Mid-Infrared Spectroscopic Assessment of Plasticity Characteristics of Clay Soils
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. X-ray Diffraction (XRD) Analysis
2.3. FT-MIR Spectroscopic Analysis
2.4. Plasticity Measurements
3. Results
3.1. Mineralogical Compositions
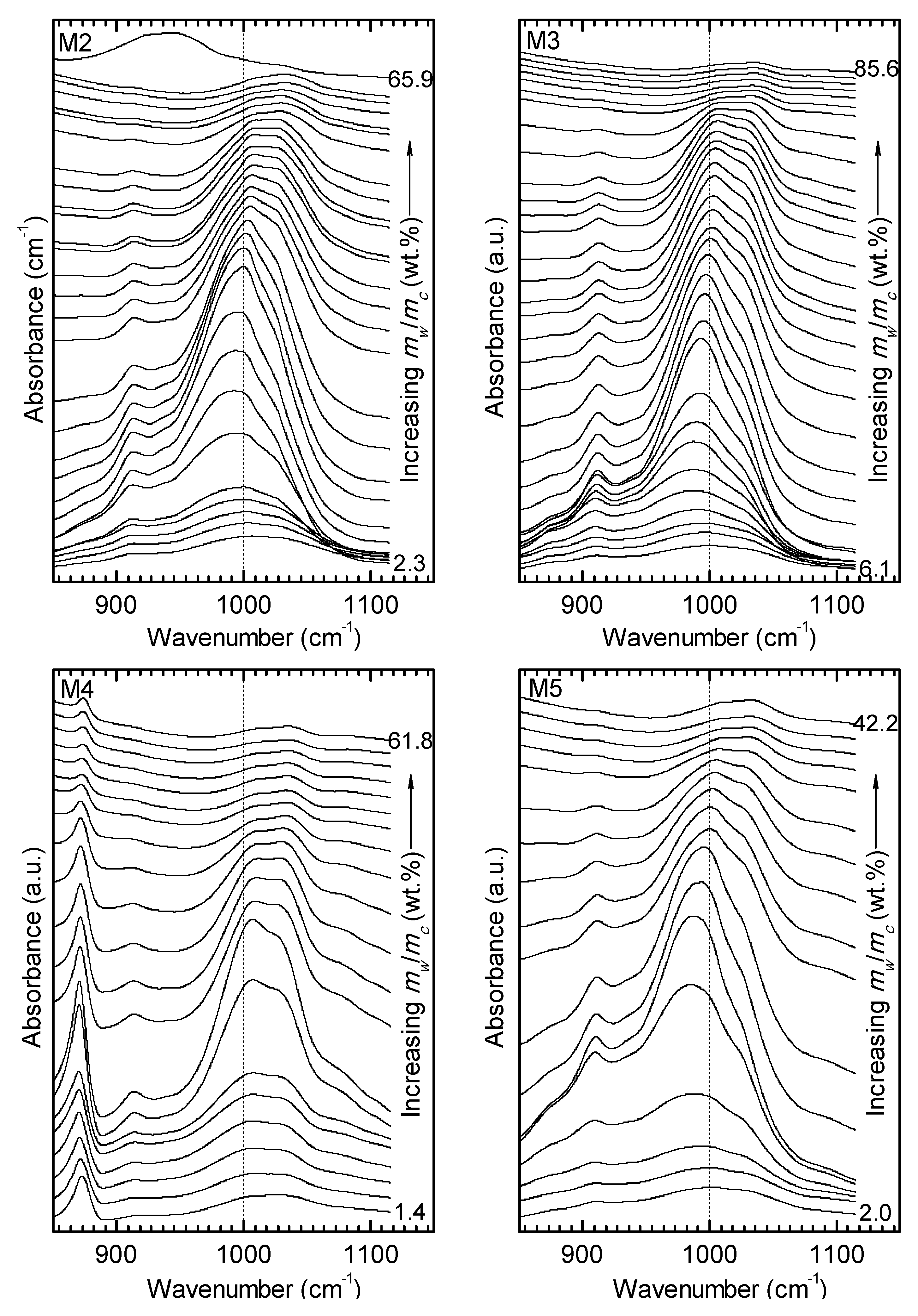
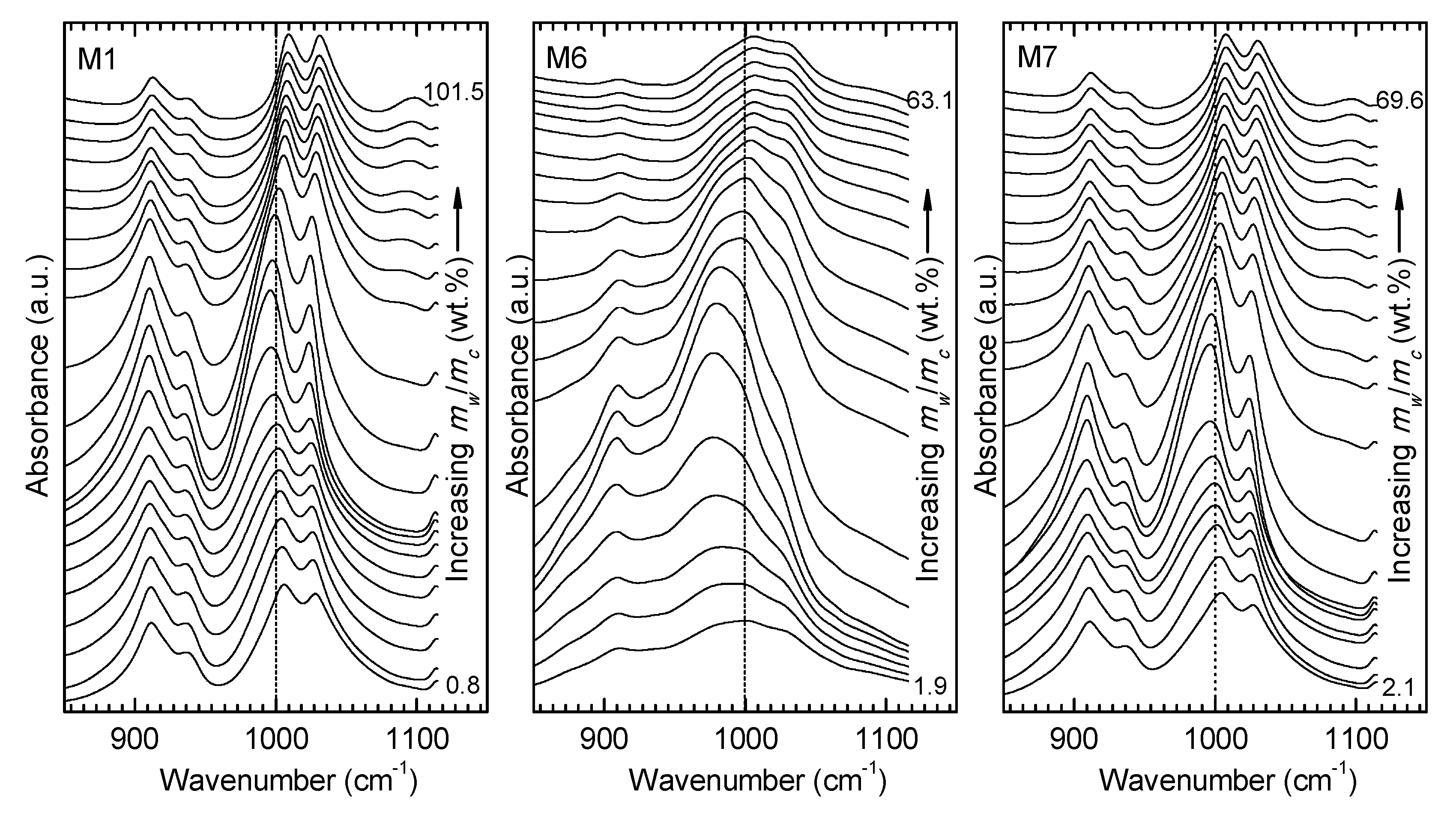
3.2. ATR FT-MIR Data
4. Discussion.
4.1. Comparison of the Spectral Behavior of Kaolinite and a Polymineral Sample
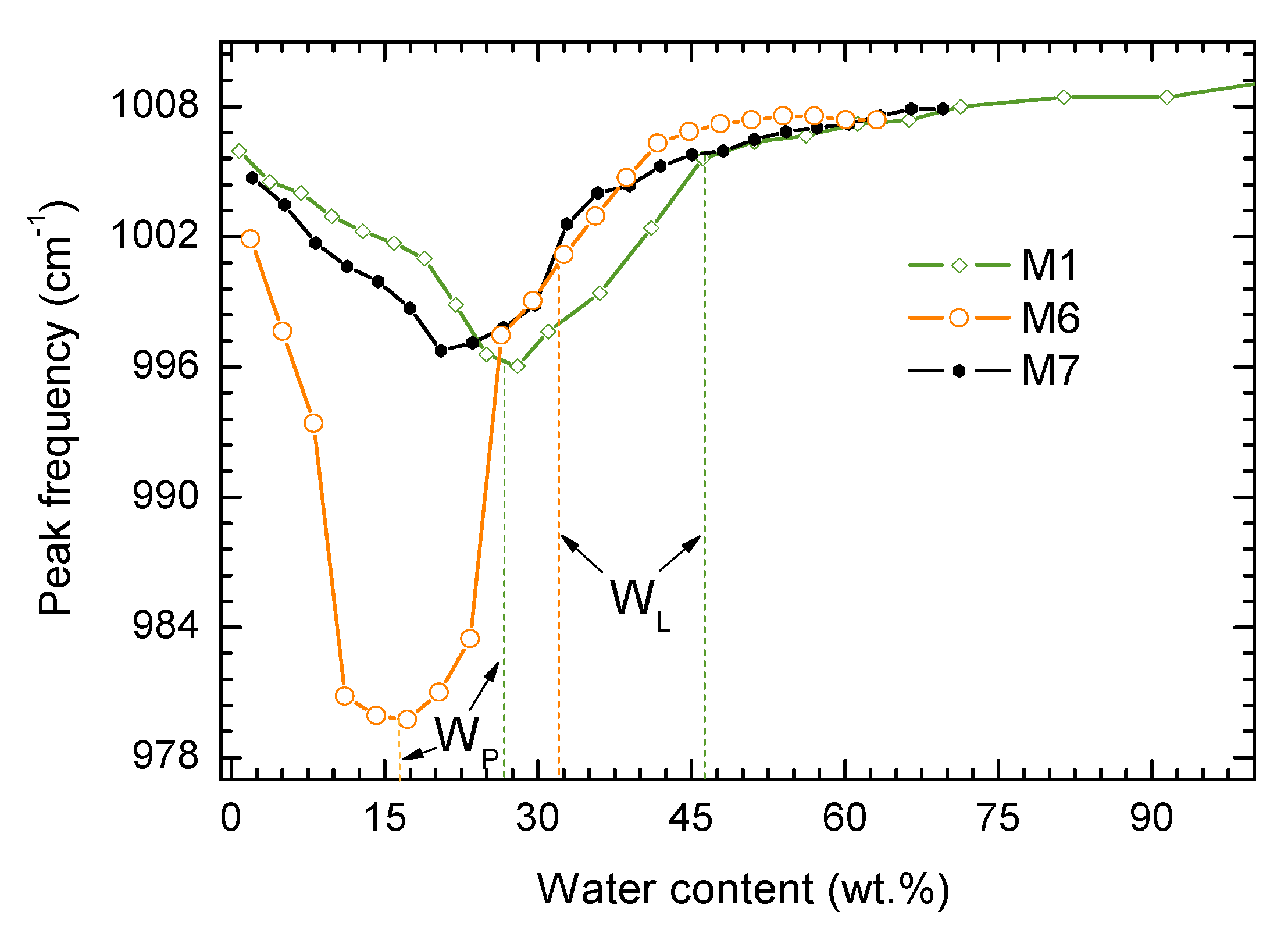
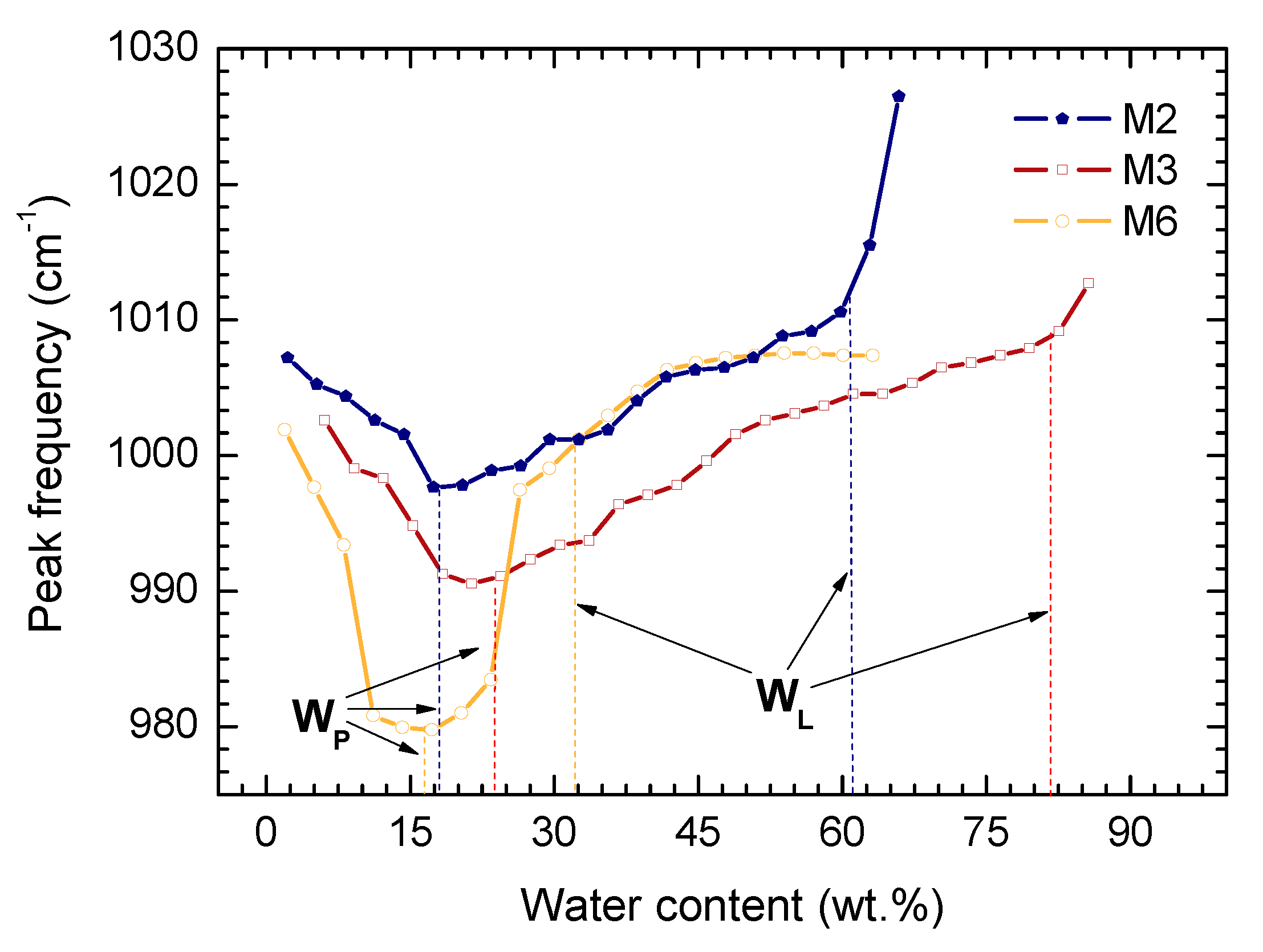
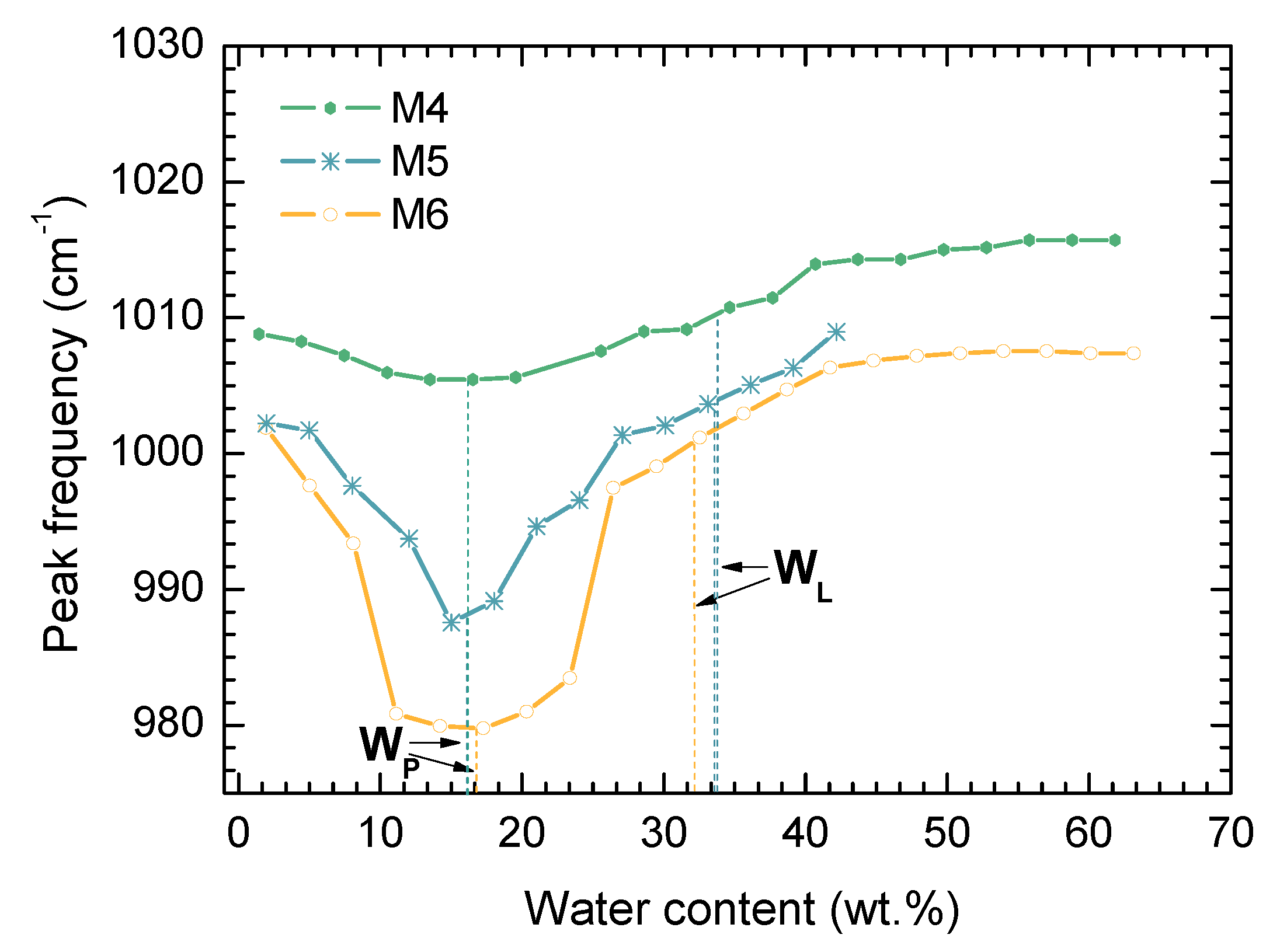
- The value PL (WP) for analyzed samples corresponds to the minimum of wavenumber in the range of moistures 0%–40% for kaolinite, and 0%–25% for polymineral soil;
- A characteristic slope-change position of the curve for sample M1 observed above LL (WL) agrees with the LL (WL) values obtained by standard methods. In the case of polymineral soil (M6) there are two characteristic slope changes coming from consecutive plastic-to-liquid transitions of soil components; the LL (WL) obtained with standard methods are between them;
- The form of the valley in the Si–O—moisture content curve is broader in a polymineral system comparing to a monomineral system. So the FTIR spectroscopy technique provides some measure of the complexity of the soil’s mineralogy.
4.2. Spectral Behavior of Kaolinite—Polymineral Sample Mixture
4.3. Influence of the Smectite Concentration on the Spectral Behavior of Polymineral Soil Samples
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Chen, W.F.; Baladi, G.Y. Soil Plasticity. Theory and Implementation. In Developments in Geotechnical Engineering, 1st ed.; Chen, W.F., Baladi, G.Y., Eds.; Elsevier Science: Amsterdam, The Netherlands, 1985; Volume 38, pp. 1–231. ISBN 9780444598363. [Google Scholar]
- Mukherjee, S. The Science of Clays, 1st ed.; Springer Science & Business Media: Dordrecht, The Netherlands, 2013; pp. 1–335. ISBN 978-94-007-6683-9. [Google Scholar]
- Interstate standard GOST 5180-2015. Soils. Laboratory methods for determination of physical characteristics; 1694-st; Standardinform: Moscow, Russia, 2016; pp. 1–18. [Google Scholar]
- ISO/TS 17892-12. Geotechnical investigation and testing. Laboratory testing of soil. Part 12: Determination of Atterberg limits; ISO: Geneva, Switzerland, 2004; pp. 1–22. [Google Scholar]
- ASTM D-4318-10. Standard Test Methods for Liquid Limit, Plastic Limit, and Plasticity Index of Soils; ASTM: West Conshohocken, PA, USA, 2010; pp. 1–16. [Google Scholar]
- Alfani, R.; Guerrini, G.L. Rheological test methods for the characterization of extrudable cement-based materials—A review. Mater. Struct. 2005, 38, 239–247. [Google Scholar] [CrossRef]
- Andrade, F.A.; Al-Qureshi, H.A.; Hotza, D. Measuring the plasticity of clays: A review. Appl. Clay Sci. 2011, 51, 1–7. [Google Scholar] [CrossRef]
- Yu, H.; Mitchell, J. Analysis of cone resistance - review of methods. J. Geotech. Geoenviron. 1998, 124, 140–149. [Google Scholar] [CrossRef]
- Modesto, C.O.; Bernardini, A.M. Determination of clay plasticity: Indentation method versus Pfefferkorn method. Appl. Clay Sci. 2008, 40, 15–19. [Google Scholar] [CrossRef]
- Gleissle, W.; Graczyk, J. Rheology and Extrudability of Ceramic Compounds. In Extrusion in Ceramics, 1st ed.; Händle, F., Ed.; Springer: Berlin, Germany, 2009; pp. 161–172. ISBN 978-3-540-27102-4. [Google Scholar]
- Baran, B.; Erturk, T.; Sarikaya, Y.; Alemdaloglu, T. Workability test method for metals applied to examine a workability measure (plastic limit) for clays. Appl. Clay Sci. 2001, 20, 53–63. [Google Scholar] [CrossRef]
- Ribeiro, M.J.; Ferreira, J.M.; Labrincha, J.A. Plastic behaviour of different ceramic pastes processed by extrusion. Ceram. Int. 2005, 31, 515–519. [Google Scholar] [CrossRef]
- Flores, O.J.; Andrade, F.A.; Hotza, D.; Al-Qureshi, H.A. Modeling of plasticity of clays submitted to compression test. World Acad. Sci. Eng. Technol. 2010, 61, 191–196. [Google Scholar]
- Haigh, S.K.; Vardanega, P.J. Fundamental basis of single-point liquid limit measurement approaches. Appl. Clay Sci. 2014, 102, 8–14. [Google Scholar] [CrossRef]
- Sivappulaiah, P.V.; Sridharan, A.; Stalin, V.K. Hydraulic conductivity of bentonite-sand mixtures. Can. Geotech. J. 2000, 37, 406–413. [Google Scholar] [CrossRef]
- Berilgen, S.A.; Berilgen, M.M.; Ozaydin, I.K. Compression and permeability relationships in high water content clays. Appl. Clay Sci. 2006, 31, 249–261. [Google Scholar] [CrossRef]
- Lawrence, W.G. Factors involved in plasticity of kaolin–water systems. J. Am. Ceram. Soc. 1958, 41, 147–150. [Google Scholar] [CrossRef]
- Dumbleton, M.J.; West, G. Some factors affecting the relation between the clay minerals in soils and their plasticity. Clay Miner. 1966, 6, 179–193. [Google Scholar] [CrossRef]
- Onoda, G.Y. Mechanism of plasticity in clay–water systems. In Science of Whitewares; Henkes, V.E., Onoda, G.Y., Carty, W.M., Eds.; American Ceramic Society: Westerville, OH, USA, 1996; pp. 79–87. ISBN 978-1-574-98011-0. [Google Scholar]
- Schmitz, R.M.; Schroeder, C.; Charlier, R. Chemo–mechanical interactions in clay: A correlation between clay mineralogy and Atterberg limits. Appl. Clay Sci. 2004, 26, 351–358. [Google Scholar] [CrossRef]
- Lagaly, G.; Dékány, I. Colloid Clay Science. In Handbook of Clay Science, 2nd ed.; Bergaya, F., Lagaly, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 5, pp. 243–345. ISBN 9780080993713. [Google Scholar]
- Jepson, W.B. Kaolins: Their properties and uses. Philos. Trans. Royal Soc. A 1984, 311, 411–432. [Google Scholar] [CrossRef]
- Grim, R.E.; Güven, N. Bentonites, Geology, Mineralogy, Properties and Uses; Elsevier Science: Amsterdam, The Netherlands, 1978; Volume 24, pp. 1–266. ISBN 978-0444416131. [Google Scholar]
- Odom, I.E. Smectite clay minerals: Properties and uses. Philos. Trans. Royal Soc. A 1984, 311, 391–409. [Google Scholar] [CrossRef]
- Janik, L.J.; Skjemstad, J.O. Characterization and analysis of soils using mid-infrared partial least squares. II. Correlations with some laboratory data. Aust. J. Soil Res. 1995, 33, 637–650. [Google Scholar] [CrossRef]
- Viscarra Rossel, R.A.; Walvoort, D.J.J.; McBratney, A.B.; Janik, L.J.; Skjemstad, J.O. Visible, near infrared, mid infrared or combined diffuse reflectance spectroscopy for simultaneous assessment of various soil properties. Geoderma 2006, 131, 59–75. [Google Scholar] [CrossRef]
- Sorensen, L.K.; Dalsgaard, S. Determination of clay and other soil properties by near infrared spectroscopy. Soil Sci. Soc. Am. J. 2005, 69, 159–167. [Google Scholar] [CrossRef]
- Brown, D.J.; Shepherd, K.D.; Walsh, M.G.; Mays, M.D.; Reinsch, T.G. Global soil characterization with VNIR diffuse reflectance spectroscopy. Geoderma 2006, 132, 273–290. [Google Scholar] [CrossRef]
- Shepherd, K.D.; Walsh, M.G. Infrared spectroscopy—enabling an evidence-based diagnostic surveillance approach to agricultural and environmental management in developing countries. J. Near Infrared Spec. 2007, 15, 1–19. [Google Scholar] [CrossRef]
- Ben-Dor, E.; Banin, A. Near-infrared analysis as a rapid method to simultaneously evaluate several soil properties. Soil Sci. Soc. Am. J. 1995, 59, 364–372. [Google Scholar] [CrossRef]
- Kariuki, P.C.; Van der Meer, F.D.; Siderius, W. Classification of soils based on engineering indices and spectral data. Int. J. Remote Sens. 2003, 24, 2567–2574. [Google Scholar] [CrossRef]
- Minasny, B.; McBratney, A.B.; Tranter, G.; Murphy, B.W. Using soil knowledge for the evaluation of mid-infrared diffuse reflectance spectroscopy for predicting soil physical and mechanical properties. Eur. J. Soil Sci. 2008, 59, 960–971. [Google Scholar] [CrossRef]
- Kariuki, P.C.; Van Der Meer, F.; Verhoef, P.N.W. Cation exchange capacity (CEC) determination from spectroscopy. Int. J. Remote Sens. 2003, 24, 161–167. [Google Scholar] [CrossRef]
- Waruru, B.K.; Shepherd, K.D.; Ndegwa, G.M.; Kamoni, P.T.; Sila, A. Rapid estimation of soil engineering properties using diffuse reflectance near infrared spectroscopy. Biosyst. Eng. 2014, 121, 177–185. [Google Scholar] [CrossRef]
- Yan, L.; Roth, C.B.; Low, P.F. Changes in the Si−O vibrations of smectite layers accompanying the sorption of interlayer water. Langmuir 1996, 12, 4421–4429. [Google Scholar] [CrossRef]
- Eberl, D.D. User’s Guide to RockJock—A program for Determining Quantitative Mineralogy from Powder X-ray Diffraction Data: U.S. Geological Survey Open-File Report 2003-78; U.S. Geological Survey: Reston, VA, USA, 2003; 47p.
- Rietveld, H.M. A Profile Refinement Method for Nuclear and Magnetic Structures. J. Appl. Crystallogr. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Omotoso, O.; McCarty, D.K.; Hillier, S.; Kleeberg, R. Some successful approaches to quantitative mineral analysis as revealed by the 3rd Reynolds cup contest. Clays Clay Miner. 2006, 54, 748–760. [Google Scholar] [CrossRef]
- Hubbard, C.R.; Evans, E.H.; Smith, D.K. The Reference Intensity Ratio for Computer Simulated Powder Patterns. J. Appl. Cryst. 1976, 9, 169–174. [Google Scholar] [CrossRef]
- Hillier, S. Accurate quantitative analysis of clay and other minerals in sandstones by XRD: Comparison of a Rietveld and a reference intensity ratio (RIR) method and the importance of sample preparation. Clay Miner. 2000, 35, 291–302. [Google Scholar] [CrossRef]
- Osacky, M.; Geramian, M.; Ivey, D.G.; Liu, Q.; Etsell, T.H. Mineralogical and chemical composition of petrologic end members of Alberta oil sands. Fuel 2013, 113, 148–157. [Google Scholar] [CrossRef]
- Bristow, T.F.; Kennedy, M.J.; Morrison, K.D.; Mrofka, D.D. The influence of authigenic clay formation on the mineralogy and stable isotopic record of lacustrine carbonates. Geochim. Cosmochim. Acta 2012, 90, 64–82. [Google Scholar] [CrossRef]
- Wagner, J.-F. Mechanical Properties of Clays and Clay Minerals. In Handbook of Clay Science, 2nd ed.; Bergaya, F., Lagaly, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 5, pp. 347–381. ISBN 9780080993645. [Google Scholar]
- Mitchell, J.K.; Soga, K. Fundamentals of Soil Behavior, 3rd ed.; John Wiley & Sons, Inc.: New York, NY, USA, 2005; pp. 1–577. ISBN 978-0-471-46302-3. [Google Scholar]
- Środoń, J.; Drits, V.A.; McCarty, D.K.; Hsieh, J.C.C.; Eberl, D.D. Quantitative X-ray diffraction analysis of clay-bearing rocks from random preparations. Clays Clay Miner. 2001, 49, 514–528. [Google Scholar] [CrossRef]
- Moore, D.M.; Reynolds, R.C., Jr. X-Ray Diffraction and the Identification and Analysis of Clay Minerals; Oxford University Press: Oxford, UK, 1989; pp. 179–201. [Google Scholar]
- Bailey, S.W. Nomenclature for regular interstratifications. Am. Mineral. 1982, 67, 394–398. [Google Scholar] [CrossRef]
- Kasprzhitskii, A.; Lazorenko, G.; Yavna, V.; Daniel, P. DFT theoretical and FT-IR spectroscopic investigations of the plasticity of clay minerals dispersions. J. Mol. Struct. 2016, 1109, 97–105. [Google Scholar] [CrossRef]
- Farmer, V.C. Differing effect of particle size and shape in the infrared and Raman spectra of kaolinite. Clay Miner. 1998, 33, 601–604. [Google Scholar] [CrossRef]
- Madejová, J.; Komadel, P. Baseline studies of the clay minerals society source clays: Infrared methods. Clays Clay Miner. 2001, 49, 410–432. [Google Scholar] [CrossRef]
- Petit, S.; Madejová, J. Fourier Transform Infrared Spectroscopy. In Handbook of Clay Science, 2nd ed.; Bergaya, F., Lagaly, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 5, pp. 213–231. ISBN 9780080993713. [Google Scholar]
- Yavna, V.A.; Kasprzhitskii, A.S.; Lazorenko, G.I.; Kochur, A.G. Study of IR spectra of a polymineral natural association of phyllosilicate minerals. Opt. Spectrosc. 2015, 118, 529–536. [Google Scholar] [CrossRef]
- Schnetzer, F.; Thissen, P.; Giraudo, N.; Emmerich, K. Unraveling the Coupled Processes of (De)hydration and Structural Changes in Na+-Saturated Montmorillonite. J. Phys. Chem. C 2016, 120, 15282–15287. [Google Scholar] [CrossRef]
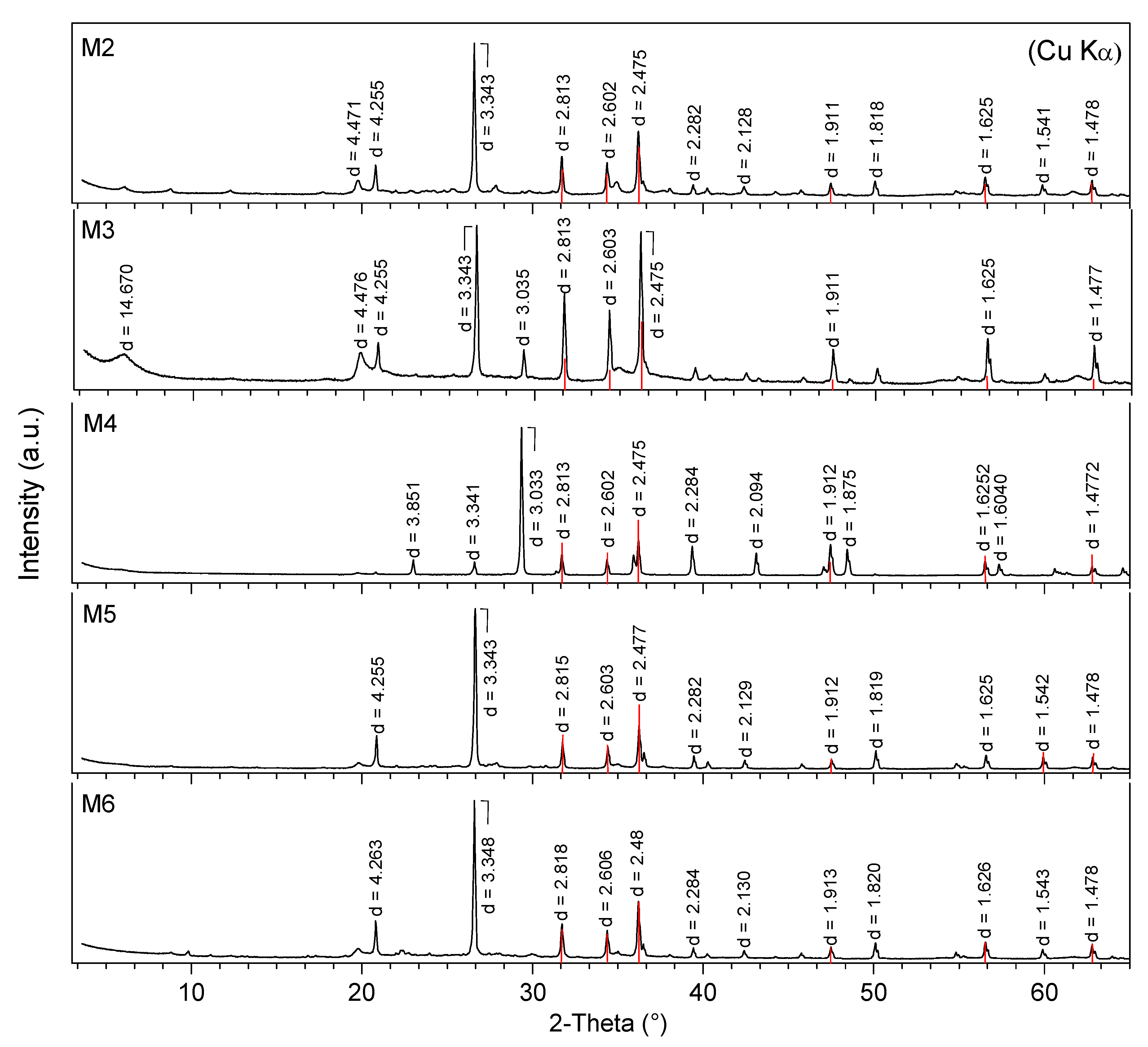
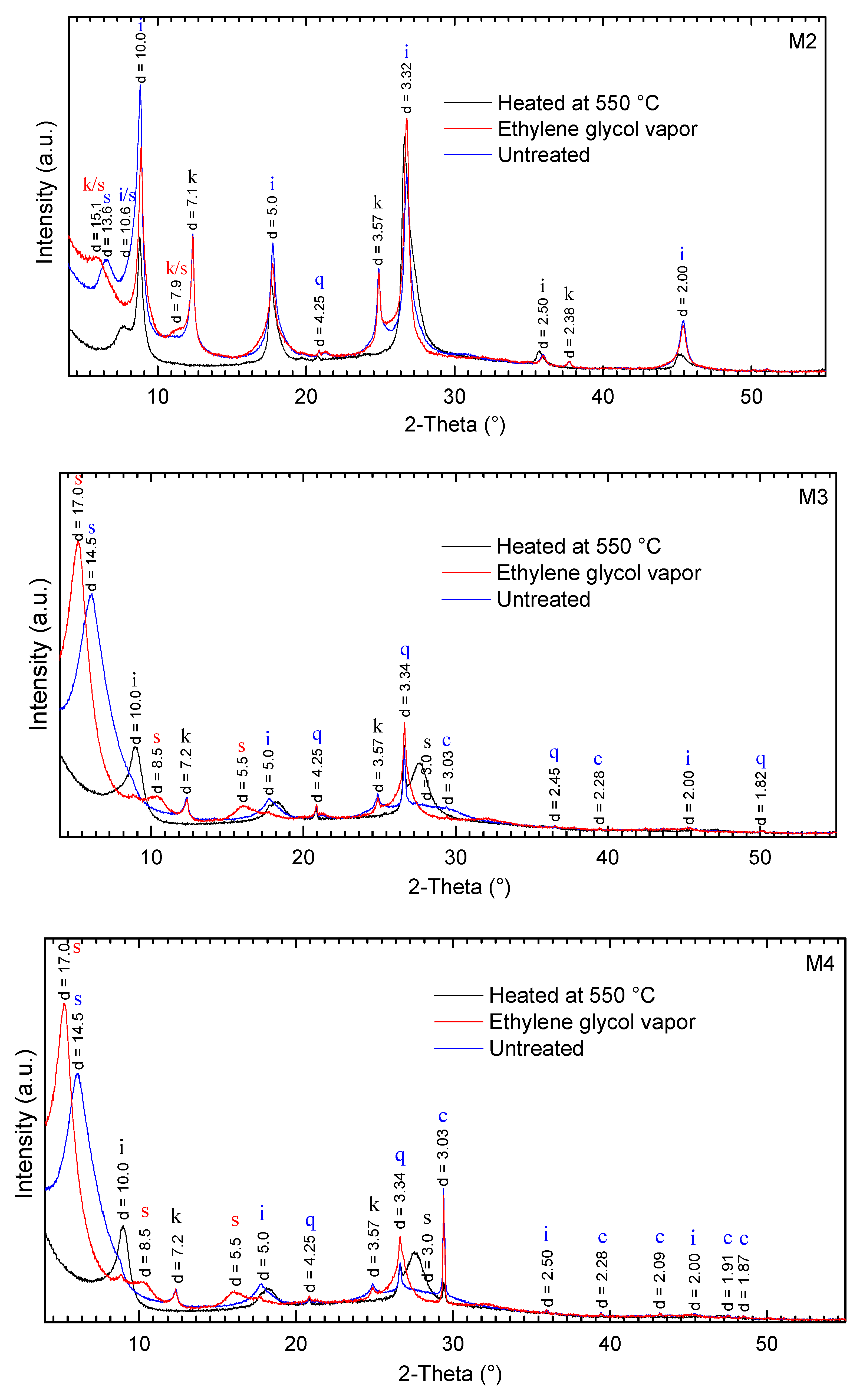
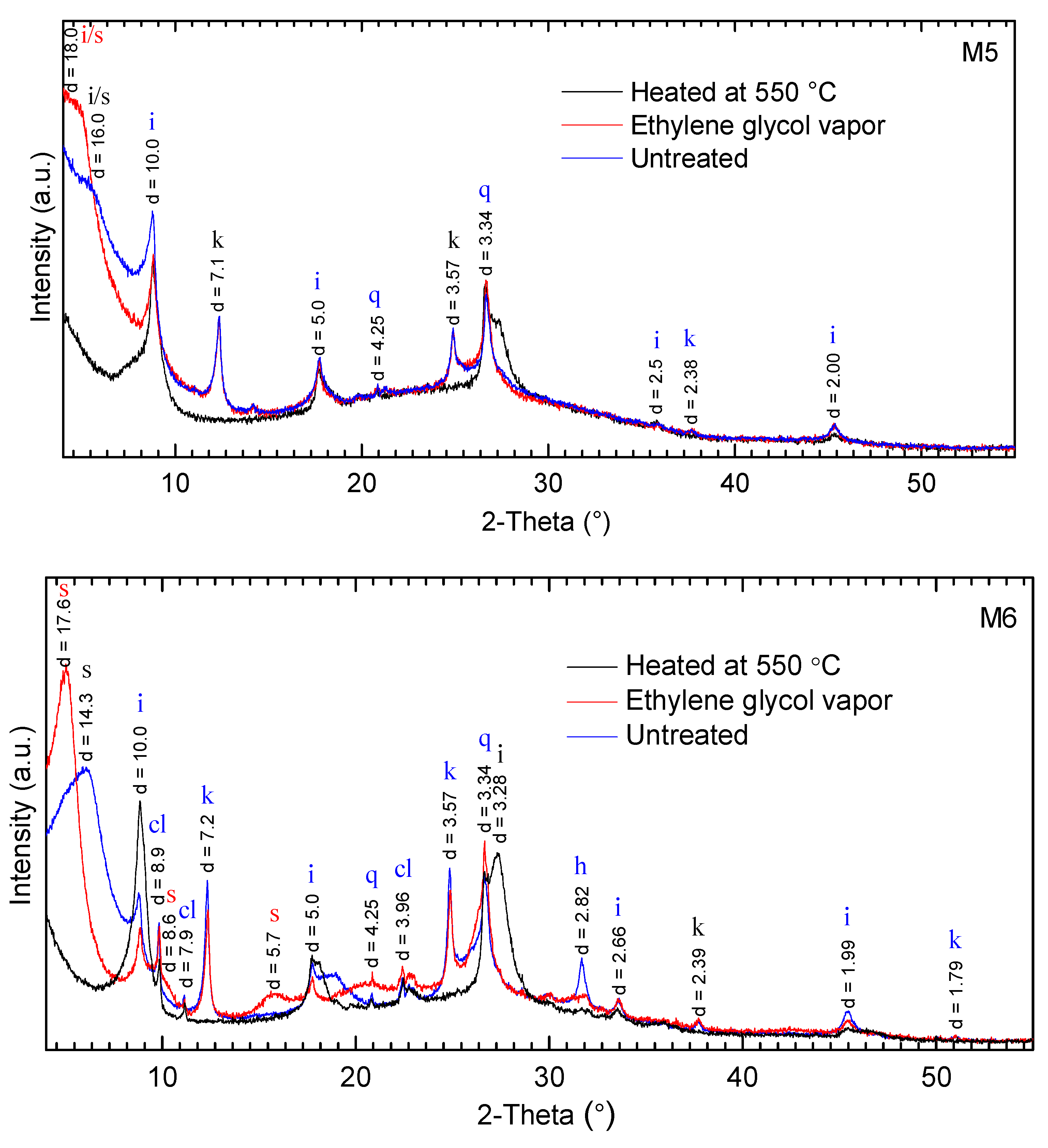
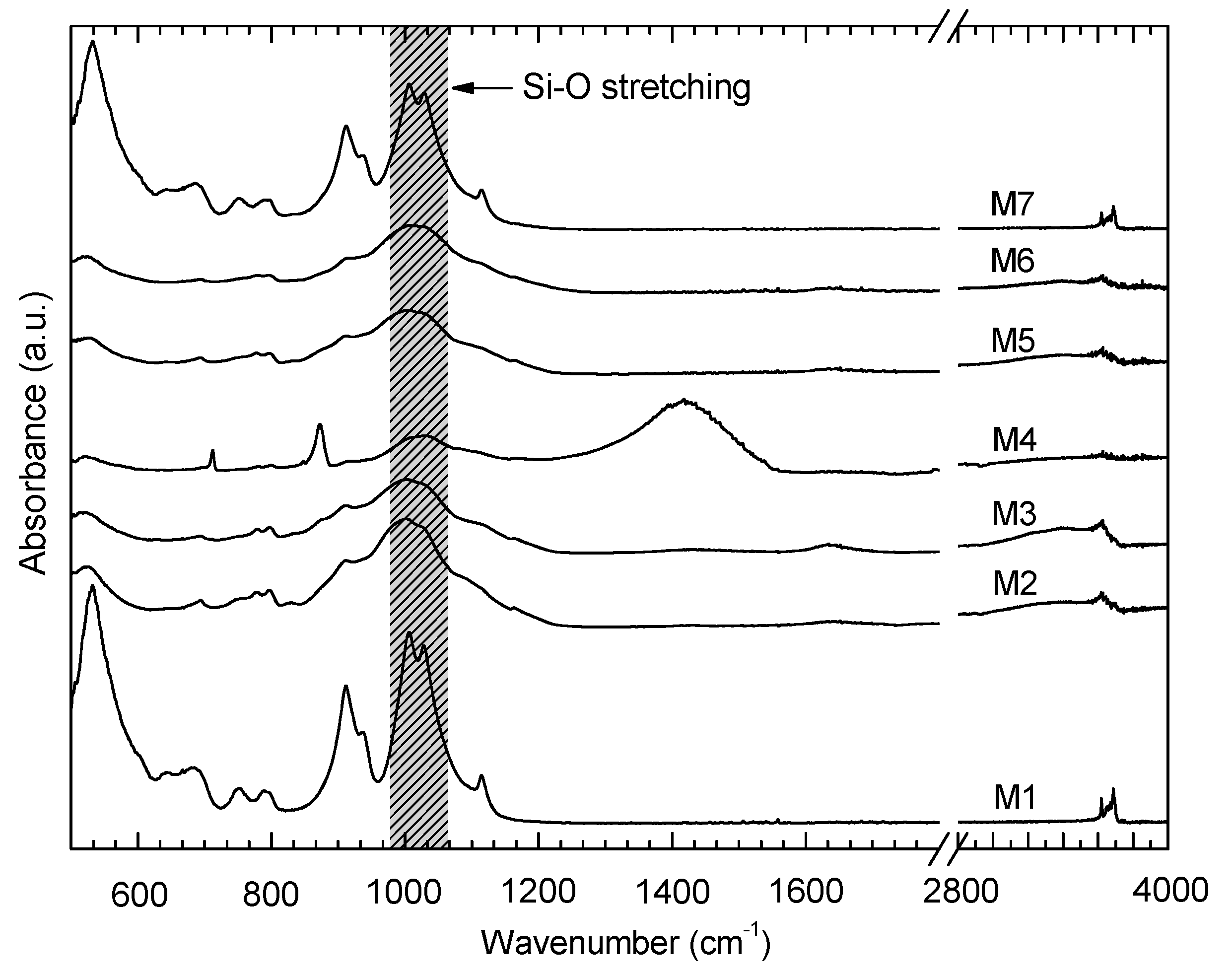

| Sample Number | Non-Clay Minerals (wt %) | Σ Non-Clay Minerals (wt %) | Clay Minerals (wt %) | Σ Clay Minerals (wt %) | Plasticity Characteristics | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Quartz | Feldspar | Albite | Calcite | Dolomite | Clinoptilolite | Kaolinite | Illite | Illite–Smectite MLM | Smectite | Kaolinite–Smectite MLM | WP/PL (wt %) | WL/LL (wt %) | |||
| M1 * | 3 | - | - | - | - | - | 3 | 97 | - | - | - | - | 97 | 26.0/28.6 | 46.3/47.7 |
| M2 | 31 | - | 7 | 1 | trace | - | 39 | 7 | 20 | 17 | 5 | 12 | 61 | 17.8 | 61.4 |
| M3 | 18 | 3 | 2 | 7 | - | - | 30 | 20 | 8 | - | 42 | - | 70 | 23.9 | 81.5 |
| M4 | 4 | - | 3 | 80 | - | - | 87 | - | 3 | - | 10 | - | 13 | 16.1 | 33.6 |
| M5 | 48 | 2 | 5 | - | 2 | - | 57 | 8 | 10 | 20 | 5 | - | 43 | 16.1 | 33.4 |
| M6 * | 35 | 2 | 3 | - | - | 25 | 65 | 4 | 9 | - | 22 | - | 35 | 17.0/21.5 | 32.2/35.6 |
| M7 | 19 | 1 | 1 | - | - | 12 | 33 | 51 | 5 | - | 11 | - | 67 | - | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasprzhitskii, A.; Lazorenko, G.; Khater, A.; Yavna, V. Mid-Infrared Spectroscopic Assessment of Plasticity Characteristics of Clay Soils. Minerals 2018, 8, 184. https://doi.org/10.3390/min8050184
Kasprzhitskii A, Lazorenko G, Khater A, Yavna V. Mid-Infrared Spectroscopic Assessment of Plasticity Characteristics of Clay Soils. Minerals. 2018; 8(5):184. https://doi.org/10.3390/min8050184
Chicago/Turabian StyleKasprzhitskii, Anton, Georgy Lazorenko, Antoine Khater, and Victor Yavna. 2018. "Mid-Infrared Spectroscopic Assessment of Plasticity Characteristics of Clay Soils" Minerals 8, no. 5: 184. https://doi.org/10.3390/min8050184
APA StyleKasprzhitskii, A., Lazorenko, G., Khater, A., & Yavna, V. (2018). Mid-Infrared Spectroscopic Assessment of Plasticity Characteristics of Clay Soils. Minerals, 8(5), 184. https://doi.org/10.3390/min8050184







