1. Introduction
Kurchatovite and clinokurchatovite are rare anhydrous Ca-Mg borates reported in skarns from several localities in Russia, Kazakhstan, Afghanistan and Japan [
1,
2,
3,
4].
Kurchatovite was discovered in a vesuvianite-garnet calc-silicate skarn in the Solongo boron and iron deposit, Buryatia (Siberia, Russia), in close association with sphalerite, magnetite and turneaurite-johnbaumite series arsenates [
1,
5,
6]. It was interpreted to have formed as an early mineral when boron was introduced with postmagmatic fluids. A wet chemical analysis after deduction of impurities gave the following formula calculated based on five oxygen atoms per formula unit (
pfu): Ca
0.955(Mg
0.816Mn
0.183Fe
0.040)
Σ1.039B
2.005O
5. Single-crystal X-ray diffraction study gave orthorhombic symmetry with
a = 11.15(0.02),
b = 36.4(0.1), and
c = 5.55(0.01) Å in the original description; whereas Yakubovich et al. [
7] gave the space group as
Pc2
1b when the structure was refined. The possibility of a monoclinic analogue was first suggested by syntheses [
8] and presence of twins [
9,
10,
11], and subsequently confirmed by single-crystal studies and crystal-structure refinements of synthetic and natural material, including a synthetic Ca-Mn analogue [
7,
12,
13,
14,
15], eventually leading to the formal description of clinokurchatovite as a new mineral [
16]. The type locality is the Sayak-IV gold and copper deposit, Balkhash Region, Kazakhstan, where clinokurchatovite occurs in skarn with calcite, harkerite, grossular-andradite garnet, magnetite and, locally, ludwigite. Other reliably reported localities of clinokurchatovite are the Novofrolovskoe copper deposit, Northern Urals, and the Titovskoe boron deposit, Polar Yakutia, Siberia, both in Russia [
1].
Kurchatovite from the Fuka mine, Okayama Prefecture, Japan, ranges in chemical composition (with negligible Mn) from the almost end member (2 mol % CaFeB
2O
5) to the Fe-dominant analogue (53 mol % CaFeB
2O
5); there is first report of a kurchatovite-type mineral with Fe > Mg [
4].
The crystal structures of kurchatovite and clinokurchatovite have been re-investigated by Callegari et al. [
17], who reported for kurchatovite the space group
Pbca. According to Yakubovich et al. [
7] and Belokoneva et al. [
18], the crystal structures of kurchatovite and clinokurchatovite could be considered as polytypes. Callegari et al. [
17] mentioned this hypothesis as a possibility, leaving it aside “to the experts in this manner”. However, if kurchatovite and clinokurchatovite are polytypes, then they should be considered as one mineral species existing in two polytypic varieties, which would warrant the discreditation of one of them and re-definition of the other.
The aim of the present paper is to report on the results of crystal-structure studies of kurchatovite and clinokurchatovite, to analyze their structural relations from the viewpoint of the concepts of polymorphism and polytypism, and to examine the validity of the two minerals as separate mineral species in the frame of actual approach accepted in mineral nomenclature.
3. Results
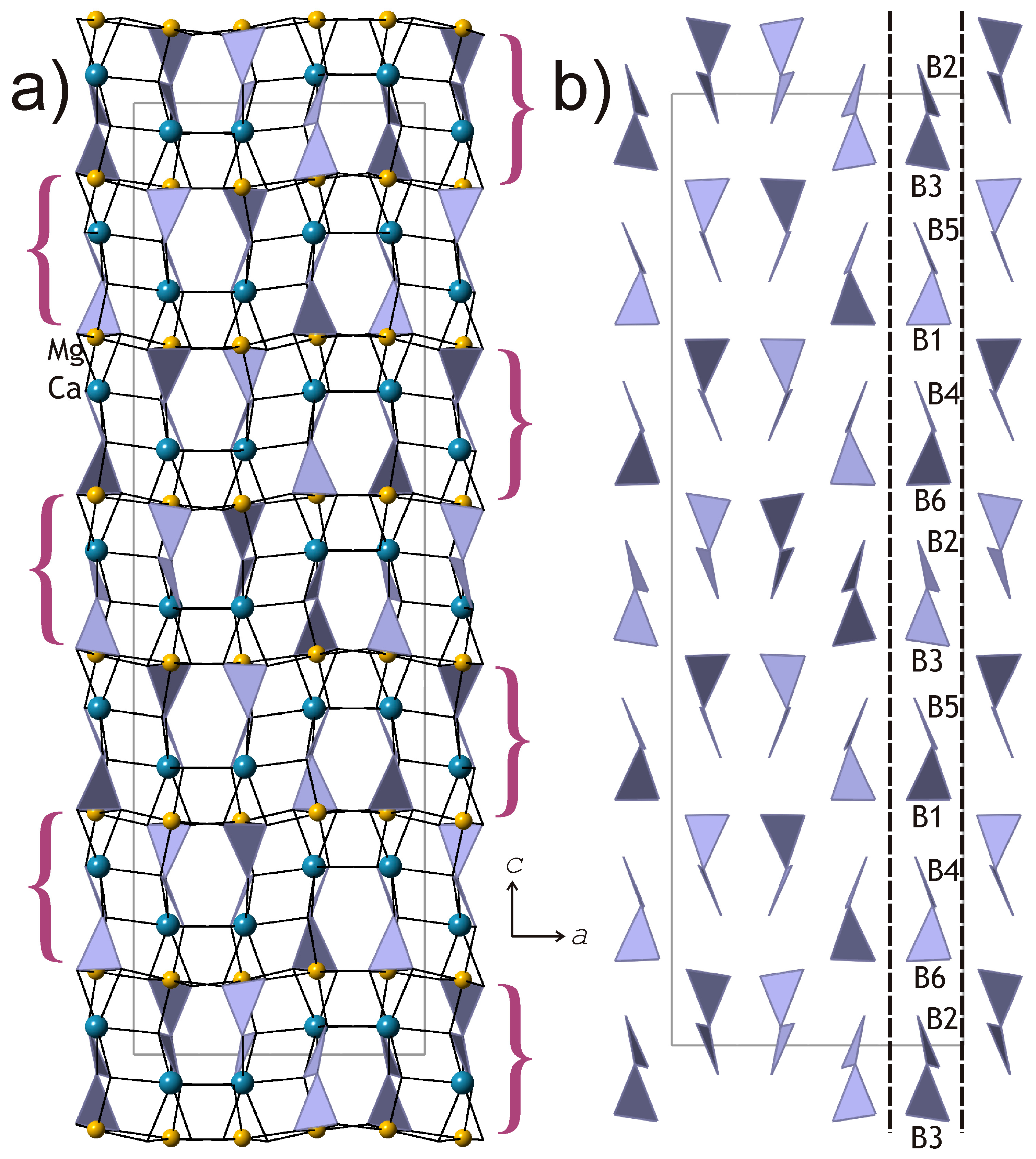
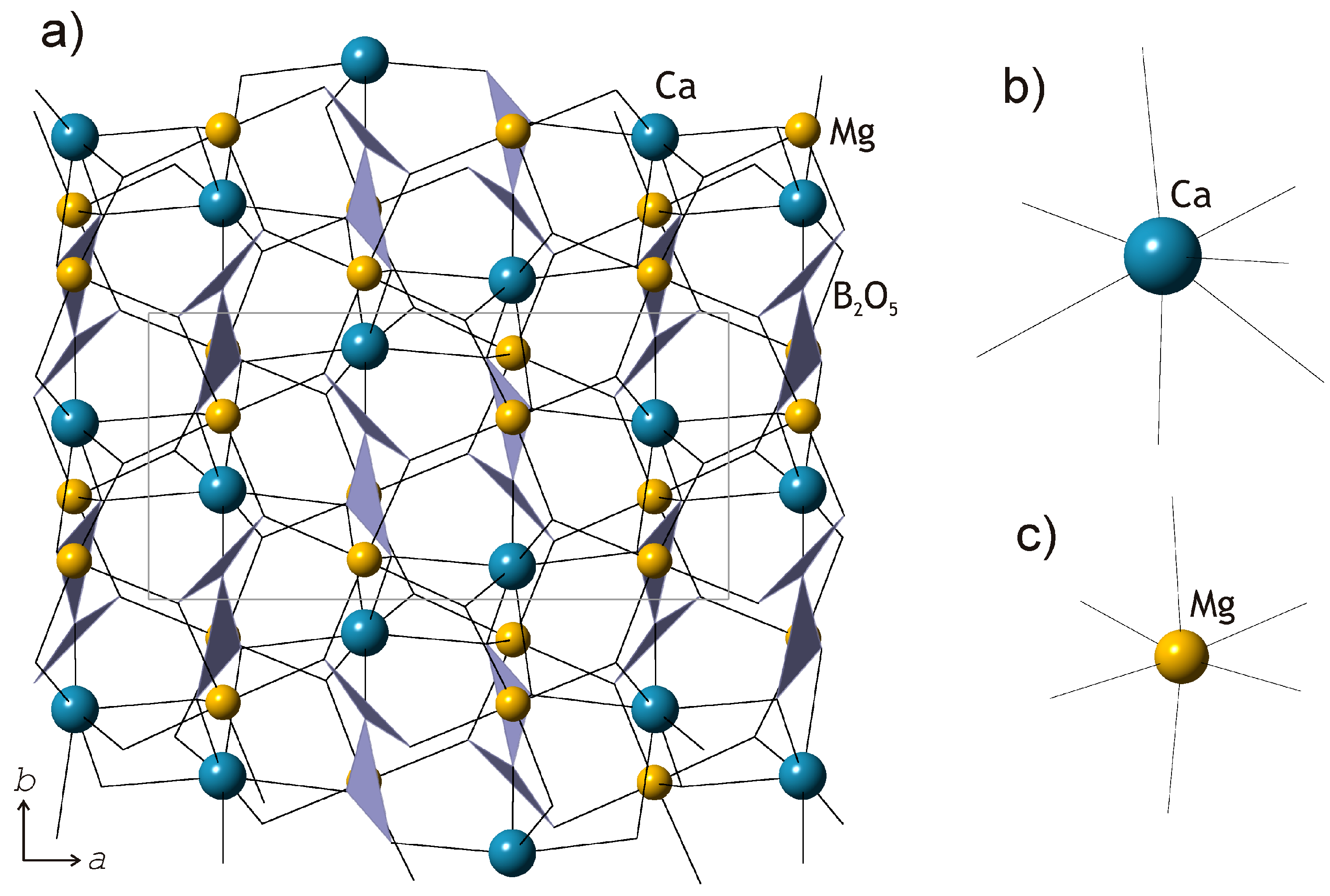
The crystal structures of kurchatovite and clinokurchatovite are shown in
Figure 1a and
Figure 2a, respectively. Their basic structural features are the same as reported by Yakubovich et al. [
7,
12,
13], Simonov et al. [
15], and Callegari et al. [
17]. Mg
2+ cations are coordinated octahedrally with the average <Mg-O> bond lengths in the range of 2.108–2.115 Å (individual bond lengths vary from 2.073 to 2.205 Å). Ca
2+ cations are coordinated by seven O atoms each with the <Ca-O> bond lengths in the range of 2.451–2.475 Å. B
3+ cations are coordinated by three O atoms each to form BO
3 triangles (<B-O> = 1.359–1.382 Å). Two BO
3 triangles share common O
br atoms (O
br = bridging O atom) to form [B
2O
5]
4− diborate group. The B-O
br bonds are essentially longer (1.407–1.425 Å) than the B-O
t bonds (1.353–1.369 Å; O
t = terminal O atoms), in agreement with the observations by Filatov and Bubnova [
20]. The B-O
br-B angles in the B
2O
5 groups are in the range 118.6–122.4°.
According to the description first developed by Yakubovich et al. [
7], the crystal structures of the two minerals are similar and based upon two-dimensional blocks of the kind shown in
Figure 3a. The blocks are about 6 Å thick and are arranged perpendicular to the
c axis in kurchatovite and parallel to (100) in clinokurchatovite. Callegari et al. [
17] proposed different approach to the structure description by splitting the crystal structures into thinner blocks (‘monoclinic modules’) consisting of B
2O
5 groups, BO
3 triangles, and Ca
2+ and Mg
2+ cations. In her detailed OD-analysis of kurchatovite and clinokurchatovite, Belokoneva [
18] subdivided in the two structures two layers, MgBO
2.5 (
L1) and CaBO
2.5 (
L2), with local symmetries
P(
b)c2
1 and
P(1)2
1/
c1, respectively. The
L1 layer contains B1O
3 triangles and Mg
2+ ions, whereas the
L2 layer contains B2O
3 triangles and Ca
2+ ions. The layers alternate to form
L1L2 pairs, which stack in the +1/4, +1/4, +1/4 … sequence in clinokurchatovite [a maximum degree of order 1 (MDO1) polytype] and in the +1/4, +1/4, +1/4, −1/4, −1/4, −1/4 … sequence in kurchatovite, where +1/4 and −1/4 symbolize shifts and orientations of the layers along the
b axis in kurchatovite and the
c axis in clinokurchatovite.
Despite their relevance and simplicity, the approaches developed by Callegari et al. [
17] and Belokoneva [
18] possess several disadvantages: (1) both approaches imply “splitting” of B
2O
5 groups into single BO
3 triangles, which is theoretically feasible, but physically unrealistic; (2) Belokoneva’s approach takes into account only the B1 and B2 sites, whereas, in clinokurchatovite, there are four B sites; (3) both approaches are unable to explain the absence of the “hypothetical protostructure” derived by Yakubovich et al. [
7], i.e., the monoclinic structure consisting of one block only, i.e., with
a ~ 6.2 Å if one uses the monoclinic setting of clinokurchatovite.
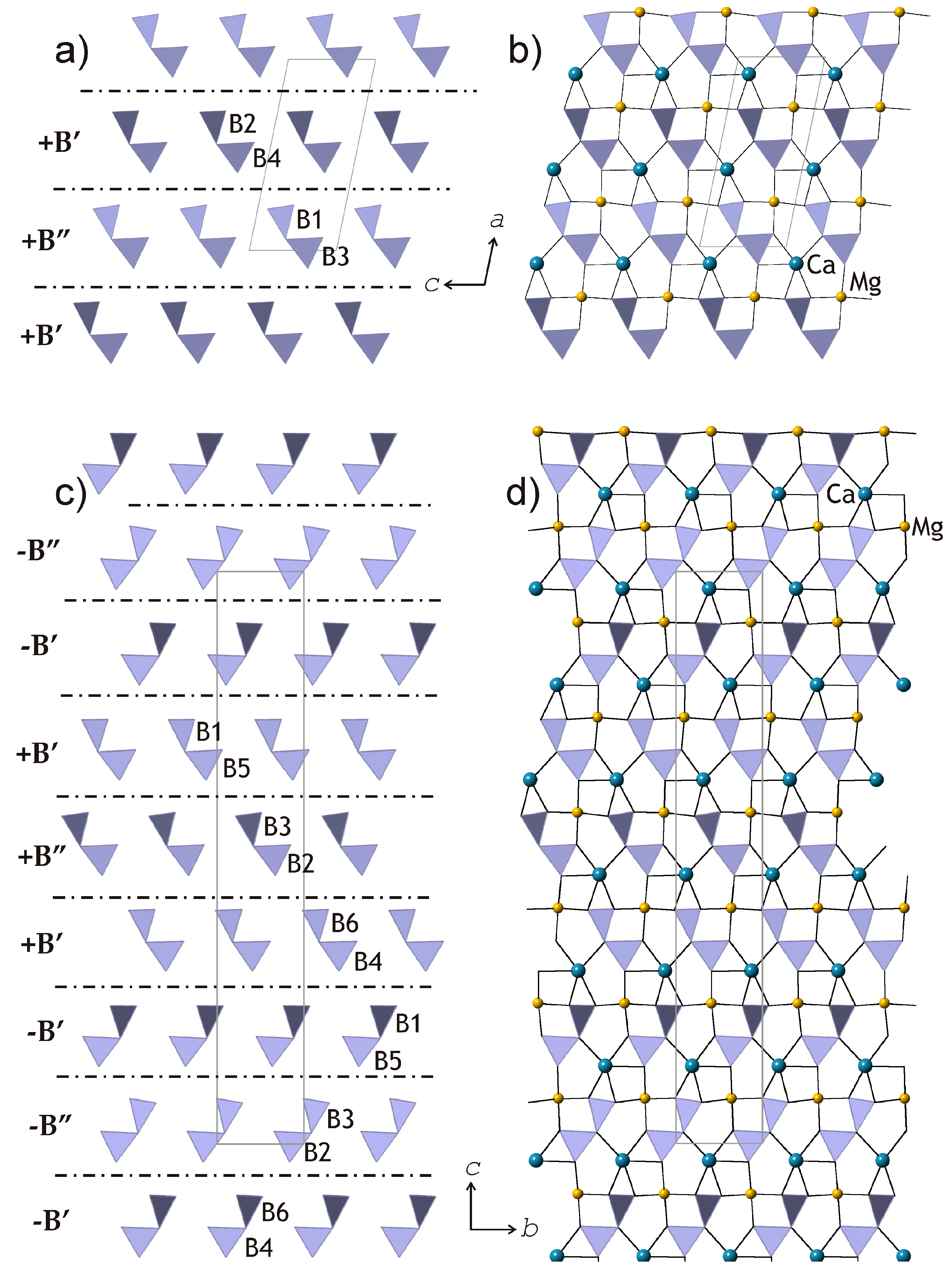
To develop a transparent and reliable method for the analysis of relationships between kurchatovite and clinokurchatovite, the two structures can first be analyzed in terms of the arrangements of the B
2O
5 groups (
Figure 1b and
Figure 2b).
In turn, the two arrangements can be split into two-dimensional slices indicated by dashed lines in
Figure 1b and
Figure 2b. The arrangement of the B
2O
5 groups in the plane of the slices (
Figure 4a,c) clearly demonstrates the relations between the two structures in terms of the sequence of the groups along the direction of the layer stacking. The Ca
2+ and Mg
2+ cations lie in the plane of the slices, possessing five- and four-fold coordination, respectively (
Figure 4b,d). The dot-and-dash lines in
Figure 4a,c show the separation of the slices into 6 Å-blocks corresponding to “protostructure” by Yakubovich et al. [
7]. The description of the two structures in terms of these blocks is physically realistic, since it does not imply hypothetical splitting of diborate groups into single BO
3 triangles.
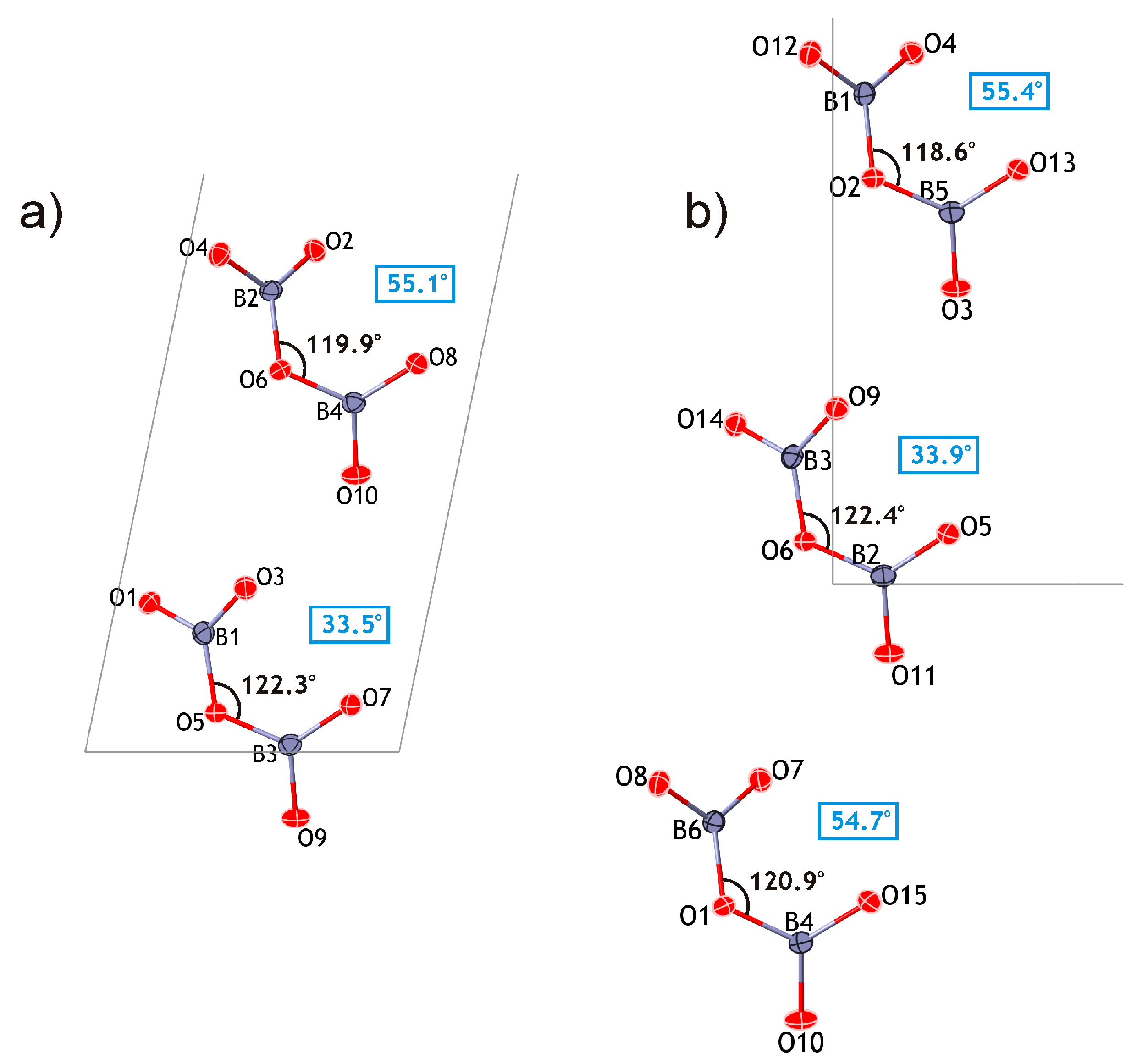
The proposed approach appears to support a polytypic relationship between kurchatovite and clinokurchatovite, since both are based upon the layers of the same kind stacked in different sequences. However, detailed analysis of geometrical parameters of the adjacent 6 Å-blocks reveals a more complicated situation. As mentioned above, the geometries of the B
2O
5 groups in the two structures are almost identical with the B–O bond lengths and valence angles varying over very narrow ranges. However, a closer investigation of symmetrically different diborate groups demonstrates that they have different degrees of conformation in terms of the δ angles between the planes of two BO
3 triangles sharing a common O atom. The diagrams in
Figure 5 show that the B
2O
5 groups belong to two discrete types with the δ values of ca. 55° and 34°, respectively. In both minerals, each 6 Å-block consists exclusively of diborate groups with the same δ value of either 55.5 ± 0.5° or 33.7 ± 0.5°. To make the distinction clear, we denote the 55° block as
B’, and the 34° block as
B”. Thus, taking into account the directions of the blocks along the
c axis in clinokurchatovite and the
b axis in kurchatovite symbolized as
+ or
− (related by the 180° rotation around the vertical axis), the stacking of the 6 Å-blocks in clinokurchatovite can be described as …
(+B’)(+B”)(+B’)(+B”)… or [
(+B’)(+B”)]. In contrast, the stacking in kurchatovite is more complex and corresponds to the sequence …
(+B’)(+B”)(+B’)(−B’)(−B”)(−B’)(+B’)(+B”)(+B’)(−B’)(−B”)(−B’)… or [
(+B’)(+B”)(+B’)(−B’)(−B”)(−B’)]. The
B’:B” ratios for clinokurchatovite and kurchatovite are 1:1 and 2:1, respectively. It is worthy to note that the proposed description of the two structures in terms of two different layers allows for the explanation of the absence of the “protostructure” by Yakubovich et al. [
7]. In fact, clinokurchatovite is the simplest structure possible in this mineral group (see also below).
4. Discussion
According to Guinier et al. [
21], “…an element or compound is polytypic if it occurs in several different structural modifications, each of which may be regarded as built up by stacking layers of (nearly) identical structure and composition, and if the modifications differ only in their stacking sequence”. This definition implies that the two structures belong to the same polytypic family if they: (1) contain nearly identical layers; (2) differ only in the stacking sequence. While the crystal structures of kurchatovite and clinokurchatovite conform to the first requirement, that is, they are built up from layers of the same kind, they do not conform to the second requirement, since the two structures cannot be obtained from one another simply by changing the stacking sequence. It is possible to obtain the crystal structure of kurchatovite from that of clinokurchatovite by the following sequence of operations: (a) splitting the structure of the latter into modules consisting of three 6 Å-blocks having the
B’:B” ratio of 2:1; (b) stacking the modules in such a way that the adjacent modules are in different orientations, which can also be described as a chemical twinning [
22,
23]. According to the modular approach [
22], this procedure for obtaining kurchatovite from clinokurchatovite means that the crystal structure of clinokurchatovite may be considered a basic structure, whereas that of kurchatovite is a derivative structure. Thus, the two minerals are not polytypes of one another; instead their relationship is better understood in terms of modular polymorphism. Re-definition of the two mineral species is not warranted. It is noteworthy that the situation would be very different if the crystal structures of kurchatovite and clinokurchatovite were to have the same
B’:B” ratio. The veatchite polytypes [
24] are an example of polytypism in which two types of layers are involved in borate minerals, but overall such polytypism is very rare.
The description in terms of the B’:B” blocks raises the important question about the potential occurrence of other members of the family consisting of the same elements. Considering the sequences of blocks in the two structures, two empirical rules can be derived:
(i) the B” blocks are always surrounded by the B’ blocks of the same directionality (i.e., of the same sign);
(ii) the
B’ blocks may occur in two combinations: either surrounded by two
B” blocks of the same sign or by one
B” block of the same sign and one
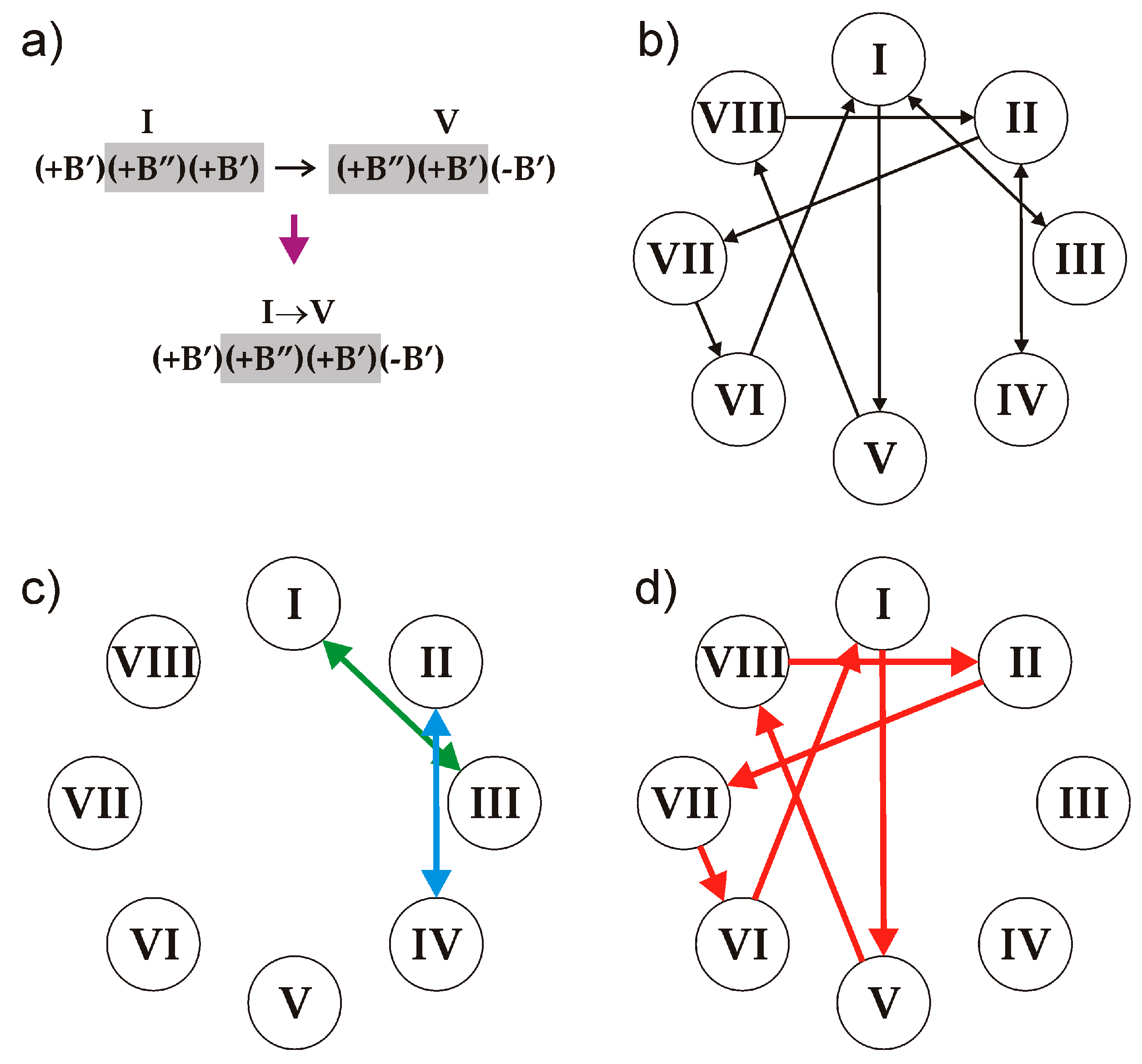
B’ block of the different sign. Thus, the following eight triplets are allowed that satisfy the (i) and (ii) conditions:
| I (+B’)(+B”)(+B’) | II (−B’)(−B”)(−B’) | III (+B”)(+B’)(+B”) | IV (−B”)(−B’)(−B”) |
| V (+B”)(+B’)(-B’) | VI (−B’)(+B’)(+B”) | VII (−B”)(−B’)(+B’) | VIII (+B’)(−B’)(−B”) |
The number of symbolic sequences that may contain these triplets is obviously infinite, and the sequences may be both periodic and aperiodic. We note that, to produce a sequence that satisfy the rules, two triplets should overlap in two symbols: two right symbols of the first (preceding) one and two left symbols of the second (following) one. Thus, all the triplets can be split into the following ten overlapping pairs (see an example in
Figure 6a):
The relations between the triplets are illustrated in
Figure 6b, which represents an analogue of de Brujin diagram for cellular automata [
25,
26,
27] that was considered as a model of crystallization processes [
28,
29]. The arrow lines in the diagram indicate the only possible sequence of triplets starting at the preceding triplet and ending at the following. Any realizable symbolic sequence is produced by the loop of arrows starting and ending at the same triplet.
Let us consider the possible symbolic sequences and their structural realizations in more detail:
1. The overlapping periodic sequences
I↔III, III↔I, II↔IV and
IV↔II (
Figure 6c) produce the structure of clinokurchatovite. This is the simplest structure possible in this group and contains two blocks within its identity period (e.g., [(
+B’)(
+B”)]).
2. Starting from triplet I and going to triplet V and so forth results in the following 6-membered sequence …→
VI→[
I→
V→
VIII→
II→
VII→
VI]→
I→… (
Figure 6d), which corresponds exactly to the 6-membered sequence of blocks observed in the crystal structure of kurchatovite.
3. An infinite number of periodic and aperiodic sequences can be obtained by inserting into the 6-membered sequence of triplets given above any even subsequence from the sequences I↔III and II↔IV. For instance, the sequence [I→V→VIII→II→(IV→II)→VII→VI] is produced by the inserting of the (IV→II) element into the sequence [I→V→VIII→II→VII→VI] after the triplet II. However, all such derivative sequences are at least two blocks longer than the sequence producing kurchatovite.
Therefore, the following important conclusion is in order: given the rules (i) and (ii), the sequences of blocks observed in the crystal structures of clinokurchatovite and kurchatovite contain the minimal number of blocks per identity period. All other sequences are either aperiodic or contain at least two more blocks within their identity periods. It is remarkable that the natural structures are observed for the minimal possible periodic sequences only.
The information-theoretic analysis of structural complexity of the two minerals [
23,
30] shows that clinokurchatovite (
IG = 4.170 bits/atom and
IG,total = 300.235 bits/cell) is structurally simpler than kurchatovite (
IG = 4.755 bits/atom and
IG,total = 1027.056 bits/cell). A greater complexity for the derivative structure is to be expected given the proposed modular relationship between the two structures and the fact that the sequence of blocks in kurchatovite is 6-membered, whereas that in clinokurchatovite is 2-membered. According to the complexity-based classification of minerals proposed by Krivovichev [
30], clinokurchatovite is intermediate, whereas kurchatovite is very complex. As it has been shown above, all other periodic structures in the “kurchatovite family” would contain at least 8-membered sequences of blocks and therefore would be even more structurally complex than kurchatovite.
Grew et al. [
31] examined structural complexity of boron minerals in detail and reported the average Shannon information content per unit cell for this class of minerals as 340 bits/cell (i.e., close to the value found for clinokurchatovite). In fact, 46% of all boron minerals are intermediate in complexity (100 to 500 bits/cell), whereas only 3% are very complex (>1000 bits/cell). The reasons for the high structural complexity of kurchatovite can be inferred from the modular character of its structure, which is one of the most common mechanisms for the formation of complex crystal structures [
30,
31].