The Distribution of Rare Metals in the LCT Pegmatites from the Giraúl Field, Angola
Abstract
1. Introduction
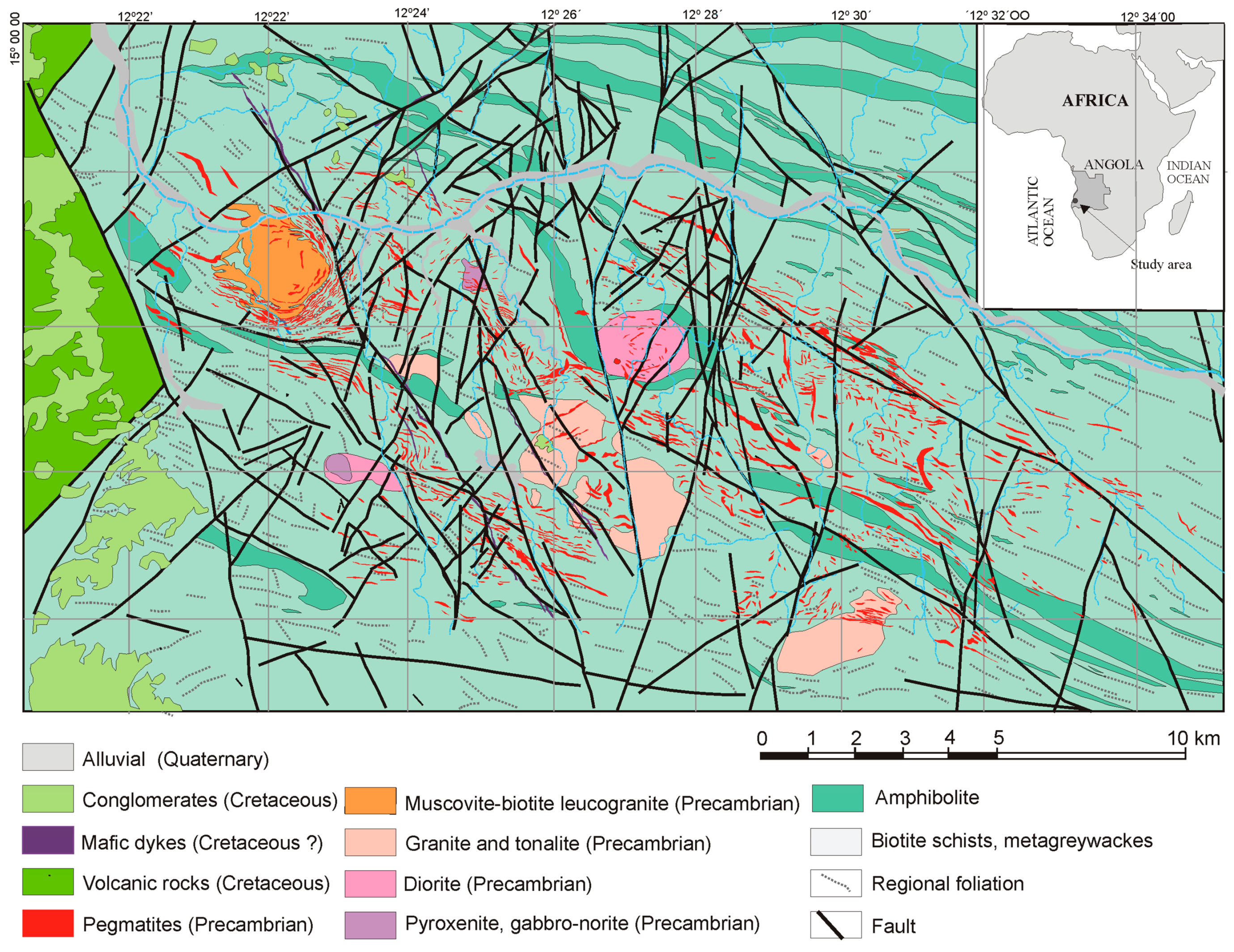
2. Geology
2.1. Regional Geology
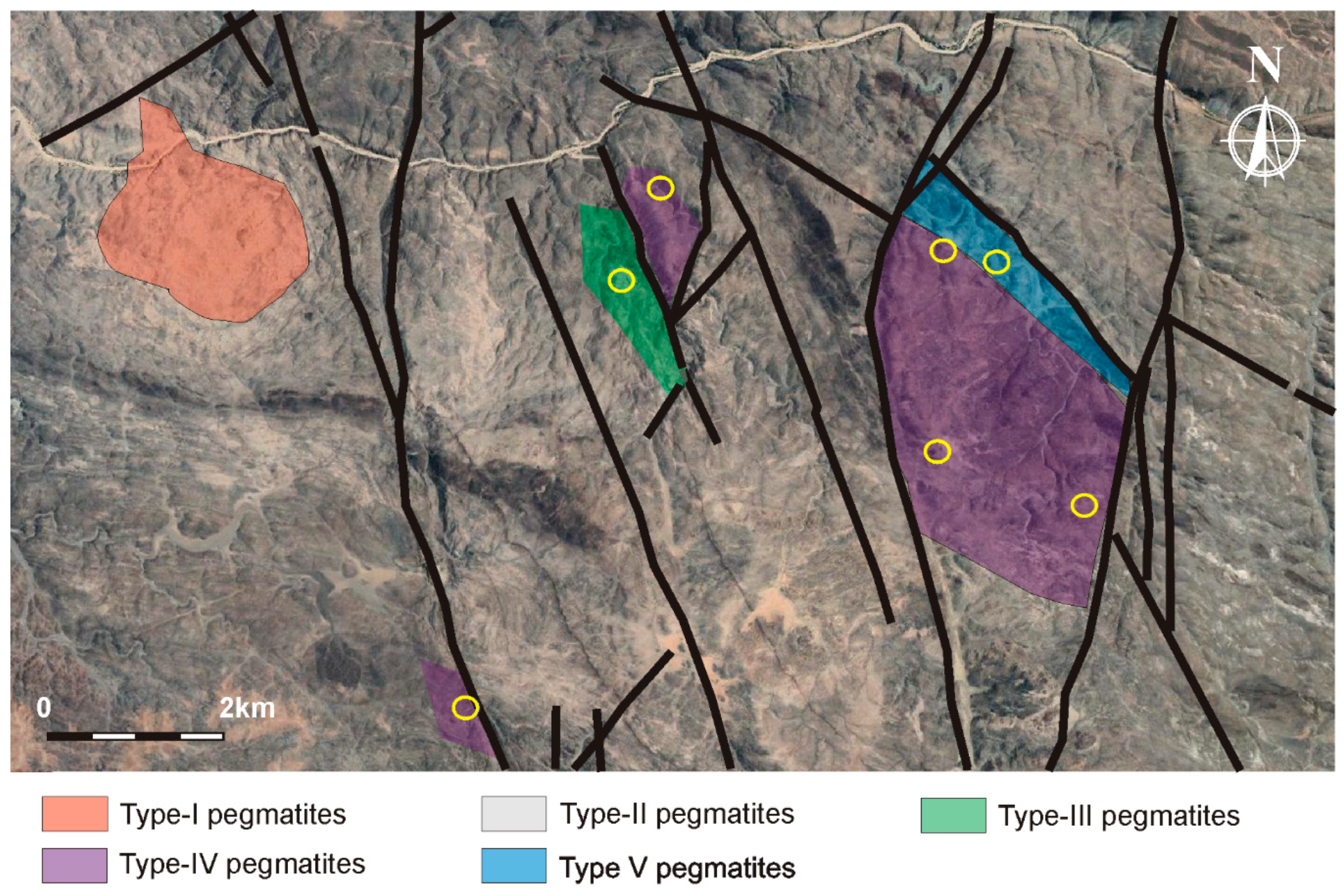
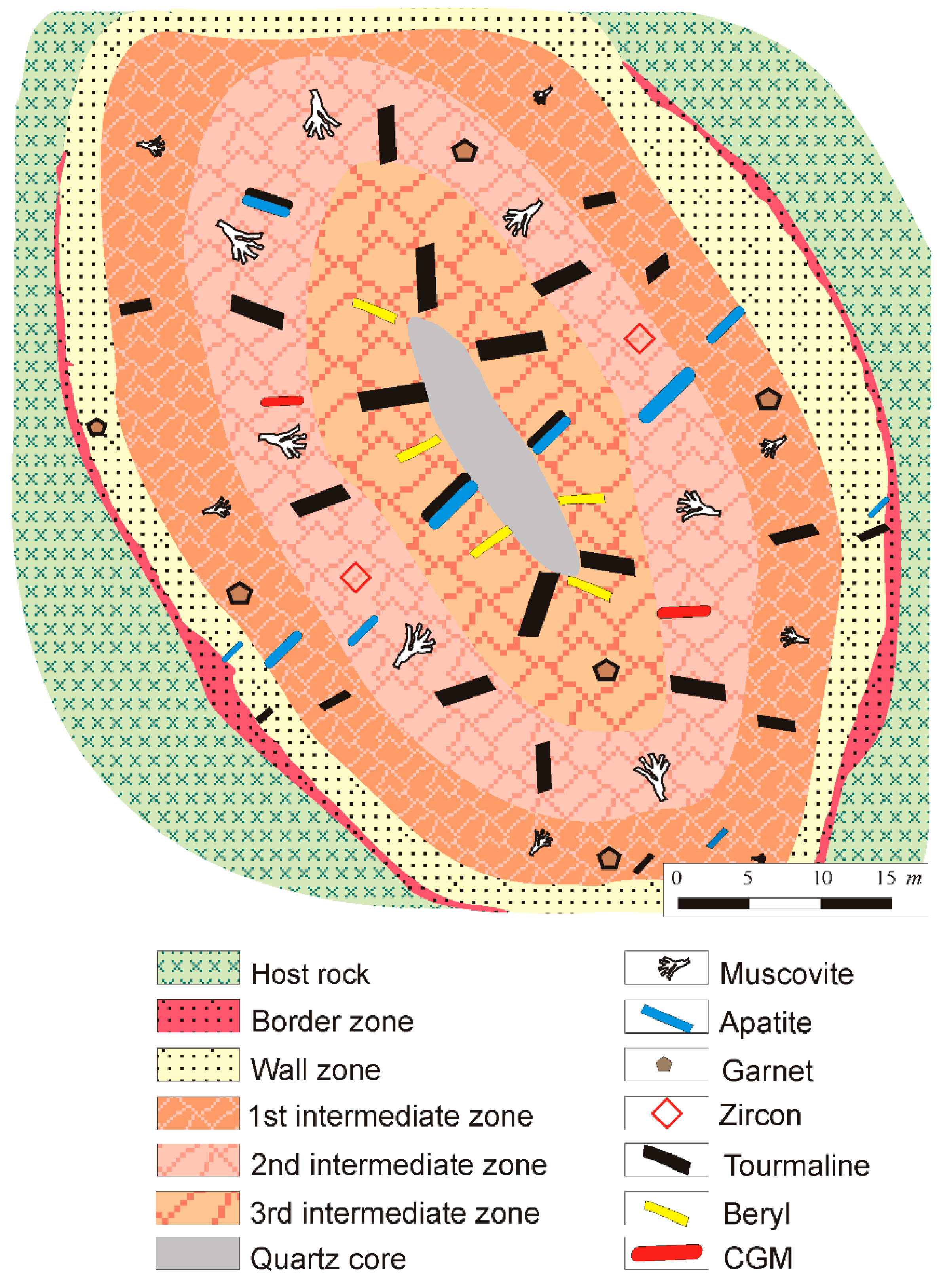
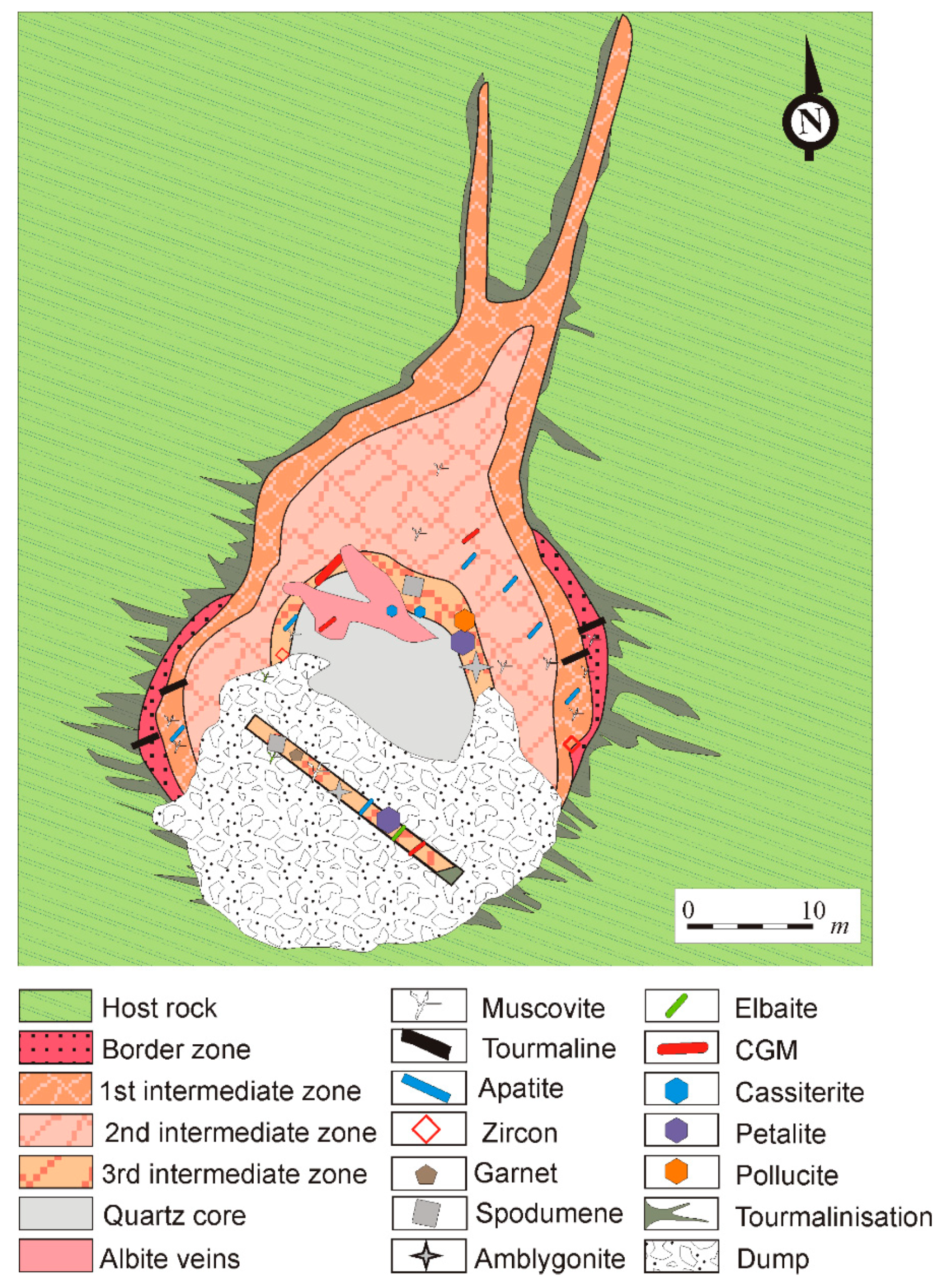
2.2. Pegmatite Types
2.2.1. Type-I Pegmatites
2.2.2. Type-II Pegmatites
2.2.3. Type-III Pegmatites
2.2.4. Type-IV Pegmatites
2.2.5. Type-V Pegmatites
3. Analytical Methods
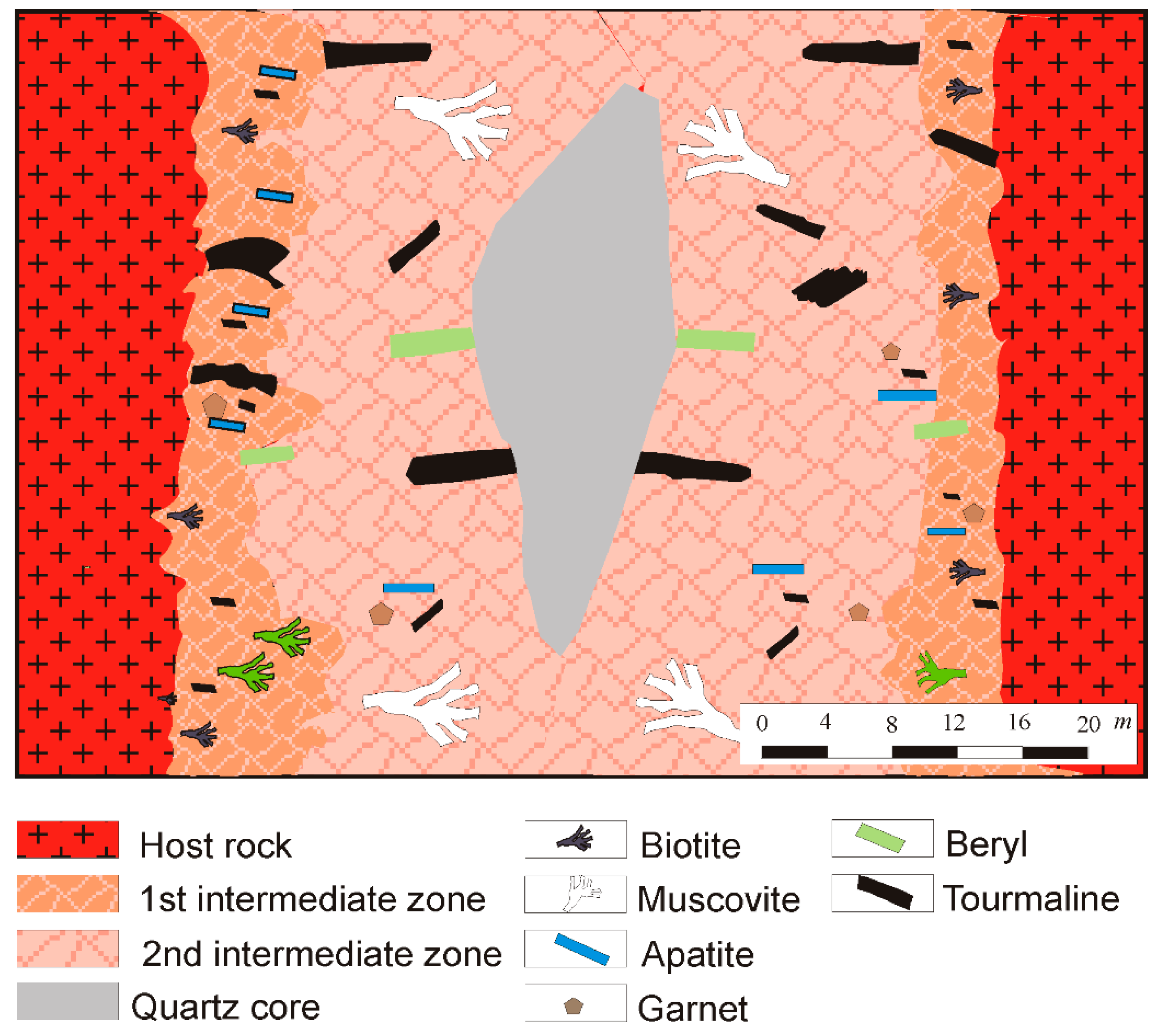
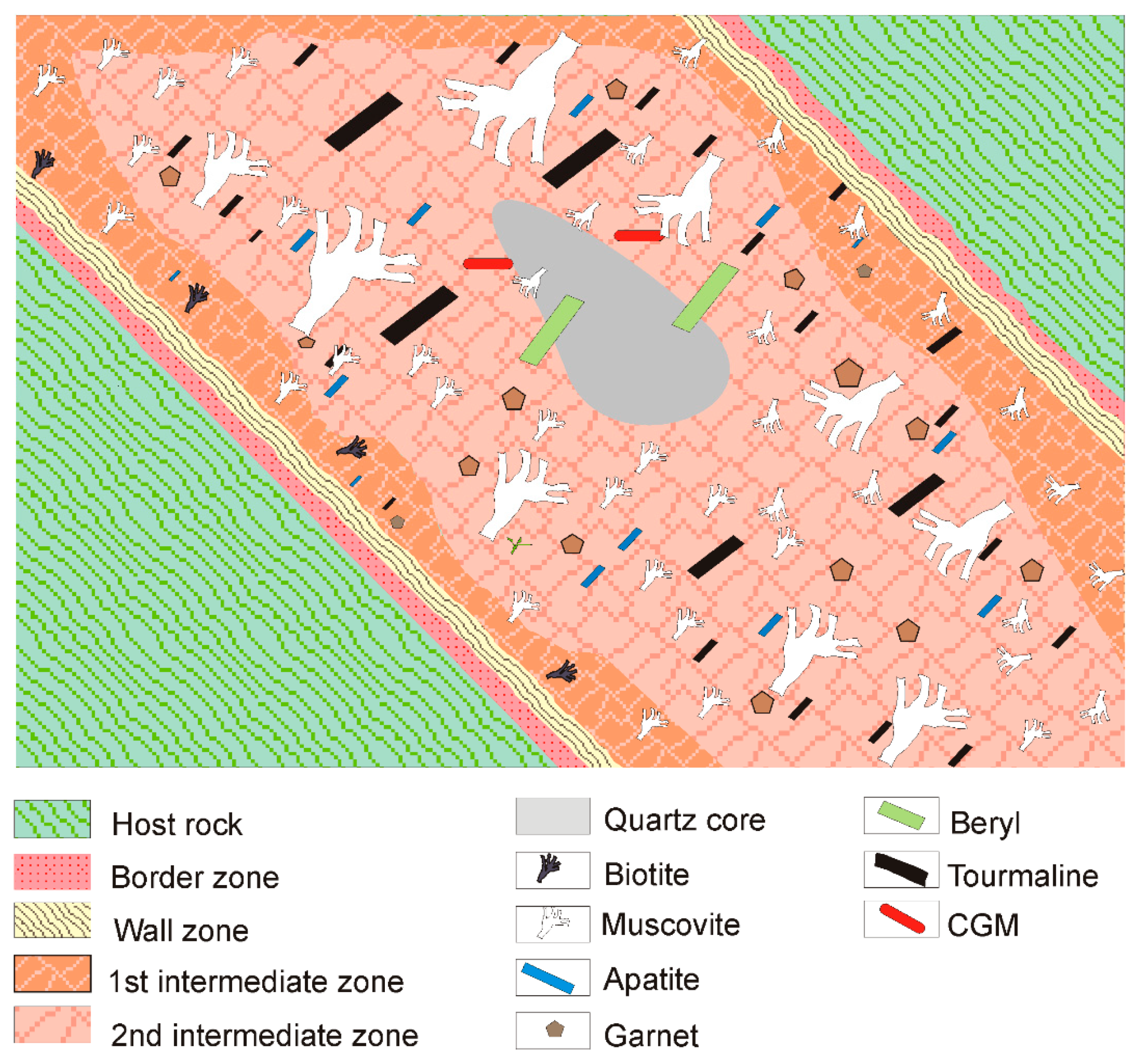
4. Mineralogy of the Giraúl Pegmatites
4.1. Mineralogical Composition and Textural Patterns
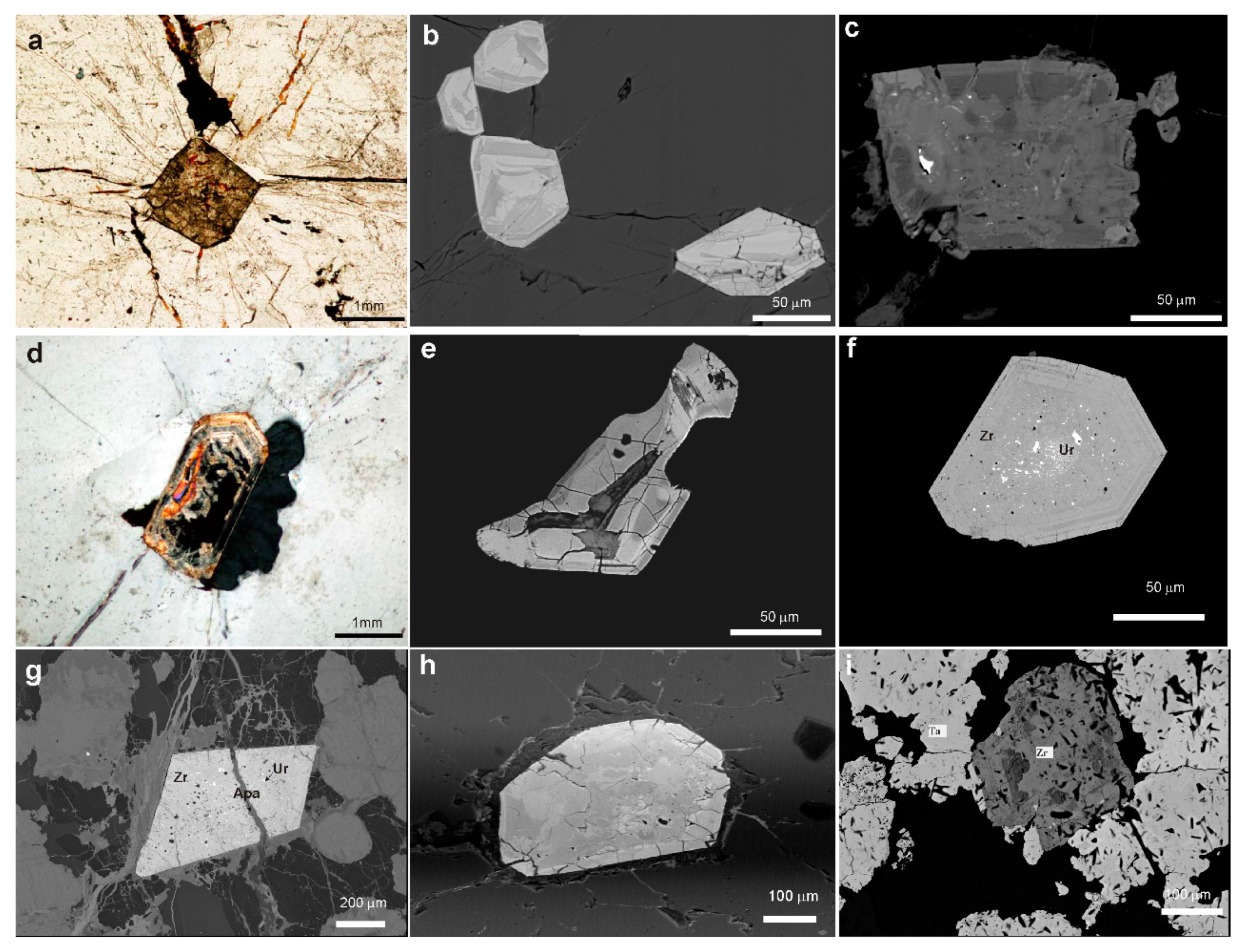
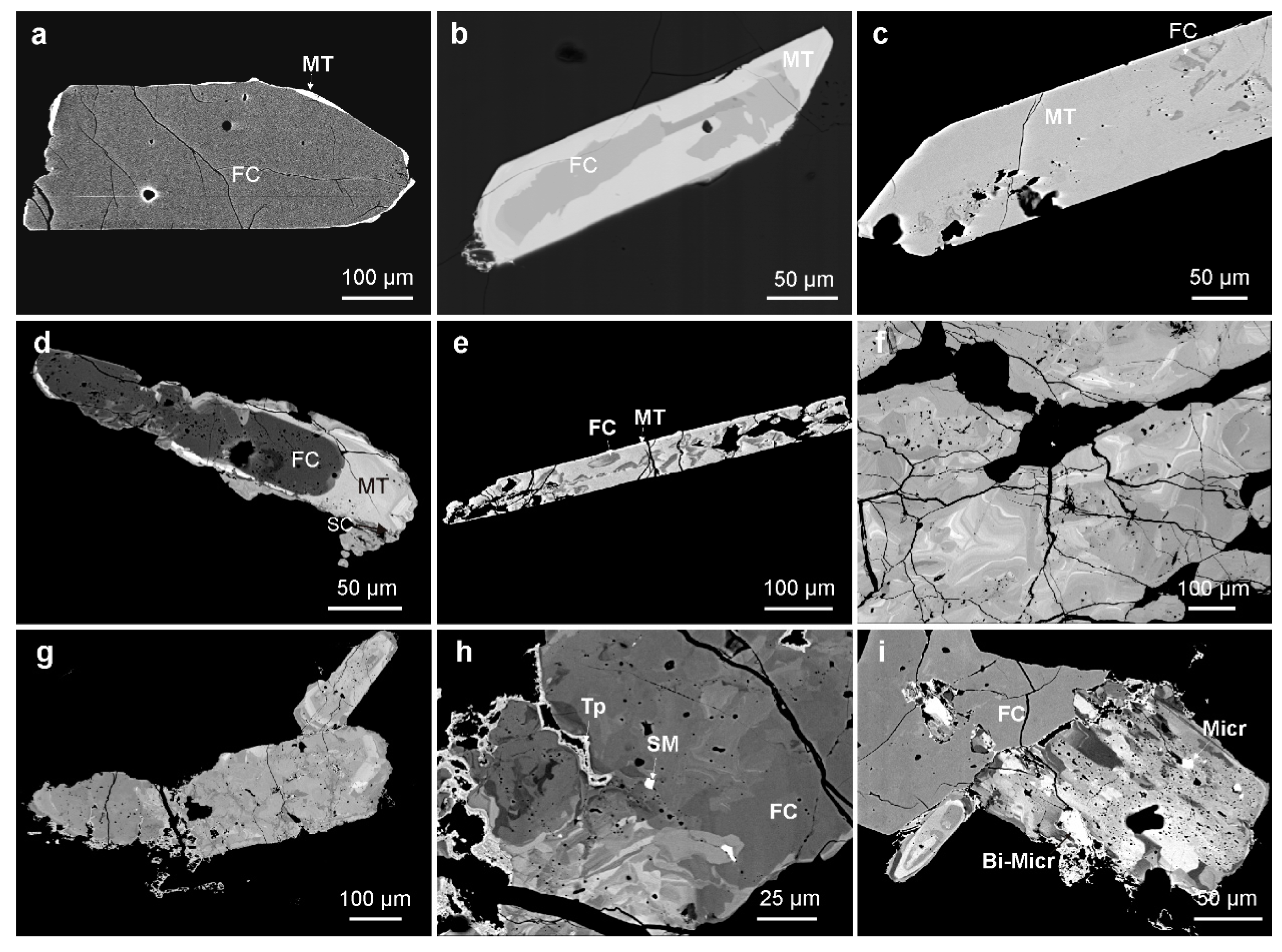
4.1.1. Textural Patterns of Zircon
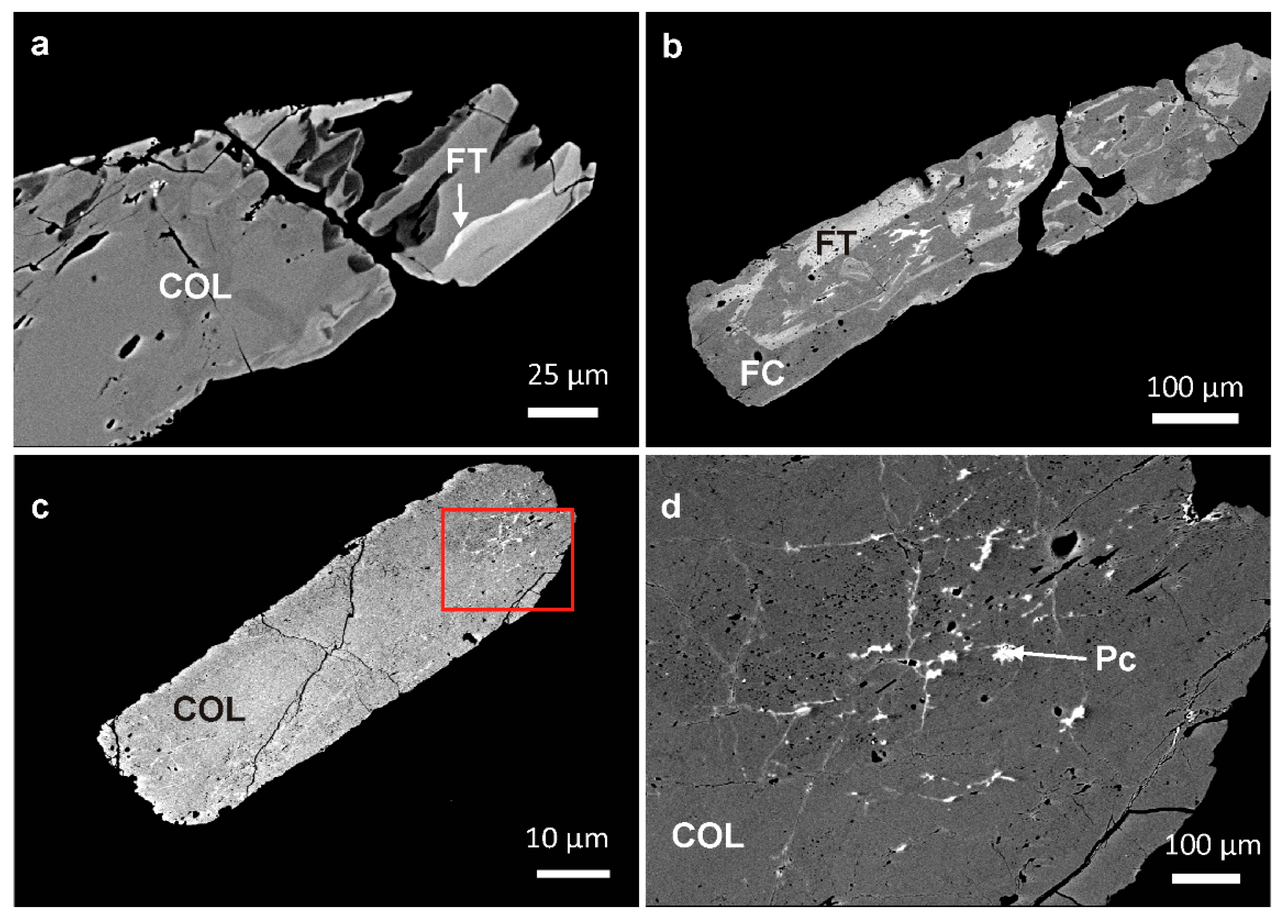
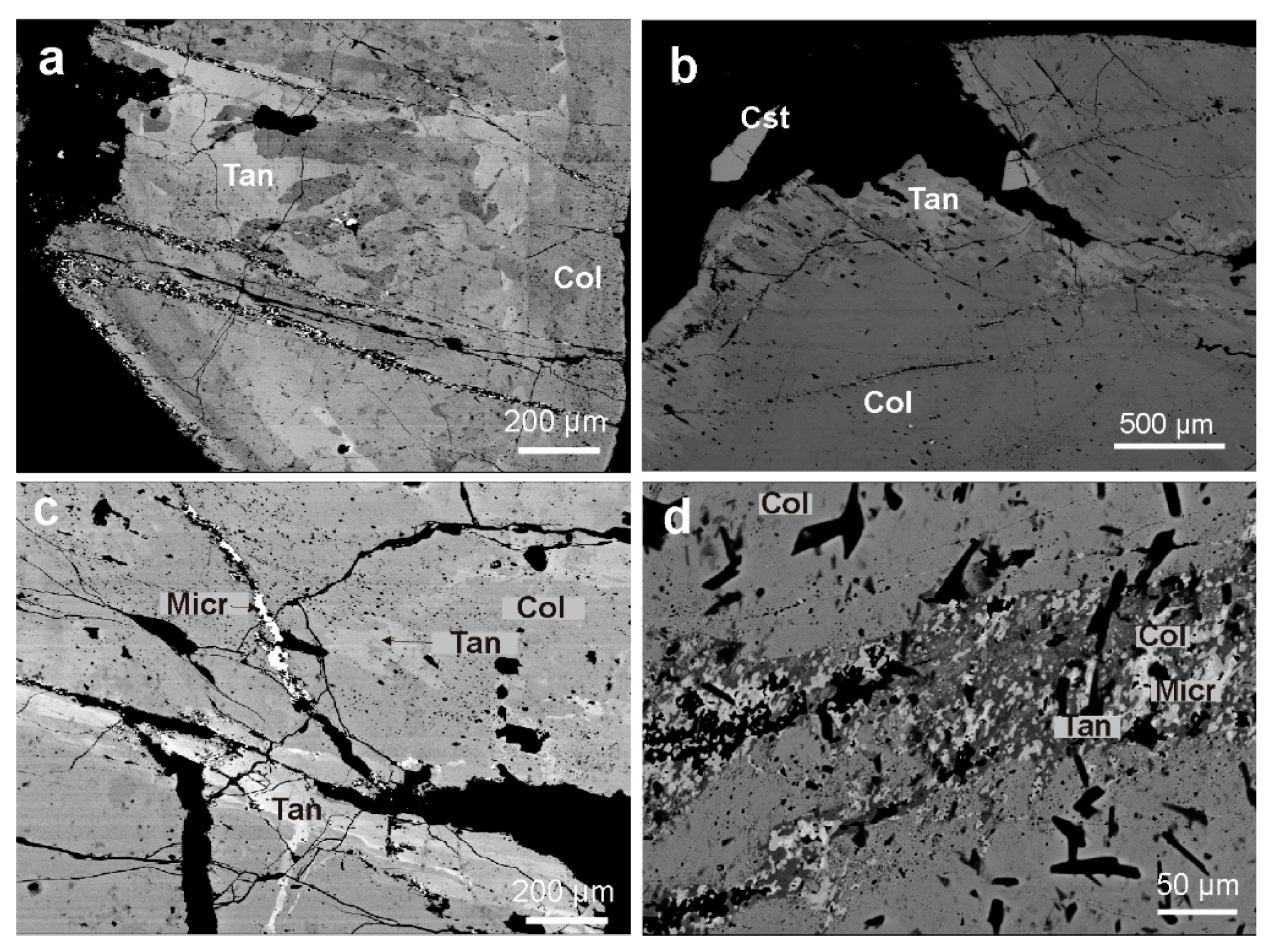
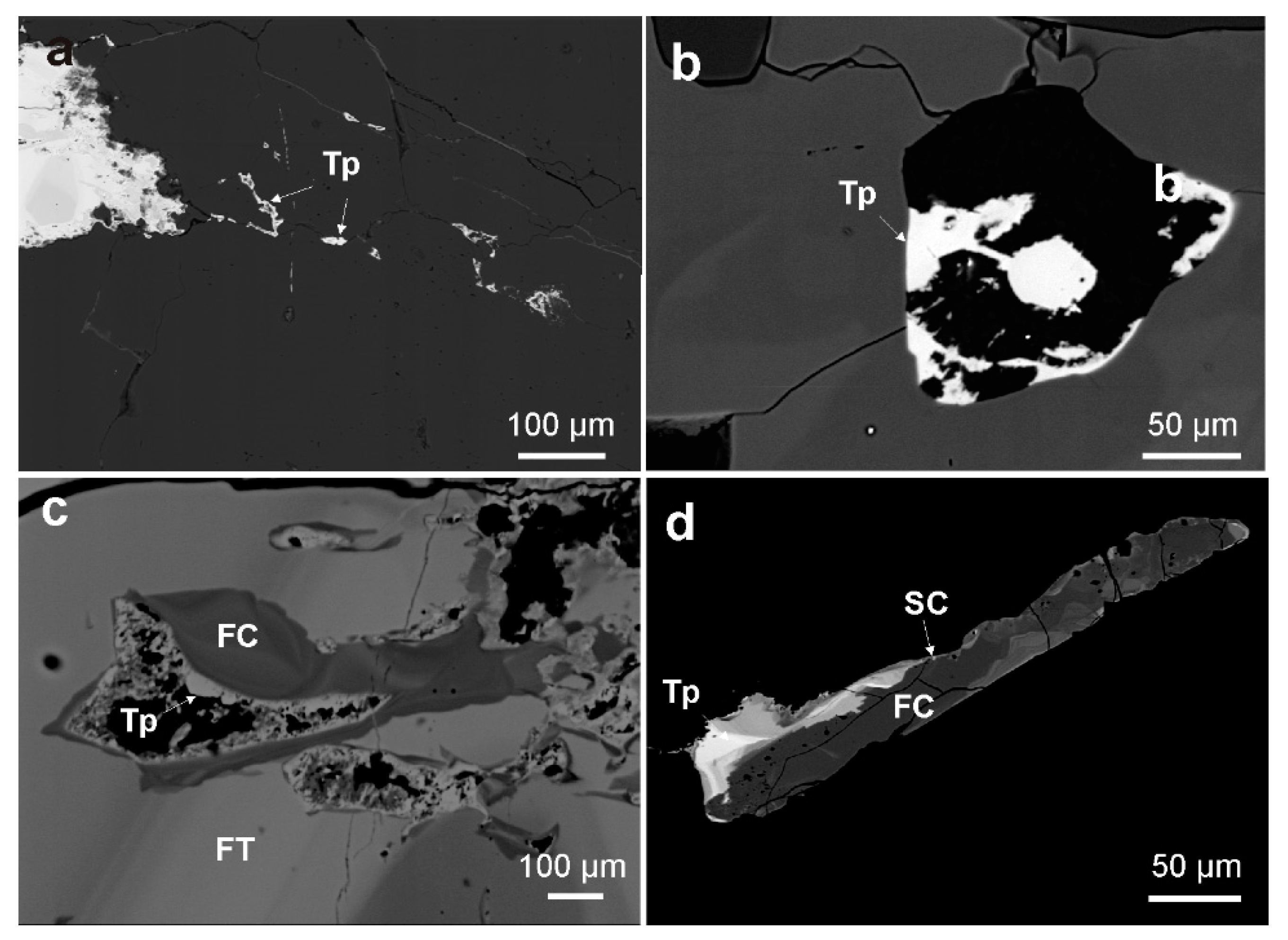
4.1.2. Textural Patterns of the Nb-Ta Oxide Minerals
4.1.3. Textural Patterns of Cassiterite
4.2. Mineral Chemistry
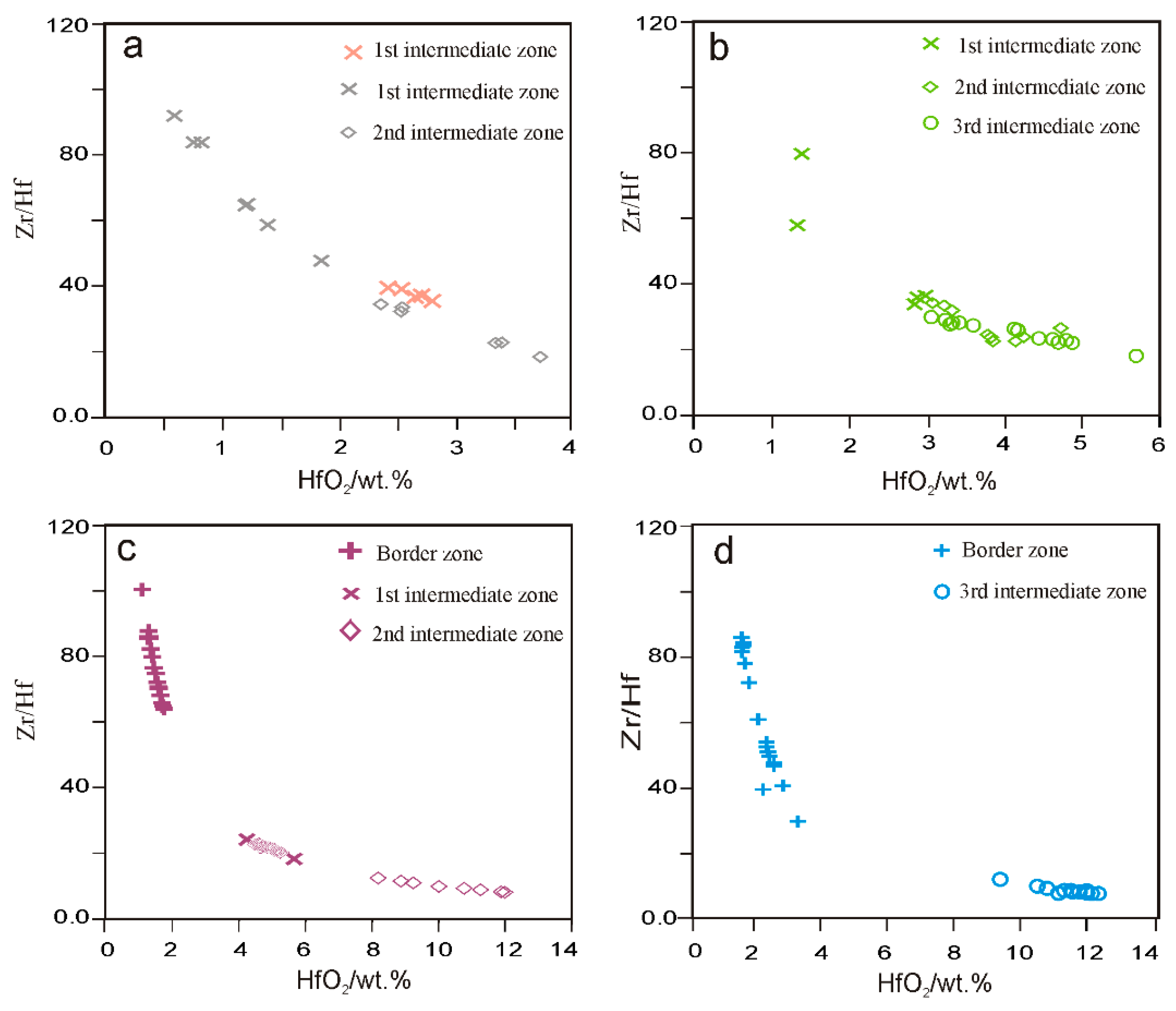
4.2.1. Zircon
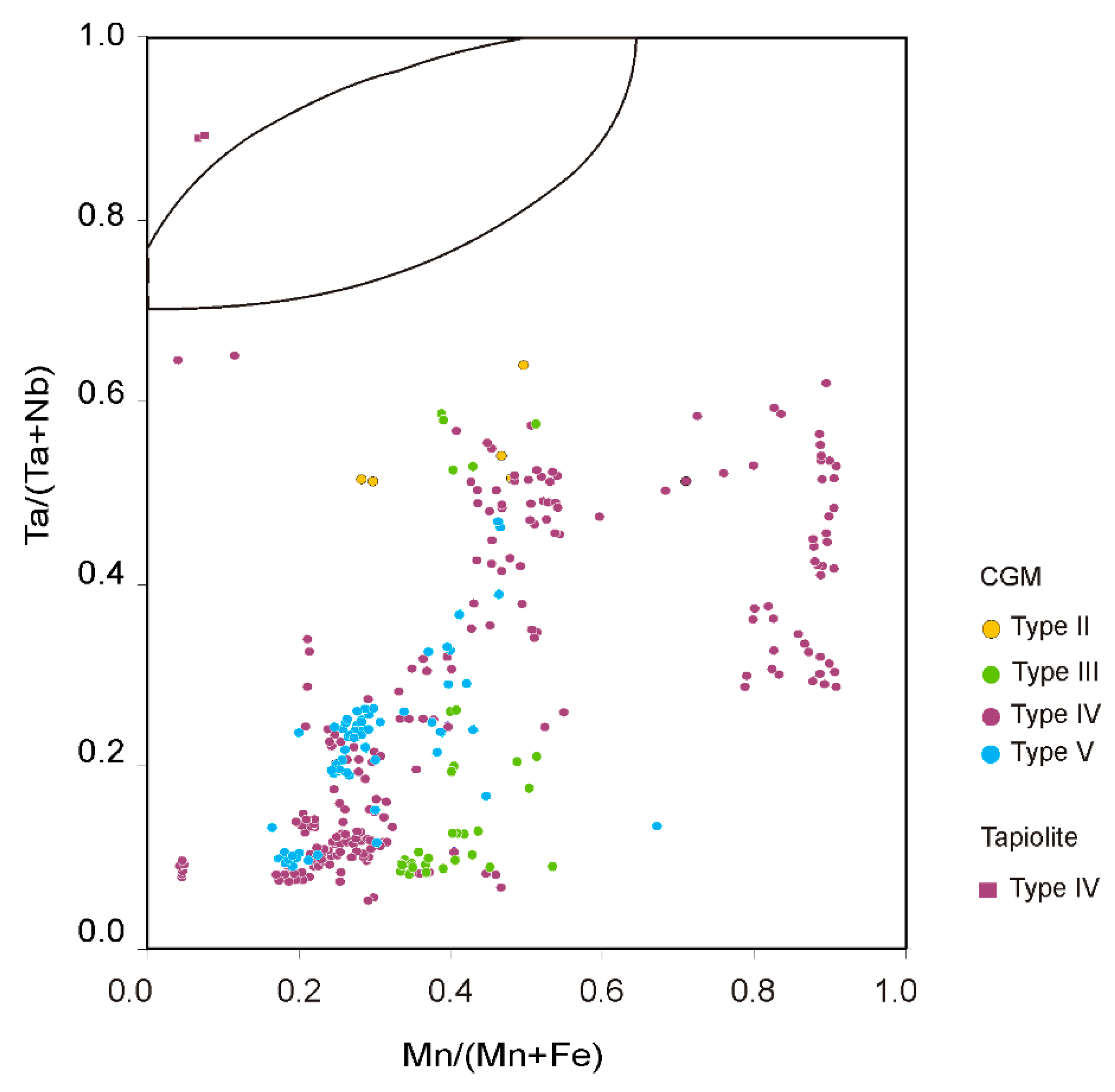
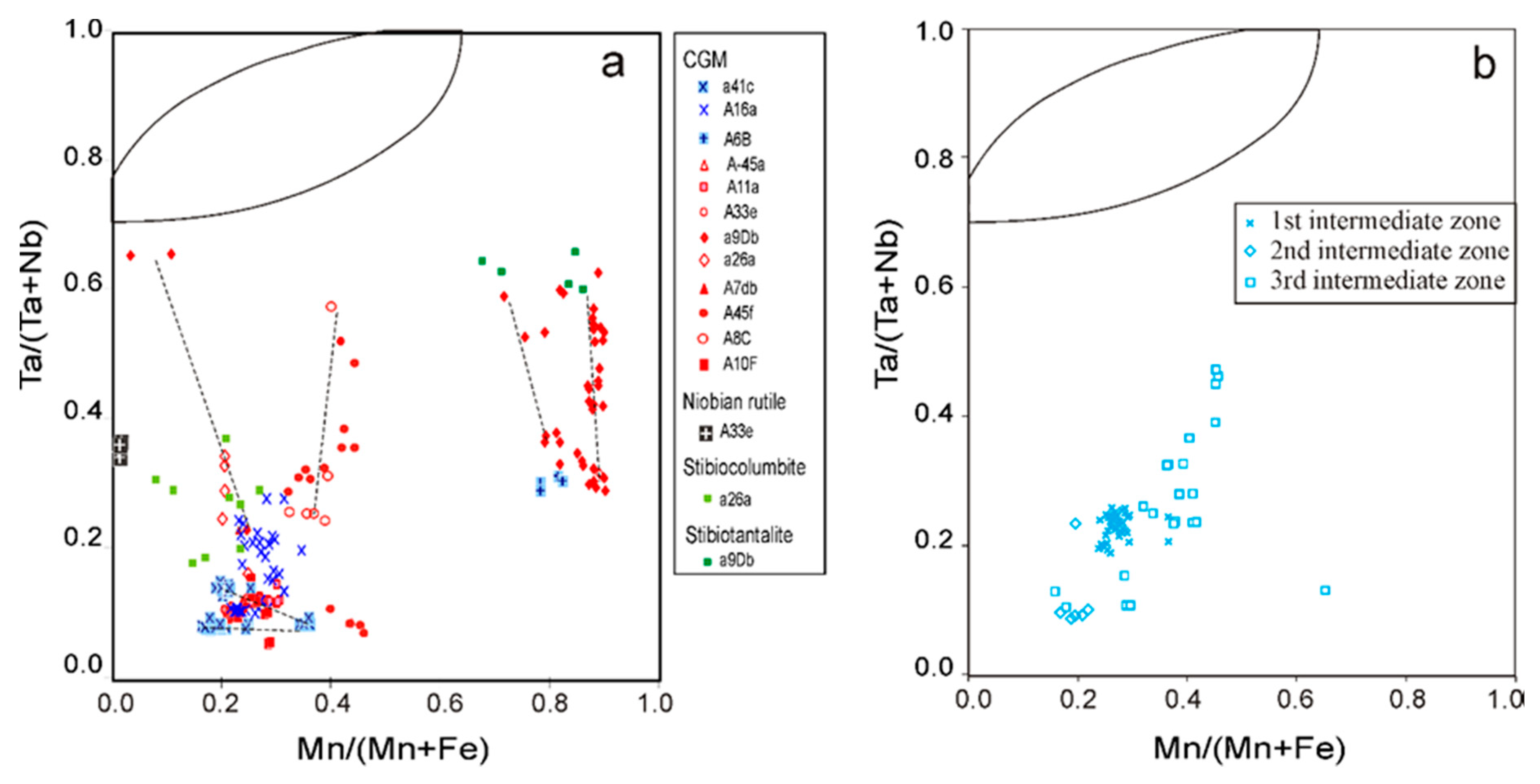
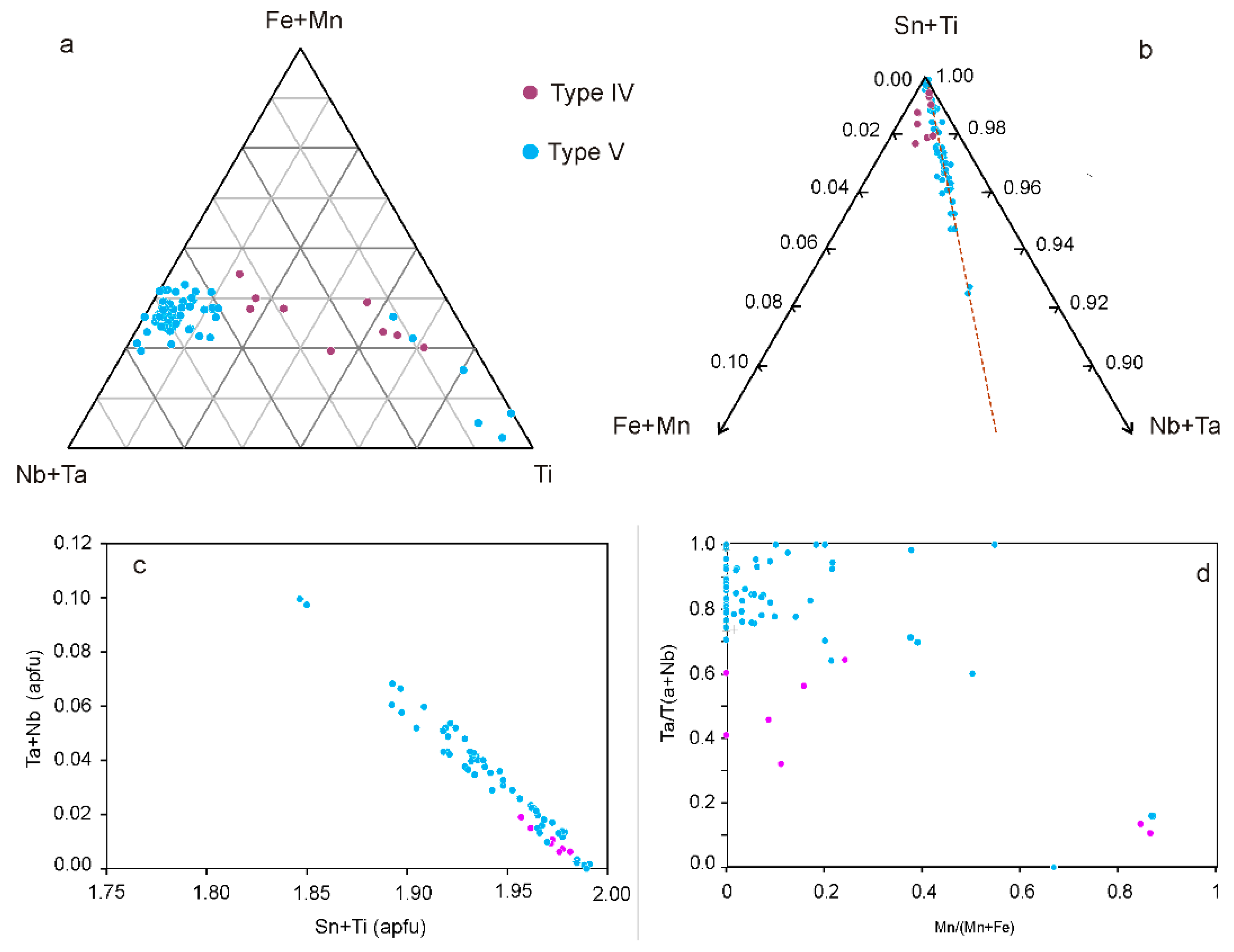
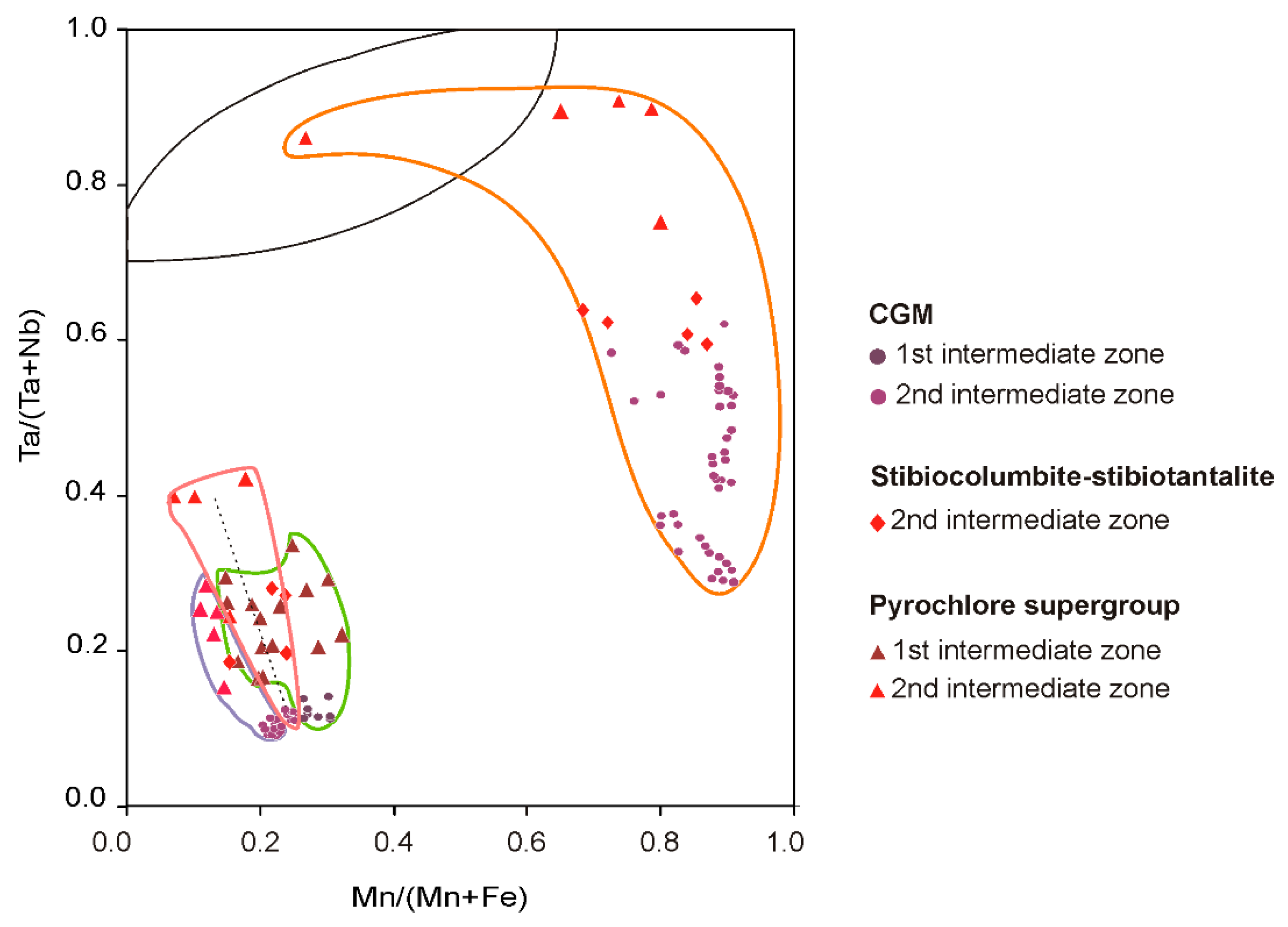
4.2.2. Columbite-Group Minerals
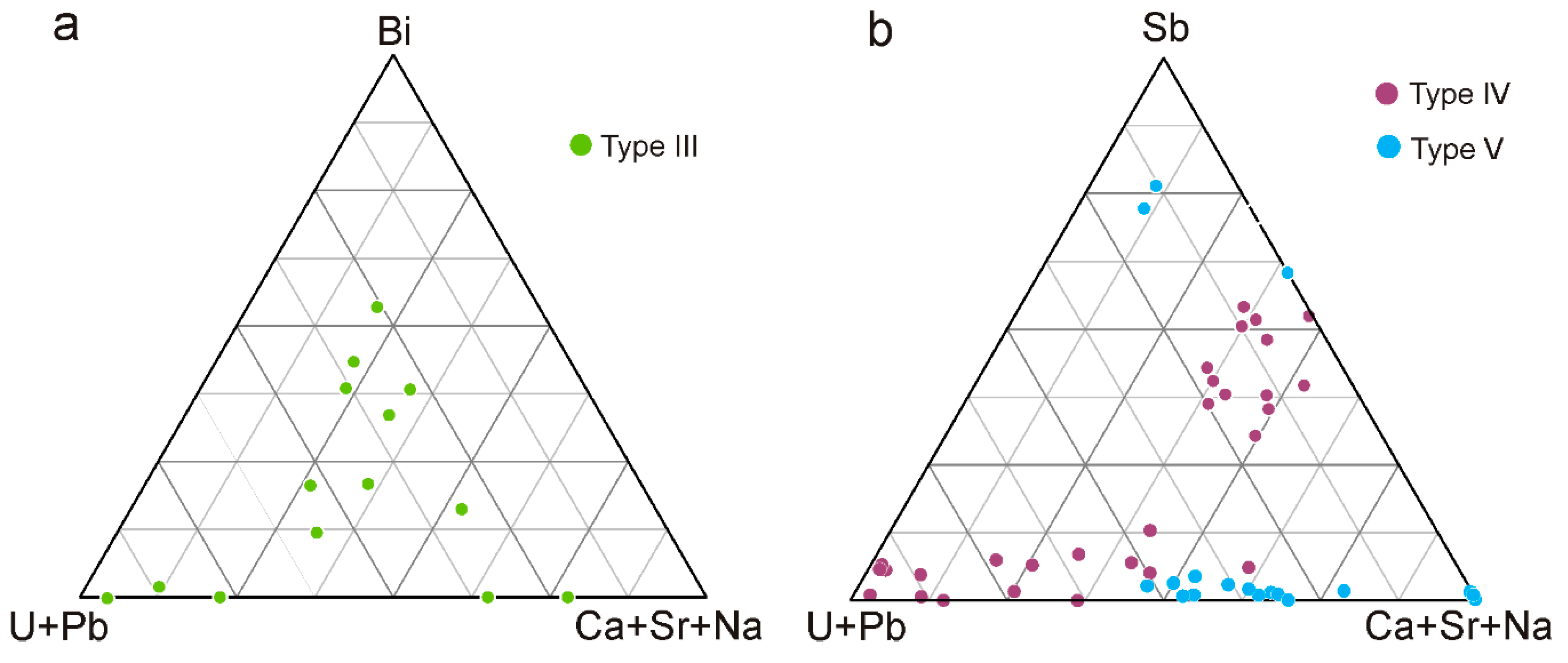
4.2.3. Stibiocolumbite and Stibiotantalite
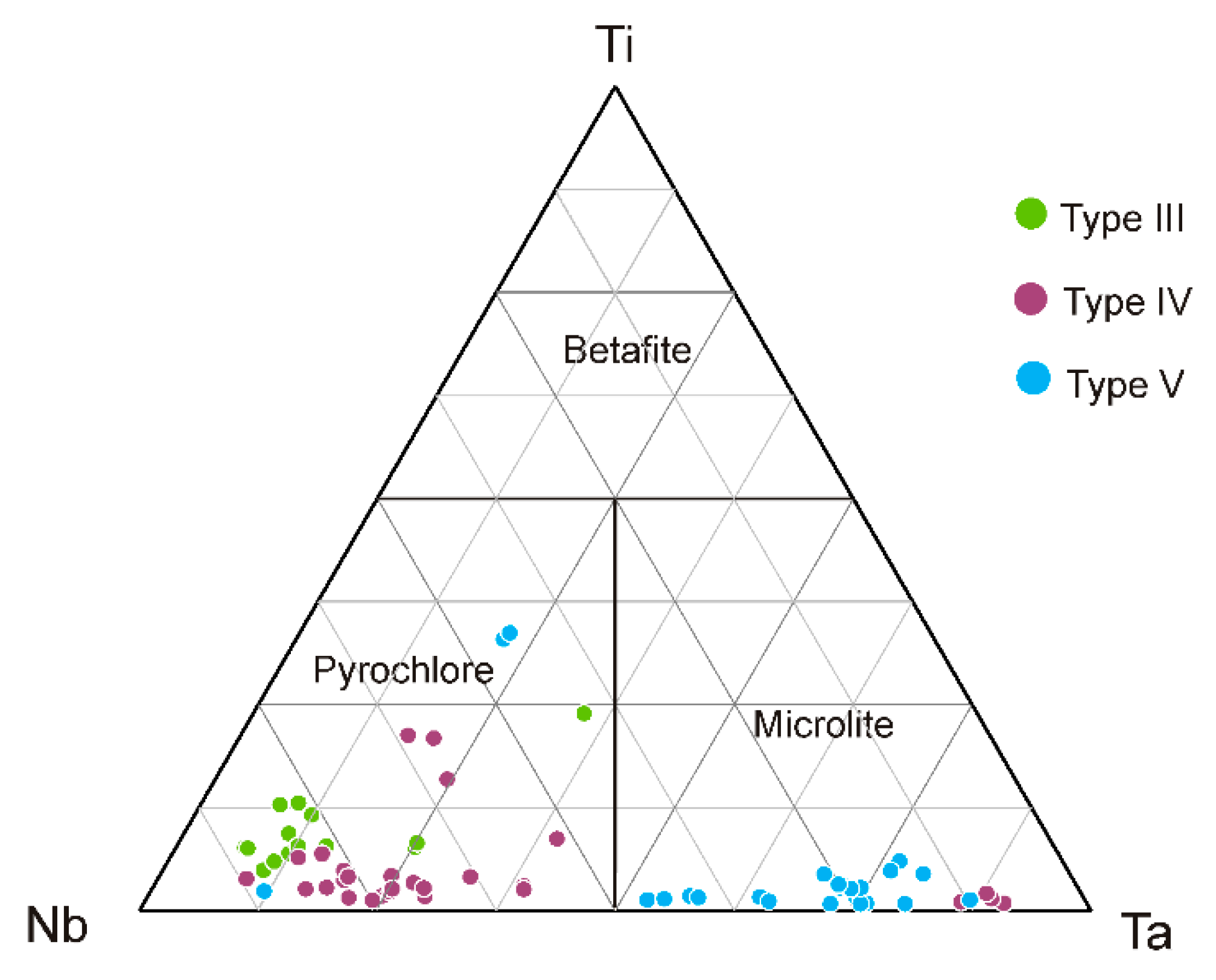
4.2.4. Pyrochlore Supergroup
4.2.5. Rutile Group
4.2.6. Cassiterite
4.2.7. Uraninite
5. Discusion
5.1. Evolution of Zircon in the Giraúl Pegmatites
5.2. Evolution of the Nb-Ta Oxide Minerals in the Giraúl Pegmatites at Crystal Scale
5.3. Evolution in the Composition of the Nb-Ta Oxides at Field Scale
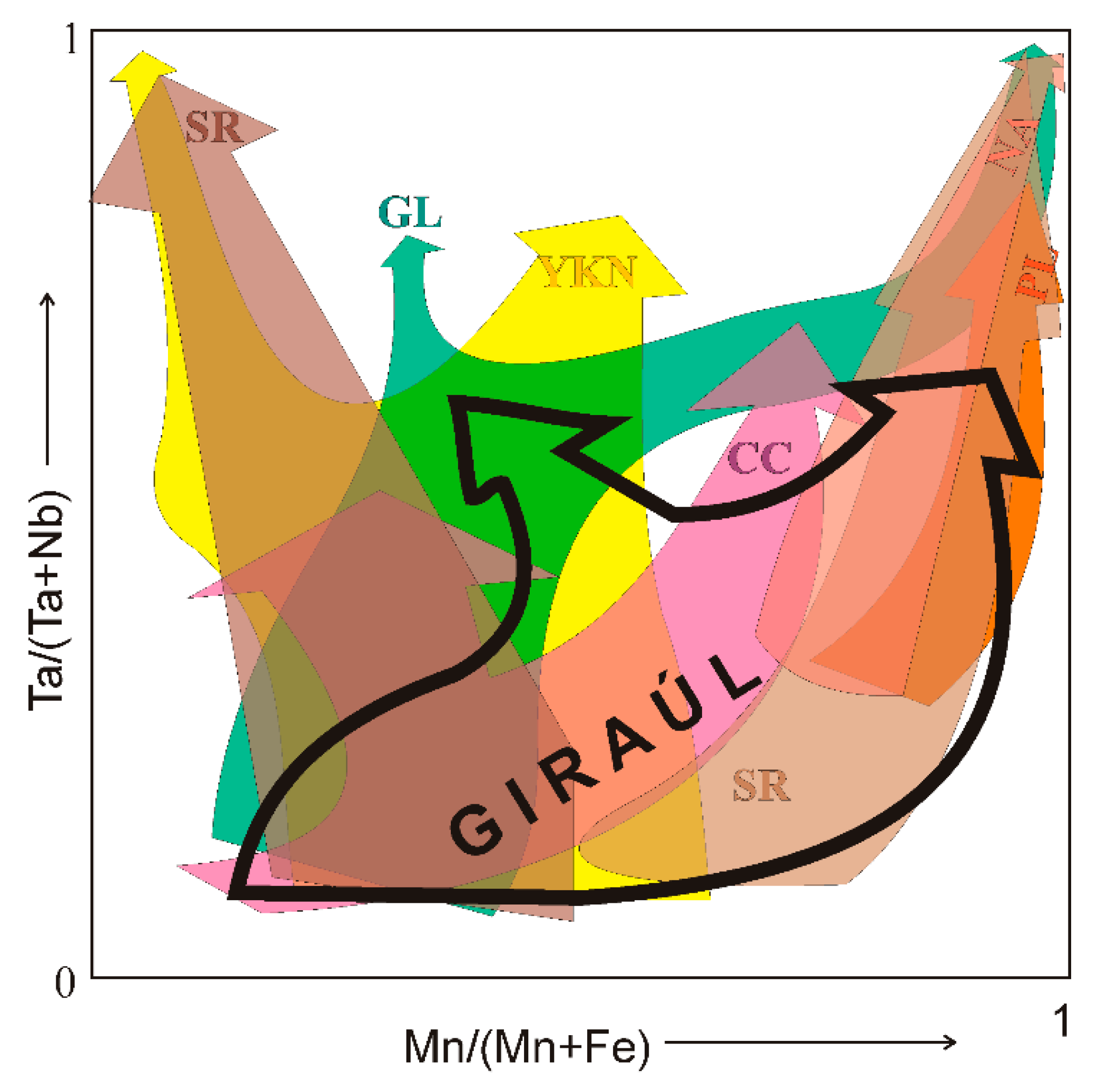
5.4. Comparison of the Nb-Ta Fractionation Trend with Other Pegmatite Fields
5.5. Mechanisms of Concentration of Nb and Ta
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Linnen, R.L. Granitic Pegmatites as Sources of Strategic Metals. Elements 2012, 8, 275–280. [Google Scholar] [CrossRef]
- London, D. Pegmatites. Can. Mineral. 2008, 10, 347. [Google Scholar]
- Mackay, A.R.; Simandl, G.J. Geology, market and supply chain of niobium and tantalum—A review. Mineral. Depos. 2014, 49, 1025–1047. [Google Scholar] [CrossRef]
- Nikishina, E.E.; Drobot, D.V.; Lebedeva, E.N. Niobium and tantalum: State of the world market, application fields, and sources of raw materials. Part 2. Russ. J. Non-Ferr. Met. 2014, 55, 130–140. [Google Scholar] [CrossRef]
- Černý, P.; Meintzer, R.E.; Anderson, A.J. Extreme fractionation in rare-element granitic pegmatites: Selected examples of data and mechanisms. Can. Mineral. 1985, 23, 381–421. [Google Scholar]
- Mulja, T.; Williams-Jones, A.E.; Martin, R.F.; Wood, S.A. Compositional variation and structural state of columbite-tantalite in rare-element granitic pegmatites of the Preissac-Lacorne batholith, Quebec, Canada. Am. Mineral. 1996, 81, 146–157. [Google Scholar] [CrossRef]
- Ercit, T.S. REE-enriched granitic pegmatites. In Rare-Element Geochemistry and Ore Deposits: Geological Association of Canada Short Course Notes; Linnen, R.L., Samson, I.M., Eds.; Mineralogical Association of Canada: Québec, QC, Canada, 2005; Volume 17, pp. 257–296. [Google Scholar]
- Raimbault, L.; Burnol, L. The Richemont rhyolite dyke, Massif Central, France; a subvolcanic equivalent of rare-metal granites. Can. Mineral. 1998, 36, 265–282. [Google Scholar]
- Stepanov, A.; Mavrogenes, J.A.; Meffre, S.; Davidson, P. The key role of mica during igneous concentration of tantalum. Contrib. Mineral. Petrol. 2014, 167, 1009. [Google Scholar] [CrossRef]
- Škoda, R.; Novák, M. Y, REE, Nb, Ta, Ti-oxide (AB2O6) minerals from REL–REE euxenite-subtype pegmatites of the Třebíč Pluton, Czech Republic; substitutions and fractionation trends. Lithos 2007, 95, 43–57. [Google Scholar] [CrossRef]
- Kaeter, D.; Barros, R.; Menuge, J.F.; Chew, D.M. The magmatic–hydrothermal transition in rare-element pegmatites from southeast Ireland: LA-ICP-MS chemical mapping of muscovite and columbite–tantalite. Geochim. Cosmochim. Acta 2018, 240, 98–130. [Google Scholar] [CrossRef]
- Deetman, S.; van Oers, L.; van der Voet, E.; Tukker, A. Deriving European tantalum flows using trade and production statistics. J. Ind. Ecol. 2018, 22, 166–179. [Google Scholar] [CrossRef]
- Anderson, M.O.; Lentz, D.R.; McFarlane, M.; Falck, H. A geological, geochemical and textural study of a LCT pegmatite: Implications for the magmatic versus metasomatic origin of Nb–Ta mineralization in the Moose II pegmatite, Northwest Territories. J. Geosci. 2013, 58, 299–320. [Google Scholar] [CrossRef]
- Linnen, R.L.; Keppler, H. Columbite solubility in granitic melts: Consequences for the enrichment and fractionation of Nb and Ta in the Earth’s crust. Contrib. Mineral. Petrol. 1997, 128, 213–227. [Google Scholar] [CrossRef]
- Linnen, R.L. The solubility of Nb-Ta-Zr-Hf-W in granitic melts with Li and Li + F: Constraints for mineralization in rare metal granites and pegmatites. Econ. Geol. 1998, 93, 1013–1025. [Google Scholar] [CrossRef]
- Belkasmi, M.; Cuney, M.; Pollard, P.J.; Bastoul, A. Chemistry of the Ta-Nb-Sn-W oxide minerals from the Yichun rare metal granite (SE China): Genetic implications and comparison with Moroccan and French Hercynian examples. Mineral. Mag. 2000, 64, 507–523. [Google Scholar] [CrossRef]
- Akoh, J.U.; Ogunleye, P.O.; Ibrahim, A.A. Geochemical evolution of micas and Sn-, Nb-, Ta-mineralization associated with the rare metal pegmatite in Angwan Doka, central Nigeria. J. Afr. Earth Sci. 2015, 112, 24–36. [Google Scholar] [CrossRef]
- Dostal, J.; Chaterjee, A.K. Contrasting behaviour of Nb/Ta and Zr/Hf ratios in a peraluminous granitic pluton (Nova Scotia, Canada). Chem. Geol. 2000, 163, 207–218. [Google Scholar] [CrossRef]
- Neiva, A.M.R.; Gomes, M.E.P.; Silva, P.B. Two generations of zoned crystals of columbite-group minerals from granitic aplite–pegmatite in the Gouveia area, central Portugal. Eur. J. Mineral. 2015, 27, 771–782. [Google Scholar] [CrossRef]
- Zhu, Z.Y.; Wang, R.C.; Che, X.D.; Zhu, J.C.; Wei, X.L.; Huang, X. Magmatic–hydrothermal rare-element mineralization in the Songshugang granite (northeastern Jiangxi, China): Insights from an electron-microprobe study of Nb–Ta–Zr minerals. Ore Geol. Rev. 2015, 65, 749–760. [Google Scholar] [CrossRef]
- Alfonso, P.; Hamid, S.A.; García-Vallès, M.; Llorens, T.; Moro, F.L.; Tomasa, O.; Calvo, D.; Oliva, J.; Parcerisa, D.; García-Polonio, F. Textural and mineral-chemistry constraints on columbite-group minerals in the Penouta deposit: Evidence from magmatic and fluid-related processes. Mineral. Mag. 2018, 82, S199–S222. [Google Scholar] [CrossRef]
- Soares de Carvalho, G. Geologia do Deserto de Moçâmedes (Angola). Mem. Junta Investig. Ultramar 1961, 26, 227. [Google Scholar]
- Černý, P.; Ercit, T.S. The classification of granitic pegmatites revisited. Can. Mineral. 2005, 43, 2005–2026. [Google Scholar] [CrossRef]
- Černý, P. (Ed.) Anatomy and classification of granitic pegmatites. In Granitic Pegmatites in Science and Industry; Short Course Handbook; Mineralogical Association of Canada: Québec, QC, Canada, 1982; Volume 8, pp. 1–39. [Google Scholar]
- Černý, P.; Chapman, R.; Ferreira, K.; Smeds, S.A. Geochemistry of oxide minerals of Nb, Ta, Sn, and Sb in the Varuträsk granitic pegmatite, Sweden: The case of an “anomalous” columbite-tantalite trend. Am. Mineral. 2004, 89, 505–518. [Google Scholar] [CrossRef]
- Goreva, J.S.; Ma, C.; Rossman, G.R. Fibrous nanoinclusions in massive rose quartz: The origin of rose coloration. Am. Mineral. 2001, 86, 466–472. [Google Scholar] [CrossRef]
- Larsen, R.B.; Henderson, I.; Ihlen, P.M.; Jacamon, F. Distribution and petrogenetic behaviour of trace elements in granitic pegmatite quartz from South Norway. Contrib. Mineral. Petrol. 2004, 147, 615–628. [Google Scholar] [CrossRef]
- Ma, C.; Goreva, J.S.; Rossmann, G.R. Fibrous nano-inclusions in massive rose quartz: HRTEM and AEM investigations. Am. Mineral. 2002, 87, 269–276. [Google Scholar] [CrossRef]
- Keller, P. The occurrence of Li-Fe-Mn phosphate minerals in granitic pegmatites of Namibia. Commun. Geol. Surv. Namib. 1991, 7, 21–34. [Google Scholar]
- Roda, E.; Pesquera, A.; Fontan, F.; Keller, P. Phosphate mineral associations in the Canada pegmatite (Salamanca, Spain): Paragenetic relationships, chemical compositions, and implications for pegmatite evolution. Am. Mineral. 2004, 89, 110–125. [Google Scholar] [CrossRef]
- Alfonso, P.; Corbella, M.; Melgarejo, J.C. Nb-Ta- minerals from the Cap de Creus pegmatite Field, eastern Pyrenees: Distribution and geochemical trends. Mineral. Petrol. 1995, 55, 53–69. [Google Scholar] [CrossRef]
- Simmons, W.B.S.; Webber, K.L. Pegmatite genesis: State of the art. Eur. J. Mineral. 2008, 20, 421–438. [Google Scholar] [CrossRef]
- Quensel, P. Minerals of the Varuträsk pegmatite. I: The lithium manganese phosphates. Geol. Fören. Förhandl. 1937, 59, 77–96. [Google Scholar] [CrossRef]
- Mason, B. Minerals of the Varuträsk pegmatite. XXIII. Some ironmanganese phosphate minerals and their alteration products, with special reference to material from Varuträsk. Geol. Fören Förhandl. 1941, 63, 117–165. [Google Scholar] [CrossRef]
- Fransolet, A.M.; Keller, P.; Fontan, F. The phosphate mineral associations of the Tsaobismund pegmatite, Namibia. Contrib. Mineral. Petrol. 1986, 92, 502–517. [Google Scholar] [CrossRef]
- Malló, A.; Fontan, F.; Melgarejo, J.C.; Mata, J.M. The Albera zoned pegmatite field, eastern Pyrenees, France. Mineral. Petrol. 1995, 55, 103–116. [Google Scholar] [CrossRef]
- Baijot, M.; Hatert, F.; Dal Bo, F.; Philippo, S. Mineralogy and petrography of phosphate mineral associations from the Jocão pegmatite, Minas Gerais, Brazil. Can. Mineral. 2014, 52, 373–397. [Google Scholar] [CrossRef]
- Sebastian, A.; Lagache, M. Experimental study of lithium-rich granitic pegmatites; Part I, Petalite + albite + quartz equilibrium. Am. Mineral. 1991, 76, 205–210. [Google Scholar]
- Thomas, R.J.; Bühmann, D.; Bullen, W.D.; Scogings, A.J.; De Bruin, D. Unusual spodumene pegmatites from the late Kibaran of southern Natal, South Africa. Ore Geol. Rev. 1994, 9, 161–182. [Google Scholar] [CrossRef]
- Stilling, A.; Černý, P.; Vanstone, P.J. The Tanco Pegmatite at Bernic Lake, Manitoba; XVI, Zonal and bulk compositions and their petrogenetic significance. Can. Mineral. 2006, 44, 599–623. [Google Scholar] [CrossRef]
- Selway, J.B.; Breaks, F.W.; Tindle, A.G. A review of rare-element (Li-Cs-Ta) pegmatite exploration techniques for the Superior Province, Canada, and large worldwide tantalum deposits. Explor. Min. Geol. 2005, 14, 1–30. [Google Scholar] [CrossRef]
- Demartis, M.; Melgarejo, J.C.; Colombo, F.; Alfonso, P.; Coniglio, J.E.; Pinotti, L.P.; D’Eramo, F.J. Extreme F activities in late pegmatitic events as a key factor for LILE and HFSE enrichment: The Ángel pegmatite, central Argentina. Can. Mineral. 2014, 52, 247–269. [Google Scholar] [CrossRef]
- Peretyazhko, I.S.; Zagorsky, V.Y.; Smirnov, S.Z.; Mikhailov, M.Y. Conditions of pocket formation in the Oktyabrskaya tourmaline-rich gem pegmatite (the Malkhan field, Central Transbaikalia, Russia). Chem. Geol. 2004, 210, 91–111. [Google Scholar] [CrossRef]
- Novák, M.; Černý, P.; Cempirek, J.; Srein, V.; Filip, J. Ferrotapiolite as a pseudomorph of stibiotantalite from the Lăstovičky lepidolite pegmatite, czech republic; an example of hydrothermal alteration at constant Ta/(Ta + Nb). Can. Mineral. 2004, 42, 1117–1128. [Google Scholar] [CrossRef][Green Version]
- Atencio, D.; Andrade, M.B.; Christy, A.G.; Gieré, R.; Kartashov, P.M. The pyrochlore supergroup of minerals nomenclature. Can. Mineral. 2010, 48, 673–698. [Google Scholar] [CrossRef]
- Chukanov, N.V.; Kuzmina, O.V.; Zadov, A.E. Bismutopyrochlore (Bi,U,Ca,Pb)1+x(Nb,Ta)2O6(OH)·nH2O—A new mineral from the pegmatites of the Mika vein (eastern Pamirs). Zap. Vsesoyuznoye Mineral. Obs. 1999, 128, 36–41. (In Russian) [Google Scholar]
- Lumpkin, G.R.; Ewing, R.C.; Williams, C.T.; Mariano, A.N. An overview of the crystal chemistry, durability, and radiation damage effects of natural pyrochlore. MRS Online Proc. Libr. Arch. 2000, 663, 921. [Google Scholar] [CrossRef]
- Matyszczak, W. Liandratite from Karkonosze pegmatites, Sudetes, Southwestern Poland. Mineral. Petrol. 2018, 112, 357–370. [Google Scholar] [CrossRef]
- Černý, P.; Novák, M.; Chapman, R.; Ferreira, K.J. Subsolidus behavior of niobian rutile from the Písek region, Czech Republic: A model for exsolution in W-and Fe2+ >> Fe3+-rich phases. J. Geosci. 2007, 52, 143–159. [Google Scholar] [CrossRef]
- Neiva, A.M.R. Geochemistry of cassiterite and its inclusions and exsolution products from tin and tungsten deposits in Portugal. Can. Mineral. 1996, 34, 745–768. [Google Scholar]
- Černý, P. Rare-element granitic pegmatites, part 1. Anatomy and internal evolution of pegmatite deposits. Geosci. Can. 1991, 18, 49–67. [Google Scholar]
- Černý, P.; Roberts, W.L.; Ercit, T.S.; Chapman, R. Zirconium and hafnium in minerals of the columbite and wodginite groups from granitic pegmatites. Can. Mineral. 1985, 45, 185–202. [Google Scholar] [CrossRef]
- Correia Neves, J.C.; Nunes, J.L.; Sahama, T.G. High hafnium members of the zircon-hafnon series from the granite pegmatites of Zambézia, Mozambique. Contrib. Mineral. Petrol. 1974, 48, 73–80. [Google Scholar] [CrossRef]
- Yin, R.; Wang, R.C.; Zhang, A.C.; Hu, H.; Zhu, J.C.; Rao, C.; Zhang, H. Extreme fractionation from zircon to hafnon in the Koktokay No. 1 granitic pegmatite, Altai, northwestern China. Am. Mineral. 2013, 98, 1714–1724. [Google Scholar] [CrossRef]
- Kudryashov, N.M.; Skublov, S.G.; Galankina, O.L.; Udoratina, O.V.; Voloshin, A.V. Abnormally high-hafnium zircon from rare-metal pegmatites of the Vasin-Mylk deposit (the northeastern part of the Kola Peninsula). Geochemistry 2019, 2019. [Google Scholar] [CrossRef]
- Fontan, F.; Monchoux, P.; Autefage, F. Présence de zircons hafnifères dans des pegmatites granitiques des Pyrénées ariégeoises; leur relation avec les niobotantalates. Bull. Mineral. 1980, 103, 88–91. [Google Scholar]
- Wang, R.C.; Fontan, F.; Xu, S.J.; Chen, X.M.; Monchoux, P. Hafnian zircon from the apical part of the Suzhou granite, China. Can. Mineral. 1996, 34, 1001–1010. [Google Scholar]
- Zaraisky, G.P.; Aksyuk, A.M.; Devyatova, V.N.; Udoratina, O.V.; Chevychelov, V.Y. The Zr/Hf ratio as a fractionation indicator of rare-metal granites. Petrology 2009, 17, 25. [Google Scholar] [CrossRef]
- Van Lichtervelde, M.; Holtz, F.; Hanchar, J.M. Solubility of manganotantalite, zircon and hafnon in highly fluxed peralkaline to peraluminous pegmatitic melts. Contrib. Mineral. Petrol. 2010, 160, 17–32. [Google Scholar] [CrossRef]
- Barsanov, G.P.; Yeremin, N.I.; Sergeyeva, N.Y.E. Columbite-tantalite zoning as revealed by electron-probe microanalysis. Dokl. Akad. Nauk SSSR 1971, 201, 174–176. (In Russian) [Google Scholar]
- Černý, P.; Ercit, T.S.; Wise, M.A. The tantalite-tapiolite gap: Natural assemblages versus experimental data. Can. Mineral. 1992, 30, 587–596. [Google Scholar]
- Novák, M.; Chládek, Š.; Uher, P.; Gadas, P. Complex magmatic and subsolidus compositional trends of columbite-tantalite in the beryl-columbite Sejby granitic pegmatite, Czech Republic: Role of crystal-structural constraints and associated minerals. J. Geosci. 2018, 63, 253–263. [Google Scholar] [CrossRef]
- Lahti, S.I. Zoning in columbite-tantalite crystals from the granitic pegmatites of the Erajarvi area, southern Finland. Geochim. Cosmochim. Acta 1987, 51, 509–517. [Google Scholar] [CrossRef]
- Putnis, A.; Fernaández-Diaz, L.; Prieto, M. Experimentally produced oscillatory zoning in the (Ba,Sr)SO4 solid solution. Nature 1992, 358, 743–745. [Google Scholar] [CrossRef]
- Černý, P. Geochemical and petrogenitic features of mineralisation in rare-element granitic pegmatites in the light of current research. Appl. Geochem. 1992, 7, 393–416. [Google Scholar]
- Putnis, A. Mineral replacement reactions: From macroscopic observations to microscopic mechanisms. Mineral. Mag. 2002, 66, 689–708. [Google Scholar] [CrossRef]
- Černý, P.; Novák, M.; Chapman, R. Effects of sillimanite-grade metamorphism and shearing on Nb-Ta oxide minerals in granitic pegmatites: Marsikov, Northern Moravia, Czechoslovakia. Can. Mineral. 1992, 30, 699–718. [Google Scholar]
- Tindle, A.G.; Breaks, F.W. Oxide minerals of the Separation Rapids rare-element granitic pegmatite group, Northwestern Ontario. Can. Mineral. 2000, 36, 609–635. [Google Scholar]
- Tindle, A.G.; Breaks, F.W.; Webb, P.C. Wodginite-group minerals from the Separation Rapids rare-element granitic pegmatite group, Northwestern Ontario. Can. Mineral. 1998, 36, 637–658. [Google Scholar]
- Černý, P.; Ercit, T.S. Some recent advances in the mineralogy and geochemistry of Nb and Ta in rare-element granitic pegmatites. Bull. Mineral. 1985, 108, 499–532. [Google Scholar] [CrossRef]
- Černý, P. Characteristics of pegmatite deposits of tantalum. In Lanthanides, Tantalum and Niobium; Möller, P., Černý, P., Saupe, F., Eds.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1989; pp. 139–195. [Google Scholar]
- Černý, P.; Goad, B.E.; Hawthorne, F.C.; Chapman, R. Fractionation trends of the Nb- and Ta- bearing oxide minerals in the Greer Lake pegmatitic granite and its pegmatite aureole, southeastern Manitoba. Am. Mineral. 1986, 71, 501–517. [Google Scholar]
- Keppler, H. Infuence of fuorine on the enrichment of high field strength trace elements in granitic rocks. Contrib. Mineral. Petrol. 1993, 114, 479–488. [Google Scholar] [CrossRef]
- Zaraisky, G.P.; Korzhinskaya, V.; Kotova, N. Experimental studies of Ta2O5 and columbite–tantalite solubility in fluoride solutions from 300 to 550 C and 50 to 100 MPa. Miner. Petrol. 2010, 99, 287–300. [Google Scholar] [CrossRef]
- Aseri, A.A.; Linnen, R.L.; Che, X.D.; Thibault, Y.; Holtz, F. Effects of fluorine on the solubilities of Nb, Ta, Zr and Hf minerals in highly fluxed water-saturated haplogranitic melts. Ore Geol. Rev. 2015, 64, 736–746. [Google Scholar] [CrossRef]
- Wise, M.A.; Meintzer, R.E.; Černý, P. The Yellowknife pegmatite Field: Mineralogy and geochemistry of Nb-Ta-Sn oxide minerals. Contrib. Geol. NWT 1986, 2, 15–25. [Google Scholar]
- Pal, D.C.; Biswajit, M.; Bernhardt, H.J. Contrasting fluid inclusion characteristics of staniferous and non-staniferous pegmatites of Southeast Bastar, Central India. Ore Geol. Rev. 2007, 30, 30–35. [Google Scholar] [CrossRef]
- Ogunleye, P.O.; Garba, I.; Ike, E.C. Factors contributing to enrichment and crystallization of niobium in pyrochlore in the Kaffo albite arfvedsonite granite, Ririwai Complex, Younger Granites province of Nigeria. J. Afr. Earth Sci. 2006, 44, 372–382. [Google Scholar] [CrossRef]
- Tadesse, S.; Zerihun, D. Composition, fractionation trend and zoning accretion of the columbite-tantalite group of minerals in the Kenticha rare-metal field (Adola, southern Ethiopia). J. Afr. Earth Sci. 1996, 23, 411–431. [Google Scholar] [CrossRef]
- Smith, S.R.; Foster, G.L.; Romer, R.L.; Tindle, A.G.; Kelley, S.P.; Noble, S.R.; Horstwood, M.; Breaks, F.W. U-Pb columbite-tantalite chronology of rare-element pegmatites using TIMS and Laser Ablation-Multi Collector-ICP-MS. Contrib. Mineral. Petrol. 2004, 147, 549–564. [Google Scholar] [CrossRef]
- Breaks, F.W.; Selway, J.B.; Tindle, A.G. Fertile Peraluminous Granites and Related Rare Element Mineralization in Pegmatites, Superior Province, Northwest and Northeast Ontario: Operation Treasure Hunt; Open File Report 6099; Ontario Geological Survey: Sudbury, ON, Canada, 2003; 179p.
- Timofeev, A.; Migdisov, A.A.; Williams-Jones, A.E. An experimental study of the solubility and speciation of tantalum in fluoride-bearing aqueous solutions at elevated temperature. Geochim. Cosmochim. Acta 2017, 197, 294–304. [Google Scholar] [CrossRef]
- Linnen, R.L. Ferrocolumbite-manganotantalite trends in granites and pegmatites: Experimental and natural constraints. Geol. Soc. Amer. Abstr. Prog. 2004, 36, 115. [Google Scholar]
- Linnen, R.L.; Samson, I.M.; Williams-Jones, A.E.; Chakhmouradian, A.R. Geochemistry of the Rare-Earth Element, Nb, Ta, Hf, and Zr Deposits. In Treatise on Geochemistry, 2nd ed.; Holland, H.D., Turekian, K.K., Eds.; Elsevier: Oxford, UK, 2014; pp. 543–564. [Google Scholar]
- Kamenetsky, V.S.; Naumov, V.B.; Davidson, P.; van Achterbergh, E.; Ryan, C.G. Immiscibility between silicate magmas and aqueous fluids: A melt inclusion pursuit into the magmatic-hydrothermal transition in the Omsukchan Granite (NE Russia). Chem. Geol. 2004, 210, 73–90. [Google Scholar] [CrossRef]
- Alfonso, P.; Melgarejo, J.C. Fluid evolution in the zoned rare-element pegmatite field at Cap de Creus, Catalonia, Spain. Can. Mineral. 2008, 46, 597–617. [Google Scholar] [CrossRef]
- Mulja, T.; Williams-Jones, A.E. The physical and chemical evolution of fluids in rare-element granitic pegmatites associated with the Lacorne pluton, Québec, Canada. Chem. Geol. 2018, 493, 281–297. [Google Scholar] [CrossRef]
- Wu, M.; Samson, I.M.; Zhang, D. Textural Features and Chemical Evolution in Ta-Nb Oxides: Implications for Deuteric Rare-Metal Mineralization in the Yichun Granite-Marginal Pegmatite, Southeastern China. Econ. Geol. 2018, 113, 937–960. [Google Scholar] [CrossRef]
- Siegel, K.; Wagner, T.; Trumbull, R.B.; Jonsson, E.; Matalin, G.; Wälle, M.; Heinrich, C.A. Stable isotope (B, H, O) and mineral-chemistry constraints on the magmatic to hydrothermal evolution of the Varuträsk rare-element pegmatite (Northern Sweden). Chem. Geol. 2016, 421, 1–16. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, H. Lanthanide tetrads in normalized rare element patterns of zircon from the Koktokay No. 3 granitic pegmatite, Altay, NW China. Am. Mineral. 2015, 100, 2630–2636. [Google Scholar] [CrossRef]
- Ballouard, C.; Poujol, M.; Boulvais, P.; Branquet, Y.; Tartèse, R.; Vigneresse, J.L. Nb-Ta fractionation in peraluminous granites: A marker of the magmatic-hydrothermal transition. Geology 2016, 44, 231–234. [Google Scholar] [CrossRef]
- Fuchsloch, W.C.; Nex, P.A.; Kinnaird, J.A. The geochemical evolution of Nb–Ta–Sn oxides from pegmatites of the Cape Cross–Uis pegmatite belt, Namibia. Mineral. Mag. 2019, 83, 161–179. [Google Scholar] [CrossRef]
- Baldwin, J.R. Replacement phenomena in tantalum minerals from rare-metal pegmatites in South Africa and Namibia. Mineral. Mag. 1989, 53, 571–581. [Google Scholar] [CrossRef]
- Uher, P.; Černý, P.; Chapman, R.; Határ, F.; Miko, O. Evolution of Nb,Ta-oxide minerals in the Prăsivá granitic pegmatites, Slovakia. II. External hydrothermal Pb,Sb overprint. Can. Mineral. 1998, 36, 535–545. [Google Scholar]
- Martin, R.F.; De Vito, C. The late-stage miniflood of Ca in granitic pegmatites: An open-system acid-reflux model involving plagioclase in the exocontact. Can. Mineral. 2014, 52, 165–181. [Google Scholar] [CrossRef]
- Duran, C.J.; Seydoux-Guillaume, A.M.; Bingen, B.; Gouy, S.; De Parseval, P.; Ingrin, J.; Guillaume, D. Fluid-mediated alteration of (Y, REE, U, Th)–(Nb, Ta, Ti) oxide minerals in granitic pegmatite from the Evje-Iveland district, southern Norway. Mineral. Petrol. 2016, 110, 581–599. [Google Scholar] [CrossRef]
- Mysen, B.O.; Holtz, F.; Pichavant, M.; Beny, J.M.; Montel, J.M. The effect of temperature and bulk composition on the solution mechanism of phosphorus in peraluminous haplogranitic magma. Am. Mineral. 1999, 84, 1336–1345. [Google Scholar] [CrossRef]






| Type | I | II | III | III | III | IV | IV | IV | V | V |
|---|---|---|---|---|---|---|---|---|---|---|
| Zone | 1st I | 1st I | 1st | 2nd I | 3rd I | Border | 1st | 2nd I | Border | 3rd I |
| Sample | X2B | GP13 | Ibep3 | I + 4B | pa8–4 | a21–10 | a6a | A10ap17 | k100q | K100C |
| SiO2 | 31.22 | 31.57 | 32.27 | 31.67 | 30.91 | 31.13 | 31.75 | 31.15 | 31.21 | 31.03 |
| TiO2 | 0.02 | 0.00 | 0.01 | 0.02 | 0.00 | 0.03 | 0.05 | 0.04 | 0.03 | 0.00 |
| ZrO2 | 61.38 | 65.86 | 65.29 | 63.17 | 63.71 | 64.72 | 60.33 | 56.93 | 67.05 | 56.12 |
| HfO2 | 2.56 | 2.15 | 1.39 | 3.32 | 4.69 | 1.68 | 5.66 | 11.89 | 1.38 | 11.54 |
| ThO2 | 0.00 | 0.00 | 0.07 | 0.04 | 0.08 | 0.00 | 0.00 | 0.04 | 0.09 | 0.00 |
| Al2O3 | 0.53 | 0.00 | 0.00 | 0.00 | 0.00 | 0.05 | 0.25 | 0.02 | 0.00 | 0.00 |
| Y2O3 | 0.29 | 0.17 | 0.10 | 1.38 | 0.00 | 0.21 | 1.31 | 0.00 | 0.00 | 0.00 |
| U2O3 | 0.88 | 0.12 | 0.16 | 0.00 | 0.35 | 0.17 | 0.00 | 0.01 | 0.00 | 0.00 |
| CaO | 0.05 | 0.01 | 0.02 | 0.03 | 0.00 | 0.10 | 0.21 | 0.00 | 0.03 | 0.09 |
| FeO | 2.07 | 0.07 | 0.26 | 0.01 | 0.03 | 0.42 | 0.15 | 0.00 | 0.16 | 0.09 |
| Total | 99.91 | 99.95 | 99.57 | 99.64 | 99.77 | 98.51 | 99.71 | 100.08 | 99.95 | 98.87 |
| Apfu * | ||||||||||
| Si | 0.53 | 0.98 | 1.00 | 0.99 | 0.97 | 0.98 | 1.00 | 1.00 | 0.97 | 1.00 |
| Zr | 0.51 | 1.00 | 0.98 | 0.96 | 0.98 | 0.99 | 0.92 | 0.89 | 1.01 | 0.89 |
| Hf | 0.01 | 0.02 | 0.01 | 0.03 | 0.04 | 0.02 | 0.05 | 0.11 | 0.01 | 0.11 |
| Al | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 |
| Y | 0.00 | 0.00 | 0.00 | 0.02 | 0.00 | 0.00 | 0.02 | 0.00 | 0.00 | 0.00 |
| U | 0.92 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Ca | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 |
| Fe | 0.03 | 0.00 | 0.01 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 |
| Zr/Hf | 40.96 | 52.33 | 80.24 | 32.50 | 23.21 | 65.81 | 18.21 | 8.18 | 83.00 | 8.31 |
| Type | II | III | III | III | IV | IV | IV | IV | V | V | V | V | V |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zone | 2nd I | 3rd I | 3rd I | 3rd I | 1st I | 1st I | 2nd I | 2nd I | 1st I | 1st I | 2nd I | 3rd I | 3rd I |
| Sample | GP1 | PA8 | P8 | P8 | D1e | A41c | A9Db | A10F | k40 | k40 | K100C | K100L | K100R |
| Mineral | FT | MC | MC | MT | MT | FC | MT | FC | FC | FC | FC | FC | FC |
| WO3 | 0.30 | 2.40 | 0.52 | 0.40 | n.a. | 1.49 | 0.35 | 0.00 | 0.67 | 0.50 | n.a. | 1.03 | 1.08 |
| Ta2O5 | 53.32 | 10.81 | 24.61 | 56.93 | 52.26 | 9.28 | 58.75 | 12.65 | 26.35 | 42.03 | 39.97 | 26.62 | 11.86 |
| Nb2O5 | 27.08 | 65.53 | 54.93 | 25.06 | 29.34 | 68.25 | 24.29 | 67.16 | 52.86 | 39.65 | 41.70 | 52.20 | 64.86 |
| TiO2 | 0.29 | 1.70 | 0.95 | 0.51 | 1.23 | 1.23 | 0.26 | 0.22 | 0.87 | 0.33 | 0.32 | 1.05 | 1.77 |
| UO2 | 0.02 | 0.13 | 0.00 | 0.03 | n.a | 0.12 | n.a. | 0.08 | 0.07 | n.a. | 0.00 | n.a. | n.a. |
| Y2O3 | 0.00 | n.a. | n.a. | n.a. | 0.01 | 0.09 | n.a. | 1.02 | n.a. | n.a. | 0.37 | 0.73 | 0.72 |
| SnO2 | 0.10 | 0.38 | 0.20 | 0.26 | 0.01 | 0.14 | 0.21 | 0.05 | 0.57 | 0.17 | 0.16 | 0.24 | 0.07 |
| Fe2O3 | 0.63 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 | 0.06 | 0.00 | 0.00 | 0.00 | 0.70 | 1.71 |
| FeO | 8.13 | 9.00 | 8.75 | 7.48 | 7.79 | 14.48 | 2.71 | 14.04 | 13.24 | 9.43 | 10.49 | 14.19 | 14.53 |
| MnO | 7.57 | 10.28 | 9.11 | 7.74 | 8.46 | 4.65 | 12.71 | 5.50 | 4.79 | 7.60 | 6.90 | 3.59 | 3.69 |
| MgO | 0.00 | 0.03 | 0.03 | 0.16 | n.a. | 0.06 | 0.03 | n.a. | 0.04 | 0.00 | n.a. | 0.13 | 0.18 |
| Sb2O3 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.02 | 0.00 | 0.06 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| PbO | 0.00 | 0.18 | |||||||||||
| Total | 97.44 | 100.26 | 99.10 | 98.57 | 99.29 | 99.81 | 99.31 | 100.78 | 99.46 | 99.71 | 99.91 | 100.48 | 100.47 |
| Atomic | contents | ||||||||||||
| W6+ | 0.023 | 0.019 | 0.034 | 0.031 | - | 0.090 | 0.027 | - | 0.044 | 0.035 | - | 0.067 | 0.065 |
| Ta5+ | 4.268 | 0.699 | 1.675 | 4.556 | 4.046 | 0.589 | 4.715 | 0.808 | 1.806 | 3.092 | 2.904 | 1.805 | 0.750 |
| Nb5+ | 3.604 | 7.049 | 6.214 | 3.334 | 3.776 | 7.196 | 3.240 | 7.132 | 6.024 | 4.848 | 5.037 | 5.883 | 6.822 |
| Ti2+ | 0.064 | 0.304 | 0.179 | 0.113 | 0.263 | 0.216 | 0.058 | 0.039 | 0.165 | 0.067 | 0.064 | 0.197 | 0.310 |
| U4+ | 0.001 | 0.007 | 0.000 | 0.002 | - | 0.006 | - | 0.004 | 0.004 | - | 0.000 | - | - |
| Y3+ | 0.000 | - | - | - | 0.002 | 0.011 | - | 0.128 | - | - | 0.053 | 0.097 | 0.089 |
| Sn4+ | 0.012 | 0.036 | 0.020 | 0.034 | 0.001 | 0.013 | 0.025 | 0.005 | 0.057 | 0.018 | 0.017 | 0.024 | 0.006 |
| Fe3+ | 0.140 | 0.000 | 0.000 | 0.000 | 0.002 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.131 | 0.299 |
| Fe2+ | 2.001 | 1.791 | 1.831 | 1.841 | 1.855 | 2.824 | 0.669 | 2.758 | 2.791 | 2.133 | 2.344 | 2.958 | 2.827 |
| Mn2+ | 1.887 | 2.072 | 1.931 | 1.929 | 2.040 | 0.919 | 3.177 | 1.094 | 1.023 | 1.741 | 1.562 | 0.758 | 0.727 |
| Mg2+ | 0.000 | 0.018 | 0.019 | 0.116 | - | 0.035 | 0.022 | - | 0.025 | 0.000 | 0.000 | 0.080 | 0.104 |
| Sb3+ | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.002 | 0.000 | 0.006 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Pb2+ | 0.000 | 0.000 | 0.000 | 0.000 | 0.014 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| CATSUM | 12.000 | 11.994 | 11.901 | 11.955 | 12.000 | 11.901 | 11.932 | 11.974 | 11.940 | 11.935 | 11.981 | 12.000 | 12.000 |
| Ta/(Ta + Nb) | 0.542 | 0.090 | 0.212 | 0.577 | 0.517 | 0.076 | 0.593 | 0.102 | 0.231 | 0.389 | 0.366 | 0.235 | 0.099 |
| Mn/(Mn + Fe) | 0.469 | 0.536 | 0.513 | 0.512 | 0.523 | 0.245 | 0.826 | 0.284 | 0.268 | 0.449 | 0.400 | 0.197 | 0.189 |
| Type | III | III | III | IV | IV | V | V | V | V |
|---|---|---|---|---|---|---|---|---|---|
| Mineral | OBP | HPP | OUP | HPP | HKP | FNM | ONM | FNM | HSP |
| Sample | P-5 | P-10 | P-11 | A33b | A11A | K100-20 | K100C46 | K100C32 | K100L |
| WO3 | 0.00 | 2.59 | 0.79 | 1.10 | 4.14 | n.a. | n.a. | n.a. | 1.19 |
| Nb2O5 | 35.04 | 16.37 | 30.16 | 26.59 | 45.49 | 18.53 | 11.24 | 25.40 | 24.23 |
| Ta2O5 | 5.23 | 22.86 | 5.32 | 18.20 | 17.80 | 58.29 | 55.74 | 48.42 | 19.45 |
| TiO2 | 1.88 | 5.70 | 2.96 | 4.40 | 1.11 | 0.40 | 0.71 | 0.30 | 10.51 |
| SnO0 | 0.31 | 0.72 | 0.38 | 0.17 | 0.24 | 0.19 | 3.08 | 0.29 | 0.72 |
| ThO2 | 0.00 | n.a. | n.a. | 0.07 | 0.00 | n.a. | n.a. | n.a. | 0.00 |
| UO2 | 8.39 | 0.00 | 36.31 | 1.90 | 0.08 | 0.15 | 14.88 | 0.25 | 0.00 |
| Sc2O3 | 0.00 | 0.06 | 0.11 | 0.10 | 0.36 | n.a. | n.a. | n.a. | 0.32 |
| Y2O3 | 0.23 | 0.00 | 0.00 | n.a. | 0.80 | 0.00 | 0.00 | 0.31 | 0.00 |
| Sb2O3 | 0.34 | 0.60 | 0.00 | 0.48 | 19.98 | 0.55 | 0.65 | 0.92 | 25.92 |
| Bi2O3 | 21.04 | 0.00 | 0.00 | n.a. | 0.00 | 0.04 | 0.20 | 0.00 | 0.00 |
| CaO | 1.73 | 1.07 | 2.19 | 0.03 | 0.00 | 9.50 | 5.65 | 10.45 | 0.03 |
| MnO | 0.56 | 0.69 | 0.48 | 0.15 | 0.11 | 0.17 | 0.00 | 0.14 | 0.10 |
| FeO | 4.35 | 1.56 | 1.84 | 1.09 | 0.55 | 0.65 | 0.04 | 0.40 | 1.32 |
| SrO | 0.00 | 0.00 | 0.00 | n.a. | 0.00 | 2.71 | 0.85 | 1.96 | 0.81 |
| BaO | 0.13 | n.a. | n.a. | 0.14 | 0.00 | n.a. | n.a. | n.a. | 0.00 |
| PbO | 3.06 | 46.11 | 8.37 | 44.56 | 0.29 | 0.09 | 1.34 | 0.08 | 9.34 |
| Na2O | 0.05 | 0.22 | 0.20 | 0.14 | 3.72 | 5.52 | 3.87 | 6.47 | 0.56 |
| K2O | 0.16 | 0.00 | 0.19 | 0.03 | 0.14 | 0.00 | 0.01 | 0.05 | 0.00 |
| F | 0.01 | 0.00 | 0.30 | 0.23 | 0.00 | 5.23 | 0.79 | 5.84 | 0.16 |
| OH | 1.19 | 0.00 | 2.12 | 2.79 | 0.00 | 0.18 | 0.00 | 1.95 | |
| O=F | −0.01 | 0.00 | −0.15 | −0.12 | 0.00 | −2.62 | −0.40 | −2.92 | −0.08 |
| Total | 82.51 | 99.74 | 89.45 | 101.38 | 97.60 | 99.40 | 98.84 | 98.36 | 96.53 |
| apfu | |||||||||
| W6+ | 0.000 | 0.077 | 0.025 | 0.030 | 0.084 | - | - | - | 0.027 |
| Nb | 1.696 | 0.795 | 1.555 | 1.168 | 1.501 | 0.683 | 0.489 | 0.923 | 0.895 |
| Ta | 0.152 | 0.668 | 0.165 | 0.481 | 0.353 | 1.292 | 1.460 | 1.059 | 0.432 |
| Ti | 0.151 | 0.460 | 0.254 | 0.321 | 0.061 | 0.025 | 0.051 | 0.018 | 0.646 |
| ΣB site | 2.000 | 2.000 | 2.000 | 2.000 | 2.000 | 2.000 | 2.000 | 2.000 | 2.000 |
| Sn4+ | 0.015 | 0.035 | 0.019 | 0.007 | 0.008 | 0.007 | 0.132 | 0.010 | 0.026 |
| Th | 0.000 | - | - | 0.002 | 0.000 | - | - | - | 0.000 |
| U | 0.200 | 0.000 | 0.922 | 0.041 | 0.001 | 0.003 | 0.319 | 0.004 | 0.000 |
| Sc | 0.000 | 0.006 | 0.011 | 0.008 | 0.023 | - | - | - | 0.023 |
| Y | 0.013 | 0.000 | 0.000 | 0.000 | 0.031 | 0.000 | 0.000 | 0.013 | 0.000 |
| Sb | 0.015 | 0.027 | 0.000 | 0.019 | 0.601 | 0.018 | 0.026 | 0.030 | 0.873 |
| Bi | 0.581 | 0.000 | 0.000 | 0.000 | 0.000 | 0.001 | 0.005 | 0.000 | 0.000 |
| Ca | 0.198 | 0.123 | 0.268 | 0.003 | 0.000 | 0.830 | 0.583 | 0.900 | 0.003 |
| Mn2+ | 0.051 | 0.063 | 0.046 | 0.012 | 0.007 | 0.012 | 0.000 | 0.010 | 0.007 |
| Fe2+ | 0.389 | 0.140 | 0.176 | 0.089 | 0.034 | 0.044 | 0.003 | 0.027 | 0.090 |
| Sr | 0.000 | 0.000 | 0.000 | - | 0.000 | 0.128 | 0.047 | 0.091 | 0.038 |
| Ba | 0.006 | - | - | 0.005 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Pb | 0.088 | 1.333 | 0.257 | 1.165 | 0.006 | 0.002 | 0.035 | 0.002 | 0.205 |
| Na | 0.010 | 0.046 | 0.044 | 0.026 | 0.527 | 0.873 | 0.723 | 1.009 | 0.089 |
| K | 0.022 | 0.000 | 0.028 | 0.004 | 0.013 | 0.000 | 0.001 | 0.005 | 0.000 |
| ΣA site | 1.589 | 1.772 | 1.770 | 1.382 | 1.250 | 1.917 | 1.874 | 2.102 | 1.354 |
| F | 0.003 | 0.000 | 0.108 | 0.071 | 0.000 | 1.348 | 0.241 | 1.485 | 0.041 |
| OH | 0.000 | 0.853 | 0.000 | 1.375 | 1.360 | 0.000 | 0.116 | 0.000 | 1.064 |
| oxygen | 6.997 | 6.147 | 7.492 | 5.554 | 5.640 | 5.807 | 6.644 | 5.871 | 5.895 |
| ΣYsite | 7.000 | 7.000 | 7.600 | 7.000 | 7.000 | 7.155 | 7.000 | 7.356 | 7.000 |
| Zone | 1st I | 2nd I | ||||
|---|---|---|---|---|---|---|
| Sample | A33a | A33b | D1E | D1E | D1E | D1E |
| Mineral | Nbrt | Nbrt | Nbrt | Nbrt | Nbrt | Tart |
| WO3 | n.a. | n.a. | 3.36 | 1.89 | 1.08 | 2.40 |
| Nb2O5 | 7.35 | 10.32 | 21.56 | 13.32 | 16.79 | 9.78 |
| Ta2O5 | 6.90 | 8.94 | 33.47 | 19.87 | 10.54 | 26.14 |
| TiO2 | 79.94 | 74.20 | 28.48 | 55.81 | 62.88 | 49.17 |
| SnO2 | 0.00 | 0.00 | 0.00 | 0.04 | 0.00 | 0.05 |
| UO2 | 0.00 | 0.00 | 0.12 | 0.01 | 0.09 | 0.05 |
| Y2O3 | 0.00 | 0.00 | 0.04 | 0.00 | 0.14 | 0.43 |
| MgO | 0.00 | 0.00 | 0.10 | 0.02 | 0.02 | 0.15 |
| MnO | 0.02 | 0.03 | 4.35 | 0.31 | 0.33 | 1.19 |
| Fe2O3 | 4.80 | 5.33 | 9.85 | 9.72 | 9.18 | 10.23 |
| Total | 99.01 | 98.82 | 101.33 | 100.99 | 101.05 | 99.59 |
| apfu | ||||||
| W6+ | - | - | 0.052 | 0.025 | 0.014 | 0.034 |
| Nb | 0.144 | 0.207 | 0.542 | 0.288 | 0.344 | 0.224 |
| Ta | 0.081 | 0.108 | 0.506 | 0.259 | 0.130 | 0.360 |
| Ti | 2.601 | 2.473 | 1.192 | 2.008 | 2.140 | 1.875 |
| Sn4+ | 0.000 | 0.000 | 0.000 | 0.001 | 0.000 | 0.001 |
| U4+ | 0.000 | 0.000 | 0.001 | 0.000 | 0.001 | 0.001 |
| Y3+ | 0.000 | 0.000 | 0.001 | 0.000 | 0.003 | 0.012 |
| Mg | 0.000 | 0.000 | 0.008 | 0.001 | 0.001 | 0.011 |
| Mn2+ | 0.001 | 0.001 | 0.205 | 0.013 | 0.013 | 0.051 |
| Fe3+ | 0.156 | 0.178 | 0.413 | 0.350 | 0.313 | 0.391 |
| Cat.Sum | 2.983 | 2.966 | 2.921 | 2.945 | 2.958 | 2.960 |
| Type | IV | V | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Zone | 2nd I | 1st I | 2nd I | 3rd I | |||||
| Sample | A11a | i4 + b | K40 | K40 | K100B | K100B | K100d | kK00d | K100L |
| WO3 | 0.04 | 0.00 | 0.00 | 0.00 | n.a. | n.a | 0.3 | 0.53 | 0.19 |
| Nb2O5 | 0.37 | 0.05 | 0.37 | 0.37 | 1.04 | 0.11 | 0.00 | 0.44 | 0.00 |
| Ta2O5 | 0.79 | 0.38 | 2.32 | 1.79 | 5.53 | 3.38 | 0.98 | 3.76 | 0.19 |
| TiO2 | 0.23 | 1.32 | 0.2 | 0.26 | 0.13 | 0.08 | 0.08 | 0.12 | 0.27 |
| SnO2 | 98.59 | 98.48 | 96.53 | 97.32 | 91.63 | 95.51 | 99.48 | 95.68 | 99.36 |
| UO2 | 0.11 | 0.06 | n.a | n.a | n.a. | n.a | n.a | 0.00 | 0.00 |
| MgO | 0.20 | 0.18 | 0.05 | 0.08 | 0.09 | 0.05 | 0.17 | 0.12 | 0.14 |
| MnO | 0.08 | 0.04 | 0 | 0 | 0.04 | 0.07 | 0.05 | 0.06 | 0.00 |
| Fe2O3 | 0.47 | 0.45 | 0.52 | 0.43 | 1.32 | 0.79 | 0.2 | 0.86 | 0.16 |
| Total | 100.88 | 100.97 | 99.99 | 100.25 | 99.78 | 99.99 | 101.26 | 101.57 | 100.31 |
| apfu | |||||||||
| W6+ | 0.001 | 0.000 | 0.000 | 0.000 | - | - | 0.004 | 0.007 | 0.003 |
| Nb | 0.008 | 0.001 | 0.008 | 0.008 | 0.024 | 0.003 | 0.000 | 0.010 | 0.000 |
| Ta | 0.011 | 0.005 | 0.032 | 0.024 | 0.076 | 0.046 | 0.013 | 0.051 | 0.003 |
| Ti | 0.009 | 0.049 | 0.008 | 0.010 | 0.005 | 0.003 | 0.003 | 0.004 | 0.010 |
| Sn4+ | 1.948 | 1.927 | 1.930 | 1.938 | 1.842 | 1.917 | 1.963 | 1.888 | 1.974 |
| U4+ | 0.003 | 0.001 | - | - | - | - | - | 0.000 | 0.000 |
| Mg | 0.015 | 0.013 | 0.004 | 0.006 | 0.007 | 0.004 | 0.013 | 0.009 | 0.010 |
| Mn2+ | 0.003 | 0.002 | 0.000 | 0.000 | 0.002 | 0.003 | 0.002 | 0.003 | 0.000 |
| Fe2+ | 0.018 | 0.017 | 0.020 | 0.016 | 0.050 | 0.030 | 0.008 | 0.032 | 0.006 |
| Cat, Sum | 2.015 | 2.015 | 2.002 | 2.003 | 2.004 | 2.006 | 2.006 | 2.003 | 2.006 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonçalves, A.O.; Melgarejo, J.-C.; Alfonso, P.; Amores, S.; Paniagua, A.; Neto, A.B.; Morais, E.A.; Camprubí, A. The Distribution of Rare Metals in the LCT Pegmatites from the Giraúl Field, Angola. Minerals 2019, 9, 580. https://doi.org/10.3390/min9100580
Gonçalves AO, Melgarejo J-C, Alfonso P, Amores S, Paniagua A, Neto AB, Morais EA, Camprubí A. The Distribution of Rare Metals in the LCT Pegmatites from the Giraúl Field, Angola. Minerals. 2019; 9(10):580. https://doi.org/10.3390/min9100580
Chicago/Turabian StyleGonçalves, Antonio Olimpio, Joan-Carles Melgarejo, Pura Alfonso, Sandra Amores, Andrés Paniagua, Andrés Buta Neto, Eduardo Alves Morais, and Antoni Camprubí. 2019. "The Distribution of Rare Metals in the LCT Pegmatites from the Giraúl Field, Angola" Minerals 9, no. 10: 580. https://doi.org/10.3390/min9100580
APA StyleGonçalves, A. O., Melgarejo, J.-C., Alfonso, P., Amores, S., Paniagua, A., Neto, A. B., Morais, E. A., & Camprubí, A. (2019). The Distribution of Rare Metals in the LCT Pegmatites from the Giraúl Field, Angola. Minerals, 9(10), 580. https://doi.org/10.3390/min9100580







