Abstract
Phosphorite, or phosphate rock, is the raw material of phosphoric acid production. It has also been regarded as the most important secondary rare earth element (REE) resource due to low contents of rare earth elements contained in the ore. In Florida, there is about 19 Mt of phosphate rock mined annually. After beneficiation, the phosphate rock concentrate is utilized to produce phosphoric acid via a wet-process in which sulfuric acid is used to digest phosphate. During these processes, REEs and some phosphorus get lost in the byproducts including phosphatic clay, flotation tailings, phosphogypsum (PG), and phosphoric sludge. Recovering REEs and phosphorus from these wastes is beneficial to maximize the utilization of these valuable resources. This study focused on the effects of wet-process operating conditions on REE and phosphorus leaching from a kind of flotation tailing of Florida phosphate rock. The tailings were first beneficiated with a shaking table, and then a series of leaching tests were conducted on the shaking table concentrate. The results indicated that REEs had similar trends of leaching efficiency to those of phosphorus. Under the conditions of 16% phosphoric acid concentration in the initial pulp, a temperature of 75 °C, a stoichiometric ratio of sulfuric acid (H2SO4) to calcium oxide (CaO) of 1.1, and a weight ratio of liquid to solid of 3.5, REE and phosphorus leaching efficiencies reached relatively high values of approximately 61% and 91%, respectively. Analyses indicated that the phosphate ions (PO43−) in the leaching solution tended to combine with REE ions to form REE phosphates which precipitated into PG, but the other large amount of anions such as sulfate ions (SO42−) and fluoride ions (F−) took effect of steric hindrance to prevent PO43− from combining with REE cations. These two opposite effects determined the REE distribution between the leaching solution and PG.
1. Introduction
Phosphorite, or phosphate rock, is a kind of igneous or sedimentary rock in which a high amount of phosphate presents as fluorapatite (Ca5(PO4)3F), or as hydroxystalline (Ca5(PO4)3OH). In the current practice of the phosphate industry, phosphorite is usually upgraded from natural phosphate ore, and then the phosphate concentrate is input to an industrial process, named a wet-process, reacting with mineral acid to produce phosphoric acid and mainly further converted into phosphate fertilizers [1]. Taking fluorapatite reacting with sulfuric acid (H2SO4) as an example, the decomposition process can be written as follows [2]:
Ca5(PO4)3F + 5H2SO4 + 5nH2O = 5CaSO4·nH2O↓+ 3H3PO4 + HF exothermic
Under different reaction temperatures, the n in Equation (1) has a different value. Accordingly, the process is named as dihydrate (n = 2) (72–83 °C), hemihydrate (n = 1/2) (90–110 °C), or anhydrate (n = 0) [1].
It is well known that there are also some rare earth elements (REEs) which coexist in phosphate rock. Most of them are present in the form of isomorphous substitutions for Ca2+ as REE-francolite, and a small amount of REEs is hosted in monazite ((Ce,La,Nd,Th)PO4), xenotime (YPO4), etc. [3,4]. In the wet-process, these REEs are also leached out with the decomposition of phosphate minerals and enter into the leaching product of phosphoric acid and residue of phosphogypsum (PG). Similar to phosphate rock, monazite and xenotime react with sulfuric acid and can be expressed as:
2(Ce,La,Nd,Th)PO4 + 3H2SO4 = (Ce,La,Nd,Th)2(SO4)3 + 2H3PO4
2YPO4 + 3H2SO4 = Y2(SO4)3 + 2H3PO4
Due to their low contents (usually lower than 1%), these REEs are not economically recovered currently. However, in recent years phosphorite has been increasingly considered as one of the most promising potential secondary resources of REEs, and recovering REEs from the wet-process of phosphoric acid production has attracted more and more attention, mainly due to the following reasons:
(1) There is a large amount of REEs in the approximately 250 million tons of phosphate rock mined per year for phosphate fertilizer production in the world [5].
(2) The cost of mining and leaching of rare-earth containing minerals is covered by the phosphate fertilizer production [6].
(3) Recovering or removing REEs as well as other hazardous components from the wet-process is beneficial to meeting the increasing demand for these elements and reducing their impact on the environment [7,8,9,10,11,12,13,14,15,16,17,18,19].
It was estimated that about 50,000,000 tons of rare earths are stored in phosphate resources worldwide, nearly 100,000 tons of which are mined annually in the production of phosphate rock [20]. Moreover, the evaluation indicated that in the U.S. the concentration and quantities of heavy REEs hosted in phosphorites are higher than those in all known primary resources, and the easy-to-extract REEs existing in phosphate rocks can meet the global demand [21].
In the central areas of Florida, there is a large quantity of marine sedimentary phosphate deposits. Nowadays, about 19 Mt of phosphate rock is mined annually in these regions to produce fertilizer [5]. The unearthed phosphate ore is first washed to remove fine clay minerals, and then the deslimed fraction is sized to produce a flotation feed. After that, the flotation feed goes through a “Crago” double flotation process (a direct flotation with fatty acid to concentrate phosphates, and then an inverse flotation with amine to remove silica from the rougher phosphate concentrate) to separate phosphate rock from sand tailings. Finally, the phosphate concentrate is used in a dihydrate process to produce phosphoric acid [22].
Several studies demonstrated that there are many kinds of REEs in Florida phosphate rocks. Although their contents are low in the phosphate matrix, the total REE amount in the reserves is rather considerable [2,15,23,24]. An investigation by Kremer and Chokshi [25] indicated that the total content of REEs in the phosphate matrix was analyzed to be 282 ppm (part per million). During ore beneficiation, 40% of the REEs get into the waste clay (phosphatic clay or slimes), 10% into sand tailings, and the remaining 50% into phosphate concentrate. In the wet-process, 37.5% of the total REEs end up in PG, and 12.5% enters into phosphoric acid and fertilizer.
The Florida Industrial and Phosphate Research Institute, as a member of the Critical Materials Institute, is conducting a systematic study on recovering REEs from phosphate rock mining and processing streams: phosphatic clay, flotation tailings, phosphoric acid, PG, and phosphoric sludge. This paper focused on the leaching of REEs and phosphorus from flotation tailings of Florida phosphate rock.
2. Materials and Methods
2.1. Flotation Tailings Sample and Chemical Reagents
Approximately 1000 kg of representative flotation tailings were provided by Mosaic Company, the largest manufacturer of phosphate fertilizer in Florida. The flotation tailings were first completely mixed, and then they were divided repeatedly by the mixing-coning-quartering method to get an aliquot portion of approximately 1.2 kg. The portion was ground using a ball mill to ensure all the particle sizes were below 200 mesh. Finally, three duplicate analysis samples were taken from the ground tailings. Analyses showed that the total REE and phosphorus contents in the tailings were 162.80 ppm and 2.92% (P2O5), respectively.
In order to reduce the amount of gangue in the tailings, the sample was beneficiated first using a shaking table in our laboratory, and a concentrate was obtained. After being mixed thoroughly, the shaking table concentrate was divided into aliquot portions of 400 g each for the leaching experiment. Results of the shaking table separation are presented in Table 1.

Table 1.
Results of shaking table separation.
Mineralogical examination of the shaking table concentrate indicated that the main phosphorus-bearing mineral was francolite, which was white to tan and dark gray and contained nearly 95% of the phosphorus in the sample. Quartz was the principle gangue mineral, and small quantities of calcite, dolomite, albite, and potassium feldspar were also found. Monazite and xenotime existed in the sample in a minute amount. Contents of the main chemical components and REEs in the concentrate are shown in Table 2, and particle-size distribution is presented in Table 3.

Table 2.
Main chemical components and rare earths in the shaking table concentrate.

Table 3.
Particle-size distribution of the shaking table concentrate.
REE stock solutions for analysis were purchased from Fisher (REE concentration in each solution 1000 ppm, Hampton, NH, USA). Deionized water was used to prepare the leaching solution and wash residue. All reagents used in the leaching test were reagent grade.
2.2. Leaching Studies
The dihydrate process of phosphoric acid production was simulated in a 2.5 L water-bath batch reactor, and the effects of four main factors on REE and phosphorus leaching efficiencies were investigated, including phosphoric acid addition in the initial leaching pulp, leaching temperature, stoichiometric ratio of H2SO4 to calcium oxide (CaO), and the weight ratio of liquid to solid. In each leaching test, 400 g of the shaking table concentrate was used, and the solution was sampled every 15 min to monitor the leaching development through chemical analysis. After leaching, the pulp was filtered and the residue was washed three times. An Inductively Coupled Plasma Mass Spectrometry (ICP-MS, PerkinElmer, Waltham, MA, USA) was used to analyze the REEs, CaO, magnesium oxide (MgO), iron oxide (Fe2O3), and aluminum oxide (Al2O3) in both solution and residue; P2O5 was determined using aspectrophotometer. For any leaching test, if the relative deviations of the mass balances for total REEs and P2O5 were more than 3.0% and 2.0% respectively, the test had to be re-conducted.
3. Results
3.1. Effect of Leaching Time
First, three leaching experiments were conducted under different conditions (among the ranges to test) to determine the proper leaching time for each test. It was found that after leaching for 165 min the concentrations of the main REEs, Y (Yttrium), La (Lanthanum), Ce (Cerium), and Nd (Neodymium), as well as REEs (total REEs) and P2O5 in the pulp has all almost reached their maximums; after leaching for 180 min, all concentrations of these species leveled off. Thus, the leaching time for subsequent tests was set at 180 min.
3.2. Effect of Initial Phosphoric Acid Concentration in Pulp
Phosphoric acid can attack phosphate rock to produce soluble monocalcium phosphate, resulting in the calcium and phosphorus being released from ore into solution. Studies [14,18] showed that adding phosphoric acid to pulp is beneficial for both P2O5 and REE leaching. Therefore, in this study, phosphoric acid was used as an auxiliary lixiviant, and a series of tests were carried out under the condition of a P2O5 concentration ranging from 0 to 20% in the initial pulps. The results in Figure 1 show that the leaching efficiencies for REEs and phosphorus increased first from approximately 26 to 62% and from 81 to 91%, respectively, with the initial phosphoric acid content ranging from 0 to 16%, and accordingly the REE concentration rose from about 65 to 161 ppm in the leachate. After this, all the leaching efficiencies dropped, which can be attributed to the impedance of the high phosphoric acid concentration in the solution to the sulfuric acid attacking on phosphorite. Thus, the phosphoric acid addition was set at a P2O5 content of 16% in the initial pulp in the following leaching experiments.
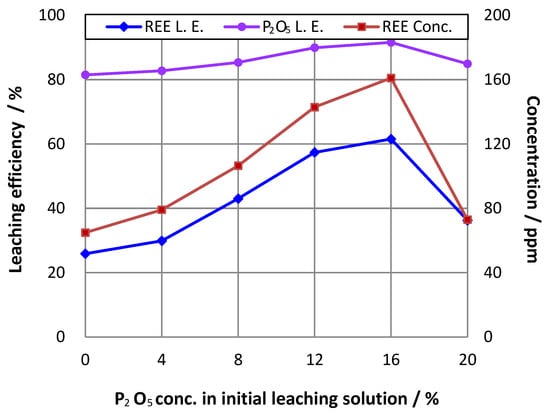
Figure 1.
Effect of phosphoric acid concentration in initial pulp on leaching efficiency (L.E.) (leaching temperature = 75 °C; stoichiometric ratio of H2SO4 to CaO = 1.2; Ratio of liquid to solid = 3.5).
3.3. Effect of Temperature
Increasing the temperature can accelerate chemical reactions in the leaching system, but to get high leaching efficiencies, the fluorapatite digestion rate should match well with the gypsum precipitation rate. With the leaching taking place, more and more Ca2+ ions were released from phosphate minerals. When the ion product of Ca2+ and SO42− exceeded the solubility product of gypsum, they start to build up gypsum crystals. In the case of too high a temperature, the crystallization speed inevitably reached a high level, which led to more REE and HPO42− ions wrapped into the gypsum crystal lattice, resulting in low leaching efficiencies of REEs and P2O5. Moreover, if the mineral decomposition rate is higher than the crystallization rate, Ca2+ will gradually accumulate in the solution to a high concentration, which will impede the migration of newly released Ca2+ from phosphate minerals and cause a serious problem that Ca2+ and SO42− form a gypsum crystal coating on phosphate mineral particle surfaces to prevent the mineral from being further attacked by sulfuric acid. Conversely, a low temperature is not conducive to sulfuric acid attacking the refractory phosphate minerals, also leading to low leaching efficiencies.
The effect of temperature on REE and phosphorus leaching efficiencies was tested within the range of 65–85 °C. It can be seen in Figure 2, that the REE leaching efficiency shows a similar trend as that of P2O5, and as the temperature increased, their leaching efficiencies initially rose to high values of approximately 62% and 91%, respectively, at 75 °C, and then dropped. As a result, the leaching temperature was set at 75 °C in the following tests.
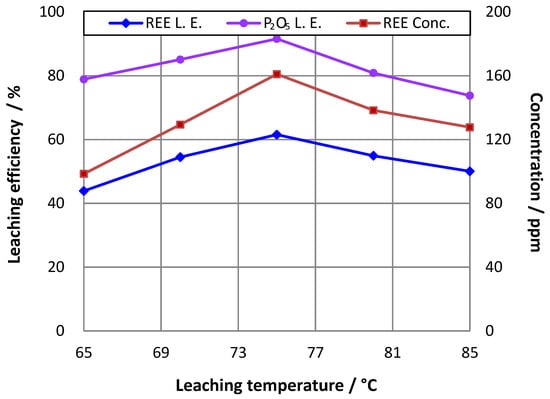
Figure 2.
Effect of temperature on rare earth leaching efficiency (L.E.) (16% P2O5 in initial leaching pulp; stoichiometric ratio of H2SO4 to CaO = 1.2; ratio of liquid to solid = 3.5).
3.4. Effect of H2SO4/CaO Ratio
As is shown in Equations (1)–(3), H2SO4 plays an essential role in fluorapatite and REE mineral decompositions. Therefore, it is necessary to add enough H2SO4 to the system to make sure that the phosphate is digested as much as possible. However, increasing the stoichiometric ratio of H2SO4 to CaO enhances the sulfuric acid concentration in the system, which will speed up mineral digestion and thus influence calcium sulfate crystallization, just as temperature does. With better crystallization, there is less eutectic crystallization and a smaller loss of REEs and phosphorus in the calcium sulfate crystal lattice [1].
The testing results in Figure 3 show that at a ratio of H2SO4 to CaO of 1.1, both REE and phosphorus leaching efficiencies reached high levels, very close to the maximums of approximately 62% and 91% at the ratio of 1.2, respectively. In order to avoid wasting of sulfuric acid, in the following experiments, the stoichiometric ratio of H2SO4 to CaO was set at 1.1 instead of 1.2.
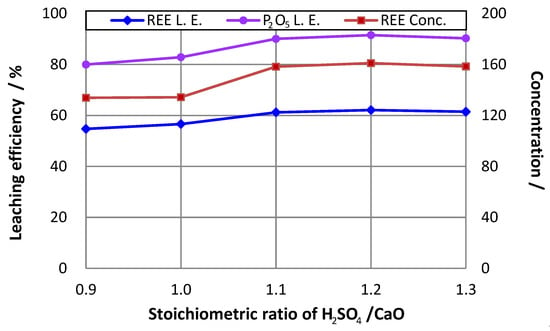
Figure 3.
Effect of stoichiometric ratio of H2SO4 to CaO on rare earth leaching efficiency (L.E.) (16% P2O5 in the initial leaching pulp; leaching temperature = 75 °C; ratio of liquid to solid = 3.5).
3.5. Effect of Weight Ratio of Liquid to Solid
Increasing the ratio of liquid to solid will lead to two competing effects on the leaching efficiency. On one hand, it can dilute the solution thereby accelerating the diffusion of leached products. This avoids the formation of a gypsum crystal coating on the surface of phosphate mineral to prevent further digestion, which is helpful for increasing leaching efficiency. On the other hand, increasing the ratio of liquid to solid will lower the sulfuric acid concentration in solution, which can reduce the ability of the acid to dissolve the phosphate minerals.
The influence of the liquid to solid weight ratio on REE and phosphorus leaching efficiencies was tested within the range of 2.5–4.5. The results in Figure 4 show that the REE and phosphorus leaching efficiencies increased initially as the ratio rose until they reached maximums of approximately 61% and 91%, respectively, at a ratio of liquid to solid of 3.5, before both following the opposite trend.
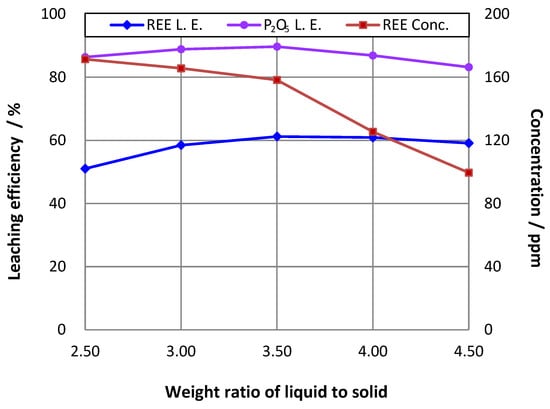
Figure 4.
Effect of ratio of liquid to solid on rare earth leaching efficiency (L.E.) (16% P2O5 in the initial leaching pulp; leaching temperature = 75 °C; stoichiometric ratio of H2SO4 to CaO = 1.1).
4. Discussion
Our tests indicated that the REEs and phosphorus in the flotation tailings of Florida phosphate rock could be highly efficiently concentrated using a shaking table, with their enrichment ratio reaching 5.4 (REEs) and 3.9 (P2O5). Under certain conditions of the dihydrate wet-process, the REEs and phosphorus in the shaking table concentrate were leached out with high leaching efficiencies of approximately 61% and 90%, respectively. During leaching, the REEs showed similar leaching efficiency to each other, especially for the four high-content elements, lanthanum (La), cerium (Ce), neodymium (Nd), and yttrium (Y), and their leaching efficiencies presented similar trends to those of phosphorus.
The REE state of existence in the leaching solution is complicated. Analyses by Liang et al. [18] demonstrated that in wet-process phosphoric acid production, the amount of REEs leached out from the original phosphate minerals were actually much higher than that detected in the leaching solution, but some of them got into the PG crystal lattice as substitutes for Ca2+ and some combined with PO43− or F− to form LnPO4 or LnF3 (Ln donates any REE) and finally precipitated into the leaching residue. In the leaching solution, the main anions include SO42−, F− and PO43−; PO43− is one of the most important determining anions for REE existence in leaching solution, because LnPO4 has a poor solubility and its solubility product (Ksp = [Ln3+][PO43−]) is as low as the level of 10−25–10−27.
The concentration of PO43− in the leaching solution depends on the ionization equilibrium of phosphoric acid. In aqueous solution, phosphoric acid ionizes in the following three steps (25 °C) [26]:
H3PO4(aq) + H2O(l) ⇄ H3O+(aq) + H2PO4−(aq) Ka1 = 7.11 × 10−3
H2PO4−(aq) + H2O(l) ⇄ H3O+(aq) + HPO42−(aq) Ka2 = 6.34 × 10−8
HPO42−(aq) + H2O(l) ⇄ H3O+(aq) + PO43−(aq) Ka3 = 4.17 × 10−13
The relationship of the phosphoric acid dissociation constant Ka1 >> Ka2 >> Ka3 indicates that in aqueous solution phosphorous exists mostly as H3PO4 and H2PO4−. Therefore, the concentrations of [H3PO4], [H2PO4−], [HPO42−], [PO43−], and [H3O+] in solution can be expressed as follows:
where, CT is the total phosphorus concentration in mol/L. In order to derive [PO43−], the contents of CT and [H3O+] need to first be determined.
[H3PO4] = CT − [H2PO4−] − [HPO42−] − [PO43−] ≈ CT − [H2PO4−]
[H2PO4−] = Ka1 × [H3PO4]/[H3O+]
[HPO42−] = Ka2 × [H2PO4−]/[H3O+]
[PO43−] = Ka3 × [HPO42−]/[H3O+]
Taking the test with better leaching results as an example (initial phosphoric acid concentration 16%, temperature 75 °C, stoichiometric ratio of H2SO4 to CaO = 1.1, weight ratio of liquid/solid = 3.5), the CT in the final solution was analyzed as 3.248 mol/L (P2O5 19.81 wt %; solution density 1.164 g/cm3), and P2O5 leaching efficiency reached 90.04%. Since the Ka1, Ka2, and Ka3 in Equation (4)–(6) are relatively small, almost all of the H3O+ in the leaching solution came from the redundant of sulfuric acid. Analysis results showed that some MgO, Fe2O3, and Al2O3 (respectively representing actual Mg-bearing minerals, Fe-bearing minerals, and Al-bearing minerals) reacted during the leaching test and they consumed 0.056 mol of sulfuric acid in total. Although the consumption of sulfuric acid in phosphate rock digestion can be calculated based on Equation (1) and the phosphorus leaching efficiency, it is hard to accurately determine the total amount of sulfuric acid that reacted with calcium-bearing minerals during leaching, because there was about 0.094 mol CaO (accounting for 7.07% of the total) which did not exist in fluorapatite (calculated on the basis of stoichiometric ratio of CaO to P2O5 in fluorapatite, and the analyzed results in Table 1). Assuming that all the Ca-bearing minerals were digested, there would be 0.201 mol sulfuric acid remaining in the solution. If only the CaO in phosphorite was leached out with phosphorus, the sulfuric acid left would be 0.295 mol. Based on Equation (1), it can be calculated that the solution weight was reduced from an initial 1400 g to about 1330 g after leaching (a phosphorus leaching efficiency of 90%). Therefore, the H3O+ concentration in the leaching solution should be in the range of 0.301 to 0.442 mol/L. Inputting the values for CT, ka1, ka2, ka3, and [H3O+] into the simultaneous Equations (9)–(12), the [PO43−] was calculated to be in the range of 0.70 × 10−20 to 2.18 × 10−20 mol/L.
By applying solubility products of LnPO4, the REE equilibrium concentration was calculated in a LnPO4-H3PO4 aqueous solution with the same [PO43−] as the leaching solution, i.e., in the range of 0.70 × 10−20 to 2.18 × 10−20 mol/L, the results of which are listed in Table 4 with the analysis results of REE concentration in our leaching solution.

Table 4.
Rare earth element (REE) concentration in different dissociation-precipitation equilibrium solution (25 °C).
Comparing the calculated values of REEs with the analyzed results in Table 4, it can be seen that the concentration for most REEs in the leaching solution is much higher than that in a LnPO4-H3PO4 aqueous solution except for the elements of Sm, Tb, Ho, Tm, and Lu due to their very low contents in both the shaking table concentrate and the leaching solution. For those high content elements Y, La, Ce, and Nd, their concentration in leaching solution is up to more than 40, 67, 402, and 297 times higher, respectively, than that in a LnPO4-H3PO4 aqueous solution. These discrepancies can be attributed to the large amount of anions in the leaching solution such as SO42−, F−, etc. that could present a strong steric hindrance to prevent PO43− from contacting and combining with REE ions. As a result, a greater proportion of REE ions remained in the leaching solution. However, the concentration of REEs in the solution was only 155.6 ppm. As an inevitable by-product in wet-process phosphoric acid production, in recent years the recovery of REEs in the phosphate rock leaching solution has attracted more and more attention.
On the other hand, the P2O5 concentration in our leaching solution was about 18%, much lower than the common concentration in the leaching solution of current wet-process phosphoric acid production (~30%). However, it is still possible to recover this part of phosphoric acid in an economically viable way. As we know, in wet-process phosphoric acid production practice, the leaching residue is usually washed three times to recover the phosphoric acid left in it, and the washing liquid containing 16 to 18% P2O5 is recycled to the leading end of the leaching tank [1]. Since the P2O5 concentration in the washing liquid is similar to that in our initial leaching pulp (16% P2O5), it can be predicted that the washing liquid with enough sulfuric acid addition can be used to leach the shaking table concentrate, and the P2O5 and REE leaching efficiencies could reach up to 90% and 61%, respectively, under certain conditions. After leaching, the solution can be returned to the industrial system. Thus, this research provides a promising pathway to recover both phosphorous and REEs from the flotation tailings by shaking table beneficiation and leaching.
In addition, the content of uranium (U) and thorium (Th) was analyzed and found to be 75.78 and 0.00 ppm by ICP-MS in the shaking table concentrate. After leaching, more than 80% of the U got into the leaching residue. No radioactivity was detected in this research, probably due to the very low contents of U and Th.
5. Conclusions
Laboratory tests showed that the REEs and phosphorus in the flotation tailings of Florida phosphate rock could be highly efficiently concentrated by shaking table separation, and REE and phosphorus leaching from the shaking table concentrate was affected significantly by four major factors including phosphoric acid concentration in the initial pulp, leaching temperature, sulfuric acid addition, and weight ratio of liquid to solid. The ranges of leaching parameters tested were as follows: Phosphoric acid concentration in the initial leaching pulp was 0–20%, temperature 65–85 °C, stoichiometric ratio of H2SO4 to CaO of 0.9–1.3, and weight ratio of liquid to solid of 2.5–4.5. High REE and phosphorus leaching efficiencies of ~61% and 90% respectively were achieved at an initial phosphoric acid concentration of 16%, temperature 75 °C, H2SO4/CaO 1.1, and weight ratio of liquid to solid of 3.5. In all the leaching tests, REE leaching efficiency showed similar trends to those of phosphorus.
Analyses indicated that PO43− in the leaching solution tended to combine with REE ions to form REE phosphates which would precipitate into the leaching residue. However, the large amount of anions in the system such as SO42−, F−, etc. took advantage of steric hindrance to prevent PO43− from contacting with REE ion. These two opposite effects determined the REE distribution between the leaching solution and residue (PG).
Authors Contributions
Conceptualization, P.Z. and D.W.D.; Methodology, P.Z. and H.L.; Validation, P.Z., H.L. and Z.J.; Formal Analysis, Z.J.; Investigation, H.L.; Resources, P.Z.; Data Curation, P.Z.; Writing-Original Draft Preparation, H.L.; Writing-Review & Editing, P.Z.; Supervision, P.Z.; Project Administration, P.Z.; Funding Acquisition, D.W.D.
Funding
This research is part of a major project of the Critical Materials Institute (CMI), under subcontract number SC-14-392 funded by the US Department of Energy, Office of Energy Efficiency and Renewable Energy, Advanced Manufacturing Office.
Acknowledgments
The guidance and leadership of this research provided by Bruce Moyer, CMI area lead, and David W. DePaoli, CMI project lead, are highly appreciated. Substantial matching fund is provided by the Florida Industrial and Phosphate Research Institute, Florida Polytechnic University. The Mosaic Co., is particularly acknowledged for their technical input, large in-kind support, and sample collection efforts. We want to express our gratitude to the following Mosaic employees and former employees for their help: Nicole Christiansen, Paul Kucera, Marcos Ortiz, Chris Dennis, Glen Oswald, Chaucer Hwang, Cameron Weed, and Gary Whitt.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Becker, P. Phosphates and Phosphoric Acid: Raw Materials, Technology, and Economics of the Wet Process; Marcel Dekker: New York, NY, USA, 1983. [Google Scholar]
- Zhang, P. Comprehensive recovery and sustainable development of phosphate resources. Procedia Eng. 2014, 83, 37–51. [Google Scholar] [CrossRef]
- Pan, Y.; Fleet, M.E.; Macrae, N.D. Oriented monazite inclusions in apatite porphyroblasts from the Hemlo gold deposit, Ontario, Canada. Mineral. Mag. 1993, 57, 697–708. [Google Scholar] [CrossRef]
- Schoneveld, L.; Spandler, C.; Hussey, K. Genesis of the central zone of the Nolans Bore rare earth element deposit, Northern Territory, Australia. Contrib. Mineral. Petrol. 2015, 170, 11. [Google Scholar] [CrossRef]
- Jasinski, S.M. Mineral Commodity Summaries 2017, Phosphate Rock; US Geological Survey: Reston, VA, USA, 2017; pp. 124–125.
- Beltrami, D.; Cote, G.; Mokhtari, H.; Courtaud, B.; Moyer, B.A.; Changes, A. Recovery of uranium from wet phosphoric acid by solvent extraction processes. Chem. Rev. 2014, 114, 12002–12023. [Google Scholar] [CrossRef] [PubMed]
- Wamser, C.; Bruen, C. Recovery of Fluorine, Uranium and Rare Earth Metal Values from Phosphoric Acid by-product Brine Raffinate. U.S. Patent 3,937,783, 21 February 1974. [Google Scholar]
- Bunus, F.; Dumitrescu, R. Simultaneous extraction of rare earth elements and uranium from phosphoric acid. Hydrometallurgy 1992, 28, 331–338. [Google Scholar] [CrossRef]
- Bunus, F.; Miu, I.; Dumitrescu, R. Simultaneous recovery and separation of uranium and rare earths from phosphoric acid in one-cycle extraction-stripping process. Hydrometallurgy 1994, 35, 375–389. [Google Scholar] [CrossRef]
- Bunus, F. Uranium and rare earth recovery from phosphate fertilizer industry by solvent extraction. Miner. Proc. Extr. Metall. Rev. 2000, 21, 381–478. [Google Scholar] [CrossRef]
- Preston, J.S.; Cole, P.M.; Craig, W.M.; Feather, A.M. The recovery of rare earth oxides from a phosphoric acid by-product. Part 1: Leaching of rare earth values and recovery of a mixed rare earth oxide by solvent extraction. Hydrometallurgy 1996, 41, 1–19. [Google Scholar]
- Koopman, C.; Witkamp, G.J. Extraction of lanthanides from the phosphoric acid production process to gain purified gypsum and a valuable lanthanide by-product. Hydrometallurgy 2000, 58, 51–60. [Google Scholar] [CrossRef]
- Krea, M.; Khalaf, H. Liquid-liquid extraction of uranium and lanthanides from phosphoric acid using a synergistic DOPPA-TOPO mixture. Hydrometallurgy 2000, 58, 215–225. [Google Scholar] [CrossRef]
- Wang, L.; Long, Z.; Huang, X.; Yu, Y.; Cui, D.; Zhang, G. Recovery of rare earths from wet-process phosphoric acid. Hydrometallurgy 2010, 101, 41–47. [Google Scholar] [CrossRef]
- Zhang, P. Recovery of critical elements from Florida phosphate: Phase 1. Characterization of rare earths. In Proceedings of the ECI International Conference: Rare earth Minerals/Metals–Sustainable Technologies for the Future, San Diego, CA, USA, 12–17 August 2012. [Google Scholar]
- Zhang, P.; Liang, H.; Jin, Z.; DePaoli, D. The ultimate mineral processing challenge: Recovery of rare earths, phosphorus and uranium from Florida phosphatic clay. Miner. Metall. Process. 2017, 34, 183–188. [Google Scholar] [CrossRef]
- Massari, S.; Ruberti, M. Rare earth elements as critical raw materials: Focus on international markets and future strategies. Resour. Policy 2013, 38, 36–43. [Google Scholar] [CrossRef]
- Liang, H.; Zhang, P.; Jin, Z.; DePaoli, D. Rare-earth leaching from Florida phosphate rock in wet-process phosphoric acid production. Miner. Metall. Process 2017, 34, 146–153. [Google Scholar] [CrossRef]
- Liang, H.; Zhang, P.; Jin, Z.; DePaoli, D.W. Rare earths recovery and gypsum upgrade from Florida phosphogypsum. Miner. Metall. Process 2017, 34, 201–206. [Google Scholar] [CrossRef]
- Kanazawa, Y.; Kamitani, M. Rare earth minerals and resources in the world. J. Alloy. Compd. 2006, 408, 1339–1343. [Google Scholar]
- Poul, E.; Mclauglin, I.P.; Breit, N.G.; Bray, A.E.; Koenig, E.A. Rare earth elements in sedimentary phosphate deposits: Solution to the global REE crisis? Gondwana Res. 2015, 27, 776–785. [Google Scholar]
- Zhang, P.; Yu, Y.; Bogan, M. Challenging the “Crago” double float process ii. Amine-type-fatty acid flotation of silicious phosphates. Miner. Eng. 1997, 10, 983–994. [Google Scholar] [CrossRef]
- Claude, L.W.; Henry, M.J.R. Method for determination of small amounts of rare earths and thorium in phosphate rocks. Anal. Chem. 1953, 25, 432–435. [Google Scholar]
- Giesekke, E.W. Florida Phosphate Rock. In SME Mineral Processing Handbook; Weiss, N.L., Ed.; Society of Mining Engineers of the American Institute of Mining, Metallurgical, and Petroleum Engineers: Englewood, IN, USA, 1985; pp. 1–18. [Google Scholar]
- Kremer, R.A.; Chokshi, J.C. Fate of Rare Earth Elements in Mining/Beneficiation of Florida Phosphate Rock and Conversion to DAP Fertilizer; Research Report; Mobil Mining and Minerals Company: Nichols, FL, USA, 1989.
- Firsching, F.H.; Brune, S.N. Solubility products of the trivalent rare-earth phosphates. J. Chem. Eng. 1991, 36, 93–95. [Google Scholar] [CrossRef]
- Liu, X.; Byrne, R.H. Rare earth and yttrium phosphate solubilities in aqueous solution. Geochim. Cosmochim. Acta 1997, 61, 1625–1633. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).