Changes in the Signature of Cobalt Colorants in Late Antique and Early Islamic Glass Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Archaeologial Materials
2.2. Analytical Methods
- the main major and minor ancient glass constituents: silicon, sodium, calcium, potassium, aluminum, magnesium, lead, chlorine, phosphorus and iron;
- most of the colouring and opacifying agents and associated impurities: cobalt, copper, antimony, tin and manganese but also zinc, nickel, arsenic, barium, chromium, vanadium, gold, silver, selenium and cadmium;
- and several other trace elements, including rubidium, strontium, cesium, zirconium, uranium, thorium and the rare earth elements.
3. Results and Discussion
3.1. Roman Cobalt Ores
3.2. Characteristics of Late Antique Cobalt Colorants
3.3. New European and Islamic Cobalt Sources
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Abe, Y.; Harimoto, R.; Kikugawa, T.; Yazawa, K.; Nishisaka, A.; Kawai, N.; Yoshimura, S.; Nakai, I. Transition in the use of cobalt-blue colorant in the New Kingdom of Egypt. J. Archaeol. Sci. 2012, 39, 1793–1808. [Google Scholar] [CrossRef]
- Shortland, A.J.; Tite, M.S.; Ewart, I. Ancient exploitation and use of cobalt alums from the Western Oases of Egypt. Archaeometry 2006, 48, 153–168. [Google Scholar] [CrossRef]
- Walton, M.; Eremin, K.; Shortland, A.; Degryse, P.; Kirk, S. Analysis of Late Bronze Age glass axes from Nippur—A new cobalt colourant. Archaeometry 2012, 54, 835–852. [Google Scholar] [CrossRef]
- Huisman, D.; van der Laan, J.; Davies, G.; van Os, B.; Roymans, N.; Fermin, B.; Karwowski, M. Purple haze: Combined geochemical and Pb-Sr isotope constraints on colourants in Celtic glass. J. Archaeol. Sci. 2017, 81, 59–78. [Google Scholar] [CrossRef]
- Rolland, J. L’artisanat du Verre Dans le Monde Celtique du Second âge du Fer, Approches Archéométriques, Technologiques et Sociales. Ph.D. Thesis, Université Paris 1 Panthéon Sorbonne, Paris, France, 2017. [Google Scholar]
- Dussubieux, L.; Gratuze, B. Origine et diffusion du verre dans le monde indien et en Asie du Sud-Est: L’importance du dosage des éléments-traces. Revue d’Archeometrie 2003, 27, 67–73. [Google Scholar] [CrossRef]
- Kaczmarczyk, A. The source of cobalt in ancient Egyptian pigments. In Proceedings of the 24th International Archaeometry Symposium; Smithsonian Institution Press: Washington, DC, USA, 1986; pp. 369–376. [Google Scholar]
- Gratuze, B.; Picon, M. Utilisation par l’industrie verrière des sels d’aluns des oasis égyptiennes au début du premier millénaire avant notre ére. In L’Alun de Mediterranie; Institut Français de Naples: Napoli, Italy, 2006; pp. 269–276. [Google Scholar]
- Gratuze, B.; Billaud, Y. Inventaire des perles en verre et en faïence de l’Age du Bronze originaires des ateliers de la région de Frattesina retrouvées en France. In Atti delle XVI Giornate Nationali di Studio sul Vetro, Adria (RO), 12–13 May 2012; Ciappi, S., Larse, A., Uboldi, M., Eds.; Nazionale Italiano AIHV: Adria, Italy, 2014; pp. 25–38. [Google Scholar]
- Dayton, J.E. Geological evidence for the discovery of cobalt blue glass in Mycenaean times as a by-product of silver smelting in the Schneeberg area of the Bohemian Erzgebirge. Revue d’Archéométrie 1981, 1, 57–61. [Google Scholar] [CrossRef]
- Gratuze, B.; Soulier, I.; Blet, M.; Vallauri, L. De l’origine du cobalt: Du verre à la céramique. Revue d’Archéométrie 1996, 20, 77–94. [Google Scholar] [CrossRef]
- Walton, M.S.; Shortland, A.; Kirk, S.; Degryse, P. Evidence for the trade of Mesopotamian and Egyptian glass to Mycenaean Greece. J. Archaeol. Sci. 2009, 36, 1496–1503. [Google Scholar] [CrossRef]
- Varberg, J.; Gratuze, B.; Kaul, F.; Hansen, A.H.; Rotea, M.; Wittenberger, M. Mesopotamian glass from Late Bronze Age Egypt, Romania, Germany, and Denmark. J. Archaeol. Sci. 2016, 74, 184–194. [Google Scholar] [CrossRef]
- Blomme, A.; Degryse, P.; Dotsika, E.; Ignatiadou, D.; Longinelli, A.; Silvestri, A. Provenance of polychrome and colourless 8th–4th century BC glass from Pieria, Greece: A chemical and isotopic approach. J. Archaeol. Sci. 2017, 78, 134–146. [Google Scholar] [CrossRef]
- Gallo, F.; Silvestri, A.; Molin, G.; Marcante, A.; Guerriero, P. Iron Age vessels from the Archaeological Museum of Adria (North-Eastern Italy): A textural, chemical and mineralogical study. In Proceedings of the 39th International Symposium on Archaeometry, Leuven, Belgium, 28 May–1 June 2012; pp. 198–207. [Google Scholar]
- Panighello, S.; Orsega, E.F.; van Elteren, J.T.; Šelih, V.S. Analysis of polychrome Iron Age glass vessels from Mediterranean I, II and III groups by LA-ICP-MS. J. Archaeol. Sci. 2012, 39, 2945–2955. [Google Scholar] [CrossRef]
- Shortland, A.; Schroeder, H. Analysis of first millennium BC glass vessels and beads from the Pichvnari necropolis, Georgia. Archaeometry 2009, 51, 947–965. [Google Scholar] [CrossRef]
- Oikonomou, A.; Henderson, J.; Gnade, M.; Chenery, S.; Zacharias, N. An archaeometric study of Hellenistic glass vessels: Evidence for multiple sources. Archaeol. Anthropol. Sci. 2018, 10, 97–110. [Google Scholar] [CrossRef]
- Cherel, A.-F.; Gratuze, B.; Patrick, S. Les perles en faïence et en verre de l’âge du Bronze découvertes en Bretagne: Nouvelles données, nouvelles approches. Étude typo-chronologique, composition, provenance. BSPF 2018, in press. [Google Scholar]
- Gratuze, B. Annexe. Étude chimique des verres de l’atelier de Beyrouth. Syria 2000, 11, 291–296. [Google Scholar] [CrossRef]
- Gratuze, B.; Soulier, I.; Barrandon, J.-N.; Roy, D. De l’origine du cobalt dans les verres. Revue d’Archéométrie 1992, 16, 97–108. [Google Scholar] [CrossRef]
- Neri, E.; Gratuze, B.; Schibille, N. Dating the mosaics of the Durres amphitheatre through interdisciplinary analysis. J. Cult. Heritage 2017, 28, 27–36. [Google Scholar] [CrossRef]
- Neri, E.; Gratuze, B.; Schibille, N. The trade of glass beads in early medieval Illyricum: Towards an Islamic monopoly. Archaeol. Anthropol. Sci. 2018, 1–16. [Google Scholar] [CrossRef]
- Pactat, I.; Hilberg, V.; Gratuze, B. The bowl glass sherds of Saint-Savin’s type discovered at Haithabu: Analytical study. 2018; in press. [Google Scholar]
- Schibille, N.; Meek, A.; Tobias, B.; Entwistle, C.; Avisseau-Broustet, M.; Da Mota, H.; Gratuze, B. Comprehensive chemical characterisation of Byzantine glass weights. PLoS ONE 2016, 11, e0168289. [Google Scholar] [CrossRef] [PubMed]
- Sode, T.; Gratuze, B. Archaeometric studies of mediaevals glass finds (beads, tesserae, glass wastes and cullets) from Ribe (Denmark). 2018; in press. [Google Scholar]
- Van Wersch, L.; Biron, I.; Neuray, B.; Mathis, F.; Chêne, G.; Strivay, D.; Sapin, C. Les vitraux alto-médiévaux de Stavelot (Belgique). ArchéoSciences 2014, 38, 219–234. [Google Scholar] [CrossRef]
- Gratuze, B. Glass characterization using laser ablation-inductively coupled plasma-mass spectrometry methods. In Recent Advances in Laser Ablation ICP-MS for Archaeology, Series: Natural Science in Archaeology; Dussubieux, L., Golitko, M., Gratuze, B., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 179–196. [Google Scholar]
- Nenna, M.-D.; Gratuze, B. Étude diachronique des compositions de verres employés dans les vases mosaïqués antiques: Résultats préliminaires. Annales du 17e Congrès de l’Association Internationale pour l’Histoire du Verre 2009, 17, 8–14. [Google Scholar]
- Cholakova, A.; Rehren, T.; Gratuze, B.; Lankton, J. Glass coloring technologies of late Roman cage cups. J. Glass Stud. 2017, 59, 117–133. [Google Scholar]
- Guérit, M.; Gratuze, B. Le mobilier en verre de deux ateliers de verriers antiques “Quartier des Prieurs” au Pègue (Drôme): Méthodologie et analyses. In L'artisanat Dans le Contexte de Recherche Archéologique Préventive: approches pluridisciplinaires. Actes de la Table-Ronde CORPUS: Etude du Mobilier Métallique et de l’instrument, Orléans, France, 2–3 Octorber 2014; Lusson, D., Roux-Capron, E., Eds.; Fédération archéologique du Loiret: Neuville-aux-Bois, France, 2016; pp. 43–50. [Google Scholar]
- Munier, C.; Brkojewitsch, C. Premiers éléments relatifs à la découverte récente d’un atelier de verrier antique à Besançon. In Echanges et Commerce du Verre Dans le Monde Antique, Proceedings of the ACTES du Colloque de l’AFAV, Aix-en-Provence et Marseille, 7–9 Juin 2001; Foy, D., Nenna, M.-D., Eds.; Editions monique mergoil: Montagnac, France, 2003; pp. 321–337. [Google Scholar]
- Mathis, F.; Vrielynck, O.; Degryse, P.; Gratuze, B. Production and exchange of glass beads in early medieval times. The case of the Bossut Gottechain (Belgium) Merovingian cemetery. 2018; in press. [Google Scholar]
- Foy, D.; Gratuze, B.; Heijmans, M.; Roussel-Ode, J. Bleus et blancs: Verres de la fin de l’époque carolingienne en Provence. J. Glass Stud. 2017, 59, 153–169. [Google Scholar]
- Schibille, N.; Meek, A.; Wypyski, M.T.; Kröger, J.; Rosser-Owen, M.; Wade Haddon, R. The glass walls of Samarra (Iraq): Ninth-century Abbasid glass production and imports. PLoS ONE 2018, in press. [Google Scholar]
- Schibille, N.; Neri, E.; Ebanista, C.; Ammar, M.R.; Bisconti, F. Something old, something new: the late antique mosaics from the catacomb of San Gennaro (Naples). 2018; under review. [Google Scholar]
- Billoin, D. Grozon (Jura). 5 rue de la Saline. Archéologie Médiévale 2015, 45, 290. [Google Scholar]
- Brill, R.H. Scientific investigations of the Jalame glass and related finds. In Excavations at Jalame: Site of a Glass Factory in Late Roman Palestine; Weinberg, G.D., Ed.; University of Missouri: Columbia, MO, USA, 1988; pp. 257–291. [Google Scholar]
- Bidegaray, A.I.; Pollard, A. Tesserae recycling in the production of medieval blue window glass. Archaeometry 2018. [Google Scholar] [CrossRef]
- Zucchiatti, A.; Bouquillon, A.; Katona, I.; D’ALESSANDRO, A. The ‘Della Robbia blue’: A case study for the use of cobalt pigments in ceramics during the Italian Renaissance. Archaeometry 2006, 48, 131–152. [Google Scholar] [CrossRef]
- Matin, M.; Pollard, A. From ore to pigment: A description of the minerals and an experimental study of cobalt ore processing from the Kāshān Mine, Iran. Archaeometry 2017, 59, 731–746. [Google Scholar] [CrossRef]
- Matin, M.; Pollard, M. Historical accounts of cobalt ore processing from the Kashan mine, Iran. Iran J. Br. Inst. Persian Stud. 2015, 53, 171–183. [Google Scholar] [CrossRef]

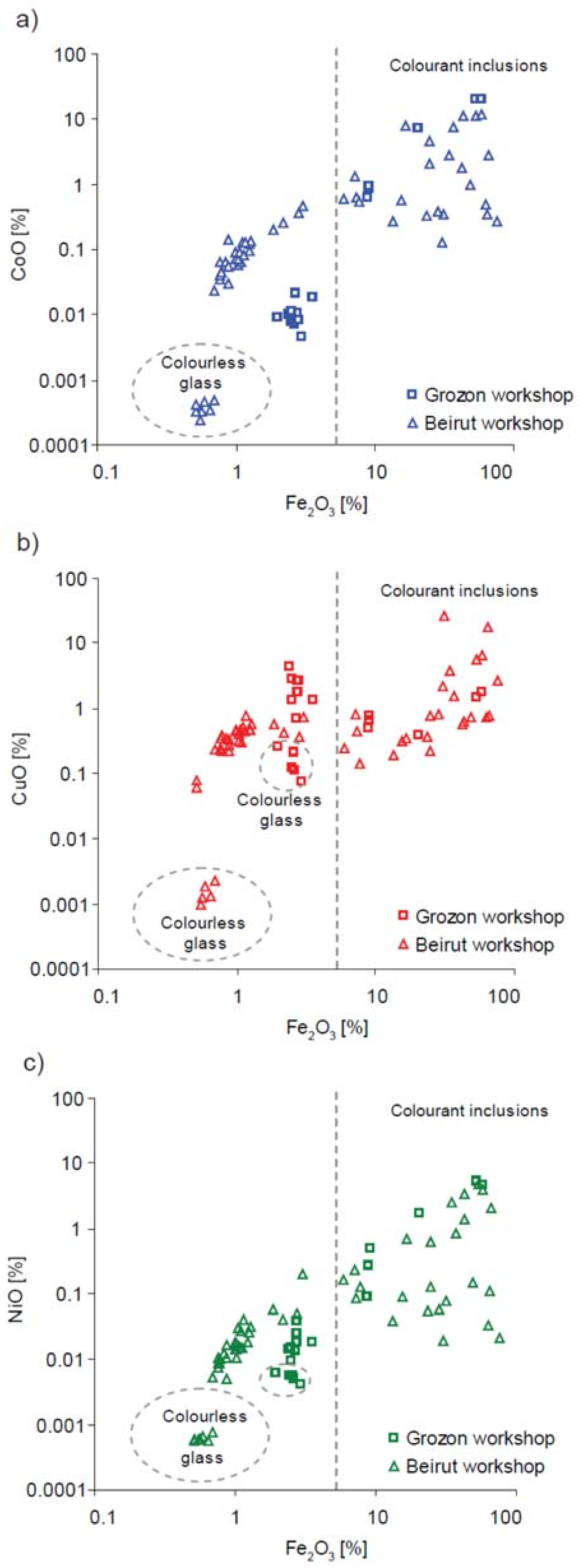
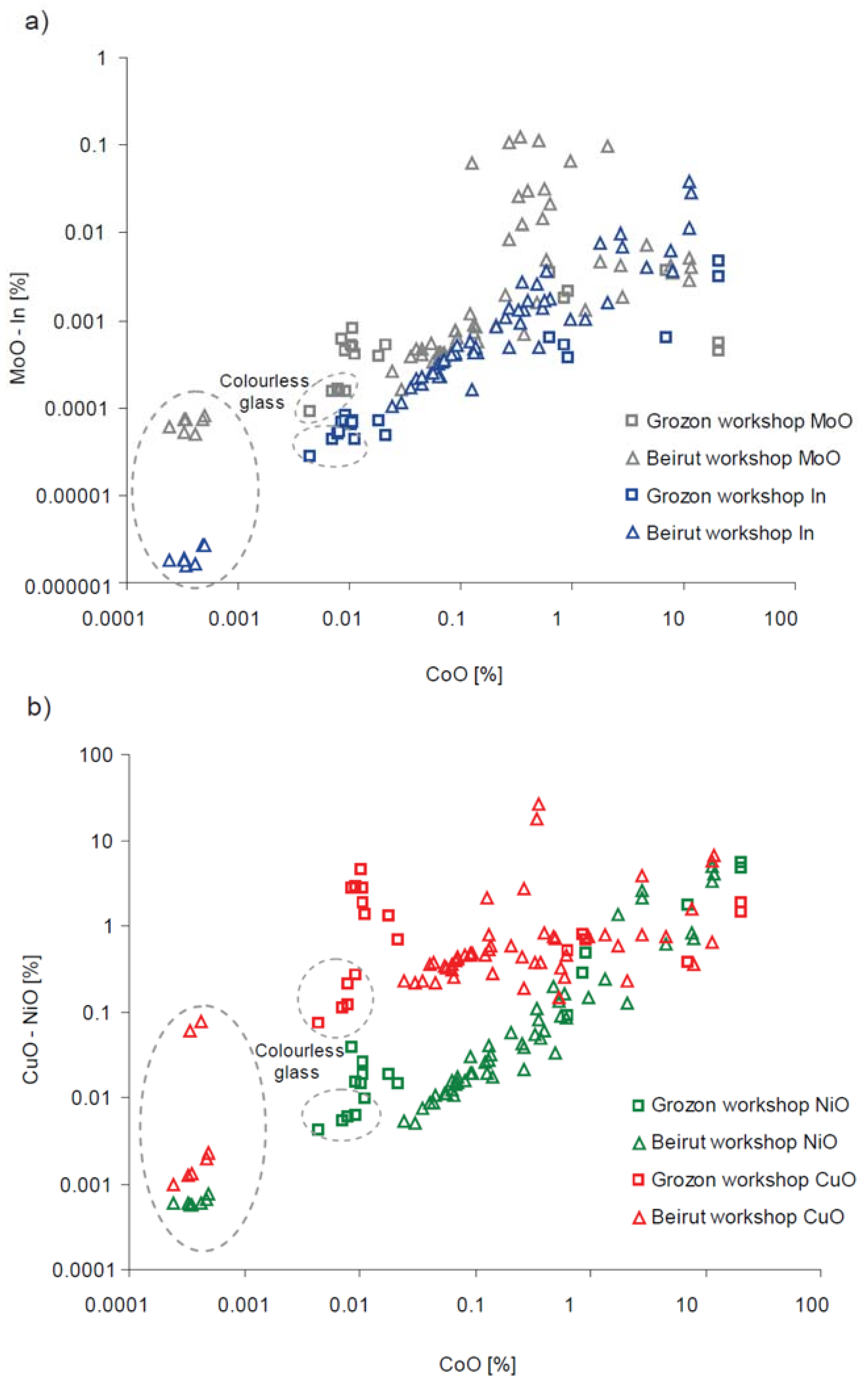
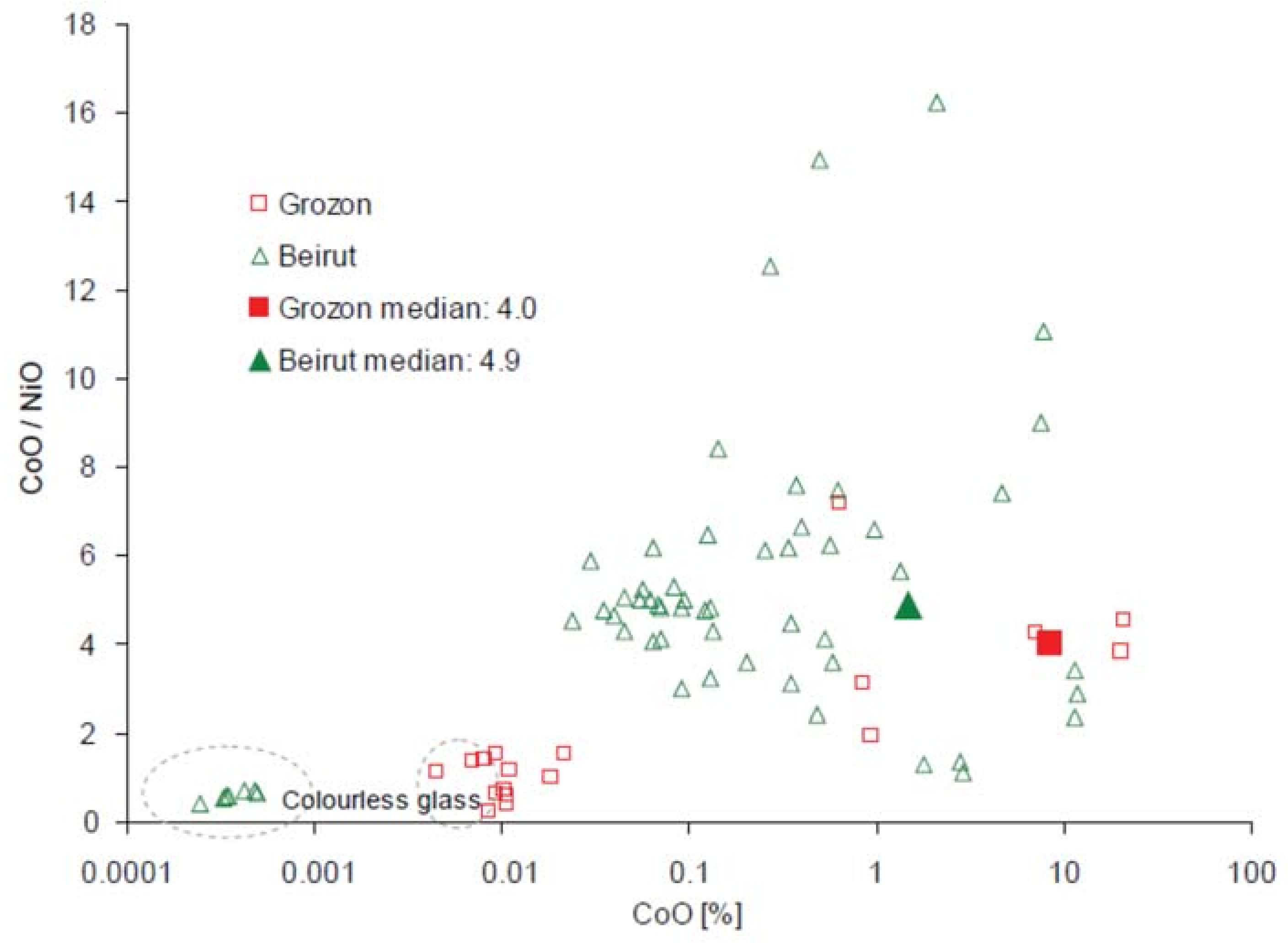
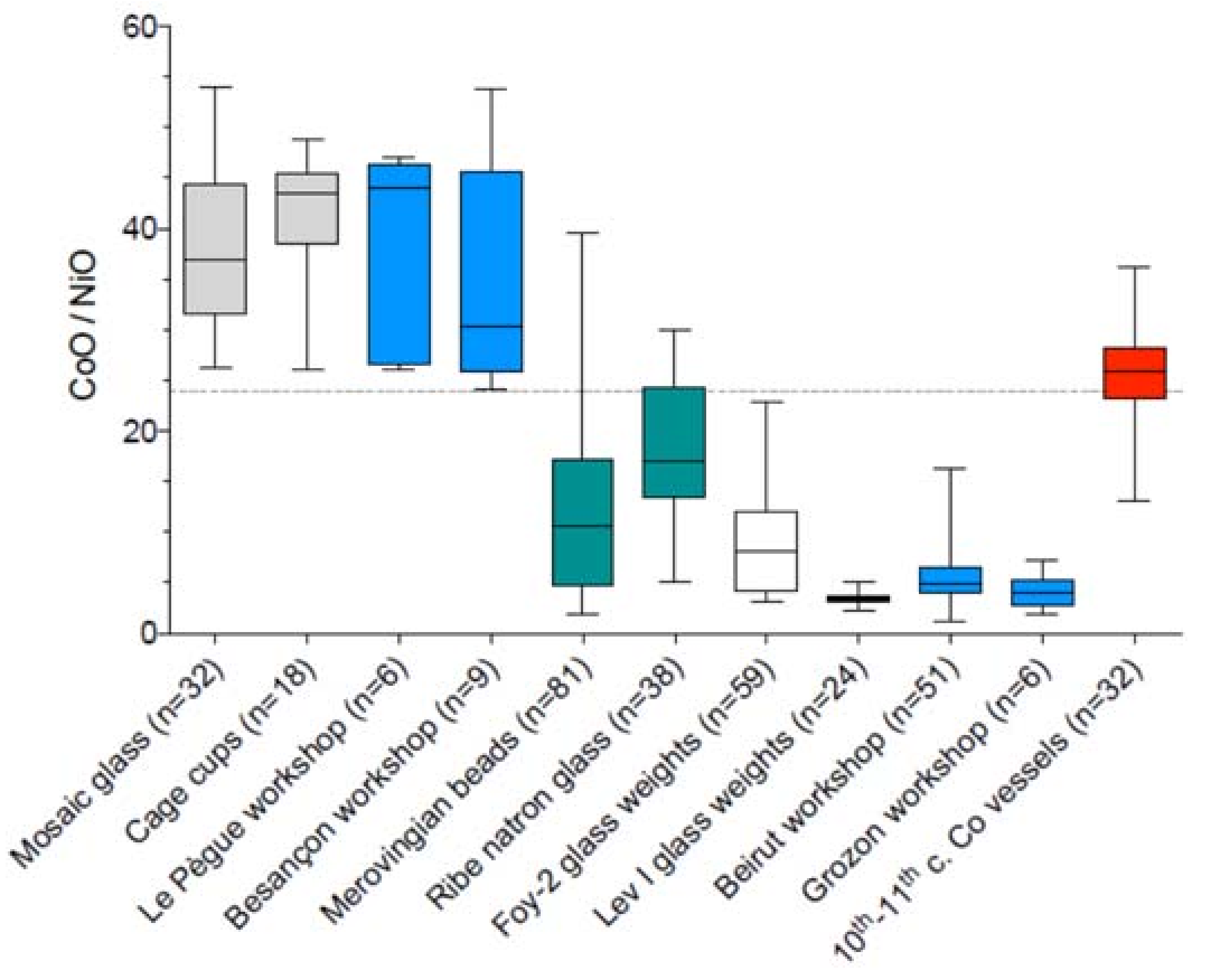
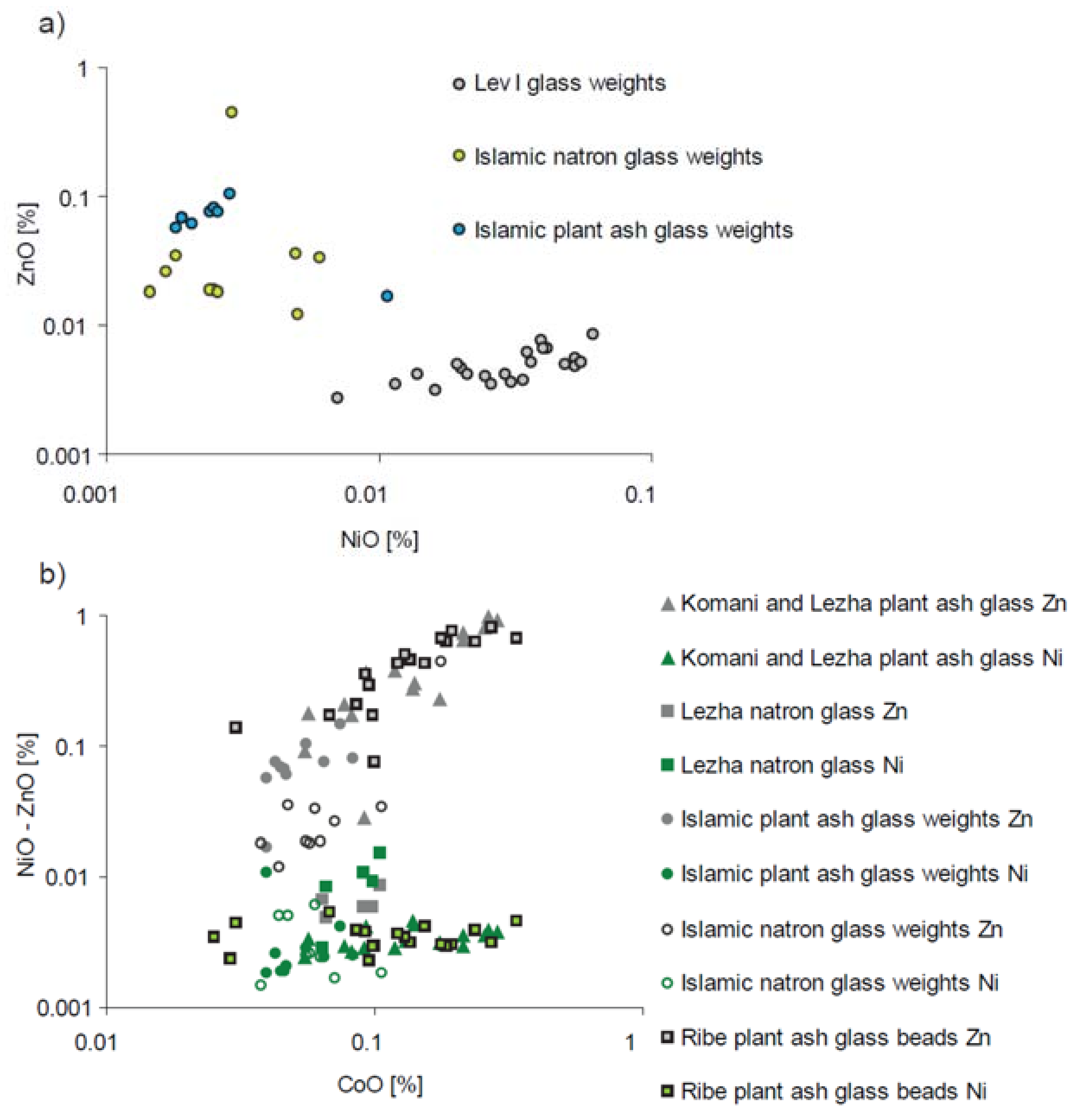
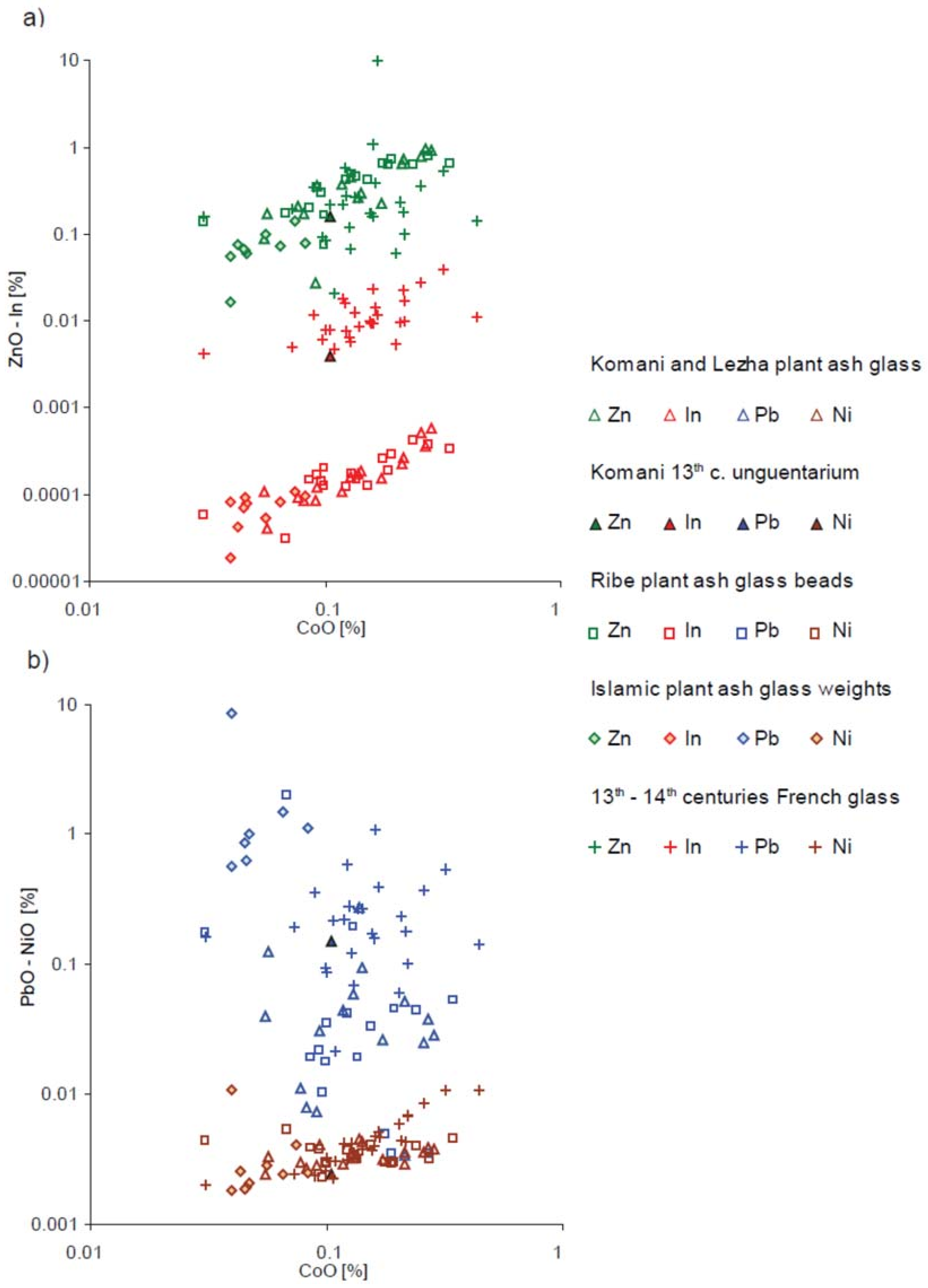
| Type of Glass | Date | Number of Analyses | Cobalt Type |
|---|---|---|---|
| Mosaic glass [29] | 1st–3rd centuries CE | 32 | Roman cobalt low nickel |
| Cage cups [30] | 4th century CE | 18 | Roman cobalt low nickel |
| Le Pègue workshop [31] | 2nd–3rd centuries CE | 6 | Roman cobalt low nickel |
| Besançon workshop [32] unpublished data | 2nd century CE | 9 | Roman cobalt low nickel |
| Hospitalet’s vessel unpublished data | 2nd–3rd centuries CE | 1 | Roman cobalt low nickel |
| Merovingian glass beads [33] | 5th–7th centuries CE | 81 | Roman cobalt low nickel late antique high nickel cobalt |
| Ribe natron glass [26] | 8th century CE | 38 | Roman cobalt low nickel late antique high nickel cobalt |
| Byzantine glass weights [25] | 6th–7th centuries CE | 59 Foy-2 24 Levantine I | Roman cobalt low nickelLate antique high nickel cobalt |
| Beirut workshop [20] | 7th century CE | 51 | Late antique high nickel cobalt |
| Grozon workshop unpublished data | 8th century CE | 19 | Late antique high nickel cobalt |
| Saint-Savin type glass [24,34] | 10th–11th centuries | 32 | Mainly Roman cobalt low nickel |
| St Denis window panel unpublished data | 12th century CE | 1 | Roman cobalt low nickel |
| Ribe Islamic plant ash glass [26] | 9th–10th centuries CE | 17 | Islamic cobalt-zinc |
| Komani and Lehza glass [23] | 7th–13th centuries CE | 16 Komani plant ash 1 Lehza plant ash 5 Lehza natron | 1 Roman cobalt low nickel 4 late antique high nickel cobalt 12 Islamic cobalt-zinc 1 European cobalt-indium-zinc |
| Samarra Co-blue flasks [35] | 9th century CE | 23 | Islamic cobalt-zinc |
| Islamic glass weights unpublished data | 9th–12th centuries CE | 10 natron 10 plant ash | Islamic cobalt-zinc |
| French glass [11] unpublished data | 13th–14th centuries CE | 28 | European cobalt-indium-zinc |
| Samples | wt % | ppm | Ratios of Metal Oxides | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Na2O | MgO | Al2O3 | SiO2 | P2O5 | Cl | K2O | CaO | Fe2O3 | Sb2O3 | CoO | NiO | CuO | SnO2 | PbO | GaO | In | Au | Fe2O3/CoO | CoO/NiO | CoO/CuO | CoO/SnO2 | CoO/PbO | |
| Cobalt glass (n = 5) | 17.1 | 0.45 | 2.18 | 70.5 | 0.036 | 1.07 | 0.57 | 6.15 | 0.82 | 0.62 | 697 | 23 | 1000 | 22 | 476 | 4.9 | 0.24 | 0.11 | 5.9 | 39.4 | 0.70 | 69.7 | 1.60 |
| SD (σ) | 1.1 | 0.02 | 0.18 | 0.7 | 0.002 | 0.07 | 0.09 | 0.44 | 0.04 | 0.02 | 39 | 4 | 81 | 4 | 86 | 0.3 | 0.01 | 0.03 | |||||
| Sb glass (n = 12) | 15.5 | 0.41 | 2.07 | 72.9 | 0.038 | 1.16 | 0.47 | 5.97 | 0.41 | 0.78 | 1.6 | 5.3 | 10 | 12 | 41 | 2.9 | 0 | 0.01 | |||||
| SD (σ) | 1.7 | 0.08 | 0.15 | 2.6 | 0.012 | 0.09 | 0.14 | 0.85 | 0.08 | 0.12 | 0.5 | 2.6 | 4 | 4 | 105 | 0.2 | 0 | 0.01 | |||||
| Co-((Sb) + 2 × σ (Sb)) | 0.25 | 694 | 13 | 982 | 2.8 | 224 | 1.5 | 0.24 | 0.08 | ||||||||||||||
| cobalt colorant [wt %] | 56.6 | 15.7 | 0.29 | 22.2 | 0.064 | 5.1 | 0.034 | 0.0053 | 0.0018 | 3.6 | 54.1 | 0.71 | 245 | 3.1 | |||||||||
| Samples | wt % | ppm | Ratios of Metal Oxides | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fe2O3 | CoO | NiO | CuO | SnO2 | PbO | Fe2O3/CoO | CoO/NiO | CoO/CuO | CoO/SnO2 | CoO/PbO | |
| Hospitalet 3320 02 blue | 1.22 | 1109 | 43 | 2319 | 126 | 4697 | 7.99 | 32.8 | 0.48 | 8.85 | 0.24 |
| Hospitalet 3320 02 colourless | 0.33 | 5.4 | 9.4 | 6.9 | 0.6 | 5.7 | |||||
| Serdica 26 blue | 1.04 | 380 | 21 | 1247 | 111 | 2201 | 10.5 | 25.3 | 0.32 | 4.00 | 0.18 |
| Serdica 27 colourless | 0.64 | 4 | 6 | 43 | 16 | 74 | |||||
| Yambol No. 1/2 | 0.88 | 539 | 24 | 848 | 72 | 2608 | 2.44 | 37.5 | 0.69 | 8.31 | 0.21 |
| Yambol No. 5/6 | 1.55 | 400 | 25 | 2793 | 130 | 932 | 20.0 | 25.9 | 0.15 | 3.24 | 0.45 |
| Yambol colourless | 0.75 | 3.9 | 9.8 | 68 | 6.9 | 44 | |||||
| CMoG CUP (55.1.143) blue | 0.88 | 429 | 24 | 949 | 27 | 1737 | 5.59 | 25.2 | 0.46 | 18.7 | 0.25 |
| CMoG CUP (55.1.143) colourless | 0.64 | 3 | 11 | 13 | 4 | 28 | |||||
| Besançon 3148 B * | 0.78 | 563 | 14 | 980 | 6.0 | 179 | 7.85 | 53.5 | 0.58 | 114 | 3.61 |
| Besançon 3148 D ** | 0.92 | 568 | 27 | 1477 | 71 | 1373 | 10.3 | 27.2 | 0.39 | 8.12 | 0.42 |
| Besançon 3239 C ** | 1.01 | 1320 | 59 | 1259 | 45 | 17208 | 5.08 | 25.3 | 1.05 | 30.2 | 0.08 |
| Besançon 3814 B ** | 0.91 | 1564 | 47 | 1337 | 3.9 | 66 | 3.67 | 38.4 | 1.18 | 552 | 36.7 |
| Besançon 3815 B ** | 0.62 | 379 | 19 | 687 | 31 | 3357 | 7.40 | 30.8 | 0.56 | 12.6 | 0.11 |
| Besancon 3828 A ** | 0.70 | 218 | 15 | 546 | 46 | 1234 | 16.7 | 24.1 | 0.40 | 4.90 | 0.18 |
| Besançon 3935 D ** | 0.95 | 902 | 36 | 1758 | 68 | 8834 | 6.81 | 30.4 | 0.52 | 13.6 | 0.10 |
| Besançon colourless | 0.34 | 1.3 | 3.1 */6.4 ** | 7.33 | 1.07 | 23.5 | |||||
| median | 7.6 | 28.8 | 0.5 | 10.7 | 0.23 | ||||||
| Samples | wt % | ppm | Ratios of Metal Oxides | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fe2O3 | CoO | NiO | CuO | SnO2 | PbO | ZnO | As2O3 | MoO | In | Fe2O3/CoO | CoO/NiO | CoO/CuO | CoO/SnO2 | CoO/PbO | |
| Levantine I cobalt (n = 24) | 1.21 | 1097 | 346 | 2575 | 116 | 5209 | 60.4 | 31.4 | 9.49 | 1.30 | 6.29 | 3.24 | 0.43 | 9.76 | 0.21 |
| SD (σ) | 0.27 | 454 | 157.5 | 1473 | 59.2 | 1946 | 56.1 | 17.3 | 4.41 | 0.47 | |||||
| Levantine I colourless (n = 25) | 0.52 | 2.82 | 7.08 | 32.7 | 3.65 | 23.1 | 16.8 | 3.09 | 0.72 | 0.02 | |||||
| SD (σ) | 0.12 | 1.32 | 2.07 | 57.3 | 4.59 | 28.2 | 12.5 | 0.95 | 0.44 | 0.02 | |||||
| Co-((colourless) + 2 × σ) | 0.47 | 1091 | 335 | 2428 | 103 | 5129 | 18.5 | 26.4 | 7.89 | 1.25 | |||||
| cobalt colorant [wt %] | 33.8 | 7.9 | 2.42 | 17.6 | 0.75 | 37.1 | 0.13 | 0.19 | 0.06 | 0.01 | 4.28 | 3.26 | 0.45 | 10.6 | 0.21 |
| Unit | Elements | Beirut | Grozon | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| inc. av n = 24 | inc. Max. | inc. Min. | Colourless Glass n = 7 | Blue Glass n = 27 | inc. av n = 6 | inc. Max. | inc. Min. | Colourless Glass n = 5 | Green Glass n = 8 | ||
| wt % | Na2O | 8.18 | 13.2 | 1.55 | 13.8 | 13.1 | 9.17 | 14.7 | 0.53 | 10.7 | 13.9 |
| MgO | 1.15 | 2.55 | 0.37 | 0.78 | 0.79 | 1.22 | 1.99 | 0.62 | 2.23 | 1.42 | |
| Al2O3 | 4.26 | 9.06 | 1.34 | 3.10 | 3.16 | 10.7 | 14.7 | 4.31 | 5.65 | 5.90 | |
| SiO2 | 37.6 | 63.2 | 7.16 | 70.5 | 69.0 | 35.9 | 55.8 | 3.4 | 64.2 | 64.0 | |
| P2O5 | 0.068 | 0.12 | 0.0000 | 0.11 | 0.11 | 0.39 | 1.42 | 0.045 | 0.33 | 0.36 | |
| Cl | 0.38 | 0.80 | 0.099 | 0.89 | 0.82 | 0.17 | 0.26 | 0.090 | 0.055 | 0.11 | |
| K2O | 0.45 | 0.93 | 0.038 | 0.75 | 0.79 | 1.50 | 2.45 | 0.19 | 5.00 | 1.95 | |
| CaO | 4.24 | 8.27 | 1.04 | 9.22 | 9.10 | 1.63 | 3.07 | 0.26 | 7.58 | 5.76 | |
| TiO2 | 0.15 | 0.28 | 0.063 | 0.084 | 0.090 | 0.41 | 0.54 | 0.30 | 0.32 | 0.30 | |
| MnO | 0.32 | 1.88 | 0.042 | 0.034 | 0.050 | 0.53 | 0.88 | 0.24 | 0.98 | 0.88 | |
| Fe2O3 | 34.2 | 76.3 | 5.88 | 0.58 | 1.19 | 26.3 | 58.0 | 8.75 | 2.52 | 2.74 | |
| CoO | 2.96 | 11.7 | 0.13 | 0.0004 | 0.11 | 8.48 | 20.8 | 0.64 | 0.0074 | 0.013 | |
| NiO | 0.91 | 4.81 | 0.020 | 0.0006 | 0.027 | 2.09 | 5.39 | 0.089 | 0.0054 | 0.019 | |
| CuO | 3.12 | 26.8 | 0.14 | 0.021 | 0.41 | 0.93 | 1.82 | 0.38 | 0.15 | 2.23 | |
| ZnO | 0.11 | 0.35 | 0.010 | 0.0014 | 0.011 | 0.19 | 0.49 | 0.023 | 0.025 | 0.020 | |
| PbO | 1.67 | 5.17 | 0.35 | 0.0074 | 1.08 | 0.062 | 0.13 | 0.025 | 0.016 | 0.14 | |
| ppm | Li2O | 8.8 | 18 | bdl | 12 | 11 | 17 | 34 | bdl | 662 | 59 |
| B2O3 | 152 | 304 | 25 | 338 | 317 | 218 | 383 | 8.2 | 158 | 361 | |
| V2O5 | 90 | 201 | 33 | 19 | 23 | 321 | 656 | 110 | 85 | 87 | |
| Cr2O3 | 88 | 266 | 2.6 | 24 | 29 | 454 | 2172 | bdl | 14 | 42 | |
| GaO | 210 | 857 | 5.6 | 4.2 | 9 | 355 | 555 | 200 | 13 | 28 | |
| As2O3 | 710 | 2809 | 107 | 2.6 | 181 | 318 | 1156 | 29 | 5.1 | 49 | |
| Rb2O | 7.7 | 14 | 1.5 | 11 | 12 | 29 | 47 | 3.6 | 129 | 39 | |
| SrO | 241 | 495 | 50 | 538 | 519 | 213 | 350 | 32 | 647 | 543 | |
| Y2O3 | 6.2 | 12 | 1.6 | 9.2 | 9.3 | 8.4 | 19 | 0.74 | 17 | 17 | |
| ZrO2 | 38 | 70 | 9.5 | 60 | 61 | 71 | 147 | 7.5 | 188 | 222 | |
| Nb2O3 | 2.3 | 6.3 | 0.83 | 2.2 | 2.3 | 4.7 | 9.7 | 0.46 | 8.0 | 8.5 | |
| MoO | 313 | 1238 | 13 | 0.66 | 6 | 20 | 36 | 4.4 | 1.4 | 5.2 | |
| Ag | 7.1 | 77 | 0.023 | 0.057 | 0.46 | 3.8 | 6.8 | 0.76 | 0.089 | 10.3 | |
| Cd | 0.10 | 0.49 | bdl | 0.027 | 0.049 | 0.088 | 0.30 | bdl | 0.59 | 0.24 | |
| In | 57 | 372 | 1.6 | 0.020 | 4.9 | 17 | 47 | 3.7 | 0.51 | 0.62 | |
| SnO2 | 100 | 439 | 5.3 | 3.9 | 21 | 1137 | 2844 | 304 | 161 | 166 | |
| Sb2O3 | 118 | 343 | 12 | 3.5 | 45 | 87 | 155 | 25 | 16 | 135 | |
| Cs2O | 0.094 | 0.51 | bdl | 0.10 | 0.12 | 1.9 | 3.6 | 0.38 | 3.8 | 2.8 | |
| BaO | 124 | 239 | 36 | 242 | 233 | 209 | 271 | 87 | 585 | 330 | |
| La2O3 | 5.1 | 12 | 1.2 | 8.4 | 8.5 | 8.5 | 20 | 0.66 | 25 | 23 | |
| CeO2 | 10.4 | 25 | 2.9 | 16 | 16 | 17 | 41 | 0.91 | 46 | 42 | |
| PrO2 | 1.2 | 2.7 | 0.26 | 1.9 | 2.0 | 1.9 | 4.5 | 0.14 | 5.5 | 5.0 | |
| Nd2O3 | 4.7 | 10.7 | 1.1 | 7.8 | 7.9 | 7.0 | 17 | 0.50 | 22 | 19 | |
| Sm2O3 | 1.03 | 2.54 | 0.31 | 1.6 | 1.6 | 1.4 | 3.3 | 0.11 | 4.2 | 3.6 | |
| Eu2O3 | 0.24 | 0.53 | bdl | 0.46 | 0.46 | 0.34 | 0.73 | 0.094 | 0.95 | 0.81 | |
| Gd2O3 | 0.84 | 2.0 | 0.0083 | 1.4 | 1.4 | 1.3 | 2.8 | 0.36 | 3.5 | 3.0 | |
| Tb2O3 | 0.16 | 0.35 | 0.045 | 0.23 | 0.23 | 0.20 | 0.47 | 0.020 | 0.50 | 0.46 | |
| Dy2O3 | 0.95 | 2.1 | 0.30 | 1.3 | 1.4 | 1.3 | 2.9 | 0.14 | 2.9 | 2.8 | |
| Ho2O3 | 0.20 | 0.43 | 0.033 | 0.28 | 0.29 | 0.26 | 0.59 | 0.022 | 0.58 | 0.56 | |
| Er2O3 | 0.55 | 1.2 | 0.16 | 0.76 | 0.76 | 0.77 | 1.7 | 0.015 | 1.6 | 1.6 | |
| Tm2O3 | 0.080 | 0.17 | 0.025 | 0.10 | 0.11 | 0.12 | 0.26 | 0.022 | 0.22 | 0.23 | |
| Yb2O3 | 0.56 | 1.16 | 0.068 | 0.71 | 0.72 | 0.78 | 1.8 | 0.065 | 1.7 | 1.6 | |
| Lu2O3 | 0.087 | 0.19 | 0.022 | 0.10 | 0.11 | 0.12 | 0.26 | bdl | 0.23 | 0.23 | |
| HfO2 | 0.90 | 1.7 | 0.23 | 1.3 | 1.4 | 1.5 | 2.9 | 0.28 | 4.2 | 4.8 | |
| Ta2O3 | 0.13 | 0.34 | 0.038 | 0.12 | 0.12 | 0.24 | 0.52 | 0.013 | 0.46 | 0.50 | |
| WO | 0.37 | 1.5 | 0.12 | 0.066 | 0.12 | 0.61 | 1.3 | 0.0080 | 1.0 | 1.5 | |
| Au | 0.14 | 1.3 | bdl | 0.0015 | 0.016 | 0.021 | 0.042 | bdl | 0.0069 | 0.10 | |
| Bi | 0.80 | 2.4 | 0.13 | 0.0074 | 0.83 | 0.54 | 1.4 | 0.12 | 0.061 | 0.45 | |
| ThO2 | 1.1 | 3.1 | 0.38 | 1.1 | 1.1 | 2.6 | 6.2 | 0.12 | 6.3 | 6.7 | |
| UO2 | 0.46 | 0.73 | 0.11 | 0.75 | 0.79 | 0.79 | 1.78 | 0.048 | 2.2 | 2.1 | |
| Samples | wt % | ppm | Average Ratios of Metal Oxides | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fe2O3 | CoO | NiO | CuO | ZnO | As2O3 | MoO | In | SnO2 | PbO | CoO/ZnO | CoO/In | |
| Komani and Lezha plant ash glass (n = 16) | 1.20 | 1492 | 34 | 1478 | 4209 | 8 | 3 | 2 | 368 | 540 | 0.58 | 852 |
| SD (σ) | 0.25 | 764 | 6 | 609 | 3004 | 4 | 1 | 2 | 517 | 674 | ||
| Komani unguentarium (n = 1) | 0.82 | 1050 | 24 | 1699 | 1605 | 11 | 1 | 40 | 459 | 1487 | 0.65 | 26.5 |
| Ribe plant ash glass beads (n = 17) | 1.39 | 1483 | 36 | 2818 | 4285 | 17 | 2 | 2 | 174 | 451 | 0.40 | 846 |
| SD (σ) | 0.42 | 794 | 8 | 4028 | 2283 | 25 | 1 | 1 | 288 | 571 | ||
| Islamic plant ash glass weights (n = 10) | 1.64 | 537 | 33 | 2108 | 738 | 20 | 3 | 1 | 484 | 3932 | 0.88 | 874 |
| SD (σ) | 0.47 | 153 | 27 | 1229 | 323 | 11 | 1 | 0 | 872 | 6261 | ||
| 13th–14th-centuries French glass (n = 28) | 1.45 | 1623 | 46 | 3248 | 4943 | 40 | 2 | 123 | 193 | 2513 | 0.44 | 15.6 |
| SD (σ) | 0.40 | 809 | 23 | 3494 | 3753 | 36 | 1 | 79 | 163 | 2199 | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gratuze, B.; Pactat, I.; Schibille, N. Changes in the Signature of Cobalt Colorants in Late Antique and Early Islamic Glass Production. Minerals 2018, 8, 225. https://doi.org/10.3390/min8060225
Gratuze B, Pactat I, Schibille N. Changes in the Signature of Cobalt Colorants in Late Antique and Early Islamic Glass Production. Minerals. 2018; 8(6):225. https://doi.org/10.3390/min8060225
Chicago/Turabian StyleGratuze, Bernard, Inès Pactat, and Nadine Schibille. 2018. "Changes in the Signature of Cobalt Colorants in Late Antique and Early Islamic Glass Production" Minerals 8, no. 6: 225. https://doi.org/10.3390/min8060225
APA StyleGratuze, B., Pactat, I., & Schibille, N. (2018). Changes in the Signature of Cobalt Colorants in Late Antique and Early Islamic Glass Production. Minerals, 8(6), 225. https://doi.org/10.3390/min8060225





