Three-D Mineralogical Mapping of the Kovdor Phoscorite-Carbonatite Complex, NW Russia: II. Sulfides
Abstract
1. Introduction
2. Materials and Methods
3. Results
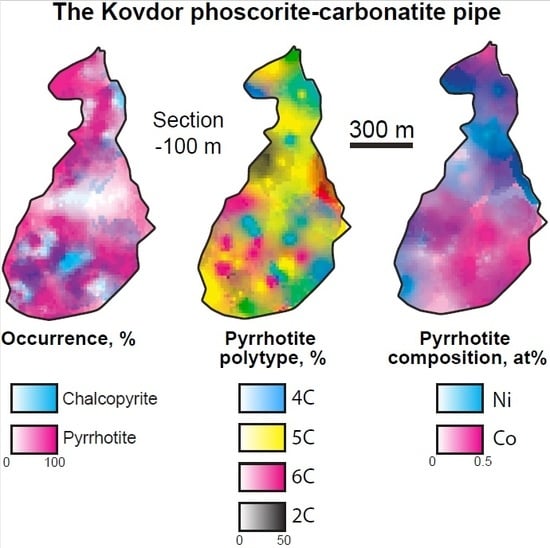
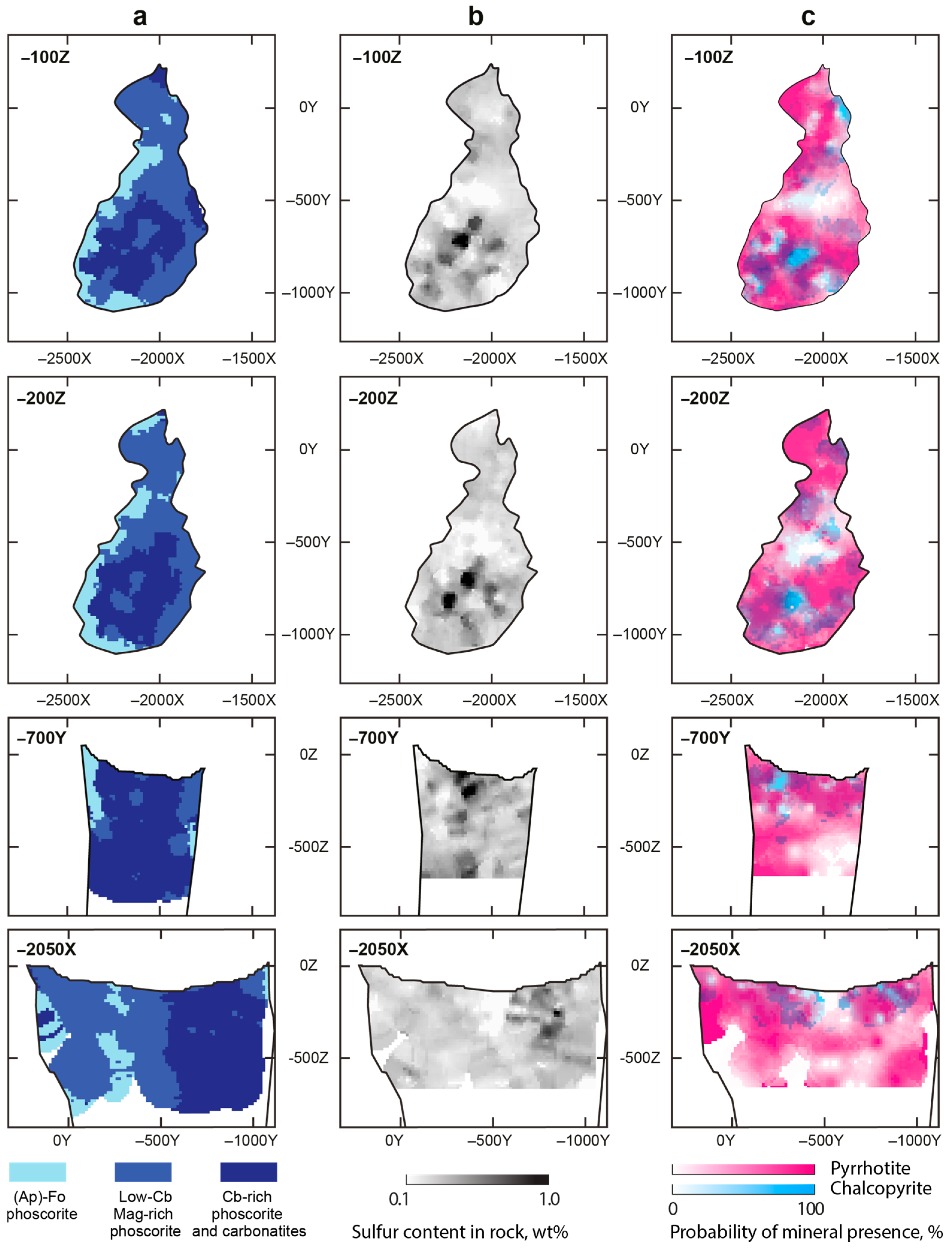
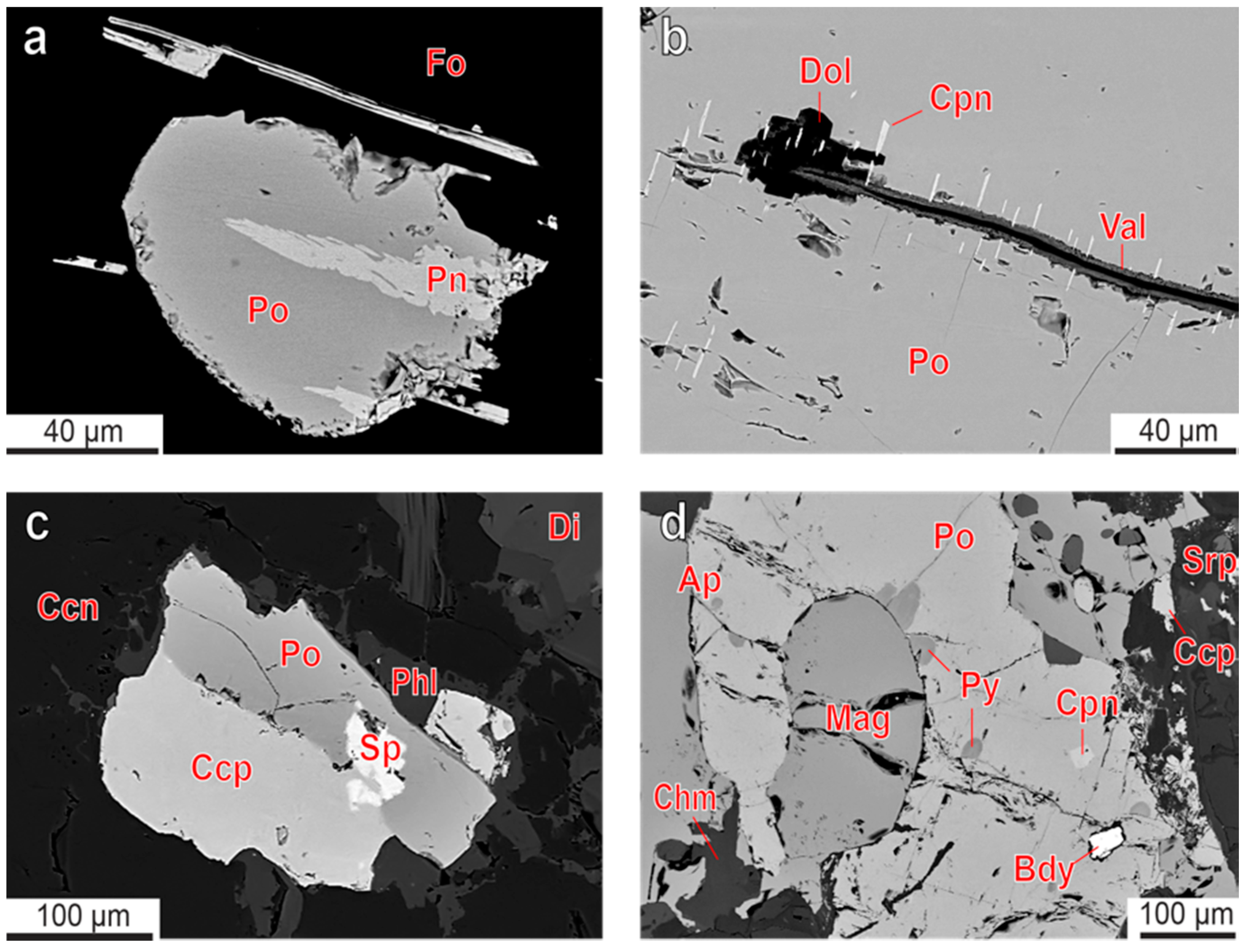
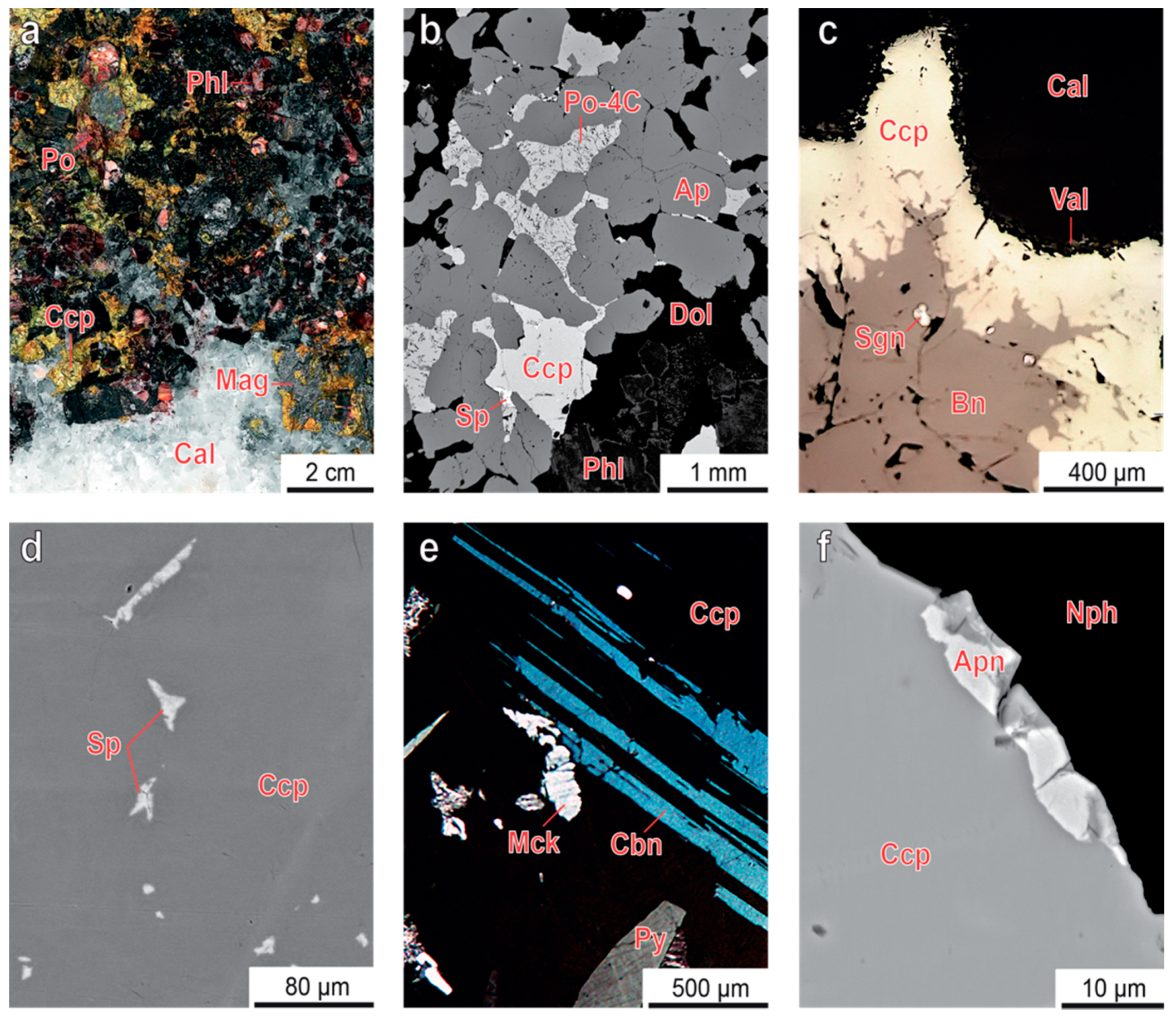
3.1. Sulfur Mineralization
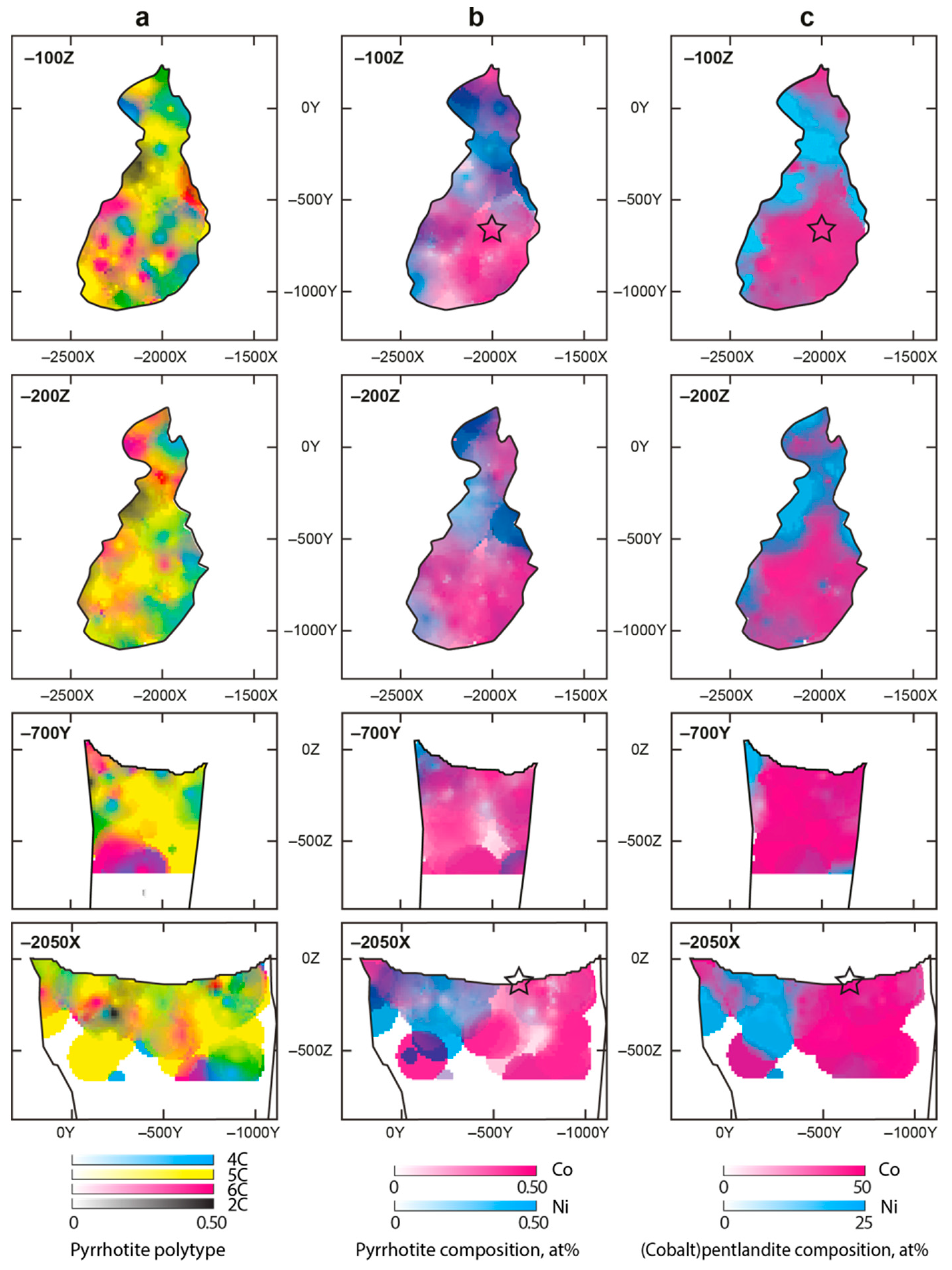
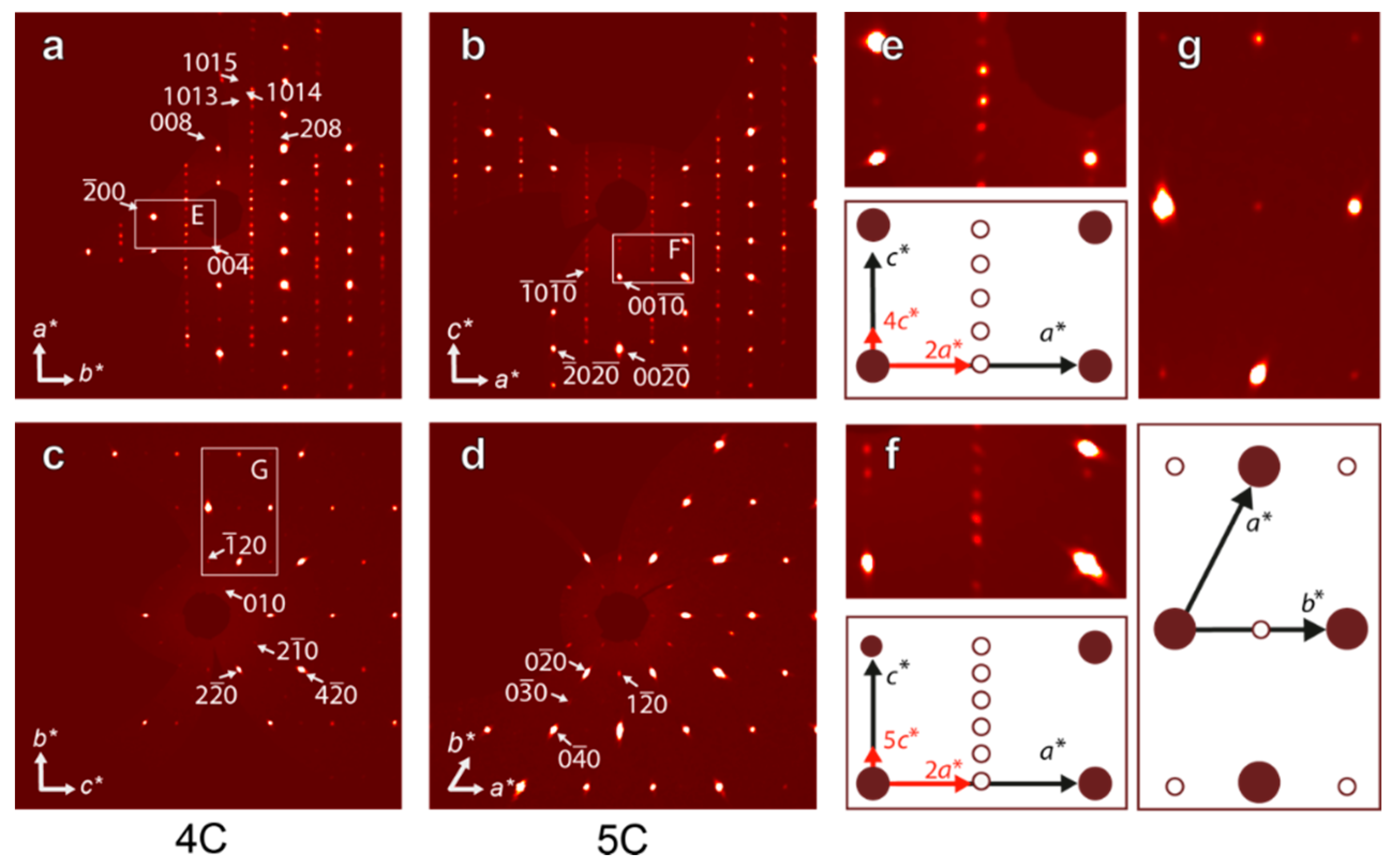
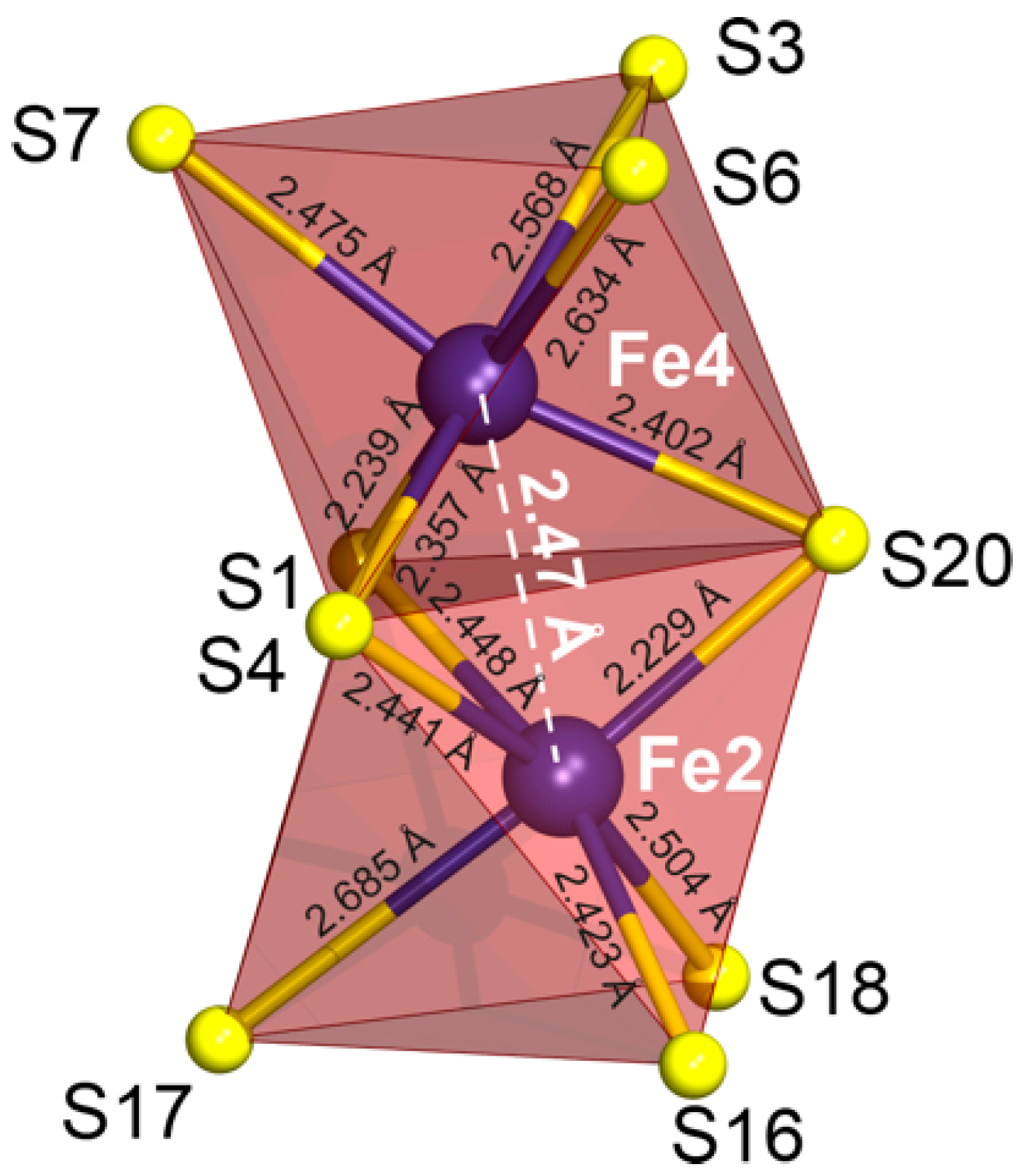
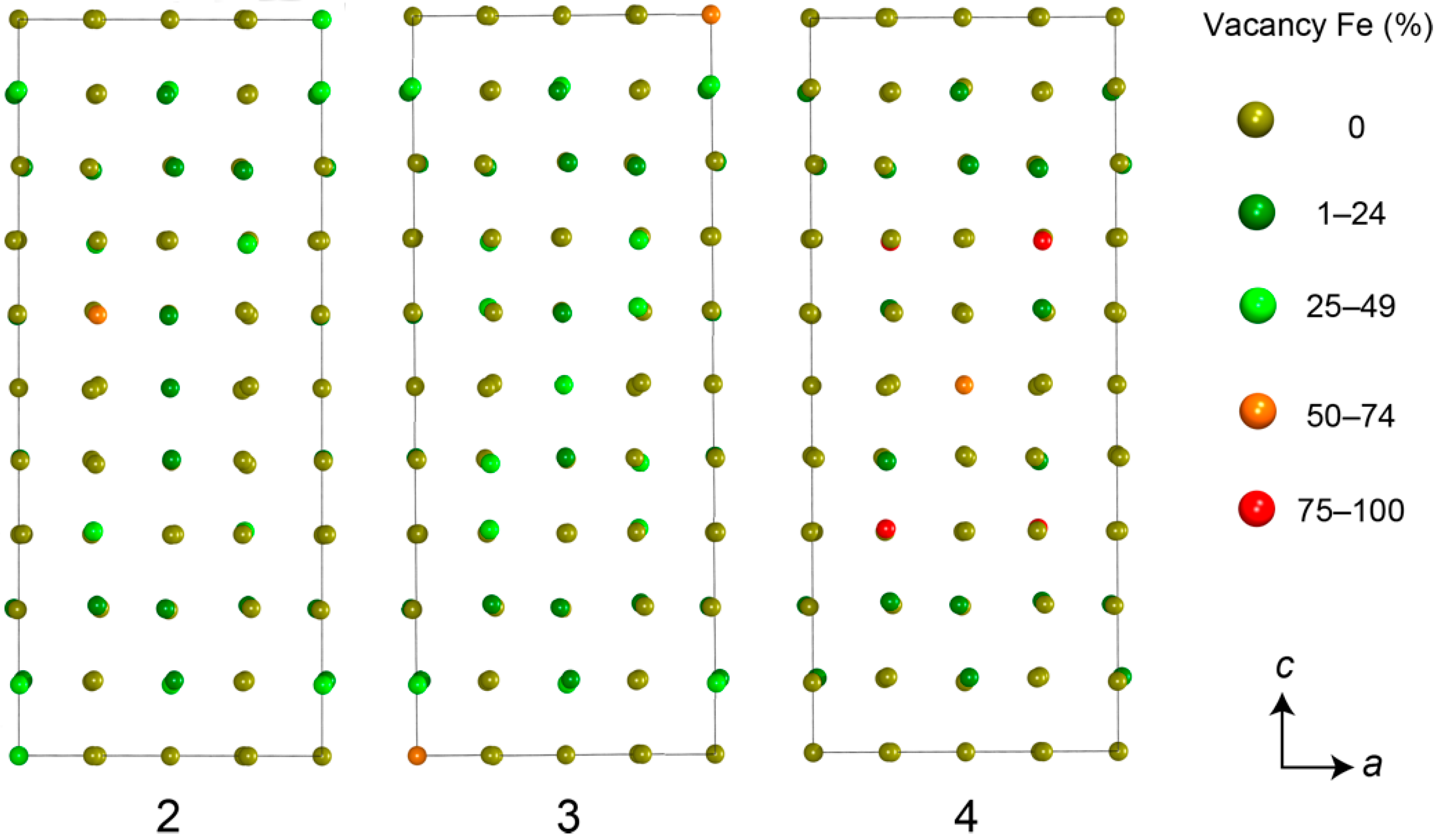
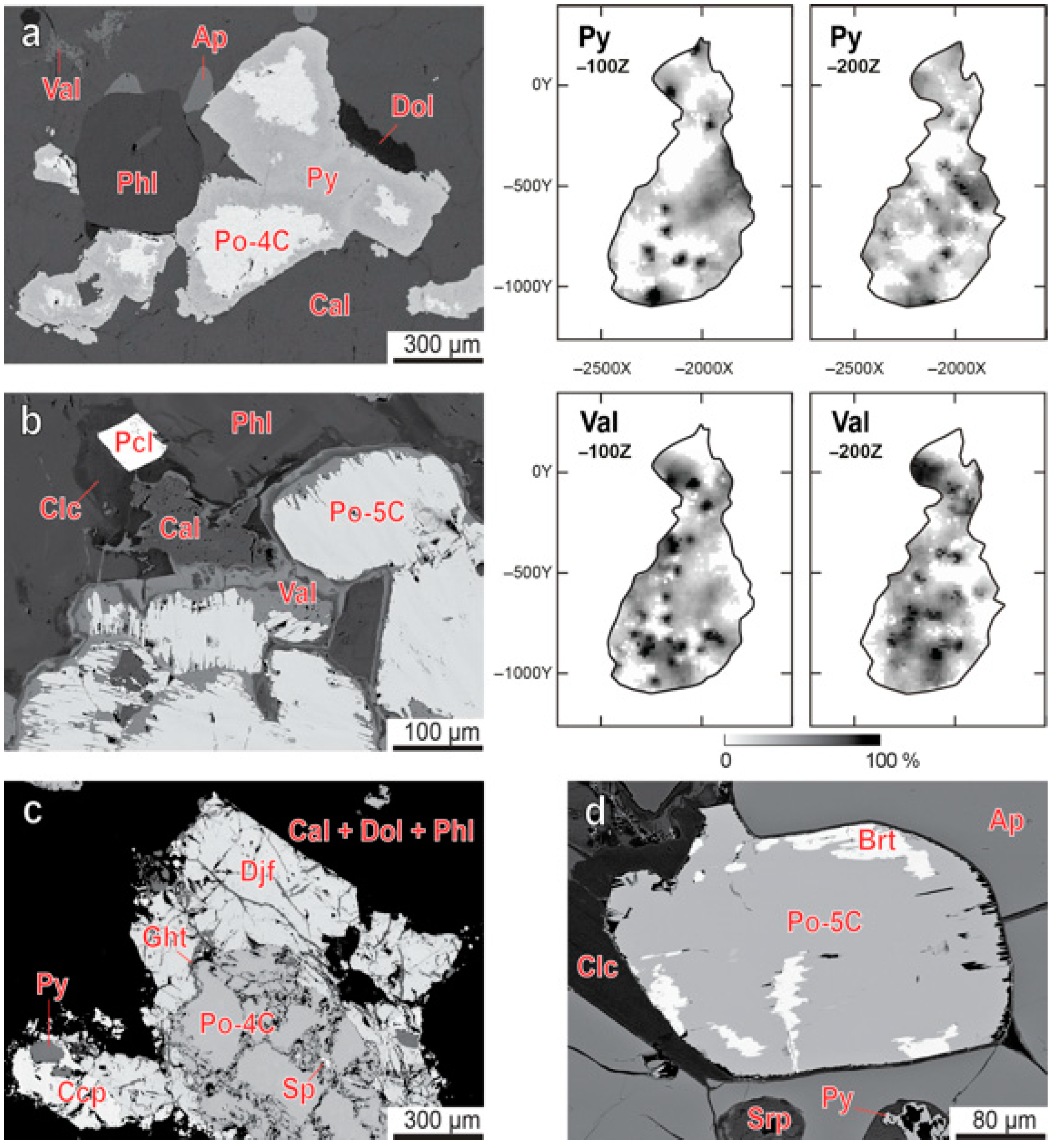
3.2. Pyrrhotite and Products of Its Alteration
3.3. Chalcopyrite and Products of Its Alteration
4. Discussion
5. Conclusions
- Primary silicate rocks of the Kovdor massif (peridotite, foidolite–melilitolite and forsteritite) are free of sulfides due to sulfur fractionation in fluid phase. For the same reason, hydrothermally altered parts of these rocks, including diopsidite, phlogopitite and skarn-like rocks, carry rich sulfide mineralization associated with secondary minerals (cancrinite, natrolite, phlogopite, clinochlore, serpentine, vesuvianite, etc.).
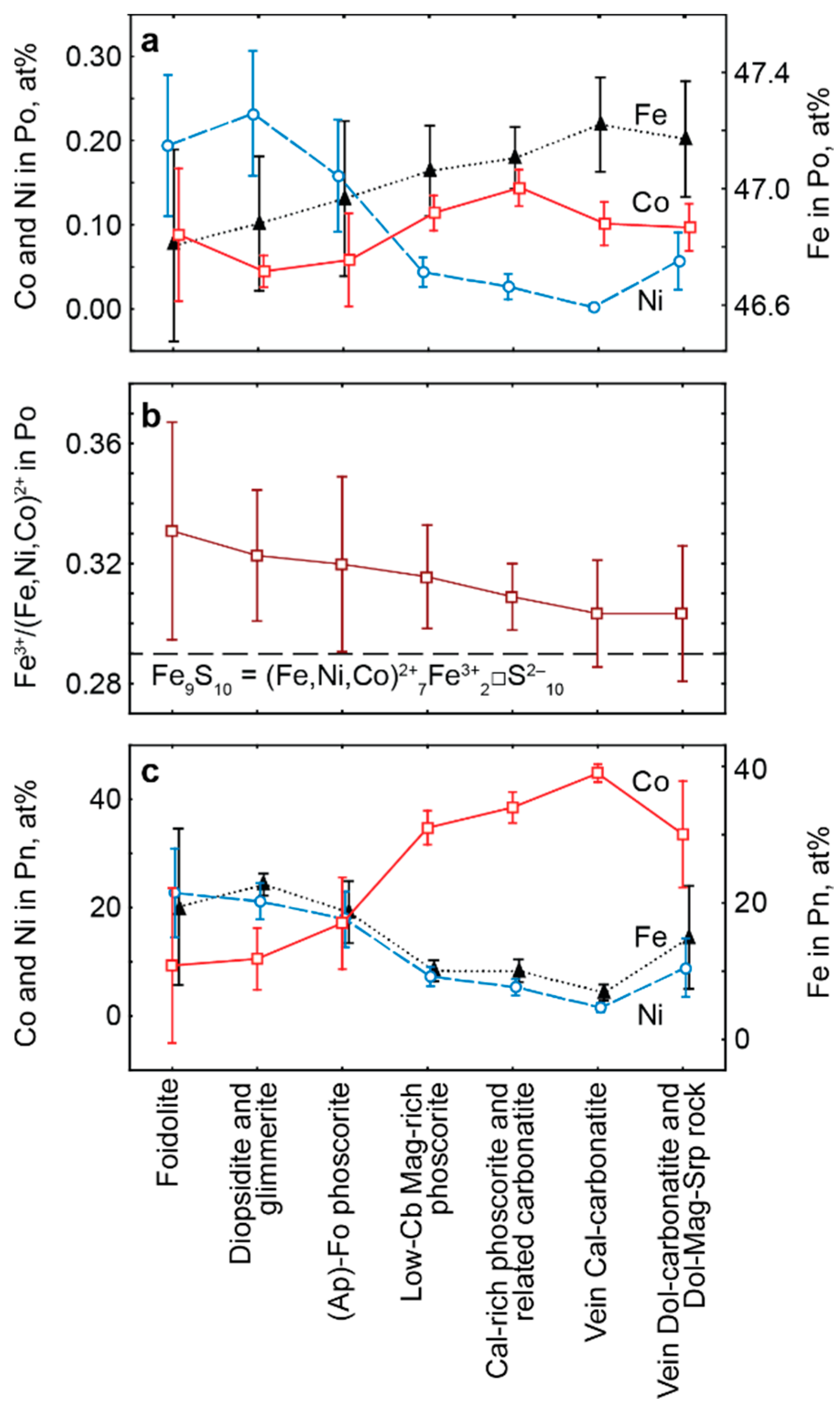
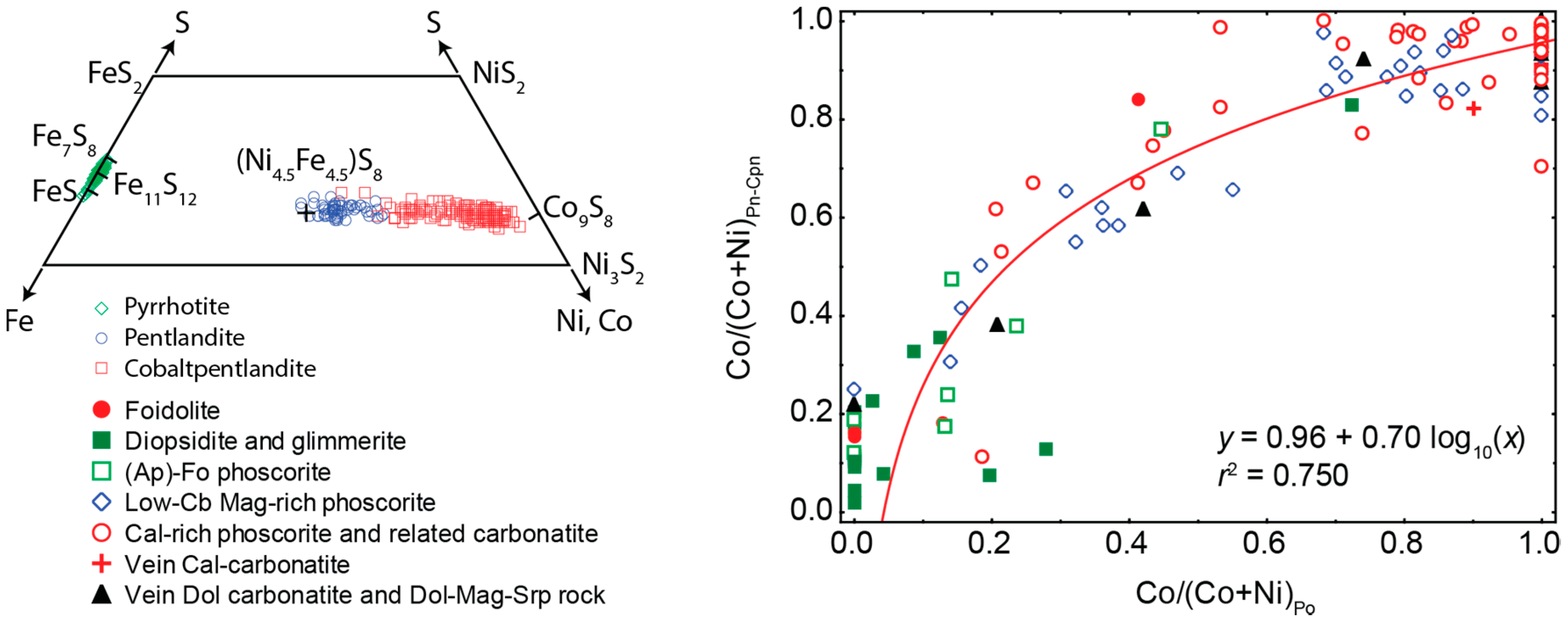
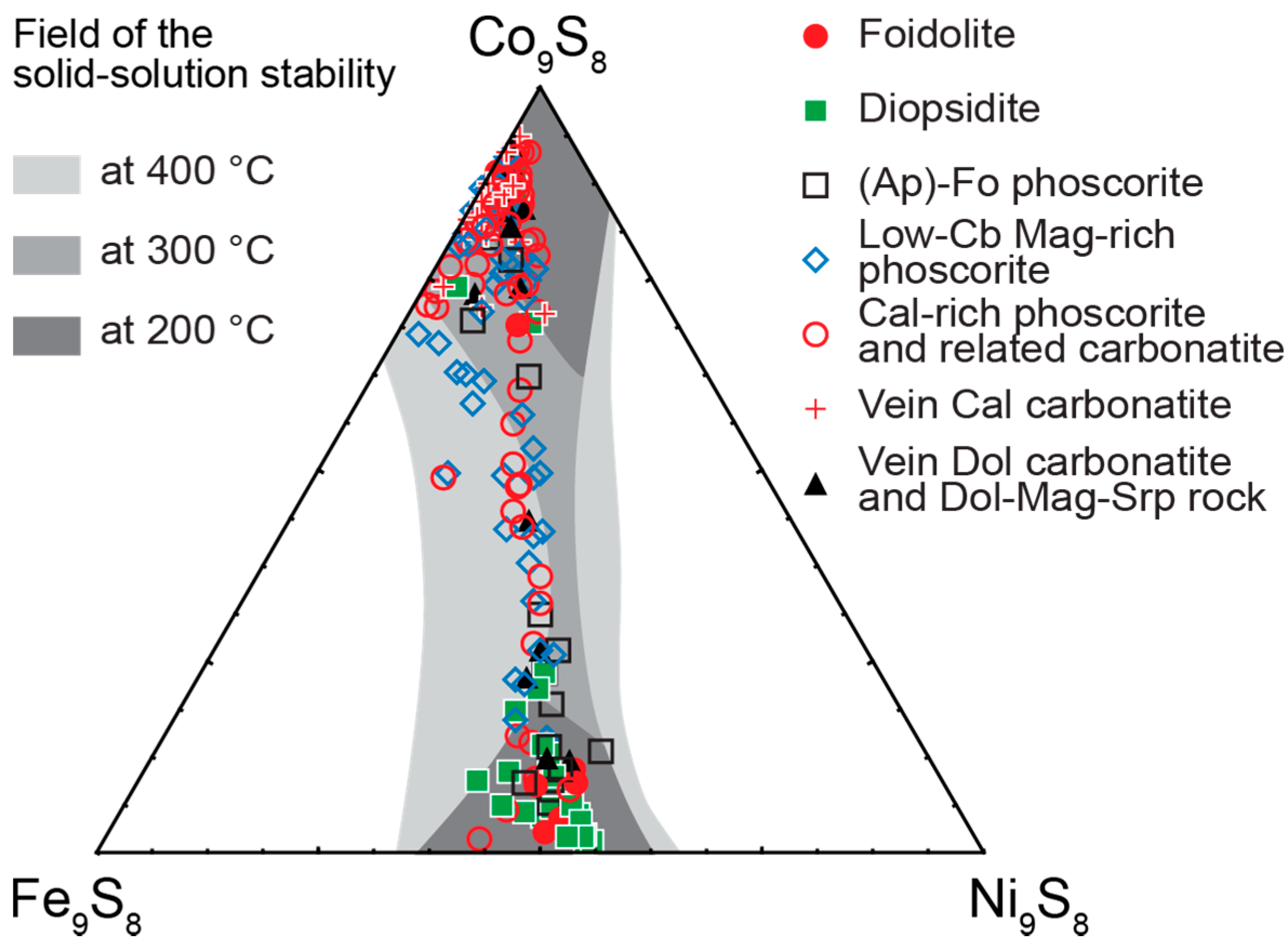
- Distribution of rock-forming sulfides within the Kovdor phoscorite-carbonatite complex reflects gradual concentric zonation of the pipe: pyrrhotite with exsolution inclusions of pentlandite in marginal (apatite)-forsterite phoscorite, pyrrhotite with exsolution inclusions of cobaltpentlandite in intermediate low-carbonate magnetite-rich phoscorite, and chalcopyrite (±pentlandite with exsolution inclusions of cobaltpentlandite) in axial carbonate-rich phoscorite and phoscorite-related carbonatite;
- Both pyrrhotite and chalcopyrite fill interstices between the main rock-forming minerals, including carbonates, and form late veinlets and irregularly shaped segregations with inclusions of surrounding minerals. Usually, chalcopyrite (with relicts of earlier bornite and exsolution inclusions of cubanite and mackinawite) crystallizes around grains of pyrrhotite (with inclusions of pentlandite-cobaltpentlandite and pyrite), and both these minerals contain common exsolution inclusions of sphalerite. Temperature of pyrite-pyrrhotite equilibration ranges from 100 °C to 400 °C, and pressure of pyrrhotite-sphalerite equilibration reaches 10 kbar;
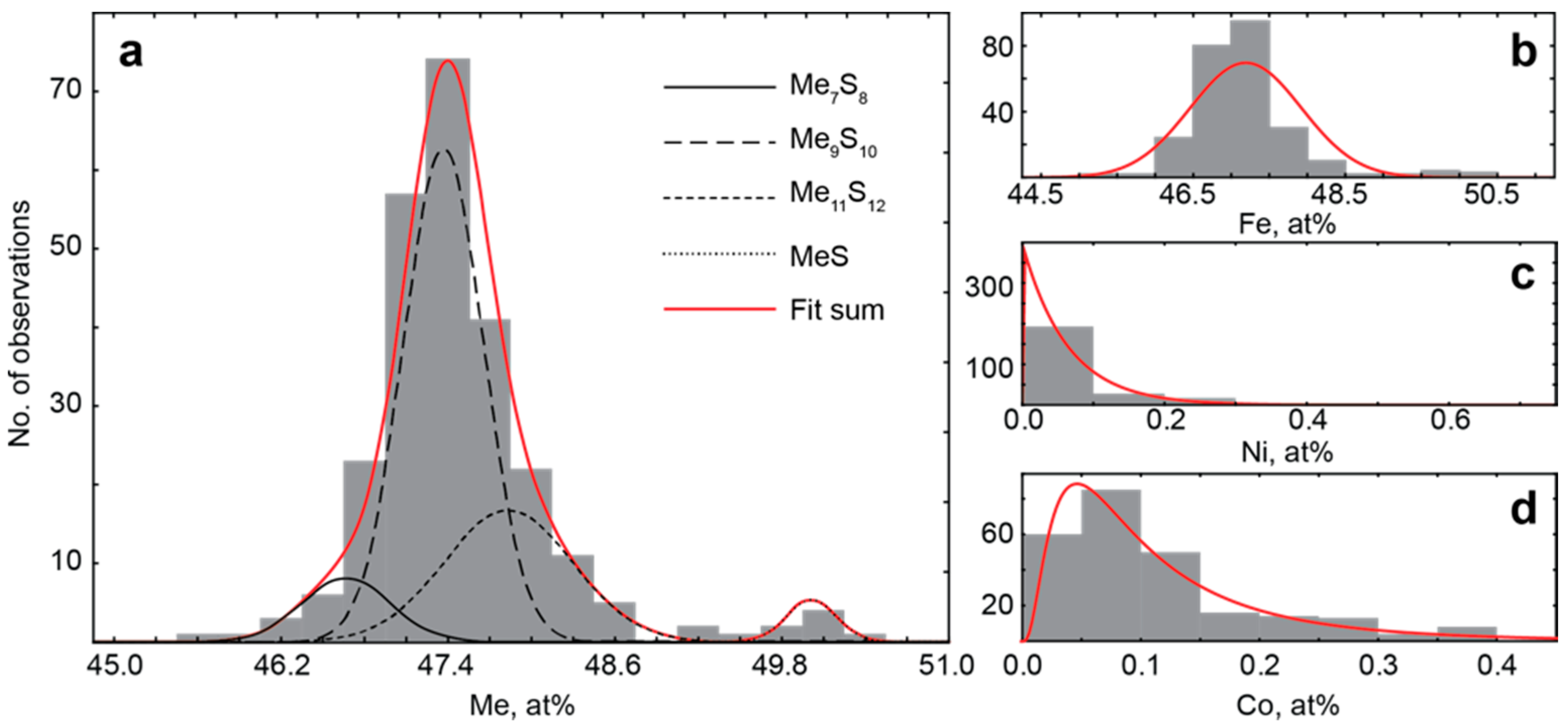
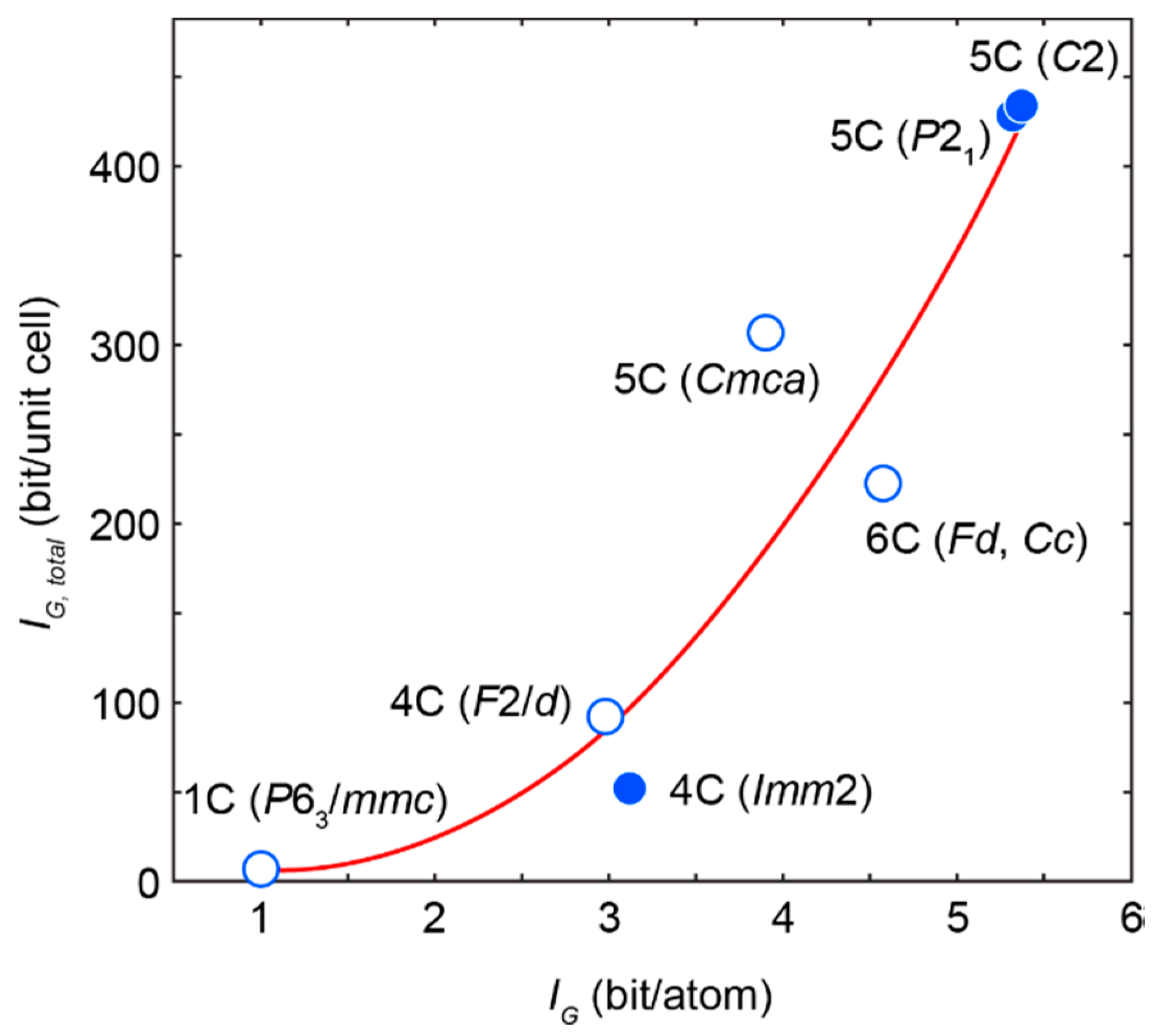
- For the most part, pyrrhotite corresponds to its non-magnetic 5C polytype. Ferrimagnetic pyrrhotite-4C forms individual grains, marginal zones of non-magnetic pyrrhotite crystals and thin lens-like inclusions in pyrrhotite-5C. Low-temperature pyrrhotite 2C (troilite) occurs as lens-like exsolution inclusions in grains of pyrrhotite-4C and -5C. In natural sequence of the Kovdor rocks, iron content in pyrrhotite gradually increases from Fe7S8 to Fe9S10 and Fe11S12 in accordance with gradual decrease of crystallization temperature and oxygen fugacity.
- High complexity of crystal structure of pyrrhotite-5C (IG,total = 429.75 bits per unit cell), which is the most common within the Kovdor massif, confirms the lowest temperature of its formation in comparison with other polytypes. Wide dissemination of this modification is associated with structural stability (variations in composition Fe8.84–8.99S10) due to different vacancies ordering mechanisms resulting in two structural modifications: P21 and C2.
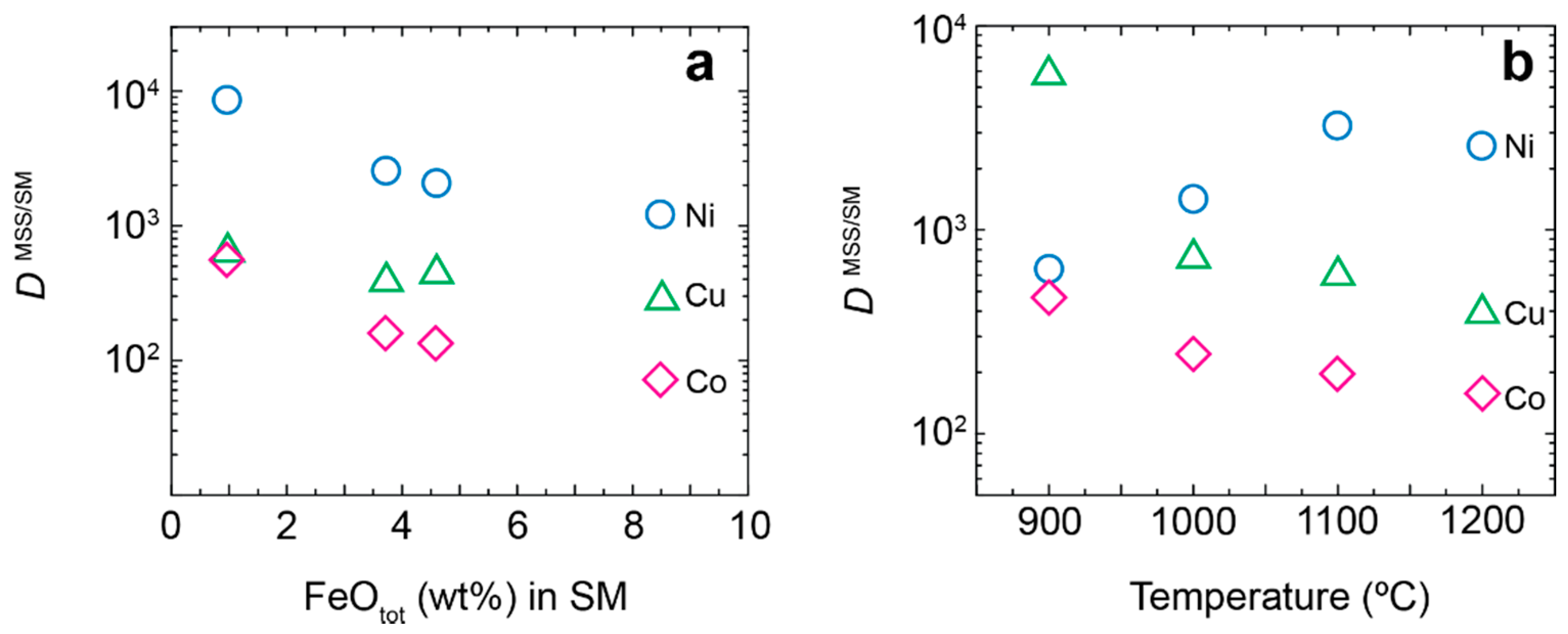
- Within the phoscorite-carbonatite complex, content of Co in pyrrhotite gradually increases from host silicate rocks and marginal forsterite-dominant phoscorite to axial carbonate-rich phoscorite and carbonatite due to Ni and Fe. This dependence probably reflects a gradual decrease of crystallyzation temperature in primary monosulfide solid solutions from the pipe margin toward its axis.
- Low-temperature hydrothermal alteration of pyrrhotite and chalcopyrite first produces partial or complete pseudomorphs of djerfisherite, pyrite, chalcocite, valleriite and goethite after the rock-forming sulfides, and then numerous phosphates and carbonates of Fe, Co and Cu (baricite, gladiusite, malachite, mitridatite, pakhomovskyite, pseudomalachite, pyroaurite, siderite, strengite, vivianite) in surrounding voids and fissures.
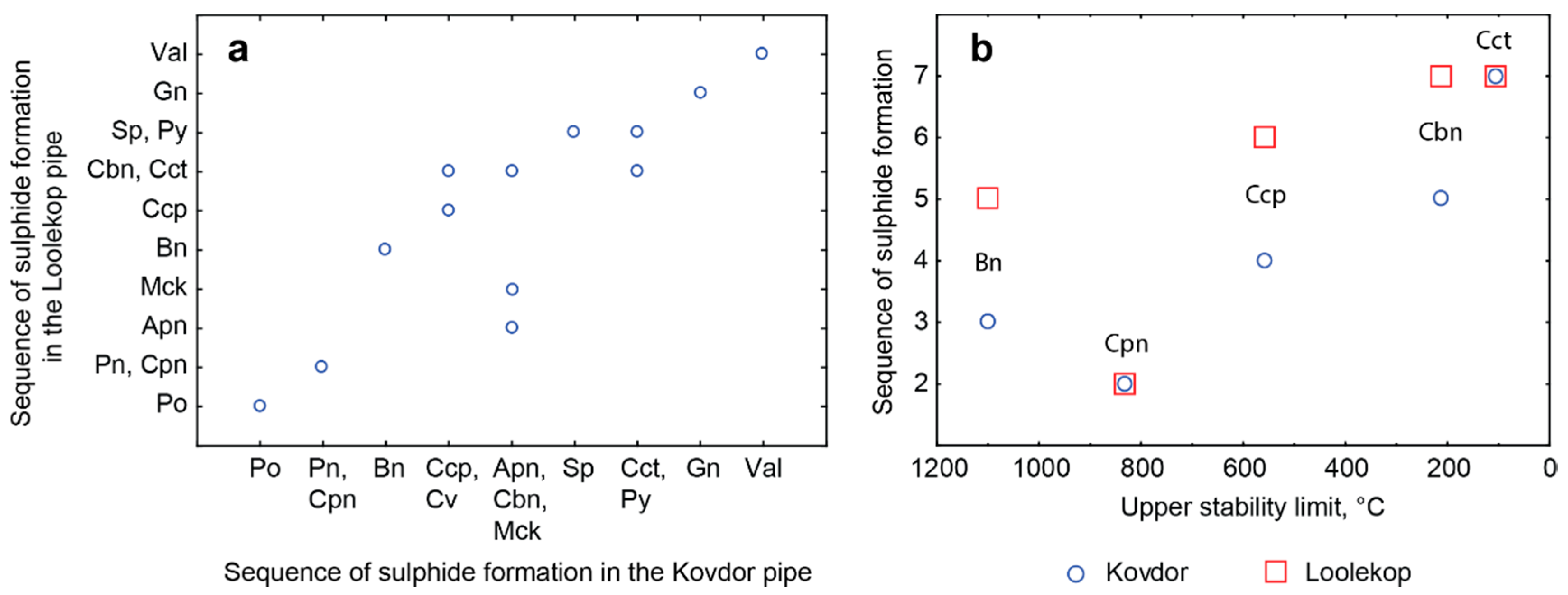
- The Kovdor and Loolekop phoscorite-carbonatite pipes have similar sequences of sulfide formation: pyrrhotite–pentlandite–cobaltpentlandite–bornite–chalcopyrite–covellite–cubanite–sphalerite–pyrite-chalcocite–galena–vallereite. This sequence corresponds to the sulfide stability limits ranged from 1100 °C (high bornite) to 103 °C (chalcocite) and even lower (for valleriite). Thus, copper specialization of the Loolekop pipe is not caused by any specific process but reflects stochastic specifics of the phoscorite-carbonatite genesis.
- Sulfide specialization of the Palabora massif can be caused by higher water content in its initial melt allowing it to dissolve much larger amounts of sulfur and, consequently, concentrate more chalcophile metals.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bailey, D.K.; Hampton, C.M. Volatiles in alkaline magmatism. Lithos 1990, 26, 157–165. [Google Scholar] [CrossRef]
- Samson, I.M.; Williams-Jones, A.E.; Liu, W. The chemistry of hydrothermal fluids in carbonatites: Evidence from leachate and SEM-decrepitate analysis of fluid inclusions from Oka, Quebec, Canada. Geochim. Cosmochim. Acta 1995, 59, 1979–1989. [Google Scholar] [CrossRef]
- Brooker, R.A.; Sparks, R.S.J.; Kavanagh, J.L.; Field, M. The volatile content of hypabyssal kimberlite magmas: Some constraints from experiments on natural rock compositions. Bull. Volcanol. 2011, 73, 959–981. [Google Scholar] [CrossRef]
- Klimm, K.; Kohn, S.C.; Botcharnikov, R.E. The dissolution mechanism of sulphur in hydrous silicate melts. II: Solubility and speciation of sulphur in hydrous silicate melts as a function of fO2. Chem. Geol. 2012, 322–323, 250–267. [Google Scholar] [CrossRef]
- Klimm, K.; Kohn, S.C.; Dell, L.A.O.; Botcharnikov, R.E.; Smith, M.E. The dissolution mechanism of sulphur in hydrous silicate melts. I: Assessment of analytical techniques in determining the sulphur speciation in iron-free to iron-poor glasses. Chem. Geol. 2012, 322–323, 237–249. [Google Scholar] [CrossRef]
- Métrich, N.; Berry, A.J.; O’Neill, H.S.C.; Susini, J. The oxidation state of sulfur in synthetic and natural glasses determined by X-ray absorption spectroscopy. Geochim. Cosmochim. Acta 2009, 73, 2382–2399. [Google Scholar] [CrossRef]
- O’Neill, H.S.C.; Mavrogenes, J.A. The Sulfide Capacity and the Sulfur Content at Sulfide Saturation of Silicate Melts at 1400 degrees C and 1 bar. J. Petrol. 2002, 43, 1049–1087. [Google Scholar] [CrossRef]
- Klimm, K.; Botcharnikov, R.E. The determination of sulfate and sulfide species in hydrous silicate glasses using Raman spectroscopy. Am. Mineral. 2010, 95, 1574–1579. [Google Scholar] [CrossRef]
- Seward, T.M.; Williams-Jones, A.E.; Migdisov, A.A. The Chemistry of Metal Transport and Deposition by Ore-Forming Hydrothermal Fluids. In Treatise on Geochemistry; Elsevier: Berlin, Germany, 2014; pp. 29–57. ISBN 9780080959757. [Google Scholar]
- Reed, M.H.; Palandri, J. Sulfide Mineral Precipitation from Hydrothermal Fluids. Rev. Mineral. Geochem. 2006, 61, 609–631. [Google Scholar] [CrossRef]
- Ivanyuk, G.Y.; Yakovenchuk, V.N.; Pakhomovsky, Y.A. Kovdor; Laplandia Minerals: Apatity, Russia, 2002; ISBN 5900395413. [Google Scholar]
- Rudashevsky, N.S.; Kretser, Y.L.; Rudashevsky, V.N.; Sukharzhevskaya, E.S. A review and comparison of PGE, noble-metal and sulphide mineralization in phoscorites and carbonatites from Kovdor and Phalaborwa. In Phoscorites and Carbonatites from Mantle to Mine: The Key Example of the Kola Alkaline Province; Wall, F., Zaitsev, A.N., Eds.; Mineralogical Society: London, UK, 2004; pp. 375–405. [Google Scholar]
- Kukharenko, A.A.; Orlova, M.P.; Bulakh, A.G.; Bagdasarov, E.A.; Rimskaya-Korsakova, O.M.; Nefedov, E.I.; Ilyinsky, G.A.; Sergeev, A.S.; Abakumova, N.B. Caledonian Complex of Ultrabasic, Alkaline Rocks and Carbonatites of Kola Peninsula and Northern Karelia (Geology, Petrology, Mineralogy and Geochemistry); Nedra: Moscow, Russia, 1965. (In Russian) [Google Scholar]
- Bykova, E. Sulphide mineralization in magnetite ores and carbonatites of the Kovdor massif. Mineral. Geochem. 1975, 5, 11–16. (In Russian) [Google Scholar]
- Subbotina, G.F.; Subbotin, V.V.; Pakhomovsky, Y.A. Some features of a sulphide mineralization of apatite-magnetite ores and carbonatites of the Kovdor deposit. In Substantial Composition of Alkaline Intrusive Complexes of the Kola Peninsula; KFAN USSR: Apatity, Russia, 1981; pp. 88–95. [Google Scholar]
- Balabonin, N.L.; Voloshin, A.V.; Pakhomovsky, Y.A. Rare sulphides in rocks of the Kovdor deposit. In Mineral Complexes and Minerals of the Kola Peninsula; KFAN USSR: Apatity, Russia, 1980; pp. 88–92. (In Russian) [Google Scholar]
- Balabonin, N.L.; Voloshin, A.V.; Pakhomovsky, Y.A.; Polyakov, K.I. Composition of djerfisherite from alkaline complexes of the Kola Peninsula. Mineral. Zhurnal 1980, 1, 90–99. (In Russian) [Google Scholar]
- Kapustin, Y.L. Mineralogy of Carbonatites; Amerind Publishing: New Delhi, India, 1980. [Google Scholar]
- Rimskaya-Korsakova, O.M.; Krasnova, N.I. Geology of Deposits of the Kovdor Massif; St. Petersburg University Press: St. Petersburg, Russia, 2002. (In Russian) [Google Scholar]
- Krasnova, N.I.; Petrov, T.G.; Balaganskaya, E.G.; García, D.; Moutte, J.; Zaitsev, A.N.; Wall, F. Introduction to phoscorites: Occurrence, composition, nomenclature and petrogenesis. In Phoscorites and Carbonatites from Mantle to Mine: The Key Example of the Kola Alkaline Province; Zaitsev, A.N., Wall, F., Eds.; Mineralogical Society: London, UK, 2004; pp. 43–72. ISBN 0903056224. [Google Scholar]
- Afanasyev, B.V. Mineral Resources of Alkaline-Ultrabasic Massifs of the Kola Peninsula; Roza Vetrov Publishing: St. Petersburg, Russia, 2011. (In Russian) [Google Scholar]
- Mikhailova, J.A.; Ivanyuk, G.Y.; Kalashnikov, A.O.; Pakhomovsky, Y.A.; Bazai, A.V.; Panikorovskii, T.L.; Yakovenchuk, V.N.; Konopleva, N.G.; Goryainov, P.M. Three-D Mineralogical Mapping of the Kovdor Phoscorite–Carbonatite Complex, NW Russia: I. Forsterite. Minerals 2018, 8, 260. [Google Scholar] [CrossRef]
- Mikhailova, J.A.; Kalashnikov, A.O.; Sokharev, V.A.; Pakhomovsky, Y.A.; Konopleva, N.G.; Yakovenchuk, V.N.; Bazai, A.V.; Goryainov, P.M.; Ivanyuk, G.Y. 3D mineralogical mapping of the Kovdor phoscorite–carbonatite complex (Russia). Miner. Depos. 2016, 51, 131–149. [Google Scholar] [CrossRef]
- Kalashnikov, A.O.; Ivanyuk, G.Y.; Mikhailova, J.A.; Sokharev, V.A. Approach of automatic 3D geological mapping: The case of the Kovdor phoscorite-carbonatite complex, NW Russia. Sci. Rep. 2017, 7, 6893. [Google Scholar] [CrossRef] [PubMed]
- Kalashnikov, A.O.; Konopleva, N.G.; Pakhomovsky, Y.A.; Ivanyuk, G.Y. Rare earth deposits of the Murmansk Region, Russia—A review. Econ. Geol. 2016, 111, 1529–1559. [Google Scholar] [CrossRef]
- Ivanyuk, G.; Kalashnikov, A.; Pakhomovsky, Y.; Bazai, A.; Goryainov, P.; Mikhailova, J.; Yakovenchuk, V.; Konopleva, N. Subsolidus Evolution of the Magnetite-Spinel-UlvöSpinel Solid Solutions in the Kovdor Phoscorite-Carbonatite Complex, NW Russia. Minerals 2017, 7, 215. [Google Scholar] [CrossRef]
- Ivanyuk, G.Y.; Kalashnikov, A.O.; Pakhomovsky, Y.A.; Mikhailova, J.A.; Yakovenchuk, V.N.; Konopleva, N.G.; Sokharev, V.A.; Bazai, A.V.; Goryainov, P.M. Economic minerals of the Kovdor baddeleyite-apatite-magnetite deposit, Russia: Mineralogy, spatial distribution and ore processing optimization. Ore Geol. Rev. 2016, 77, 279–311. [Google Scholar] [CrossRef]
- Kalashnikov, A.O.; Yakovenchuk, V.N.; Pakhomovsky, Y.A.; Bazai, A.V.; Sokharev, V.A.; Konopleva, N.G.; Mikhailova, J.A.; Goryainov, P.M.; Ivanyuk, G.Y. Scandium of the Kovdor baddeleyite-apatite-magnetite deposit (Murmansk Region, Russia): Mineralogy, spatial distribution, and potential resource. Ore Geol. Rev. 2016, 72, 532–537. [Google Scholar] [CrossRef]
- Ivanyuk, G.Y.; Pakhomovsky, Y.A.; Panikorovsky, T.L.; Mikhailova, J.A.; Kalashnikov, A.O.; Bazai, A.V.; Yakovenchuk, V.N.; Konopleva, N.G.; Goryainov, P.M. Three-D Mineralogical Mapping of the Kovdor Phoscorite-Carbonatite Complex, NW Russia: III. Pyrochlore Supergroup Minerals. Minerals 2018, 8, 277. [Google Scholar] [CrossRef]
- Krivovichev, S.V.; Yakovenchuk, V.N.; Zhitova, E.S.; Zolotarev, A.A.; Pakhomovsky, Y.A.; Ivanyuk, G.Y. Crystal chemistry of natural layered double hydroxides. 1. Quintinite-2H-3c from the Kovdor alkaline massif, Kola peninsula, Russia. Mineral. Mag. 2010, 74, 821–832. [Google Scholar] [CrossRef]
- Krivovichev, S.V.; Yakovenchuk, V.N.; Zhitova, E.S.; Zolotarev, A.A.; Pakhomovsky, Y.A.; Ivanyuk, G.Y. Crystal chemistry of natural layered double hydroxides. 2. Quintinite-1M: First evidence of a monoclinic polytype in M2+-M3+ layered double hydroxides. Mineral. Mag. 2010, 74, 833–840. [Google Scholar] [CrossRef]
- Zhitova, E.S.; Yakovenchuk, V.N.; Krivovichev, S.V.; Zolotarev, A.A.; Pakhomovsky, Y.A.; Ivanyuk, G.Y. Crystal chemistry of natural layered double hydroxides. 3. The crystal structure of Mg, Al-disordered quintinite-2H. Mineral. Mag. 2010, 74, 841–848. [Google Scholar] [CrossRef]
- Zhitova, E.S.; Ivanyuk, G.Y.; Krivovichev, S.V.; Yakovenchuk, V.N.; Pakhomovsky, Y.A.; Mikhailova, Y.A. Crystal Chemistry of Pyroaurite from the Kovdor Pluton, Kola Peninsula, Russia, and the Långban Fe–Mn deposit, Värmland, Sweden. Geol. Ore Depos. 2017, 59, 652–661. [Google Scholar] [CrossRef]
- Zhitova, E.S.; Krivovichev, S.V.; Yakovenchuk, V.N.; Ivanyuk, G.Y.; Pakhomovsky, Y.A.; Mikhailova, J.A. Crystal chemistry of natural layered double hydroxides. 4. Crystal structures and evolution of structural complexity of quintinite polytypes from the Kovdor alkaline massif, Kola peninsula, Russia. Mineral. Mag. 2018, 82, 329–346. [Google Scholar] [CrossRef]
- Yakovenchuk, V.N.; Ivanyuk, G.Y.; Mikhailova, Y.A.; Selivanova, E.A.; Krivovichev, S.V. Pakhomovskyite, Co3(PO4)2·8H2O, a new mineral species from Kovdor, Kola Peninsula, Russia. Can. Mineral. 2006, 44, 117–123. [Google Scholar] [CrossRef]
- Yakovenchuk, V.N.; Ivanyuk, G.Y.; Pakhomovsky, Y.A.; Panikorovskii, T.L.; Britvin, S.N.; Krivovichev, S.V.; Shilovskikh, V.V.; Bocharov, V.N. Kampelite, Ba3Mg1.5Sc4(PO4)6(OH)3·4H2O, a new very complex Ba-Sc phosphate mineral from the Kovdor phoscorite-carbonatite complex (Kola Peninsula, Russia). Mineral. Petrol. 2017, 4, 111–121. [Google Scholar] [CrossRef]
- Tomilin, M.G.; Ivanyuk, G.Y. The application of thin nematic liquid crystal layers to mineral analysis. Liq. Cryst. 1993, 14, 1599–1606. [Google Scholar] [CrossRef]
- UTHSCSA. ImageTool 3.0. Available online: http://www.compdent.uthscsa.edu/dig/pub/IT (accessed on 8 July 2013).
- Rosstandart FR.1.31.2016.25424. The Method for Determining the Content of Rare-Earth Elements (Y, La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, Lu), Sodium, Aluminum, Potassium, Calcium, Titanium, Iron, Thorium and Uranium in Apatite Mineral Raw Materials and Phosphogypsum by Inductively Coupled Plasma Mass Spectrometry. Available online: http://www.fundmetrology.ru/06_metod/2view_file.aspx? id=25424 (accessed on 27 June 2018).
- Dolivo-Dobrovolsky, D.D. MINAL, Free Software. Available online: http://www.dimadd.ru (accessed on 8 July 2013).
- StatSoft Inc. Statistica 8. Available online: www.statsoft.ru (accessed on 27 June 2018).
- Systat Software Inc. TableCurve 2D. Available online: www.sigmaplot.co.uk/products/tablecurve2d/tablecurve2d.php (accessed on 19 June 2018).
- Micromine Pty Ltd. Micromine 16.1. Available online: www.micromine.com (accessed on 27 June 2018).
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Agilent CrysAlis PRO 2014. Available online: https://www.rigaku.com/en/products/smc/crysalis (accessed on 19 June 2018).
- Putz, H.; Brandenburg, K. Diamond–Crystal and Molecular Structure Visualization; Crystal Impact GbR: Bonn, Germany, 2012. [Google Scholar]
- De Villiers, J.P.R.; Liles, D.C. The crystal-structure and vacancy distribution in 6C pyrrhotite. Am. Mineral. 2010, 95, 148–152. [Google Scholar] [CrossRef]
- International Tables for Crystallography. Volume C: Mathematical, Physical and Chemical Tables; Wilson, A.J.C., Ed.; Kluwer Academic: Dordrecht, The Netherlands, 1992. [Google Scholar]
- Alsén, N. Röntgenographische Untersuchung der Kristallstrukturen von Magnetkies, Breithauptit, Pentlandit, Millerit und verwandten Verbindungen. Geologiska Föreningen i Stockholm Förhandlingar 1925, 47, 19–72. [Google Scholar] [CrossRef]
- Tokonami, M.; Nishiguchi, K.; Morimoto, N. Crystal structure of a monoclinic pyrrhotite (Fe7S8). Am. Mineral. 1972, 57, 1066–1080. [Google Scholar]
- Morimoto, N.; Gyobu, A.; Mukaiyama, H.; Izawa, E. Crystallography and stability of pyrrhotites. Econ. Geol. 1975, 70, 824–833. [Google Scholar] [CrossRef]
- De Villiers, J.P.R.; Liles, D.C.; Becker, M. The crystal structure of a naturally occurring 5C pyrrhotite from Sudbury, its chemistry, and vacancy distribution. Am. Mineral. 2009, 94, 1405–1410. [Google Scholar] [CrossRef]
- Koto, K.; Morimoto, N.; Gyobu, A. The superstructure of the intermediate pyrrhotite. I. Partially disordered distribution of metal vacancy in the 6 C type, Fe 11 S 12. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1975, 31, 2759–2764. [Google Scholar] [CrossRef]
- Yamamoto, A.; Nakazawa, H. Modulated structure of the NC-type (N = 5.5) pyrrhotite, Fe1−xS. Acta Crystallogr. Sect. A 1982, 38, 79–86. [Google Scholar] [CrossRef]
- De Villiers, J. The Composition and Crystal Structures of Pyrrhotite: A Common but Poorly Understood Mineral. Available online: http://www.mintek.co.za/Mintek75/Proceedings/L01-DeVilliers.pdf (accessed on 19 June 2018).
- Liles, D.C.; de Villiers, J.P.R. Redetermination of the structure of 5C pyrrhotite at low temperature and at room temperature. Am. Mineral. 2012, 97, 257–261. [Google Scholar] [CrossRef]
- Kaneda, H.; Takenouchi, S.; Shoji, T. Stability of pentlandite in the Fe-Ni-Co-S system. Miner. Depos. 1986, 21, 169–180. [Google Scholar] [CrossRef]
- Barton, P.B.; Toulmin, P. Phase relations involving sphalerite in the Fe-Zn-S system. Econ. Geol. 1966, 61, 815–849. [Google Scholar] [CrossRef]
- Lusk, J.; Ford, C.E. Experimental extension of the sphalerite geobarometer to 10 kbar. Am. Mineral. 1978, 63, 516–519. [Google Scholar]
- Scott, S.D.; Barnes, H.L. Sphalerite geothermometry and geobarometry. Econ. Geol. 1971, 66, 653–669. [Google Scholar] [CrossRef]
- Fleet, M.E.M.E. Phase Equilibria at High Temperatures. Rev. Mineral. Geochem. 2006, 61, 365–419. [Google Scholar] [CrossRef]
- Ghiorso, M.S.; Evans, B.W. Thermodynamics of rhombohedral oxide solid solutions and a revision of the Fe-Ti two-oxide geothermometer and oxygen-barometer. Am. J. Sci. 2008, 308, 957–1039. [Google Scholar] [CrossRef]
- Yund, R.A.; Hall, H.T. Kinetics and Mechanism of Pyrite Exsolution from Pyrrhotite. J. Petrol. 1970, 11, 381–404. [Google Scholar] [CrossRef]
- Kissin, S.A.; Scott, S.D. Phase relations involving pyrrhotite below 350 degrees C. Econ. Geol. 1982, 77, 1739–1754. [Google Scholar] [CrossRef]
- Nekrasov, I.J.; Besmen, N.I. Pyrite-pyrrhotite geothermometer. Distribution of cobalt, nickel and tin. Phys. Chem. Earth 1979, 11, 767–771. [Google Scholar] [CrossRef]
- Lusk, J.; Scott, S.D.; Ford, C.E. Phase relations in the Fe-Zn-S system to 5 kbars and temperatures between 325 degrees and 150 degrees C. Econ. Geol. 1993, 88, 1880–1903. [Google Scholar] [CrossRef]
- Kapustin, Y. Mineralogy of the Weathering Crust of Carbonatites; Nedra: Moscow, Russia, 1973. (In Russian) [Google Scholar]
- Liferovich, R.P.; Pakhomovsky, Y.A.; Yakovenchuk, V.N.; Bogdanova, A.N.; Bakhchisaraitsev, A.Y. Vivianite minerals group and bobierrite from the Kovdor massif. Zap. RMO 1999, 128, 109–117. [Google Scholar]
- Liferovich, R.P.; Sokolova, E.V.; Hawthorne, F.C.; Laajoki, K.V.O.; Gehor, S.; Pakhomovsky, Y.A.; Sorokhtina, N.V. Gladiusite, Fe3+2(Fe2+,Mg)4(PO4)(OH)11(H2O), a new hydrothermal mineral species from the phoscorite carbonatite unit, Kovdor complex, Kola Peninsula, Russia. Can. Mineral. 2000, 38, 1477–1485. [Google Scholar] [CrossRef]
- Doyle, C.D.; Naldrett, A.J. Ideal mixing of divalent cations in mafic magma. II. The solution of NiO and the partitioning of nickel between coexisting olivine and liquid. Geochim. Cosmochim. Acta 1987, 51, 213–219. [Google Scholar] [CrossRef]
- Seifert, S.; O’Neill, H.S.C.; Brey, G. The partitioning of Fe, Ni and Co between olivine, metal, and basaltic liquid: An experimental and thermodynamic investigation, with application to the composition of the lunar core. Geochim. Cosmochim. Acta 1988, 52, 603–616. [Google Scholar] [CrossRef]
- Ehlers, K.; Grove, T.L.; Sisson, T.W.; Recca, S.I.; Zervas, D.A. The effect of oxygen fugacity on the partitioning of nickel and cobalt between olivine, silicate melt, and metal. Geochim. Cosmochim. Acta 1992, 56, 3733–3743. [Google Scholar] [CrossRef]
- Li, C.; Ripley, E.M.; Mathez, E.A. The effect of S on the partitioning of Ni between olivine and silicate melt in MORB. Chem. Geol. 2003, 201, 295–306. [Google Scholar] [CrossRef]
- Gaetani, G.A.; Grove, T.L. Partitioning of moderately siderophile elements among olivine, silicate melt, and sulfide melt: Constraints on core formation in the Earth and Mars. Geochim. Cosmochim. Acta 1997, 61, 1829–1846. [Google Scholar] [CrossRef]
- Kiseeva, E.S.; Wood, B.J. A simple model for chalcophile element partitioning between sulphide and silicate liquids with geochemical applications. Earth Planet. Sci. Lett. 2013, 383, 68–81. [Google Scholar] [CrossRef]
- Li, Y.; Audétat, A. Effects of temperature, silicate melt composition, and oxygen fugacity on the partitioning of V, Mn, Co, Ni, Cu, Zn, As, Mo, Ag, Sn, Sb, W, Au, Pb, and Bi between sulfide phases and silicate melt. Geochim. Cosmochim. Acta 2015, 162, 25–45. [Google Scholar] [CrossRef]
- Powell, A.V.; Vaqueiro, P.; Knight, K.S.; Chapon, L.C.; Sánchez, R.D. Structure and magnetism in synthetic pyrrhotite Fe7S8: A powder neutron-diffraction study. Phys. Rev. B 2004, 70, 014415. [Google Scholar] [CrossRef]
- Krivovichev, S.V. Topological complexity of crystal structures: Quantitative approach. Acta Crystallogr. Sect. A Found. Crystallogr. 2012, 68, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Krivovichev, S.V. Structural complexity of minerals: Information storage and processing in the mineral world. Mineral. Mag. 2013, 77, 275–326. [Google Scholar] [CrossRef]
- Holzheid, A.; Palme, H. The influence of FeO on the solubilities of cobalt and nickel in silicate melts. Geochim. Cosmochim. Acta 1996, 60, 1181–1193. [Google Scholar] [CrossRef]
- Keppler, H. Water solubility in carbonatite melts. Am. Mineral. 2003, 88, 1822–1824. [Google Scholar] [CrossRef]
- Zhong, R.; Brugger, J.; Chen, Y.; Li, W. Contrasting regimes of Cu, Zn and Pb transport in ore-forming hydrothermal fluids. Chem. Geol. 2015, 395, 154–164. [Google Scholar] [CrossRef]
- Leroy, A. Palabora—Not just another copper mine. Miner. Ind. Int. 1992, 1005, 14–19. [Google Scholar]
- Palabora_Mining_Company_Limited. The geology and the economic deposits of copper, iron, and vermiculite in the Palabora igneous complex: A brief review. Econ. Geol. 1976, 71, 177–192. [Google Scholar]
- Vielreicher, N.M.; Groves, D.I.; Vielreicher, R.M. The Phalaborwa (Palabora) Deposit and its Potential Connection to Iron-Oxide Copper-Gold Deposits of Olympic Dam Type. In Hydrothermal Iron Oxide Copper-Gold & Related Deposits: A Global Perspective, Volume 1; Porter, T.M., Ed.; PGS Publishing: Adelaide, Australia, 2000; pp. 321–329. [Google Scholar]
- Karchevsky, P.I. Sulfide, Strontium, and REE Mineralozation in Phoscorites and Carbonatites of the Turiy Complex (Russia) and Loolekop Deposit (RSA); Kolo: St. Petersburg, Russia, 2005. [Google Scholar]
- Verwoerd, W. Mineral deposits associated with carbonatites and alkaline rocks. In Mineral Deposits of Southern Africa. Volume 2; Anhaeusser, C.R., Maske, S., Eds.; Geological Society of South Africa: Johannesburg, South Africa, 1986; pp. 2173–2191. [Google Scholar]
- Ericsson, S. Phalaborwa: A saga of magmatism, metasomatism and miscibility. In Carbonatites: Genesis and Evolution; Bell, K., Ed.; Unwin Hyman: London, UK, 1989; pp. 221–250. [Google Scholar]
- Heinrich, E. The Palabora Carbonatitic Complex—A Unique Copper Deposit. Can. Mineral. 1970, 10, 585–598. [Google Scholar]
- Gaillard, F.; Scaillet, B.; Pichavant, M.; Bény, J.-M. The effect of water and fO2 on the ferric–ferrous ratio of silicic melts. Chem. Geol. 2001, 174, 255–273. [Google Scholar] [CrossRef]
- Wilke, M.; Behrens, H.; Burkhard, D.J.; Rossano, S. The oxidation state of iron in silicic melt at 500 MPa water pressure. Chem. Geol. 2002, 189, 55–67. [Google Scholar] [CrossRef]
- Schuessler, J.A.; Botcharnikov, R.E.; Behrens, H.; Misiti, V.; Freda, C. Amorphous Materials: Properties, structure, and Durability: Oxidation state of iron in hydrous phono-tephritic melts. Am. Mineral. 2008, 93, 1493–1504. [Google Scholar] [CrossRef]
- Richards, J.P. The oxidation state, and sulfur and Cu contents of arc magmas: Implications for metallogeny. Lithos 2014, 233, 27–45. [Google Scholar] [CrossRef]
- Groves, D.I.; Vielreicher, N.M. The Phalabowra (Palabora) carbonatite-hosted magnetite-copper sulfide deposit, South Africa: An end-member of the iron-oxide copper-gold-rare earth element deposit group? Miner. Depos. 2001, 36, 189–194. [Google Scholar] [CrossRef]


| Element | Detection Limit, wt % | Standards for EPMA Analyses | Element | Detection Limit, wt % | Standards for EPMA Analyses |
|---|---|---|---|---|---|
| Mg | 0.1 | Pyrope | Se | 0.08 | Synthetic PbSe |
| Al | 0.05 | Pyrope | Mo | 0.1 | Metallic molybdenum |
| Si | 0.05 | Diopside | Pd | 0.05 | Metallic palladium |
| S | 0.05 | Synthetic Fe10S11 | Ag | 0.05 | Metallic silver |
| K | 0.03 | Wadeite | Cd | 0.05 | Synthetic CdS |
| Ca | 0.03 | Diopside | Sn | 0.05 | Metallic tin |
| Mn | 0.01 | Synthetic MnCO3 | Sb | 0.05 | Antimony |
| Fe | 0.01 | Synthetic Fe10S11 | Te | 0.05 | Synthetic PbTe |
| Co | 0.01–0.03 | Metallic cobalt | Pt | 0.05 | Metallic platinum |
| Ni | 0.01 | Synthetic NiAs | Au | 0.05 | Metallic gold |
| Cu | 0.01 | Metallic copper | Pb | 0.05 | Synthetic PbSe |
| Zn | 0.01 | Synthetic ZnO | Bi | 0.06 | Bismuth |
| As | 0.05 | Synthetic NiAs |
| Rock | Host Silicate Rock | (Ap)-Fo Phoscorite | Low-Cb Mag-Rich Phoscorite | Cal-Rich Phoscorite and Related Carbonatite | Vein Calcite Carbonatite |
|---|---|---|---|---|---|
| n | 44 | 17 | 90 | 50 | 16 |
| S, wt % | 0.22 | 0.20 | 0.15 | 0.29 | 1.53 |
| Fe | 6.49 | 7.88 | 29.79 | 17.48 | 3.85 |
| Cu, ppm | 56 | 44 | 37 | 78 | 55 |
| Zn | 89 | 100 | 198 | 117 | 28 |
| Co | 40 | 65 | 92 | 70 | 29 |
| Ni | 111 | 49 | 39 | 17 | 17 |
| Ag | 2 | 1 | 8 | 6 | 1 |
| Pb | 1 | 1 | 1 | 2 | 3 |
| Rock | Host Rock | Phoscorite and Related Carbonatite | Vein Carbonatite | ||||
|---|---|---|---|---|---|---|---|
| Foidolite | Diopsidite | (Ap)-Fo | Low-Cb Mag-rich | Cal-rich | Cal | Dol | |
| n | 9 | 27 | 13 | 60 | 94 | 26 | 25 |
| D, µm | 100 ± 80 15–300 | 140 ± 90 10–300 | 120 ± 90 50–400 | 200 ± 80 50–450 | 220 ± 90 10–600 | 230 ± 90 100–600 | 200 ± 100 50–600 |
| S, wt % | 39.2 ± 0.5 38.54–40.38 | 39.1 ± 0.5 37.67–39.98 | 39 ± 1 36.72–39.82 | 39.1 ± 0.6 36.31–40.52 | 38.9 ± 0.7 35.81–40.36 | 39.0 ± 0.4 38.27–39.77 | 39.0 ± 0.5 37.74–39.77 |
| Fe | 60.5 ± 0.5 59.83–61.11 | 60.6 ± 0.9 59.31–63.01 | 61 ± 2 59.77–64.13 | 60.8 ± 0.8 58.18–63.51 | 60.9 ± 0.8 58.93–63.37 | 60.8 ± 0.5 59.77–61.87 | 60.7 ± 0.6 59.37–61.92 |
| Co | 0.1 ± 0.1 <0.01–0.43 | 0.06 ± 0.06 <0.01–0.19 | 0.1 ± 0.1 <0.01–0.39 | 0.2 ± 0.1 <0.01–0.39 | 0.2 ± 0.1 <0.01–0.54 | 0.14 ± 0.09 0.02–0.36 | 0.13 ± 0.08 <0.01–0.33 |
| Ni | 0.3 ± 0.1 0.03–0.44 | 0.3 ± 0.2 <0.01–0.82 | 0.2 ± 0.1 <0.01–0.38 | 0.06 ± 0.09 <0.01–0.36 | 0.04 ± 0.09 <0.01–0.77 | 0.01 ± 0.02 <0.01–0.08 | 0.1 ± 0.1 <0.01–0.38 |
| Fe, at. % | 46.8 ± 0.4 45.85–47.35 | 47.0 ± 0.7 46.02–48.98 | 48 ± 1 46.34–50.02 | 47.1 ± 0.7 45.26–50.10 | 47.2 ± 0.8 45.80–50.22 | 47.2 ± 0.4 46.20–47.87 | 47.2 ± 0.5 46.21–48.08 |
| Ni | 0.2 ± 0.1 0.00–0.32 | 0.2 ± 0.2 0.00–0.59 | 0.1 ± 0.1 0.00–0.26 | 0.04 ± 0.07 0.00–0.26 | 0.03 ± 0.07 0.00–0.58 | 0.00 ± 0.01 0.00–0.05 | 0.06 ± 0.08 0.00–0.31 |
| Co | 0.1 ± 0.1 0.00–0.32 | 0.04 ± 0.05 0.00–0.16 | 0.05 ± 0.07 0.00–0.27 | 0.11 ± 0.08 0.00–0.27 | 0.14 ± 0.09 0.00–0.38 | 0.10 ± 0.06 0.00–0.27 | 0.10 ± 0.07 0.00–0.27 |
| S | 52.9 ± 0.4 52.55–53.88 | 52.8 ± 0.6 51.02–53.56 | 52 ± 1 49.98–53.39 | 52.7 ± 0.7 49.90–54.47 | 52.6 ± 0.7 49.73–53.82 | 52.7 ± 0.4 52.03–53.53 | 52.7 ± 0.5 51.76–53.36 |
| Sample | 1 (00-01) | 2 (36-33) | 3 (00-10-41) | 4 (01-11-91) | 5 (00-51) |
|---|---|---|---|---|---|
| Modification | 4C | 5C | 5C | 5C | 5C |
| Refined formula | Fe6.78S8 | Fe8.91Ni0.25S10 | Fe8.99S10 | Fe8.84S10 | Fe8.88S10 |
| Formula weight | 634.86 | 833.04 | 822.41 | 814.45 | 816.41 |
| Temperature/K | 100 (2) | 100 (2) | 100 (2) | 100 (2) | 100 (2) |
| Crystal system | orthorhombic | monoclinic | monoclinic | monoclinic | monoclinic |
| Space group | Imm2 | C2 | C2 | C2 | P21 |
| a (Å) | 22.678 (4) | 11.8624 (9) | 11.8875 (11) | 11.8706 (7) | 6.8477 (5) |
| b (Å) | 3.4131 (5) | 6.8613 (5) | 6.8667 (6) | 6.8589 (5) | 28.584 (4) |
| c (Å) | 5.9083 (13) | 28.593 (2) | 28.661 (2) | 28.5953 (16) | 6.8518 (5) |
| α (°) | 90 | 90 | 90 | 90 | 90 |
| β (°) | 90 | 89.897 (8) | 90.023 (8) | 89.982 (5) | 119.972 (11) |
| γ (°) | 90 | 90 | 90 | 90 | 90 |
| Volume/Å3 | 457.31 (15) | 2327.3 (3) | 2339.6 (4) | 2328.2 (3) | 1161.8 (2) |
| Z | 2 | 8 | 8 | 8 | 4 |
| ρcalc (g/cm3) | 4.611 | 4.755 | 4.670 | 4.647 | 4.668 |
| μ (mm−1) | 12.202 | 12.914 | 12.542 | 12.432 | 12.499 |
| F (000) | 608.0 | 3190.0 | 3149.0 | 3119.0 | 1563.0 |
| Crystal size (mm3) | 0.15 × 0.15 × 0.15 | 0.18 × 0.18 × 0.10 | 0.13 × 0.13 × 0.13 | 0.13 × 0.13 × 0.05 | 0.12 × 0.12 × 0.12 |
| Radiation | MoKα (λ = 0.71073) | ||||
| 2Θ range for data collection (°) | 7.126–54.92 | 5.7–61.33 | 5.69–62.04 | 5.70–50.00 | 5.7–61.932 |
| Index ranges | −29 ≤ h ≤ 27, −3 ≤ k ≤ 4, −4 ≤ l ≤ 7 | −15 ≤ h ≤ 16, −8 ≤ k ≤ 9, −40 ≤ l ≤ 23 | −16 ≤ h ≤ 16, −6 ≤ k ≤ 9, −33 ≤ l ≤ 40 | −14 ≤ h ≤ 14, −8 ≤ k ≤ 7, −34 ≤ l ≤ 33 | −9 ≤ h ≤ 7, −38 ≤ k ≤ 32, −9 ≤ l ≤ 9 |
| Reflections collected | 1018 | 5920 | 10,932 | 7368 | 6471 |
| Independent reflections | 475 | 4494 | 4797 | 3597 | 4708 |
| Rint, Rsigma | 0.0171, 0.0189 | 0.0354, 0.0397 | 0.0396, 0.0545 | 0.0607, 0.0571 | 0.0278, 0.0271 |
| Data/restraints/parameters | 475/31/53 | 4494/127/255 | 4797/1/136 | 3597/1/135 | 4708/211/216 |
| Goodness-of-fit on F2 | 1.202 | 1.074 | 1.145 | 1.608 | 1.125 |
| Final R indexes [I>= 2σ(I)] | R1 = 0.0780 wR2 = 0.1173 | R1 = 0.0896, wR2 = 0.2586 | R1 = 0.0884, wR2 = 0.2361 | R1 = 0.1170, wR2 = 0.3522 | R1 = 0.0881, wR2 = 0.1802 |
| Final R indexes [all data] | R1 = 0.0817 wR2 = 0.1197 | R1 = 0.1009, wR2 = 0.2728 | R1 = 0.1311, wR2 = 0.2941 | R1 = 0.1286, wR2 = 0.3608 | R1 = 0.0990, wR2 = 0.1874 |
| Largest diff. peak/hole/e Å−3 | 1.61/−2.06 | 4.02/−3.57 | 5.13/−5.73 | 4.01/−4.36 | 4.93/−4.66 |
| Flack parameter | 0.49 (12) | 0.53 (19) | 0.5 (2) | 0.26 (15) | 0.09 (6) |
| Rock | Host Rock | Phoscorite and Related Carbonatite | Vein Carbonatite | ||||
|---|---|---|---|---|---|---|---|
| Foidolite | Diopsidite | (Ap)-Fo | Low-Cb Mag-rich | Cal-rich | Cal | Dol | |
| n | 6 | 24 | 13 | 51 | 67 | 17 | 8 |
| S, wt % | 33 ± 1 32.34–34.80 | 33 ± 1 27.51–34.73 | 33.0 ± 0.5 32.06–33.74 | 32.8 ± 0.7 31.84–35.24 | 32.7 ± 0.8 31.61–36.12 | 32.6 ± 0.4 31.80–33.57 | 32.4 ± 0.9 30.64–33.66 |
| Fe | 26 ± 9 11.64–30.64 | 28 ± 3 15.52–32.87 | 23 ± 7 9.25–30.94 | 14 ± 7 4.81–28.00 | 13 ± 8 3.52–35.02 | 9 ± 3 4.24–15.42 | 17 ± 9 4.58–28.42 |
| Co | 10 ± 20 1.72–47.16 | 10 ± 10 0.77–54.33 | 20 ± 20 4.50–52.27 | 40 ± 10 10.11–61.91 | 50 ± 20 1.21–61.92 | 57 ± 4 47.63–62.36 | 30 ± 20 8.16–63.16 |
| Ni | 30 ± 10 8.86–34.53 | 30 ± 8 2.65–37.12 | 20 ± 10 5.11–34.47 | 10 ± 9 0.7–30.14 | 10 ± 10 0.62–32.96 | 2 ± 3 0.25–10.26 | 20 ± 10 3.94–32.21 |
| Ni, apfu | 3 ± 2 1.20–4.64 | 4 ± 1 0.42–5.01 | 3 ± 1 0.68–4.64 | 1 ± 1 0.09–3.93 | 1 ± 1 0.08–4.31 | 0.3 ± 0.3 0.03–1.36 | 2 ± 2 0.53–4.24 |
| Fe | 4 ± 1 1.65–4.23 | 3.9 ± 0.4 2.59–4.53 | 3.2 ± 0.9 1.28–4.38 | 1.9 ± 0.9 0.68–3.87 | 2 ± 1 0.50–4.51 | 1.2 ± 0.4 0.60–2.17 | 0.65 ± 4.07 3.87–23.32 |
| Co | 2 ± 3 0.22–6.35 | 1 ± 2 0.10–8.60 | 3 ± 2 0.60–6.88 | 6 ± 2 1.31–8.38 | 6 ± 2 0.15–8.26 | 7.6 ± 0.6 6.30–8.45 | 4 ± 3 1.07–8.52 |
| S | 8 | 8 | 8 | 8 | 8 | 8 | 8 |
| Rock | Host Rock | Phoscorite and Related Carbonatite | Vein Carbonatite | ||||
|---|---|---|---|---|---|---|---|
| Foidolite | Diopsidite | (Ap)-Fo | Low-Cb Mag-rich | Cal-rich | Cal | Dol | |
| n | 3 | 13 | 3 | 14 | 24 | 9 | 17 |
| S, wt % | 31.6 ± 0.6 | 32.0 ± 0.6 | 32 ± 1 | 31.7 ± 0.9 | 31.7 ± 0.9 | 31.5 ± 0.7 | 32 ± 1 |
| 31.02–32.22 | 30.96–32.83 | 30.43–32.88 | 29.60–33.22 | 29.78–33.86 | 30.70–32.59 | 27.60–33.44 | |
| Mn | 0.5 ± 0.5 | 0.3 ± 0.5 | 0.8 ± 0.2 | 0.6 ± 0.9 | 1 ± 1 | 1 ± 1 | 0.5 ± 0.5 |
| <0.01–0.95 | <0.01–1.44 | 0.57–0.99 | <0.01–3.51 | <0.01–6.17 | <0.01–2.89 | <0.01–1.52 | |
| Fe | 10 ± 1 | 9 ± 1 | 8.8 ± 0.9 | 10 ± 2 | 10 ± 2 | 9 ± 2 | 10 ± 2 |
| 9.51–11.51 | 6.18–11.33 | 8.13–9.79 | 6.67–15.06 | 5.51–15.82 | 5.69–13.23 | 5.86–12.77 | |
| Co | <0.01 | <0.01 | 0.1 ± 0.2 | 0.2 ± 0.3 | 0.1 ± 0.2 | 0.2 ± 0.4 | 0.2 ± 0.3 |
| <0.01–0.40 | <0.01–0.99 | <0.01–0.63 | <0.01–1.15 | <0.01–1.04 | |||
| Ni | <0.01 | <0.01 | <0.01 | <0.01 | 0.01 ± 0.03 | <0.01 | <0.01 |
| <0.01–0.13 | |||||||
| Zn | 56 ± 1 | 58 ± 2 | 58 ± 2 | 57 ± 3 | 54 ± 5 | 57 ± 5 | 57 ± 3 |
| 54.52–57.01 | 54.04–61.50 | 56.78–60.27 | 51.71–62.99 | 42.41–63.11 | 49.17–62.79 | 52.99–62.12 | |
| Cd | 2 ± 1 | 1 ± 1 | <0.01 | 0.6 ± 0.8 | 3 ± 3 | 2 ± 2 | 0.1 ± 0.3 |
| 0.73–3.37 | <0.01–3.57 | <0.01–2.98 | <0.01–10.01 | <0.01–5.96 | <0.01–1.03 | ||
| Zn, apfu | 0.86 ± 0.01 | 0.89 ± 0.04 | 0.90 ± 0.06 | 0.89 ± 0.07 | 0.83 ± 0.09 | 0.88 ± 0.09 | 0.88 ± 0.07 |
| 0.85–0.87 | 0.81–0.95 | 0.86–0.97 | 0.77–1.04 | 0.63–0.99 | 0.75–1.00 | 0–0 | |
| Fe | 0.19 ± 0.02 | 0.16 ± 0.03 | 0.16 ± 0.02 | 0.17 ± 0.04 | 0.19 ± 0.04 | 0.17 ± 0.05 | 0.19 ± 0.04 |
| 0.18–0.21 | 0.11–0.20 | 0.14–0.17 | 0.12–0.28 | 0.10–0.27 | 0.11–0.25 | 0.11–0.27 | |
| Mn | 0.01 ± 0.01 | 0.01 ± 0.01 | 0.01 | 0.01 ± 0.02 | 0.03 ± 0.02 | 0.01 ± 0.02 | 0.01 ± 0.01 |
| 0.00–0.02 | 0.00–0.03 | 0.01–0.02 | 0.00–0.06 | 0.00–0.11 | 0.00–0.05 | 0.00–0.03 | |
| Cd | 0.02 ± 0.01 | 0.01 ± 0.01 | – | 0.01 ± 0.01 | 0.03 ± 0.03 | 0.02 ± 0.02 | 0.00 |
| 0.01–0.03 | 0.00–0.03 | 0.00–0.03 | 0.00–0.09 | 0.00–0.05 | 0.00–0.01 | ||
| Co | – | – | 0.00 | 0.00 ± 0.01 | 0.00 | 0.00 ± 0.01 | 0.00 ± 0.01 |
| 0.00–0.01 | 0.00–0.02 | 0.00–0.01 | 0.00–0.02 | 0.00–0.02 | |||
| S | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| P, kbar | 2.2 ± 0.1 | 4 ± 2 | 4 ± 1 | 4 ± 2 | 3 ± 2 | 5 ± 3 | 3 ± 2 |
| 2.1–2.3 | 0.5–8.9 | 2.6–5.4 | 1.1–7.6 | 0.4–9.9 | 1.8–9.4 | 0.2–9.5 | |
| Rock | Low-Cb Mag-Rich Phoscorite | Cal-Rich Phoscorite/Carbonatite | Dol-Carbonatite |
|---|---|---|---|
| n | 2 | 8 | 2 |
| S, wt % | 52.4 ± 0.6/51.94–52.80 | 53.0 ± 0.2/52.76–53.25 | 53.1 ± 0.2/52.97–53.30 |
| Fe | 48 ± 1/47.86–48.65 | 46.8 ± 0.9/45.52–48.51 | 46.1 ± 0.2/45.93–46.27 |
| Co | 0.4 ± 0.2/0.26–0.49 | 0.1 ± 0.2/<0.01–0.01 | 0.8 ± 0.6/0.35–1.21 |
| Ni | <0.01 | 0.03 ± 0.05/<0.01–0.13 | 0.02 ± 0.01/0.02–0.03 |
| Fe, apfu | 1.05 ± 0.04/1.02–1.08 | 1.02 ± 0.02/0.98–1.05 | 1.00 ± 0.01/0.99–1.00 |
| Co | 0.01 | 0.00/0.00–0.01 | 0.02 ± 0.01/0.01–0.03 |
| S | 2 | 2 | 2 |
| T, °C | 170 ± 20/159–188 | 300 ± 100/235–444 | 170 ± 90/106–228 |
| Mineral | Mck | Vlt | Cls |
| Sample | K-02-124 | 74/67.2 | 73/205.8 |
| Rock | CM-phoscorite | Mag-Dol-Srp rock | Vein Cal-carbonatite |
| S | 35.61/8.00 | 43.31/4.00 | bd |
| Mn | bd | bd | bd |
| Fe | 58.27/7.52 | 16.99/0.90 | bd |
| Co | 0.94/0.12 | 13.33/0.67 | bd |
| Ni | 5.56/0.68 | 26.40/1.33 | bd |
| Zn | bd | bd | bd |
| Se | bd | bd | 26.82/1.00 |
| Cd | bd | bd | bd |
| Pb | bd | bd | 73.98/1.05 |
| Total | 100.38/16.32 | 100.03/6.90 | 100.80/2.05 |
| Mineral | Hss | Mch | Ptz |
| Sample | 931/341.2 | ||
| Rock | Diopsidite | ||
| Ag | 56.57/1.97 | bd | 37.07/2.84 |
| Te | 34.04/1.00 | 44.54/1.54 | 30.84/2.00 |
| Pd | bd | 8.38/0.35 | bd |
| Pt | bd | 25.40/0.58 | bd |
| Au | bd | bd | 17.93/0.75 |
| Pb | 8.91/0.16 | bd | 14.40/0.58 |
| Bi | bd | 21.68/0.46 | bd |
| Total | 99.52/3.13 | 100.00/2.93 | 100.24/6.17 |
| Mineral | Py | Mrc | Djf | Val | Brt |
|---|---|---|---|---|---|
| Sample | 1011/79/6 | K-96-19-1 | 1004/656.5 | K-051 | 941/41.5 |
| Rock | AF-phoscorite | Dol-carbonatite | Cal-carbonatite | Dol-carbonatite | MAF-foscorite |
| Mg | bd | bd | bd | 11.08/2.53 | bd |
| Al | bd | bd | bd | 0.67/0.14 | bd |
| S | 53.05/2.00 | 53.34/2.00 | 32.86/26.00 | 23.16/4.00 | 13.65/1.00 |
| Cl | bd | bd | 1.43/1.02 | bd | bd |
| K | bd | bd | 9.33/6.05 | bd | bd |
| Ca | bd | bd | bd | bd | 0.37/0.02 |
| Fe | 46.19/1.00 | 46.79/1.01 | 35.21/15.99 | 31.81/3.15 | bd |
| Co | 0.39/0.01 | bd | 0.03/0.01 | bd | bd |
| Ni | 0.05/0.00 | bd | 0.05/0.02 | 2.93/0.28 | bd |
| Cu | bd | bd | 21.52/8.59 | 9.23/0.80 | bd |
| Sr | bd | bd | bd | bd | 0.87/0.02 |
| Ba | bd | bd | bd | bd | 57.02/0.96 |
| Total | 99.68/3.01 | 100.13/3.01 | 100.43/57.70 | 78.88/10.90 | 71.91/2.00 |
| Rock | Phoscorite and Related Carbonatite | Vein Carbonatite | |||
|---|---|---|---|---|---|
| (Ap)-Fo | Low-Cb Mag-Rich | Cal-Rich | Cal | Dol | |
| n | 2 | 2 | 2 | 2 | 2 |
| S, wt % | 34.1 ± 0.8 33.53–34.70 | 34.7 ± 0.1 34.60–34.79 | 35.0 ± 0.9 34.40–35.68 | 34.9 ± 0.1 34.86–35.00 | 35.1 ± 0.3 34.83–35.29 |
| Fe | 30.6 ± 0.2 30.40–30.73 | 29.9 ± 0.2 29.76–30.02 | 30.4 ± 0.3 30.22–30.65 | 30.1 ± 0.5 29.80–30.50 | 30.8 ± 0.1 30.74–30.92 |
| Co | <0.01 | 0.01 ± 0.01 <0.01–0.02 | 0.03 ± 0.1 0.02–0.04 | <0.01 | <0.01 |
| Ni | <0.01 | 0.02 ± 0.02 <0.01–0.04 | 0.01 ± 0.01 <0.01–0.02 | 0.01 ± 0.02 <0.01–0.02 | 0.02 ± 0.02 <0.01–0.03 |
| Cu | 35.0 ± 0.3 34.80–35.29 | 34.29 ± 0.05 34.26–34.32 | 34.3 ± 0.5 33.93–34.58 | 34.0 ± 0.3 33.80–34.24 | 34.5 ± 0.1 34.39–34.53 |
| Zn | <0.01 | <0.01 | <0.01 | 0.3 ± 0.3 0.00–0.50 | <0.01 |
| Cu, apfu | 1.04 ± 0.04 1.01–1.06 | 1.00 | 0.99 ± 0.04 0.96–1.01 | 0.99 ± 0.01 0.98–0.99 | 0.99 ± 0.01 0.99–1.00 |
| Fe | 1.03 ± 0.03 1.01–1.05 | 0.99 | 1.00 ± 0.02 0.99–1.01 | 0.99 ± 0.02 0.98–1.00 | 1.01 ± 0.01 1.00–1.01 |
| S | 2 | 2 | 2 | 2 | 2 |
| Mineral | Bn | Cbn | Mck | Sgn | Sp |
| Sample | KZh-25b | K-97-3 | K-97-3 | 2002/Bn2 | K-97-3 |
| Rock | CM-phoscorite | CMF-phoscorite | CMF-phoscorite | CMAF-phoscorite | CMF-phoscorite |
| S | 25.34/4.00 | 35.57/3.00 | 35.87/8.00 | 41.90/4.00 | 33.94/1.00 |
| Mn | bd | bd | bd | bd | 1.59/0.03 |
| Fe | 11.82/1.07 | 41.09/1.99 | 51.59/6.61 | 0.57/0.03 | 13.38/0.23 |
| Co | bd | bd | 12.50/1.52 | 19.72/1.02 | bd |
| Ni | bd | bd | 0.40/0.05 | 35.98/1.88 | bd |
| Cu | 62.67/4.99 | 23.70/1.01 | bd | 2.12/0.10 | 0.71/0.01 |
| Zn | bd | bd | bd | bd | 46.17/0.67 |
| Cd | bd | bd | bd | bd | 4.80/0.04 |
| Total | 99.83/10.06 | 100.36/6.00 | 100.36/16.18 | 100.29/7.03 | 100.59/1.98 |
| Mineral | Hwl | Apn | Acn | Vol | Tsu |
| Sample | 1009/121.4 | 910/348.2 | 999/76.6 | 913/57.1 | 913/57.1 |
| Rock | MF-phoscorite | Diopsidite | CMAF-phoscorite | CM-phoscorite | CM-phoscorite |
| S | 23.76/1.00 | 30.78/8.00 | 12.45/1.00 | bd | bd |
| Fe | 3.12/0.08 | 32.43/4.84 | bd | bd | 3.58/0.11 |
| Co | bd | 0.34/0.05 | bd | bd | bd |
| Ni | bd | 23.08/3.28 | bd | bd | bd |
| Cu | 6.50/0.14 | bd | bd | bd | bd |
| Zn | 8.29/0.17 | bd | bd | bd | bd |
| Ag | bd | 13.35/1.03 | 87.03/2.08 | 19.82/1.08 | bd |
| Cd | 58.11/0.70 | bd | bd | bd | bd |
| Te | bd | bd | bd | 43.52/2.00 | 38.07/1.00 |
| Bi | bd | bd | bd | 35.79/1.00 | 58.42/0.94 |
| Total | 99.78/2.09 | 99.98/17.20 | 99.48/3.08 | 99.13/4.08 | 100.07/2.05 |
| Mineral | Val | Djf | Cct | Cv | Py |
|---|---|---|---|---|---|
| Sample | KZh-25b | K-97-3 | K-01-1110 | K-02-122 | K-0042 |
| Rock | CM-phoscorite | CMF-phoscorite | CMAF-phoscorite | CM-phoscorite | Dol-carbonatite |
| Mg | 11.12/2.54 | bd | bd | bd | bd |
| Al | 3.57/0.73 | bd | bd | bd | bd |
| S | 23.14/4.00 | 32.91/26.00 | 19.64/1.00 | 31.68/1.00 | 53.20/2.00 |
| Cl | bd | 0.63/0.45 | bd | bd | bd |
| K | bd | 9.06/5.87 | bd | bd | bd |
| Fe | 25.66/2.55 | 35.55/16.13 | bd | 1.54/0.03 | 46.76/1.01 |
| Co | bd | 0.06/0.03 | bd | bd | 0.36/0.01 |
| Ni | 0.12/0.01 | 1.40/0.60 | bd | bd | 0.02/0.00 |
| Cu | 12.06/1.05 | 16.51/6.58 | 79.12/2.03 | 66.30/1.06 | bd |
| Ag | bd | 3.34/0.78 | bd | bd | bd |
| Total | 75.67/10.88 | 99.46/56.44 | 98.76/3.03 | 99.52/2.09 | 100.34/3.02 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanyuk, G.Y.; Pakhomovsky, Y.A.; Panikorovskii, T.L.; Mikhailova, J.A.; Kalashnikov, A.O.; Bazai, A.V.; Yakovenchuk, V.N.; Konopleva, N.G.; Goryainov, P.M. Three-D Mineralogical Mapping of the Kovdor Phoscorite-Carbonatite Complex, NW Russia: II. Sulfides. Minerals 2018, 8, 292. https://doi.org/10.3390/min8070292
Ivanyuk GY, Pakhomovsky YA, Panikorovskii TL, Mikhailova JA, Kalashnikov AO, Bazai AV, Yakovenchuk VN, Konopleva NG, Goryainov PM. Three-D Mineralogical Mapping of the Kovdor Phoscorite-Carbonatite Complex, NW Russia: II. Sulfides. Minerals. 2018; 8(7):292. https://doi.org/10.3390/min8070292
Chicago/Turabian StyleIvanyuk, Gregory Yu., Yakov A. Pakhomovsky, Taras L. Panikorovskii, Julia A. Mikhailova, Andrei O. Kalashnikov, Ayya V. Bazai, Victor N. Yakovenchuk, Nataly G. Konopleva, and Pavel M. Goryainov. 2018. "Three-D Mineralogical Mapping of the Kovdor Phoscorite-Carbonatite Complex, NW Russia: II. Sulfides" Minerals 8, no. 7: 292. https://doi.org/10.3390/min8070292
APA StyleIvanyuk, G. Y., Pakhomovsky, Y. A., Panikorovskii, T. L., Mikhailova, J. A., Kalashnikov, A. O., Bazai, A. V., Yakovenchuk, V. N., Konopleva, N. G., & Goryainov, P. M. (2018). Three-D Mineralogical Mapping of the Kovdor Phoscorite-Carbonatite Complex, NW Russia: II. Sulfides. Minerals, 8(7), 292. https://doi.org/10.3390/min8070292