Quantitative Mineralogical Comparison between HPGR and Ball Mill Products of a Sn-Ta Ore
Abstract
:1. Introduction
2. Study Area
3. Materials and Methods
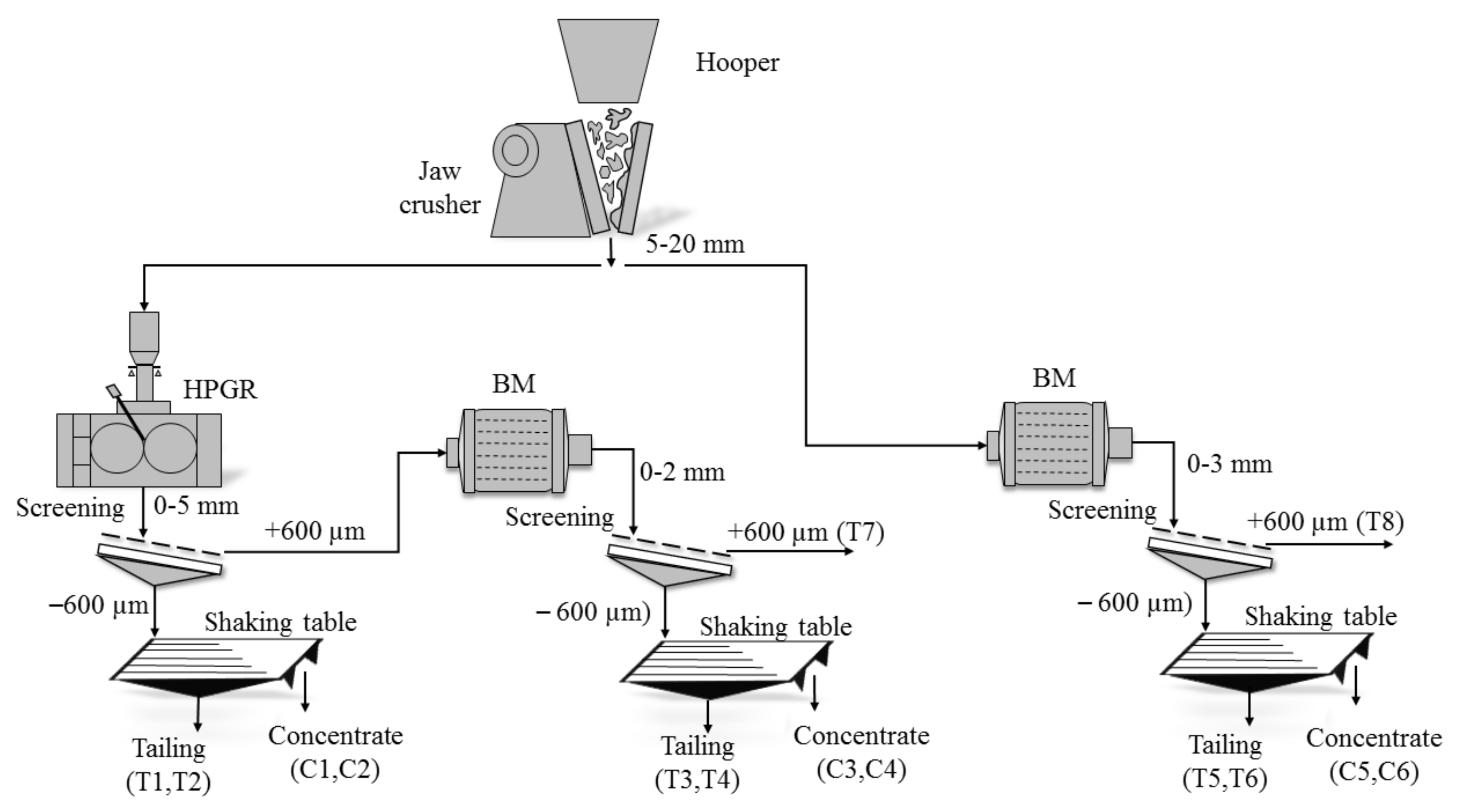
3.1. Sample Preparation
3.2. Analytical Methods
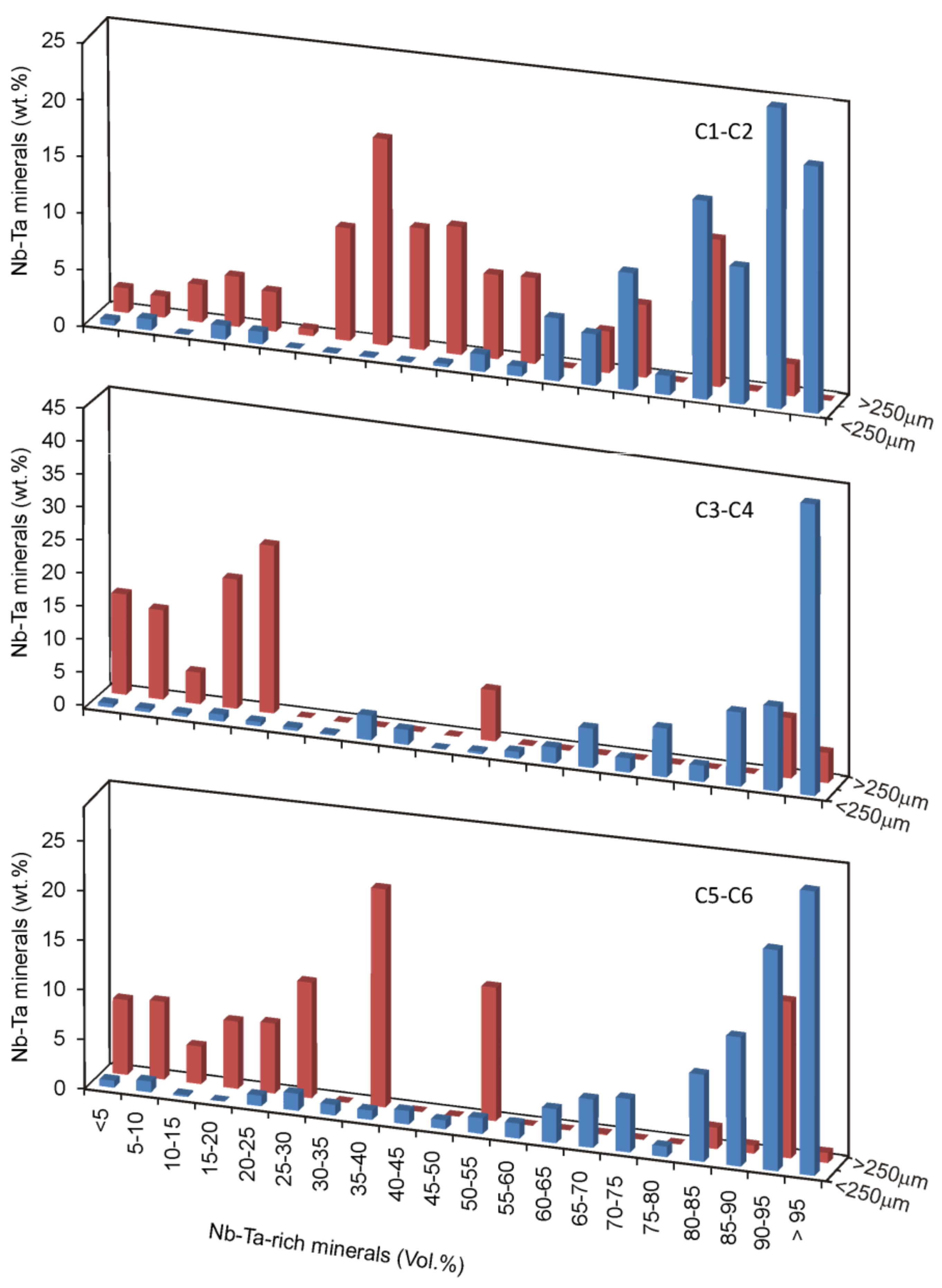
4. Results and Discussion
4.1. Chemical and Mineralogical Characterization of the Bulk Sample
4.2. Characterization of the Comminuted Products
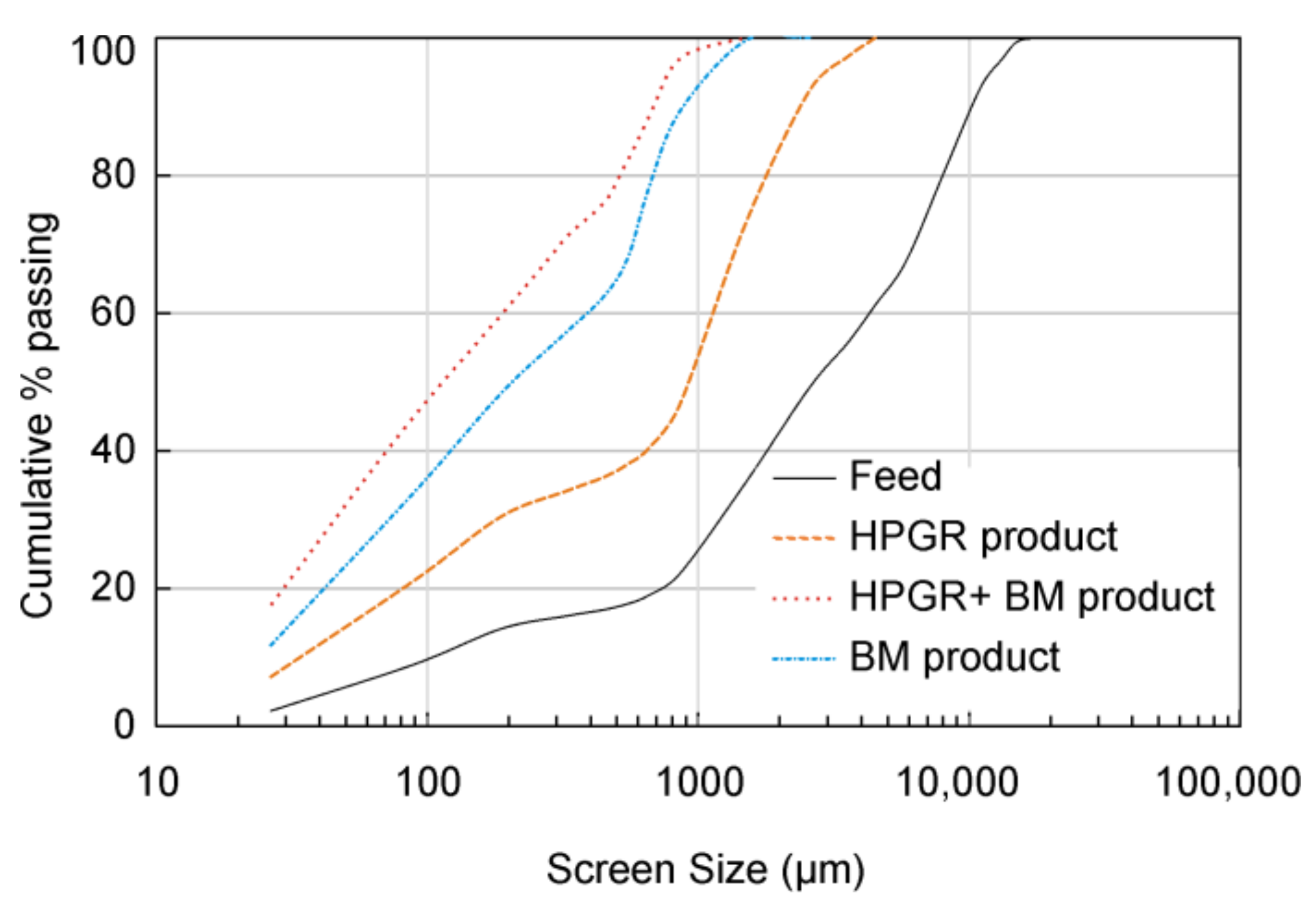
4.2.1. Particle Size Distribution (PSD)
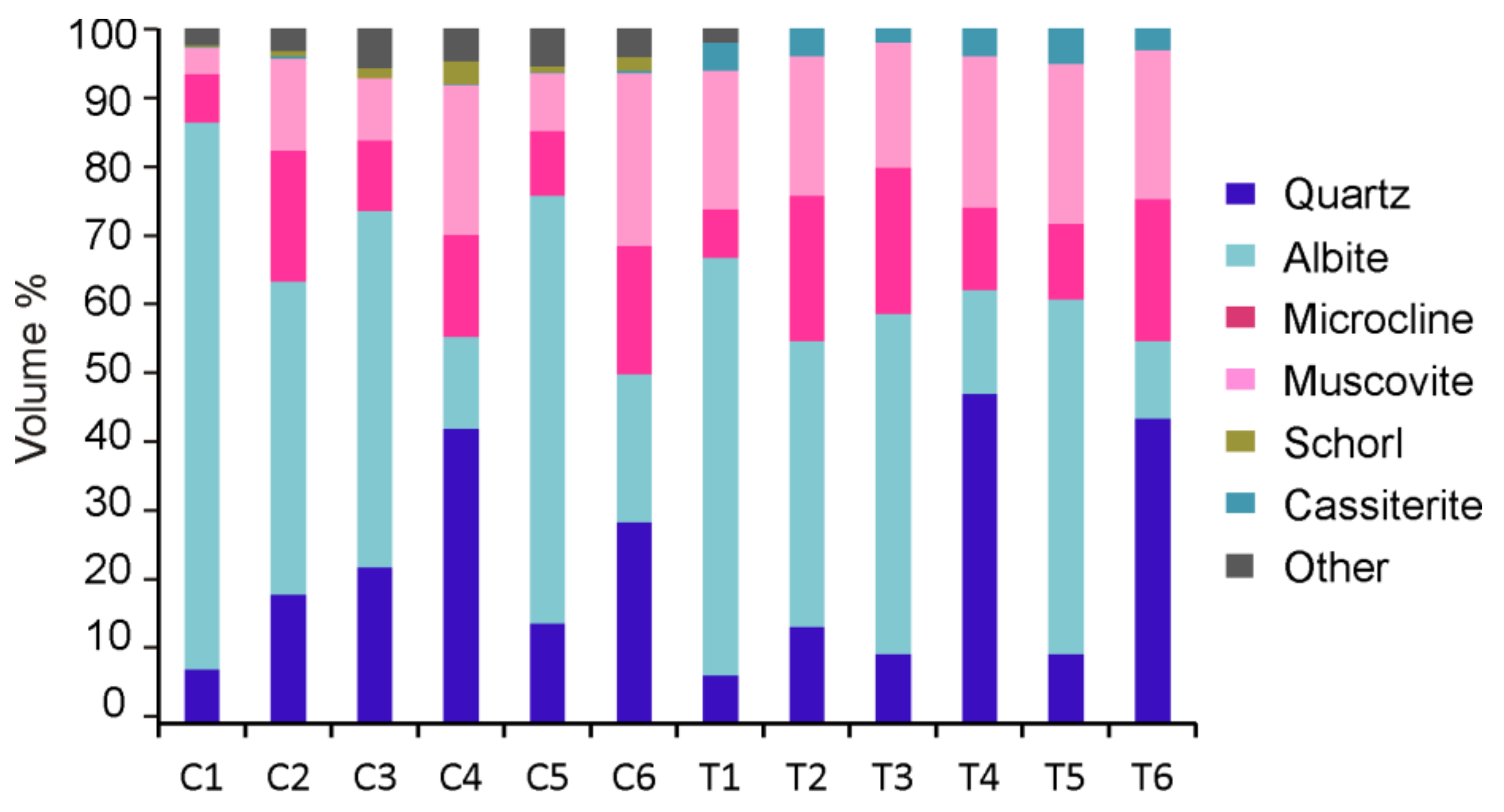
4.2.2. Modal Mineralogy
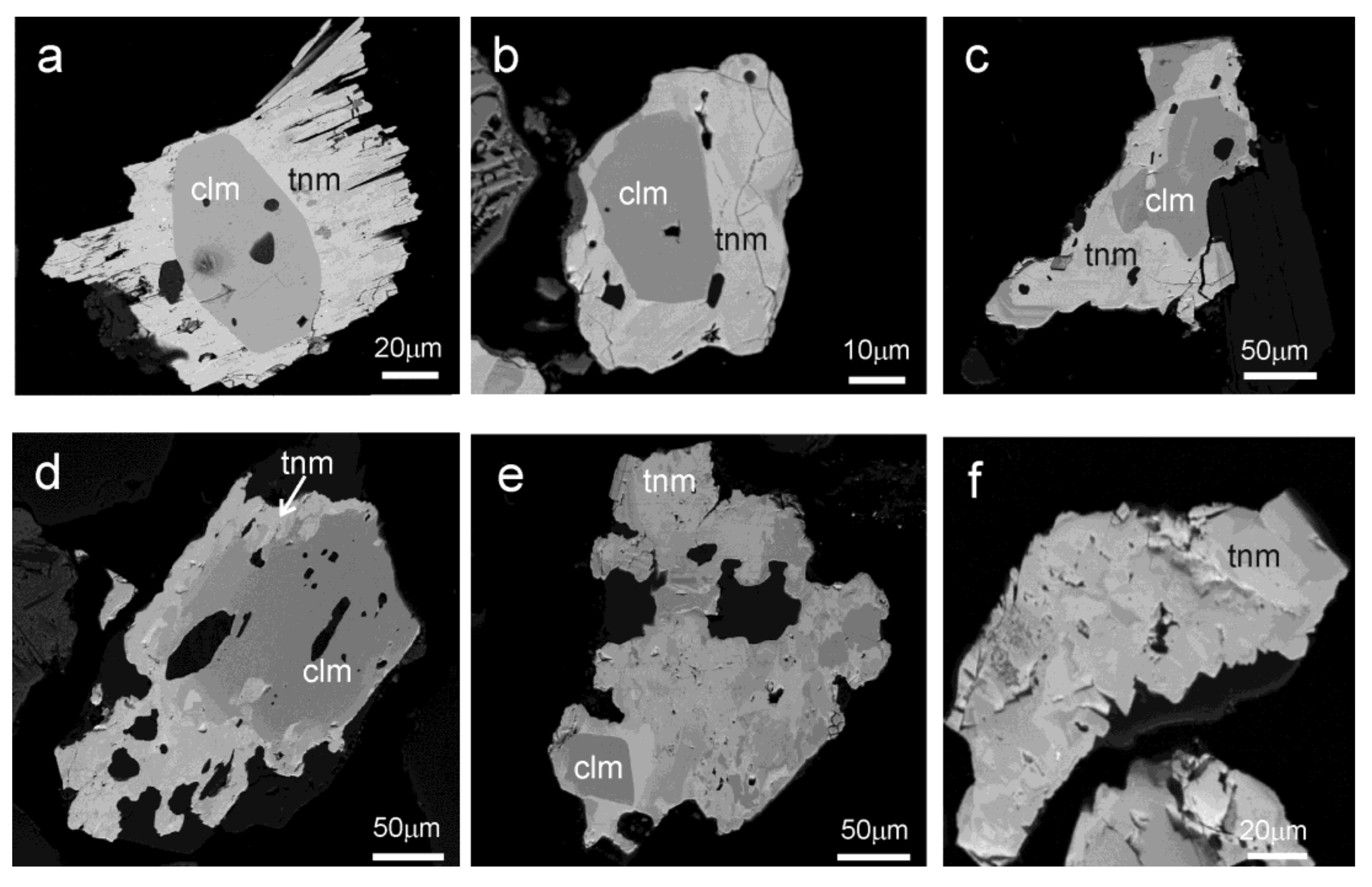
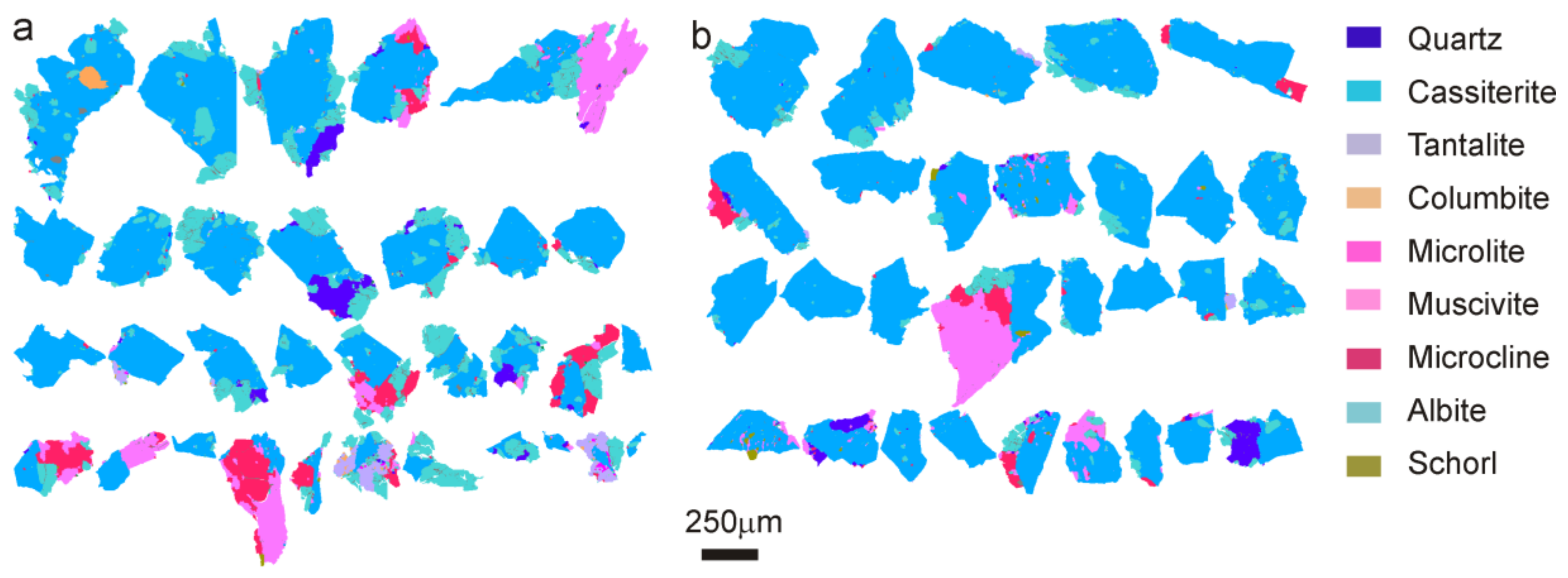
4.2.3. Morphology and Texture of Particles
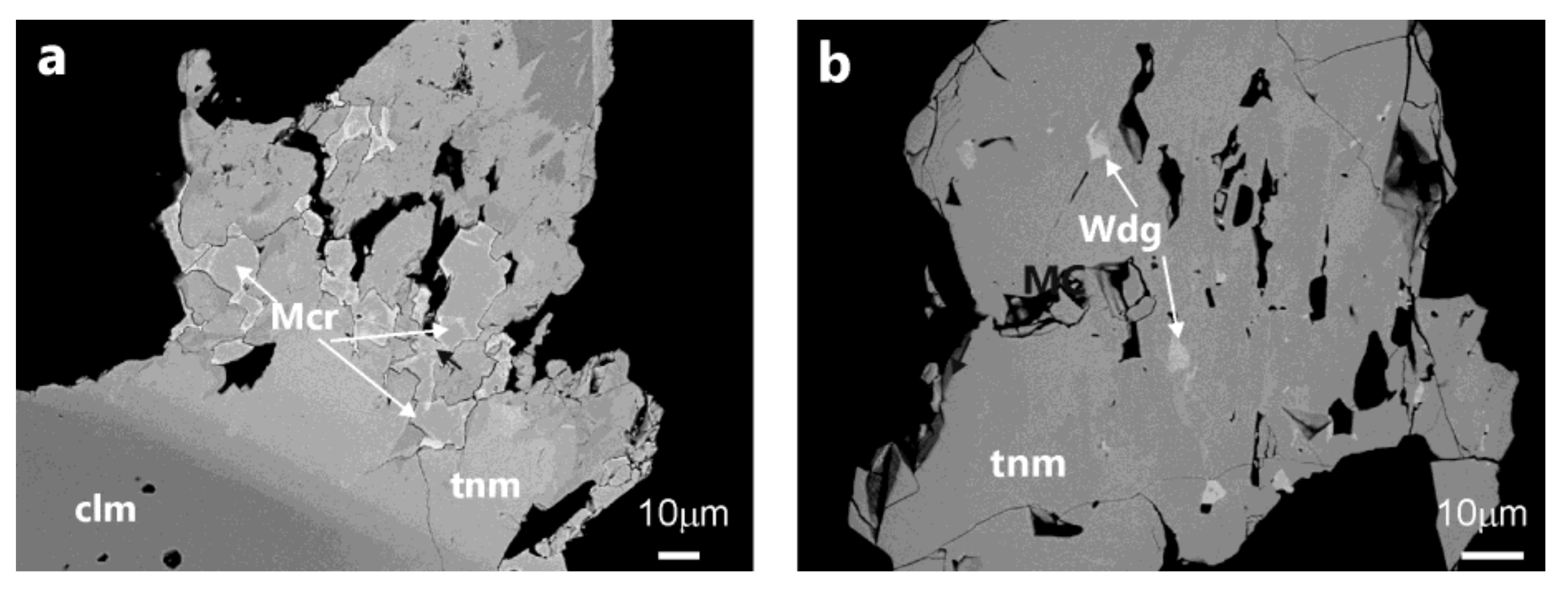
4.2.4. Mineral Associations
4.2.5. Metal Distribution
4.3. Mineral Liberation
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Deetman, S.; van Oers, L.; van der Voet, E.; Tukker, A. Deriving European tantalum flows using trade and production statistics. J. Ind. Ecol. 2017, 22, 166–179. [Google Scholar] [CrossRef]
- Linnen, R.L.; Van Lichtervelde, M.; Černý, P. Granitic pegmatites as sources of strategic metals. Elements 2012, 8, 275–280. [Google Scholar] [CrossRef]
- Mackay, D.A.R.; Simandl, G.J. Geology, market and supply chain of niobium and tantalum—A review. Miner. Deposita 2014, 49, 1025–1047. [Google Scholar] [CrossRef]
- Chakhmouradian, A.R.; Smith, M.P.; Kynicky, J. From “strategic” tungsten to “green” neodymium: A century of critical metals at a glance. Ore Geol. Rev. 2015, 64, 455–458. [Google Scholar] [CrossRef]
- European Comission. Study on the Review of the List of Critical Raw Materials. 2017. Available online: https://publications.europa.eu/en/publication-detail/-/publication/08fdab5f-9766-11e7-b92d-01aa75ed71a1/language-en (accessed on 9 February 2018).
- Černý, P.; Blevin, P.L.; Cuney, M.; London, D. Granite-related ore deposits. In Economic Geology 100th Anniversary Volume; Hedenquist, J.W., Thompson, J.F.H., Goldfarb, R.J., Richards, J.P., Eds.; Society of Economic Geologists: Littleton, CO, USA, 2005; pp. 337–370. [Google Scholar]
- Wikedzi, A.; Arinanda, M.A.; Leißner, T.; Peuker, U.A.; Mütze, T. Breakage and liberation characteristics of low grade sulphide gold ore blends. Miner. Eng. 2018, 115, 33–40. [Google Scholar] [CrossRef]
- Morrell, S. A method for predicting the specific energy requirement of comminution circuits and assessing their energy utilisation efficiency. Miner. Eng. 2008, 2, 224–233. [Google Scholar] [CrossRef]
- Aydoğan, N.A.; Benzer, H. Comparison of the overall circuit performance in the cement industry: High compression milling vs. ball milling technology. Miner. Eng. 2011, 24, 211–215. [Google Scholar] [CrossRef]
- Ballantyne, G.R.; Hilden, M.; van der Meer, F.P. Improved characterisation of ball milling energy requirements for HPGR products. Miner. Eng. 2018, 16, 72–81. [Google Scholar] [CrossRef]
- Romer, R.L.; Smeds, S.A.; Černý, P. Crystal-chemical and genetic controls of U-Pb systematics of columbite-tantalite. Mineral. Petrol. 1996, 57, 243–260. [Google Scholar] [CrossRef]
- Jones, M.P. Applied Mineralogy: A Quantitative Approach; Graham and Trotman: London, UK, 1987. [Google Scholar]
- King, R.P. Modelling and Simulation of Mineral Processing Systems; Butterworth-Heinemann: New Delhi, India, 2001; ISBN 0-7506-4884-8. [Google Scholar]
- Little, L.; Becker, M.; Wiese, J.; Mainza, A.N. Auto-SEM particle shape characterisation: Investigating fine grinding of UG2 ore. Miner. Eng. 2015, 82, 92–100. [Google Scholar] [CrossRef]
- Gaudin, A.M. Principles of Mineral Dressing; McGraw-Hill: New York, NY, USA, 1939. [Google Scholar]
- Butcher, A.R. A practical guide to some aspects of mineralogy that affect flotation. In Flotation Plant Optimisation; Spectrum Ser. Greet, C.J., Ed.; The Australasian Institute of Mining and Metallurgy: Melbourne, Australia, 2010; Volume 16, pp. 83–93. [Google Scholar]
- Djordjevic, N. Image based modelling of rock fragmentation. Miner. Eng. 2013, 46–47, 68–75. [Google Scholar] [CrossRef]
- Van der Wielen, K.P.; Rollinson, G. Texture-based analysis of liberation behaviour using Voronoi tessellations. Miner. Eng. 2016, 89, 93–107. [Google Scholar] [CrossRef]
- Schneider, C.L. The Measurement and Calculation of Liberation in Continuous Grinding Circuits. Ph.D. Thesis, University of Utah, Salt Lake City, UT, USA, 1995. [Google Scholar]
- Gu, Y. Automated Scanning Electron Microscope Based Mineral Liberation Analysis An Introduction to JKMRC/FEI Mineral Liberation Analyser. J. Miner. Mater. Charact. Eng. 2003, 2, 33–41. [Google Scholar] [CrossRef]
- Fandrich, R.; Gu, Y.; Burrows, D.; Moeller, K. Modern SEM-based mineral liberation analysis. Int. J. Miner. Process. 2007, 84, 310–320. [Google Scholar] [CrossRef]
- Hunt, J.A.; Berry, R.; Walters, S.G.; Bonnici, N.; Kamenetsky, M.; Nguyen, K.; Evans, C.L. A new look at mineral maps and the potential relationships of extracted data to mineral processing behaviours. In Proceedings of the ICAM Australia 2008, Brisbane, Australia, 8–10 September 2008; pp. 429–432. [Google Scholar]
- Ford, F.D.; Wercholaz, C.R.; Lee, A. Predicting process outcomes for Sudbury platinum-group minerals using grade-recovery modelling from Mineral Liberation Analyzer (MLA) data. Can. Mineral. 2011, 49, 1627–1642. [Google Scholar] [CrossRef]
- Sandmann, D.; Gutzmer, J. Use of Mineral Liberation Analysis (MLA) in the Characterization of Lithium-Bearing Micas. J. Miner. Mater. Charact. Eng. 2013, 1, 285–292. [Google Scholar] [CrossRef]
- Goodall, W.R.; Scales, P.J. An overview of the advantages and disadvantages of the determination of gold mineralogy by automated mineralogy. Miner. Eng. 2007, 116, 82–87. [Google Scholar] [CrossRef]
- Cook, N.J.; Ciobanu, C.L.; Ehrig, K.; Slattery, A.; Verdugo-Ihl, M.R.; Courtney-Davies, L.; Gao, W. Advances and opportunities in ore mineralogy. Minerals 2017, 7, 333. [Google Scholar] [CrossRef]
- Lotter, N.O. Modern Process Mineralogy: An integrated multi-disciplined approach to flowsheeting. Miner. Eng. 2011, 24, 1229–1237. [Google Scholar] [CrossRef]
- Lastra, R.; Paktunc, D. An estimation of the variability in automated quantitative mineralogy measurements through inter-laboratory testing. Miner. Eng. 2016, 95, 138–145. [Google Scholar] [CrossRef]
- Spencer, S.; Shutherland, D. Stereological correction of mineral liberation grade distributions estimated by single sectioning of particles. Image Anal. Stereol. 2000, 19, 175–182. [Google Scholar] [CrossRef]
- Leißner, T.; Hoang, D.H.; Rudolph, M.; Heing, T.; Bachmann, K.; Gutzmer, J.; Schubert, H.; Peuker, U.A. A mineral liberation study of grain boundary fracture based on measurements of the surface exposure after milling. Int. J. Miner. Process. 2016, 156, 3–13. [Google Scholar]
- Shutherland, D. Estimation of mineral grain size using automated mineralogy. Miner. Eng. 2007, 20, 452–460. [Google Scholar] [CrossRef]
- ADARO. Proyecto de Investigación de la Mina de Penouta. Cálculo de Reservas Para Leyes de Corte de 800 y 600 g/t; Unpublished Report; 1982; p. 69. [Google Scholar]
- López Moro, F.J.; García Polonio, F.; Llorens González, T.; Sanz Contreras, J.L.; Fernández-Fernández, A.; Moro Benito, M.C. Ta and Sn concentration by muscovite fractionation and degassing in a lens-like granite body: The case study of the Penouta rare-metal albite granite (NW Spain). Ore Geol. Rev. 2017, 82, 10–30. [Google Scholar] [CrossRef]
- Llorens González, T.; García Polonio, F.; López Moro, F.J.; Fernández-Fernández, A.; Sans Contreras, J.L.; Moro Benito, M.C. Tin-tantalum-niobium mineralization in the Penouta deposit (NW Spain): Textural features and mineral chemistry to unravel the genesis and evolution of cassiterite and columbite group minerals in a peraluminous system. Ore Geol. Rev. 2017, 81, 79–95. [Google Scholar] [CrossRef]
- Alfonso, P.; Hamid, S.; Garcia-Valles, M.; Llorens, T.; López Moro, J.; Tomasa, O.; Calvo, D.; Guasch, E.; Anticoi, H.; Oliva, J.; et al. Textural and mineral-chemistry constraints on columbite-group minerals in the Penouta deposit: Evidence from magmatic and fluid-related processes. Mineral. Mag. 2018. [Google Scholar] [CrossRef]
- Ghorbani, Y.; Fitzpatrick, R.; Kinchington, M.; Rollinson, G.; Hegarty, P. A Process Mineralogy Approach to Gravity Concentration of Tantalum Bearing Minerals. Minerals 2017, 7, 194. [Google Scholar] [CrossRef]
- Guasch, E.; Anticoi, H.; Hamid, S.A.; Oliva, J.; Alfonso, P.; Escobet, T.; Sanmiquel, L.; Bascompta, M. New approach to ball mill modelling as a piston flow process. Miner. Eng. 2018, 116, 82–87. [Google Scholar] [CrossRef]
- Kazerani Nejad, R.; Sam, A. Limitation of HPGR application. Miner. Process. Ext. Met. 2017, 126, 224–230. [Google Scholar] [CrossRef]
- Hill, R.J.; Tsambourakis, G.; Madsen, I.C. Improved petrological modal analyses from X-ray powder diffraction data by use of the rietveld method I. selected igneous, volcanic, and metamorphic rocks. J. Petrol. 1993, 34, 867–900. [Google Scholar] [CrossRef]
- Moore, D.M.; Reynolds, R.C. X-ray Diffraction and the Identification and Analysis of Clay Minerals; Oxford University Press: New York, NY, USA, 1989. [Google Scholar]
- Rahfeld, A.; Kleeberg, R.; Möckel, R.; Gutzmer, J. Quantitative mineralogical analysis of European Kupferschiefer ore. Miner. Eng. 2018, 115, 21–32. [Google Scholar] [CrossRef]
- Vizcarra, T.G.; Wightman, E.M.; Johnson, N.W.; Manlapig, E.V. The effect of breakage mechanism on the mineral liberation properties of sulphide ores. Miner. Eng. 2010, 23, 374–382. [Google Scholar] [CrossRef]
- Han, Y.X.; Liu, L.; Yuan, Z.T.; Wang, Z.H.; Zhang, P. Comparison of low-grade hematite product characteristics in a high-pressure grinding roller and jaw crusher. Miner. Metall. Process. 2012, 29, 75–80. [Google Scholar]
- Ueda, T.; Oki, T.; Koyaanaka, S. Comparison of Seven Texture Analysis Indices for Their Applicability to Stereological Correction of Mineral Liberation Assessment in Binary Particle Systems. Minerals 2017, 7, 222. [Google Scholar] [CrossRef]
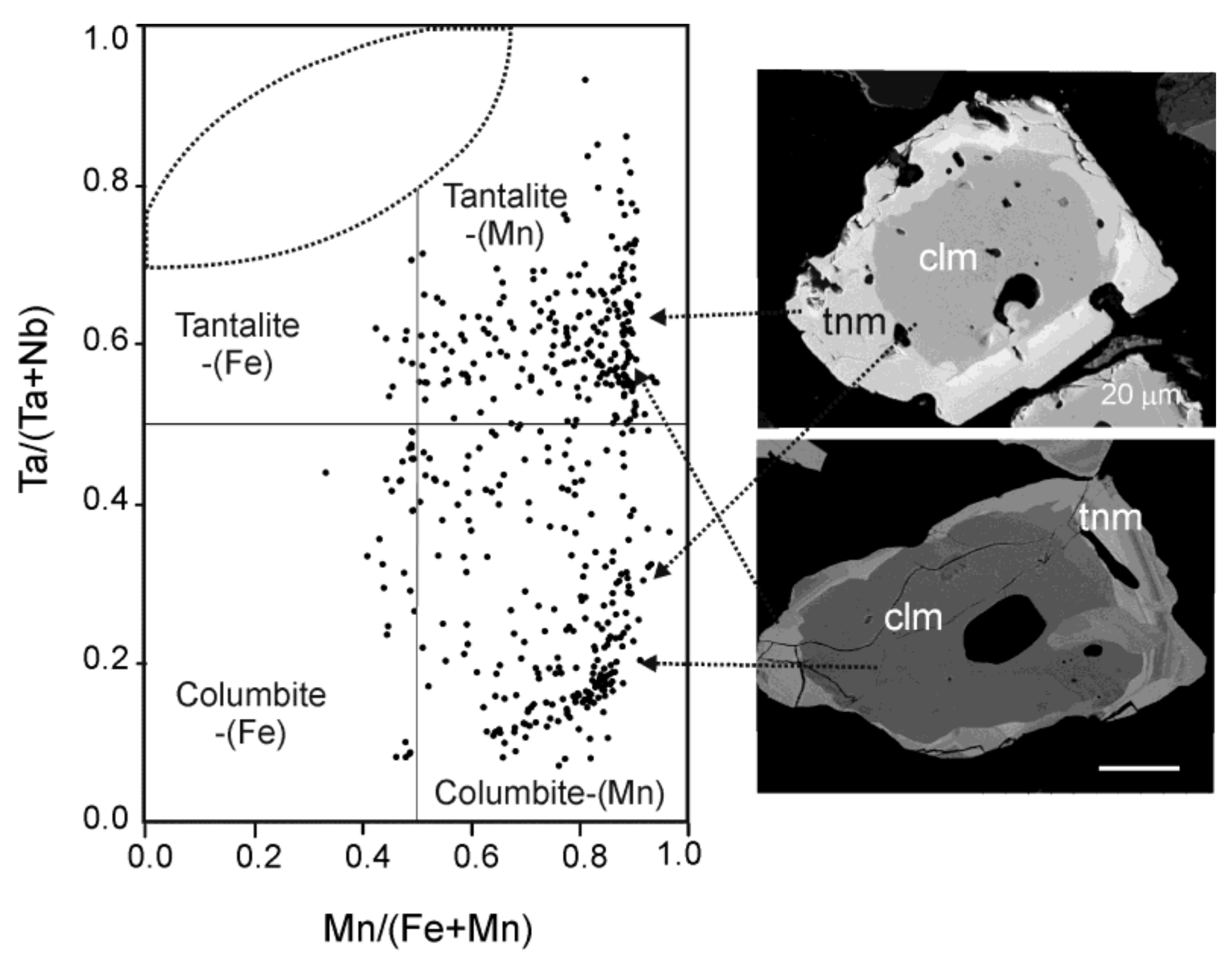


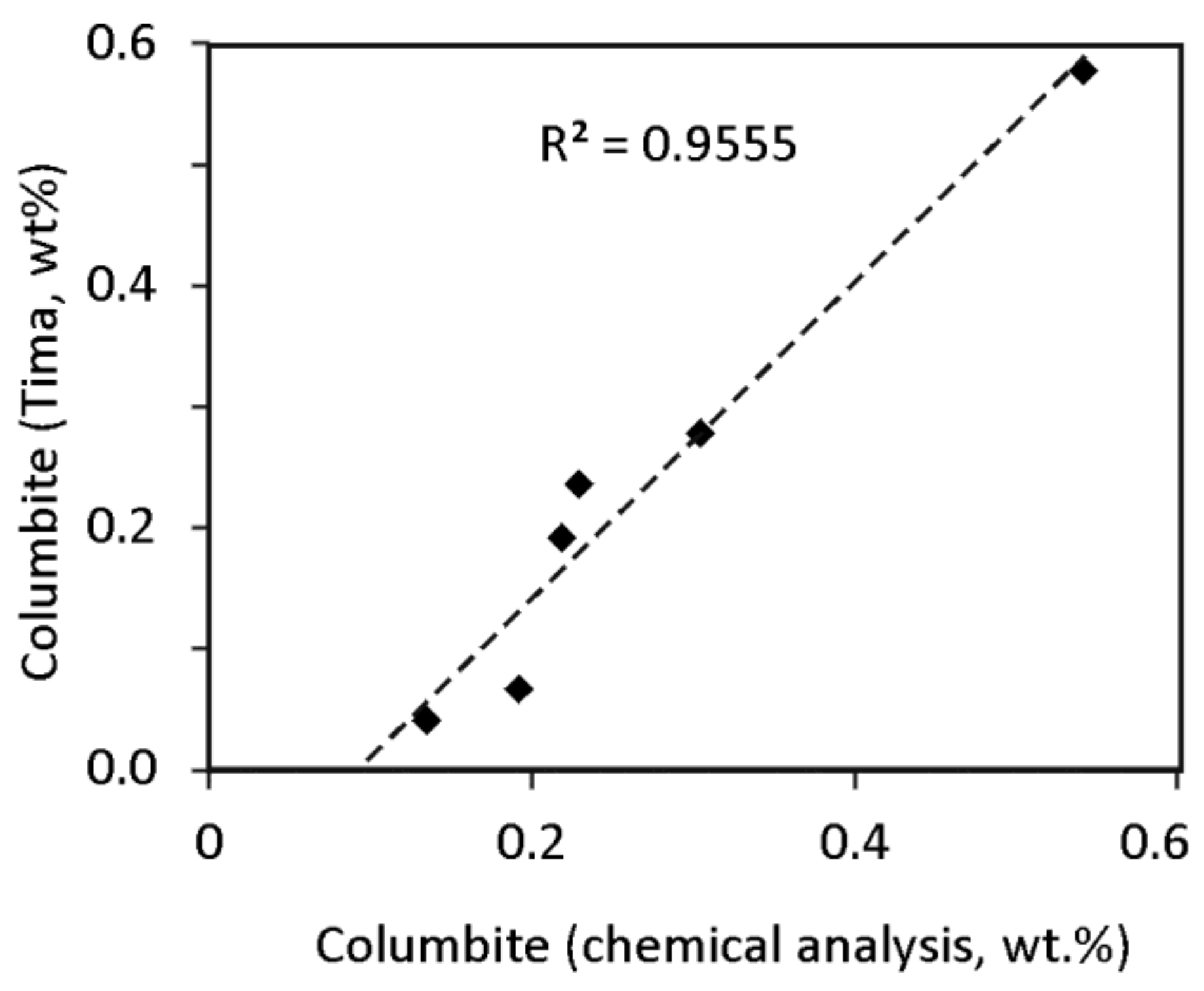
| Oxides, wt % | P1 | P2 | P3 | P94 | P5 | P6 | P7 | P8 |
|---|---|---|---|---|---|---|---|---|
| clm | clm | clf | clm | tnm | tnm | tnm | tnm | |
| WO3 | 0.23 | 0.11 | 0.52 | 0.33 | 0.19 | 0.16 | 0.33 | 0.43 |
| Ta2O5 | 19.13 | 20.47 | 30.22 | 47.29 | 55.80 | 57.69 | 63.55 | 69.87 |
| Nb2O5 | 61.06 | 60.17 | 50.46 | 33.87 | 27.33 | 24.21 | 19.52 | 13.57 |
| TiO2 | 0.04 | 0.02 | 0.06 | 0.10 | 0.00 | 0.09 | 0.12 | 0.00 |
| UO2 | 0.00 | 0.02 | 0.00 | 0.31 | 0.04 | 0.24 | 0.00 | 0.11 |
| ThO2 | 0.00 | 0.00 | 0.00 | 0.22 | 0.00 | 0.00 | 0.08 | 0.00 |
| Sc2O3 | 0.14 | 0.16 | 0.07 | 0.01 | 0.08 | 0.37 | 0.30 | 0.01 |
| SnO2 | 0.06 | 0.08 | 0.10 | 0.11 | 0.18 | 0.22 | 0.22 | 0.24 |
| Fe2O3 | 0.52 | 0.34 | 0.14 | 1.12 | 0.29 | 1.28 | 0.71 | 0.34 |
| FeO | 2.86 | 3.07 | 9.80 | 7.74 | 1.63 | 2.14 | 7.55 | 3.38 |
| MnO | 16.35 | 16.16 | 8.68 | 8.53 | 14.48 | 12.98 | 7.65 | 11.36 |
| Total | 100.41 | 100.60 | 100.05 | 99.65 | 100.02 | 99.38 | 100.03 | 99.31 |
| Atomic Contents | ||||||||
| W6+ | 0.015 | 0.007 | 0.034 | 0.024 | 0.015 | 0.012 | 0.025 | 0.035 |
| Ta5+ | 1.259 | 1.352 | 2.099 | 3.580 | 4.374 | 4.603 | 5.195 | 5.972 |
| Nb5+ | 6.673 | 6.599 | 5.827 | 4.263 | 3.560 | 3.211 | 2.653 | 1.928 |
| Ti2+ | 0.007 | 0.003 | 0.011 | 0.022 | 0.000 | 0.021 | 0.028 | 0.000 |
| U4+ | 0.000 | 0.001 | 0.000 | 0.019 | 0.003 | 0.016 | 0.000 | 0.008 |
| Th4+ | 0.000 | 0.000 | 0.000 | 0.014 | 0.000 | 0.000 | 0.006 | 0.000 |
| Sc3+ | 0.012 | 0.016 | 0.022 | 0.028 | 0.046 | 0.055 | 0.058 | 0.065 |
| Sn4+ | 0.015 | 0.017 | 0.008 | 0.001 | 0.011 | 0.048 | 0.040 | 0.000 |
| Fe3+ | 0.095 | 0.062 | 0.027 | 0.235 | 0.063 | 0.285 | 0.149 | 0.080 |
| Fe2+ | 0.577 | 0.622 | 2.094 | 1.803 | 0.393 | 0.523 | 1.898 | 0.888 |
| Mn2+ | 3.347 | 3.321 | 1.878 | 2.011 | 3.535 | 3.226 | 1.948 | 3.024 |
| CATSUM | 12.000 | 12.000 | 12.000 | 12.000 | 12.000 | 12.000 | 12.000 | 12.000 |
| Mineral (wt %) | T1 | T2 | T3 | T4 | T5 | T6 | T7 | T8 |
|---|---|---|---|---|---|---|---|---|
| Quartz | 7 | 14 | 10 | 48 | 10 | 43 | 62 | 37 |
| Albite | 60 | 41 | 51 | 15 | 51 | 11 | 15 | 30 |
| Microcline | 7 | 21 | 23 | 12 | 11 | 20 | 13 | 14 |
| Muscovite | 20 | 20 | 14 | 22 | 23 | 21 | 7 | 16 |
| Kaolinite | 4 | 4 | 2 | 4 | 5 | 3 | 1 | 1 |
| Beryl | 2 | - | - | - | - | - | 2 |
| Mineral (wt %) | C1 | C2 | C3 | C4 | C5 | C6 |
|---|---|---|---|---|---|---|
| Cassiterite | 84.06 | 48.55 | 87.97 | 71.05 | 82.38 | 67.66 |
| Columbite | 45.62 | 11.13 | 50.39 | 30.13 | 52.40 | 13.44 |
| Tantalite | 50.45 | 18.66 | 53.59 | 23.65 | 48.19 | 26.17 |
| Microlite | 8.38 | 1.96 | 26.29 | 0.39 | 26.59 | 0.00 |
| Wodginite | 5.72 | 1.07 | 11.66 | 0.18 | 14.20 | 0.43 |
| Grinding | Chemical Composition (ppm) | Mineralogy (wt %) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (Normative) | (TIMA-X) | |||||||||||||
| Method | Size (μm) | Sample | Type | Sn | Nb | Ta | Cst | tn | cl | tn/cl | Cst | tn | cl | tn/cl |
| HPGR | –250 | PN-1 | C1 | 3010 | 1080 | 1980 | 0.38 | 0.28 | 0.23 | 1.22 | 0.39 | 0.72 | 0.24 | 3.07 |
| HPGR | +250 | PN-3 | C2 | - | 1030 | 1990 | - | 0.28 | 0.22 | 1.29 | 1.81 | 0.58 | 0.19 | 3.02 |
| HPGR + BM | –250 | PN-5 | C3 | - | 1435 | 2720 | - | 0.39 | 0.30 | 1.26 | 1.30 | 0.63 | 0.28 | 2.26 |
| HPGR + BM | +250 | PN-7 | C4 | - | 635 | 1355 | - | 0.19 | 0.13 | 1.42 | 3.76 | 0.06 | 0.04 | 1.43 |
| BM | –250 | PN-9 | C5 | - | 2550 | 5000 | - | 0.71 | 0.54 | 1.31 | 1.99 | 1.13 | 0.58 | 1.96 |
| BM | +250 | PN-11 | C6 | - | 905 | 1895 | - | 0.27 | 0.19 | 1.40 | 3.23 | 0.11 | 0.07 | 1.68 |
| HPGR | –250 | PN-2 | T1 | 67 | 14 | 30 | 0.01 | 0.00 | 0.00 | 1.43 | ||||
| HPGR | +250 | PN-4 | T2 | 224 | 58 | 80 | 0.03 | 0.01 | 0.01 | 0.92 | ||||
| HPGR + BM | –250 | PN-6 | T3 | 88 | 17 | 30 | 0.01 | 0.00 | 0.00 | 1.18 | ||||
| HPGR + BM | +250 | PN-8 | T4 | 131 | 45 | 40 | 0.02 | 0.01 | 0.01 | 0.59 | ||||
| BM | –250 | PN-10 | T5 | 118 | 23 | 40 | 0.01 | 0.01 | 0.00 | 1.16 | ||||
| BM | +250 | PN-12 | T6 | 132 | 49 | 50 | 0.02 | 0.01 | 0.01 | 0.68 | ||||
| HPGR + BM | +600 | PN-19 | T7 | 528 | 42 | 30 | 0.07 | 0.00 | 0.01 | 0.48 | ||||
| BM | +600 | PN-20 | T8 | 336 | 46 | 60 | 0.04 | 0.01 | 0.01 | 0.87 | ||||
| Mineral | Casssiterite | Columbite-Group | ||||||
|---|---|---|---|---|---|---|---|---|
| HPGR + BM | BM | HPGR + BM | BM | |||||
| Grade | −250 | +250 | −250 | +250 | −250 | +250 | −250 | +250 |
| <10 | 0.08 | 0.14 | 0.10 | 0.16 | 1.20 | 17.51 | 1.79 | 15.37 |
| 10–20 | 0.00 | 0.79 | 0.00 | 0.00 | 1.39 | 16.64 | 0.18 | 10.56 |
| 20–30 | 0.44 | 1.79 | 0.55 | 4.14 | 1.07 | 15.66 | 2.79 | 18.68 |
| 30–40 | 0.00 | 3.96 | 0.88 | 1.86 | 2.25 | 11.83 | 2.06 | 22.01 |
| 40–50 | 0.11 | 4.84 | 1.96 | 2.46 | 1.43 | 9.24 | 2.30 | 0.00 |
| 50–60 | 0.34 | 4.38 | 0.63 | 0.56 | 1.77 | 10.58 | 3.10 | 13.48 |
| 60–70 | 3.53 | 16.02 | 5.42 | 8.40 | 8.85 | 1.57 | 8.37 | 0.10 |
| 70–80 | 7.35 | 16.76 | 2.71 | 4.23 | 10.32 | 2.70 | 6.49 | 0.00 |
| 80–90 | 4.21 | 22.74 | 17.98 | 40.83 | 19.82 | 5.51 | 21.88 | 2.97 |
| >90 | 83.95 | 28.59 | 69.76 | 37.36 | 51.90 | 8.76 | 51.04 | 16.83 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamid, S.A.; Alfonso, P.; Anticoi, H.; Guasch, E.; Oliva, J.; Dosbaba, M.; Garcia-Valles, M.; Chugunova, M. Quantitative Mineralogical Comparison between HPGR and Ball Mill Products of a Sn-Ta Ore. Minerals 2018, 8, 151. https://doi.org/10.3390/min8040151
Hamid SA, Alfonso P, Anticoi H, Guasch E, Oliva J, Dosbaba M, Garcia-Valles M, Chugunova M. Quantitative Mineralogical Comparison between HPGR and Ball Mill Products of a Sn-Ta Ore. Minerals. 2018; 8(4):151. https://doi.org/10.3390/min8040151
Chicago/Turabian StyleHamid, Sarbast Ahmad, Pura Alfonso, Hernan Anticoi, Eduard Guasch, Josep Oliva, Marek Dosbaba, Maite Garcia-Valles, and Marina Chugunova. 2018. "Quantitative Mineralogical Comparison between HPGR and Ball Mill Products of a Sn-Ta Ore" Minerals 8, no. 4: 151. https://doi.org/10.3390/min8040151
APA StyleHamid, S. A., Alfonso, P., Anticoi, H., Guasch, E., Oliva, J., Dosbaba, M., Garcia-Valles, M., & Chugunova, M. (2018). Quantitative Mineralogical Comparison between HPGR and Ball Mill Products of a Sn-Ta Ore. Minerals, 8(4), 151. https://doi.org/10.3390/min8040151









