Abstract
A portion of dodecyl amine (DDA) in a muscovite flotation system was replaced with alcohols with different carbon-chain lengths. These alcohols included octanol (OCT); decanol (DEC); dodecanol (DOD); and tetradecanol (TER). The muscovite adsorption behavior of the mixed DDA and alcohol systems were investigated through zeta potential; contact angle; and adsorption quantity tests. Single-mineral flotation tests showed that the muscovite-collecting power of the mixed DDA/alcohol (OCT, DEC, or DOD) system was stronger than that of the pure DDA system. The muscovite-collecting power of the collector systems decreased in the following order: DDA/DEC > DDA/OCT > DDA/DEC > DDA > DDA/TER. Zeta potential and contact angle analysis indicated that when combined with DDA; alcohols physically adsorbed on the surfaces of muscovite. This behavior improved the hydrophobicity of muscovite. Furthermore, adsorption analysis revealed that synergy between DDA and alcohol enhanced the adsorption of alcohol on muscovite. DDA has a dominant role in synergistic adsorption; whereas alcohol has a supporting role. Among all tested alcohols; DDA and DOD exhibit the highest synergetic adsorption effect because of their similar carbon-chain lengths. This similarity promotes the formation of a compact adsorption layer on the muscovite surface.
1. Instruction
Muscovite has received considerable interest because of its insulating and heat-resistant properties. It is widely applied in the fabrication of coatings, paints, plastics, and electrical components [1]. In addition, it is a crucial carrier of rare metals, such as rubidium, titanium, cesium, and vanadium [2]. Minerals can be efficiently separated from gangue through flotation [3,4,5,6]. Given that the surface of the separation solution is highly negative, cationic surfactants, such as quaternary ammonium salts, Gemini cationic compounds, and primary amines, are mainly used as collectors in muscovite flotation [6,7,8,9]. However, muscovite flotation cannot be widely applied because of its numerous shortcomings, such as sensitivity to metal ions, adverse environmental effects, and strong corrosion effect on flotation equipment [6,10]. In addition, the high sensitivity and poor selectivity of amines decrease the flotation indexes of muscovite [8,11,12,13]. Therefore, existing muscovite flotation technologies should be improved or else novel muscovite flotation technologies should be developed to derive additional benefits from low-grade muscovite ores.
Various mixed collector systems have attracted increased attention in the field of mineral processing because of their synergistic effects and low costs [14,15]. Nonionic surfactants are used as auxiliary collectors to decrease flotation costs and increase flotation efficiency [16,17]. Therefore, in recent years, various ionic and nonionic collectors have been widely studied. For example, Sis et al. [18] studied a combined collector system for phosphate flotation. They found that the addition of nonionic collectors to traditional fatty acid collectors can improve flotation results. Rybinski et al. [19] studied the selective flotation of scheelite from calcite. The flotation recovery and selectivity of combined anion–nonionic collector systems are superior to those of single-collector systems and can decrease the required collector dosage. Fuerstenau et al. [20] found that the synergistic effect of dodecanol (DOD) and the anionic collector sodium dodecyl sulfate can significantly improve emery flotation. The addition of alcohol can decrease the required amount of alkyl sulfate by 10-fold while achieving the same flotation effect.
We previously investigated the effects of a mixed dodecyl amine chloride/octanol (OCT) collector system on muscovite flotation [21]. The muscovite-collecting power of the mixed collector system is superior to that of the single dodecyl amine (DDA) system. The chain lengths of collectors have an important role in flotation [22]. Generally, flotation recovery increases with the chain length of collectors. Although the flotation behavior and adsorption of amine/alcohol on muscovite has been studied, few studies have focused on the relationship between muscovite flotation and the chain length of alcohol collectors.
In this study, we replaced a portion of DDA with alcohols of different carbon-chain lengths. These alcohols included OCT, DEC, decanol (DOD), and tetradecanol (TER). Then, the muscovite-adsorption behaviors of the mixed DDA and alcohol collector systems were investigated through contact angle, zeta potential, and adsorption measurements. We also discussed the effect of the carbon-chain length of alcohol in muscovite flotation.
2. Experiment
2.1. Materials and Reagents
The pure muscovite minerals used in this work were obtained from Lingshou, Hebei Province, China. The samples were first manually picked over to remove impurities, artificially hammered to a certain size, and ground in a pottery ball mill to −0.074 mm. Then, ground samples were wet-screened with a 200-mesh sieve to −74 + 38 μm fractions for flotation. Granule samples were washed repeatedly with deionized water and dried under low temperature for subsequent experiments. The nonionic surfactants TER, OCT, DEC, and DOD and the cationic surfactant DDA were purchased from Sinopharm Chemical Reagent Co., Ltd., Shanghai, China. All reagents had an analytical purity of 99.9%. Solution pH was adjusted with reagent-grade H2SO4 and NaOH. Deionized water was used in all processes.
2.2. Flotation Experiments
Flotation experiments were performed in a XFG5-35 flotation machine (Xinhai mining, Beijing, China) with a 40 mL effective cell volume and a spindle speed of 1600 RPM. Muscovite powder (2 g) was placed in a plexiglass cell. The cell was then filled with distilled water. After 1 min of mixing time, H2SO4 or NaOH was used to adjust the solution pH for 2 min. Collectors were added to the solution, conditioned for 3 min, and collected for 3 min. Flotation concentrates and tailings were weighed separately after filtration and drying for flotation recovery calculations. Flotation results were represented by the average value of three different measurements.
2.3. Contact Angle Measurements
The contact angles of minerals treated with different mixed collectors were quantified through the sessile drop method with MiniLab ILMS (GBX, Romans-sur-Isere, France). The experiment was performed in air, and deionized water was used as the liquid phase. Reagent concentration was gradually increased during the measurement. New muscovite surfaces were obtained by using adhesive tape to peel off old surfaces from the mineral sample. The muscovite sample was immersed for 3 min in a beaker containing certain concentrations of reagents. The mineral surface and the molecular reagent were allowed to fully react with each other. Afterward, the muscovite sample was washed thrice to remove remaining surface impurities and dried with nitrogen. Finally, contact angles were measured through the sessile drop method.
Measurements were repeated by dropping water on different sampling locations on the same muscovite section. Contact angle was represented by the average value of three different measurements.
2.4. Zeta Potential Measurement
The zeta potentials of muscovite samples treated with different mixed collectors were measured by using a JS94H microelectrophoresis instrument (Shanghai Zhongchen Digital Technic Apparatus Co., Shanghai, China). A 20 mg sample was added to a 100-mL breaker with 40 mL of distilled water. The solution was stirred for 3 min by using a magnetic stirrer, and the pH of the suspension was adjusted. The measurement temperature was maintained at 20 °C. The reagents were added to the suspension, which was then stirred for 5 min to allow the reagents and mineral surface to fully react. The agitated slurry was allowed to rest for 1 min to allow large particles in the solution to settle. The supernatant was injected into the cell of the zeta potential analyzer for zeta potential measurement. Each sample was measured three successive times, and the results were presented as the averaged values of three measurements.
2.5. Adsorption Measurement
The traditional residual concentration method was used to measure adsorption. The adsorption amount on solid surfaces was obtained by changing the initial and equilibrium solution concentrations. Therefore, the key to this experiment was the measurement of surfactant concentration in the solution.
Surfactant amount was measured with Elementar liquid TOCII (Elementar Company, Langenselbold, Germany), which can simultaneously measure the total organic carbon (TOC) and total organic nitrogen (TON) concentration of a solution. This method was selected because (a) it is simple, rapid, environmentally friendly, and nontoxic; and (b) it is necessary for measuring the adsorption capacity of DDA and alcohol molecules on the muscovite surface after mixing. In the mixed collector system, DDA and alcohol molecules contribute to TOC but only DDA contributes to TON. Therefore, this method can differentiate between DDA and alcohol. A series of DDA standard solutions were prepared for TOC and TON analysis. The actual DDA content in the solution was calculated on the basis of TOC content. A standard curve was obtained from the above data and designated as the DDA-N content standard curve. The results are shown in Figure 1.
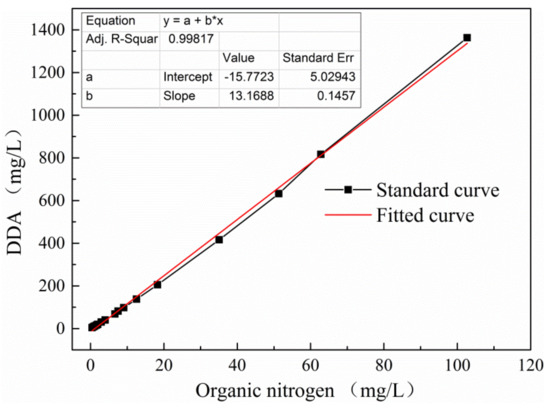
Figure 1.
Standard curve of dodecyl amine (DDA)-N content obtained through total organic nitrogen (TON) analysis.
Figure 1 shows that TON and real DDA concentrations are linearly related with a correlation coefficient of 0.99817. The curve indicates that DDA solution concentration can be reliably calculated on the basis of TON concentration. Error is reduced by direct positioning in the standard curve. TOC and TON can be measured separately in the mixed collector system of DDA/alcohol (OCT, DEC, DOD, or TER). The DDA concentration of the sample solution was determined in accordance with the corresponding relationship shown in Figure 1, and alcohol concentration was calculated by subtracting the TOC of DDA from that of DDA/alcohol.
The process for sample preparation was similar to that for the flotation experiment. Pure mineral particles (2.0 g) were added to a beaker (100 mL) with 40 mL of deionized water. The measurement temperature was maintained at 20 °C. pH was adjusted through the addition of H2SO4 and NaOH and was maintained at 6 ± 0.5. Reagents were added at certain concentrations to the beaker in accordance with the test requirement. The mixture was then placed on an oscillator for 30 min. The pulp obtained through the above process was centrifuged at 8000 rpm for 15 min, and the TOC and TON contents of the supernatant were analyzed. The number of surfactants adsorbed on muscovite particles was calculated as:
where C0 and C are the initial and supernatant concentrations, respectively; V is the solution volume; m is the amount of the particles per sample; and A is the specific surface area of the mineral.
3. Results
3.1. Flotation Performance of Single Minerals
Figure 2 shows the influence of the total concentration of alcohols with different carbon lengths (OCT, DEC, DOD, or TER) and DDA on muscovite recovery under pH = 6 ± 0.5. The results show that muscovite recovery increases with increasing collector concentration and gradually reaches equilibrium. The carbon-chain length of the alcohol in the collector system does not affect this trend. Nevertheless, combined collector systems comprising alcohols with different carbon lengths have different muscovite recovery rates. At the same concentration, the muscovite flotation efficiency of DDA combined with OCT, DEC, or DOD is higher than that of DDA alone. This finding indicates that different degrees of synergy exist among OCT, DEC, DOD, and DDA. The muscovite flotation effect of TER and DDA is lower than that of the single DDA system, indicating that TER and DDA exert negligible effects on muscovite recovery rates. Under treatment with 10−4 mol/L mixed OCT/DDA and mixed DEC/DDA, the recovery rate exceeds 80% and reaches saturation. The muscovite recovery rate of the combined DOD and DDA collector system is 49%. The muscovite recovery rate of the TER and DDA system is lower (33%) than that of DDA alone (41%).
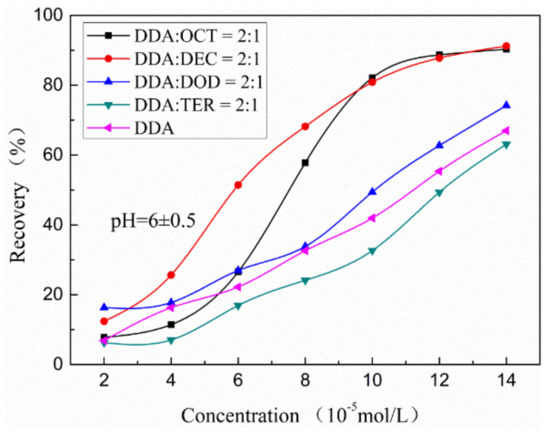
Figure 2.
Muscovite recovery as a function of mixed collector dosage.
3.2. Zeta Potential Analysis
Zeta potential data have been used to interpret the trend of flotation efficiency and the modification of flotation behavior by reagents [23,24,25]. The zeta potentials of muscovite as a function of pH and under treatment with 10−4 mol/L 2:1 DDA/OCT, 2:1 DDA/DEC, 2:1 DDA/DOD, 2:1 DDA/TER, and pure DDA are depicted in Figure 3. Figure 3 shows that under treatment with mixed collector systems, the zeta potentials of muscovite exhibit the same trend with increasing pH. Nevertheless, the values of the two parameters exhibit some differences. Under the same conditions, the zeta potentials of muscovite follow the order of mixed DDA/DOD > pure DDA ≈ mixed DDA/TER > mixed DDA/DEC ≈ mixed DDA/OCT. Given that alcohol itself is uncharged, increasing the zeta potentials of muscovite increases the adsorption of DDA on the muscovite surface. Therefore, the intensification of amine adsorption on the muscovite surface by alcohols with different carbon-chain lengths should also follow the order of DOD > TER > DEC ≈ OCT. This sequence represents the order of the strength of the synergistic adsorption of the amine and alcohol on the muscovite surface. This finding is consistent with the conclusion of Vidyadhar [16,26], who stated that the synergistic adsorption of amine and alcohol on the muscovite surface can be attributed to the similar carbon-chain lengths of DDA and DOD; this similarity induces the formation of a compact adsorption layer. However, this effect is not the same as that observed in pure mineral flotation. This phenomenon indicates that the synergistic adsorption of amine and alcohol on the muscovite surface may not be the only factor that determines flotation recovery. The solubility and effect of alcohol on solution and froth properties may also be important factors that affect flotation recovery.
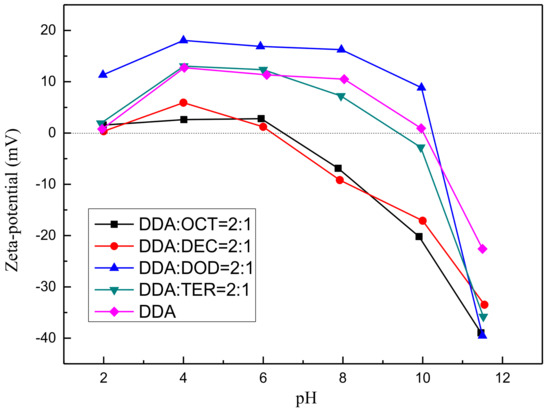
Figure 3.
Zeta potentials of muscovite as a function of pH.
3.3. Contact Angle Analysis
Contact angle measurements were obtained to study the effects of the wettability of each mixed collector on the mineral surface and thereby confirm that synergistic effects exist between alcohols with different carbon-chain lengths and DDA on the solid–liquid interface [27,28]. The contact angle of muscovite as a function of mixed collector dosage under pH 6 ± 0.5 and treatment with 2:1 DDA/OCT, 2:1 DDA/DEC, 2:1 DDA/DOD, 2:1 DDA/TER, and pure DDA was observed. The results are presented in Figure 4 and indicate that the contact angle of muscovite first increases, then maximizes, and finally remains steady under increasing collector concentrations. Under relatively low collector concentrations, different combinations of different reagents exert different effects on the contact angle of muscovite, and contact angle increases in the order of mixed DDA/DOD > mixed DDA/TER > mixed DDA/DEC > mixed DDA/OCT > pure DDA. This order reflects the hydrophobicity degree of the mineral surface and the strength of the synergistic adsorption of different kinds of alcohols and amines. The high synergistic adsorption strength of the mixed DDA/DOD system may be related to their similar carbon-chain lengths. Under relatively high collector concentrations, the contact angles of muscovite in each mixed system reach saturation, and the contact angle value increases in the order of mixed DDA/DEC > mixed DDA/OCT > mixed DDA/DOD > mixed DDA/TER > pure DDA. This result is consistent with the results for muscovite flotation.
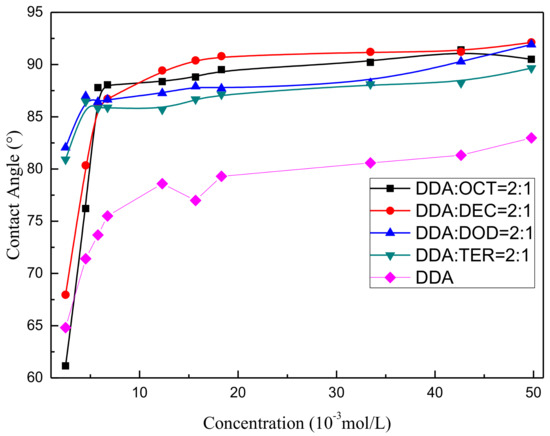
Figure 4.
Contact angle of muscovite as a function of mixed collector dosage.
3.4. Adsorption Amount
The adsorption amount of DDA collectors as a function of pH is discussed in this section. The adsorption amounts at the given initial DDA concentration (CDDA = 10−4 mol/L) under increasing pH are presented in Figure 5. Figure 5a shows that the adsorption amount of DDA increases sharply with increasing pH and is maintained at approximately 1.4 × 10−6 mol/m2 when the solution pH exceeds 6.
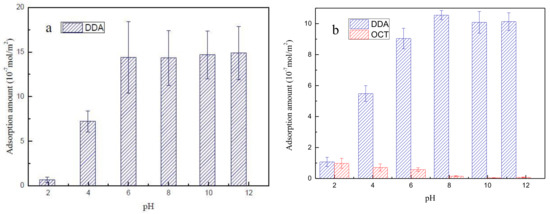
Figure 5.
DDA adsorption on muscovite as a function of pH under treatment with (a) DDA alone and (b) mixed collector systems.
Muscovite is negatively charged throughout the pH range given its low zero charge point, and its negative surface charges increase under increasing pH. DDA exists in ionic form under acidic conditions. Therefore, the amounts of DDA that have adsorbed on muscovite increases because the electrostatic interaction between the negatively charged mineral surface and the positively charged DDA is enhanced. Under strong alkali conditions, DDA mainly exists in molecular instead of ionic form, which can form double-layer adsorption at the muscovite surface [29]. The adsorption amounts of DDA and OCT on muscovite under a given initial DDA/OCT concentration (CDDA+OCT = 10−4 mol/L and CDDA/COCT = 2) and increasing pH are presented in Figure 5b. The figure shows that the trends of the adsorption amounts of DDA as a function of increasing pH values under combined collection systems are similar to those of DDA under single DDA systems. When the pH exceeds 8, the adsorption amount of DDA is maintained at approximately 1.0 × 10−6 mol/m2. OCT alone does not adsorb on muscovite. However, OCT can adsorb on muscovite in the presence of DDA, especially under acidic conditions. The adsorption amount of OCT gradually decreases with increasing pH values. The above phenomenon shows that OCT interacts with DDA to adsorb on the surface of muscovite and that the synergistic adsorption of DDA/OCT occurs specifically under acidic to neutral conditions. These phenomena may account for the higher muscovite recovery efficiency of the mixed DDA/OCT system than that of the single DDA system and are consistent with the results of zeta potential and contact angle analyses.
The muscovite-adsorbed amounts of DDA as a function of the initial dosages of 2:1 DDA/OCT, 2:1 DDA/DEC, 2:1 DDA/DOD, 2:1 DDA/TER, and DDA under pH 6 ± 0.5 are presented in Figure 6a. The results show that the muscovite-adsorbed amount of DDA increases as the initial concentrations of mixed collectors increases. In addition, under the same reagent concentration, the amount of muscovite-adsorbed DDA under each mixed collector system is significantly higher than that of DDA alone. These findings indicate that the addition of alcohol enhances DDA adsorption on muscovite. Nevertheless, the different types of alcohols in the mixed collector systems do not significantly affect the amount of muscovite-adsorbed DDA.
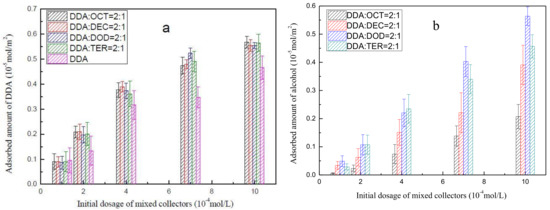
Figure 6.
Adsorbed amount of DDA (a) and alcohol (b) on muscovite as a function of the initial dosage of mixed collectors.
The adsorption amount of alcohol on muscovite as a function of the dosage of 2:1 DDA/OCT, 2:1 DDA/DEC, 2:1 DDA/DOD, 2:1 DDA/TER, and DDA under pH 6 ± 0.5 are presented in Figure 6b. The figure shows that alcohol molecules alone do not adsorb on muscovite and exhibit different degrees of adsorption on muscovite in the presence of DDA. The adsorption amounts of the alcohols decrease in the order of DOD > TER > DEC > OCT. This finding is consistent with zeta potential and contact angle measurements. That is, among all mixed collector systems, the mixed collector system of DDA and DOD exhibit the strongest synergistic adsorption.
The isotherm of DDA adsorption on muscovite under treatment with DDA alone and the mixed DDA/OCT collector system at pH 6 ± 0.5 and 25 °C are presented in Figure 7. The trend of DDA adsorption on muscovite agrees with the typical four-step adsorption theory: Adsorption initially increases, plateaus, and then sharply increases until the final saturated adsorption state is reached. At low concentrations (I), the positively charged DDA and negatively charged muscovite interact through ion exchange and electrostatic adsorption. Thus, the adsorption isotherm sharply increases. As the equilibrium concentration of DDA increases (II), negative charges on the muscovite surface are neutralized, consequently weakening electrostatic interactions between muscovite and DDA. The adsorption force between DDA and muscovite is dominated by weak van der Waals forces. This phenomenon is represented by the weak curve of the adsorption isotherm. When the DDA concentration continues to increase to the critical hemimiceller concentration (HMC) value (III), the adsorption amount of DDA increases sharply as a result of hydrophobic associations among nonpolar carbon chains. Finally, the DDA concentration reaches the critical miceller concentration (CMC) value (IV), which tends to reach saturation as a result of micelle formation. Comparing the measured DDA adsorption isotherm with its standard adsorption isotherm model reveals that that the inflection point concentration (CMC value) reaches saturation adsorption at approximately 2 × 10−2 mol/L.
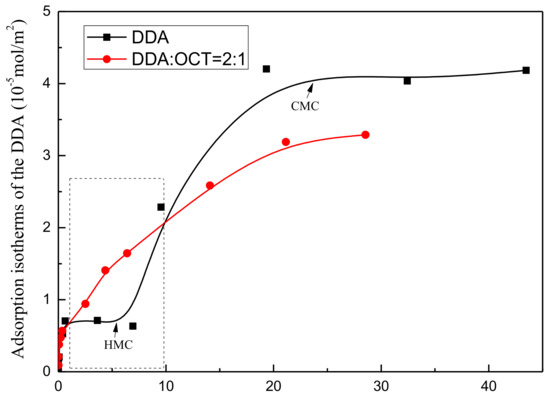
Figure 7.
Comparison between the adsorption isotherms of the single DDA and mixed DDA/ octanol (OCT) systems.
In the mixed DDA/OCT system, low concentrations of OCT significantly increase the adsorption strength of DDA on muscovite. This phenomenon indicates that DDA and OCT exhibit synergistic adsorption, and the presence of OCT promotes the adsorption of DDA. However, the adsorption strength of DDA under high concentrations of mixed DDA/OCT is weaker than that under DDA. This result can be attributed to the transition of the synergistic adsorption of DDA and OCT to competitive adsorption after adsorption reaches a certain saturation degree.
4. Conclusions
This study investigated the synergistic adsorption of mixed DDA/alcohol (OCT, DEC, DOD, or TER) collector systems on muscovite. The following are the main conclusions.
The muscovite-collecting power of mixed DDA/alcohol (OCT or DEC) collector systems is stronger than that of the single DDA collector system. Mixed DDA/alcohol (OCT, DEC, or DOD) collector systems can attain a muscovite recovery rate of 80%. Saturation can be obtained with 10−4 mol/L 2:1 DDA/OCT or 2:1 DDA/DEC. This result indicates that out of all mixed collector systems, the DAA/OCT and DDA/DEC mixed collector systems exhibit the most significantly positive synergistic effects on muscovite flotation. The mixed DDA/DOD recovery system has a muscovite recovery rate of 49%, which is slightly higher than that of the single DDA system. However, muscovite recovery by the mixed DDA/TER system (33%) is lower than that by the mixed DDA system (41%).
The zeta potential test shows that muscovite is negatively charged almost over the entirety of the pH range. DDA adsorbs on muscovite, which is negatively charged, through electrostatic force. This phenomenon shifts the zeta potential of muscovite to a positive value. Under the DDA concentration of 10−4 mol/L, the presence of OCT can promote DDA adsorption on the muscovite surface. DOD has the strongest promoting effect on the adsorption of DDA on the muscovite surface. This effect may be attributed to the similar carbon-chain lengths of the two compounds that enable the formation of tightly adsorbed layers through synergistic adsorption.
Contact angle measurements show that the contact angle of muscovite significantly increases with the dosage of the mixed DDA/OCT collector system. This effect is not observed under the single DDA system and can be attributed to the promotion of the surface hydrophobicity of muscovite through the synergistic adsorption of DDA and OCT. OCT by itself does not absorb on muscovite, and the presence of DDA is a prerequisite for its adsorption on muscovite. DDA plays a dominant role in the synergistic adsorption of OCT and DDA, whereas OCT plays a supporting role. Low concentrations of the mixed DDA/DOD system can improve the surface hydrophobicity of muscovite. This result is consistent with zeta potential measurements.
OCT is adsorbed on the muscovite surface through coadsorption with DDA, indicating that the synergetic adsorption of amine and alcohol occurs during muscovite flotation, especially under acidic and neutral conditions. DDA mixes with alcohol molecules, and DDA adsorption on the mineral surface can promote alcohol adsorption and vice versa. In addition, the synergetic adsorption of DDA and DOD is relatively strong, as indicated by the results of zeta potential and contact angle analyses. The synergy between DDA and alcohol molecules is apparent. Adsorption saturation also increases with increasing collector concentration, and amine and alcohol adsorption on muscovite adsorption sites shifts from synergetic to competitive adsorption.
Acknowledgments
We gratefully appreciate the financial support provided by the Natural Science Foundation of China (NSFC, 51704329), the project of Sublimation Scholar’s Distinguished Professor of Central South University, the National 111 Project (No. B14034), and Collaborative Innovation Center for Clean and Efficient Utilization of Strategic Metal Mineral Resources.
Author Contributions
Li Wang, Yuehua Hu and Wei Sun conceived and designed the experiments; Ning Sun, Jiapeng Liu and Honghu Tang performed the experiments and analyzed the date; Runqing Liu, Haisheng Han and Li Wang contributed reagents and materials; Li Wang and Ning Sun wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Barlow, S.G.; Manning, D.A.C. Influence of time and temperature on reactions and transformations of muscovite mica. Br. Ceram. Trans. 1999, 98, 122–126. [Google Scholar] [CrossRef]
- Ssuerr, N.B.M. Composition and structural state of K-feldspars from some US pegmatites. Am. Mineral. 1979, 64, 49–56. [Google Scholar]
- Mehrabani, J.V.; Noaparast, M.; Mousavi, S.M.; Dehghan, R.; Ghorbani, A. Process optimization and modelling of sphalerite flotation from a low-grade Zn-Pb ore using response surface methodology. Sep. Purif. Technol. 2010, 72, 242–249. [Google Scholar] [CrossRef]
- Xu, L.; Wu, H.; Dong, F.; Wang, Z.; Xiao, J. Flotation and adsorption of mixed cationic/anionic collectors on muscovite mica. Miner. Eng. 2013, 41, 41–45. [Google Scholar] [CrossRef]
- Santana, R.C.; Ribeiro, J.A.; Santos, M.A.; Reis, A.S.; Ataíde, C.H.; Barrozo, M.A. Flotation of fine apatitic ore using microbubbles. Sep. Purif. Technol. 2012, 98, 402–409. [Google Scholar]
- Wang, L.; Sun, W.; Hu, Y.H.; Xu, L.H. Adsorption mechanism of mixed anionic/cationic collectors in Muscovite–Quartz flotation system. Miner. Eng. 2014, 64, 44–50. [Google Scholar] [CrossRef]
- Nishimura, S.; Tateyama, H.; Tsunematsu, K.; Jinnai, K. Zeta potential measurement of muscovite mica basal plane-aqueous solution interface by means of plane interface technique. J. Colloid Interface Sci. 1992, 152, 359–367. [Google Scholar] [CrossRef]
- Pugh, R.J.; Rutland, M.W.; Manev, E.; Jinnai, K. Dodecylamine collector—pH effect on mica flotation and correlation with thin aqueous foam film and surface force measurements. Int. J. Miner. Process. 1996, 46, 245–262. [Google Scholar] [CrossRef]
- Sekulić, Ž.; Canić, N.; Bartulović, Z.; Daković, A. Application of different collectors in the flotation concentration of feldspar, mica and quartz sand. Miner. Eng. 20004, 17, 77–80. [Google Scholar] [CrossRef]
- Bayraktar, I.; Ersayin, S.; Gülsoy, Ö.Y. Upgrading titanium bearing Na-feldspar by flotation using sulphonates, succinamate and soaps of vegetable oils. Miner. Eng. 1997, 10, 1363–1374. [Google Scholar] [CrossRef]
- Al-Thyabat, S. Evaluation of mechanical flotation of non-slimed Jordanian siliceous phosphate. Arab. J. Sci. Eng. 2012, 37, 877–887. [Google Scholar] [CrossRef]
- Smith, R.W.; Scott, J.L. Mechanisms of dodecylamine flotation of quartz. Miner. Process. Extr. Metall. Rev. 1990, 7, 81–94. [Google Scholar] [CrossRef]
- Vieira, A.M.; Peres, A.E.C. The effect of amine type, pH, and size range in the flotation of quartz. Miner. Eng. 2007, 20, 1008–1013. [Google Scholar] [CrossRef]
- Wang, L.; Liu, R.; Hu, Y.; Liu, J.; Sun, W. Adsorption behavior of mixed cationic/anionic surfactants and their depression mechanism on the flotation of quartz. Powder Technol. 2016, 302, 15–20. [Google Scholar] [CrossRef]
- Xu, L.; Hu, Y.; Tian, J.; Wu, H.; Wang, L.; Yang, Y.; Wang, Z. Synergistic effect of mixed cationic/anionic collectors on flotation and adsorption of muscovite. Colloids Surf. 2016, 492, 181–189. [Google Scholar] [CrossRef]
- Vidyadhar, A.; Kumari, N.; Bhagat, R.P. Adsorption mechanism of mixed collector systems on hematite flotation. Miner. Eng. 2012, 26, 102–104. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, W.; Han, C.; Hao, H. Effects of monohydric alcohols on the flotation of magnesite and dolomite by sodium oleate. J. Mol. Liq. 2018, 249, 1060–1067. [Google Scholar] [CrossRef]
- Sis, H.; Chander, S. Improving froth characteristics and flotation recovery of phosphate ores with nonionic surfactants. Miner. Eng. 2003, 16, 587–595. [Google Scholar] [CrossRef]
- Von Rybinski, W.; Schwuger, M.J.; Dobias, B. Surfactant mixtures as collectors in flotation. Colloids Surf. 1987, 26, 291–304. [Google Scholar] [CrossRef]
- Fuerstenau, D.W.; Yamada, B.J. Neutral molecules in flotation collection. Trans. AIME 1962, 223, 50–52. [Google Scholar]
- Wang, L.; Hu, Y.; Liu, J.; Sun, Y.; Sun, W. Flotation and adsorption of muscovite using mixed cationic–nonionic surfactants as collector. Powder Technol. 2015, 276, 26–33. [Google Scholar] [CrossRef]
- Xu, L.; Hu, Y.; Dong, F.; Hao, J.; Wu, H.; Wang, Z.; Liu, R. Effects of particle size and chain length on flotation of quaternary ammonium salts onto kaolinite. Miner. Petrol. 2015, 109, 309–316. [Google Scholar] [CrossRef]
- Zouboulis, A.I.; Avranas, A. Treatment of oil-in-water emulsions by coagulation and dissolved-air flotation. Colloids Surf. A. 2000, 172, 153–161. [Google Scholar] [CrossRef]
- Fuerstenau, D.W. Zeta potentials in the flotation of oxide and silicate minerals. Adv. Colloid Interface Sci. 2005, 114, 9–26. [Google Scholar] [CrossRef] [PubMed]
- Kirby, B.J.; Hasselbrink, E.F. Zeta potential of microfluidic substrates: 2. Data for polymers. Electrophor. 2004, 25, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Vidyadhar, A.; Rao, K.H. Adsorption mechanism of mixed cationic/anionic collectors in feldspar-quartz flotation system. J. Colloid Interface Sci. 2007, 306, 195–204. [Google Scholar] [PubMed]
- Crawford, R.; Ralston, J. The influence of particle size and contact angle in mineral flotation. Int. J. Miner. Process. 1988, 23, 1–24. [Google Scholar] [CrossRef]
- Chau, T.T. A review of techniques for measurement of contact angles and their applicability on mineral surfaces. Miner. Eng. 2009, 22, 213–219. [Google Scholar] [CrossRef]
- Wang, L.; Liu, R.; Hu, Y.; Sun, W. pH effects on adsorption behavior and self-aggregation of dodecylamine at muscovite/aqueous interfaces. J. Mol. Graph. Modell. 2016, 67, 62–68. [Google Scholar]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).