Rare Earth and Phosphorus Leaching from a Flotation Tailings of Florida Phosphate Rock
Abstract
:1. Introduction
2. Materials and Methods
2.1. Flotation Tailings Sample and Chemical Reagents
2.2. Leaching Studies
3. Results
3.1. Effect of Leaching Time
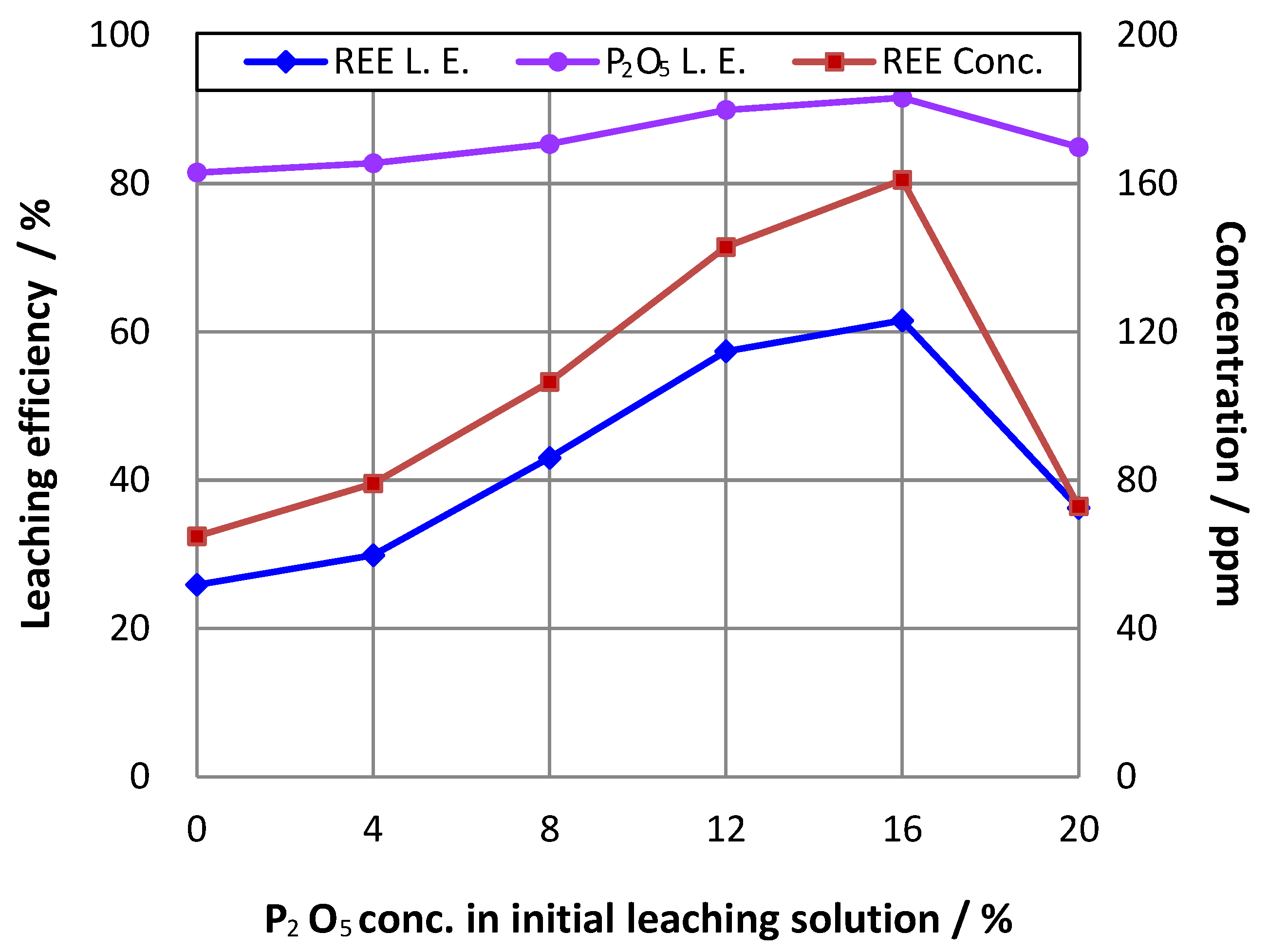
3.2. Effect of Initial Phosphoric Acid Concentration in Pulp
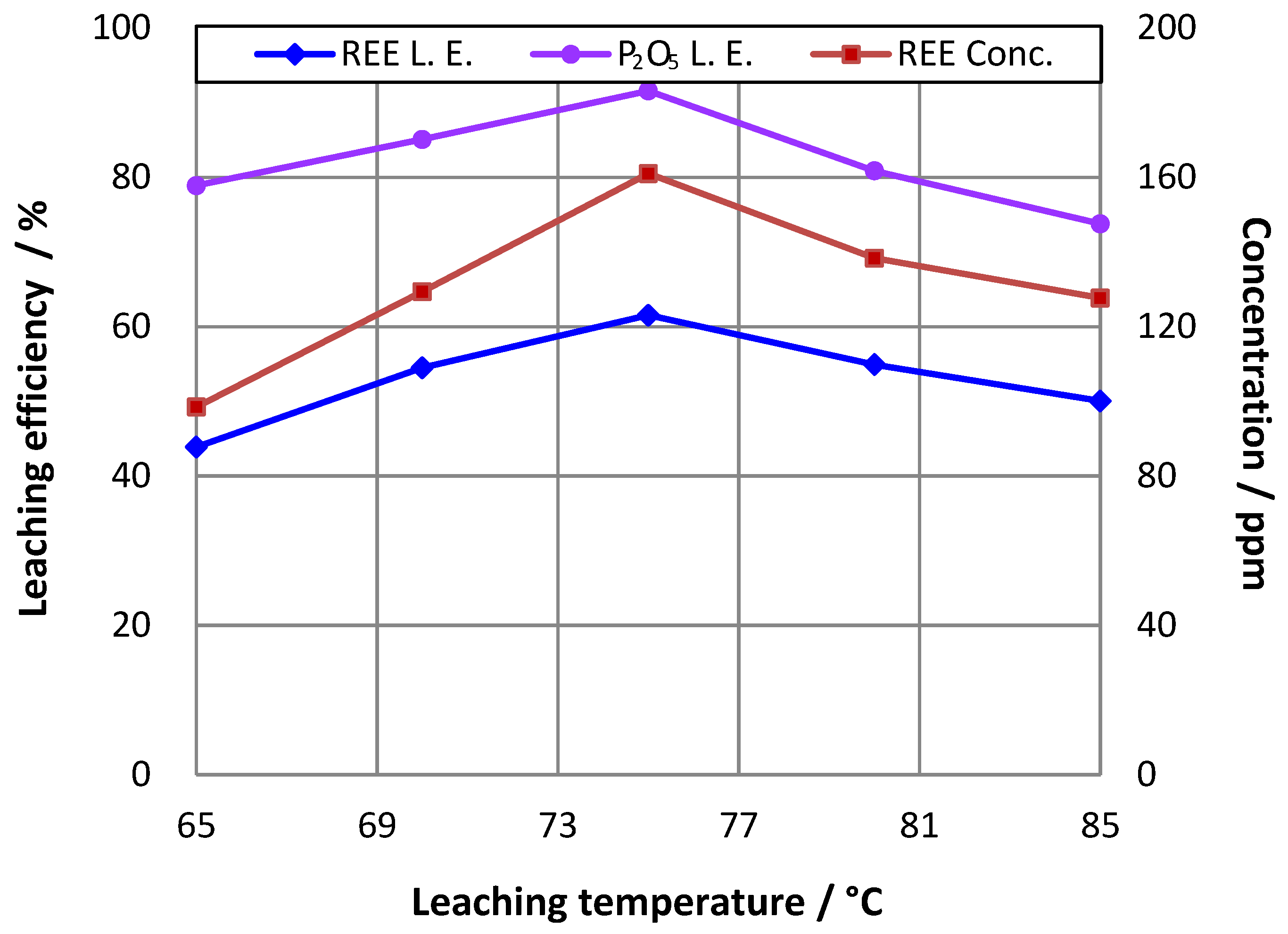
3.3. Effect of Temperature
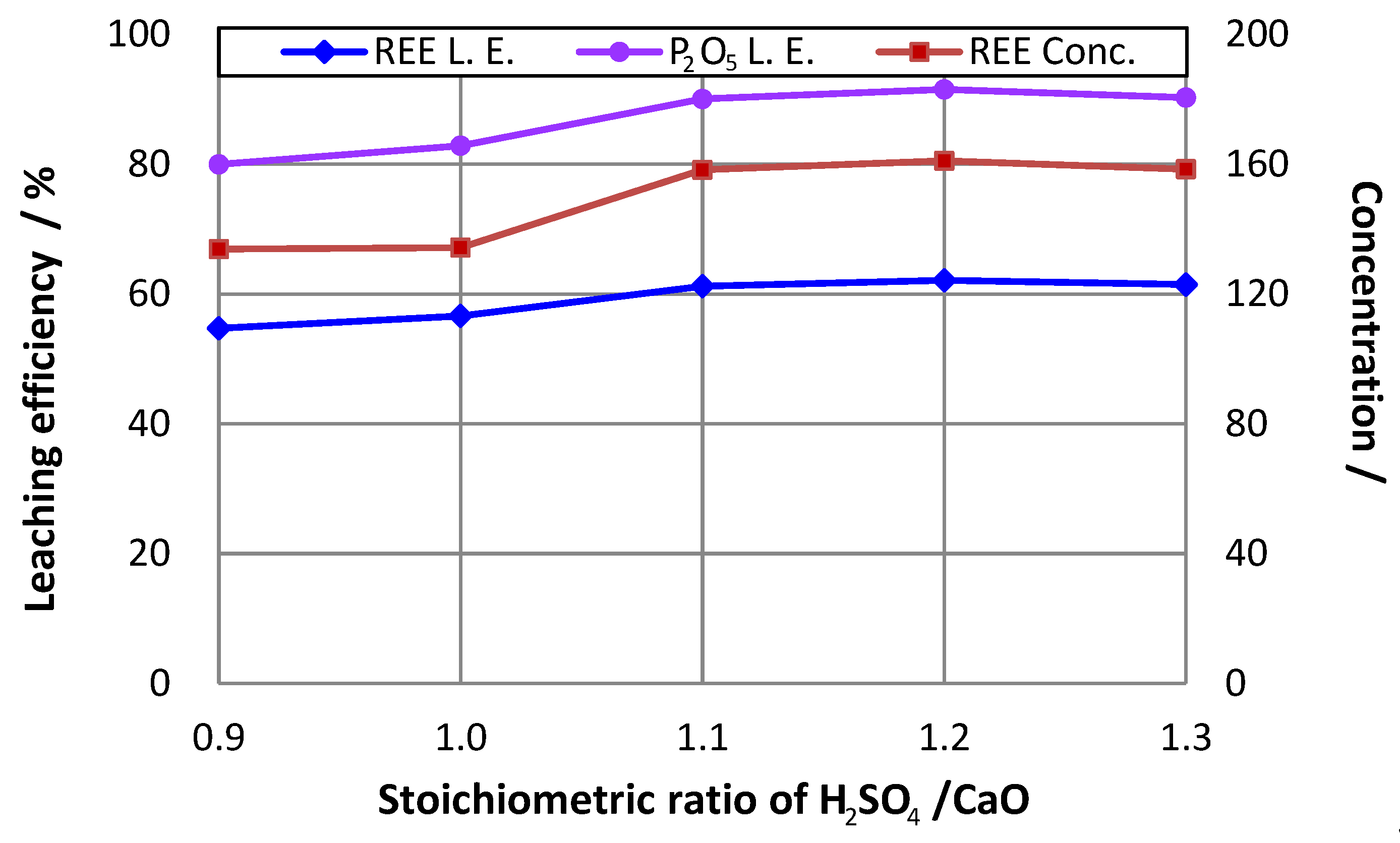
3.4. Effect of H2SO4/CaO Ratio
3.5. Effect of Weight Ratio of Liquid to Solid
4. Discussion
5. Conclusions
Authors Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Becker, P. Phosphates and Phosphoric Acid: Raw Materials, Technology, and Economics of the Wet Process; Marcel Dekker: New York, NY, USA, 1983. [Google Scholar]
- Zhang, P. Comprehensive recovery and sustainable development of phosphate resources. Procedia Eng. 2014, 83, 37–51. [Google Scholar] [CrossRef]
- Pan, Y.; Fleet, M.E.; Macrae, N.D. Oriented monazite inclusions in apatite porphyroblasts from the Hemlo gold deposit, Ontario, Canada. Mineral. Mag. 1993, 57, 697–708. [Google Scholar] [CrossRef]
- Schoneveld, L.; Spandler, C.; Hussey, K. Genesis of the central zone of the Nolans Bore rare earth element deposit, Northern Territory, Australia. Contrib. Mineral. Petrol. 2015, 170, 11. [Google Scholar] [CrossRef]
- Jasinski, S.M. Mineral Commodity Summaries 2017, Phosphate Rock; US Geological Survey: Reston, VA, USA, 2017; pp. 124–125.
- Beltrami, D.; Cote, G.; Mokhtari, H.; Courtaud, B.; Moyer, B.A.; Changes, A. Recovery of uranium from wet phosphoric acid by solvent extraction processes. Chem. Rev. 2014, 114, 12002–12023. [Google Scholar] [CrossRef] [PubMed]
- Wamser, C.; Bruen, C. Recovery of Fluorine, Uranium and Rare Earth Metal Values from Phosphoric Acid by-product Brine Raffinate. U.S. Patent 3,937,783, 21 February 1974. [Google Scholar]
- Bunus, F.; Dumitrescu, R. Simultaneous extraction of rare earth elements and uranium from phosphoric acid. Hydrometallurgy 1992, 28, 331–338. [Google Scholar] [CrossRef]
- Bunus, F.; Miu, I.; Dumitrescu, R. Simultaneous recovery and separation of uranium and rare earths from phosphoric acid in one-cycle extraction-stripping process. Hydrometallurgy 1994, 35, 375–389. [Google Scholar] [CrossRef]
- Bunus, F. Uranium and rare earth recovery from phosphate fertilizer industry by solvent extraction. Miner. Proc. Extr. Metall. Rev. 2000, 21, 381–478. [Google Scholar] [CrossRef]
- Preston, J.S.; Cole, P.M.; Craig, W.M.; Feather, A.M. The recovery of rare earth oxides from a phosphoric acid by-product. Part 1: Leaching of rare earth values and recovery of a mixed rare earth oxide by solvent extraction. Hydrometallurgy 1996, 41, 1–19. [Google Scholar]
- Koopman, C.; Witkamp, G.J. Extraction of lanthanides from the phosphoric acid production process to gain purified gypsum and a valuable lanthanide by-product. Hydrometallurgy 2000, 58, 51–60. [Google Scholar] [CrossRef]
- Krea, M.; Khalaf, H. Liquid-liquid extraction of uranium and lanthanides from phosphoric acid using a synergistic DOPPA-TOPO mixture. Hydrometallurgy 2000, 58, 215–225. [Google Scholar] [CrossRef]
- Wang, L.; Long, Z.; Huang, X.; Yu, Y.; Cui, D.; Zhang, G. Recovery of rare earths from wet-process phosphoric acid. Hydrometallurgy 2010, 101, 41–47. [Google Scholar] [CrossRef]
- Zhang, P. Recovery of critical elements from Florida phosphate: Phase 1. Characterization of rare earths. In Proceedings of the ECI International Conference: Rare earth Minerals/Metals–Sustainable Technologies for the Future, San Diego, CA, USA, 12–17 August 2012. [Google Scholar]
- Zhang, P.; Liang, H.; Jin, Z.; DePaoli, D. The ultimate mineral processing challenge: Recovery of rare earths, phosphorus and uranium from Florida phosphatic clay. Miner. Metall. Process. 2017, 34, 183–188. [Google Scholar] [CrossRef]
- Massari, S.; Ruberti, M. Rare earth elements as critical raw materials: Focus on international markets and future strategies. Resour. Policy 2013, 38, 36–43. [Google Scholar] [CrossRef]
- Liang, H.; Zhang, P.; Jin, Z.; DePaoli, D. Rare-earth leaching from Florida phosphate rock in wet-process phosphoric acid production. Miner. Metall. Process 2017, 34, 146–153. [Google Scholar] [CrossRef]
- Liang, H.; Zhang, P.; Jin, Z.; DePaoli, D.W. Rare earths recovery and gypsum upgrade from Florida phosphogypsum. Miner. Metall. Process 2017, 34, 201–206. [Google Scholar] [CrossRef]
- Kanazawa, Y.; Kamitani, M. Rare earth minerals and resources in the world. J. Alloy. Compd. 2006, 408, 1339–1343. [Google Scholar]
- Poul, E.; Mclauglin, I.P.; Breit, N.G.; Bray, A.E.; Koenig, E.A. Rare earth elements in sedimentary phosphate deposits: Solution to the global REE crisis? Gondwana Res. 2015, 27, 776–785. [Google Scholar]
- Zhang, P.; Yu, Y.; Bogan, M. Challenging the “Crago” double float process ii. Amine-type-fatty acid flotation of silicious phosphates. Miner. Eng. 1997, 10, 983–994. [Google Scholar] [CrossRef]
- Claude, L.W.; Henry, M.J.R. Method for determination of small amounts of rare earths and thorium in phosphate rocks. Anal. Chem. 1953, 25, 432–435. [Google Scholar]
- Giesekke, E.W. Florida Phosphate Rock. In SME Mineral Processing Handbook; Weiss, N.L., Ed.; Society of Mining Engineers of the American Institute of Mining, Metallurgical, and Petroleum Engineers: Englewood, IN, USA, 1985; pp. 1–18. [Google Scholar]
- Kremer, R.A.; Chokshi, J.C. Fate of Rare Earth Elements in Mining/Beneficiation of Florida Phosphate Rock and Conversion to DAP Fertilizer; Research Report; Mobil Mining and Minerals Company: Nichols, FL, USA, 1989.
- Firsching, F.H.; Brune, S.N. Solubility products of the trivalent rare-earth phosphates. J. Chem. Eng. 1991, 36, 93–95. [Google Scholar] [CrossRef]
- Liu, X.; Byrne, R.H. Rare earth and yttrium phosphate solubilities in aqueous solution. Geochim. Cosmochim. Acta 1997, 61, 1625–1633. [Google Scholar] [CrossRef]

| Product | Yield/% | REE Content/ppm | P2O5 Content/% | Recovery/% | |
|---|---|---|---|---|---|
| REEs | P2O5 | ||||
| Concentrate | 10.43 | 866.31 | 11.74 | 55.84 | 40.97 |
| Tailings | 89.57 | 79.79 | 1.97 | 44.16 | 59.03 |
| Calculated feed | 100.00 | 161.82 | 2.99 | 100.00 | 100.00 |
| Component | P2O5 | CaO | Fe2O3 | MgO | Al2O3 | ||||
|---|---|---|---|---|---|---|---|---|---|
| Content (%) | 11.74 | 18.62 | 1.64 | 0.12 | 0.44 | ||||
| Element | Sc | Y | La | Ce | Pr | Nd | Sm | Eu | Gd |
| Content (ppm) | 9.18 | 187.38 | 131.86 | 251.23 | 29.96 | 164.23 | 0.00 | 3.87 | 28.02 |
| Element | Tb | Dy | Ho | Er | Tm | Yb | Lu | Total REEs | |
| Content (ppm) | 2.44 | 23.23 | 4.11 | 10.93 | 6.38 | 12.03 | 1.46 | 866.31 | |
| Screen Mesh | Size (um) | Yield (%) | Content of Chemical Component (%) | |||||
|---|---|---|---|---|---|---|---|---|
| P2O5 | CaO | MgO | Fe2O3 | Al2O3 | Total REEs (ppm) | |||
| +35 | +500 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| −35 + 60 | −500 + 250 | 0.40 | 8.27 | 11.10 | 0.23 | 1.52 | 1.25 | 246.32 |
| −60 + 80 | −250 + 180 | 1.84 | 7.87 | 8.93 | 0.12 | 1.02 | 0.63 | 239.54 |
| −80 + 120 | −180 + 125 | 23.59 | 10.14 | 22.05 | 0.12 | 0.98 | 0.41 | 707.56 |
| −120 + 150 | −125 + 100 | 21.93 | 17.74 | 26.81 | 0.16 | 1.36 | 0.48 | 910.16 |
| −150 + 200 | −100 + 75 | 42.34 | 10.65 | 15.25 | 0.12 | 1.68 | 0.43 | 794.47 |
| −200 + 270 | −75 + 53 | 8.51 | 7.04 | 10.10 | 0.11 | 3.25 | 0.34 | 1436.11 |
| −270 + 350 | −53 + 45 | 0.85 | 6.21 | 7.80 | 0.12 | 5.11 | 0.29 | 1799.29 |
| −350 | −45 | 0.54 | 4.35 | 4.28 | 0.14 | 7.12 | 0.18 | 3404.82 |
| Total/Weighted average | 100.00 | 11.64 | 18.70 | 0.13 | 1.62 | 0.43 | 864.18 | |
| REE | Ksp0 of LnPO4 | Calculated REE Concentration in LnPO4-H3PO4Aqueous Solution with [PO43−] = (0.70–2.18) × 10−20 mol/L | Analyzed REE Concentration in Leaching Solution | |
|---|---|---|---|---|
| ×10−6 mol/L | ppm | ×10−6 mol/L | ||
| Y | 9.55 × 10−26 | 4.38–13.74 | 41.71 | 551.72 |
| La | 1.78 × 10−26 | 0.82–2.56 | 20.40 | 172.72 |
| Ce | 5.37 × 10−27 | 0.25–0.77 | 36.95 | 310.12 |
| Pr | 3.72 × 10−27 | 0.17–0.53 | 2.78 | 23.20 |
| Nd | 6.31 × 10−27 | 0.29–0.91 | 33.26 | 271.17 |
| Sm | 6.46 × 10−27 | 0.30–0.93 | 0.00 | 0.00 |
| Eu | 1.10 × 10−26 | 0.50–1.58 | 1.18 | 9.13 |
| Gd | 2.40 × 10−26 | 1.10–3.45 | 4.38 | 32.76 |
| Tb | 4.07 × 10−26 | 1.87–5.86 | 0.63 | 4.66 |
| Dy | 6.61 × 10−26 | 3.03–9.50 | 4.63 | 33.51 |
| Ho | 8.51 × 10−26 | 3.90–12.24 | 0.77 | 5.49 |
| Er | 7.41 × 10−26 | 3.40–10.66 | 4.15 | 29.18 |
| Tm | 9.33 × 10−26 | 4.28–13.42 | 1.06 | 7.38 |
| Yb | 1.29 × 10−25 | 5.91–18.53 | 3.19 | 21.68 |
| Lu | 1.78 × 10−25 | 8.16–25.58 | 0.51 | 3.43 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, H.; Zhang, P.; Jin, Z.; DePaoli, D.W. Rare Earth and Phosphorus Leaching from a Flotation Tailings of Florida Phosphate Rock. Minerals 2018, 8, 416. https://doi.org/10.3390/min8090416
Liang H, Zhang P, Jin Z, DePaoli DW. Rare Earth and Phosphorus Leaching from a Flotation Tailings of Florida Phosphate Rock. Minerals. 2018; 8(9):416. https://doi.org/10.3390/min8090416
Chicago/Turabian StyleLiang, Haijun, Patrick Zhang, Zhen Jin, and David W. DePaoli. 2018. "Rare Earth and Phosphorus Leaching from a Flotation Tailings of Florida Phosphate Rock" Minerals 8, no. 9: 416. https://doi.org/10.3390/min8090416
APA StyleLiang, H., Zhang, P., Jin, Z., & DePaoli, D. W. (2018). Rare Earth and Phosphorus Leaching from a Flotation Tailings of Florida Phosphate Rock. Minerals, 8(9), 416. https://doi.org/10.3390/min8090416




