Abstract
The need to explore more complex and low-grade silver ores and to develop novel and cost-effective processes to recover silver from waste is becoming an important challenge. This paper aims to characterize old, low-grade, silver tailings generated by the former Zgounder silver mine, located in Morocco. Understanding the mineralogical composition, particularly the silver deportment, was critical to allow the recovery of silver from these tailings. More than 88 samples of low grade tailings were sampled and characterized using chemical and mineralogical techniques. Froth flotation was used to recover silver bearing minerals using a combination of different collectors (dithiophosphate, dialkyl dithiophosphinates, Aero 7518, Aero 7640, alkyl dithiophosphates and potassium butyl-xanthate). The main goal was to optimize the flotation process at a laboratory scale through the testing of different parameters, such as collectors and frother types and dosage, activators and sulphidizing agents, and pH conditions. The characterization results showed that silver content varied between 30 and 440 ppm with an overall average content of 148 ppm. Silver occurs mainly in the form of native silver as well as in association with sulphides, such as acanthite and pyrite. Minor amounts of sphalerite, chalcopyrite, arsenopyrite, and hematite were identified. The flotation results showed the following optimum conditions: particle size of 63 µm, conditioning pH of 8.5, a combination of butyl-xanthate and dithiophosphate as collectors at a dosage of 80 g/t each, a concentration of 200 g/t of the activating agent (CuSO4), 30 g/t of methyl isobutyl carbonyl (MIBC) frother and a duration time of 8 min with slow kinetics. With these optimal conditions, it was possible to achieve a maximum silver recovery yield of 84% with 1745 ppm Ag grade to be cyanided. Moreover, the environmental behavior of the final clean tailings was demonstrated to be inert using Toxicity characteristic leaching procedure (TCLP) leaching tests.
1. Introduction
Recently, the demand for silver has increased exponentially due to the growing development of green technologies and nanotechnologies where silver is greatly required. Paradoxically to this high demand, the price of silver has increased, and world reserves are becoming scarcer. Worldwide silver reserves are significantly decreasing, and their price is continuously growing [1,2]. Silver price has more than tripled in the last two decades [3], and in the near future, silver will potentially be more expensive than gold. Therefore, the processing of complex low-grade ores has become a critical issue which the mining industry is facing. Many challenges and opportunities need to be addressed. Further research should be carried out in order to develop processing methods that take into account the effects of mineralogy, pre-processing methods, surface chemistry in regard to flotation rates, the activation and passivation of gangue minerals, the liberation degree of valuable minerals, etc. These aspects have been widely studied for gold, lead, zinc, and copper minerals. However, the literature shows that only few studies have dealt with the recovery of silver bearing minerals by froth flotation.
Regarding mineralogy, silver might be associated with hundreds of different silver bearing minerals with a wide range of compositions. This natural issue makes the recovery of silver bearing minerals a more complex and challenging task. Researchers have classified silver bearing minerals into eight different groups: silver alloys [4,5], sulphides [6], selenides [7,8], antimonides [9], tellurides [8,10], sulphosalts [11,12], halides [13], and solid solution [14]. Acanthite and argentite are the main silver sulphide minerals reported in the literature [14,15,16]. Silver may be associated with halide group minerals and sulphosalts. In some sulphosalts, Ag does not appear in the chemical formula because it occurs in the lattice sites. Ag can also be associated with telluride group minerals. It is highly associated with Au and sulphides. The naumannite mineral is the main Ag bearing mineral in the group of selenides. It is generally found in association with quartz and carbonates in hydrothermal veins. Silver can also take the form of a solid solution when some sulphides, such as chalcopyrite, sphalerite, galena and pyrite, are present in the silver ore. Special attention should be given to these minerals, because silver can replace iron, zinc, palladium, nickel, and cobalt [14].
Concerning silver beneficiation, different metallurgical processing techniques have been developed in order to recover silver from complex ores. Two main parameters need to be understood before selecting the appropriate recovery process: (i) silver deportment among Ag minerals (sulfosalts) and as a trace substitution within other minerals (pyrite, galena, etc.); and (ii) silver occurrence in terms of particle size distribution. These parameters can help to better choose the appropriate treatment method to recover silver bearing minerals of interest.
The flotation technique has generally been used to selectively separate valuable minerals from gangue non-valuable materials using the hydrophobicity properties [17,18,19,20,21,22]. It has been widely used to recover silver minerals from different ore deposits [23,24,25,26]. Silver minerals can be recovered either by selective or bulk flotation depending on the amount of minerals of interest within the ore [11]. Various types of reagents could be used to recover silver, based on the surface properties and the nature of present minerals. Activators, pH modifiers, depressants, collectors, frothers, and dispersants in different commercial nomenclatures are commonly used. Dorr and Bosqui [25] were among the first researchers interested in the effects of different reagents on the efficiency of silver mineral recovery. They described the reagents used in Mochito mine collectors (Aerofloat 25/31, amyl and butyl xanthates, Aerofloat 208 and 404); pH modifiers (sodium carbonate); activators (copper sulphate); depressants (starch); dispersants (sodium silicate); and sulphidising reagents. A study performed by Thompson [27] investigated the most appropriate reagents to use for the recovery of silver from an ore containing 350 ppm of Ag. Silver was associated with sulphosalts (freibergite, pyrargyrite), sulphides (proustite, argentite), and in solid solution with pyrite. It was shown that it is possible to float 82% of silver by using a combination of collectors (thionocarbamate with ethyl xanthate) to produce a concentrate containing 17,500 ppm of Ag. In another study, xanthates and dithiophosphates as collectors with MIBC, pine oil, or polypropylene glycol as frothers were used to recover Cu–Ag, Co–Ag, or Cu–Ag–Bi bearing minerals [6]. Lime was used to achieve the neutral pH. The flotation recovery yields might also depend on the grain particle size. Indeed, it was demonstrated in the literature that low flotation rates are obtained when particles are finely ground due to the low collision efficiency between particles and bubbles [28,29,30]. Therefore, multiple flotation processes have been developed in order to enhance the bubble–particle collision efficiency, either by decreasing the bubble size or by increasing the apparent particle size [31].
However, it is important to mention that, to the best of knowledge of the current paper’s authors, no study related to the recovery of silver from old mine tailings using flotation has yet been reported. Pioneer studies should be carried out in order to understand the reactional mechanisms affecting the flotation of silver bearing minerals. The recovery of silver from old, weathered tailings may present a challenge (since mineral surfaces are oxidized), and at the same time, could be a profitable opportunity if it is economically feasible and environmentally viable.
Morocco is one of the main world producers of silver, classified 16th according to 2014 statistics [32]. In this study, complex low-grade and weathered tailings from the Zgounder mine, one of the oldest silver mines in Morocco, are tested for the recovery of residual silver bearing minerals using froth flotation as a pretreatment before cyanidation. The unexpectedly high silver values in the Zgounder old tailings storage facility (TSF) are believed to be due to insufficient residence time in the reaction tanks; the presence of coarse silver particles; and an insufficiently fine grind during previous operations. Therefore, this study aimed to recover silver from Zgounder low grade tailings using froth flotation. The effect of several parameters on the silver recovery rate, such as particle size, pH, types and amounts of collectors, frothers, activation, and sulphidization were investigated. The main objective was to optimize the parameters affecting silver recovery conditions.
2. Materials and Methods
2.1. Sampling Campaign
Zgounder mine is located in the Western part of the central Anti-Atlas Mountain Range, 150 km south of Marrakech, Morocco. Silver in Zgounder ores is enriched at narrow mineralized veins of chlorite, quartz, siderite, and disseminated ore minerals [33]. It generally occurs in the form of native silver, mercurian silver, silver bearing sulphides and sulphosalts. Silver ore was processed by crushing, milling and cyanidation. The mine had undergone two mine exploitation phases. In the first phase, silver was extracted from 1982 to 1990 by crushing and milling, followed by cyanidation. The site was closed in 1990 and the mine was reopened again in July 2014 by Zgounder Millenium Silver Mining company. The north tailings pond of the Zgounder mine was selected in this study due to its significant volume of tailings produced between 1982 and 1990 (more than 400,000 tons) and its content of Ag (an average grade of 125 g/t silver) [34]. A quantity of 88 samples (approximately 6 kg for each sample) was taken from the tailings pond at a depth of 1.6 m, as presented in Figure 1. The samples were homogenized and stored in sealed bottles to ensure minimal contact with air. All of the samples were dried at a low temperature, disaggregated, homogenized, separated, and sealed in plastic bags until testing.
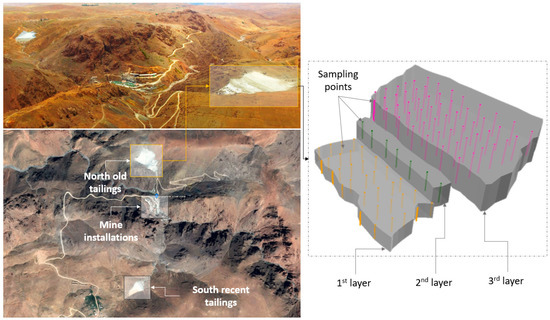
Figure 1.
Location of the Zgounder mine tailings storage facilities and the used sampling procedure.
2.2. Tailing Characterization
The volumetric particle size distribution of the tailings was determined using a laser analyzer (Malvern Mastersizer; ISO-13320, Panalytical, Almelo, The Netherlands). The specific gravity (Gs) measurement was performed with a helium gas pycnometer (Micromeritics Accupyc 1330, Micromeritics, Norcross, GA, USA). The chemical composition of the tailings was determined with atomic absorption equipment (Thermo Fisher Scientific, Waltham, MA, USA) following HNO3/HCl digestion. The inorganic carbon content (Cin) was determined using a LECO furnace with a ±0.05 to 0.1 wt % precision. The crystalline minerals performed only for the composite sample were determined by X-ray diffraction (Bruker AXS Advance D8, Bruker, Billerica, MA, USA), using CuKα radiation with a fixed counting time of 4 s in the range from 5° to 70° with steps of 0.02° in 2θ. The quantitative phase analysis of the tailings was performed using the TOPAS 4.2 program (Bruker, Billerica, MA, USA), based on the Rietveld analysis and data reconciliation against chemical analysis. Scanning electron microscopy (SEM) observations using backscattered electrons (BSE) were performed on polished sections prepared with the bulk samples and an epoxy resin using a Hitachi S-3500N microscope equipped with an X-ray energy dispersive spectrometer (Silicon Drift Detector Bruker, Bruker, Billerica, MA, USA) with ESPRIT 2® software (Bruker, Billerica, MA, USA). The static tests were also performed to evaluate the environmental behavior of Zgounder mine tailing (ZMT) waste. This test was conducted using acid-base accounting, according to the Sobek method modified by Lawrence and Scheske [35].
2.3. Flotation Tests
Before flotation, the samples were tested as raw tailings or were regrinded at two levels (less 105 and 63 microns) in order to liberate silver and refresh mineral surfaces. Numerous flotation tests were performed in triplicate on Zgounder mine tailings (ZMT) using a Denver D-12 lab flotation machine (911 Metallurgist Corporation, Kamloops, BC, Canada) with a cell volume of 4 L. All of the samples were dried, mixed, homogenized, and divided into representative samples for the flotation tests. The objective was to assess the effects of the main parameters on silver recovery and grade. Many reagents were used. It is known that the reagent types and their dosages highly affect the recovery of silver bearing minerals. The effectiveness of these reagents depends mainly on their functionalities and the minerals’ particle compositions and surfaces [36,37]. The synthesized methodology and the reagents used in this study are illustrated in Figure 2.
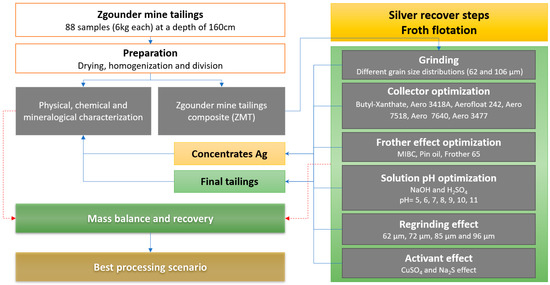
Figure 2.
The methodology scheme used in this study.
Figure 3 presents the flotation setup used to recover silver bearing minerals. During all rougher and scavenging flotation experiments, the solid content was maintained between 30% and 32%, as commonly used in the industry. The speed of the rotor-stator was adjusted to 1500 rpm, and airflow varied between at 2 and 3 L·min−1. Combinations of different families of collectors were used, such as dithiophosphate (Aerofloat 242), dialkyl dithiophosphinates (Aero 3418A), alkyl dithiophosphates (Aero 3477) and potassium butyl-xanthate (PBX), as well as other special formulations, such as Aero 7518 and Aero 7640 [38]. Sodium sulfide (Na2S) was used as a sulphidising reagent, while CuSO4 was used as an activator. Sodium hydroxide (NaOH) and sulphuric acid (H2SO4) were used as pH modifiers. Methyl isobutyl carbonyl (MIBC), pin oil and polypropylene glycol frother (Aerofroth 65) were used as frothers. The test flotation procedure involved a series of tests with two roughing stages followed by one stage of cleaning and scavenging. Flotation optimization was based on testing different parameters classified in a priority order as well as using industrial experience. The flotation kinetics; effect of pH; effect of grinding; and the effects of several types of collectors and frothers were verified in the study (Table 1).
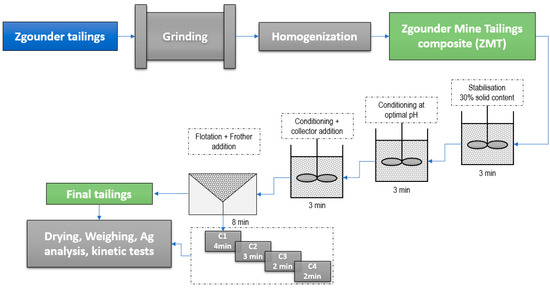
Figure 3.
Zgounder mine tailing (ZMT) preparation and flotation test scheme.

Table 1.
Synthesis of all the studied parameters and related conditions.
3. Results and Discussion
3.1. Tailing Characterization Results
The chemical properties obtained by atomic absorption spectrometry (AAS) of all of the samples are presented in Figure 4. The results showed that the content of silver in old Zgounder mine tailings varied between 30 and 440 ppm. The general average of the samples was about 142 ppm. After a composite sample was prepared from the different samples, the chemical analysis showed that silver content in these samples was about 148 ppm.
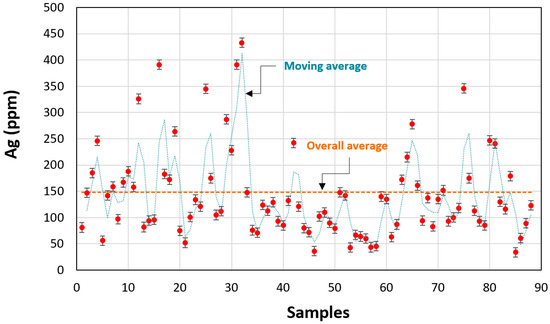
Figure 4.
Variation of silver grade in the different samples taken from Zgounder mine tailings (TSF).
Table 2 presents the physical, chemical, mineralogical, and environmental characteristics of the prepared ZMT composite sample. The particle size distribution results show the fine-grained particle size distribution of the tailings with a D80 of 150 µm. Some traces of heavy metals (Pb, Zn, Cu) and metalloids (As and Sb) were also detected. The sulphur content was about 0.58% in the ZMT composite. The results of the static tests showed that the net neutralization potential (NNP) of ZMT was around −9.8 kg CaCO3/t, thus classifying the ZMT as having an uncertain acid generation potential (−20 kg CaCO3/t < NNP < +20 kg CaCO3/t). This result was expected due to the mineralogical composition of Zgounder tailings mainly comprising non-sulfidic gangue minerals (more than 95 wt %). The XRD results showed that the ZMT composite is mainly composed of gangue minerals, muscovite, quartz, albite, actinolite, and orthoclase. Minor amounts of pyrite, sphalerite, chalcopyrite, arsenopyrite, and hematite were identified by SEM observations. Figure 5 shows some particles of these minerals and their associations.

Table 2.
Physical, chemical, and mineralogical characteristics of the ZMT composite sample.
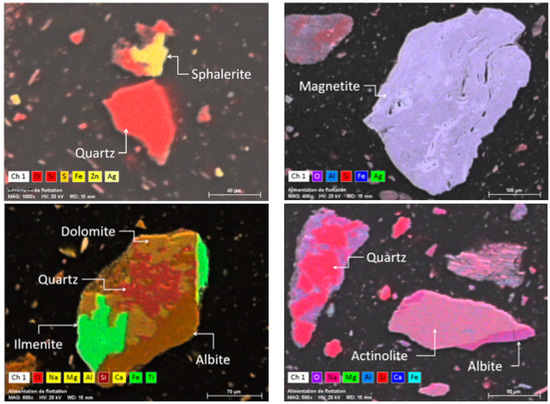
Figure 5.
Combined BSE SEM and X-mapping images of some minerals in the ZMT composite sample.
3.2. Size-by-Size Chemistry Analysis
The Zgounder mine tailing sample was homogenized and sieved into different grain size fractions: +300 µm, 212–300 µm, 150–212 µm, 106–150 µm, 75 + 106 µm, 45–75 µm, and 0–45 µm. The objective was to define the fraction that is richest in silver. The distributions and grades of silver in these fractions were determined and are presented in Figure 6. The measured silver content in the ZMT composite sample was 148 ppm. An Ag analysis on a size-by-size basis indicated that silver varied between 100 and 200 ppm in the finer and intermediate size fractions (0–300 µm), while the coarse fraction recorded the highest silver content: 540 ppm.
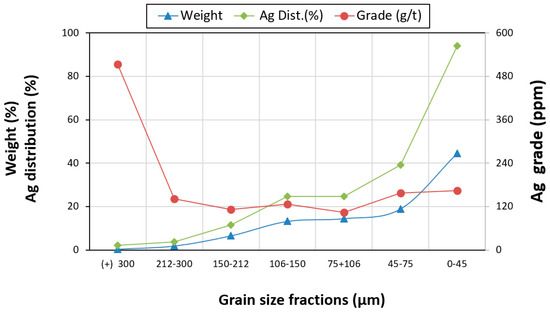
Figure 6.
Distributions and grades of silver in different grain size fractions.
3.3. Flotation Kinetics
Preliminary tests were conducted in order to determine the optimal conditions of flotation in terms of flotation residence time and grain size distribution. Two rougher kinetic flotation tests were conducted on the composite following the conditions described below. The ZMT composite sample was ground to prepare distinct samples with D90 of 62 µm and 106 µm to be floated as well as the raw ZMT sample. Butyl-xanthate (60 g/t) and dithiophosphate (100 g/t) were used as collectors, MIBC (35 g/t) as collector. This combination of reagents is widely recognized as being a strong and selective Ag bearing mineral collector in the industry. The rougher kinetics of silver (Ag) flotation at two different grinding levels are graphically shown in Figure 7.
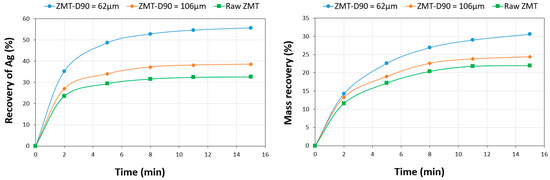
Figure 7.
Flotation kinetics of mineral silver at two grain sizes: (a) silver recovery and (b) mass recovery as a function of flotation residence time.
The results of the rougher testing proved that the recovery of Ag is higher when the particle size distribution is finer. A rougher recovery rate of nearly 52 wt % was achieved when a D90 of 62 µm was used, while only 38 wt % and 32 wt % (106 µm and 150 µm, respectively) were achieved for coarser tailings. The maximum mass recovery was also obtained with the fine grinded material (31 wt %). The silver recovery reached a recovery plateau after a flotation duration of around 8 min. Under these conditions, to obtain a greater Ag recovery yield, particular attention should be given to the effects of reagents (type and dosage). Therefore, the following tests were all carried out with a particle size of D90 = 62 µm and a flotation time of 8 min.
3.4. Selection of Collector and Optimal pH
The next flotation experiments were carried out in order to select the optimal collector mix and conditioning pH. Combinations of butyl-xanthate and five other collectors were tested. A summary of the conducted tests is highlighted in Table 1-1. The dosage of each collector was 60 g/t for collector 1 (butyl-xanthate) and 100 g/t for collector 2 (dialkyl dithiophosphinates (Aero 3418A), dithiophosphate (Aerofloat 242), Aero7518, Aer7640 and alkyl dithiophosphates (Aero 3477)). The results presented in Figure 8 show that the best Ag recovery yields were obtained when butyl-xanthate was mixed with dithiophosphate or dialkyl dithiophosphinates. It was possible to reach a silver recovery greater than 50 wt % with these collectors, while a recovery yield of 30 wt % was achieved with the other collector combinations at each of the three pHs (7.5, 9 and 10.5). At a pH of 7.5, recovery yields and silver grades of 56 wt % and 337 ppm and 53 wt % and 295 ppm were achieved, respectively, with dithiophosphate and dialkyl dithiophosphinates. It was also observed that the best recovery yield was obtained with pH values of 7.5 and 9. Even though an increase in silver grade was recorded at a pH of 10 for the same collector combinations, recovery yields decreased significantly to less than 50 wt %. Out of these two combinations, butyl-xanthate/dithiophosphate gave the best rates. Also, from a price/efficiency ratio perspectives, dithiophosphate was chosen for the rest of experiments in this study.
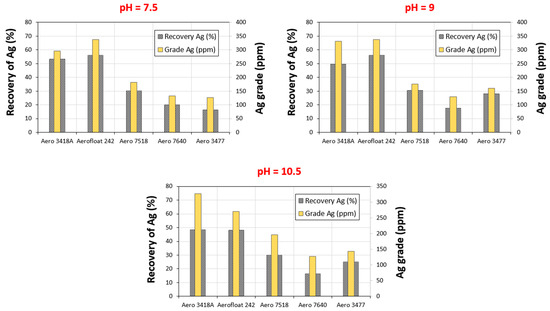
Figure 8.
Grade and recovery of silver for different collector combinations and under different pH conditions.
3.5. Selection of the Frother
Several frothers were tested using the previous optimized conditions in terms of grain size, residence time, and collector combinations. More details about the tests that were carried out are given in Table 1-2. The results are graphically highlighted in Figure 9. The best silver recovery yield and grade were obtained with MIBC at the different pH conditions. The overall yield for MIBC at the four pH levels was greater than 40 wt %. Therefore, the MIBC frother was selected for the other tests due the froth quality, silver recovery, and concentrate quality.
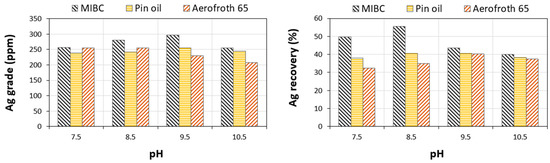
Figure 9.
Grade and recovery of silver for different types of frothers and under different pH levels.
3.6. Effect of Collector Dosage
During this stage, the ZMT composite sample was floated at the previously determined optimal (Table 1-3). A series of flotation tests with varying doses of butyl-xanthate and dithiophosphate were carried out under a pH of 8.5. These tests were conducted in three stages: roughing, cleaning, and scavenging. The results (Figure 10) show that an increase in the collector dosage led to an increase in the silver recovery yield and a decrease in the silver grade. Based on the obtained results, it could be concluded that the best flotation can be obtained with a dosage of 80 g/t of each collector (butyl-xanthate and dithiophosphate).
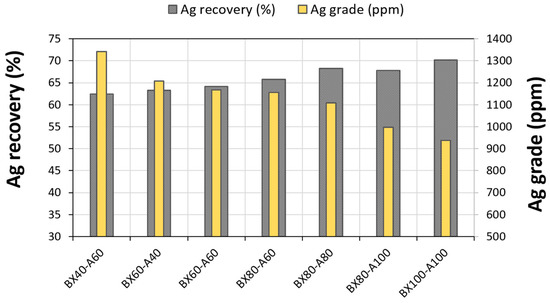
Figure 10.
The evolution of silver grade and recovery as a function of the dosage of collector combinations given; BX = butyl-xanthate and A = Dithiophosphate (Aerofloat-242).
3.7. Effects of Other Parameters
Multiple flotation tests were performed under various pH levels: 5, 6, 7, 8, 9, 10 and 11. The previously optimized conditions were used in these tests (Table 1-4). The pH prior to flotation was measured, and sulphuric acid or lime was used as a regulator to set the desired pH. Figure 11a highlights the obtained results. The silver recovery reached its maximum (64 to 67 wt %) at a pH interval of 6 to 8. The best silver grade was obtained at a pH of 8 (Ag grade concentrate = 1210 ppm).
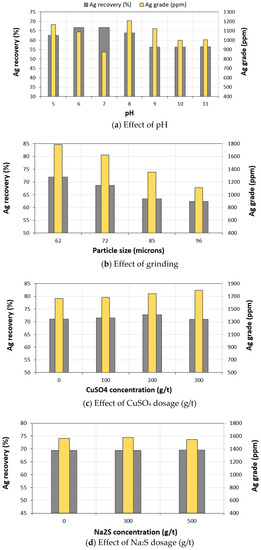
Figure 11.
Evolution of silver grade and recovery as a function of: (a) pH; (b) grain size fraction (D90); (c) CuSO4 dosage (g/t) and (d) Na2S dosage (g/t).
In order to enhance the recovery of silver, the effect of grinding on mineral liberation and Ag recovery was re-evaluated. The goal was to reduce the grinding time and consequently, reduce the treatment costs to achieve the best particle size distribution. Also, it is well known that very fine particles present a big challenge due to their difficult flotation and entrainment abilities. Therefore, four different grain size distributions were evaluated (62, 72, 85 and 96 µm), as illustrated in Table 1-5. The overall recovery yield and silver grade are highlighted in Figure 11b. These results indicate that as fineness increased, the recovery yield and silver grade increased. An increase of 38 wt % of silver grade was reached when the ZMT sample was ground from a D90 of 96 µm (1110 ppm) to 62 µm (1784 ppm). An overall silver recovery yield of 72 wt % was achieved.
Due to the possible association of silver with other sulphide minerals and their probable surface alterations, it was important to evaluate the effects of activating agents on silver recovery yield, and grade. CuSO4 was used to activate sulphides, in particular, the sphalerite mineral. A series of tests was completed in three stages: roughing, cleaning, and scavenging, and the CuSO4 dosage was from 0 to 300 g/t (Table 1-6). The results are graphically highlighted in Figure 11c. It was observed that CuSO4 significantly affected the recovery and grade of silver. The silver grade was increased from 1660 ppm to 1800 ppm when a CuSO4 dosage of 300 g/t was used. Recovery yields varied within the range of 71–73 wt %. Based on these results, the optimal dosage of CuSO4 is 200 g/t. the silver grade is around 1742 g/t and the overall recovery yield is around 72.8 wt %.
As it is possible to have some oxidized minerals in the ZMT tailings, it was important to use sulphidizing agents to recover them. Sulphidization enables the recovery of this type of mineral by flotation. In this study, sodium sulphide (Na2S) was used to recover the oxidized sulphide minerals. Before doing so, all of the sulphide minerals were first floated, and then the sulphidizing agent was added. Figure 11d presents the effects of the addition of Na2S on the recovery and grade of silver. It was observed that the addition of the sulphidizing agent did not lead to any increase or decrease in silver grade or recovery yield.
3.8. Flotation Tests with Optimized Parameters (Flotation Open Circuit Tests)
Three flotation tests (triplicates) were conducted using the optimized conditions for each parameter (Table 3). The results are graphically highlighted in Figure 12. Under these conditions and with two rounds of roughing, three rounds of scavenging and one round of concentration, it was possible to reach a recovery yield of 83 wt % with an average silver grade of 1745 ppm. Figure 13 shows some SEM images of silver bearing minerals found in the final concentrate. It was observed that silver was found mainly in three forms: free silver, Ag associated with acanthite, and Ag as a substitution within pyrite (refractory silver, 1200 ppm Ag in FeS2). It was also observed that acanthite was associated with pyrite particles.

Table 3.
Final flotation tests under the optimized conditions.
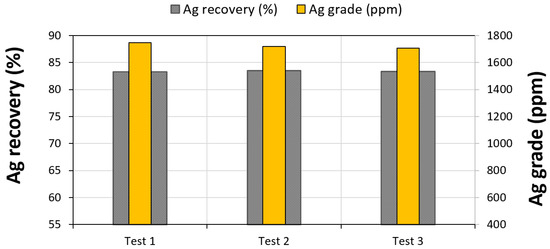
Figure 12.
Silver recovery and grade under the optimized conditions.
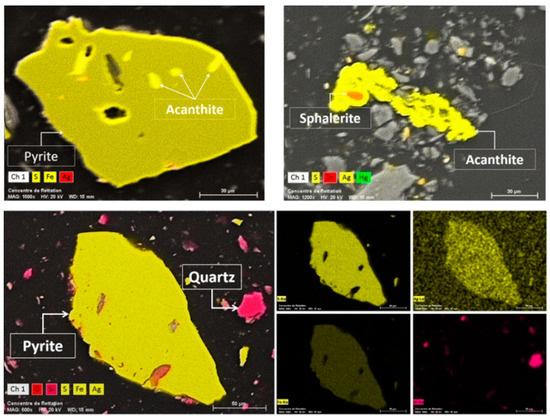
Figure 13.
Combined BSE SEM and X-mapping images of the main silver bearing minerals.
3.9. Environmental Behavior of Cleaned Tailings
The environmental behavior of cleaned tailings was inspected using two tests: a static test and a toxicity characteristic leaching procedure test. The results presented in Table 4 show that the flotation allowed the acid generation potential of tailings to be reduced from 18.1 to 2.6 kg CaCO3/t and the neutralization potential was increased from 8.3 to 16.7 kg CaCO3/t. The net neutralization potential therefore increased from −9.8 (ZMT) to 14.1 CaCO3/t. according to the TCLP test results. It was observed that pollutants, such as As, Ba, Cd, Cr, Mo, and Pb, were in accordance with United States Environmental Protection Agency (US-EPA) limits, while the Zn concentration was superior to the requested limit. The presence of Zn in cleaned tailings is associated with sphalerite mineral. In general, the released concentrations of Cu and Zn may present an ecological risk to the environment. It is unsafe to deposit tailings that contain these elements unless implemented techniques aim at controlling the mine drainage generation. The use of covers over these tailings should protect them from reactivity (sulfide oxidation). In addition, the reprocessed tailings may be valorized/recycled during the production of ceramic materials.

Table 4.
Static and toxicity characteristic leaching procedure tests results.
4. Conclusions
The aim of this paper was to optimize the recovery of silver from old, low-grade tailings based on laboratory regrinding and flotation tests. Tailings were sampled in the TSF of Zgounder mine, taking into account the need for a representative sample. Mineralogical characterization results obtained by a combination of SEM-EDS examination and XRD analysis showed that silver is mainly associated with acanthite (Ag2S) and within pyrite as a trace element. The chemical results showed that the overall grade of silver in tailings (88 samples) was about 148 ppm. Flotation tests occurred with different parameters being optimized, namely, particle size distribution (regrinding), collector type and dosage, pH, D80, activator, and sulphidizing agents, to achieve a silver concentrate grade of 1745 ppm with a silver recovery grade of 84 wt % and a mass recovery of around 40 wt %. These were obtained with two rounds of roughing, three rounds of scavenging and one round of cleaning. The particle grain size was reduced to a D90 of 63 microns, the conditioning pH was fixed at 8.5, a combination of butyl-xanthate and dithiophosphate was used at a dosage of 80 g/t each, the dosage of the activating agent (CuSO4) was fixed at 200 g/t and the frother (MIBC) dosage was maintained at 30 g/t. Our perspective (paper in progress) is that the obtained concentrate should be submitted to cyanidation for further silver concentration, requiring a full technico-economical and feasibility study to be carried out. The cleaned tailings could be valorized as secondary raw materials for ceramic manufacturing. This could lead to a potential stabilization of released elements, such as Zn and Cu.
Author Contributions
B.D. conducted all flotation tests besides the physical and chemical characterizations. The interpretation of results and paper writing were done by B.D. and T.Y. under the supervision of M.B. and R.H.
Acknowledgments
The authors greatly acknowledge the Zgounder Millenium Silver Mining for the great help concerning the sampling, flotation tests, and chemical analyses to valorize their wastes. The authors are also grateful to the staff of URSTM at the University of Quebec in Abitibi Temiscamingue for their valuable contribution to the solid samples analysis.
Conflicts of Interest
The authors declare no conflict of interest.
Acronyms and Abbreviations
| AAS | Atomic absorption spectroscopy |
| AP | Acidity potential |
| BSE | Back Scattered Electrons |
| Cin | Inorganic carbon content |
| EDS | Energy dispersive spectroscopy |
| Gs | Specific gravity |
| MIB | Methyl isobutyl carbinol |
| NNP | Net neutralization potential |
| NP | Neutralization potential |
| SEM | Scanning electron microscopy |
| TCLP | Toxicity characteristic leaching procedure |
| TSF | Tailing storage facility |
| XRD | X-ray diffraction |
| ZMT | Zgounder Mine Tailings |
References
- Schweikert, K. Are gold and silver cointegrated? New evidence from quantile cointegrating regressions. J. Bank. Financ. 2018, 88, 44–51. [Google Scholar] [CrossRef]
- Lucey, M.E.; O’Connor, F.A. Mind the gap: Psychological barriers in gold and silver prices. Financ. Res. Lett. 2016, 17, 135–140. [Google Scholar] [CrossRef]
- Eryiğit, M. Short-term and long-term relationships between gold prices and precious metal (palladium, silver and platinum) and energy (crude oil and gasoline) prices. Econ. Res. 2017, 30, 499–510. [Google Scholar] [CrossRef]
- Wallace, T.C.; Barton, M.; Wilson, W.E. Silver & silver-bearing minerals. Rocks Miner. 1994, 69, 16–38. [Google Scholar]
- Allan, G.; Woodcock, J. A review of the flotation of native gold and electrum. Miner. Eng. 2001, 14, 931–962. [Google Scholar] [CrossRef]
- Quinteros, J.; Wightman, E.; Bradshaw, D. Applying process mineralogy to complex low-grade silver ores. In Proceedings of the XXVII International Mineral Processing Congress-IMPC, Santiago, Chile, 20–24 October 2014. [Google Scholar]
- Wiegers, G. Crystal-structure of low-temperature form of silver selenide. Am. Mineral. 1971, 56, 1882. [Google Scholar]
- Cook, N.J.; Ciobanu, C.L.; Spry, P.G.; Voudouris, P. Understanding gold-(silver)-telluride-(selenide) mineral deposits. Episodes 2009, 32, 249–263. [Google Scholar]
- Celep, O.; Alp, İ.; Deveci, H. Improved gold and silver extraction from a refractory antimony ore by pretreatment with alkaline sulphide leach. Hydrometallurgy 2011, 105, 234–239. [Google Scholar] [CrossRef]
- Mueller, A.G.; Muhling, J.R. Silver-rich telluride mineralization at Mount Charlotte and Au–Ag zonation in the giant Golden Mile deposit, Kalgoorlie, Western Australia. Miner. Depos. 2013, 48, 295–311. [Google Scholar] [CrossRef]
- Woodcock, J.T.; Henley, K.; Cathro, K. Metallurgy of Gold and Silver with Reference to Other Precious Metals; Course Notes for an Australian Mineral Foundation Workshop Course; Australian Mineral Foundation: Glenside, Australia, 1976. [Google Scholar]
- Sack, R.; Brackebusch, F. Fahlore as an indicator of mineralization temperature and gold fineness. Can. Min. Metall. Bull. 2004, 97, 78–83. [Google Scholar]
- Viñals, J.; Roca, A.; Cruells, M.; Núñez, C. Characterization and cyanidation of Rio Tinto gossan ores. Can. Metall. Q. 1995, 34, 115–122. [Google Scholar] [CrossRef]
- Gasparrini, C. Gold and Other Precious Metals: From Ore to Market; Springer Science & Business Media: Berlin, Germany, 2012. [Google Scholar]
- Klein, C.; Hurlbut, C.S.; Dana, J.D. The 22nd Edition of the Manual of Mineral Science: (After James D. Dana); Wiley: New York, NY, USA, 2002. [Google Scholar]
- Fleischer, M.; Mandarino, J.A. Glossary of Mineral Species, 1995; Mineralogical Record Incorporated: Tucson, AZ, USA, 1995. [Google Scholar]
- Horwood, E.J. Process of Treating and Subsequently Separating Sulfid Ores, &C. U.S. Patent US1020353A, 12 March 1912. [Google Scholar]
- Hoover, T.J. Concentrating Ores by Flotation; Mining Magazine: London, UK, 1914. [Google Scholar]
- Gaudin, A. Flotation; McGraw-Hill Book Co., Inc.: New York, NY, USA, 1957; Volume 463, p. 31. [Google Scholar]
- Fuerstenau, D.W. Fine Particle Flotation. In Proceedings of the International Symposium on Fine Particles Processing, Las Vegas, NV, USA, 24–28 February 1980; pp. 669–705. [Google Scholar]
- King, R. Modeling & Simulation of Mineral Processing Systems Butterworth; Heinemann: Oxford, UK, 2001; 403p. [Google Scholar]
- Wills, B.A.; Napier-Munn, T. Wills’ Mineral Processing Technology: An Introduction to the Practical Aspects of Ore Treatment and Mineral Recovery; Elsevier Science and Technology: New York, NY, USA, 2006. [Google Scholar]
- Leaver, E.; Woolf, J. Flotation of Silver Minerals; Research Investigation 3436; US Bureau of Mines: Washington, DC, USA, 1939.
- Taggart, A.F. Handbook of Mineral Dressing; Wiley: New York, NY, USA, 1945. [Google Scholar]
- Dorr, J.; Bosqui, F. Cyanidation and Concentration of Gold and Silver Ores, 2nd ed.; McGraw Hill: New York, NY, USA, 1950. [Google Scholar]
- Malhotra, D.; Harris, L. Review of plant practice of flotation of gold and silver ores. In Advances in Flotation Technology; Parekh, B., Miller, J., Eds.; SME: Southfield, MI, USA, 1999; pp. 167–181. [Google Scholar]
- Thompson, P. The selection of flotation reagents via batch flotation test. In Mineral Processing Plant Design, Practice, and Control: Proceedings; SME: Southfield, MI, USA, 2002; Volume 1. [Google Scholar]
- Trahar, W.; Warren, L. The flotability of very fine particles—A review. Int. J. Min. Process. 1976, 3, 103–131. [Google Scholar] [CrossRef]
- Yoon, R.; Luttrell, G. The effect of bubble size on fine particle flotation. Min. Process. Extr. Metall. Rev. 1989, 5, 101–122. [Google Scholar] [CrossRef]
- Dai, Z.; Fornasiero, D.; Ralston, J. Particle–bubble collision models—A review. Adv. Colloid Interface Sci. 2000, 85, 231–256. [Google Scholar] [CrossRef]
- Miettinen, T.; Ralston, J.; Fornasiero, D. The limits of fine particle flotation. Min. Eng. 2010, 23, 420–437. [Google Scholar] [CrossRef]
- Institute, S. World Silver Survey 2014; The Silver Institute: Washington, DC, USA, 2014. [Google Scholar]
- Petruk, W. Mineralogy and geology of the Zgounder silver deposit in Morocco. Can. Mineral. 1975, 13, 43–54. [Google Scholar]
- El Adnani, M.; Plante, B.; Benzaazoua, M.; Hakkou, R.; Bouzahzah, H. Tailings Weathering and Arsenic Mobility at the Abandoned Zgounder Silver Mine, Morocco. Mine Water Environ. 2016, 35, 508–524. [Google Scholar] [CrossRef]
- Lawrence, R.W.; Scheske, M. A method to calculate the neutralization potential of mining wastes. Environ. Geol. 1997, 32, 100–106. [Google Scholar] [CrossRef]
- Welsby, S.D.D. On the Interpretation of Floatability Using the Bubble Load. Ph.D. Thesis, The University of Queensland, Brisbane, Australia, 2009. [Google Scholar]
- Vianna, S.M.S. The Effect of Particle Size Collector Coverage and Liberation on the Floatability of Galena Particles in an Ore. Ph.D. Thesis, The University of Queensland, Brisbane, Australia, 2004. [Google Scholar]
- Thomas, W. Mining Chemical Handbook; Cytec Industries Inc.: Woodland Park, NJ, USA, 2010. [Google Scholar]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).