Acid Rock Drainage or Not—Oxidative vs. Reductive Biofilms—A Microbial Question
Abstract
1. Introduction
1.1. General Mine Waste Management Practices
1.2. Challenges in Mine Waste Management
1.3. From an Iron Phosphate Coating to a Biofilm or from Stoichiometry to Geomicrobiology
2. Approaching the Geo-Microbiological Challenge
2.1. Reproducable and Replicable Outdoor and Laoratory Experiments
2.2. LUNEs, Large Un-Replicable Natural Experiments
2.3. Characteristics and Composition of CPMW
2.4. Which Microbial Groups Form the Organic Coating or Improve the Biofilm?
3. General Characteristics of Biofilms and Functions
3.1. Fundamentals of Biofilm Formation
- have adapted to all types of terrestrial/subaerial stresses such as desiccation, extreme temperatures, low nutrient availability, and intense solar radiation;
- interact with minerals that serve both as a dwelling and a source of nutrients and trace elements, and;
- enhance weathering of rocks and soil formation.
3.2. Acidophilic, Mineral-Oxidizing Biofilms on Metal Sulfide Surfaces
3.3. Potential Effects of CPMW on Oxidative Biofilms on Metal Sulfides Surfaces
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Agricola, C. De Re Metallica, First English ed.; Hoover, H.; Hoover, H.L., Translators; Dover Publications, Inc.: New York, NY, USA, 1912; Available online: Available online: http://farlang.com/books/agricola-hoover-de-re-metallica (accessed on 3 May 2018).
- Jakubick, A.; McKenna, G. Stabilization of tailings deposits: International experience. In Proceedings of the Mining in the Environment III, Sudbury, ON, Canada, 25–28 May 2003; pp. 1–9. [Google Scholar]
- Kalin, M.; Wheeler, W.N.; Sudbury, M.P.; Harris, B. Mining, Ecological Engineering and Metals Extraction for the 21st Century. Subject: Environmental Issues and Problems, Environmental Engineering, Sustainability and Solutions. In Oxford Research Encyclopedias; Oxford University Press: Oxford, UK, 2018. [Google Scholar]
- Gusek, J.; Plocus, V.G. Case study 20 years of acid rock drainage chemistry improvements after bactericide application. J. Am. Soc. Min. Reclam. 2016, 67–85. [Google Scholar] [CrossRef]
- INAP. The International Network of Acid Prevention, Global Acid Rock Drainage Guide. Chapter 6.0, Prevention and Mitigation. 6.6.4. pp. 272–275. Available online: http://www.gardguide.com/images/5/5f/TheGlobalAcidRockDrainageGuide.pdf (accessed on 4 May 2018).
- Mauric, A.; Lottermoser, B.G. Phosphate amendment of metalliferous waste rocks, Century Pb–Zn mine, Australia: Laboratory and field trials. Appl. Geochem. 2011, 26, 45–56. [Google Scholar] [CrossRef]
- Meek, F.A. Research into the Use of Apatite Rock for Acidic Drainage Prevention. Available online: https://wvmdtaskforce.files.wordpress.com/2015/12/84-meek.pdf (accessed on 3 May 2018).
- Meek, F.A. Assessment of Acid Preventative Techniques Employed at the Island Creek Mining Company Tenmile Site. Available online: https://wvmdtaskforce.files.wordpress.com/2015/12/91-meek.pdf (accessed on 3 May 2018).
- Spotts, E.; Dollhopf, O.J. Evaluation of phosphate materials for control of acid production in pyritic mine overburden. J. Environ. Qual. 1992, 21, 627–634. [Google Scholar] [CrossRef]
- Renton, J.J.; Stiller, A.H.; Rymer, T.E. Use of phosphate materials as ameliorants for acid mine drainage. J. Am. Soc. Min. Reclam. 1987, 67–75. [Google Scholar] [CrossRef]
- Stiller, A.; Renton, J.; Rymer, T.E. An experimental evaluation of the use of rock phosphate (apatite) for the amelioration of acid-producing coal mine waste. Min. Sci. Technol. 1989, 9, 283–287. [Google Scholar] [CrossRef]
- Hart, W.; Stiller, A.; Rymer, T.; Skousen, J.; Sencindiver, J.; Samuel, D. The use of phosphate refuse as a potential AMD ameliorant. In Proceedings of the Mining and Reclamation Conference and Exhibition, Charleston, WV, USA, 23–26 April 1990; West Virginia University Publications Service: Morgantown, WV, USA, 1990; pp. 43–49. [Google Scholar]
- Ziemkiewicz, P. Advances in the prediction and control of acid mine drainage. In Proceedings of the Mining and Reclamation Conference and Exhibition, Charleston, WV, USA, 23–26 April 1990; pp. 51–54. [Google Scholar]
- Georgopoulou, Z.; Fytas, K.; Soto, H.; Evangelou, B. Feasibility and cost of creating an iron-phosphate coating on pyrrhotite to prevent oxidation. Environ. Geol. 1996, 28, 61–69. [Google Scholar] [CrossRef]
- Belzile, N.; Maki, S.; Chen, Y.-W.; Goldsack, D. Inhibition of pyrite oxidation by surface treatment. Sci. Total Environ. 1997, 196, 177–186. [Google Scholar] [CrossRef]
- Evangelou, V.P. Pyrite Oxidation and its Control; CRC Press: Boca Raton, FL, USA, 1995. [Google Scholar]
- Olson, G.J.; Clark, T.R.; Mudder, T.I.; Logsdon, M. Toward source control of acid rock drainage. In Proceedings of the Seventh International Conference on Acid Rock Drainage, St. Louis, MO, USA, 2006; Barnhisel, R.I., Ed.; American Society of Mining and Reclamation: Lexington, KY, USA, 2006; pp. 1435–1452. [Google Scholar]
- Kalin, M. Ecological Engineering and Biological Polishing: Methods to Economize Waste Management in Hard Rock Mining. In Ecological Engineering: An Introduction to Ecotechnology; Mitsch, W.J., Jorgensen, S.E., Eds.; Wiley & Sons, Inc.: New York, NY, USA, 1989; pp. 443–461. ISBN 0-471-62559-0. [Google Scholar]
- Wilson, G.W. Why are we still struggling with acid rock drainage? Geotech. News 2008, 26, 52–56. [Google Scholar]
- Kuenzer, C.; Stracher, G.B. Geomorphology of coal seam fires. Geomorphology 2012, 138, 209–222. [Google Scholar] [CrossRef]
- Rosenblum, G.; Finch, J.A.; Waters, K.E.; Nesset, J.E. A test apparatus for studying the effects of weathering on self-heating sulphides. In Proceedings of the Conference of Metallurgists, Canadian Institute of Mining, Metallurgy and Petroleum, Toronto, ON, Canada, 23–26 August 2015; p. 9. [Google Scholar]
- Lüttge, A.; Arvidson, R.S. The mineral-water interface. In Kinetics of Water-Rock Interaction; Brantley, S.L., Kubicki, J.D., White, A.F., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 73–107. [Google Scholar]
- Bellenberg, S.; Kalin, M.; Sand, W. Microbial Community Composition on Lignite before and after the Addition of Phosphate Mining Wastes. Adv. Mater. Res. 2013, 825, 42–45. [Google Scholar] [CrossRef]
- Erwin, D.H. Macroevolution of ecosystem engineering, niche construction and diversity. Trends Ecol. Evol. 2008, 23, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Laland, K.; Mathews, B.; Feldman, M.W. An introduction to niche construction theory. Evol. Ecol. 2016, 30, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Scott, D. Gibraltar’s dump leaching—An insight into acid effluent control. North. Min. 1991, 77, 1–7. [Google Scholar]
- Kalin, M.; Harris, B. Chemical precipitation within pyritic waste rock. Hydrometallurgy 2005, 78, 213–229. [Google Scholar] [CrossRef]
- Kalin, M.; Ferris, G.; Paulo, C. Reducing sulphide oxidation in pyritic mining wastes-phosphate mining wastes stimulate biofilm formation on mineral surface. In Proceedings of the “Securing the Future/8th ICARD”, Skellefteå, Sweden, 23–26 2009. [Google Scholar]
- Ueshima, M.; Fortin, D.; Kalin, M. Development of iron-phosphate biofilms on pyritic mine waste rock surfaces previously treated with natural phosphate rocks. Geomicrobiol. J. 2004, 21, 313–323. [Google Scholar] [CrossRef]
- AMIRA. 2017. Available online: http://www.amira.com.au/web/site.asp?section=projects&page=projectdetails&ProjectLink=2861&Source_ID=1 (accessed on 3 May 2018).
- Kalin, M.; Paulo, C.; Smart, R.; Wheeler, W. Phosphate mining reduces microbial oxidation of sulphidic minerals: A proposed mechanism. In Proceedings of the International Conference on Acid Rock Drainage, Ottawa, QC, Canada, 20–26 May 2012; pp. 585–593. [Google Scholar]
- Barley, S.; Meeuwig, J. The Power and the Pitfalls of Large-scale, Un-replicated Natural Experiments. Ecosystems 2017, 20, 1–9. [Google Scholar] [CrossRef]
- Kalin, M.; Smith, M.P.; Fyson, A. The use of natural phosphate rock to reduce drainage from pyritic waste rock. In Proceedings of the International Conference on Acid Rock Drainage, Vancouver, BC, Canada, 31 May–6 June 1997. [Google Scholar]
- Kalin, M.; Smith, M.P.; Fyson, A. The Role of Phosphate in Applied Biotechnology in Mine Waste Management: Reduction in AMD from Pyritic Waste Rock. In Proceedings of the International Symposium, the Metallurgical Society of the Canadian Institute for Mining, ‘Waste Processing and Recycling’, Calgary, AB, Canada, 16–19 August 1998; pp. 15–29. [Google Scholar]
- Kalin, M. Improving pore water quality in reactive tailings with phosphate mining wastes. In Proceedings of the “The Conference of Metallurgists”, COM 2004, Materials: The future of manufacturing in a sustainable environment, Hamilton, ON, Canada, 22–25 August 2004; pp. 427–437. [Google Scholar]
- Kalin, M.; Wheeler, W.N. A review of the role of phosphate mining waste: A chemical or biological reagent for AMD prevention. In Proceedings of the Twelfth Annual West Virginia Surface Mine Drainage Task Force Symposium, Morgantown, WV, USA, 29–30 March 2011; pp. 1–12. [Google Scholar]
- Kalin, M.; Paulo, C.; Sleep, B. Proactive prevention of acid generation: Reduction/inhibition of sulphide oxidation. In Proceedings of the International Mine Water Association Symposium, Sydney, NS, Canada, 5–9 September 2010; pp. 479–482. [Google Scholar]
- Kalin, M.; Fyson, A.; Smith, M.P.; Werker, A. Tailings surface cover development through integration of reactive phosphate and organic matter. In Proceedings of the Mining and Environment III and Annual Meeting of the CLRA, Sudbury, ON, USA, 25–28 May 2003. [Google Scholar]
- Kalin, M.; Paulo, C.; Sudbury, M.P.; Wheeler, W.N. Reducing sulphide oxidation in mining wastes by recognizing the geomicrobiology role of phosphate mining wastes. J. Am. Soc. Min. Reclam. 2015, 4, 102–121. [Google Scholar] [CrossRef]
- Costerton, J.W.; Lewandowski, Z.; Caldwell, D.E.; Korber, D.R.; Lappin-Scott, H.M. Microbial biofilms. Annu. Rev. Microbiol. 1995, 49, 711–745. [Google Scholar] [CrossRef] [PubMed]
- Stoodley, P.; Sauer, K.; Davies, D.G.; Costerton, J.W. Biofilms as complex differentiated communities. Annu. Rev. Microbiol. 2002, 56, 187–209. [Google Scholar] [CrossRef] [PubMed]
- Kolenbrander, P.E.; Andersen, R.N.; Moore, L.V. Intrageneric coaggregation among strains of human oral bacteria: Potential role in primary colonization of the tooth surface. Appl. Environ. Microbiol. 1990, 56, 3890–3894. [Google Scholar] [PubMed]
- Dang, H.; Lovell, C.R. Bacterial Primary Colonization and Early Succession on Surfaces in Marine Waters as Determined by Amplified rRNA Gene Restriction Analysis and Sequence Analysis of 16S rRNA Genes. Appl. Environ. Microbiol. 2000, 66, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Sand, W.; Bock, E. Concrete corrosion in the Hamburg Sewer system. Environ. Technol. Lett. 1984, 5, 517–528. [Google Scholar] [CrossRef]
- Kemmling, A.; Kämper, M.; Flies, C.; Schieweck, O.; Hoppert, M. Biofilms and extracellular matrices on geomaterials. Environ. Geol. 2004, 46, 429–435. [Google Scholar] [CrossRef]
- Kip, N.; van Veen, J.A. The dual role of microbes in corrosion. ISME J. 2015, 9, 542–551. [Google Scholar] [CrossRef] [PubMed]
- Gorbushina, A.A.; Broughton, W.J. Microbiology of the atmosphere-rock interface: How biological interactions and physical stresses modulate a sophisticated microbial ecosystem. Annu. Rev. Microbiol. 2009, 63, 431–450. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Rawlings, D.E.; Johnson, D.B. The microbiology of biomining: Development and optimization of mineral-oxidizing microbial consortia. Microbiology 2007, 153, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Nordstrom, D.K.; Alpers, C.N. Geochemistry of acid mine waters. Rev. Econ. Geol. 1999, 6, 133–160. [Google Scholar]
- Bennett, J.C.; Tributsch, H. Bacterial leaching patterns on pyrite crystal surfaces. J. Bacteriol. 1978, 134, 310–317. [Google Scholar] [PubMed]
- Farah, C.; Vera, M.; Morin, D.; Haras, D.; Jerez, C.A.; Guiliani, N. Evidence for a functional quorum-sensing type AI-1 system in the extremophilic bacterium Acidithiobacillus ferrooxidans. Appl. Environ. Microbiol. 2005, 71, 7033–7040. [Google Scholar] [CrossRef] [PubMed]
- Hammer, B.K.; Bassler, B.L. Quorum sensing controls biofilm formation in Vibrio cholerae. Mol. Microbiol. 2003, 50, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Parsek, M.R.; Greenberg, E.P. Sociomicrobiology: The connections between quorum sensing and biofilms. Trends Microbiol. 2005, 13, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Waters, C.M.; Lu, W.; Rabinowitz, J.D.; Bassler, B.L. Quorum sensing controls biofilm formation in Vibrio cholerae through modulation of cyclic di-GMP levels and repressio of vpsT. J. Bacteriol. 2008, 190, 2527–2536. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.; Bellenberg, S.; Mamani, S.; Ruiz, L.; Echeverria, A.; Soulère, L.; Doutheau, A.; Demergasso, C.; Sand, W.; Queneau, Y.; et al. AHL signaling molecules with a large acyl chain enhance biofilm formation on sulfur and metal sulfides by the bioleaching bacterium Acidithiobacillus ferrooxidans. Appl. Microbiol. Biotechnol. 2013, 97, 3729–3737. [Google Scholar] [CrossRef] [PubMed]
- Bellenberg, S.; Diaz, M.; Noel, N.; Sand, W.; Poetsch, A.; Guiliani, N.; Vera, M. Biofilm formation, communication and interactions of leaching bacteria during colonization of pyrite and sulfur surfaces. Res. Microbiol. 2014, 165, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Garrett, T.R.; Bhakoo, M.; Zhang, Z. Bacterial adhesion and biofilms on surfaces. Progress Nat. Sci. 2008, 18, 1049–1056. [Google Scholar] [CrossRef]
- Nöel, N.; Florian, B.; Sand, W. AFM & EFM study on attachment of acidophilic leaching organisms. Hydrometallury 2010, 104, 370–375. [Google Scholar]
- Bellenberg, S.; Barthen, R.; Boretska, M.; Zhang, R.; Sand, W.; Vera, M. Manipulation of pyrite colonization and leaching by iron-oxidizing Acidithiobacillus species. Appl. Microbiol. Biotechnol. 2015, 99, 1435–1449. [Google Scholar] [CrossRef] [PubMed]
- Gehrke, T.; Telegdi, J.; Thierry, D.; Sand, W. Importance of Extracellular Polymeric Substances from Thiobacillus ferrooxidans for Bioleaching. Appl. Environ. Microbiol. 1998, 64, 2743–2747. [Google Scholar] [PubMed]
- Gehrke, T.; Hallmann, R.; Kinzler, K.; Sand, W. The EPS of Acidithiobacillus ferrooxidans—A model for structure-function relationships of attached bacteria and their physiology. Water Sci. Technol. 2001, 43, 159–167. [Google Scholar] [PubMed]
- Harneit, K.; Göksel, A.; Kock, D.; Klock, J.H.; Gehrke, T.; Sand, W. Adhesion to metal sulphide surfaces by cells of Acidithiobacillus ferrooxidans, Acidithiobacillus thiooxidans and Leptospirillum ferrooxidans. Hydrometallurgy 2006, 83, 245–254. [Google Scholar] [CrossRef]
- Solari, J.A.; Huerta, G.; Escobar, B.; Vargas, T.; Badilla-Ohlbaum, R.; Rubio, J. Interfacial phenomena affecting the adhesion of Thiobacillus ferrooxidans to sulphide mineral surfaces. Colloids Surf. 1992, 69, 159–166. [Google Scholar] [CrossRef]
- Sampson, M.I.; Phillips, C.V.; Blake, R.C., II. Influence of the attachment of acidophilic bacteria during the oxidation of mineral sulfides. Min. Eng. 2000, 13, 373–389. [Google Scholar] [CrossRef]
- Schippers, A.; Jozsa, P.; Sand, W. Sulfur chemistry in bacterial leaching of pyrite. Appl. Environ. Microbiol. 1996, 62, 3424–3431. [Google Scholar] [PubMed]
- Barreto, M.; Gehrke, T.; Harneit, K.; Sand, W.; Jedlicki, E.; Holmes, D. Unexpected insights into biofilm formation by Acidithiobacillus Ferrooxidans revealed by genome analysis and experimental approaches. In Proceedings of the 16th International Biohydrometallurgy Symposium Compress, Cape Town, South Africa, 25–29 September 2005; pp. 817–825. [Google Scholar]
- Bellenberg, S.; Leon-Morales, C-F.; Sand, W.; Vera, M. Visualization of capsular polysaccharide induction in Acidithiobacillus ferrooxidans. Hydrometallurgy 2012, 129–130, 82–89. [Google Scholar] [CrossRef]
- Africa, C.-J.; Harrison, S.T.L.; Becker, M.; Hille, R.P. In situ investigation and visualisation of microbial attachment and colonisation in a heap bioleach environment: The novel biofilm reactor. Miner. Eng. 2010, 23, 486–491. [Google Scholar]
- Afzal Ghauri, M.; Okibe, N.; Johnson, D.B. Attachment of acidophilic bacteria to solid surfaces: The significance of species and strain variations. Hydrometallurgy 2007, 85, 72–80. [Google Scholar] [CrossRef]
- Bellenberg, S.; Florian, B.; Vera, M.; Rohwerder, T.; Sand, W. Comparative study of planktonic and sessile cells from pure and mixed cultures of Acidithiobacillus ferrooxidans and Acidiphilium cryptum growing on pyrite. Adv. Mater. Res. 2009, 71, 333–336. [Google Scholar] [CrossRef]
- Hallmann, R. Einfluss extrazellulärer, polymerer Substanzen von Leptospirillum ferrooxidans auf dessen Bioconenose mit Acidiphilium spec. RB8. Ph.D. Thesis, University of Hamburg, Hamburg, Germany, 1996. [Google Scholar]
- Caiazza, N.C.; O’Toole, G.A. Alpha-toxin is required for biofilm formation by Staphylococcus aureus. J. Bacteriol. 2003, 185, 3214–3217. [Google Scholar] [CrossRef] [PubMed]
- McDougald, D.; Rice, S.A.; Barraud, N.; Steinberg, P.D.; Kjelleberg, S. Should we stay or should we go: Mechanisms and ecological consequences for biofilm dispersal. Nat. Rev. Microbiol. 2012, 10, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, D.; McDermott, F.; Clipson, N. Understanding microbially active biogeochemical environments. Adv. Appl. Microbiol. 2007, 62, 81–104. [Google Scholar] [PubMed]
- Xavier, J.B.; Foster, K.R. Cooperation and conflict in microbial biofilms. Proc. Natl. Acad. Sci. USA 2007, 104, 876–881. [Google Scholar] [CrossRef] [PubMed]
- De Beer, D.; Stoodley, P.; Roe, F.; Lewandowski, Z. Effects of biofilm structures on oxygen and mass transport. Biotechnol. Bioeng. 1994, 43, 1131–1138. [Google Scholar] [CrossRef] [PubMed]

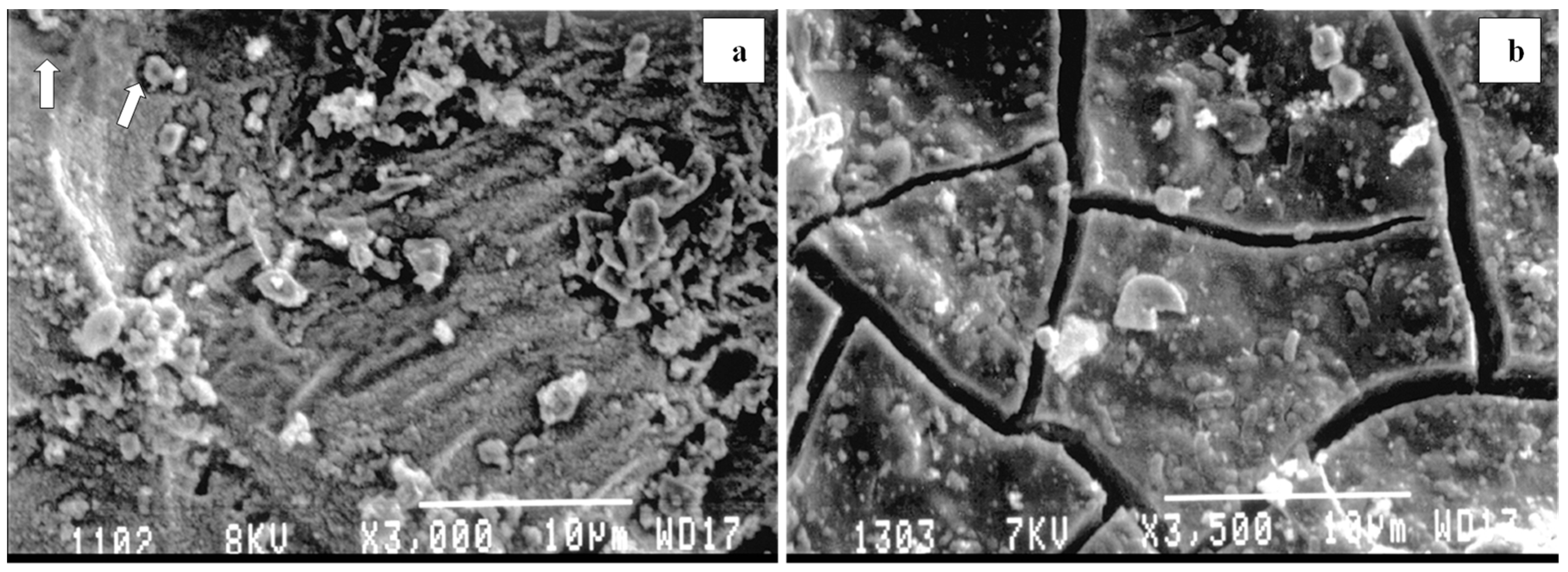

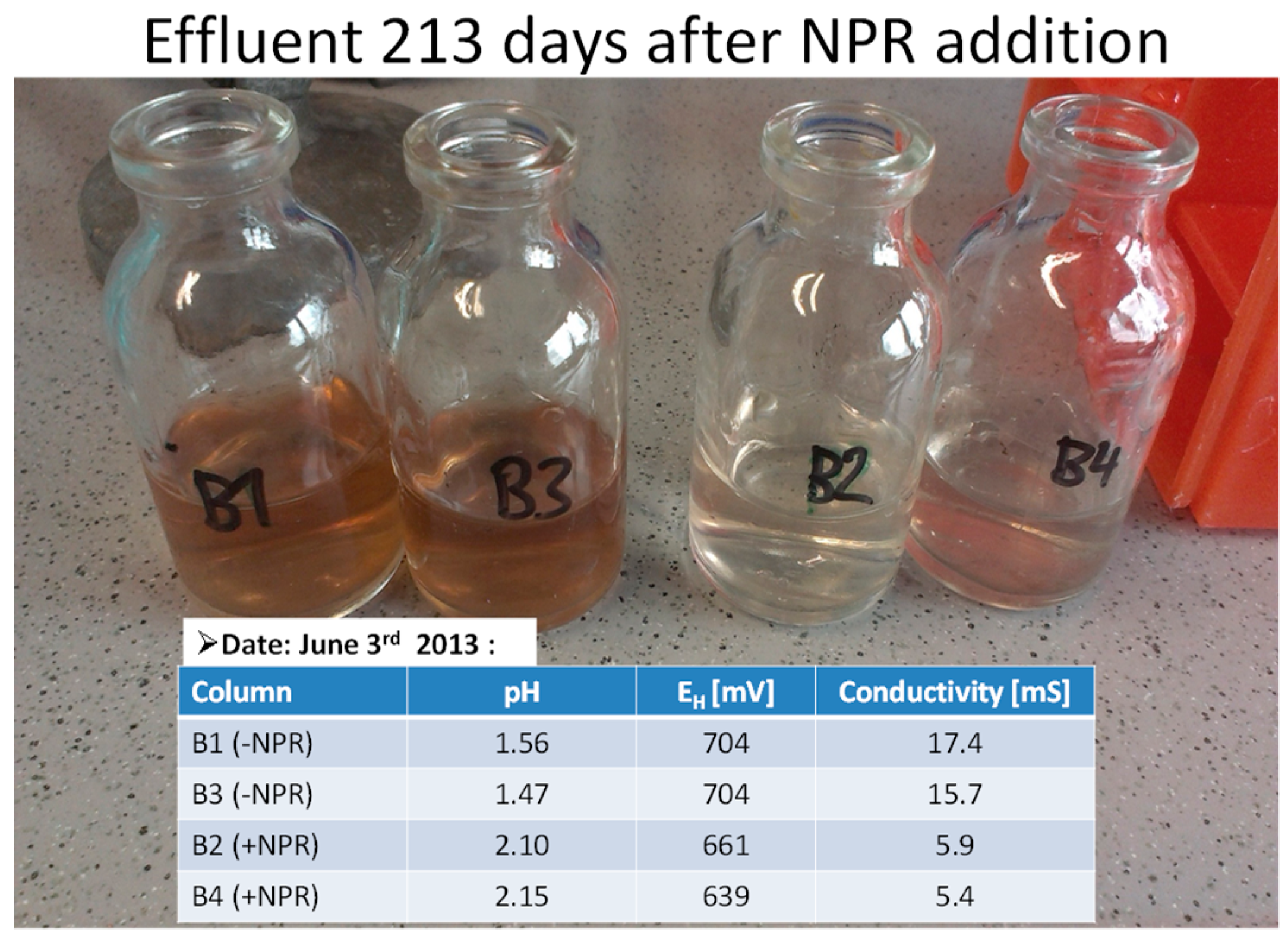
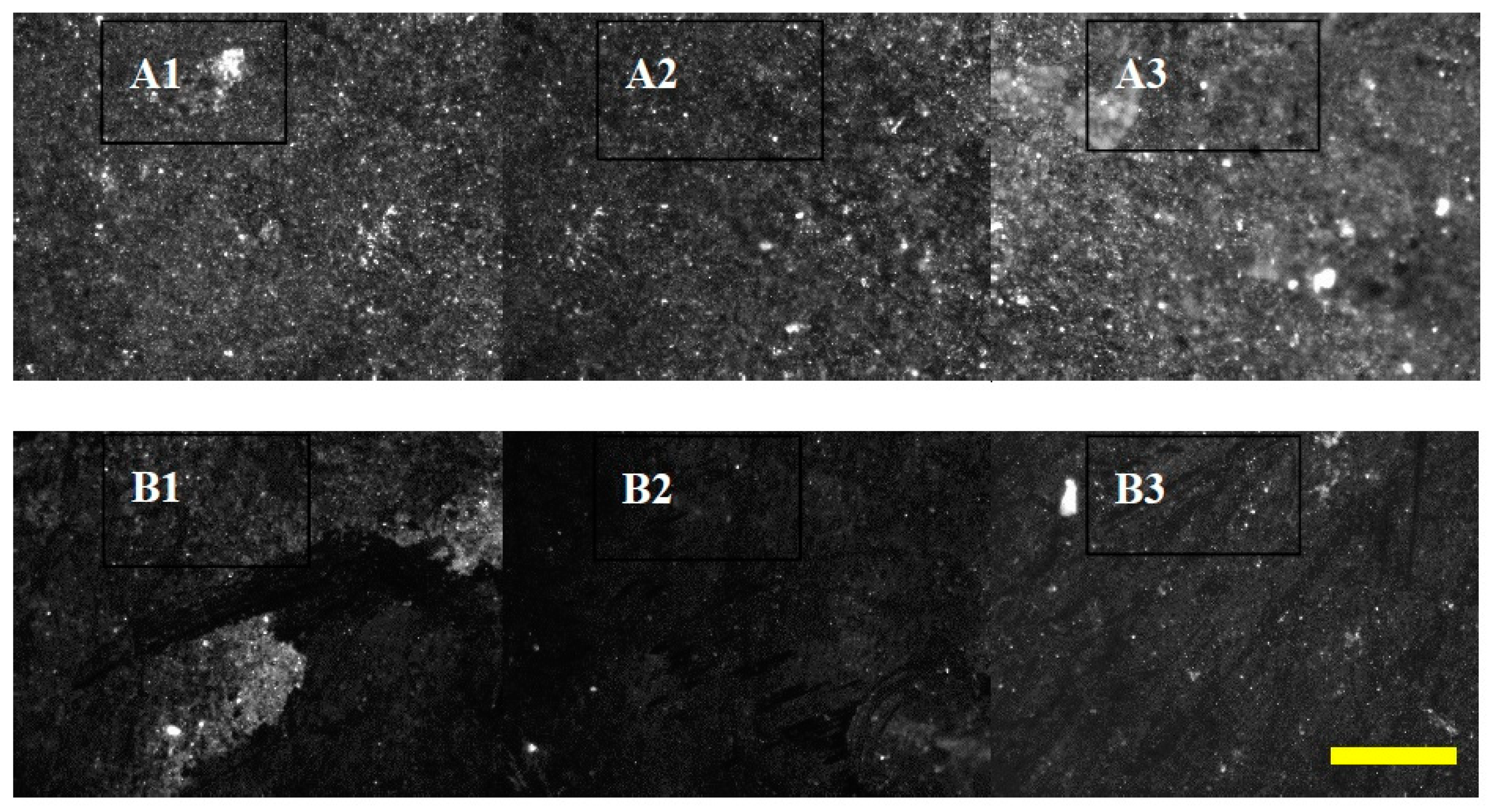
| Description | Uranium Tailings | Pyrrhotite Tailings | Polymetallic Tailings | Waste Rock |
|---|---|---|---|---|
| Length of time (years) | ||||
| First Exposure (field/outdoors) | 3.75 | 3.25 | 3.18 | 2.7 |
| Storage indoors | 6.5 | 5.5 | 0.5 | 4.5 |
| Monitoring of Effluent/sol (1:5 w/v) | 1.83 | 1.83 | 2.87 | 2.7 |
| CPMW application dosage | 30 kg·m−2 | 1:4 (w/w) | 115 kg·t−1 | |
| CPMW grain size | 4 to <0.04 mm | 4 to <0.04 mm | ||
| Number of measurements (a) | 7/7 | 7/7 | 8/8 | 58/115 |
| General Site Characteristics | ||||
| Hydraulic conductivity (cm·s−1) | 10−5 | 10−8 | 10−5 | 1 |
| Site specific sulphide (%) | 2 | 85 | 6–8 | 4–15 |
| Uranium Tailings | Pyrrhotite Tailings | Polymetallic Tailings | Waste Rock (Cu/Zn) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Elements | Units | Control | NPR | Control | NPR | Control | NPR | Control | NPR |
| N = 1 | N = 1 | N = 1 | N = 1 | N = 2 | N = 2 | N = 3 | N = 6 | ||
| Al | mg·L−1 | 50 | <0.005 | 870 | 120 | 89 | 5 | 3.98 | 0.42 |
| Ca | mg·L−1 | 560 | 630 | 500 | 490 | 485 | 510 | 41 | 122 |
| Cu | mg·L−1 | 0.59 | 0.001 | 0.68 | 17 | 86 | 0.07 | 14 | 4.13 |
| Fe | mg·L−1 | 18 | 0.01 | 43 | 0.1 | 1053 | 0.02 | 3.77 | 0.32 |
| P | mg·L−1 | 0.03 | 0.05 | 0.22 | 6.9 | 0.16 | 0.04 | 0.20 | 0.13 |
| S | mg·L−1 | 630 | 510 | 4460 | 1060 | 3020 | 500 | 126 | 123 |
| Zn | mg·L−1 | 0.98 | <0.005 | 9.3 | 5.3 | 2085 | 22 | 78 | 22 |
| pH | 2.67 | 6.751 | 3.061 | 3.84 | 3 | 5 | 4.06 | 6.09 | |
| Conductivity | µS·cm−1 | 3410 | 1682 | 7180 | 4030 | 6725 | 1690 | n.a. | n.a. |
| Eh | mV | 734 | 584 | 758 | 661 | 784 | 467 | n.a. | n.a. |
| Acidity | Mg (CaCO3)·L−1 | 656 | 39 | 6715.4 | 1090 | 5544 | 87 | 257 | 76 |
| Fraction | Ca | P | Fe | Mg | K | Zn | Mn | Cu | Mo | Pb | Co |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fine Gravel 45% | 68400 | 24400 | 1550 | 600 | 400 | 15.7 | 4.5 | 1.8 | 2.5 | 0.45 | 0.11 |
| Coarse Sand 52% | 37400 | 13300 | 1230 | 650 | 200 | 8.7 | 3.1 | 1 | 1.4 | 0.27 | 0.07 |
| Medium Sand 2% | 1.600 | 433 | 300 | 0.2 | 0.08 | 1.1 | 1.0 | 1.6 | 0.04 | 0.04 | 0.01 |
| Fine Sand 0.7% | 533 | 200 | 100 | 0.12 | 0.12 | 0.41 | 0.36 | 0.49 | 0.02 | 0.02 | <0.01 |
| Silt 0.3% | 150 | 3 | 4.3 | 1.0 | 0.16 | 0.24 | 0.16 | 0.84 | 0.01 | 0.02 | <0.01 |
| Unfractionated | 304000 | 47100 | 5900 | 3630 | 158 | 388 | 28.6 | 29.7 | n.a. | n.a. | n.a. |
| Leached Elements | [mg/L] | Sum of Decants 0–8 | H2SO4 | dH2O | Rain |
|---|---|---|---|---|---|
| Major Nutrients | Ca | 620 | 680 | 510 | 6.65 |
| P | 400 | 490 | 9.8 | 06 | |
| Fe | 5.37 | 11.9 | 0.21 | 0.052 | |
| Mg | 18 | 31 | 0.6 | 1.23 | |
| K | 3.7 | 8.9 | 0.7 | 8 | |
| Cofactors/trace elements | Zn | 0.25 | 0.5 | 0.026 | 0.04 |
| Mn | 0.094 | 0.16 | 0.003 | 0.015 | |
| Cu | 0.017 | 0.09 | >0.001 | −0.003 | |
| Mo | 0.015 | 0.029 | 0.018 | n.a. | |
| Pb | >0.002 | >0.002 | >0.002 | n.a. | |
| Co | 1.58 | 0.035 | −0.001 | n.a. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalin, M.; Wheeler, W.N.; Bellenberg, S. Acid Rock Drainage or Not—Oxidative vs. Reductive Biofilms—A Microbial Question. Minerals 2018, 8, 199. https://doi.org/10.3390/min8050199
Kalin M, Wheeler WN, Bellenberg S. Acid Rock Drainage or Not—Oxidative vs. Reductive Biofilms—A Microbial Question. Minerals. 2018; 8(5):199. https://doi.org/10.3390/min8050199
Chicago/Turabian StyleKalin, Margarete, William N. Wheeler, and Sören Bellenberg. 2018. "Acid Rock Drainage or Not—Oxidative vs. Reductive Biofilms—A Microbial Question" Minerals 8, no. 5: 199. https://doi.org/10.3390/min8050199
APA StyleKalin, M., Wheeler, W. N., & Bellenberg, S. (2018). Acid Rock Drainage or Not—Oxidative vs. Reductive Biofilms—A Microbial Question. Minerals, 8(5), 199. https://doi.org/10.3390/min8050199




