 | FeS1.944 | 0.514 | |
| 11 | - | - | - | 7 | Fe1.019S1.969As0.011 | 0.515 | 0.006 | 6 | FeS1.935 | 0.517 | |
| 12 | - | - | - | 6 | Fe1.015S1.944As0.040 | 0.512 | 0.021 | 4 | FeS1.941 | 0.515 | |
| Sample Number | Arsenopyrite (Monoclinic, P21/c) | Pyrite (Cubic, Pa3) | |||||
|---|---|---|---|---|---|---|---|
| a | b | c | ßo | D | a | D | |
| Nat-10 | 0.57693 | 0.56766 | 0.57395 | 112.087 | 61 | 0.54208 | 76 |
| UV-3/13 | 0.57425 | 0.56669 | 0.57591 | 111.898 | 56 | 0.54227 | 65 |
| DG-10/14 | 0.57592 | 0.56636 | 0.57327 | 111.881 | 58 | 0.54192 | 71 |
| ZR-10/13 | 0.57357 | 0.56753 | 0.57648 | 111.979 | 54 | 0.54141 | 60 |
| Sample Number | Element | Element Content (ppm) | DPy/Asp | Matrix Composition (at. %) Asp/As–Py * | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pyrite | Arsenopyrite | Str. | Sur. | As | S | Fe | ||||
| Str. | Sur. | Str. | Sur. | |||||||
| Nat-10 | Au | 1.8 | 2.7 | 1.2 | 1.9 | 1.5 | 1.4 | 35.83/0.60 | 31.23/66.12 | 32.94/33.28 |
| Ag | 12 | 220 | 120 | 250 | 0.1 | 0.9 | ||||
| Pt | 3.6 | 35 | 4.2 | 89 | 0.9 | 0.4 | ||||
| Pd | 1.1 | 4.9 | 0.4 | 8.1 | 2.8 | 0.6 | ||||
| Ru | 4.9 | 30 | 5.7 | 79 | 0.9 | 0.4 | ||||
| UV-3/13 | Au | 0.29 | 1.4 | 0.12 | 1.3 | 2.4 | 1.1 | 36.57/0.53 | 30.53/65.54 | 32.90/33.93 |
| Ag | 450 | 800 | 30 | 450 | 15 | 1.8 | ||||
| Pt | 37 | 189 | 14 | 190 | 2.6 | 1.0 | ||||
| Pd | 1.0 | 18.4 | 0.8 | 24.5 | 1.2 | 0.8 | ||||
| Ru | 10 | 330 | 19 | 218 | 0.5 | 1.5 | ||||
| DG-10/14 | Au | 0.13 | 1.1 | 0.03 | 0.48 | 4.3 | 2.3 | 34.75/0.47 | 32.19/66.36 | 33.06/33.17 |
| Ag | 120 | 200 | 7 | 60 | 17 | 3.3 | ||||
| Pt | 5.1 | 103 | 2.1 | 29 | 2.4 | 3.6 | ||||
| Pd | 1.2 | 8.2 | 0.23 | 3.7 | 5.2 | 2.2 | ||||
| Ru | 8.8 | 97 | 3.9 | 35 | 2.3 | 2.8 | ||||
| ZR-10/13 | Au | 6.8 ** | n/d | 13.7 | 47 | 0.5 | n/d | 35.70/0.57 | 31.20/65.52 | 33.10/33.91 |
| Ag | 1100 | 7000 | 59 | 400 | 19 | 17.5 | ||||
| Pt | 12 | 259 | 37 | 228 | 0.3 | 1.1 | ||||
| Pd | 3.2 | 30 | 4.1 | 38 | 0.8 | 0.8 | ||||
| Ru | 25 | 458 | 7.7 | 369 | 3.2 | 1.2 | ||||
| Sample No. | Ti | V | Mn | Co | Ni | Cu | Pb | Bi | Sb | Se | Mo | W | Y | Nb | La | Ce | Nd | Yb | Au |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nat-10 | 8.4 | 5.3 | 6.2 | 8.0 | 56.1 | 36.4 | 23.4 | 1.1 | 11.5 | bdl | bdl | 9.7 | 2.3 | 4.2 | 1.2 | 1.6 | 4.1 | 3.6 | 1.2 |
| 1.8 | 2.6 | 0.8 | 1.5 | 37.1 | 15.4 | 9.8 | 0.4 | 7.4 | - | - | 9.1 | 1.7 | 2.8 | 1.0 | 1.0 | 0.9 | 2.5 | 0.9 | |
| UV-3/13 | 73.9 | 3.8 | 6.7 | 7.1 | 26.0 | 57.7 | 84.5 | 2.8 | 67.2 | bdl | bdl | 5.4 | 14.4 | 1.6 | 1.5 | 2.9 | 6.2 | 4.1 | bdl |
| 23.8 | 2.2 | 1.8 | 2.2 | 10.3 | 20.6 | 19.5 | 1.0 | 18.1 | - | - | 2.8 | 7.4 | 0.7 | 1.0 | 2.8 | 2.5 | 1.7 | - | |
| DG-10/14 | 23.6 | 4.3 | 7.9 | 9.2 | 72.3 | 18.2 | 43.6 | 4.5 | 47.5 | 68.8 | 2.8 | 5.4 | 0.7 | 7.2 | 0.4 | 0.6 | bdl | 3.3 | 0.6 |
| 10.3 | 3.3 | 2.7 | 2.6 | 51.5 | 9.5 | 29.3 | 2.7 | 17.0 | 12.4 | 2.7 | 3.4 | 0.5 | 2.6 | 0.2 | 0.1 | - | 0.2 | 0.2 | |
| ZR-10/13 | 25.8 | 1.7 | 6.1 | 1.3 | 15.7 | 5.6 | 10.7 | 0.8 | 7.7 | bdl | bdl | 14.7 | 0.4 | 1.7 | 1.8 | 3.6 | 2.2 | 1.5 | 6.8 |
| 3.1 | 0.4 | 2.7 | 0.6 | 7.2 | 1.8 | 4.3 | 0.2 | 2.2 | - | - | 3.1 | 0.2 | 1.4 | 1.0 | 2.5 | 1.0 | 0.9 | 2.1 |
| Sample No. | Ti | V | Co | Ni | Cu | Pb | Bi | Sb | Se | Mo | W | Y | Nb | Yb | Au |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nat-10 | 3.8 | 0.7 | 10.7 | 10.7 | 10.5 | 7.7 | 1.1 | 133 | 81.2 | 1.8 | bdl | 0.6 | bdl | bdl | 1.6 |
| 0.6 | 0.2 | 2.4 | 3.6 | 1.8 | 4.1 | 0.5 | 45 | 26.9 | 0.8 | - | 0.6 | - | - | 0.9 | |
| UV-3/13 | 6.1 | 0.6 | 4.2 | 6.1 | 3.8 | 8.5 | 0.7 | 89.7 | 105 | 2.7 | bdl | 0.3 | bdl | bdl | 0.9 |
| 0.4 | 0.1 | 3.3 | 1.5 | 1.3 | 1.4 | 0.2 | 12.0 | 17 | 0.5 | - | 0.1 | - | - | 0.2 | |
| DG-10/14 | 71 | 1.0 | 18.8 | 24.4 | 12.4 | 16.4 | 3.5 | 146 | 187 | 2.6 | 2.5 | 0.4 | 7.2 | 1.5 | 0.6 |
| 50 | 0.4 | 9.6 | 8.5 | 6.5 | 6.9 | 1.5 | 27 | 26 | 0.9 | 0.8 | 0.1 | 1.2 | 0.4 | 0.2 | |
| ZR-10/13 | 7.2 | 0.7 | 5.6 | 5.1 | 4.4 | 7.5 | 1.3 | 115 | 53.7 | 2.3 | bdl | 0.5 | bdl | bdl | 8.9 |
| 0.6 | 0.1 | 4.9 | 1.8 | 0.8 | 1.3 | 0.4 | 20 | 10.8 | 0.6 | - | 0.3 | - | - | 1.8 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tauson, V.; Lipko, S.; Kravtsova, R.; Smagunov, N.; Belozerova, O.; Voronova, I. Distribution of “Invisible” Noble Metals between Pyrite and Arsenopyrite Exemplified by Minerals Coexisting in Orogenic Au Deposits of North-Eastern Russia. Minerals 2019, 9, 660. https://doi.org/10.3390/min9110660
Tauson V, Lipko S, Kravtsova R, Smagunov N, Belozerova O, Voronova I. Distribution of “Invisible” Noble Metals between Pyrite and Arsenopyrite Exemplified by Minerals Coexisting in Orogenic Au Deposits of North-Eastern Russia. Minerals. 2019; 9(11):660. https://doi.org/10.3390/min9110660
Chicago/Turabian StyleTauson, Vladimir, Sergey Lipko, Raisa Kravtsova, Nikolay Smagunov, Olga Belozerova, and Irina Voronova. 2019. "Distribution of “Invisible” Noble Metals between Pyrite and Arsenopyrite Exemplified by Minerals Coexisting in Orogenic Au Deposits of North-Eastern Russia" Minerals 9, no. 11: 660. https://doi.org/10.3390/min9110660
APA StyleTauson, V., Lipko, S., Kravtsova, R., Smagunov, N., Belozerova, O., & Voronova, I. (2019). Distribution of “Invisible” Noble Metals between Pyrite and Arsenopyrite Exemplified by Minerals Coexisting in Orogenic Au Deposits of North-Eastern Russia. Minerals, 9(11), 660. https://doi.org/10.3390/min9110660






