Experimental Investigation of Evolution of Pore Structure in Longmaxi Marine Shale Using an Anhydrous Pyrolysis Technique
Abstract
:1. Introduction
2. Samples and Methods
3. Results and Discussion
3.1. Mineral Composition and Organic Geochemistry
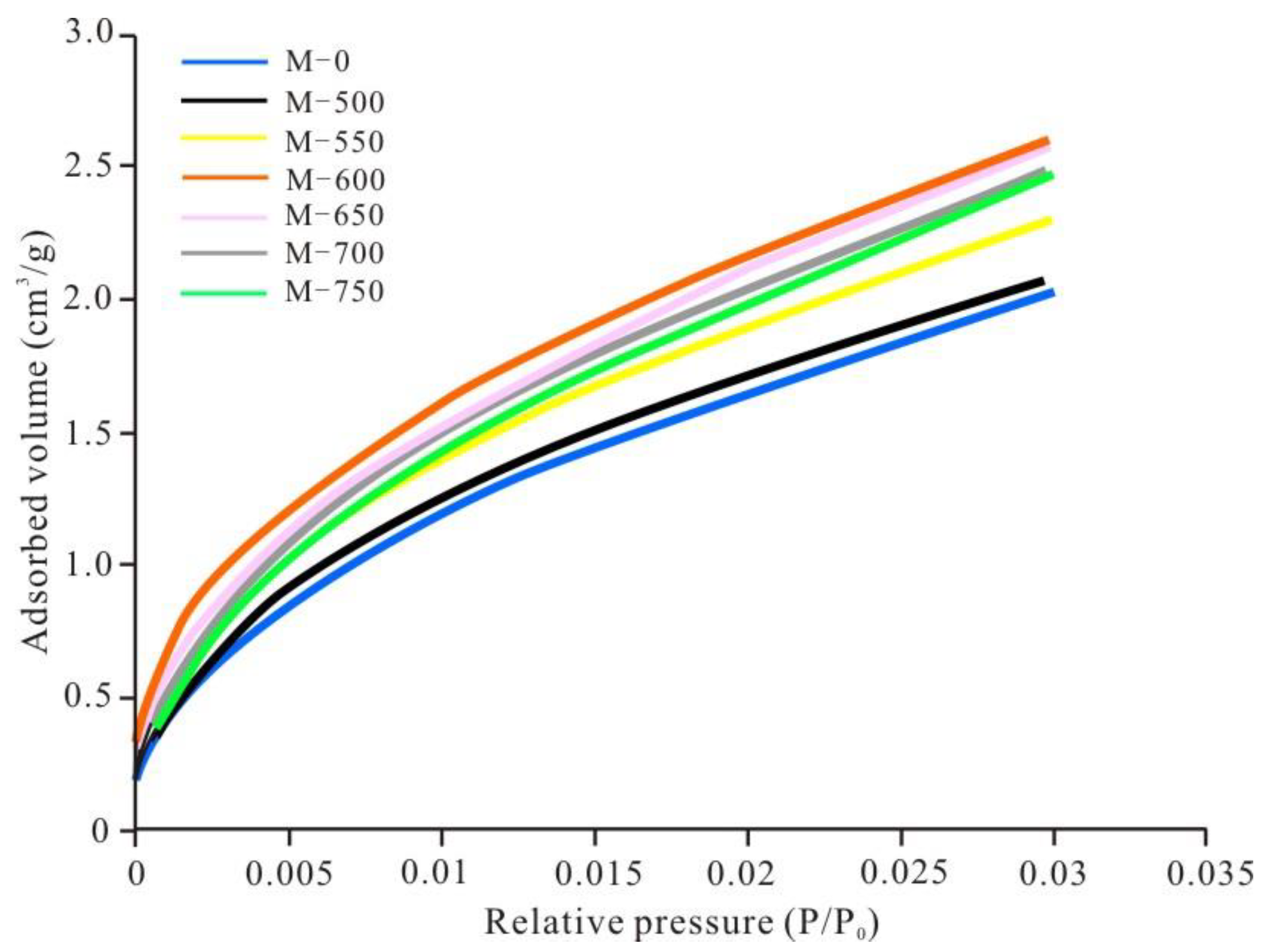
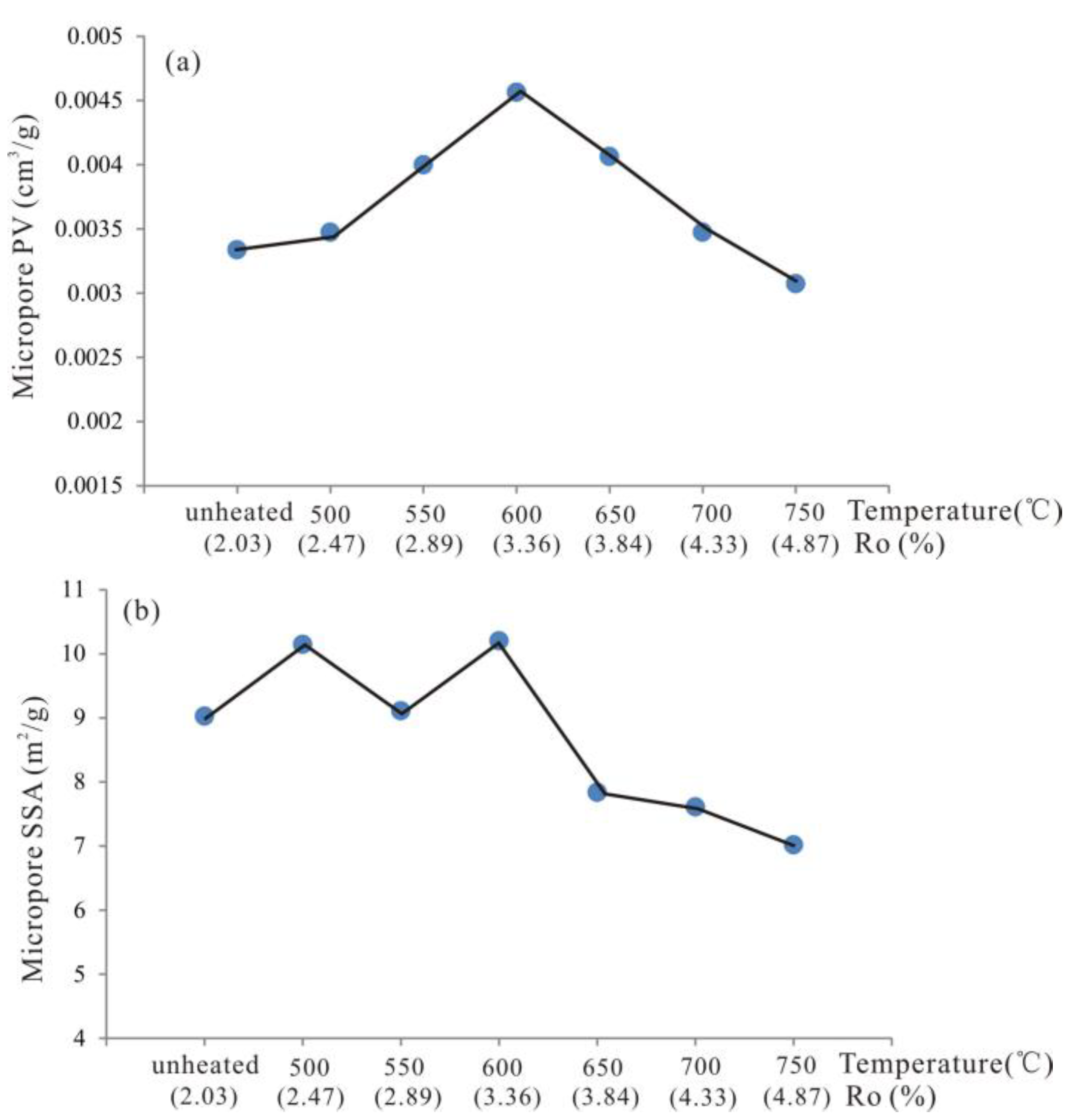
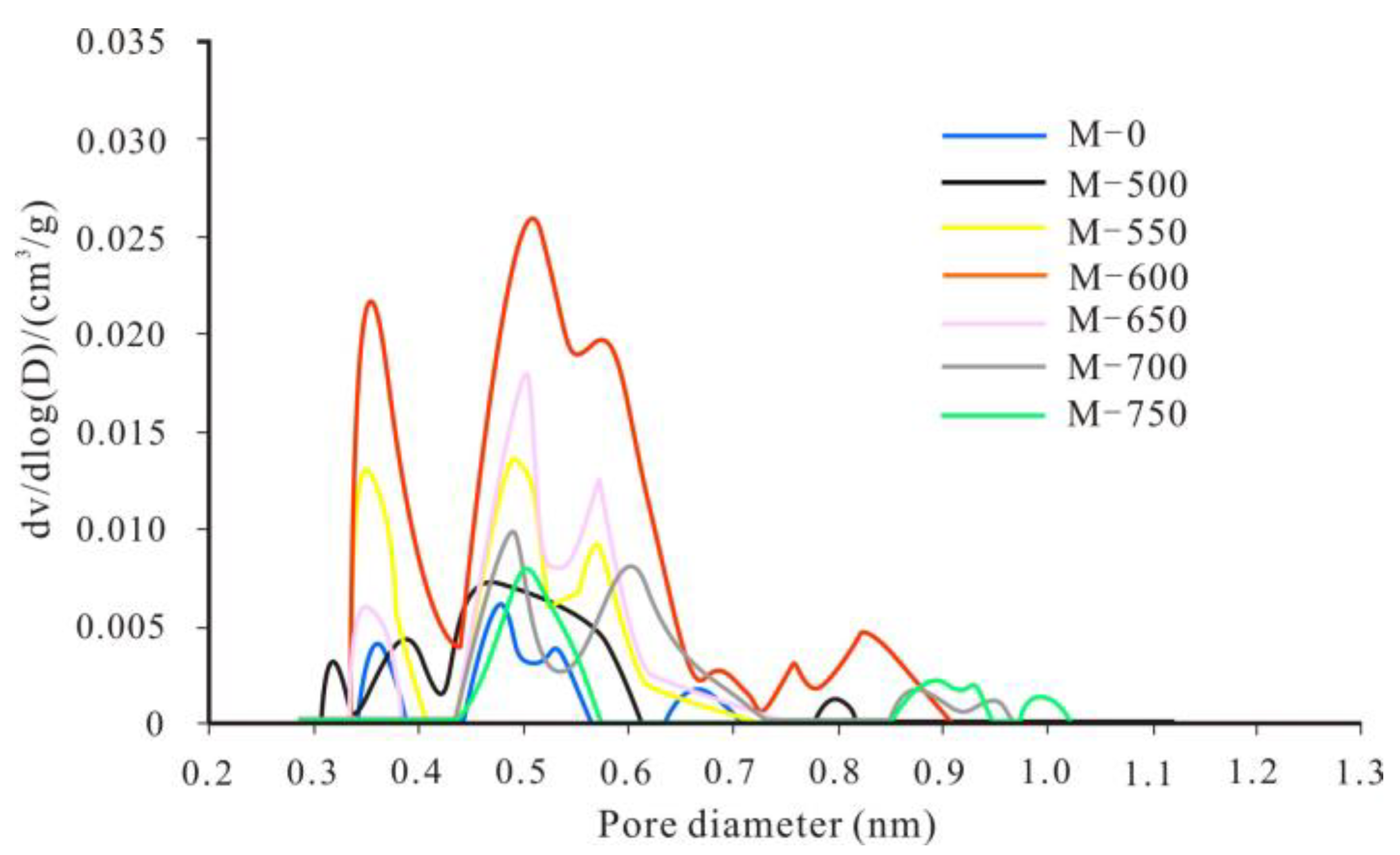
3.2. Evolutionary Characteristics of Micropores
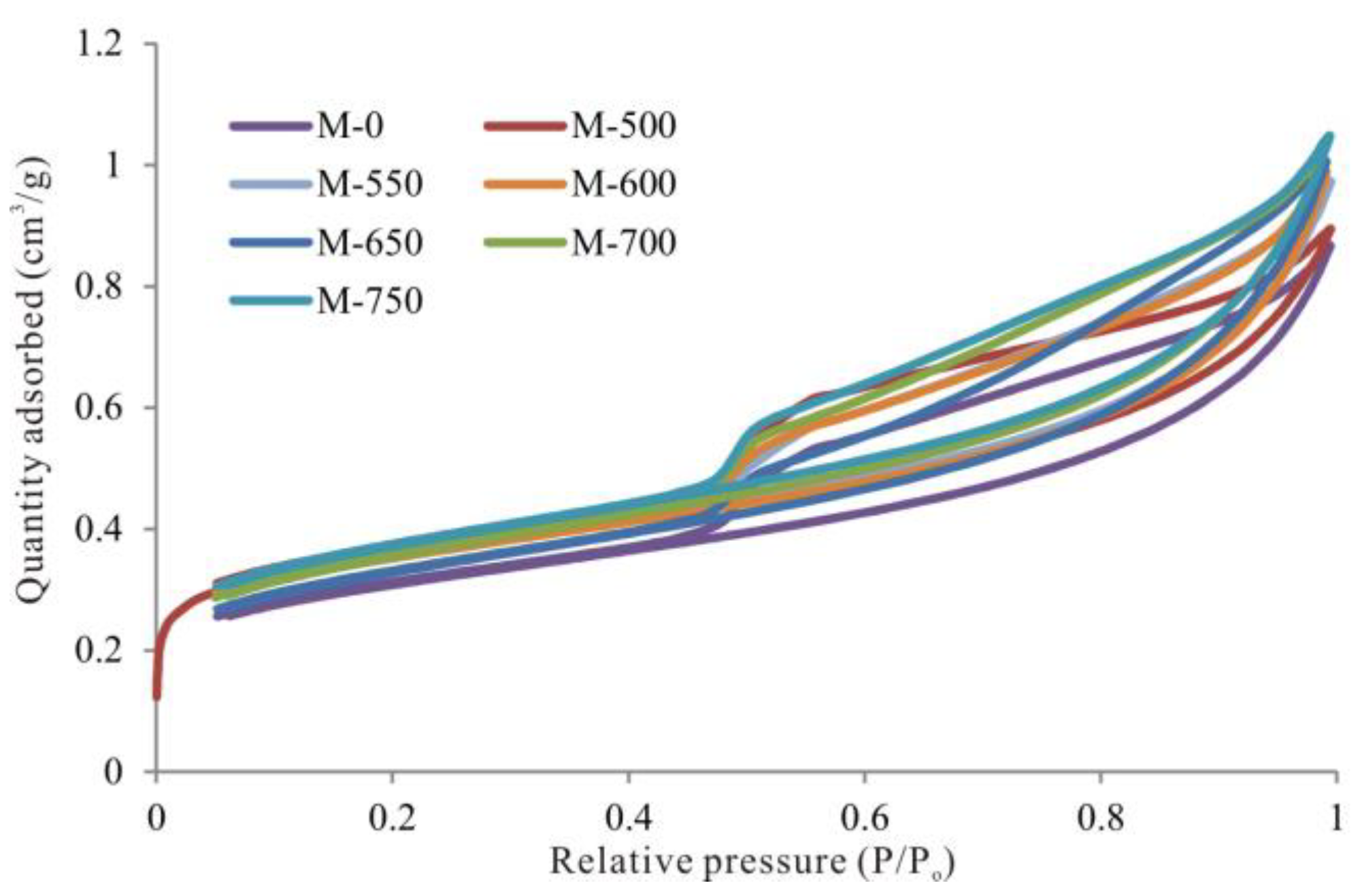
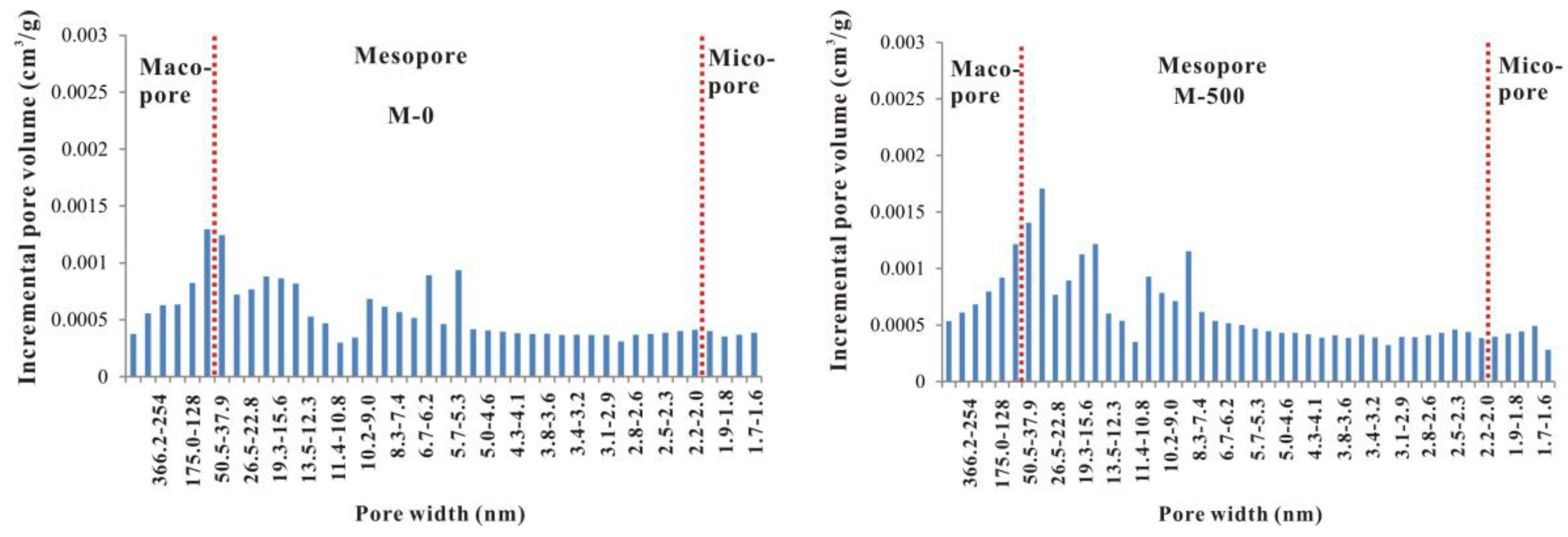
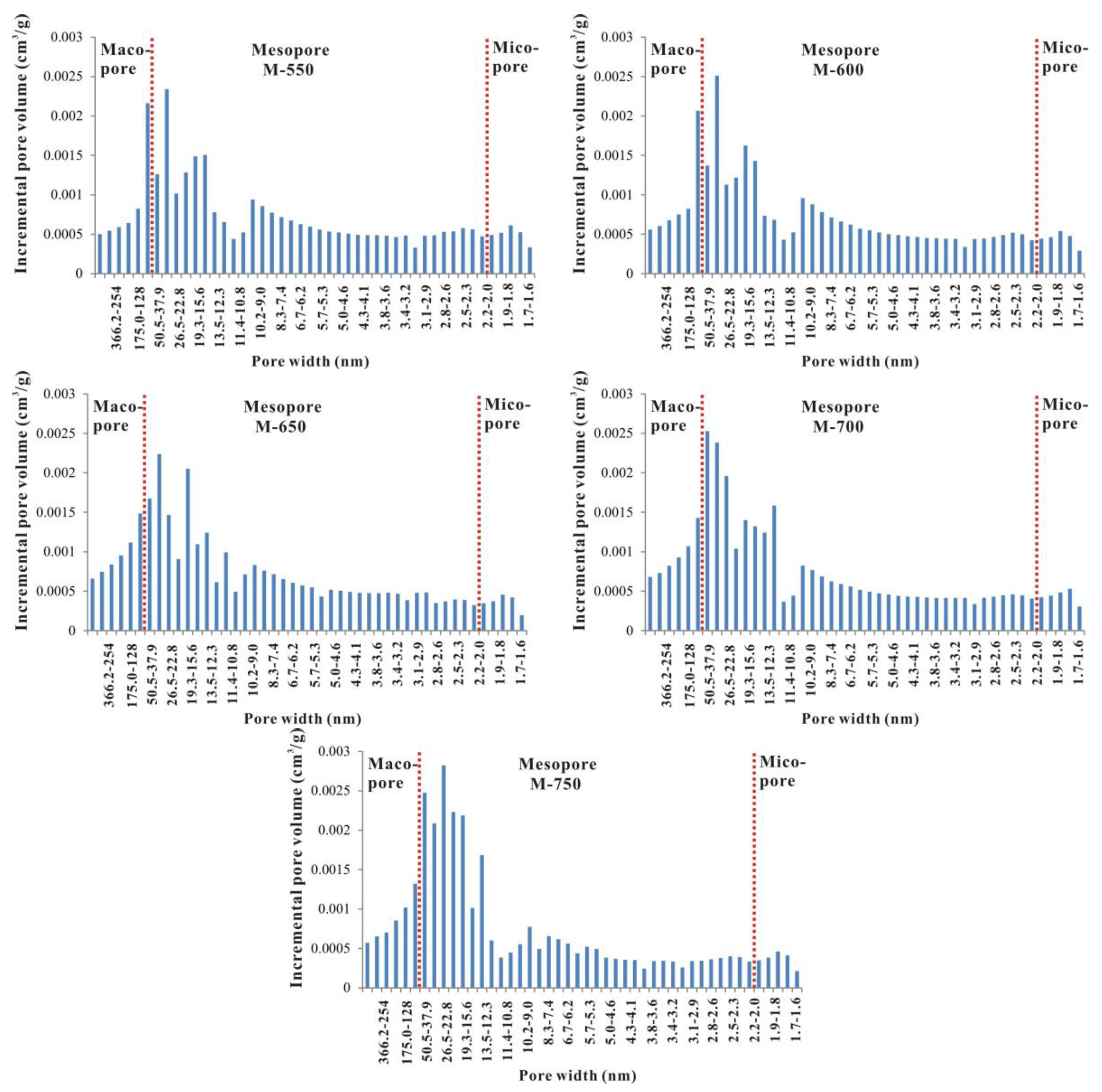
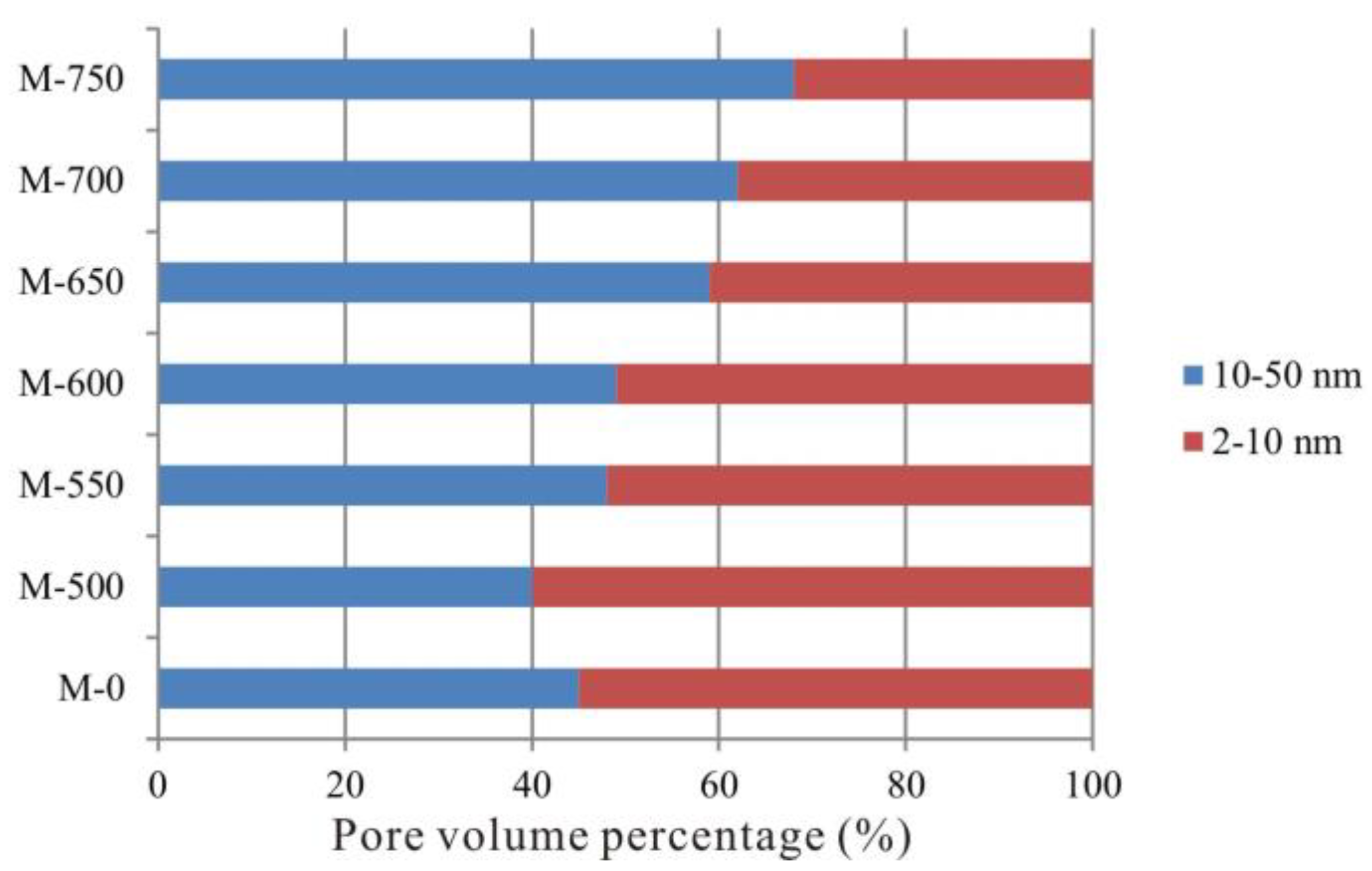
3.3. Evolutionary Characteristics of Mesopores
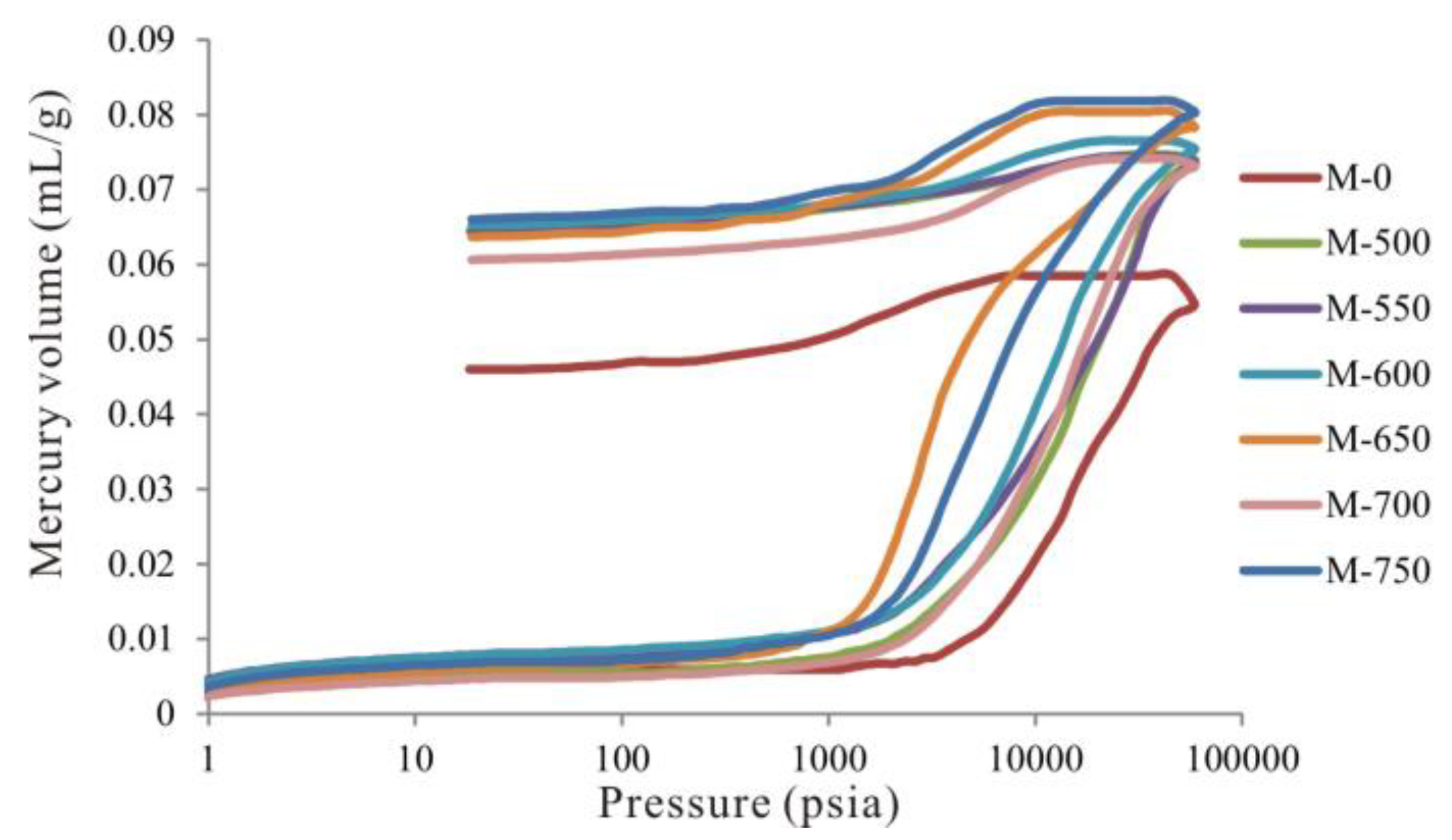
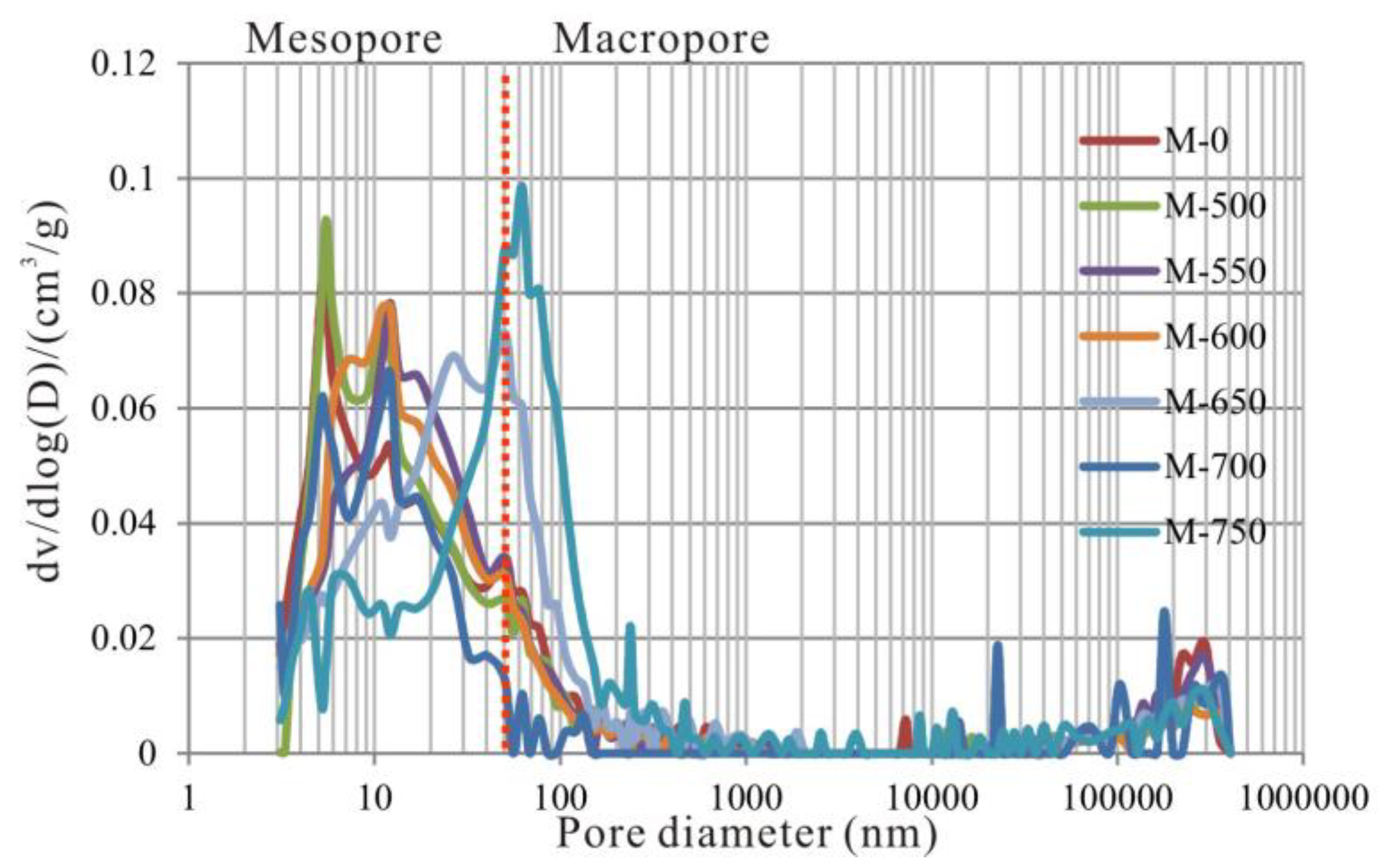
3.4. Evolutionary Characteristics of Macropores
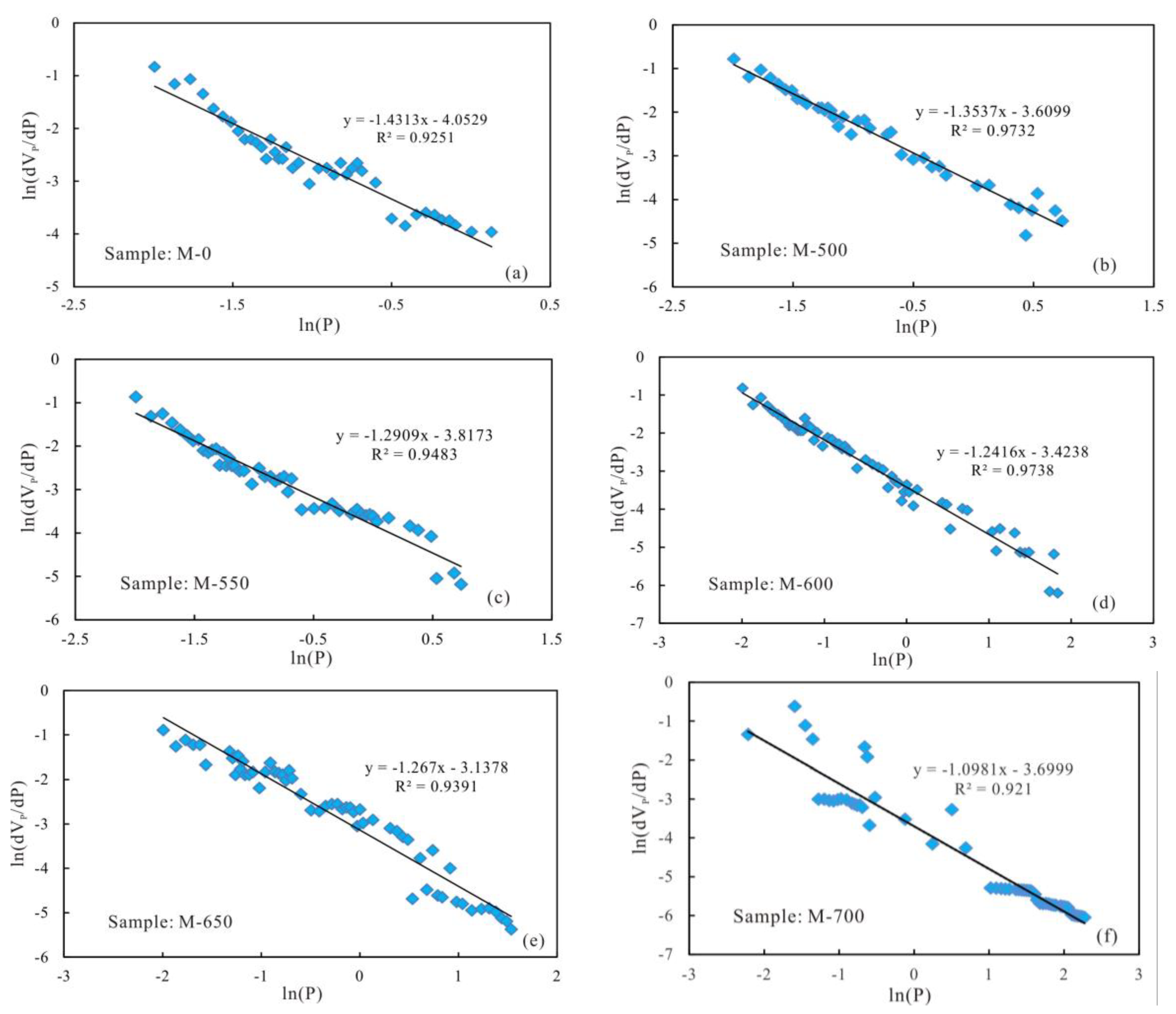
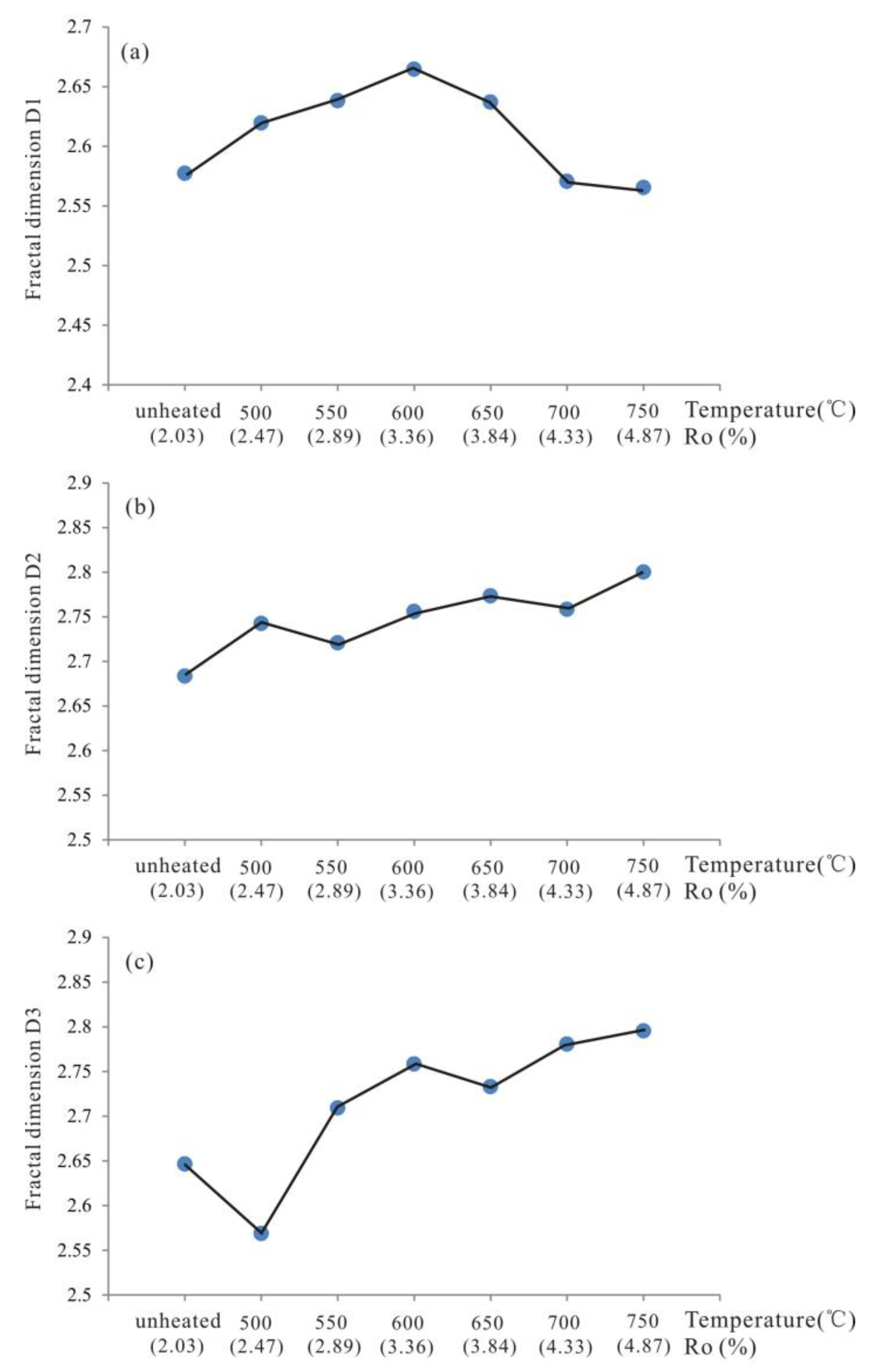
3.5. Fractal Dimension of Pore Structure
3.6. Evolution Mechanism of Pore Structure during Pyrolysis
- (1)
- From unheated to 600 °C, micropore, mesopore and macropore PV values increased with increasing temperature, especially for the micropores and fine mesopores (2–10 nm). The increase of pore content can be attributed to further OM transformation. Mastalerz et al. revealed that there were still a lot of pores filled by liquid hydrocarbon in over-mature shale because of a marked increase in pore volume after extraction by dichloromethane [15]. Previous studies have also suggested that the remaining gas generation potential of OM in over-mature shale is still considerable [18], indicating that OM can still continue to break bonds and crack to form shale gas. Therefore, further OM transformation can unblock pre-existing pores and create new pores, resulting in pore volume increases.
- (2)
- From 600 to 750 °C, the number of pores with small diameter was reduced and pores with large diameter slightly increased. After 600 °C, OM was carbonized and the OM structure became more orderly and inorganic minerals also decomposed, which led to widening diameter in small pores (for example, through coalescence of adjacent pores), resulting in a reduction in micropores and fine mesopores, and an increase in macropores and large mesopores. Bai et al. also revealed that pore diameter enlarged and small pores transformed to large pores when the simulation temperature was above 600 °C [17]. In fact, however, macropores and large mesopores may be difficult to preserve under geological conditions in this stage due to post-compaction.
4. Conclusions
- (1)
- The preset temperature and Ro values of the synthetic Longmaxi marine shale samples (pyrolysis product) had a good linear relationship, and pore structure parameters changed during pyrolysis, indicating the temperature can represent maturity and promote the evolution of pore structure in the samples.
- (2)
- Micropore are the most developed from unheated to 600 °C and then the micropore content decreased from 600 to 750 °C. The evolutionary characteristics of mesopore and macropore PV are different from that of SSA. Mesopore and macropore PV increased with increasing temperature, whereas mesopore SSA decreased from 600 to 750 °C. Fractal dimensions of the shale samples increased from unheated to 600 °C, indicating that the pore structure became more heterogeneous at higher maturity levels. From 600 to 750 °C, fractal analysis showed that larger pore sizes meant more complicated pore structure.
- (3)
- The evolution of pore structure in marine shale with high thermal maturity could be divided into two stages. (1) The increase in micropore, mesopore, and macropore content during the transition from unheated to 600 °C was associated with the further transformation of OM into hydrocarbons; (2) the decrease in small diameter pores (<10 nm) and increase in large diameter pores volumes (>10 nm) caused a rearrangement in PSD from 600 to 750 °C, which was associated with the expansion of micropores, mesopores, and macropores.
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Milliken, K.L.; Rudnicki, M.; Awwiller, D.N.; Zhang, T.W. Organic matter-hosted pore system, marcellus formation (Devonian), Pennsylvania. AAPG Bull. 2013, 97, 177–200. [Google Scholar] [CrossRef]
- Zou, C.N.; Dong, D.Z.; Wang, Y.M.; Li, X.J.; Huang, J.L.; Wang, S.F.; Guan, Q.Z.; Zhang, C.C.; Wang, H.Y.; Liu, H.L.; et al. Shale gas in China: Characteristics, challenges and prospects (II). Petro. Explor. Dev. 2015, 42, 753–767. [Google Scholar] [CrossRef]
- Hu, J.G.; Tang, S.H.; Zhang, S.H. Investigation of pore structure and fractal characteristics of the Lower Silurian Longmaxi shales in western Hunan and Hubei Provinces in China. J. Nat. Gas. Sci. Eng. 2016, 28, 522–535. [Google Scholar] [CrossRef]
- Wan, Y.; Tang, S.H.; Pan, Z.J. Evaluation of the shale gas potential of the lower Silurian Longmaxi Formation in northwest Hunan Province, China. Mar. Petrol. Geol. 2017, 79, 159–175. [Google Scholar] [CrossRef]
- Xi, Z.D.; Tang, S.H.; Wang, J.; Yi, J.J.; Guo, Y.Y.; Wang, K.F. Pore Structure and Fractal Characteristics of Niutitang Shale from China. Minerals 2018, 8, 163. [Google Scholar] [CrossRef]
- Zhao, W.Z.; Li, J.Z.; Yang, T.; Wang, S.F.; Huang, J.L. Geological difference and its significance of marine shale gases in South China. Petrol. Explor. Dev. 2016, 43, 547–559. [Google Scholar] [CrossRef]
- Loucks, R.G.; Reed, R.M.; Ruppel, S.C.; Jarvie, D.M. Morphology, genesis, and distribution of nanometer-scale pores in siliceous mudstones of the Mississippian Barnett shale. J. Sediment. Res. 2009, 79, 848–861. [Google Scholar] [CrossRef]
- Loucks, R.G.; Reed, R.M.; Ruppel, S.C.; Hammes, U. Spectrum of pore types and networks in mudrocks and a descriptive classification for matrix-related mudrock pores. AAPG Bull. 2012, 96, 1071–1098. [Google Scholar] [CrossRef]
- Zhang, T.W.; Ellis, G.S.; Rupple, S.C.; Milliken, K.; Yang, R. Effect of organic matter type and thermal maturity on methane adsorption in shale-gas systems. Org. Geochem. 2012, 47, 120–131. [Google Scholar] [CrossRef]
- Ross, D.J.K.; Bustin, R.M. Characterizing the shale gas resource potential of Devonian-Mississippian strata in the Western Canada sedimentary basin: Application of an integrated formation evaluation. AAPG Bull. 2008, 92, 87–125. [Google Scholar] [CrossRef]
- Ross, D.J.K.; Bustin, R.M. The importance of shale composition and pore structure upon gas storage potential of shale gas reservoirs. Mar. Pet. Geol. 2009, 26, 916–927. [Google Scholar] [CrossRef]
- Xi, Z.D.; Tang, S.H.; Zhang, S.H.; Li, J. Nano-Scale Pore Structure of Marine-Continental Transitional Shale from Liulin Area, the Eastern Margin of Ordos Basin, China. J. Nanosci. Nanotechno. 2017, 17, 6109–6123. [Google Scholar] [CrossRef]
- Clackson, C.R.; Haghshenas, B.; Ghanizadeh, A.; Qanbari, F.; Williams-Kovacs, J.D.; Riazi, N.; Debuhr, C.; Deglint, H.J. Nanopores to megafractures: Current challenges and methods for shale gas reservoir and hydraulic fracture characterization. J. Nat. Gas. Sci. Eng. 2016, 31, 612–657. [Google Scholar] [CrossRef]
- Curtis, M.E.; Cardott, B.J.; Sondergeld, C.H.; Rai, C.S. Development of organic porosity in the Woodford Shale with increasing thermal maturity. Int. J. Coal Geol. 2012, 103, 26–31. [Google Scholar] [CrossRef]
- Mastalerz, M.; Schimmelmann, A.; Drobniak, A.; Chen, Y.Y. Porosity of Devonian and Mississippian New Albany Shale across a maturation gradient: Insights from organic petrology, gas adsorption, and mercury intrusion. AAPG Bull. 2013, 97, 1621–1643. [Google Scholar] [CrossRef]
- Sun, L.N.; Tuo, J.C.; Zhang, M.F.; Wu, C.J.; Wang, Z.X.; Zheng, Y.W. Formation and development of the pore structure in Chang 7 member oil-shale from Ordos Basin during organic matter evolution induced by hydrous pyrolysis. Fuel 2018, 158, 549–557. [Google Scholar] [CrossRef]
- Bai, F.T.; Sun, Y.H.; Liu, Y.M.; Guo, M.Y. Evaluation of the porous structure of Huadian oil shale during pyrolysis using multiple approaches. Fuel 2017, 187, 1–8. [Google Scholar] [CrossRef]
- Chen, J.; Xiao, X. Evolution of nanoporosity in organic-rich shales during thermal maturation. Fuel 2014, 129, 173–181. [Google Scholar] [CrossRef]
- Jiang, S.; Tang, X.L.; Cai, D.S.; Xue, G.; He, Z.L.; Long, S.X.; Peng, Y.M.; Gao, B.; Xu, Z.Y.; Dahdah, N. Comparison of marine, transitional, and lacustrine shales: A case study from the Sichuan Basin in China. J. Petrol. Sci. Eng. 2017, 150, 334–347. [Google Scholar] [CrossRef]
- Liu, W.P.; Liu, J.; Cai, M.L.; Luo, C.; Shi, X.W.; Zhang, J. Pore evolution characteristic of shale in the Longmaxi Formation, Sichuan Basin. Pet. Res. 2017, 2, 291–300. [Google Scholar] [CrossRef]
- Xi, Z.D.; Tang, S.H.; Zhang, S.H.; Sun, K. Pore structure characteristics of marine–continental transitional shale: A case study in the Qinshui Basin, China. Energy Fuels 2017, 31, 7854–7866. [Google Scholar] [CrossRef]
- Li, J.; Tang, S.H.; Zhang, S.H.; Xi, Z.D.; Yang, N.; Yang, G.Q.; Li, L.; Li, Y.P. Paleo-environmental conditions of the Early Cambrian Niutitang Formation in the Fenggang area, the southwestern margin of the Yangtze Platform, southern China: Evidence from major elements, trace elements and other proxies. J. Asian Earth Sci. 2018, 159, 81–97. [Google Scholar] [CrossRef]
- Hunt, J.M. Petroleum Geochemistry and Geology; W.H. Freeman and Company: New York, NY, USA, 1996; p. 743. [Google Scholar]
- Tuo, J.C.; Wu, C.J.; Zhang, M.F. Organic matter properties and shale gas potential of Paleozoic shales in Sichuan Basin, China. J. Nat. Gas. Sci. Eng. 2015, 28, 434–446. [Google Scholar] [CrossRef]
- Waples, D.W. Time and temperature in petroleum formation: Application of Lopatin’s method to petroleum exploration. AAPG Bull. 1980, 64, 916–926. [Google Scholar]
- Sweeney, J.J.; Burnham, A.K. Evaluation of a simple model of vitrinite reflectance based on chemical kinetics. AAPG Bull. 1990, 74, 1559–1570. [Google Scholar]
- Dang, W.; Zhang, J.C.; Tang, X.; Chen, Q.; Han, S.B.; Li, Z.M.; Du, X.R.; Wei, X.L.; Zhang, M.Q.; Liu, J.; et al. Shale gas potential of Lower Permian marine-continental transitional black shales in the Southern North China Basin, central China: Characterization of organic geochemistry. J. Nat. Gas. Sci. Eng. 2016, 28, 639–650. [Google Scholar] [CrossRef]
- Stahl, W.J.; Wollanke, G.; Boigk, H. Carbon and nitrogen isotope data of Upper Carboniferous and Rotliegend natural gases from North Germany and their relationship to the maturity of the organic source material. In Proceedings of the 7th International Meeting on Organic Geochemistry Advances in Organic Geochemistry, Madrid, Spain, 16–19 September 1975; pp. 539–560. [Google Scholar]
- Chalmers, G.R.L.; Bustin, R.M. Geologicalevaluation of Halfway-Doig-Montney hybrid gas shale-tight gas reservoir, northeastern British Columbia. Mar. Petro. Geo. 2012, 38, 53–72. [Google Scholar] [CrossRef]
- Bu, H.J.; Ju, Y.W.; Tan, J.Q.; Wang, G.C.; Li, X.S. Fractal characteristics of pores in non-marine shales from the Huainan coalfield, eastern China. J. Nat. Gas. Sci. Eng. 2015, 24, 166–177. [Google Scholar] [CrossRef]
- Jiang, F.J.; Chen, D.; Chen, J.; Li, Q.W.; Liu, Y.; Shao, X.H.; Hu, T.; Dai, J.X. Fractal Analysis of Shale Pore Structure of Continental Gas Shale Reservoir in the Ordos Basin, NW China. Energy Fuels 2016, 30, 4676–4689. [Google Scholar] [CrossRef]
- Valenza, J.J.; Drenzek, N.; Marques, F.; Pagels, M.; Mastalerz, M. Geochemical controls on shale microstructure. Geology 2013, 41, 611–614. [Google Scholar] [CrossRef]
- Groen, J.C.; Peffer, L.A.A.; Perez-Ramírez, J. Pore size determination in modified micro- and mesoporous materials. Pitfalls and limitations in gas adsorption data analysis. Microporous Mesoporous Mater. 2003, 60, 1–17. [Google Scholar] [CrossRef]
- Yang, R.; Hao, F.; He, S.; He, C.C.; Guo, X.S.; Yi, J.Z.; Hu, H.Y.; Zhang, S.W.; Hu, Q.H. Experimental investigations on the geometry and connectivity of pore space in organic-rich Wufeng and Longmaxi shales. Mar. Pet. Geol. 2017, 84, 225–242. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.H.; Wang, J.Q.; Jiang, Z.; Jin, C.; Wang, Y.F. Characterization of organic matter pores in typical marine and terrestrial shales, China. J. Nat. Gas Sci. Eng. 2018, 49, 56–65. [Google Scholar] [CrossRef]
- Liu, X.J.; Xiong, J.; Liang, L.X. Investigation of pore structure and fractal characteristics of organic-rich Yanchang formation shale in central China by nitrogen adsorption/desorption analysis. J. Nat. Gas Sci. Eng. 2015, 22, 62–72. [Google Scholar] [CrossRef]
- Hao, F.; Zou, H.Y.; Lu, Y.C. Mechanisms of shale gas storage: Implications for shale gas exploration in China. AAPG Bull. 2013, 97, 1325–1346. [Google Scholar] [CrossRef]
- Mishra, S.; Mendhe, V.A.; Varma, A.K.; Kamble, A.D.; Sharma, S.; Bannerjee, M.; Kalpana, M.S. Influence of organic and inorganic content on fractal dimensions of Barakar and Barren Measures shale gas reservoirs of Raniganj basin, India. J. Nat. Gas Sci. Eng. 2018, 49, 393–409. [Google Scholar] [CrossRef]
- Yao, Y.B.; Liu, D.M.; Tang, D.Z.; Tang, S.H.; Huang, W.H. Fractal characterization of adsorption-pores of coals from North China: An investigation on CH4 adsorption capacity of coals. Int. J. Coal Geol. 2008, 73, 27–42. [Google Scholar] [CrossRef]
- Yao, Y.B.; Liu, D.M.; Tang, D.Z.; Tang, S.H.; Huang, W.H.; Liu, Z.; Che, Y. Fractal characterization of seepage-pores of coals from China: An investigation on permeability of coals. Comput. Geosci. 2009, 35, 1159–1166. [Google Scholar] [CrossRef]
- Pfeifer, P.; Avnir, D. Chemistry in noninteger dimensions between 2 and 3, I: Fractal theory of heterogeneous surface. J. Chem. Phys. 1983, 79, 3558–3565. [Google Scholar] [CrossRef]
- Tang, X.L.; Jiang, Z.X.; Li, Z.; Gao, Z.Y.; Bai, Y.Q.; Zhao, S.; Feng, J. The effect of the variation in material composition on the heterogeneous pore structure of high-maturity shale of the Silurian Longmaxi formation in the southeastern Sichuan Basin, China. J. Nat. Gas Sci. Eng. 2015, 23, 464–473. [Google Scholar] [CrossRef]
- Zhang, S.H.; Tang, S.H.; Zhang, J.P.; Pan, Z.J. Pore structure characteristics of China sapropelic coal and their development influence factors. J. Nat. Gas Sci. Eng. 2018, 53, 370–384. [Google Scholar] [CrossRef]
- Yang, F.; Ning, Z.F.; Liu, H. Fractal characteristics of shales from a shale gas reservoir in the Sichuan Basin, China. Fuel 2014, 115, 378–384. [Google Scholar] [CrossRef]




| Sample ID | Formation | Age | Ro (%) | TOC (%) | Amorphinite (%) | Vitrinite (%) | Inertinite (%) | TI | δ13CPDB (‰) | OM Type (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| M-0 | Longmaxi | Silurian | 2.03 | 3.84 | 92 | 5 | 3 | 85.25 | −30.7 | I |
| Sample ID | Mineral Composition (%) | Clay (%) | |||||
|---|---|---|---|---|---|---|---|
| Quartz | Feldspar | Calcite | Clay | Illite | Illite/Smectite | Chlorite | |
| M-0 | 62.5 | 7.3 | / | 30.2 | 82 | 15 | 3 |
| Sample ID | Temperature (°C) | Ro (%) | Sample ID | Temperature (°C) | Ro (%) |
|---|---|---|---|---|---|
| M-500 | 500 | 2.47 | M-650 | 650 | 3.84 |
| M-550 | 550 | 2.89 | M-700 | 700 | 4.33 |
| M-600 | 600 | 3.36 | M-750 | 750 | 4.87 |
| Sample ID | Less Than 4.5 nm in Diameter | 4.5–50 nm in Diameter | Greater Than 50 nm in Diameter | |||
|---|---|---|---|---|---|---|
| D1 | R2 | D2 | R2 | D3 | R2 | |
| M-0 | 2.5772 | 0.9981 | 2.6833 | 0.9944 | 2.6463 | 0.9251 |
| M-500 | 2.6194 | 0.9982 | 2.7424 | 0.9952 | 2.5687 | 0.9732 |
| M-550 | 2.6382 | 0.9976 | 2.7207 | 0.9896 | 2.7091 | 0.9483 |
| M-600 | 2.6646 | 0.9985 | 2.7559 | 0.9962 | 2.7584 | 0.9738 |
| M-650 | 2.6369 | 0.9934 | 2.7733 | 0.9941 | 2.733 | 0.9391 |
| M-700 | 2.5706 | 0.9963 | 2.7582 | 0.9875 | 2.7806 | 0.921 |
| M-750 | 2.5654 | 0.9962 | 2.8002 | 0.9573 | 2.7953 | 0.9706 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xi, Z.; Wang, J.; Hu, J.; Tang, S.; Xiao, H.; Zhang, Z.; Xing, Y. Experimental Investigation of Evolution of Pore Structure in Longmaxi Marine Shale Using an Anhydrous Pyrolysis Technique. Minerals 2018, 8, 226. https://doi.org/10.3390/min8060226
Xi Z, Wang J, Hu J, Tang S, Xiao H, Zhang Z, Xing Y. Experimental Investigation of Evolution of Pore Structure in Longmaxi Marine Shale Using an Anhydrous Pyrolysis Technique. Minerals. 2018; 8(6):226. https://doi.org/10.3390/min8060226
Chicago/Turabian StyleXi, Zhaodong, Jing Wang, Jingang Hu, Shuheng Tang, Heqi Xiao, Zhongyao Zhang, and Yanan Xing. 2018. "Experimental Investigation of Evolution of Pore Structure in Longmaxi Marine Shale Using an Anhydrous Pyrolysis Technique" Minerals 8, no. 6: 226. https://doi.org/10.3390/min8060226
APA StyleXi, Z., Wang, J., Hu, J., Tang, S., Xiao, H., Zhang, Z., & Xing, Y. (2018). Experimental Investigation of Evolution of Pore Structure in Longmaxi Marine Shale Using an Anhydrous Pyrolysis Technique. Minerals, 8(6), 226. https://doi.org/10.3390/min8060226




