The Influencing Mechanisms of Sodium Hexametaphosphate on Chalcopyrite Flotation in the Presence of MgCl2 and CaCl2
Abstract
:1. Introduction
2. Materials and Methods
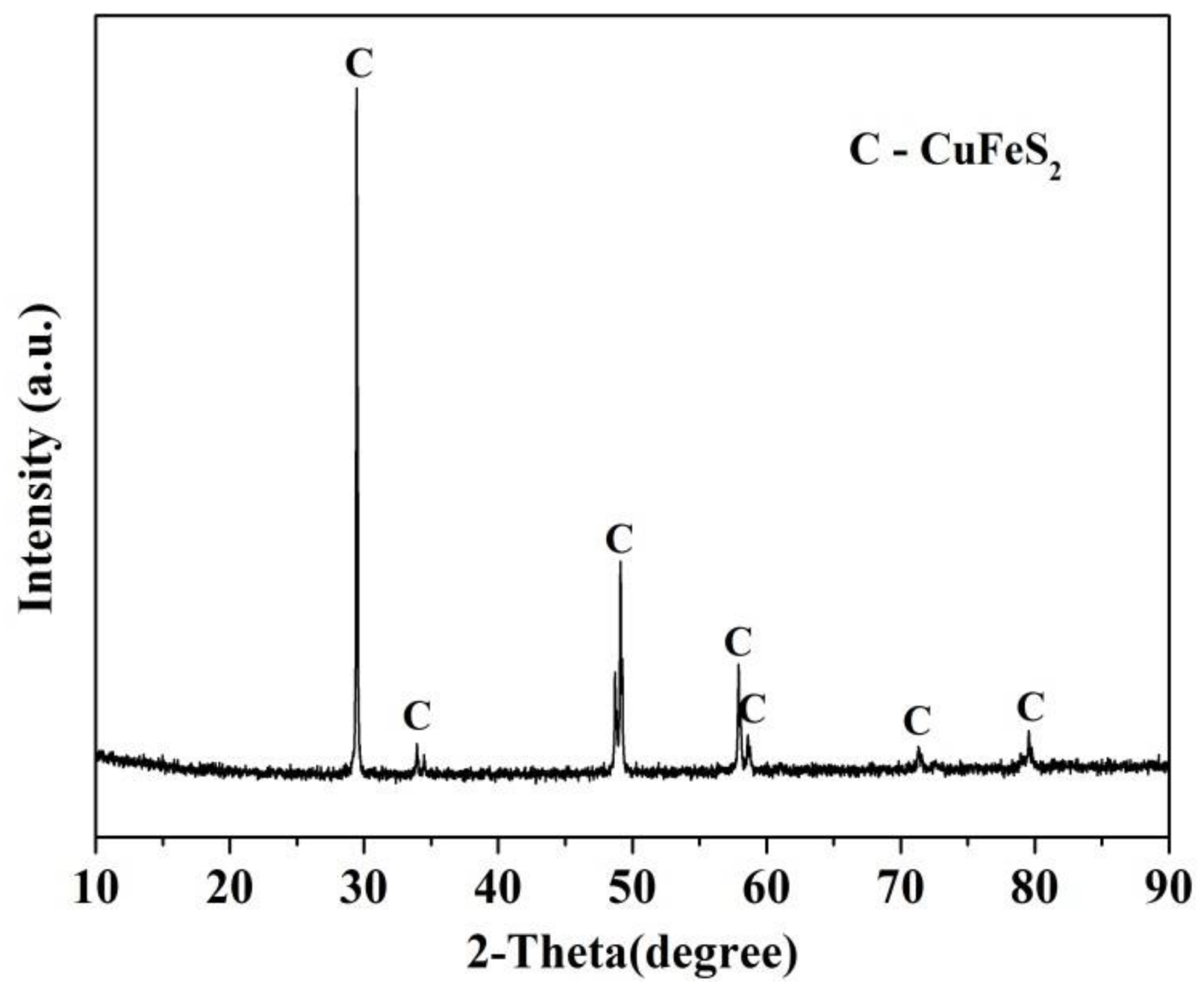
2.1. Samples and Reagents
2.2. Flotation Experiments
2.3. Contact Angle Measurements
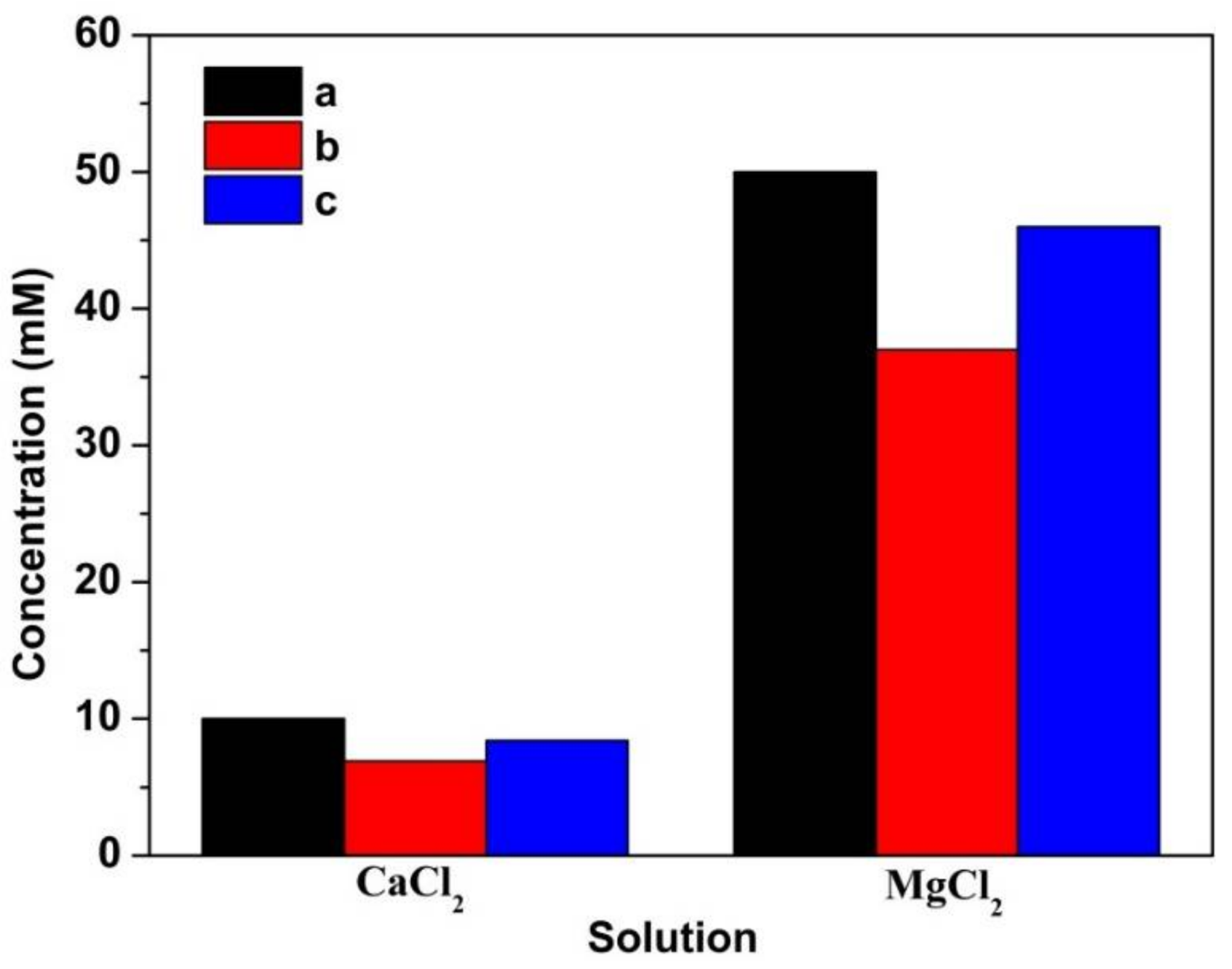
2.4. Ion Concentration Analyses
2.5. Zeta Potential Measurements
2.6. FTIR Spectra
2.7. XPS Measurements
3. Results and discussion
3.1. Flotation Results
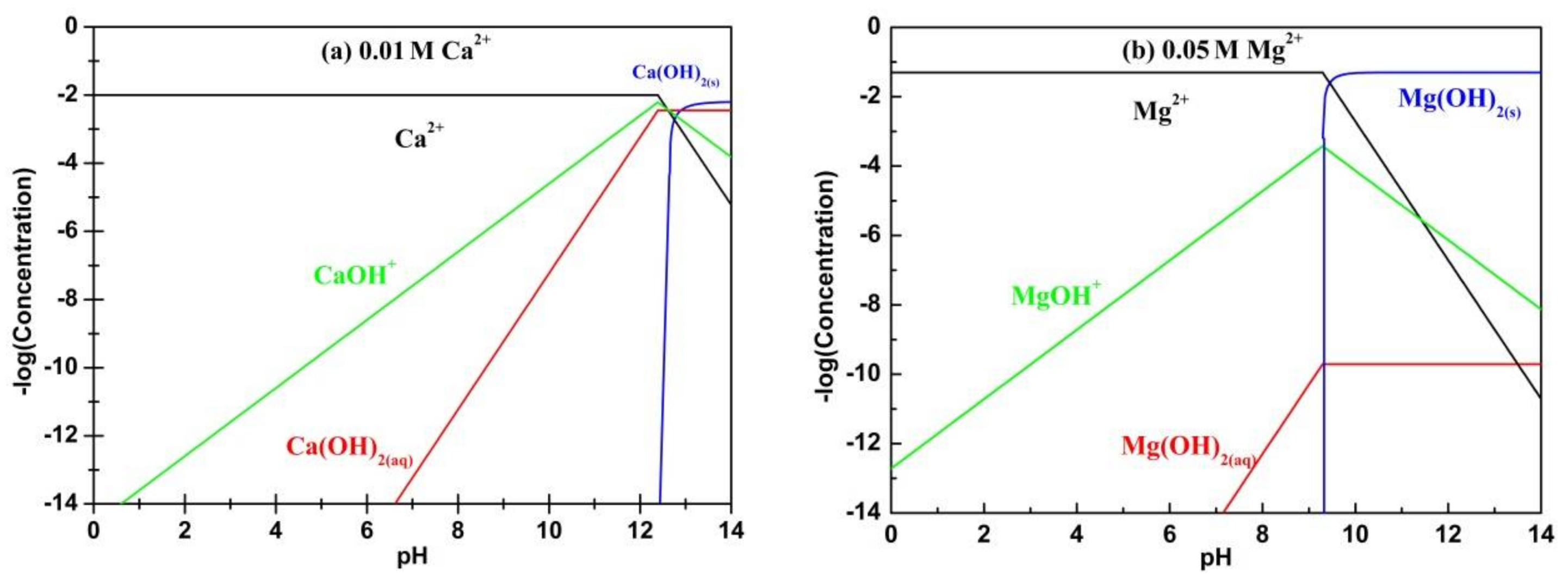
3.2. Species Calculation
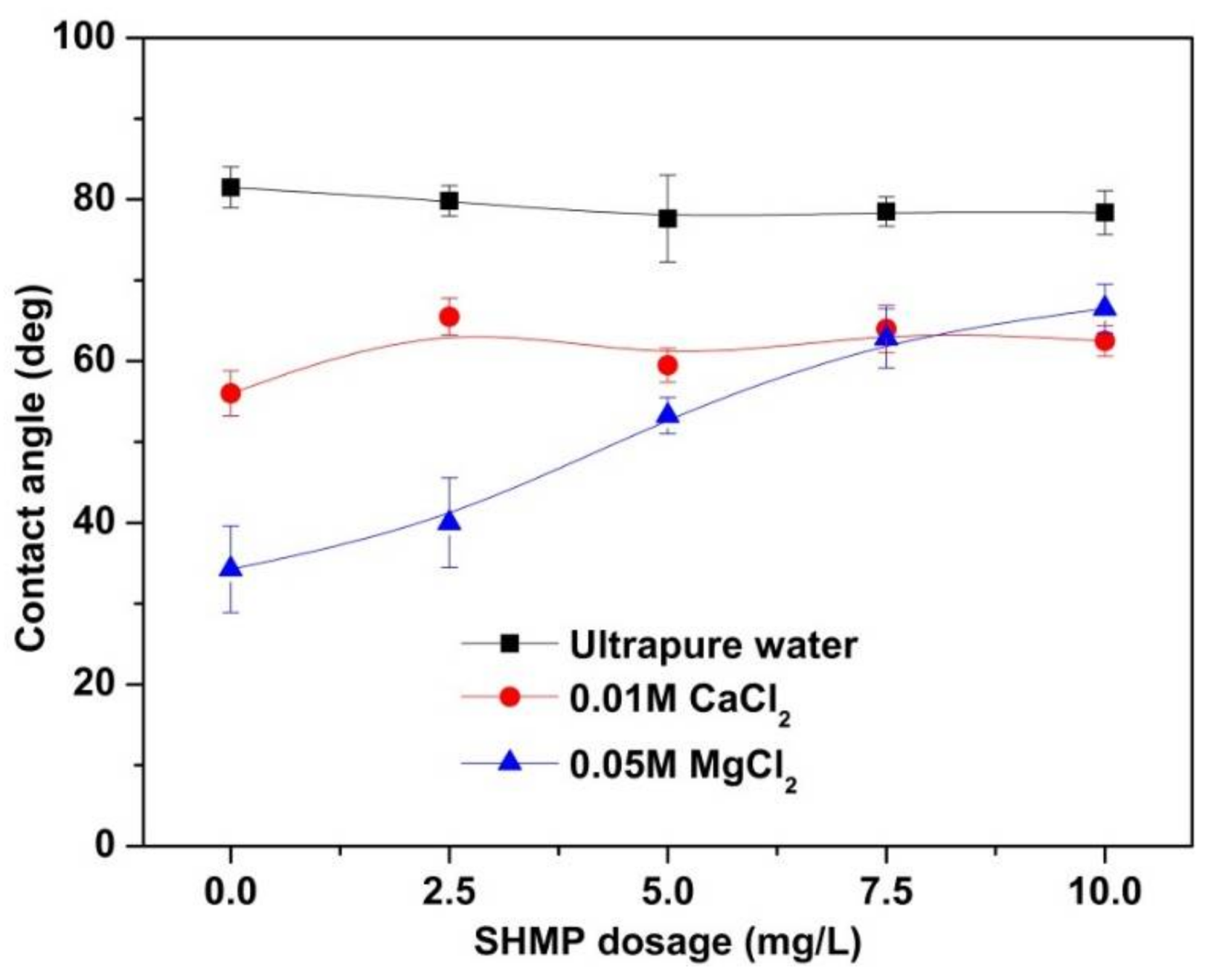
3.3. Contact Angle Analyses
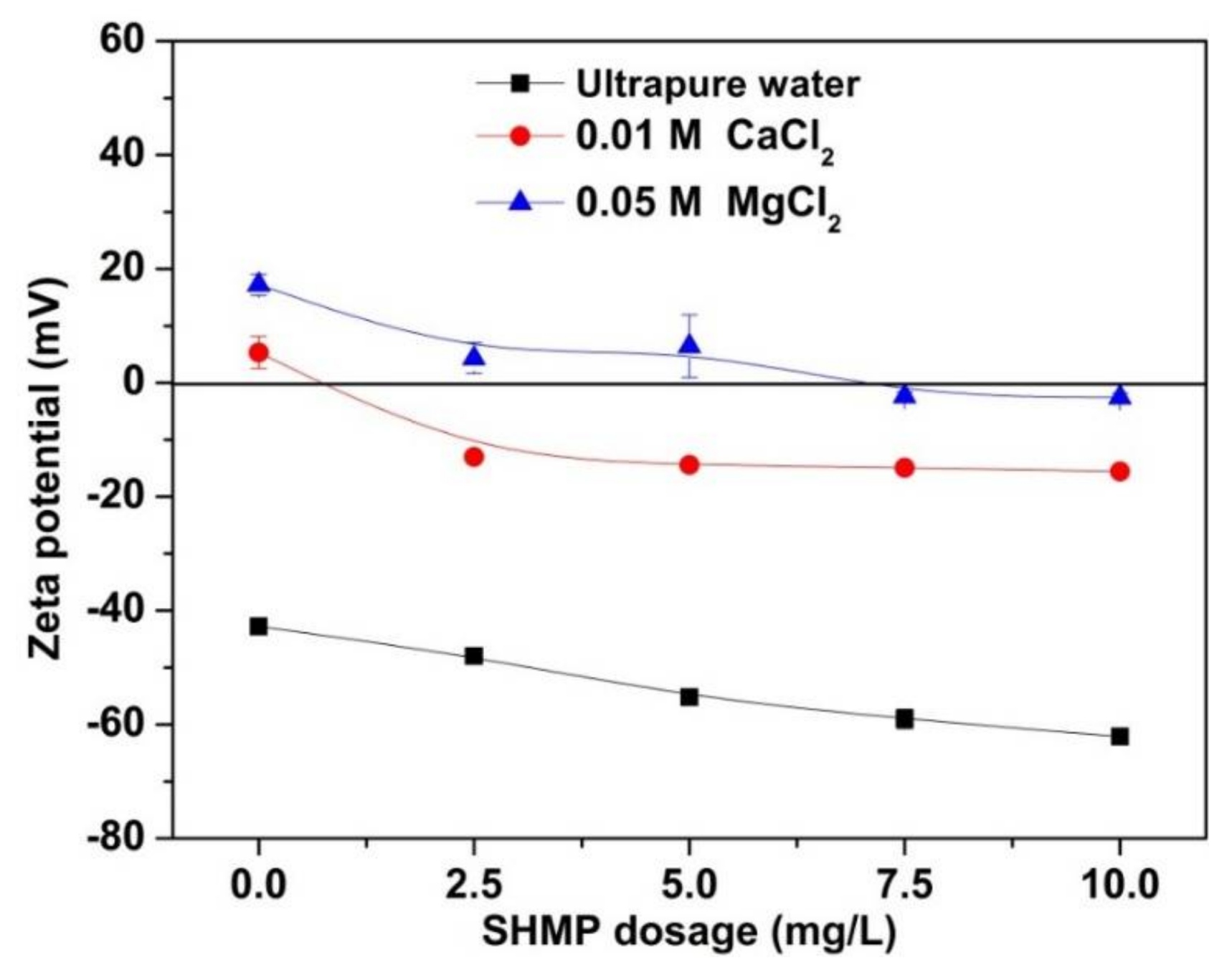
3.4. Zeta Potential Analyses
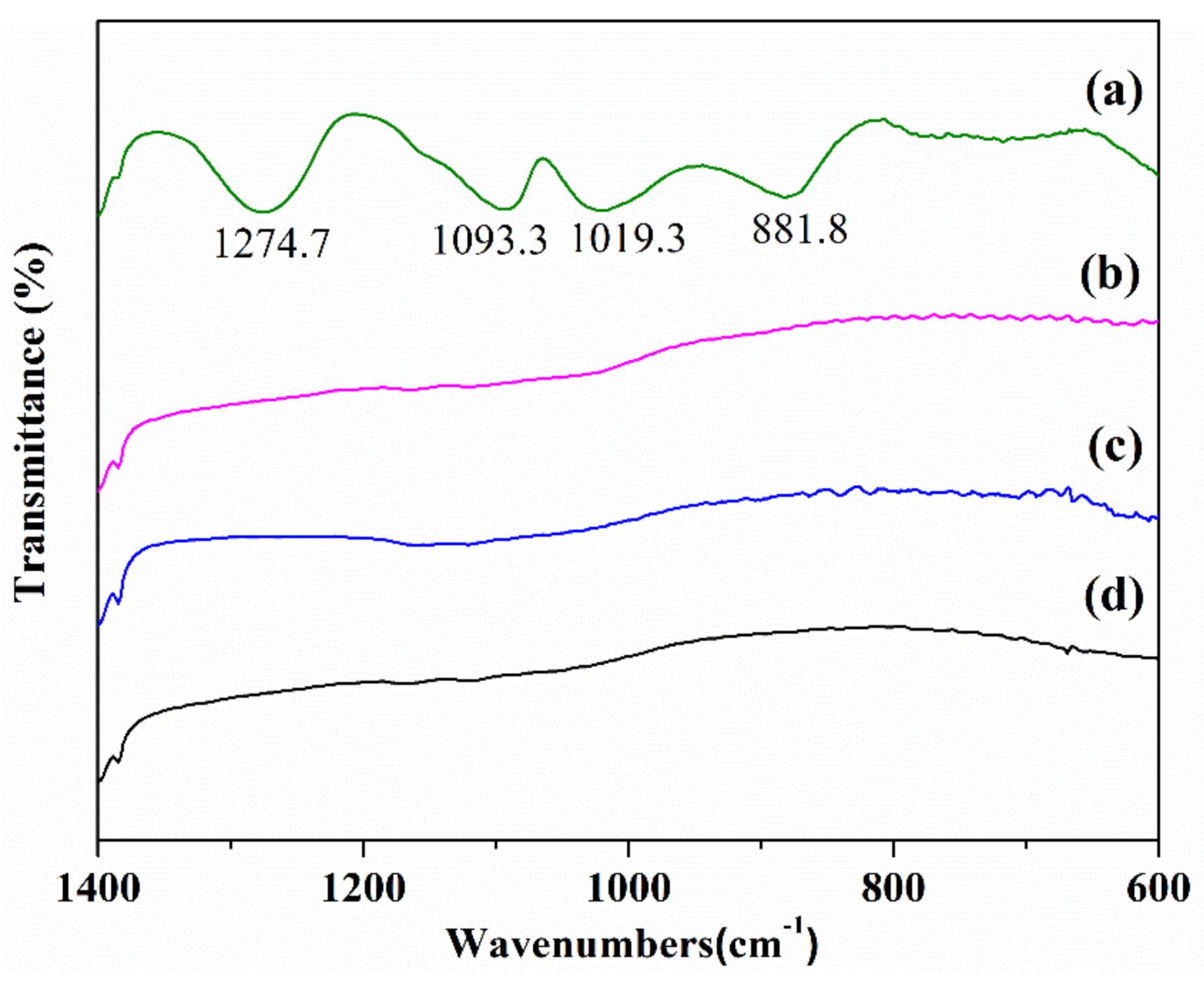
3.5. FTIR Analyses
3.6. XPS Analyses
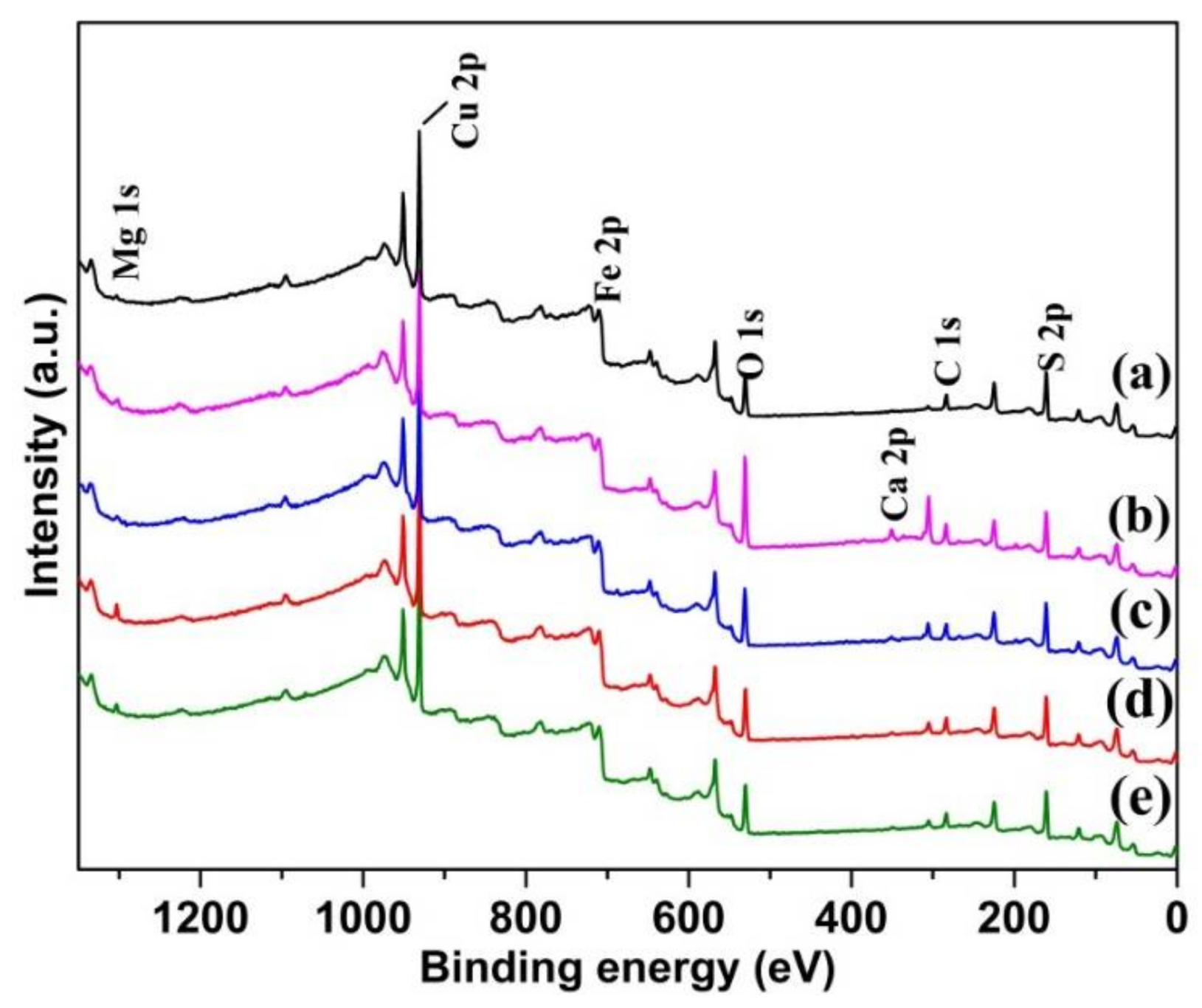
3.6.1. Survey Spectra
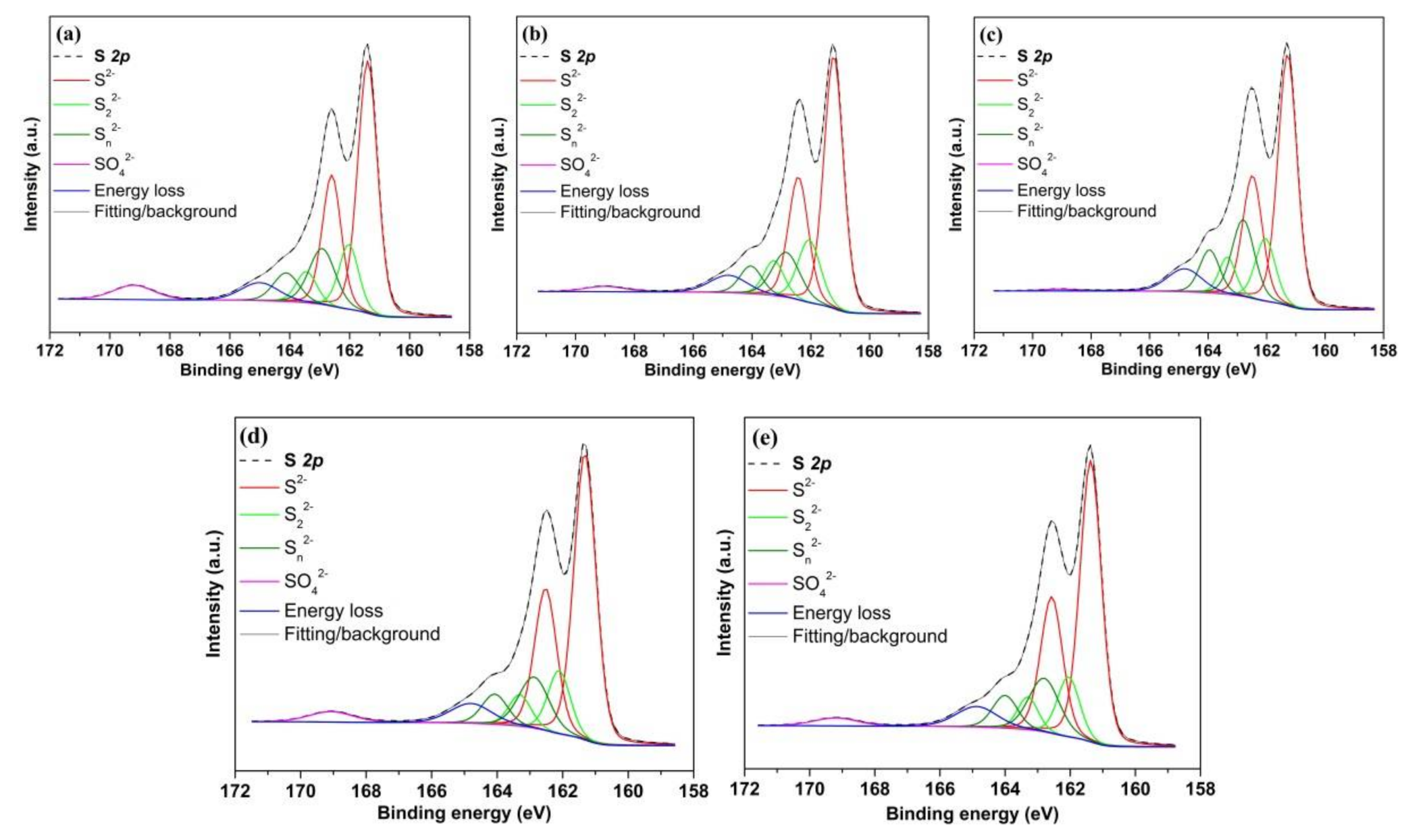
3.6.2. S 2p Spectra
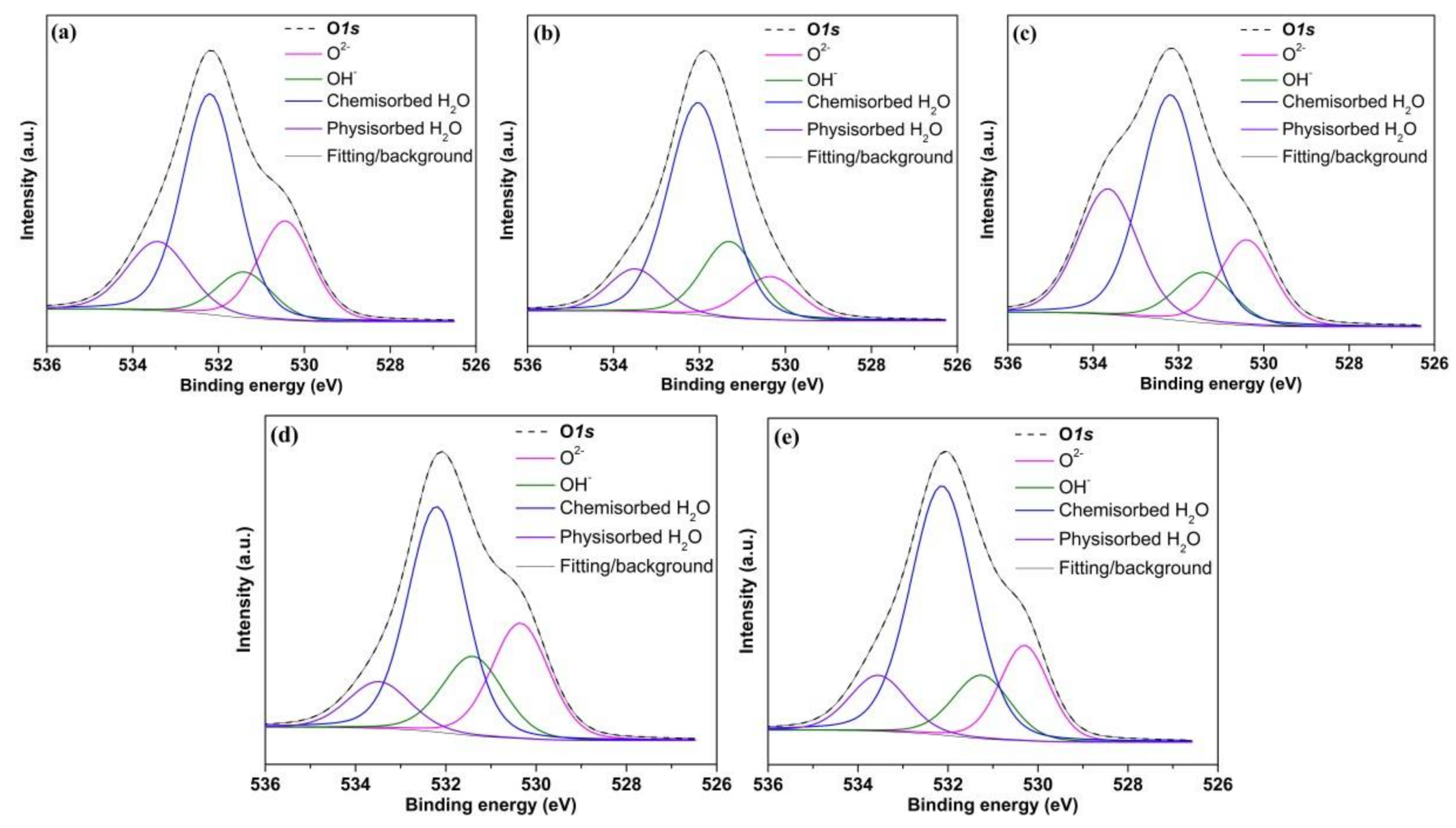
3.6.3. O 1s Spectra
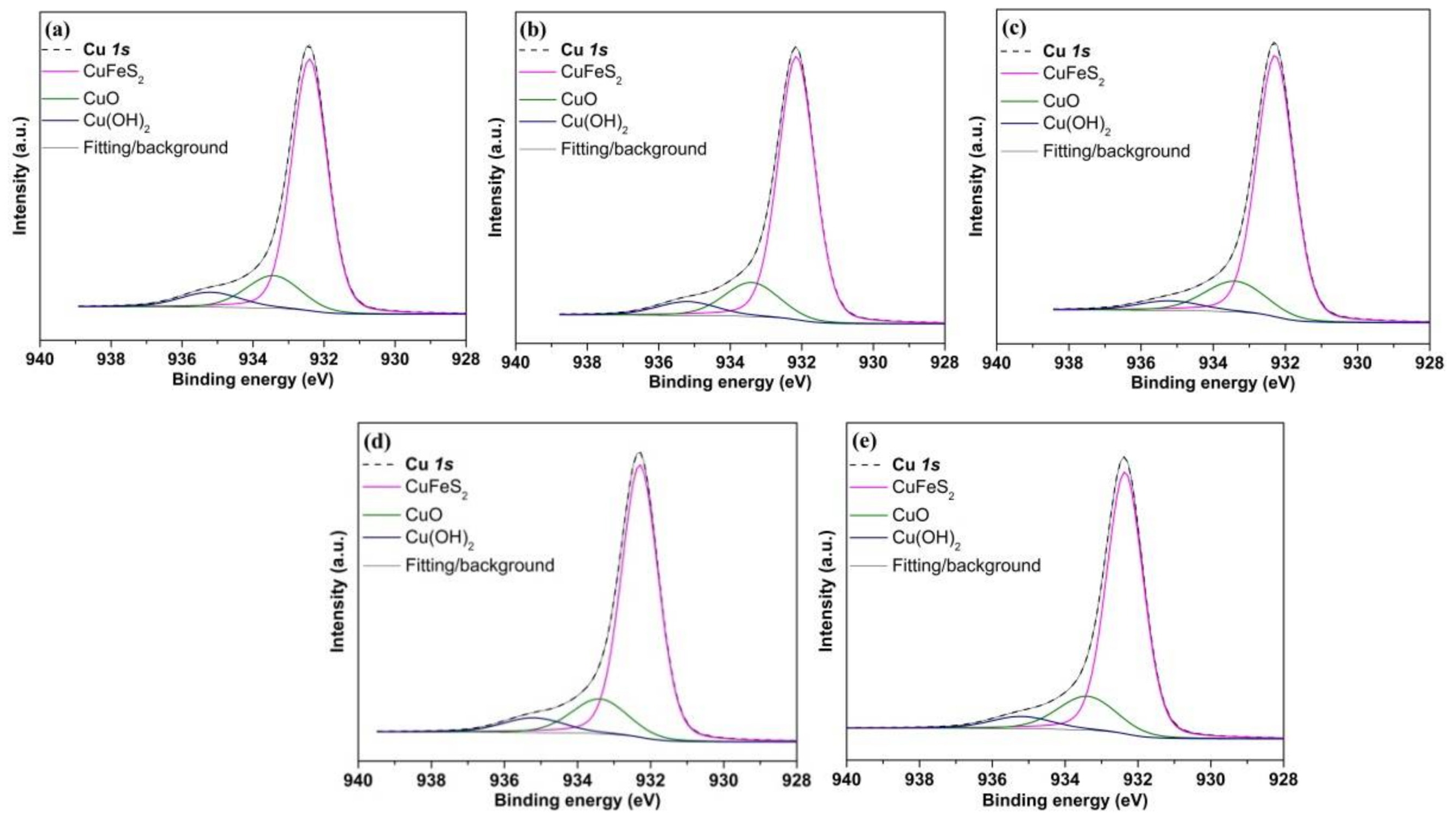
3.6.4. Cu 2p Spectra
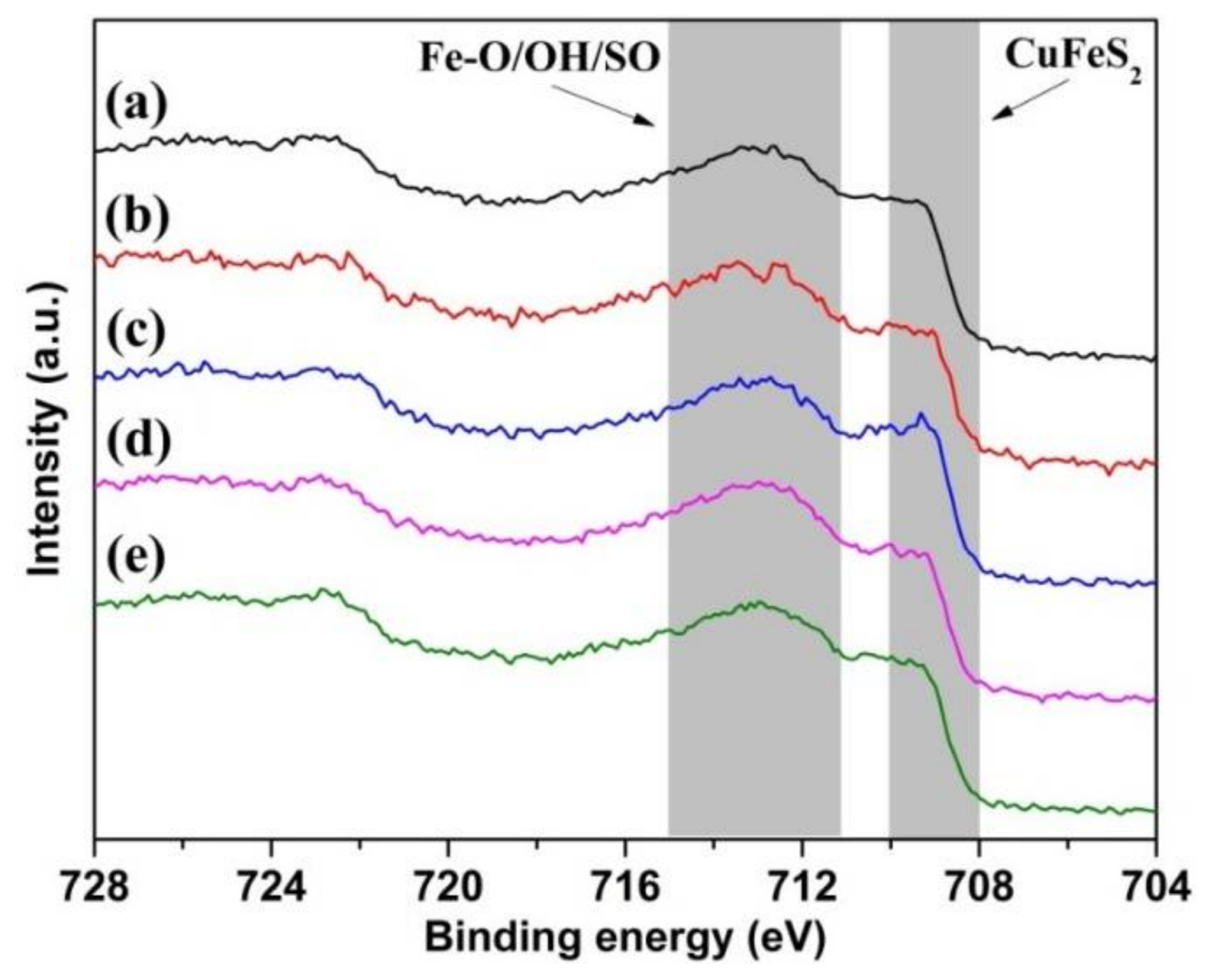
3.6.5. Fe 2p Spectra
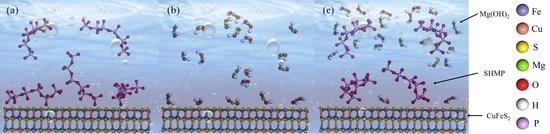
3.7. Mechanisms
4. Conclusions
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Farrokhpay, S. The significance of froth stability in mineral flotation—A review. Adv. Colloid Interface Sci. 2011, 166, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Peng, Y. The effect of saline water on mineral flotation—A critical review. Miner. Eng. 2014, 66–68, 13–24. [Google Scholar] [CrossRef]
- Liu, W.; Moran, C.; Vink, S. A review of the effect of water quality on flotation. Miner. Eng. 2013, 53, 91–100. [Google Scholar] [CrossRef]
- Castro, S.; Laskowski, J.S. Froth flotation in saline water. Kona Powder Part. J. 2011, 29, 4–15. [Google Scholar] [CrossRef]
- Greenlee, L.; Lawler, D.; Freeman, B.; Marrot, B.; Moulin, P. Reverse osmosis desalination: Water sources, technology, and today’s challenges. Water Res. 2009, 43, 2317–2348. [Google Scholar] [CrossRef] [PubMed]
- Moreno, P.A.; Aral, H.; Cuevas, J.; Monardes, A.; Adaro, M.; Norgate, T.; Bruckard, W. The use of seawater as process water at Las Luces copper–molybdenum beneficiation plant in Taltal (Chile). Miner. Eng. 2011, 24, 852–858. [Google Scholar] [CrossRef]
- Castro, S. Challenges in flotation of Cu–Mo sulfide ores in sea water. In The First International Symposium on Water in Mineral Processing; Drelich, J., Ed.; Society for Mining, Metallurgy, and Exploration: Seattle, WA, USA, 2012. [Google Scholar]
- Bıçak, Ö.; Ekmekçi, Z.; Can, M.; Öztürk, Y. The effect of water chemistry on froth stability and surface chemistry of the flotation of a Cu–Zn sulfide ore. Int. J. Miner. Process. 2012, 102, 32–37. [Google Scholar] [CrossRef]
- Peng, Y.; Seaman, D. The flotation of slime–fine fractions of Mt. Keith pentlandite ore in de-ionised and saline water. Miner. Eng. 2011, 5, 479–481. [Google Scholar] [CrossRef]
- Quinn, J.J.; Kracht, W.; Gomez, C.O.; Gagnon, C.; Finch, J.A. Comparing the effect of salts and frother (MIBC) on gas dispersion and froth properties. Miner. Eng. 2007, 20, 1296–1302. [Google Scholar] [CrossRef]
- Cao, Q.; Wang, X.; Miller, J.D.; Cheng, F.; Jiao, Y. Bubble attachment time and FTIR analysis of water structure in the flotation of sylvite, bischofite and carnallite. Miner. Eng. 2011, 24, 108–114. [Google Scholar] [CrossRef]
- Castro, S.; Miranda, C.; Toledo, P.; Laskowski, J.S. Effect of frothers on bubble coalescence and foaming in electrolyte solutions and seawater. Int. J. Miner. Process. 2013, 124, 8–14. [Google Scholar] [CrossRef]
- Corin, K.C.; Reddy, A.; Miyen, L.; Wiese, J.G.; Harris, P.J. The effect of ionic strength of plant water on valuable mineral and gangue recovery in a platinum bearing ore from the Merensky reef. Miner. Eng. 2011, 24, 131–137. [Google Scholar] [CrossRef]
- Choi, J.; Choi, S.Q.; Park, K.; Han, Y.; Kim, H. Flotation behaviour of malachite in mono- and di-valent salt solutions using sodium oleate as a collector. Int. J. Miner. Process. 2016, 146, 38–45. [Google Scholar] [CrossRef]
- Jeldres, R.I.; Forbes, L.; Cisternas, L.A. Effect of seawater on sulfide ore flotation: A review. Miner. Process. Extr. Metall. Rev. 2016, 37, 369–384. [Google Scholar] [CrossRef]
- Li, Y.; Li, W.; Xiao, Q.; He, N.; Ren, Z.; Lartey, C.; Gerson, A. The influence of common monovalent and divalent chlorides on chalcopyrite flotation. Minerals 2017, 7, 111. [Google Scholar] [CrossRef]
- Hirajima, T.; Suyantara, G.P.W.; Ichikawa, O.; Elmahdy, A.M.; Miki, H.; Sasaki, K. Effect of Mg2+ and Ca2+ as divalent seawater cations on the floatability of molybdenite and chalcopyrite. Miner. Eng. 2016, 96–97, 83–93. [Google Scholar] [CrossRef]
- Laskowski, J.S.; Castro, S. Hydrolyzing Ions in Flotation Circuits: Sea Water Flotation. In Proceedings of the 13th International Mineral Processing Symposium, Bodrum, Turkey, 10–12 October 2012; pp. 219–228. [Google Scholar]
- Suyantara, G.P.W.; Hirajima, T.; Elmahdy, A.M.; Miki, H.; Sasaki, K. Effect of kerosene emulsion in MgCl2 solution on the kinetics of bubble interactions with molybdenite and chalcopyrite. Colloids Surf. A Physicochem. Eng. Asp. 2016, 501, 98–113. [Google Scholar] [CrossRef]
- Li, Y.; Kawashima, N.; Li, J.; Chandra, A.P.; Gerson, A.R. A review of the structure, and fundamental mechanisms and kinetics of the leaching of chalcopyrite. Adv. Colloid Interface Sci. 2013, 197–198, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Haga, K.; Nishioka, K.; Altansukh, B.; Shibayama, A. Floatability and bubble behavior in seawater flotation for the recovering copper mineral. Int. J. Soc. Mater. Eng. Resour. 2014, 20, 82–86. [Google Scholar] [CrossRef]
- Wang, Y.; Peng, Y.; Nicholson, T.; Lauten, R.A. The role of cations in copper flotation in the presence of bentonite. Miner. Eng. 2016, 96–97, 108–112. [Google Scholar] [CrossRef]
- Nagaraj, D.R.; Farinato, R. Chemical factor effects in saline and hypersaline waters in the flotation of Cu and Cu-Mo ores. In Proceedings of the XXVII International Mineral Processing Congress, Santiago, Chile, 20–24 October 2014. [Google Scholar]
- Jeldres, R.I.; Arancibia-Bravo, M.P.; Reyes, A.; Aguirre, C.E.; Cortes, L.; Cisternas, L.A. The impact of seawater with calcium and magnesium removal for the flotation of copper-molybdenum sulphide ores. Miner. Eng. 2017, 109, 10–13. [Google Scholar] [CrossRef]
- Qiu, Z.; Liu, G.; Liu, Q.; Zhong, H.; Zhang, M. Separation of pyrite from chalcopyrite and molybdenite by using selective collector of N-isopropoxypropyl-N′-ethoxycarbonyl thiourea in high salinity water. Miner. Eng. 2017, 100, 93–98. [Google Scholar] [CrossRef]
- Rebolledo, E.; Laskowski, J.S.; Gutierrez, L.; Castro, S. Use of dispersants in flotation of molybdenite in seawater. Miner. Eng. 2017, 100, 71–74. [Google Scholar] [CrossRef]
- Wan, H.; Yang, W.; He, T.; Yang, J.; Guo, L.; Peng, Y. The influence of Ca2+ and pH on the interaction between PAHs and molybdenite edges. Minerals 2017, 7, 104. [Google Scholar] [CrossRef]
- Suyantara, G.P.W.; Hirajima, T.; Miki, H.; Sasaki, K. Floatability of molybdenite and chalcopyrite in artificial seawater. Miner. Eng. 2018, 115, 117–130. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, X.; Hu, Y.; Lan, Y. Influences of phosphates on dispersion of fine alumin-silicate minerals. Cent. South Univ. Sci. Technol. 2007, 38, 238–244. [Google Scholar]
- Xu, D.; Zhu, S.; Cao, G.; Cu, H. Influences of sodium hexametaphosphate on dispersion of fine montmorillonite in coal flotation. J. China Coal Soc. 2016, 41, 192–198. [Google Scholar]
- Feng, B.; Wang, P.; Lu, Y.; Feng, Q. Role of sodium hexametaphosphate in flotation of a nickel ore. Physicochem. Probl. Miner. Process. 2015, 51, 170–181. [Google Scholar]
- Feng, Q.; Zhou, Q.; Zhang, G.; Lu, Y.; Yang, S. Inhibition mechanism of sodium hexametaphosphate on calcite. Chin. J. Nonferr. Met. 2011, 21, 436–441. [Google Scholar]
- Long, T.; Feng, Q.; Lu, Y. Dispersive mechanism of sodium hexametaphosphate on flotation of copper-nickel sulphide. Chin. J. Nonferr. Met. 2012, 22, 1763–1769. [Google Scholar]
- Lu, Y.; Zhang, M.; Feng, Q.; Long, T.; Ou, L.; Zhang, G. Effect of sodium hexametaphosphate on separation of serpentine from pyrite. Trans. Nonferr. Met. Soc. China 2011, 21, 208–213. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, J.; Liu, H.; Wang, Y. Effects of water hardness on the dispersion of fine coal and montmorillonite. J. China Univ. Min. Technol. 2009, 38, 114–118. [Google Scholar]
- Luo, N.; Wei, D.; Shen, Y.; Han, C.; Zhang, C. Elimination of the adverse effect of calcium ion on the flotation separation of magnesite from dolomite. Minerals 2017, 7, 150. [Google Scholar] [CrossRef]
- Zhang, G.; zhu, Y.; Cui, M. Effect of sodium hexametaphosphate on flotation separation of rhodochrosite from calcite. Chin. J. Nonferr. Met. 2012, 22, 3214–3220. [Google Scholar]
- Ghahremaninezhad, A.; Dixon, D.G.; Asselin, E. Electrochemical and XPS analysis of chalcopyrite (CuFeS2) dissolution in sulfuric acid solution. Electrochim. Acta 2013, 87, 97–112. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, Y. Effect of calcium ions and citric acid on the flotation separation of chalcopyrite from galena using dextrin. Miner. Eng. 2000, 13, 1405–1416. [Google Scholar] [CrossRef]
- Ramos, O.; Castro, S.; Laskowski, J.S. Copper–molybdenum ores flotation in sea water: Floatability and frothability. Miner. Eng. 2013, 53, 108–112. [Google Scholar] [CrossRef]
- Ni, X.; Liu, Q. Adsorption behaviour of sodium hexametaphosphate on pyrochlore and calcite. Can. Metall. Q. 2013, 52, 473–478. [Google Scholar] [CrossRef]
- Ding, H.; Lin, H.; Deng, Y. Depressing effect of sodium hexametaphosphate on apatite in flotation of rutile. J. Univ. Sci. Technol. Beijing 2007, 14, 200–203. [Google Scholar] [CrossRef]
- Schott, H. Electrokinetic studies of magnesium hydroxide. J. Pharm. Sci. 1981, 70, 486–489. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Han, Y.; Li, Y.; Gao, P. Effect of serpentine and sodium hexametaphosphate on ascharite flotation. Trans. Nonferr. Met. Soc. China 2017, 27, 1841–1848. [Google Scholar] [CrossRef]
- Xia, Q.; Li, Z.; Qiu, X.; Dai, Z. Invesitgation of action mechanism between sodium hexametaphosphate and serpenine. Min. Metall. Eng. 2002, 22, 53–56. [Google Scholar]
- Li, Y.; Chandra, A.P.; Gerson, A.R. Scanning photoelectron microscopy studies of freshly fractured chalcopyrite exposed to O2 and H2O. Geochim. Cosmochim. Acta 2014, 133, 372–386. [Google Scholar] [CrossRef]
- Li, Y.; Qian, G.; Brown, P.L.; Gerson, A.R. Chalcopyrite dissolution: Scanning photoelectron microscopy examination of the evolution of sulfur species with and without added iron or pyrite. Geochim. Cosmochim. Acta 2017, 212, 33–47. [Google Scholar] [CrossRef]
- Li, Y.; Qian, G.; Li, J.; Gerson, A.R. Kinetics and roles of solution and surface species of chalcopyrite dissolution at 650 mV. Geochim. Cosmochim. Acta 2015, 161, 188–202. [Google Scholar] [CrossRef]
- Vizcarra, T.G.; Harmer, S.L.; Wightman, E.M.; Johnson, N.W.; Manlapig, E.V. The influence of particle shape properties and associated surface chemistry on the flotation kinetics of chalcopyrite. Miner. Eng. 2011, 24, 807–816. [Google Scholar] [CrossRef]
- Acres, R.G.; Harmer, S.L.; Beattie, D.A. Synchrotron XPS, NEXAFS, and ToF-SIMS studies of solution exposed chalcopyrite and heterogeneous chalcopyrite with pyrite. Miner. Eng. 2010, 23, 928–936. [Google Scholar] [CrossRef]
- Yin, Z.; Sun, W.; Hu, Y.; Zhang, C.; Guan, Q.; Liu, R.; Chen, P.; Tian, M. Utilization of acetic acid-[(hydrazinylthioxomethyl)thio]-sodium as a novel selective depressant for chalcopyrite in the flotation separation of molybdenite. Sep. Purif. Technol. 2017, 179, 248–256. [Google Scholar] [CrossRef]
- Nesbitt, H.W.; Muir, I.J. Oxidation states and speciation of secondary products on pyrite and arsenopyrite reacted with mine waste waters and air. Mineral. Petrol. 1998, 62, 123–144. [Google Scholar] [CrossRef]
- Fujisawa, M.; Suga, S.; Mizokawa, T.; Fujimori, A.; Sato, K. Electronic structures of CuFeS2 and CuAl0.9Fe0.1S2 studied by electron and optical spectroscopies. Phys. Rev. B 1994, 49, 7155–7164. [Google Scholar] [CrossRef]
- Guo, Y.; Cui, K.; Hu, M.; Jin, S. Fe(III) ions enhanced catalytic properties of (BiO)2CO3 nanowires and mechanism study for complete degradation of xanthate. Chemosphere 2017, 181, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Harmer, S.L.; Thomas, J.E.; Fornasiero, D.; Gerson, A.R. The evolution of surface layers formed during chalcopyrite leaching. Geochim. Cosmochim. Acta 2006, 70, 4392–4402. [Google Scholar] [CrossRef]
- Wang, J.; Xie, L.; Liu, Q.; Zeng, H. Effects of salinity on xanthate adsorption on sphalerite and bubble–sphalerite interactions. Miner. Eng. 2015, 77, 34–41. [Google Scholar] [CrossRef]
- Buckley, A.N.; Skinner, W.M.; Harmer, S.L.; Pring, A.; Lamb, R.N.; Fan, L.J.; Yang, Y.W. Examination of the proposition that Cu(II) can be required for charge neutrality in a sulfide lattice—Cu in tetrahedrites and sphalerite. Can. J. Chem. 2007, 85, 767–781. [Google Scholar] [CrossRef]
- Hirajima, T.; Miki, H.; Suyantara, G.P.W.; Matsuoka, H.; Elmahdy, A.M.; Sasaki, K.; Imaizumi, Y.; Kuroiwa, S. Selective flotation of chalcopyrite and molybdenite with H2O2 oxidation. Miner. Eng. 2017, 100, 83–92. [Google Scholar] [CrossRef]
- Kalegowda, Y.; Chan, Y.; Wei, D.; Harmer, S.L. X-PEEM, XPS and ToF-SIMS characterisation of xanthate induced chalcopyrite flotation: Effect of pulp potential. Surf. Sci. 2015, 635, 70–77. [Google Scholar] [CrossRef]
- Owusu, C.; Fornasiero, D.; Addai-Mensah, J.; Zanin, M. Influence of pulp aeration on the flotation of chalcopyrite with xanthate in chalcopyrite/pyrite mixtures. Int. J. Miner. Process. 2015, 134, 50–57. [Google Scholar] [CrossRef]
- Adamczyk, Z.; Weroński, P. Application of the DLVO theory for particle deposition problems. Adv. Colloid Interface Sci. 1999, 83, 137–226. [Google Scholar] [CrossRef]
- Missana, T.; Adell, A. On the applicability of DLVO theory to the prediction of clay colloids stability. J. Colloid Interface Sci. 2000, 230, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Shen, J.; Luo, S. Particle Dispersion Science and Technology; Chemical Industry Publisher: Beijing, China, 2005. [Google Scholar]
- Sharma, P.K.; Rao, K.H. Adhesion of paenibacillus polymyxa on chalcopyrite and pyrite: Surface thermodynamics and extended DLVO theory. Colloids Surf. B Biointerfaces 2003, 29, 21–38. [Google Scholar] [CrossRef]
- Van Oss, C.J.; Giese, R.F.; Costanzo, P.M. DLVO and non-DLVO interactions in hectorite. Clays Clay Miner. 1990, 38, 151–159. [Google Scholar] [CrossRef]
- Feng, B.; Lu, Y.; Feng, Q.; Li, H. Solution chemistry of sodium silicate and implications for pyrite flotation. Ind. Eng. Chem. Res. 2012, 51, 12089–12094. [Google Scholar] [CrossRef]



| Concentrator | Location | Processing Content | References |
|---|---|---|---|
| Batu Hijau | Indonesia | Using sea water to process a gold-rich porphyry copper ore | [7] |
| Las luses | Chile | Grinding and flotation using mixed sea water and tailing dam water | [6] |
| Çayeli Bakır Is letmeleri A.S. | Turkey | Processing Cu–Zn sulfide ore using dissolved metal ions and sulfide ions (SO42− and S2O32−) | [8] |
| Mt Keith | Australia | Processing nickel minerals using bore water with high ionic strength | [9] |
| Raglan | Canada | Flotation without frother using saline water with salt levels ranging 20,000~35,000 ppm | [10] |
| Element | BE (eV) | Conditions | ||||
|---|---|---|---|---|---|---|
| Untreated | CaCl2 | CaCl2 + SHMP | MgCl2 | MgCl2 + SHMP | ||
| C 1s | 284.8 | 18 | 20 | 18 | 17 | 18 |
| S 2p | 161.5 | 27 | 22 | 26 | 25 | 26 |
| O 1s | 532.1 | 19 | 29 | 23 | 23 | 21 |
| Fe 2p | 710.8 | 16 | 12 | 15 | 15 | 15 |
| Cu 2p | 932.6 | 19 | 14 | 17 | 17 | 18 |
| Ca 2p | 352.0 | 0 | 2 | 0 | 0 | 0 |
| Mg 1s | 1304.7 | 1 | 1 | 1 | 3 | 2 |
| Species | BE (eV) | FWHM (eV) | Conditions | ||||
|---|---|---|---|---|---|---|---|
| Untreated | CaCl2 | CaCl2 + SHMP | MgCl2 | MgCl2 + SHMP | |||
| S2− | 161.3 | 0.7–0.8 | 58 | 61 | 59 | 62 | 61 |
| S22− | 162.0 | 0.7–0.9 | 15 | 17 | 15 | 14 | 14 |
| Sn2− | 162.8 | 0.8–1.0 | 17 | 15 | 20 | 15 | 17 |
| SO42− | 169.1 | 1.5–1.6 | 5 | 2 | 0 | 3 | 2 |
| Energy loss | 164.9 | 1.4–1.6 | 5 | 5 | 6 | 6 | 6 |
| Species | BE (eV) | FWHM (eV) | Conditions | ||||
|---|---|---|---|---|---|---|---|
| Untreated | CaCl2 | CaCl2 + SHMP | MgCl2 | MgCl2 + SHMP | |||
| O2− | 530.4 | 1.4–1.5 | 27 | 15 | 18 | 24 | 18 |
| OH−/SO42− | 531.3 | 1.5–1.6 | 10 | 16 | 11 | 20 | 13 |
| Chemisorbed H2O | 532.1 | 1.5–1.7 | 47 | 58 | 47 | 45 | 57 |
| Physisorbed H2O | 533.5 | 1.5–1.7 | 16 | 11 | 24 | 11 | 12 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Li, Y.; Xiao, Q.; Wei, Z.; Song, S. The Influencing Mechanisms of Sodium Hexametaphosphate on Chalcopyrite Flotation in the Presence of MgCl2 and CaCl2. Minerals 2018, 8, 150. https://doi.org/10.3390/min8040150
Li W, Li Y, Xiao Q, Wei Z, Song S. The Influencing Mechanisms of Sodium Hexametaphosphate on Chalcopyrite Flotation in the Presence of MgCl2 and CaCl2. Minerals. 2018; 8(4):150. https://doi.org/10.3390/min8040150
Chicago/Turabian StyleLi, Wanqing, Yubiao Li, Qing Xiao, Zhenlun Wei, and Shaoxian Song. 2018. "The Influencing Mechanisms of Sodium Hexametaphosphate on Chalcopyrite Flotation in the Presence of MgCl2 and CaCl2" Minerals 8, no. 4: 150. https://doi.org/10.3390/min8040150
APA StyleLi, W., Li, Y., Xiao, Q., Wei, Z., & Song, S. (2018). The Influencing Mechanisms of Sodium Hexametaphosphate on Chalcopyrite Flotation in the Presence of MgCl2 and CaCl2. Minerals, 8(4), 150. https://doi.org/10.3390/min8040150