Effect of Quartz on the Preparation of Sodium Stannate from Cassiterite Concentrates by Soda Roasting Process
Abstract
:1. Introduction
2. Materials and Methods
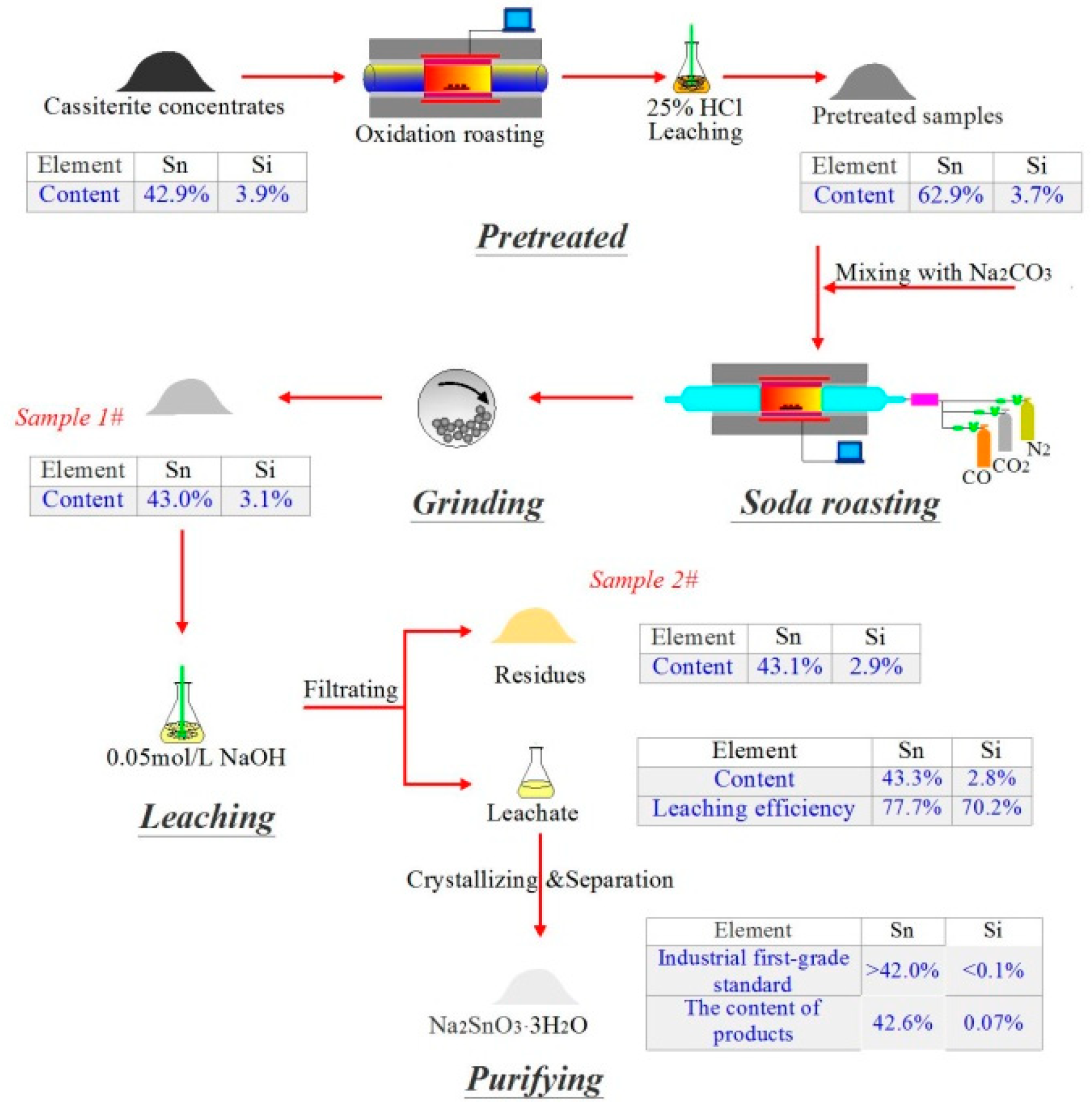
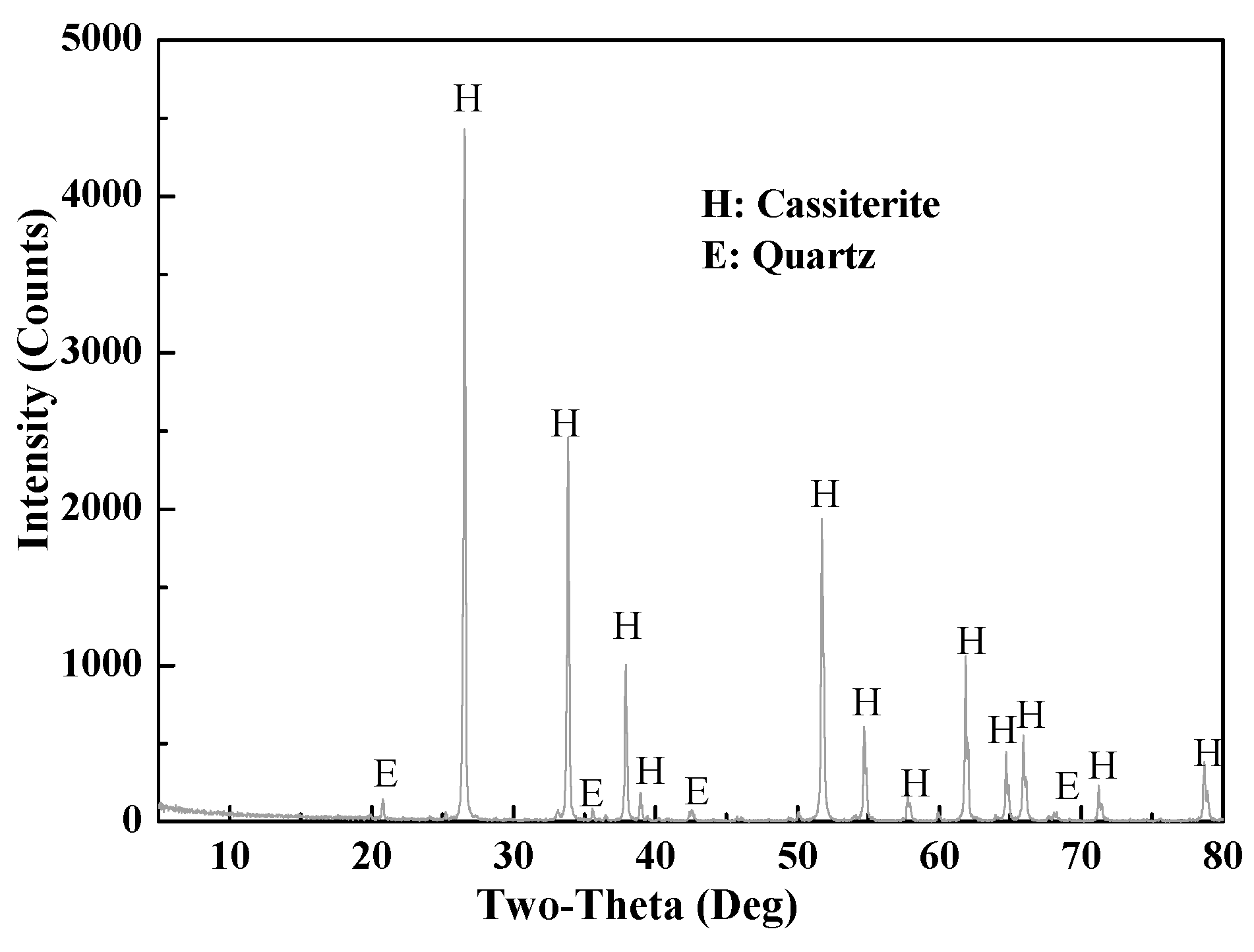
2.1. Materials
2.2. Methods
2.2.1. Roasting Process
2.2.2. Leaching Process
2.2.3. Instrument Techniques
3. Results and Discussion
3.1. Behaviour of Si during the Soda Roasting-Leaching Processs
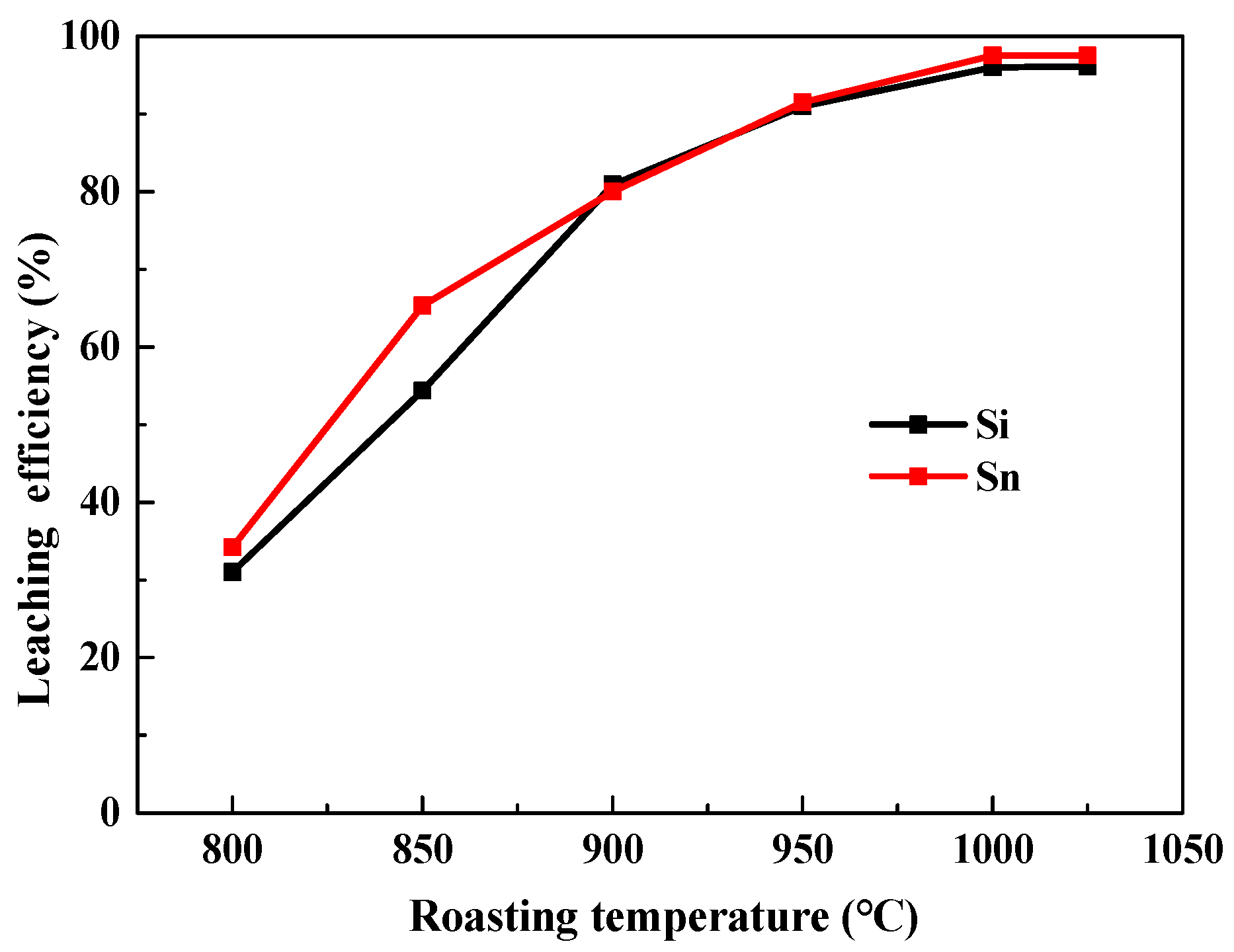
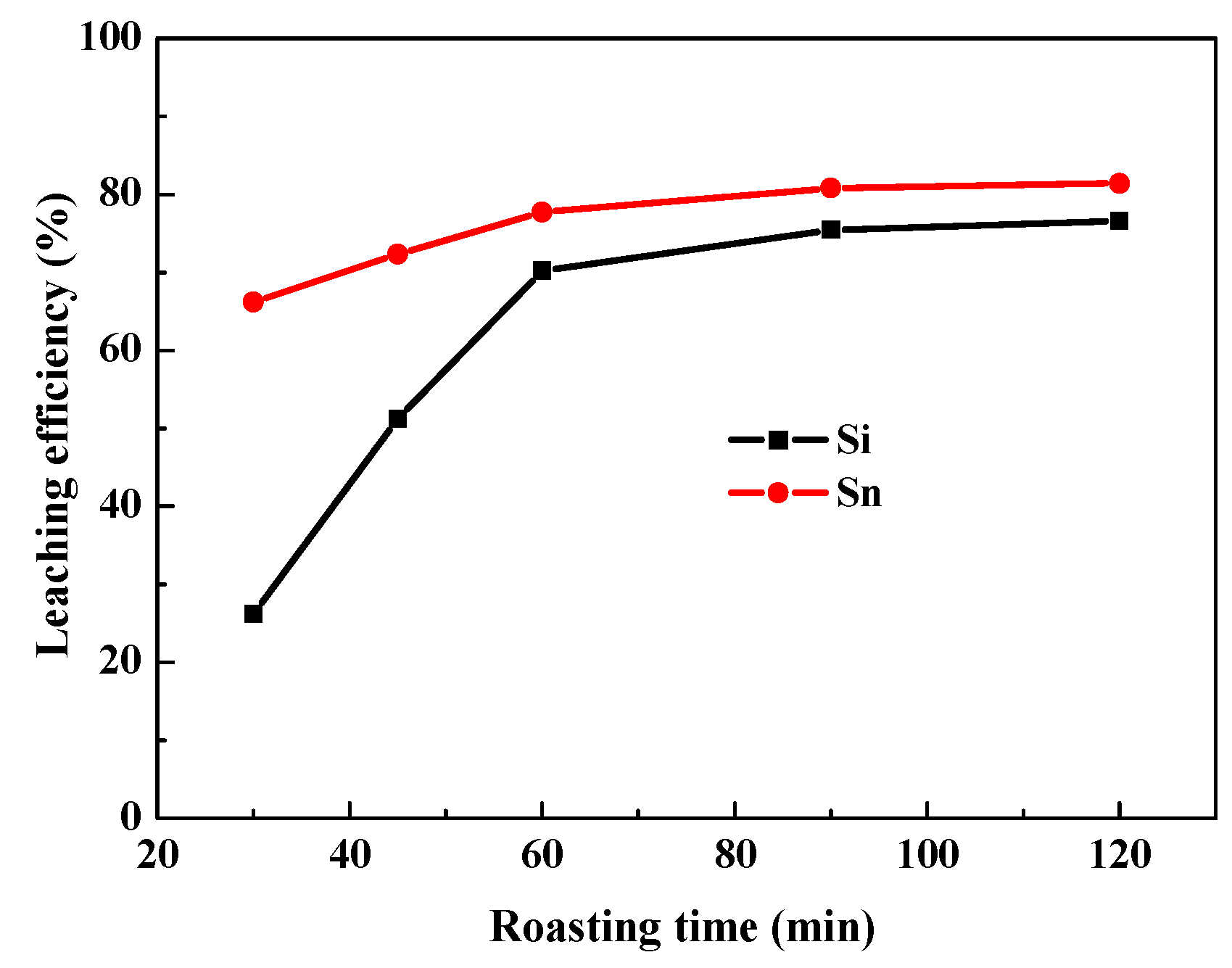
3.1.1. Effect of Roasting Temperatures and Time
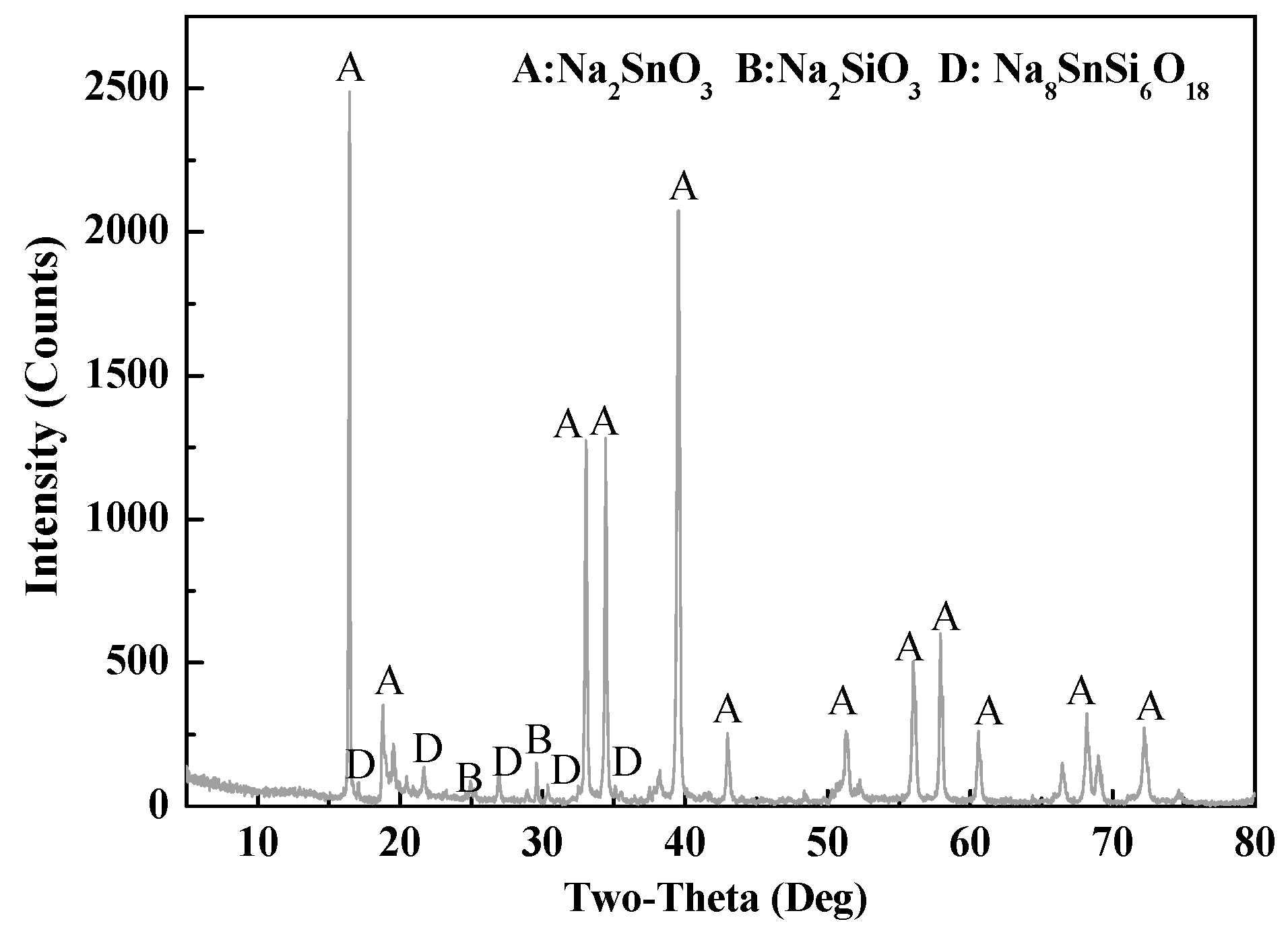
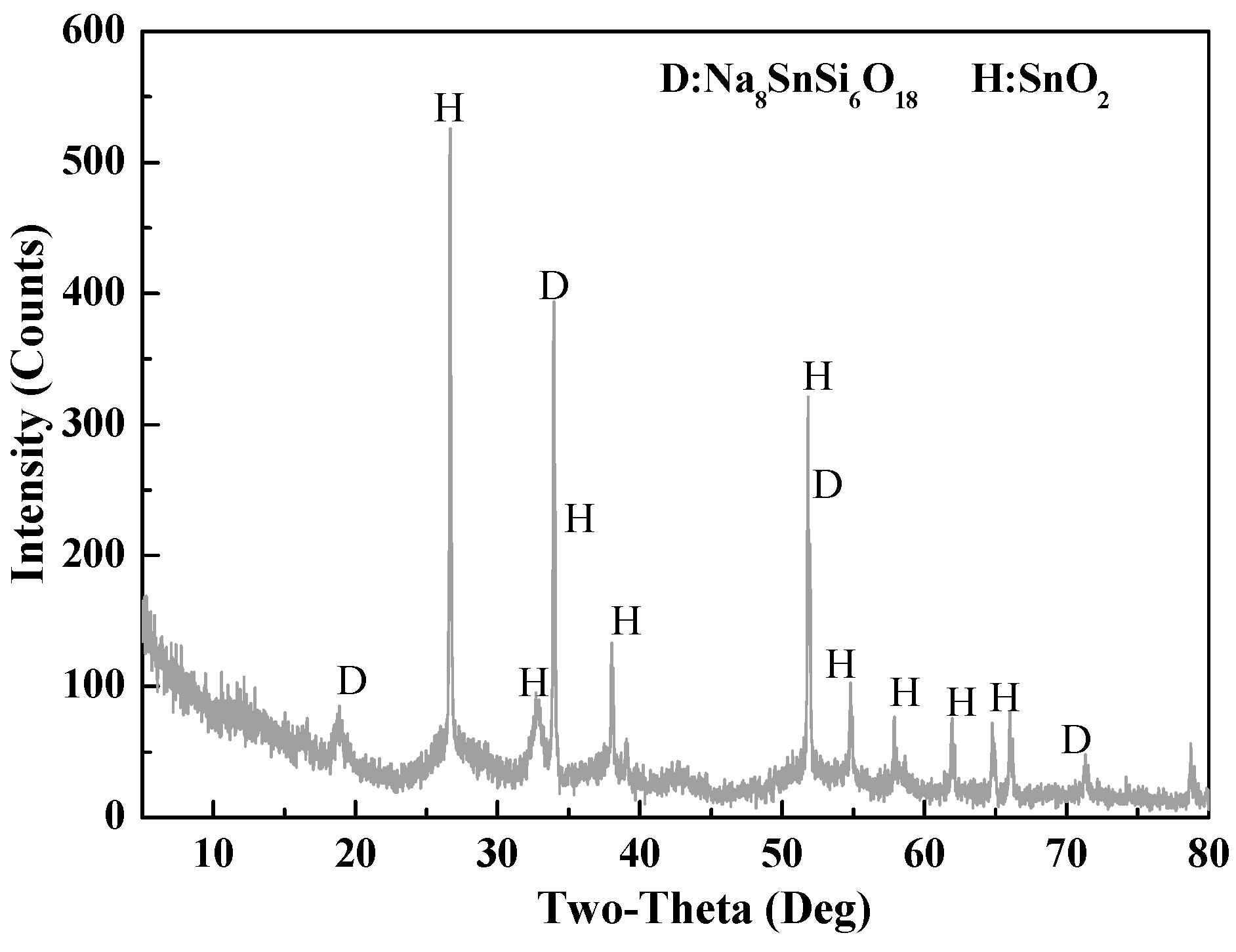
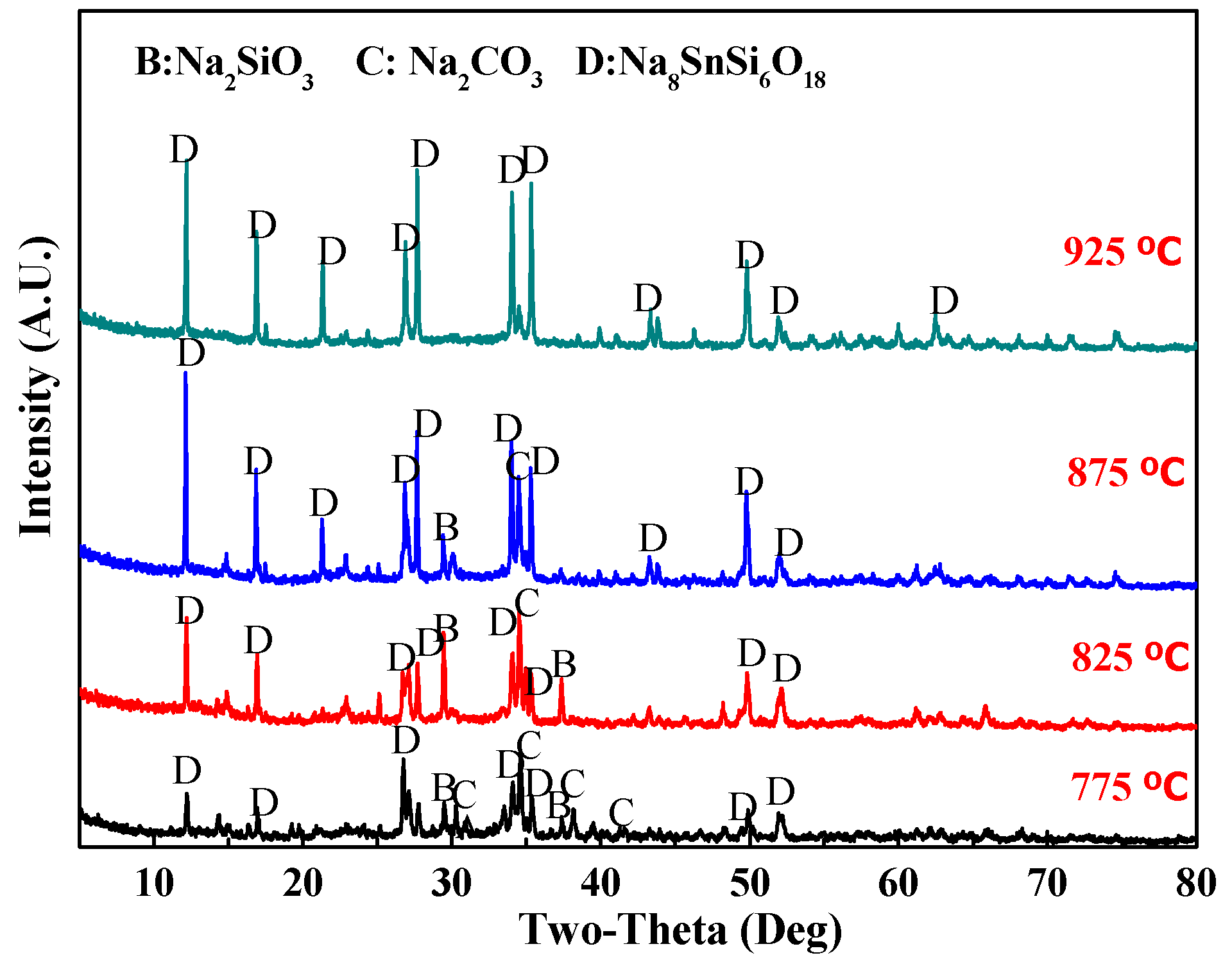
3.1.2. Phase Analysis of Roasted Samples and Leach Residues
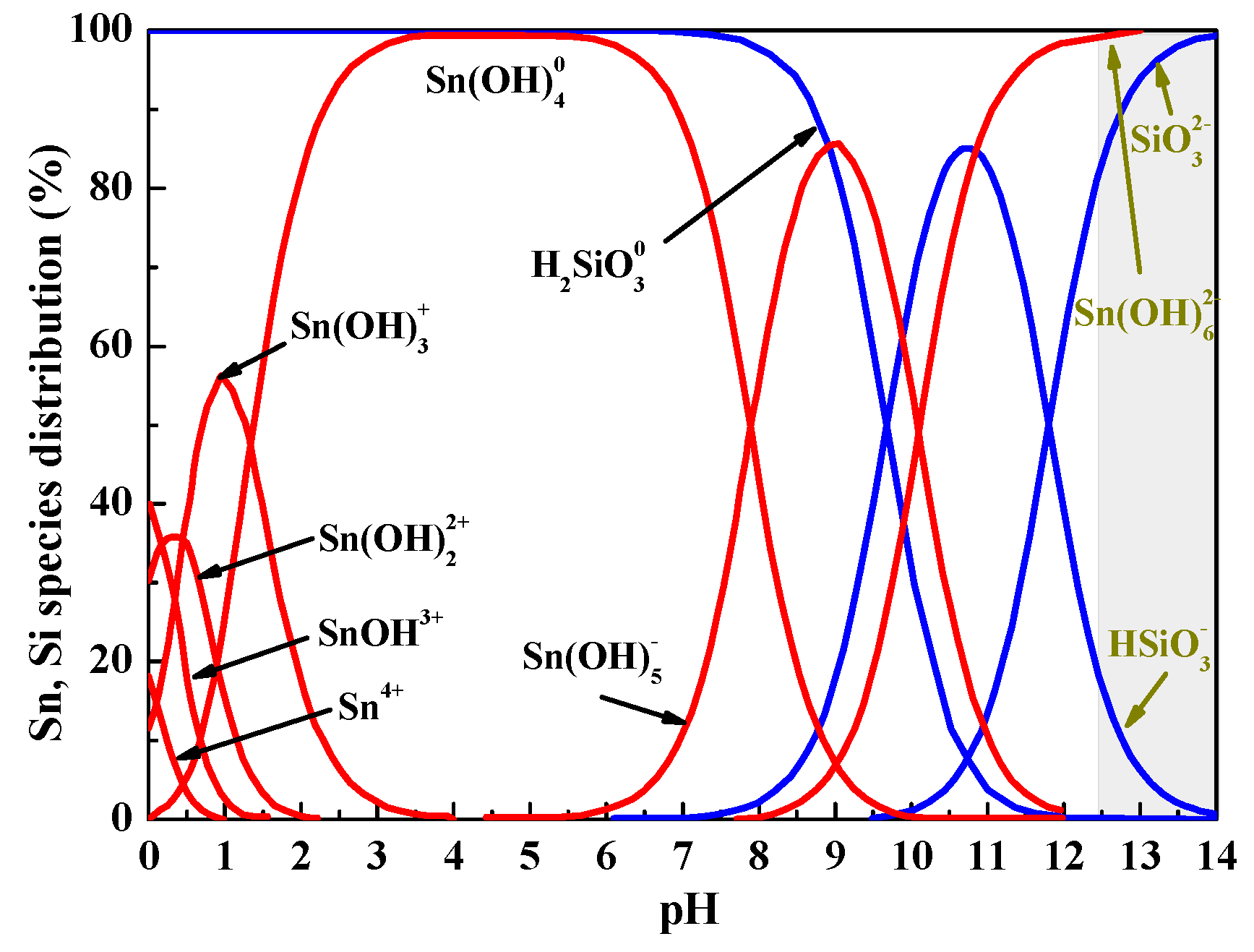
3.1.3. Solution Chemistry of Metasilicic Acid and Tin
3.2. Effect of SiO2 on Phase Evolution of SnO2–Na2CO3 System
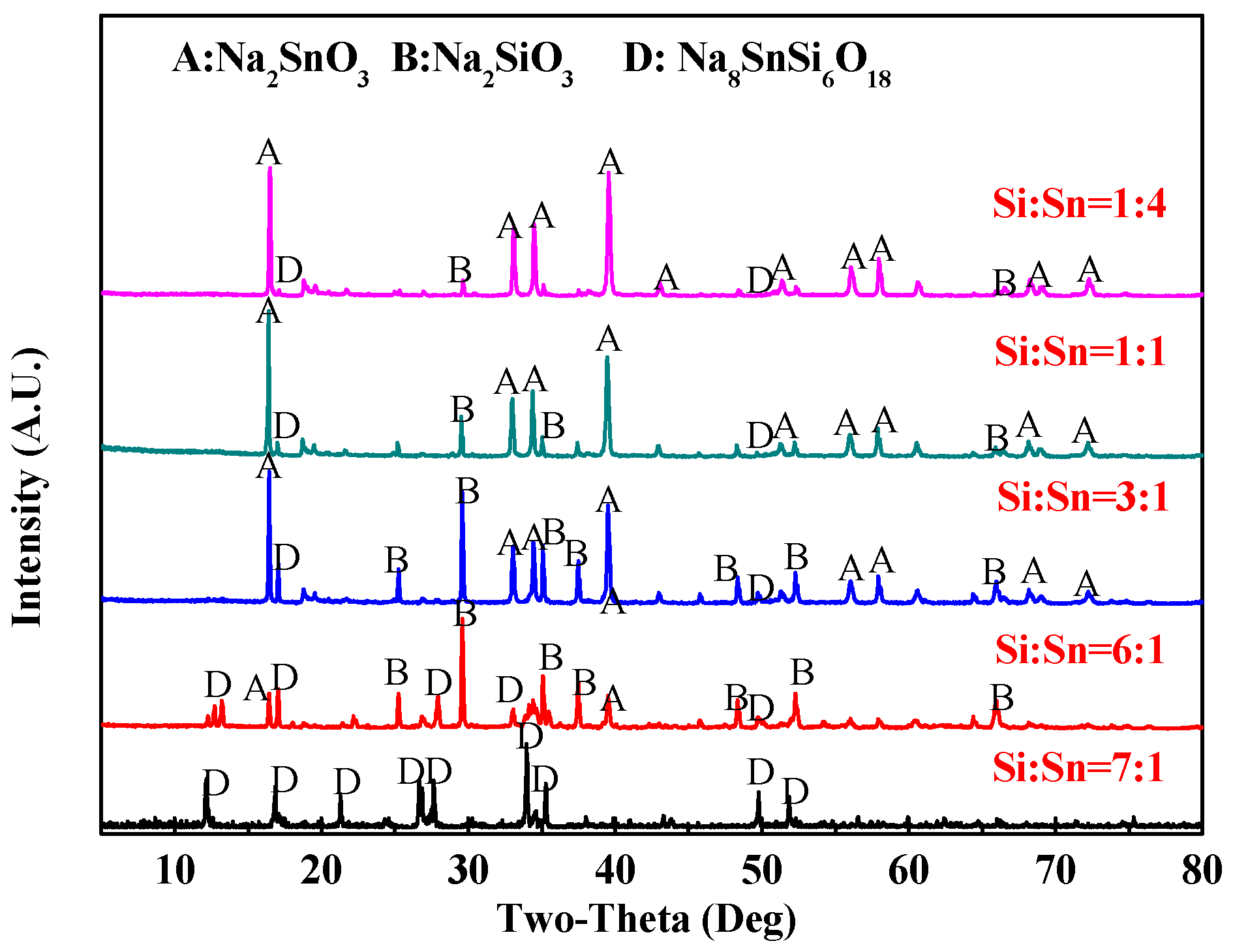
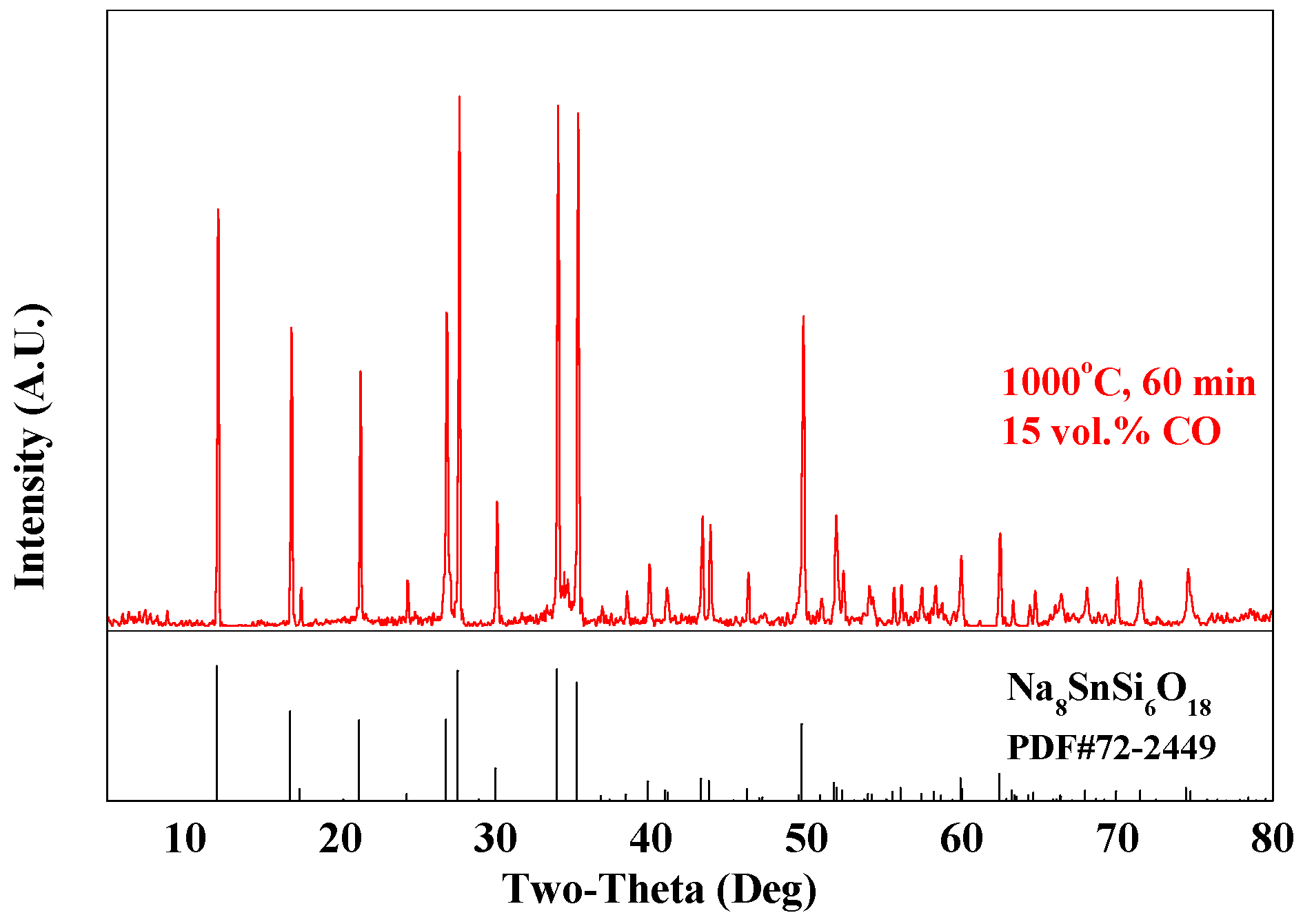
3.2.1. Effect of SiO2/SnO2 Mole Ratio
3.2.2. Effect of Roasting Temperature
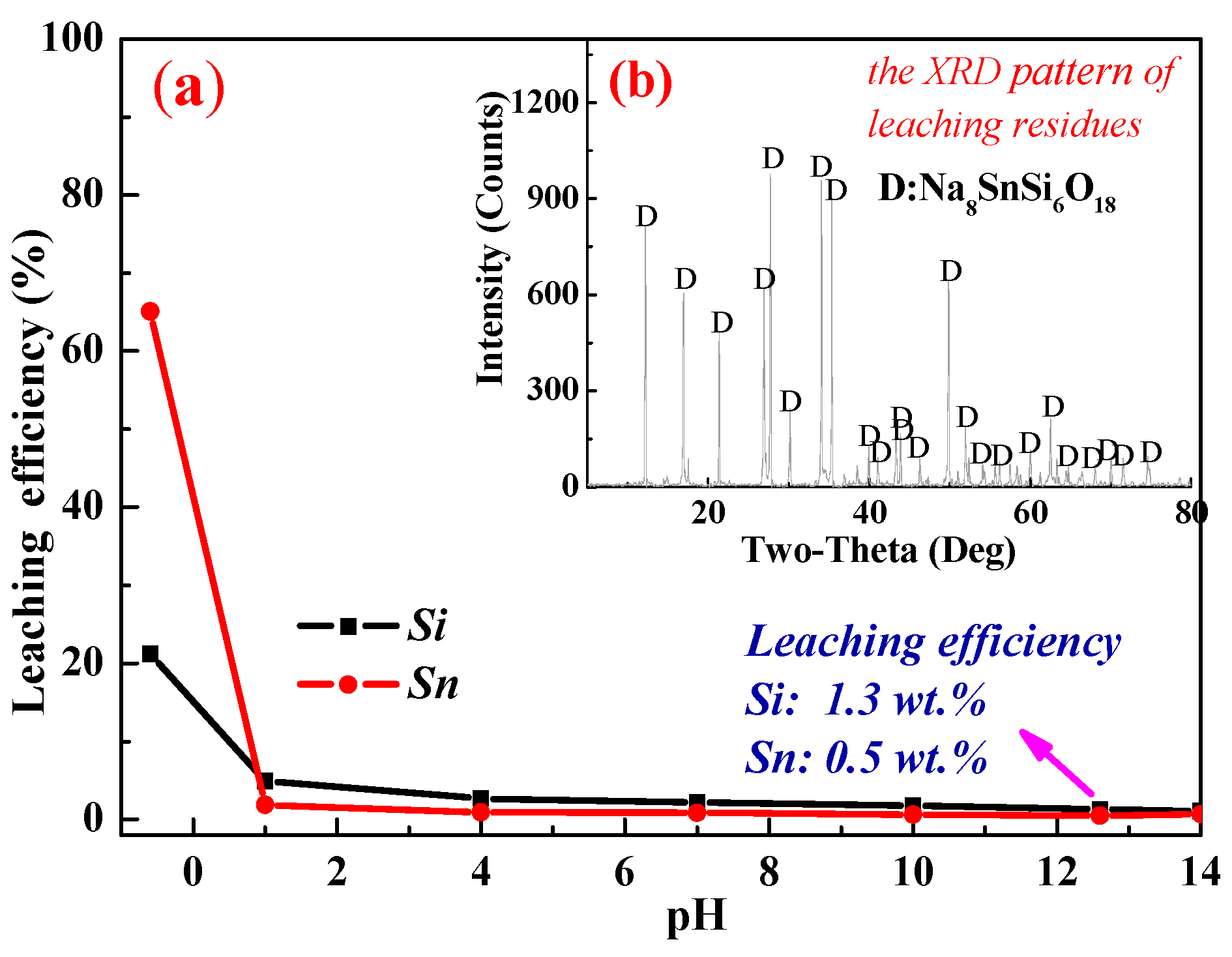
3.3. Leaching Behavior of Na8SnSi6O18
4. Conclusions
- Na2SnO3, Na2SiO3, and Na8SnSi6O18 were easily formed when the Na2CO3 + SnO2 + SiO2 mixtures were roasted under CO–CO2 atmosphere. Na2SnO3 and Na2SiO3 were easily dissolved into the leachate during the leaching process, while Na8SnSi6O18 enriched into the leach residues.
- Na8SnSi6O18 was inevitably formed in the roasting process of preparation of Na2SnO3. Roasting temperature and Si/Sn mole ratio were the two critical factors affecting the formation of Na8SnSi6O18, which was more easily formed at higher roasting temperature and Si/Sn mole ratio.
- The leaching behavior of synthetic Na8SnSi6O18 indicated that Na8SnSi6O18 was almost insoluble in the leachate at the pH range of 1–14. Therefore, the loss of tin in the residues was mainly attributed to the insoluble Na8SnSi6O18 formed during the roasting process.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sharma, A.; Das, S.; Das, K. Effect of different electrolytes on the microstructure, corrosion and whisker growth of pulse plated tin coatings. Microelectron. Eng. 2017, 170, 59–68. [Google Scholar] [CrossRef]
- Yang, W.; Xu, D.; Yao, X. Stable preparation and characterization of yellow micro arc oxidation coating on magnesium alloy. J. Alloy. Compd. 2018, 745, 609–616. [Google Scholar] [CrossRef]
- Zhang, S.G.; Wei, Y.D.; Yin, S.F.; Luo, S.L.; Au, C.T. Superbasic sodium stannate as catalyst for dehydrogenation, Michael addition and transesterification reactions. Appl. Catal. A Gen. 2011, 406, 113–118. [Google Scholar] [CrossRef]
- Sang, B.; Li, Z.; Yu, L.; Li, X.; Zhang, Z. Preparation of zinc hydroxystannate-titanate nanotube flame retardant and evaluation its smoke suppression efficiency for flexible polyvinyl chloride matrix. Mater. Lett. 2017, 204, 133–137. [Google Scholar] [CrossRef]
- Butova, V.V.; Shukaev, I.L. Ion exchange conversion of solid electrolyte, potassium sodiostannate, into isomorphous metastable sodium stannate. Mendeleev Commun. 2016, 26, 246–247. [Google Scholar] [CrossRef]
- Wang, L.; Pu, S. Direct preparation of sodium stannate from tin concentrates. J. Cent. South Inst. Min. Metall. 1987, 18, 427–431. [Google Scholar]
- Liu, W.; Li, W.; Han, J.; Wu, D.; Li, Z.; Gu, K.; Qin, W. Preparation of calcium stannate from lead refining slag by alkaline leaching-purification-causticization process. Sep. Purif. Technol. 2019, 212, 119–125. [Google Scholar] [CrossRef]
- Wu, D.; Liu, W.; Han, J.; Jiao, F.; Xu, J.; Gu, K.; Qin, W. Direct preparation of sodium stannate from lead refining dross after NaOH roasting-water leaching. Sep. Purif. Technol. 2019, 227, 115683. [Google Scholar] [CrossRef]
- Liu, W.; Li, Z.; Han, J.; Li, W.; Wang, X.; Wang, N.; Qin, W. Selective Separation of Arsenic from Lead Smelter Flue Dust by Alkaline Pressure Oxidative Leaching. Minerals 2019, 9, 308. [Google Scholar] [CrossRef]
- Wu, D.; Han, J.; Liu, W.; Jiao, F.; Qin, W. Preparation of Calcium Stannate from Lead Refining Dross by Roast–Leach–Precipitation Process. Minerals 2019, 9, 283. [Google Scholar] [CrossRef]
- Akimoto, Y.; Iizuka, A.; Shibata, E. High-voltage pulse crushing and physical separation of polycrystalline silicon photovoltaic panels. Miner. Eng. 2018, 125, 1–9. [Google Scholar] [CrossRef]
- Yang, C.; Li, J.; Tan, Q.; Liu, L.; Dong, Q. Green process of metal recycling: Coprocessing waste printed circuit boards and spent tin stripping solution. ACS Sustain. Chem. Eng. 2017, 5, 3524–3534. [Google Scholar] [CrossRef]
- Yang, C.; Tan, Q.; Liu, L.; Dong, Q.; Li, J. Recycling Tin from electronic waste: A problem that needs more attention. ACS Sustain. Chem. Eng. 2017, 5, 9586–9598. [Google Scholar] [CrossRef]
- Zhang, Y.; Su, Z.; Liu, B.; You, Z.; Yang, G.; Li, G.; Jiang, T. Sodium stannate preparation from stannic oxide by a novel soda roasting–leaching process. Hydrometallurgy 2014, 146, 82–88. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, Y.; Su, Z.; Li, G.; Jiang, T. Phase Evolution of Tin Oxides Roasted Under CO–CO2 Atmospheres in the Presence of Na2CO3. Miner. Process. Extr. Metall. Rev. 2016, 37, 264–273. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, Y.; Su, Z.; Li, G.; Jiang, T. Function mechanism of CO-CO2 atmosphere on the formation of Na2SnO3 from SnO2 and Na2CO3 during the roasting process. Powder Technol. 2016, 301, 102–109. [Google Scholar] [CrossRef]
- Su, Z.; Zhang, Y.; Liu, B.; Chen, J.; Li, G.; Jiang, T. Effect of CaCO3 on the Gaseous Reduction of Tin Oxide Under CO-CO2 Atmosphere. Miner. Process. Extr. Metall. Rev. 2016, 37, 179–186. [Google Scholar] [CrossRef]
- Su, Z.; Zhang, Y.; Liu, B.; Chen, Y.; Li, G.; Jiang, T. Effect of CaF2 On the Reduction Volatilization of Tin Oxide Under CO-CO2 Atmosphere. Miner. Process. Extr. Metall. Rev. 2017, 38, 207–213. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, Y.; Su, Z.; Li, G.; Jiang, T. Formation kinetics of Na2SnO3 from SnO2 and Na2CO3 roasted under CO-CO2 atmosphere. Int. J. Miner. Process. 2017, 165, 34–40. [Google Scholar] [CrossRef]
- Zhang, Y.; Su, Z.; You, Z.; Liu, B.; Yang, G.; Li, G.; Jiang, T. Sodium stannate preparation from cassiterite concentrate and sodium carbonate by roasting under a CO–CO2 atmosphere. In Proceedings of the Rare Metal Technology, Conference Proceeding, TMS, San Diego, CA, USA, 16–20 February 2014; pp. 163–169. [Google Scholar]
- Feng, Q.; Wen, S.; Zhao, W.; Chen, Y. Effect of calcium ions on adsorption of sodium oleate onto cassiterite and quartz surfaces and implications for their flotation separation. Sep. Purif. Technol. 2018, 200, 300–306. [Google Scholar] [CrossRef]
- Tian, M.; Zhang, C.; Han, H.; Liu, R.; Gao, Z.; Chen, P.; He, J.; Hu, Y.; Sun, W.; Yuan, D. Novel insights into adsorption mechanism of benzohydroxamic acid on lead (II)-activated cassiterite surface: An integrated experimental and computational study. Miner. Eng. 2018, 122, 327–338. [Google Scholar] [CrossRef]
- Wright, P.A. Extractive Metallurgy of Tin, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 1982. [Google Scholar]
- Song, X.C. Tin Metallurgy; Metallurgical Industry Press: Beijing, China, 2011. [Google Scholar]
- Paparoni, G.; Webster, J.D.; Walker, D. Experimental techniques for determining tin solubility in silicate melts using silica capsules in 1 atm furnaces and rhenium capsules in thepiston cylinder. Am. Mineral. 2010, 95, 776–783. [Google Scholar] [CrossRef]
- Zhang, Y.; Su, Z.; Liu, B.; Zhou, Y.; Jiang, T.; Li, G. Reduction behavior of SnO2 in the tin-bearing iron concentrates under CO-CO2 atmosphere. Part II: Effect of quartz. Powder Technol. 2016, 291, 337–343. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, B.; Su, Z.; Chen, J.; Li, G.; Jiang, T. Effect of Na2CO3 on the preparation of metallic tin from cassiterite roasted under strong reductive atmosphere. J. Min. Metall. 2016, 52, 9–15. [Google Scholar] [CrossRef]
- Sanchez-Segado, S.; Monti, T.; Katrib, J.; Kingman, S.; Dodds, C.; Jha, A. Towards sustainable processing of columbite group minerals: Elucidating the relation between dielectric properties and physico-chemical transformations in the mineral phase. Sci. Rep. 2017, 7, 18016. [Google Scholar] [CrossRef]
- Escudero-Castejon, L.; Sanchez-Segado, S.; Parirenyatwa, S.; Hara, Y.; Jha, A. A Cr6+-free extraction of chromium oxide from chromite ores using carbothermic reduction in the presence of alkali. In Applications of Process Engineering Principles in Materials Processing, Energy and Environmental Technologies; Springer: Berlin/Heidelberg, Germany, 2017; pp. 179–188. [Google Scholar]
- Parirenyatwa, S.; Escudero-Castejon, L.; Sanchez-Segado, S.; Hara, Y.; Jha, A. An investigation on the kinetics and mechanism of alkali reduction of mine waste containing titaniferous minerals for the recovery of metals. In Applications of Process Engineering Principles in Materials Processing, Energy and Environmental Technologies; Springer: Berlin/Heidelberg, Germany, 2017; pp. 465–474. [Google Scholar]
- Sanchez-Segado, S.; Lahiri, A.; Jha, A. Alkali roasting of bomar ilmenite: Rare earths recovery and physico-chemical changes. Open Chem. 2015, 13, 270–278. [Google Scholar] [CrossRef]
- Su, Z.; Zhang, Y.; Han, B.; Liu, B.; Lu, M.; Peng, Z.; Li, G.; Jiang, T. Synthesis, characterization, and catalytic properties of nano-SnO by chemical vapor transport (CVT) process under CO–CO2 atmosphere. Mater. Des. 2017, 121, 280–287. [Google Scholar] [CrossRef]
- Knutsson, P.; Lai, H.; Stiller, K. A method for investigation of hot corrosion by gaseous Na2SO4. Corros. Sci. 2013, 73, 230–236. [Google Scholar] [CrossRef]
- Li, Y.; He, J.B.; Zhang, M.; He, X.L. Corrosion inhibition effect of sodium phytate on brass in NaOH media. Potential-resolved formation of soluble corrosion products. Corros. Sci. 2013, 74, 116–122. [Google Scholar] [CrossRef]
- Séby, F.; Potin-Gautier, M.; Giffaut, E.; Donard, O.F.X. A critical review of thermodynamic data for inorganic tin species. Geochim. Cosmochim. Acta 2001, 65, 3041–3053. [Google Scholar] [CrossRef]
- Park, H.; Englezos, P. Osmotic coefficient data for Na2SiO3 and Na2SiO3-NaOH by an isopiestic method and modeling using Pitzer’s model. Fluid Phase Equilib. 1998, 153, 87–104. [Google Scholar] [CrossRef]
- Kim, E.; Osseo-Asare, K. Dissolution windows for hydrometallurgical purification of metallurgical-grade silicon to solar-grade silicon: Eh-pH diagrams for Fe silicides. Hydrometallurgy 2012, 127, 178–186. [Google Scholar] [CrossRef]
- Zhao, Z.; Shuai, W.; Zhang, J.; Chen, X. Sn (IV) anions adsorption onto ferric hydroxide: A speciation-based model. Hydrometallurgy 2013, 140, 135–143. [Google Scholar] [CrossRef]
- Lindsay, W.L. Chemical Equilibria in Soils; Wiley: New York, NY, USA, 1979; pp. 79–85. [Google Scholar]
- Hummel, W.; Berner, U.; Curti, E.; Pearson, F.J.; Thoenen, T. Nagra/PSI chemical thermodynamic data base 01/01. Radiochim. Acta 2002, 90, 805–813. [Google Scholar] [CrossRef]
- Angadi, S.I.; Sreenivas, T.; Jeon, H.S.; Baek, S.H.; Mishra, B.K. A review of cassiterite beneficiation fundamentals and plant practices. Miner. Eng. 2015, 70, 178–200. [Google Scholar] [CrossRef]
- Choi, Y.I.; Salman, S.; Kuroda, K.; Okido, M. Synergistic corrosion protection for AZ31 Mg alloy by anodizing and stannate post-sealing treatments. Electrochim. Acta 2013, 97, 313–319. [Google Scholar] [CrossRef]
| Element | Sn | Si | Fe | CaO | S | Al2O3 | Zn | As | Pb |
|---|---|---|---|---|---|---|---|---|---|
| Raw material | 42.9 | 3.9 | 8.86 | 8.31 | 5.11 | 1.16 | 1.21 | 0.50 | 0.38 |
| Pretreated | 62.9 | 3.7 | 0.11 | 0.17 | 0.04 | 0.28 | 0.02 | 0.03 | 0.03 |
| Equation | Reaction Equation | Equilibrium Constants (K) |
|---|---|---|
| (4) | Sn4+ + H2O = Sn(OH) 3+ + H+ | Kα1 = 103.73 |
| (5) | Sn4+ + 2H2O = Sn(OH)22+ + 2H+ | Kα1 = 101.29 |
| (6) | Sn4+ + 3H2O = Sn(OH)3+ + 3H+ | Kα1 = 100.47 |
| (7) | Sn4+ + 4H2O = Sn(OH)4 + 4H+ | Kα1 = 100.4 |
| (8) | Sn4+ + 5H2O = Sn(OH)5− + 5H+ | Kα2 = 10−7.7 |
| (9) | Sn4+ + 6H2O = Sn(OH)62− + 6H+ | Kα3 = 10−18.1 |
| (10) | SiO32− + H+ = HSiO3− | Kβ1 = 10−11.82 |
| (11) | HSiO3− + H+ = H2SiO3 | Kβ2 = 10−9.69 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Han, B.; Su, Z.; Chen, X.; Lu, M.; Liu, S.; Liu, J.; Jiang, T. Effect of Quartz on the Preparation of Sodium Stannate from Cassiterite Concentrates by Soda Roasting Process. Minerals 2019, 9, 605. https://doi.org/10.3390/min9100605
Zhang Y, Han B, Su Z, Chen X, Lu M, Liu S, Liu J, Jiang T. Effect of Quartz on the Preparation of Sodium Stannate from Cassiterite Concentrates by Soda Roasting Process. Minerals. 2019; 9(10):605. https://doi.org/10.3390/min9100605
Chicago/Turabian StyleZhang, Yuanbo, Benlai Han, Zijian Su, Xijun Chen, Manman Lu, Shuo Liu, Jicheng Liu, and Tao Jiang. 2019. "Effect of Quartz on the Preparation of Sodium Stannate from Cassiterite Concentrates by Soda Roasting Process" Minerals 9, no. 10: 605. https://doi.org/10.3390/min9100605
APA StyleZhang, Y., Han, B., Su, Z., Chen, X., Lu, M., Liu, S., Liu, J., & Jiang, T. (2019). Effect of Quartz on the Preparation of Sodium Stannate from Cassiterite Concentrates by Soda Roasting Process. Minerals, 9(10), 605. https://doi.org/10.3390/min9100605





