Directional Oxidation of Pyrite in Acid Solution
Abstract
:1. Introduction
2. Sample and Methods
2.1. Sample Preparation
2.2. Experimental
3. Result and Discussion
3.1. Pyrite Surface Characterization
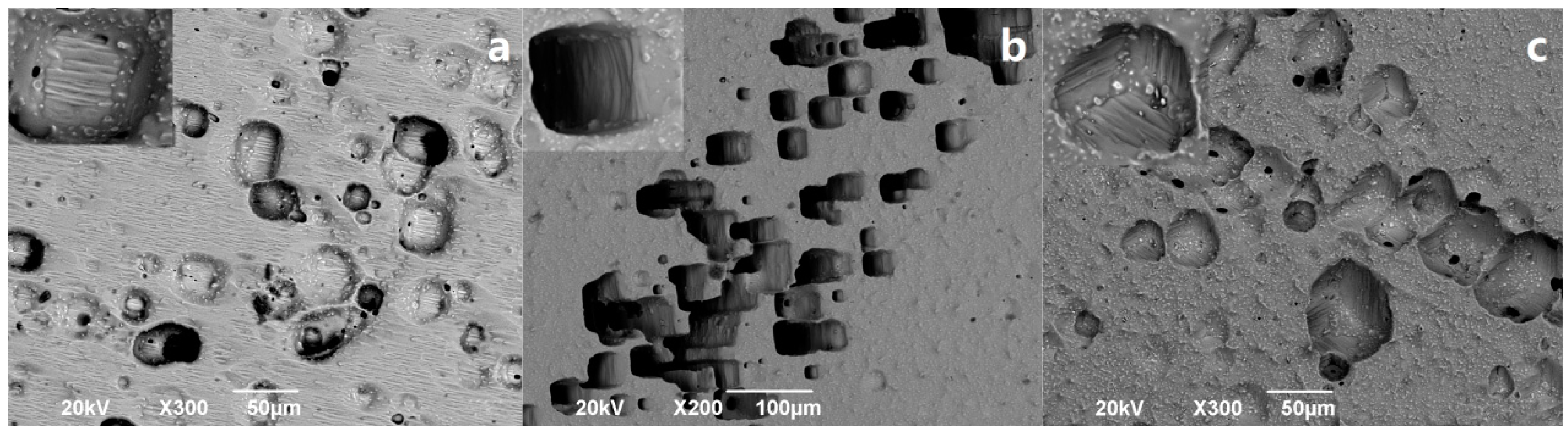
3.2. Surface Topography Change
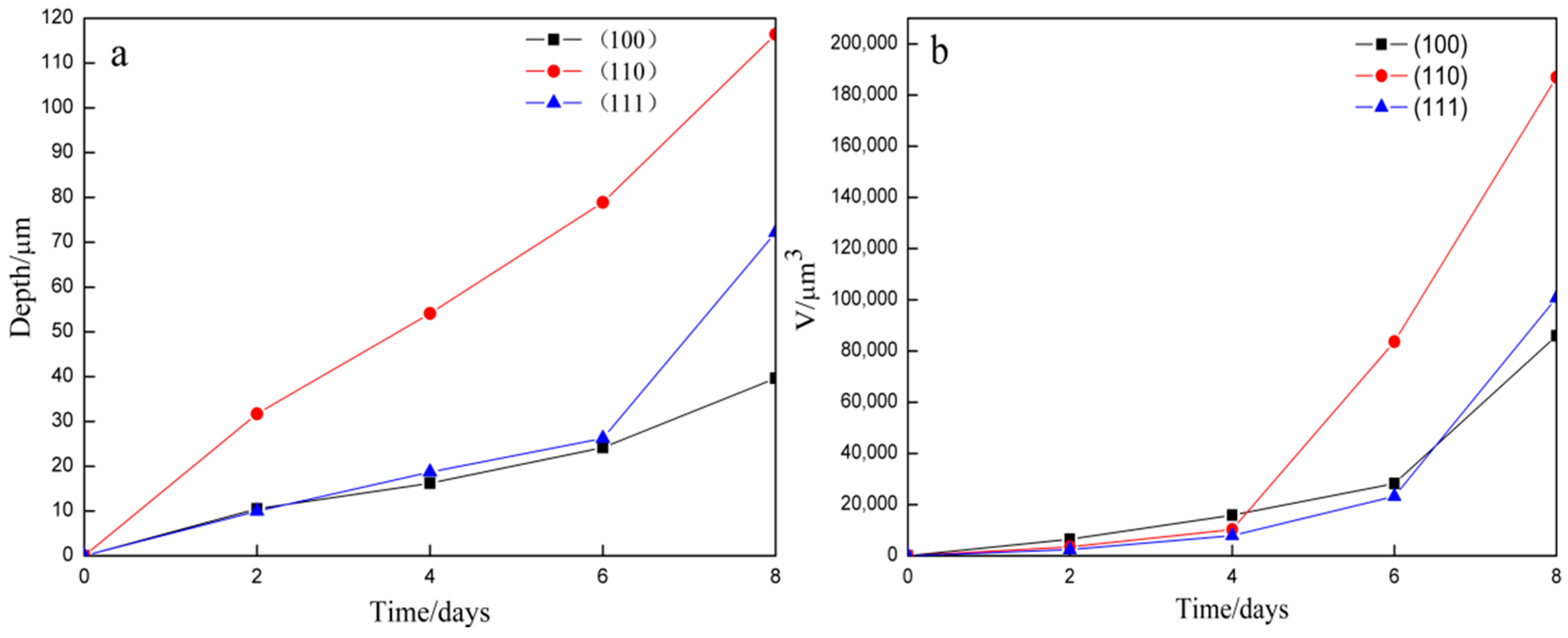
3.3. Depth and Volume Analysis of Etching Pits
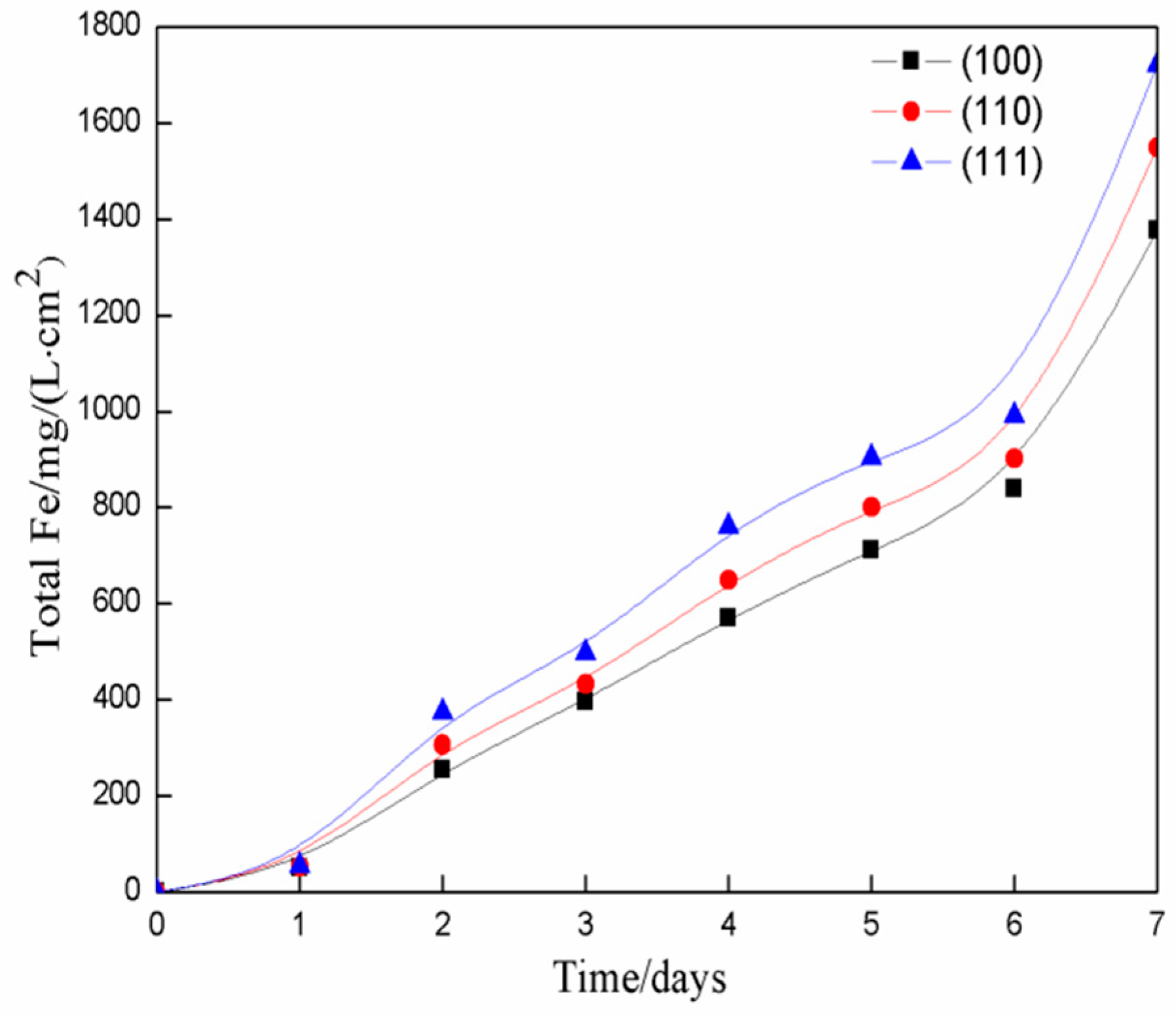
3.4. Fe Ion Concentration
3.5. Raman Spectra Analysis
3.6. XPS Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nesbitt, H.W.; Muir, I.J. X-ray photoelectron spectroscopic study of a pristine pyrite surface reacted with water vapour and air. Geochim. Cosmochim. Acta. 1994, 58, 4667–4679. [Google Scholar] [CrossRef]
- Rickard, D. Pyrite: A Natural History of Fool’s Gold; Oxford University Press: Oxford, UK, 2015. [Google Scholar]
- Tauson, V.L.; Kravtsova, R.G.; Grebenshchikova, V.I.; Lustenberg, E.E.; Lipko, S.V. Surface typochemistry of hydrothermal pyrite: Electron spectroscopic and scanning probe microscopic data. II. Natural pyrite. Geochem. Int. 2009, 47, 231–243. [Google Scholar] [CrossRef]
- Abraitis, P.K.; Pattrick, R.A.D.; Vaughan, D.J. Variations in the compositional, textural and electrical properties of natural pyrite: A review. Int. J. Miner. Process. 2004, 74, 41–59. [Google Scholar] [CrossRef]
- Sasaki, K.; Tsunekawa, M.; Ohtsuka, T.; Konno, H. Confirmation of a sulfur-rich layer on pyrite after oxidative dissolution by Fe(III) ions around pH 2. Geochim. Cosmochim. Acta 1995, 59, 3155–3158. [Google Scholar] [CrossRef]
- Mckibben, M.A.; Barnes, H.L. Oxidation of pyrite in low temperature acidic solutions: Rate laws and surface textures. Geochim. Cosmochim. Acta 1986, 50, 1509–1520. [Google Scholar] [CrossRef]
- Woods, A.N.B. The surface oxidation of pyrite. Appl. Surf. Sci. 1987, 27, 437–452. [Google Scholar]
- De Leeuw, N.H.; Parker, S.C.; Sithole, H.M.; Ngoepe, P.E. Modeling the Surface Structure and Reactivity of Pyrite: Introducing a Potential Model for FeS2. J. Phys. Chem. B 2000, 104, 7969–7976. [Google Scholar] [CrossRef]
- Hung, A.; Muscat, J.; Yarovsky, I.; Russo, S.P. Density-functional theory studies of pyrite FeS2(100) and (110) surfaces. Surf. Sci. 2002, 513, 511–524. [Google Scholar] [CrossRef]
- Qiu, G.; Gao, T.; Hong, J.; Tan, W.; Liu, F.; Zheng, L. Mechanisms of arsenic-containing pyrite oxidation by aqueous arsenate under anoxic conditions. Geochim. Cosmochim. Acta 2017, 217. [Google Scholar] [CrossRef]
- Chandra, A.P.; Gerson, A.R. The mechanisms of pyrite oxidation and leaching: A fundamental perspective. Surf. Sci. Rep. 2010, 65, 293–315. [Google Scholar] [CrossRef]
- Long, H.; Dixon, D.G. Pressure oxidation of pyrite in sulfuric acid media: A kinetic study. Hydrometallurgy 2004, 73, 335–349. [Google Scholar] [CrossRef]
- Tu, Z.; Guo, C.; Zhang, T.; Lu, G.; Wan, J.; Liao, C.; Dang, Z. Investigation of intermediate sulfur species during pyrite oxidation in the presence and absence of Acidithiobacillus ferrooxidans. Hydrometallurgy 2016, 167, 58–65. [Google Scholar] [CrossRef]
- Kelsall, G.H.; Yin, Q.; Vaughan, D.J.; England, K.E.R.; Brandon, N.P. Electrochemical oxidation of pyrite (FeS2) in aqueous electrolytes. J. Electroanal. Chem. 1999, 471, 116–125. [Google Scholar] [CrossRef]
- Sun, H.; Chen, M.; Zou, L.; Shu, R.; Ruan, R. Study of the kinetics of pyrite oxidation under controlled redox potential. Hydrometallurgy 2015, 155, 13–19. [Google Scholar] [CrossRef]
- Tu, Z.; Wan, J.; Guo, C.; Fan, C.; Zhang, T.; Lu, G.; Reinfelder, J.R.; Dang, Z. Electrochemical oxidation of pyrite in pH 2 electrolyte. Electrochim. Acta 2017, 239, 25–35. [Google Scholar] [CrossRef]
- Paszkowicz, W.; Leiro, J.A. Rietveld refinement study of pyrite crystals. J. Alloys Compd. 2005, 401, 289–295. [Google Scholar] [CrossRef]
- Vaughan, D.J.; Craig, J.R. Mineral Chemistry of Metal Sulfides; Cambridge University Press: Cambridge, UK, 1987; pp. 410–411. [Google Scholar]
- Chen, G.Y.; Sun, D.S.; Zhang, L.; Zang, W.S.; Wang, J.; Lu, A.H. Morphogenesis of pyrite. Geoscience 1987, 1, 60–76. [Google Scholar]
- Sit, P.H.; Cohen, M.H.; Selloni, A. Interaction of Oxygen and Water with the (100) Surface of Pyrite: Mechanism of Sulfur Oxidation. J. Phys. Chem. Lett. 2012, 3, 2409–2414. [Google Scholar] [CrossRef]
- Zhu, J.; Xian, H.; Lin, X.; Tang, H.; Du, R.; Yang, Y.; Zhu, R.; Liang, X.; Wei, J.; Teng, H.H. Surface structure-dependent pyrite oxidation in relatively dry and moist air: Implications for the reaction mechanism and sulfur evolution. Geochim. Cosmochim. Acta 2018, 228, 259–274. [Google Scholar] [CrossRef]
- Ndlovu, S.; Monhemius, A.J. The influence of crystal orientation on the bacterial dissolution of pyrite. Hydrometallurgy 2005, 78, 187–197. [Google Scholar] [CrossRef]
- Zhao, S.; Xu, C.; Zhang, G.; Li, K. Etch Figures Related to the Symmetry and Structure of the Crystal Faces. Earth Sci. 2013, 38, 211–217. [Google Scholar]
- Nesbitt, H.W.; Bancroft, G.M.; Pratt, A.R.; Scaini, M.J. Sulfur and iron surface states on fractured pyrite surfaces. Am. Mineral. 1998, 83, 1067–1076. [Google Scholar] [CrossRef]
- Wang, W.; Wang, J.; Zhao, S. Crystal Morphology; China University of Geosciences Press: Wuhan, China, 2001. [Google Scholar]
- Descostes, M.; Mercier, F.; Thromat, N.; Beaucaire, C.; Gautier-Soyer, M. Use of XPS in the determination of chemical environment and oxidation state of iron and sulfur samples: constitution of a data basis in binding energies for Fe and S reference compounds and applications to the evidence of surface species of an oxidized pyrite in a carbonate medium. Appl. Surf. Sci. 2000, 165, 288–302. [Google Scholar]
- Eggleston, C.M.; Ehrhardt, J.J.; Stumm, W. Surface structural controls on pyrite oxidation kinetics; an XPS-UPS, STM, and modeling study. Am. Mineral. 1996, 81, 1036–1056. [Google Scholar] [CrossRef]
- Sang, Y.L.; Kim, D.H.; Choi, S.C.; Lee, D.J.; Ji, Y.C.; Kim, H.D. Porous multi-walled carbon nanotubes by using catalytic oxidation via transition metal oxide. Microporous Mesoporous Mater. 2014, 194, 46–51. [Google Scholar]
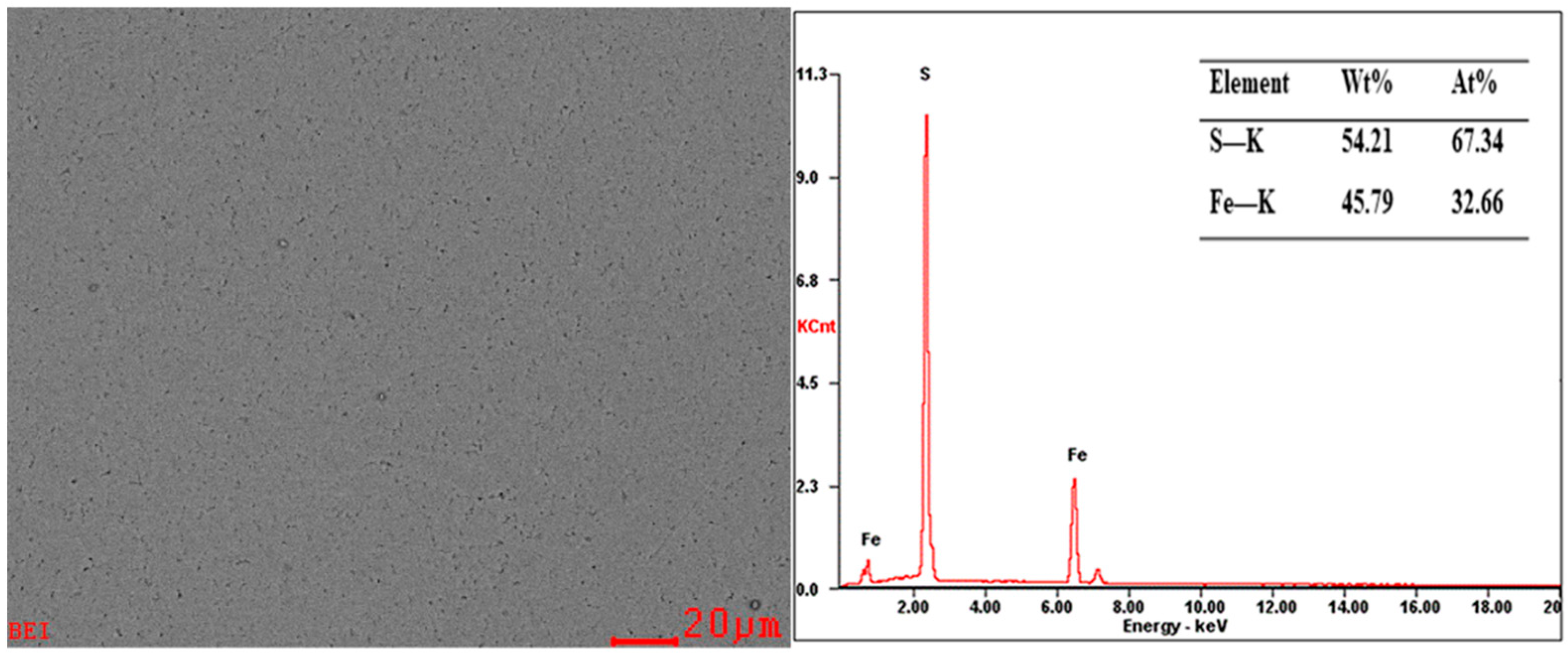

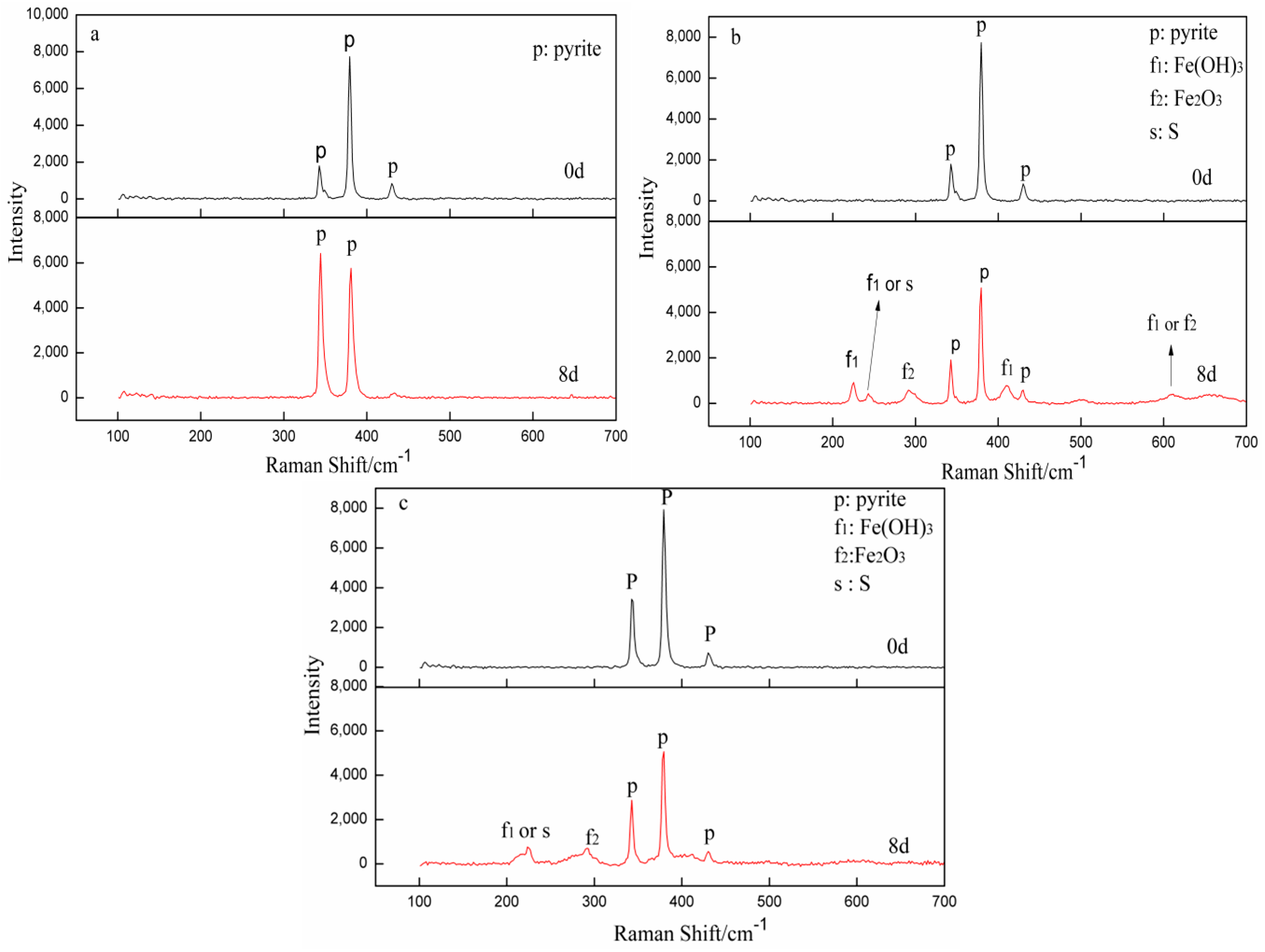
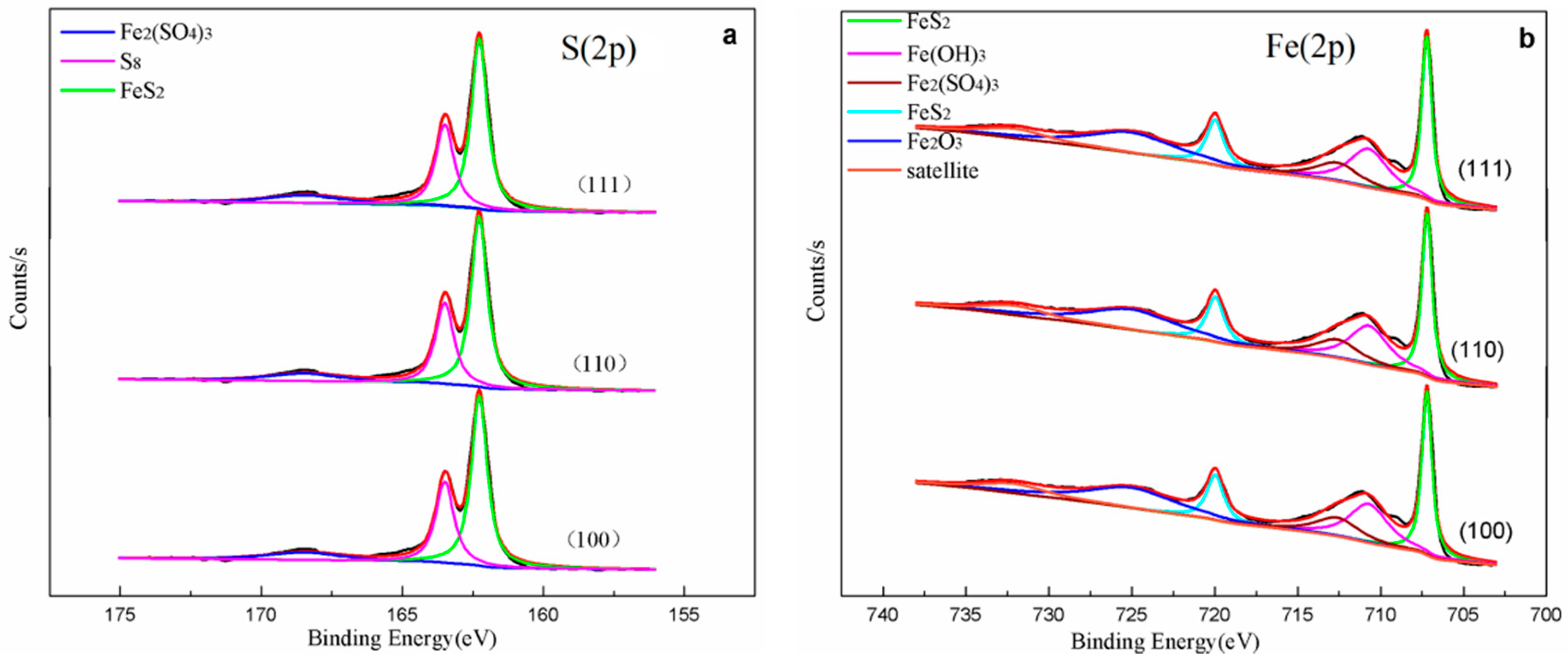
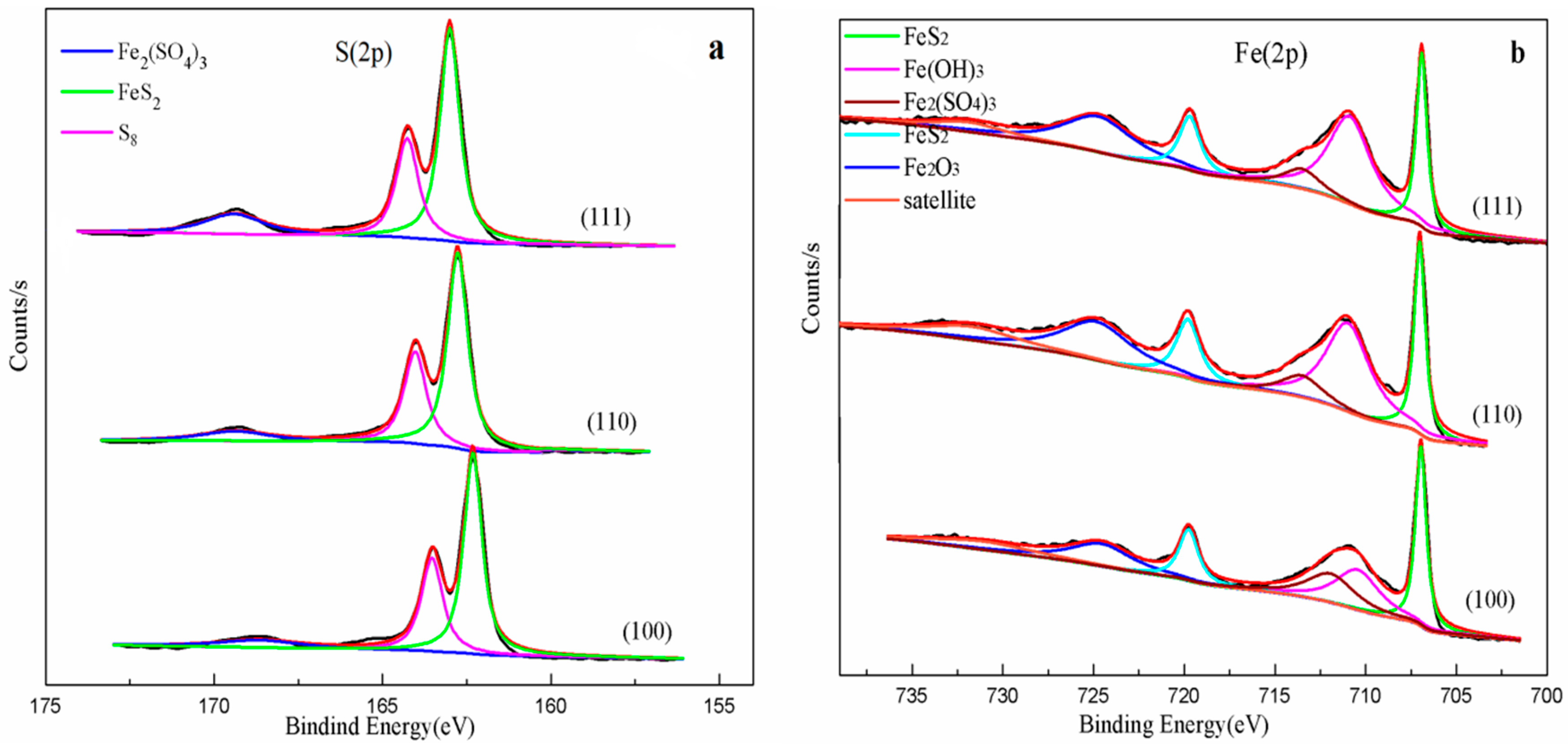
| Element | Fe | S | O | Si | Al | K | Ti | Ca | Zn | Ru | Mg | P | Ni | Cr |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Composition (wt%) | 44.86 | 44.06 | 8.45 | 1.07 | 0.72 | 0.33 | 0.12 | 0.09 | 0.09 | 0.09 | 0.07 | 0.02 | 0.02 | 0.01 |
| Spectrum | Unreacted Pyrite | Binding Energy(eV) (2p) | Species | ||
|---|---|---|---|---|---|
| (100) | (110) | (111) | |||
| S2p | 162.3(2p3/2) | 162.3(2p3/2) | 162.3(2p3/2) | 162.3(2p3/2) | FeS2 [16,26] |
| 163.4(2p3/2) | 163.5(2p3/2) | 163.5(2p3/2) | 163.5(2p3/2) | S8 [7,16] | |
| 168.5(2p3/2) | 168.6(2p3/2) | 168.6(2p3/2) | 168.4(2p3/2) | Fe2(SO4)3 [16,26] | |
| Fe2p | 707.2(2p3/2) 720.0(2p1/2) | 707.0(2p3/2) 719.8(2p1/2) | 707.0(2p3/2) 720.0(2p1/2) | 706.9(2p3/2) 719.7(2p1/2) | FeS2 [16,26,27] |
| Satellite | 710.7(2p3/2) | 710.3(2p3/2) | 711.1(2p3/2) | 710.8(2p3/2) | Fe(OH)3 [16] |
| 724.7(2p1/2) | 724.7(2p1/2) | 724.8(2p1/2) | 724.7(2p1/2) | Fe2O3 [26,28] | |
| 732.7(2p1/2) | 732.2(2p1/2) | 732.1(2p1/2) | 731.8(2p1/2) | ||
| 713.2(2p3/2) | 713.2(2p3/2) | 713.5(2p3/2) | 713.5(2p3/2) | Fe2(SO4)3 [26] | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, J.; Tian, H.; Huang, Y.; Ding, Z.; Yin, Z. Directional Oxidation of Pyrite in Acid Solution. Minerals 2019, 9, 7. https://doi.org/10.3390/min9010007
Feng J, Tian H, Huang Y, Ding Z, Yin Z. Directional Oxidation of Pyrite in Acid Solution. Minerals. 2019; 9(1):7. https://doi.org/10.3390/min9010007
Chicago/Turabian StyleFeng, Jiling, Hua Tian, Yaling Huang, Zhiying Ding, and Zhoulan Yin. 2019. "Directional Oxidation of Pyrite in Acid Solution" Minerals 9, no. 1: 7. https://doi.org/10.3390/min9010007
APA StyleFeng, J., Tian, H., Huang, Y., Ding, Z., & Yin, Z. (2019). Directional Oxidation of Pyrite in Acid Solution. Minerals, 9(1), 7. https://doi.org/10.3390/min9010007




