Aurihydrargyrumite, a Natural Au6Hg5 Phase from Japan
Abstract
:1. Introduction
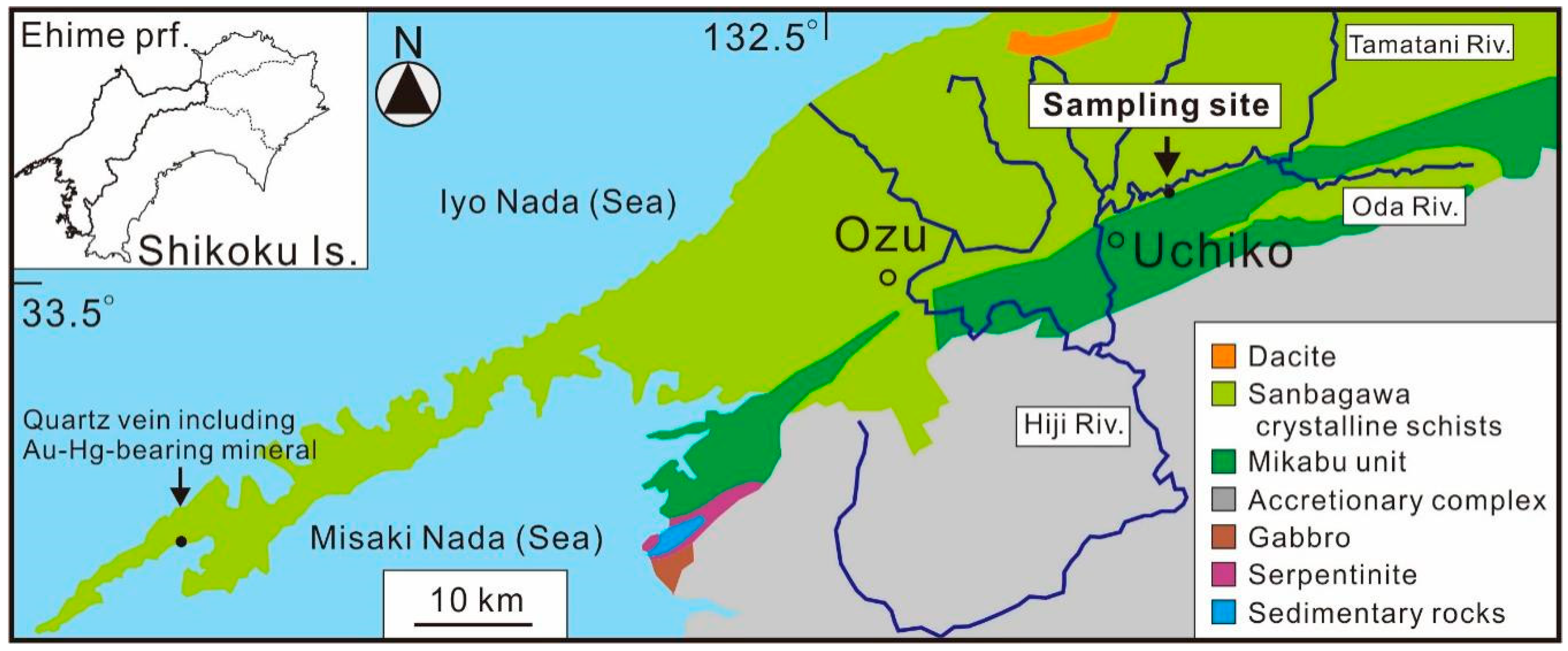
2. Occurrence
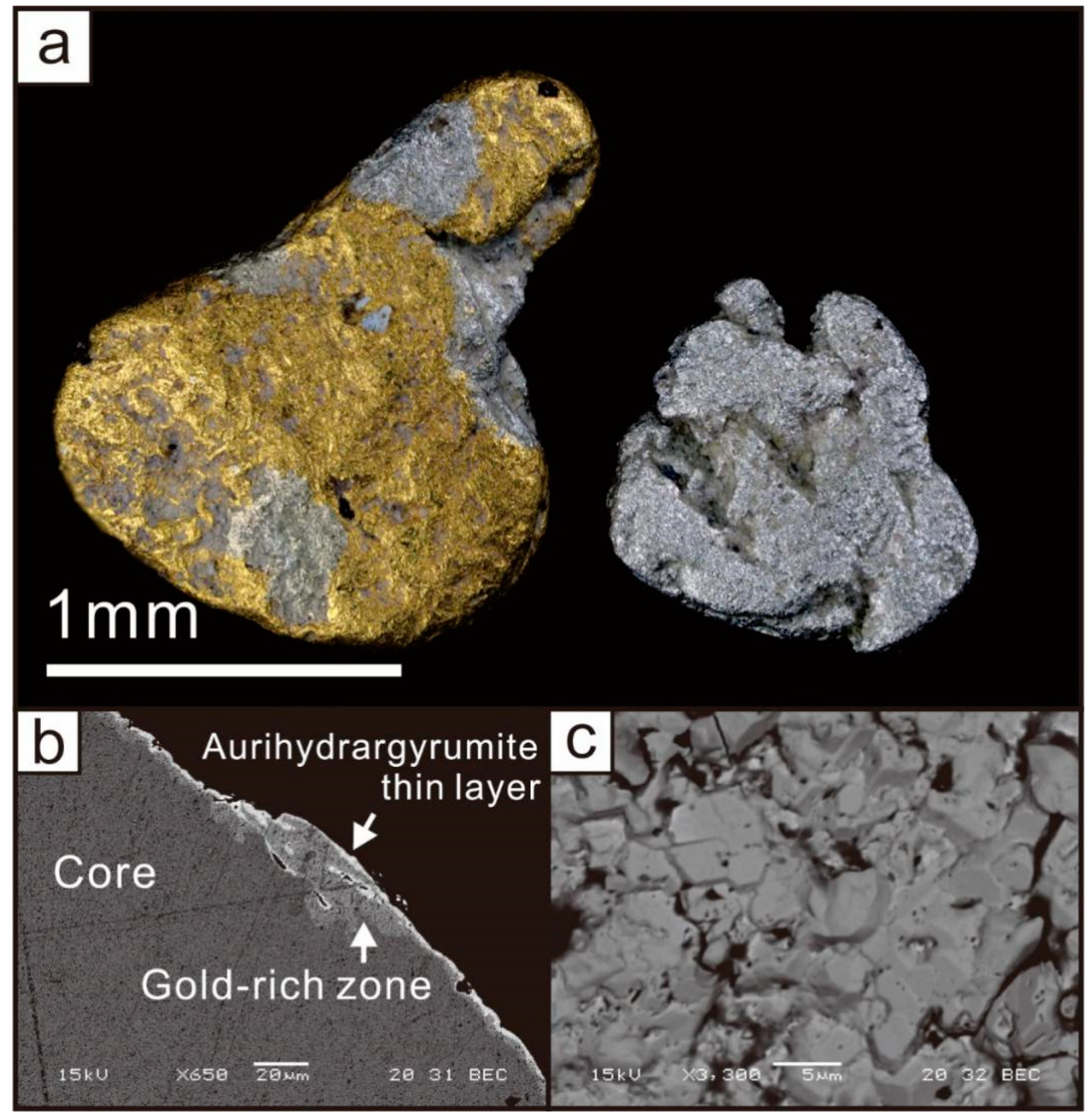
3. Appearance and Physical Properties
4. Chemical Composition
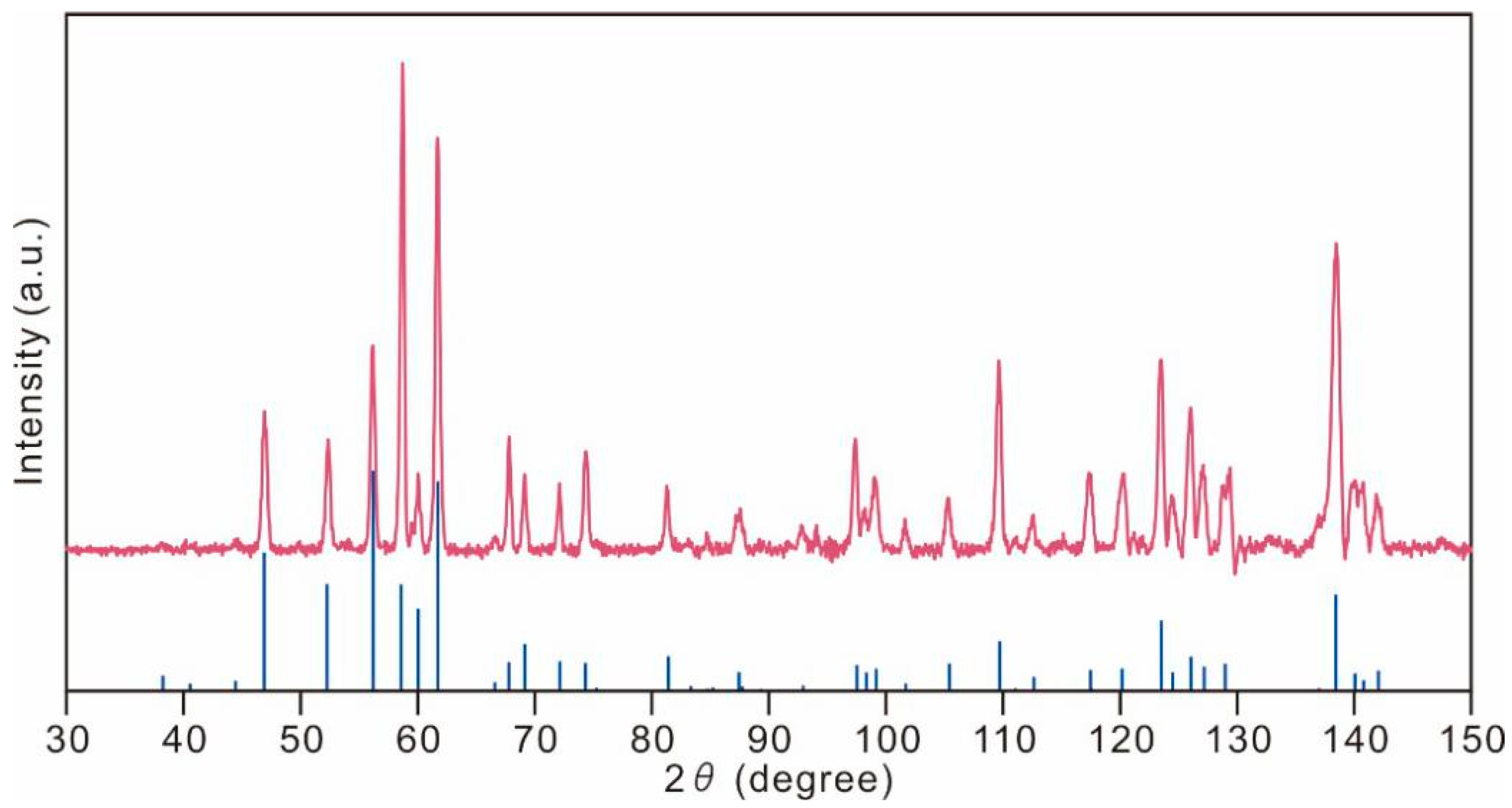
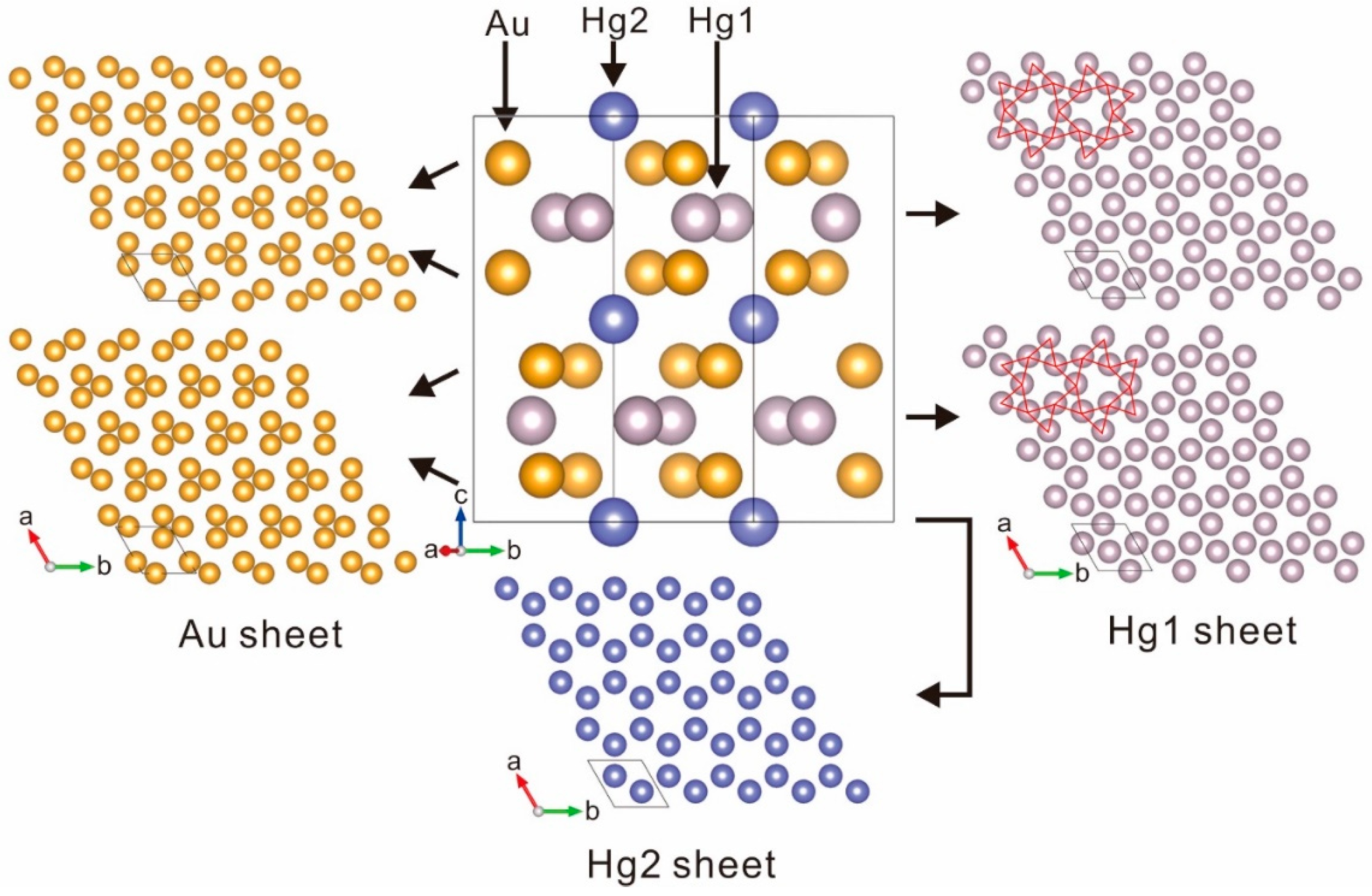
5. X-ray Crystallography
6. Relation to Other Species
7. Discussion
8. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Okamoto, H.; Massalski, T.B. Au-Hg (Gold-Mercury). In Binary Alloy Phase Diagrams, 2nd ed.; Massalski, T.B., Ed.; ASM International: Materials Park, OH, USA, 1990; Volume 1, pp. 376–379. [Google Scholar]
- Lindahl, T. Crystal structure of Au6Hg5. Acta Chem. Scand. 1970, 24, 946–952. [Google Scholar] [CrossRef]
- Atanasov, V.; Iordanov, I. Amalgams of gold from the Palakharya River alluvial sands, Distinct of Sofia. Doklady Bolgarskoj Akademii Nauk. 1983, 36, 465–468. [Google Scholar]
- Atanasov, V.; Jordanov, J.A.; Vitov, O.C.; Atanasov, A.V. Part I: Geology. In Amalgams of Gold in Some of the Bulgarian Alluvial Sands; Higher Institute of Mining and Geology: Sofia, Bulgaria, 1988; Volume 34, pp. 227–238. [Google Scholar]
- Li, Y.; Ouyang, S.; Tian, P. Weishanite—A new gold-bearing mineral. Acta Mineral. Sin. 1984, 4, 102–105. [Google Scholar]
- Desborough, G.A.; Foord, E.E. A monoclinic, pseudo-orthorhombic Au-Hg mineral of potential economic significance in Pleistocene Snake River alluvial deposits of southeastern Idaho. Can. Mineral. 1992, 30, 1033–1038. [Google Scholar]
- Barkov, A.Y.; Nixon, G.T.; Levson, V.M.; Martin, R.F. A cryptically zoned amalgam (Au1.5-1.9Ag1.1-1.4)Σ2.8-3.0Hg1.0-1.2 from a placer deposit in the Tulameen-Similkameen river system, British Columbia, Canada: Natural or Man-made? Can. Mineral. 2009, 47, 433–440. [Google Scholar] [CrossRef]
- Svetlitskaya, T.V.; Nevolko, P.A.; Kolpakov, V.V.; Tolstykh, N.D. Native gold from the Inagli Pt-Au placer deposit (the Aldan Shield, Russia): Geochemical characteristics and implications for possible bedrock sources. Miner. Depoz. 2018, 53, 323–338. [Google Scholar] [CrossRef]
- Shikazono, N.; Shimizu, M. Electrum: Chemical Composition, Mode of Occurrence, and Depositional Environment; The University Museum, University of Tokyo: Tokyo, Japan, 1988; Volume 32, pp. 1–81. [Google Scholar]
- Yokoyama, K.; Takeuchi, S.; Nakai, I.; Tsutsumi, Y.; Sano, T.; Shigeoka, M.; Miyawaki, M.; Matsubara, M. Chemical Compositions of Electrum Grains in Ore and Placer Deposits in the Japanese Islands; National Museum of Nature and Science Monographs: Tokyo, Japan, 2011; Volume 42, pp. 1–80. [Google Scholar]
- Miyahisa, M.; Higaki, J. Gold-Silver Mineral Resource of Ehime Prefecture. Ehime-Ken. Chika. Shigen. Shiryo. 1981, 10, 11–24. [Google Scholar]
- Rolfe, C.; Hume-Rothery, W. The constitution of alloys of gold and mercury. J. Less-Common Met. 1967, 13, 1–10. [Google Scholar] [CrossRef]
- Groen, J.C.; Craig, J.R.; Rimstidt, J.D. Gold-rich rim formation on electrum grains in placers. Can. Mineral. 1990, 28, 207–228. [Google Scholar]
| Aurihydrargyrumite | Gold-Rich Zone | Core | ||
|---|---|---|---|---|
| Mean 5 (Range) wt.% | Ideal | Mean 5 (Range) wt.% | Mean 5 (Range) wt.% | |
| Au | 54.92 (54.26–55.76) | 54.09 | 96.82 (95.47–98.73) | 88.20 (88.15–88.87) |
| Ag | - | - | - | 9.90 (9.83–10.04) |
| Hg | 47.50 (46.54–48.91) | 45.91 | 2.96 (1.41–4.60) | 1.69 (1.28–2.17) |
| Total wt.% | 102.42 | 100 | 99.78 | 99.79 |
| pfu | pfu | pfu | ||
| Au | 5.95 | 6 | 97.09 | 81.72 |
| Ag | - | - | - | 16.75 |
| Hg | 5.05 | 5 | 2.91 | 1.53 |
| Σ | 11 | 11 | 100 | 100 |
| Aurihydrargyrumite | Synthetic Au6Hg5 [2] | ||||
|---|---|---|---|---|---|
| hkl | dobs. (Å) | dcalc. (Å) | I/I0 | dcalc. (Å) | Icalc. |
| 100 | 6.059 | 6.057 | 4 | ||
| 002 | 5.077 | 5.074 | <1 | ||
| 102 | 3.891 | 3.889 | <1 | ||
| 110 | 3.502 | 3.498 | 1 | 3.497 | 5 |
| 111 | 3.309 | 3.307 | 1 | 3.306 | 2 |
| 200 | 3.025 | 3.029 | 2 | 3.028 | 4 |
| 112 | 2.877 | 2.881 | 29 | 2.879 | 52 |
| 202 | 2.597 | 2.602 | 23 | 2.600 | 49 |
| 004 | 2.539 | 2.537 | <1 | ||
| 113 | 2.434 | 2.432 | 42 | 2.431 | 100 |
| 104 | 2.337 | 2.341 | 100 | 2.340 | 46 |
| 210 | 2.290 | 2.290 | 13 | 2.289 | 38 |
| 211 | 2.234 | 2.234 | 87 | 2.233 | 91 |
| 212 | 2.087 | 2.088 | 3 | 2.087 | 5 |
| 114 | 2.053 | 2.055 | 22 | 2.053 | 12 |
| 300 | 2.019 | 2.020 | 15 | 2.019 | 21 |
| 204 | 1.947 | 1.946 | 13 | 1.945 | 14 |
| 213 | 1.895 | 1.897 | 21 | 1.896 | 18 |
| 302 | 1.876 | 1.877 | 2 | 1.876 | 2 |
| 115 | 1.758 | 1.756 | 13 | 1.755 | 13 |
| 220 | 1.749 | 1.748 | <1 | ||
| 221 | 1.724 | 1.723 | 3 | ||
| 214 | 1.700 | 1.700 | 1 | ||
| 006 | 1.692 | 1.691 | 4 | ||
| 310 | 1.680 | 1.680 | <1 | ||
| 311 | 1.658 | 1.658 | 8 | 1.657 | 10 |
| 222 | 1.654 | 1.653 | 2 | ||
| 106 | 1.630 | 1.629 | 3 | ||
| 312 | 1.595 | 1.595 | 1 | ||
| 304 | 1.581 | 1.580 | 5 | 1.580 | 4 |
| 223 | 1.554 | 1.553 | <1 | ||
| 116 | 1.525 | 1.523 | 23 | 1.523 | 18 |
| 215 | 1.519 | 1.519 | <1 | ||
| 400 | 1.516 | 1.515 | 8 | 1.514 | 12 |
| 313 | 1.506 | 1.505 | 15 | 1.505 | 15 |
| 206 | 1.478 | 1.477 | 5 | 1.477 | 3 |
| 402 | 1.452 | 1.451 | <1 | ||
| 224 | 1.441 | 1.440 | 11 | 1.440 | 18 |
| 314 | 1.401 | 1.401 | 39 | 1.401 | 31 |
| 320 | 1.390 | 1.390 | 2 | ||
| 321 | 1.378 | 1.377 | 7 | 1.377 | 8 |
| 216 | 1.361 | 1.360 | 1 | ||
| 322 | 1.341 | 1.341 | 16 | 1.340 | 16 |
| 117 | 1.340 | 1.339 | 4 | ||
| 225 | 1.325 | 1.325 | 1 | ||
| 410 | 1.321 | 1.322 | 16 | 1.322 | 18 |
| 411 | 0.131 | 1.311 | 1 | ||
| 404 | 1.301 | 1.301 | 41 | 1.300 | 49 |
| 306 | 1.297 | 1.296 | <1 | ||
| 315 | 1.294 | 1.295 | 11 | 1.294 | 14 |
| 323 | 1.286 | 1.286 | 29 | 1.285 | 29 |
| 412 | 1.280 | 1.280 | 18 | 1.279 | 21 |
| 008 | 1.269 | 1.269 | 15 | 1.269 | 18 |
| 108 | 1.242 | 1.242 | <1 | ||
| 413 | 1.232 | 1.231 | 3 | ||
| 217 | 1.225 | 1.225 | 65 | 1.225 | 89 |
| 324 | 1.219 | 1.219 | 15 | 1.219 | 13 |
| 226 | 1.216 | 1.216 | 14 | 1.216 | 11 |
| 500 | 1.211 | 1.212 | 11 | 1.211 | 19 |
| 118 | 1.193 | 1.192 | 1 | ||
| Minerals | ||||||||||
| Name | Composition | Symmetry | a (Å) | b (Å) | c (Å) | α (°) | β (°) | γ (°) | V (Å3) | Reference |
| Aurihydrargyrumite | Au6Hg5 | P63/mcm | 6.996 | = a | 10.154 | 90 | 90 | 120 | 430.4 | This study |
| Weishanite | (Au,Ag)3Hg2 | P63/mmc | 2.9265 | = a | 4.8176 | 90 | 90 | 120 | 35.7 | [5] |
| UM1992-08-E:AuHg | Au94-88Hg6-12 | Monoclinic | 4.729 | 5.243 | 4.546 | 90 | 90.9 | 90 | 112.7 | [6] |
| Synthetic Phases | ||||||||||
| Name | Composition | Symmetry | a (Å) | b (Å) | c (Å) | α (°) | β (°) | γ (°) | V (Å3) | Reference |
| - | Au6Hg5 | P63/mcm | 6.9937 | = a | 10.148 | 90 | 90 | 120 | 430.4 | [2] |
| - | Au4Hg | Hexagonal | 8.736 | = a | 9.577 | 90 | 90 | 120 | 633 | [12] |
| - | Au3Hg | P63/mmc | 2.918 | = a | 4.8113 | 90 | 90 | 120 | 35.7 | [12] |
| - | Au2Hg | Hexagonal | 13.98 | = a | 17.2 | 90 | 90 | 120 | 2911.2 | [12] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishio-Hamane, D.; Tanaka, T.; Minakawa, T. Aurihydrargyrumite, a Natural Au6Hg5 Phase from Japan. Minerals 2018, 8, 415. https://doi.org/10.3390/min8090415
Nishio-Hamane D, Tanaka T, Minakawa T. Aurihydrargyrumite, a Natural Au6Hg5 Phase from Japan. Minerals. 2018; 8(9):415. https://doi.org/10.3390/min8090415
Chicago/Turabian StyleNishio-Hamane, Daisuke, Takahiro Tanaka, and Tetsuo Minakawa. 2018. "Aurihydrargyrumite, a Natural Au6Hg5 Phase from Japan" Minerals 8, no. 9: 415. https://doi.org/10.3390/min8090415
APA StyleNishio-Hamane, D., Tanaka, T., & Minakawa, T. (2018). Aurihydrargyrumite, a Natural Au6Hg5 Phase from Japan. Minerals, 8(9), 415. https://doi.org/10.3390/min8090415




