Integrated Mineral Carbonation of Ultramafic Mine Deposits—A Review
Abstract
1. Introduction
2. Mineral Carbonation
2.1. Process Routes
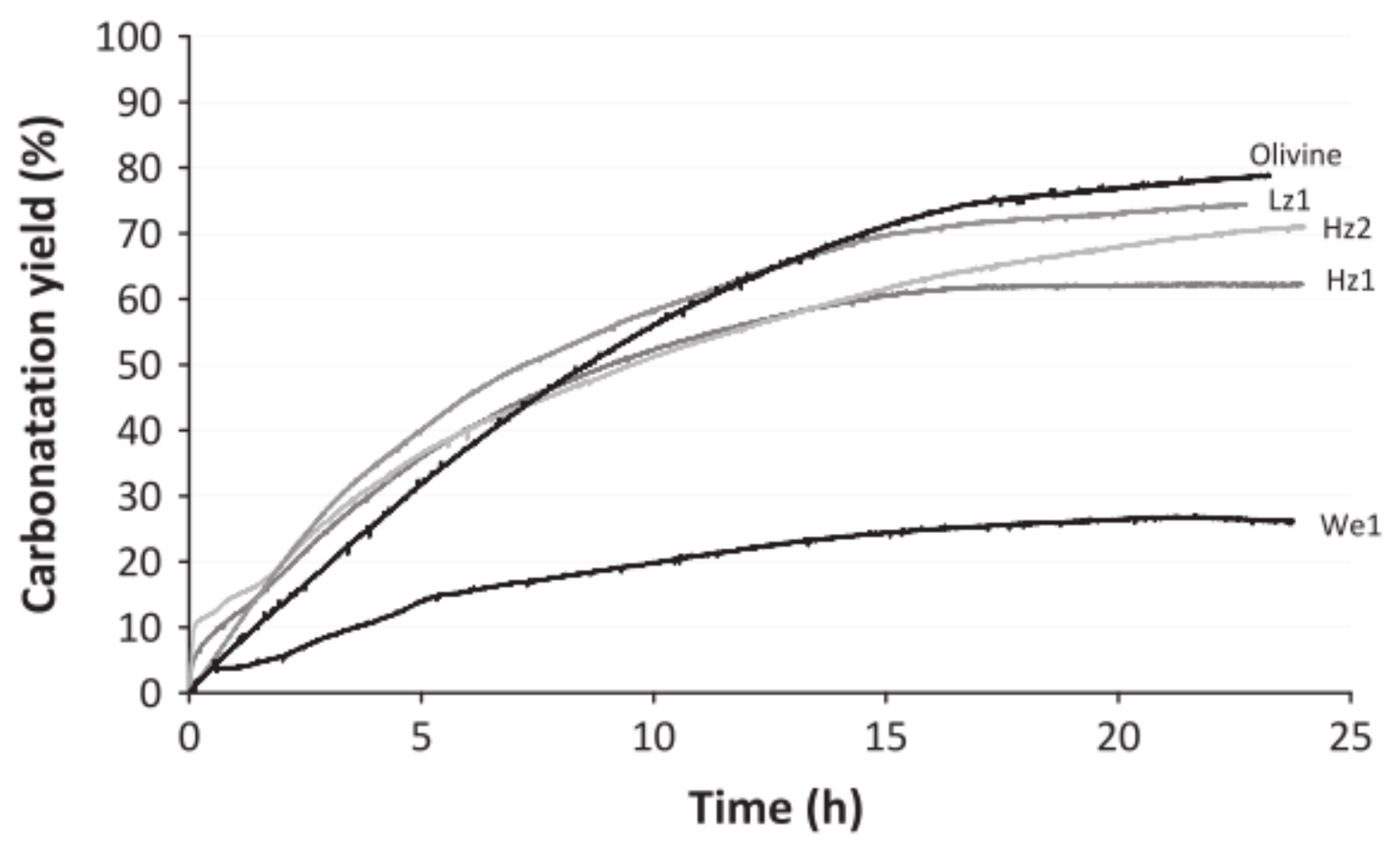
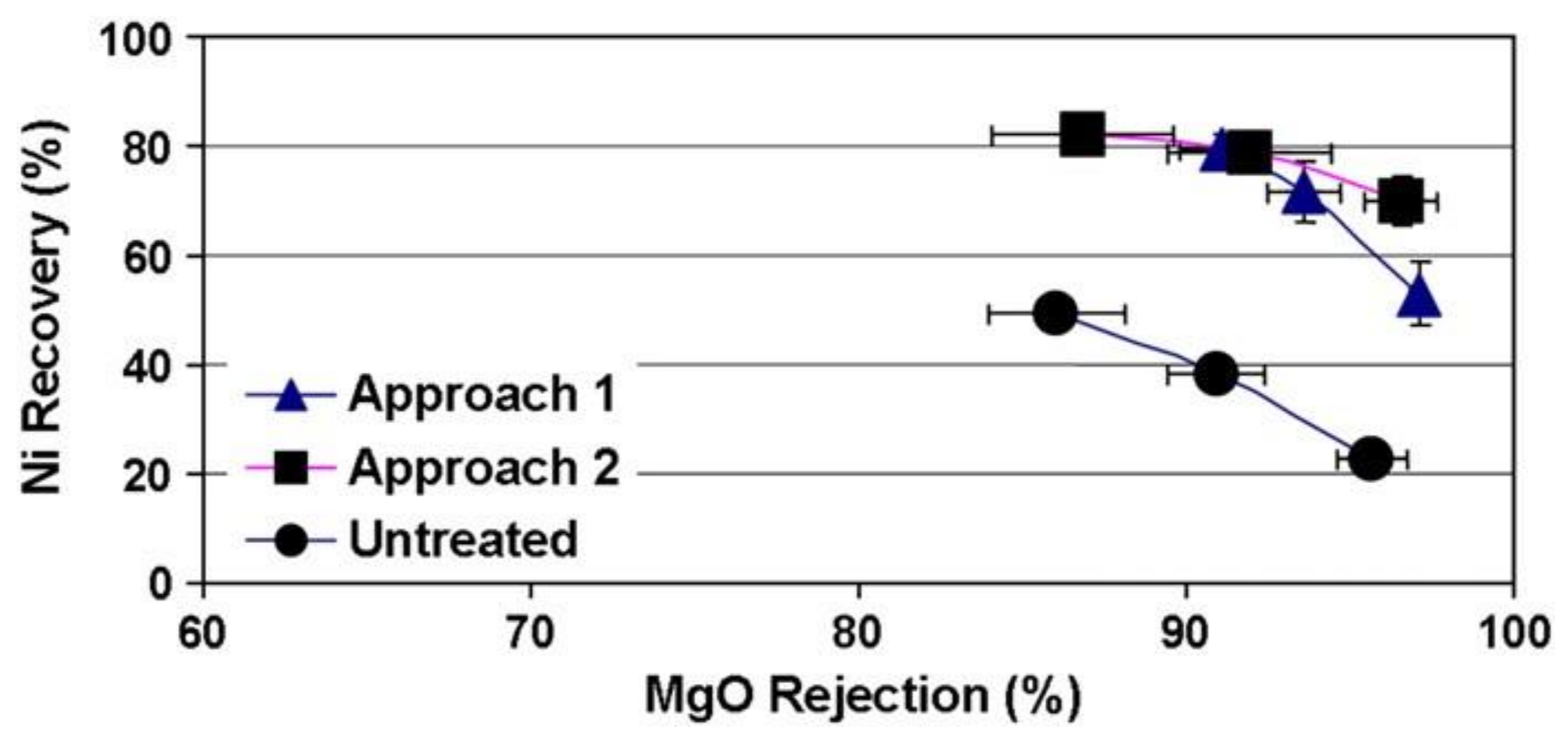
2.2. Pre-Treatment
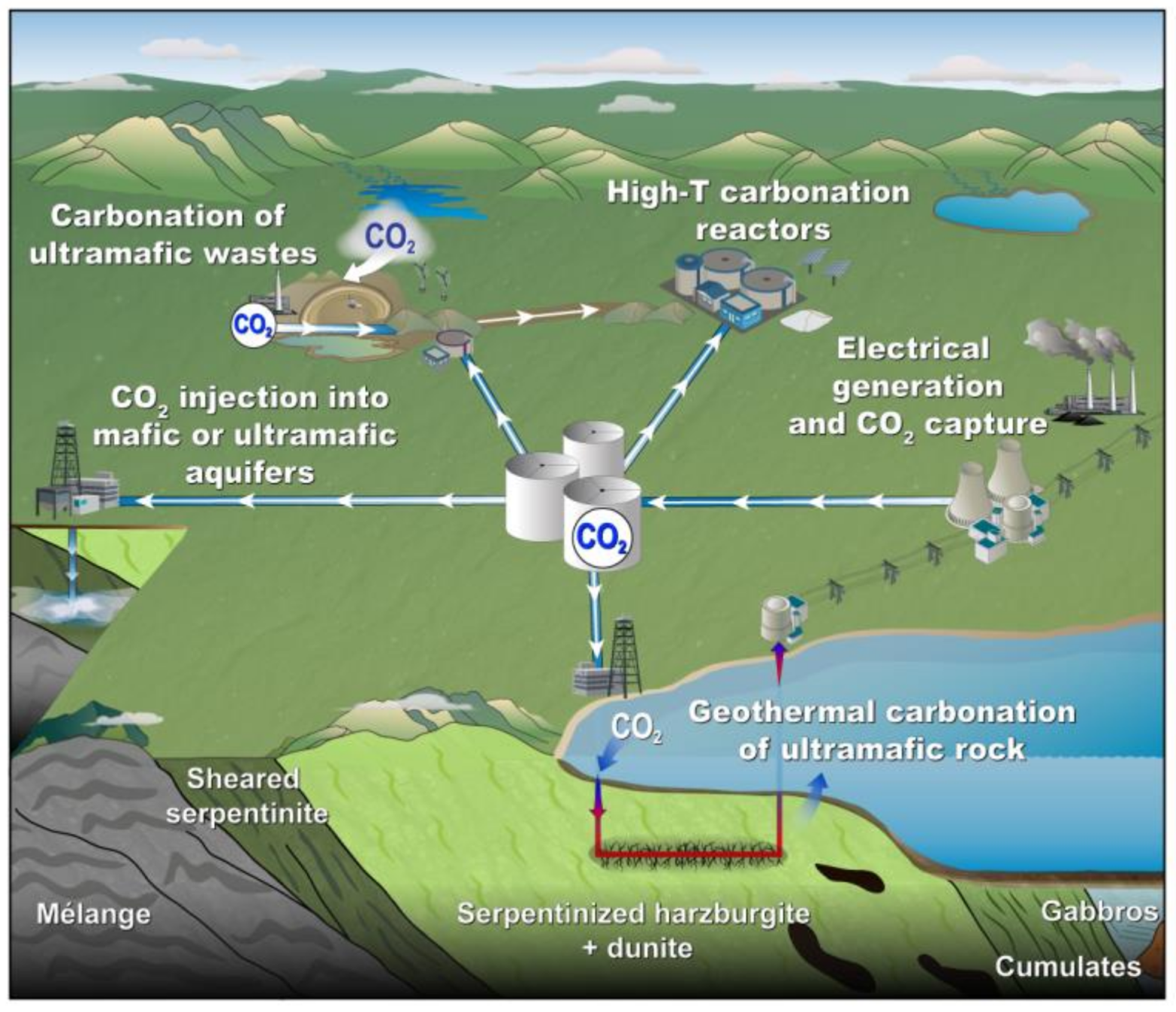
3. Integrated Mineral Carbonation in Mining
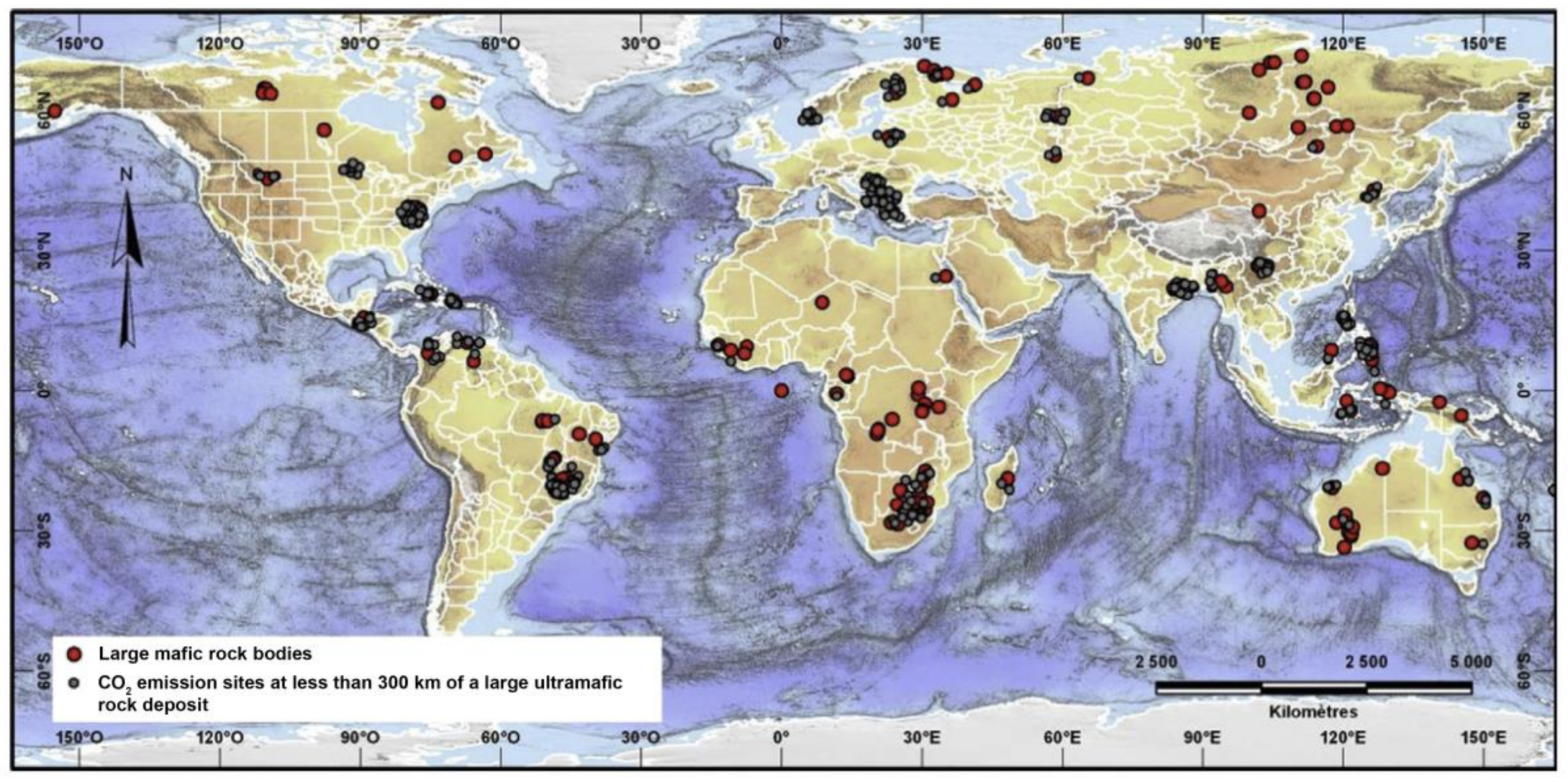
3.1. Suitable Mine for Mineral Carbonation
3.2. Modified Passive Carbonation Method
3.3. Ex-Situ Mineral Carbonation
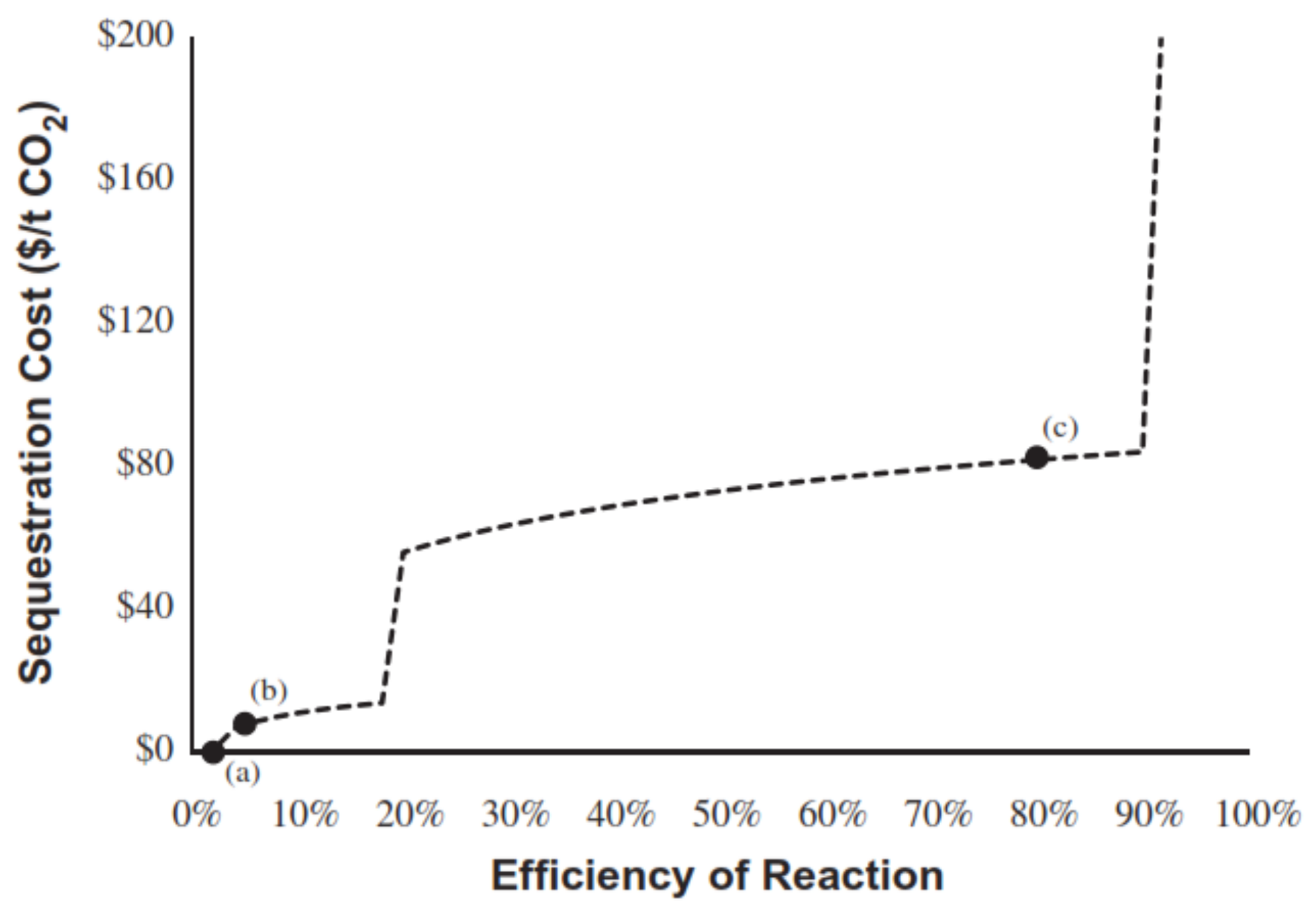
4. Techno-Economic Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- IPCC (Intergovernmental Panel on Climate Change). Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Pachauri, R.K., Meyer, L.A., Eds.; IPCC: Geneva, Switzerland, 2014. [Google Scholar]
- Hänchen, M. CO2 Storage by Aqueous Mineral Carbonation: Olivine Dissolution and Precipitation of Mg-Carbonates; ETH Zurich: Zurich, Switzerland, 2007. [Google Scholar]
- GMD. Trends in Atomospheric Carbon Dioxide. Available online: http://www.esrl.noaa.gov/gmd/ccgg/trends/data.html (accessed on 10 February 2018).
- Ray, P.K. Hi-Tech Horticulture and Climate Change: Principle and Applications. In Climate Dynamics in Horticultural Science; Choudhary, M.L., Patel, V.B., Siddiqui, M.W., Mahdi, S.S., Eds.; Apple Academic Press: Waretown, NJ, USA, 2015; pp. 1–22. [Google Scholar]
- UNFCCC (United Nations Framework Convention on Climate Change). United Nations Framework Convention on Climate Change. 1992. Available online: http://unfccc.int/files/essential_background/background_publications_htmlpdf/application/pdf/conveng.pdf (accessed on 8 April 2018).
- UNFCCC (United Nations Framework Convention on Climate Change). Paris Agreement. 2015. Available online: http://unfccc.int/files/essential_background/convention/application/pdf/english_paris_agreement.pdf (accessed on 8 April 2018).
- Leung, D.Y.C.; Caramanna, G.; Maroto-Valer, M.M. An overview of current status of carbon dioxide capture and storage technologies. Renew. Sustain. Energy Rev. 2014, 39, 426–443. [Google Scholar] [CrossRef]
- Boot-Handford, M.E.; Abanades, J.C.; Anthony, E.J.; Blunt, M.J.; Brandani, S.; Mac Dowell, N.; Fernandez, J.R.; Ferrari, M.-C.; Gross, R.; Hallett, J.P.; et al. Carbon capture and storage update. Energy Environ. Sci. 2014, 7, 130–189. [Google Scholar] [CrossRef]
- Pan, S.; Chung, T.; Ho, C.; Hou, C.; Chen, Y.; Chiang, P.-C. CO2 Mineralization and Utilization using Steel Slag for Establishing a Waste-to-Resource Supply Chain. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lackner, K.S. A Guide to CO2 Sequestration. Science 2003, 300, 1677–1678. [Google Scholar] [CrossRef] [PubMed]
- Bobicki, E.R. Pre-Treatment of Ultramafic Nickel Ores for Improved Mineral Carbon Sequestration. Ph.D. Thesis, University of Alberta, Edmonton, AB, Canada, 2014. [Google Scholar]
- Lackner, K.; Wendt, C.; Butt, D.; Joycejr, E.; Sharp, D. Carbon dioxide disposal in carbonate minerals. Energy 1995, 20, 1153–1170. [Google Scholar] [CrossRef]
- Bobicki, E.R.; Liu, Q.; Xu, Z.; Zeng, H. Carbon capture and storage using alkaline industrial wastes. Prog. Energy Combust. Sci. 2012, 38, 302–320. [Google Scholar] [CrossRef]
- Romanov, V.; Soong, Y.; Carney, C.; Rush, G.E.; Nielsen, B.; O’Connor, W. Mineralization of carbon dioxide: A literature review. ChemBioEng Rev. 2015, 2, 231–256. [Google Scholar] [CrossRef]
- Seifritz, W. CO2 disposal by means of silicates. Nature 1990, 345, 486. [Google Scholar] [CrossRef]
- Yuen, Y.T.; Sharratt, P.N.; Jie, B. Carbon dioxide mineralization process design and evaluation: Concepts, case studies, and considerations. Environ. Sci. Pollut. Res. 2016, 23, 22309–22330. [Google Scholar] [CrossRef] [PubMed]
- Geerlings, H.; Zevenhoven, R. CO2 mineralization-bridge between storage and utilization of CO2. Annu. Rev. Chem. Biomol. Eng. 2013, 4, 103–117. [Google Scholar] [CrossRef] [PubMed]
- Gadikota, G.; Fricker, K.; Jang, S.-H.; Park, A.-H.A. Carbonation of silicate minerals and industrial wastes and their potential use as sustainable construction materials. In Advances in CO2 Capture, Sequestration, and Conversion; American Chemical Society: Washington, DC, USA, 2015; pp. 295–322. [Google Scholar]
- Markewitz, P.; Kuckshinrichs, W.; Leitner, W.; Linssen, J.; Zapp, P.; Bongartz, R.; Schreiber, A.; Müller, T.E. Worldwide innovations in the development of carbon capture technologies and the utilization of CO2. Energy Environ. Sci. 2012, 5, 7281–7305. [Google Scholar] [CrossRef]
- Azdarpour, A.; Asadullah, M.; Mohammadian, E.; Hamidi, H.; Junin, R.; Karaei, M.A. A review on carbon dioxide mineral carbonation through pH-swing process. Chem. Eng. J. 2015, 279, 615–630. [Google Scholar] [CrossRef]
- Sanna, A.; Uibu, M.; Caramanna, G.; Kuusik, R.; Maroto-Valer, M.M. A review of mineral carbonation technologies to sequester CO2. Chem. Soc. Rev. 2014, 43, 8049–8080. [Google Scholar] [CrossRef] [PubMed]
- Power, I.M.; Wilson, S.A.; Dipple, G.M. Serpentinite Carbonation for CO2 Sequestration. Elements 2013, 9, 115–121. [Google Scholar] [CrossRef]
- O’Connor, W.K.; Dahlin, D.C.; Rush, G.E.; Gerdemann, S.J.; Penner, L.R.; Nilsen, D.N. Aqueous Mineral Carbonation: Mineral Availability, Pretreatment, Reaction Parametrics, and Process Studies; Report No. DOE/ARC-TR-04-002; National Energy Technology Laboratory US DOE: Albany, OR, USA, 2005.
- Kwon, S.; Fan, M.; Dacosta, H.F.M.; Russell, A.G.; Berchtold, K.A.; Dubey, M.K. CO2 sorption. In Coal Gasification and Its Applications; Bell, D.A., Towler, B.F., Fan, M., Eds.; William Andrew Publishing, Elsevier Inc.: New York, NY, USA, 2011; pp. 293–339. [Google Scholar]
- Guthrie, G.D.; Carey, J.W.; Bergfeld, D.; Byler, D.; Chipera, S.; Ziock, H.; Lackner, K.S. Geochemical Aspects of the Carbonation of Magnesium Silicates in an Aqueious Medium; Los Alamos National Laboratory: Los Alamos, NM, USA, 1999.
- Doucet, F.J. Scoping Study on CO2 Mineralization Technologies; CGS: Pretoria, South Africa, 2011. [Google Scholar]
- Matter, J.M.; Stute, M.; Snæbjörnsdottir, S.Ó.; Oelkers, E.H.; Gislason, S.R.; Aradottir, E.S.; Sigfusson, B.; Gunnarsson, I.; Sigurdardottir, H.; Gunnlaugsson, E.; Axelsson, G.; et al. Rapid carbon mineralization for permanent diposal of anthropogenic carbon dioxide emissions. Science 2016, 352, 1312–1314. [Google Scholar] [CrossRef] [PubMed]
- Nduagu, E.; Bergerson, J.; Zevenhoven, R. Life cycle assessment of CO2 sequestration in magnesium silicate rock—A comparative study. Energy Convers. Manag. 2012, 55, 116–126. [Google Scholar] [CrossRef]
- Huijgen, W.J.J.; Comans, R.N.J. Carbon Dioxide Sequestration by Mineral Carbonation, Literature Review Update 2003–2004; Report No. 2005/11 ECN-C-05-022; Energy Research Centre of the Netherlands: Petten, The Netherlands, 2005. [Google Scholar]
- Hrsak, D.; Malina, J.; Hadzipasic, A.B. The decompostion of serpentine by thermal treatment. Mater. Technol. 2005, 39, 225–227. [Google Scholar]
- Balucan, R.D.; Dlugogorski, B.Z. Thermal activation of antigorite for mineralization of CO2. Environ. Sci. Technol. 2013, 47, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Vega, F.; Sanna, A.; Navarrete, B.; Maroto-Valer, M.M.; Cortés, V.J. Degradation of amine-based solvents in CO2 capture process by chemical absorption. Greenh. Gases Sci. Technol. 2014, 4, 707–733. [Google Scholar] [CrossRef]
- Sanna, A.; Gaubert, J.; Maroto-Valer, M.M. Alternative regeneration of chemicals employed in mineral carbonation towards technology cost reduction. Chem. Eng. J. 2016, 306, 1049–1057. [Google Scholar] [CrossRef]
- Haug, T.A. Dissolution and Carbonation of Mechanically Activated Olivine. Ph.D. Thesis, Norwegian University of Science and Technology, Trondheim, Norway, 2010. [Google Scholar]
- Fabian, M.; Shopska, M.; Paneva, D.; Kadinov, G.; Kostova, N.; Turianicová, E.; Briančin, J.; Mitov, I.; Kleiv, R.A.; Baláž, P. The influence of attrition milling on carbon dioxide sequestration on magnesium-iron silicate. Miner. Eng. 2010, 23, 616–620. [Google Scholar] [CrossRef]
- Kim, D.-J.; Sohn, J.-S.; Ahn, J.-G.; Chung, H.-S. Extraction of metals from mechanically milled serpentine. Geosyst. Eng. 2008, 11, 25–28. [Google Scholar] [CrossRef]
- Gualtieri, A.F.; Giacobbe, C.; Viti, C. The dehydroxylation of serpentine group minerals. Am. Miner. 2012, 97, 666–680. [Google Scholar] [CrossRef]
- O’Connor, W.K.; Dahlin, D.C.; Dahlin, C.L.; Collins, W.K. Carbon dioxide sequestration by direct carbonation: Process minerology of feed and products. In Proceedings of the SME Annual Meeting & Exhibit, Denver, CO, USA, 23–25 February 2001; pp. 1–8. [Google Scholar]
- Wang, X.; Maroto-Valer, M.M. Integration of CO2 capture and mineral carbonation by using recyclable ammonium salts. ChemSusChem 2011, 4, 1291–1300. [Google Scholar] [CrossRef] [PubMed]
- Baláž, P.; Turianicová, E.; Fabián, M.; Kleiv, R.A.; Briancin, J.; Obut, A. Structural changes in olivine (Mg, Fe)2SiO4 mechanically activated in high-energy mills. Int. J. Miner. Process. 2008, 88, 1–6. [Google Scholar] [CrossRef]
- Kleiv, R.A.; Thornhill, M. Mechanical activation of olivine. Miner. Eng. 2006, 19, 340–347. [Google Scholar] [CrossRef]
- Zhang, Q.; Sugiyama, K.; Saito, F. Enhancement of acid extraction of magnesium and silicon from serpentine by mechanochemical treatment. Hydrometallurgy 1997, 45, 323–331. [Google Scholar] [CrossRef]
- Rigopoulos, I.; Petallidou, K.C.; Vasiliades, M.A.; Delimitis, A.; Ioannou, I.; Efstathiou, A.M.; Kyratsi, T. Carbon dioxide storage in olivine basalts: Effect of ball milling process. Powder Technol. 2015, 273, 220–229. [Google Scholar] [CrossRef]
- Atashin, S.; Wen, J.Z.; Varin, R.A. Investigation of milling energy input on structural variations of processed olivine powders for CO2 sequestration. J. Alloys Compd. 2015, 618, 555–561. [Google Scholar] [CrossRef]
- Atashin, S.; Wen, J.Z.; Varin, R.A. Optimizing milling energy for enhancement of solid-state magnesium sulfate (MgSO4) thermal extraction for permanent CO2 storage. RSC Adv. 2016, 6, 68860–68869. [Google Scholar] [CrossRef]
- Turianicová, E.; Baláž, P.; Tuček, Ľ.; Zorkovská, A.; Zeleňák, V.; Németh, Z.; Šatka, A.; Kováč, J. A comparison of the reactivity of activated and non-activated olivine with CO2. Int. J. Miner. Process. 2013, 123, 73–77. [Google Scholar] [CrossRef]
- Sandvik, K.L.; Kleiv, R.A.; Haug, T.A. Mechanically activated minerals as a sink for CO2. Adv. Powder Technol. 2011, 22, 416–421. [Google Scholar] [CrossRef]
- Trapasso, F.; Croci, D.; Plescia, P.; Tempesta, E. Asbestos waste carbonation: A new asbestos treatment with CO2 recovery. In Proceedings of the 3rd International Conference on Industrial and Hazardous Waste Management, Chania, Greece, 12–14 September 2012; pp. 1–8. [Google Scholar]
- O’Connor, W.K.; Dahlin, D.C.; Nilsen, D.N. Research status on the sequestration of carbon dioxide by direct aqueous mineral carbonation. In Proceedings of the 18th Annual International Pittsburgh Coal Conference, Newcastle, Austrilia, 3–7 December 2001; pp. 1–12. [Google Scholar]
- Uddin, S.; Rao, S.R.; Mirnezami, M.; Finch, J.A. Processing an ultramafic ore using fiber disintegration by acid attack. Int. J. Miner. Process. 2012, 102–103, 38–44. [Google Scholar] [CrossRef]
- McKelvy, M.J.; Chizmeshya, A.V.G.; Squires, K.; Carpenter, R.W.; Béarat, H. A Novel Approach to Mienral Carbonation: Enhanceing Carbonation while Avoiding Mineral Pretreatment Process Cost; Report Number: DE-FG26-04NT42124; Arizona State University: Tempe, AZ, USA, 2005. [Google Scholar]
- Pan, S.-Y.; Chiang, A.; Chang, E.-E.; Lin, Y.-P.; Kim, H.; Chiang, P.-C. An innovative approach to integrated carbon mineralization and waste utilization: A review. Aerosol Air Qual. Res. 2015, 15, 1072–1091. [Google Scholar] [CrossRef]
- Chang, E.E.; Chiu, A.C.; Pan, S.Y.; Chen, Y.H.; Tan, C.S.; Chiang, P.C. Carbonation of basic oxygen furnace slag with metalworking wastewater in a slurry reactor. Int. J. Greenh. Gas Control 2013, 12, 382–389. [Google Scholar] [CrossRef]
- Power, I.M.; Harrison, A.L.; Dipple, G.M.; Southam, G. Carbon sequestration via carbonic anhydrase facilitated magnesium carbonate precipitation. Int. J. Greenh. Gas Control 2013, 16, 145–155. [Google Scholar] [CrossRef]
- Pan, S.Y.; Chiang, P.C.; Chen, Y.H.; Tan, C.S.; Chang, E.E. Kinetics of carbonation reaction of basic oxygen furnace slags in a rotating packed bed using the surface coverage model: Maximization of carbonation conversion. Appl. Energy 2014, 113, 267–276. [Google Scholar] [CrossRef]
- Santos, R.M.; François, D.; Mertens, G.; Elsen, J.; Van Gerven, T. Ultrasound-intensified mineral carbonation. Appl. Therm. Eng. 2013, 57, 154–163. [Google Scholar] [CrossRef]
- Li, W.; Li, W.; Li, B.; Bai, Z. Electrolysis and heat pretreatment methods to promote CO2 sequestration by mineral carbonation. Chem. Eng. Res. Des. 2009, 87, 210–215. [Google Scholar] [CrossRef]
- Ballantyne, S.M. Greenhouse Gas Emissions in Mining Operations: Challenges and Opportunities in British Columbia, Canada. Ph.D. Thesis, Univerisity of British Columbia, Vancouver, BC, Canada, 2010. [Google Scholar]
- Kruse, N.A.; Strosnider, W.H.J. Carbon dioxide dynamics and sequestration in mine water and waste. Mine Water Environ. 2015, 34, 3–9. [Google Scholar] [CrossRef]
- McCutcheon, J.; Power, I.M.; Harrison, A.L.; Dipple, G.M.; Southam, G. A Greenhouse-Scale photosynthetic microbial bioreactor for carbon sequestration in magnesium carbonate minerals. Environ. Sci. Technol. 2014, 48, 9142–9151. [Google Scholar] [CrossRef] [PubMed]
- Hitch, M.; Ballantyne, S.M.; Hindle, S.R. Revaluing mine waste rock for carbon capture and storage. Int. J. Min. Reclam. Environ. 2010, 24, 64–79. [Google Scholar] [CrossRef]
- Hindle, S.R. Feasibility and Sensitivity Analysis of Integrating Mining and Mineral Carbonation: A Case Study of the Turnagain Nickel Project. Ph.D. Thesis, University of British Columbia, Vancouver, BC, Canada, 2011. [Google Scholar]
- Larachi, F.; Daldoul, I.; Beaudoin, G. Fixation of CO2 by chrysotile in low-pressure dry and moist carbonation: Ex-situ and in-situ characterizations. Geochim. Cosmochim. Acta 2010, 74, 3051–3075. [Google Scholar] [CrossRef]
- Bobicki, E.R.; Liu, Q.; Xu, Z. Mineral carbon storage in pre-treated ultramafic ores. Miner. Eng. 2015, 70, 43–54. [Google Scholar] [CrossRef]
- Jacobs, A.D.; Hitch, M. Experimental mineral carbonation: Approaches to accelerate CO2 sequestration in mine waste materials. Int. J. Min. Reclam. Environ. 2011, 25, 321–331. [Google Scholar] [CrossRef]
- Sarvaramini, A.; Assima, G.P.; Beaudoin, G.; Larachi, F. Biomass torrefaction and CO2 capture using mining wastes – A new approach for reducing greenhouse gas emissions of co-firing plants. Fuel 2014, 115, 749–757. [Google Scholar] [CrossRef]
- Pasquier, L.-C.; Mercier, G.; Blais, J.-F.F.; Cecchi, E.; Kentish, S. Reaction mechanism for the aqueous-phase mineral carbonation of heat-activated serpentine at low temperatures and pressures in flue gas conditions. Environ. Sci. Technol. 2014, 48, 5163–5170. [Google Scholar] [CrossRef] [PubMed]
- Veetil, S.P.; Mercier, G.; Blais, J.-F.; Cecchi, E.; Kentish, S. Magnetic separation of serpentinite mining residue as a precursor to mineral carbonation. Int. J. Miner. Process. 2015, 140, 19–25. [Google Scholar] [CrossRef]
- Wilson, S.A.; Dipple, G.M.; Power, I.M.; Barker, S.L.L.; Fallon, S.J.; Southam, G. Subarctic weathering of mineral wastes provides a sink for atmospheric CO2. Environ. Sci. Technol. 2011, 45, 7727–7736. [Google Scholar] [CrossRef] [PubMed]
- Meyer, N.A.; Vögeli, J.U.; Becker, M.; Broadhurst, J.L.; Reid, D.L.; Franzidis, J.P. Mineral carbonation of PGM mine tailings for CO2 storage in South Africa: A case study. Miner. Eng. 2014, 59, 45–51. [Google Scholar] [CrossRef]
- Vogeli, J.; Reid, D.L.; Becker, M.; Broadhurst, J.; Franzidis, J.P. Investigation of the potential for mineral carbonation of PGM tailings in South Africa. Miner. Eng. 2011, 24, 1348–1356. [Google Scholar] [CrossRef]
- Picot, J.C.; Cassard, D.; Maldan, F.; Greffié, C.; Bodénan, F. Worldwide potential for ex-situ mineral carbonation. Energy Procedia 2011, 4, 2971–2977. [Google Scholar] [CrossRef][Green Version]
- Power, I.M.; Harrison, A.L.; Dipple, G.M.; Wilson, S.A.; Kelemen, P.B.; Hitch, M.; Southam, G. Carbon mineralization: From natural analogues to engineered systems. Rev. Mineral. Geochem. 2013, 77, 305–360. [Google Scholar] [CrossRef]
- Bodénan, F.; Bourgeois, F.; Petiot, C.; Augé, T.; Bonfils, B.; Julcour-Lebigue, C.; Guyot, F.; Boukary, A.; Tremosa, J.; Lassin, A.; et al. Ex situ mineral carbonation for CO2 mitigation: Evaluation of mining waste resources, aqueous carbonation processability and life cycle assessment (Carmex project). Miner. Eng. 2014, 59, 52–63. [Google Scholar] [CrossRef]
- Wilson, S.A. Carbon Sequestration in Chrysotile Mine Tailings. Ph.D. Thesis, University of British Columbia, Vancouver, BC, Canada, 2005. [Google Scholar]
- Wilson, S.A.; Harrison, A.L.; Dipple, G.M.; Power, I.M.; Barker, S.L.L.; Ulrich Mayer, K.; Fallon, S.J.; Raudsepp, M.; Southam, G. Offsetting of CO2 emissions by air capture in mine tailings at the Mount Keith Nickel Mine, Western Australia: Rates, controls and prospects for carbon neutral mining. Int. J. Greenh. Gas Control 2014, 25, 121–140. [Google Scholar] [CrossRef]
- Power, I.M.; Wilson, S.A.; Harrison, A.L.; Dipple, G.M.; McCutcheon, J.; Southam, G.; Kenward, P.A. A depositional model for hydromagnesite-magnesite playas near Atlin, British Columbia, Canada. Sedimentology 2014, 61, 1701–1733. [Google Scholar] [CrossRef]
- Wilson, S.A.; Dipple, G.M.; Power, I.M.; Thom, J.M.; Anderson, R.G.; Raupsepp, M.; Gabites, J.E.; Southam, G. Carbon dioxide fixation within mine wastes of ultramafic-hosted ore deposits: Examples from the Clinton Creek and Cassiar Chrysotile Deposits, Canada. Econ. Geol. 2009, 104, 95–112. [Google Scholar] [CrossRef]
- Beinlich, A.; Austrheim, H. In situ sequestration of atmospheric CO2 at low temperature and surface cracking of serpentinized peridotite in mine shafts. Chem. Geol. 2012, 332–333, 32–44. [Google Scholar] [CrossRef]
- Power, I.M.; McCutcheon, J.; Harrison, A.; Wilson, S.; Dipple, G.; Kelly, S.; Southam, C.; Southam, G. Strategizing carbon-neutral mines: A case for pilot projects. Minerals 2014, 4, 399–436. [Google Scholar] [CrossRef]
- Thom, J.G.M.; Dipple, G.M.; Power, I.M.; Harrison, A.L. Chrysotile dissolution rates: Implications for carbon sequestration. Appl. Geochem. 2013, 35, 244–254. [Google Scholar] [CrossRef]
- Power, I.M.; Dipple, G.M.; Southam, G. Bioleaching of ultramafic tailings by acidithiobacillus spp. for CO2 sequestration. Environ. Sci. Technol. 2010, 44, 456–462. [Google Scholar] [CrossRef] [PubMed]
- McCutcheon, J.; Dipple, G.M.; Wilson, S.A.; Southam, G. Production of magnesium-rich solutions by acid leaching of chrysotile: A precursor to field-scale deployment of microbially enabled carbonate mineral precipitation. Chem. Geol. 2015, 413, 119–131. [Google Scholar] [CrossRef]
- Li, J.; Hitch, M. Ultra-fine grinding and mechanical activation of mine waste rock using a high-speed stirred mill for mineral carbonation. Int. J. Miner. Metall. Mater. 2015, 22, 1005–1016. [Google Scholar] [CrossRef]
- Li, J.; Hitch, M. Mechanical activation of ultramafic mine waste rock in dry condition for enhanced mineral carbonation. Miner. Eng. 2016, 95, 1–4. [Google Scholar] [CrossRef]
- Li, J.; Hitch, M. Ultra-fine grinding and mechanical activation of mine waste rock using a planetary mill for mineral carbonation. Int. J. Miner. Process. 2017, 158, 18–26. [Google Scholar] [CrossRef]
- Renforth, P. The potential of enhanced weathering in the UK. Int. J. Greenh. Gas Control 2012, 10, 229–243. [Google Scholar] [CrossRef]
- Schuiling, R.D.; Wilson, S.A.; Power, I.M. Enhanced silicate weathering is not limited by silicic acid saturation. Proc. Natl. Acad. Sci. USA 2011, 108, E41. [Google Scholar] [CrossRef] [PubMed]
- Harrison, A.L.; Power, I.M.; Dipple, G.M. Accelerated carbonation of brucite in mine tailings for carbon sequestration. Environ. Sci. Technol. 2013, 47, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Power, I.M.; Harrison, A.L.; Dipple, G.M. Accelerating mineral carbonation using carbonic anhydrase. Environ. Sci. Technol. 2016, 50, 2610–2618. [Google Scholar] [CrossRef] [PubMed]
- Harrison, A.L. Mechanisms of Carbon Mineralization form the Pore to Field Scale: Implications for CO2 Sequestration. Ph.D. Thesis, University of British Columbia, Vancouver, BC, Canada, 2014. [Google Scholar]
- Hänchen, M.; Prigiobbe, V.; Baciocchi, R.; Mazzotti, M. Precipitation in the Mg-carbonate system—Effects of temperature and CO2 pressure. Chem. Eng. Sci. 2008, 63, 1012–1028. [Google Scholar] [CrossRef]
- Power, I.M.; Kenward, P.A.; Dipple, G.M.; Raudsepp, M. Room Temperature Magnesite Precipitation. Cryst. Growth Des. 2017, 17, 5652–5659. [Google Scholar] [CrossRef]
- Power, I.M.; Wilson, S.A.; Small, D.P.; Dipple, G.M.; Wan, W.; Southam, G. Microbially mediated mineral carbonation: Roles of phototrophy and heterotrophy. Environ. Sci. Technol. 2011, 45, 9061–9068. [Google Scholar] [CrossRef] [PubMed]
- Zarandi, A.E.; Larachi, F.; Beaudoin, G.; Plante, B.; Sciortino, M. Multivariate study of the dynamics of CO2 reaction with brucite-rich ultramafic mine tailings. Int. J. Greenh. Gas Control 2016, 52, 110–119. [Google Scholar] [CrossRef]
- Assima, G.P.; Larachi, F.; Beaudoin, G.; Molson, J. Dynamics of carbon dioxide uptake in chrysotile mining residues – Effect of mineralogy and liquid saturation. Int. J. Greenh. Gas Control 2013, 12, 124–135. [Google Scholar] [CrossRef]
- Harrison, A.L.; Dipple, G.M.; Power, I.M.; Mayer, U.K. The impact of evolving mineral–water–gas interfacial areas on mineral–fluid reaction rates in unsaturated porous media. Chem. Geol. 2016, 421, 65–80. [Google Scholar] [CrossRef]
- Assima, G.P.; Larachi, F.; Molson, J.; Beaudoin, G. Comparative study of five Québec ultramafic mining residues for use in direct ambient carbon dioxide mineral sequestration. Chem. Eng. J. 2014, 245, 56–64. [Google Scholar] [CrossRef]
- Veetil, S.P.; Pasquier, L.-C.; Blais, J.-F.; Cecchi, E.; Kentish, S.; Mercier, G. Direct gas-solid carbonation of serpentinite residues in the absence and presence of water vapor: A feasibility study for carbon dioxide sequestration. Environ. Sci. Pollut. Res. 2015, 22, 13486–13495. [Google Scholar] [CrossRef] [PubMed]
- Ben Ghacham, A.; Cecchi, E.; Pasquier, L.-C.; Blais, J.-F.; Mercier, G. CO2 sequestration using waste concrete and anorthosite tailings by direct mineral carbonation in gas-solid-liquid and gas-solid routes. J. Environ. Manag. 2015, 163, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Pasquier, L.-C.; Mercier, G.; Blais, J.-F.; Cecchi, E.; Kentish, S. Parameters optimization for direct flue gas CO2 capture and sequestration by aqueous mineral carbonation using activated serpentinite based mining residue. Appl. Geochem. 2014, 50, 66–73. [Google Scholar] [CrossRef]
- Veetil, S.P.; Mercier, G.; Blais, J.-F.; Cecchi, E.; Kentish, S. CO2 sequestration by direct dry gas-solid contact of serpentinite mining residues: A solution for industrial CO2 emission. Int. J. Environ. Pollut. Remediat. 2014, 2, 52–59. [Google Scholar] [CrossRef]
- Jacobs, A.D. Quantifying the Mineral Carbonation Potential of Mine Waste Mineral: A New Parameter for Geospatial Estimation. Ph.D. Thesis, University of British Columbia, Vancouver, BC, Canada, 2014. [Google Scholar]
- Li, J.; Hitch, M. Carbon dioxide adsorption isotherm study on mine waste for integrated CO2 capture and sequestration processes. Powder Technol. 2016, 291, 408–413. [Google Scholar] [CrossRef]
- Li, J.; Hitch, M. Characterization of the microstructure of mechanically-activated olivine using X-ray diffraction pattern analysis. Miner. Eng. 2016, 86, 24–33. [Google Scholar] [CrossRef]
- Rigopoulos, I.; Petallidou, K.C.; Vasiliades, M.A.; Delimitis, A.; Ioannou, I.; Efstathiou, A.M.; Kyratsi, T. On the potential use of quarry waste material for CO2 sequestration. J. CO2 Util. 2016, 16, 361–370. [Google Scholar] [CrossRef]
- Teir, S.; Kuusik, R.; Fogelholm, C.; Zevenhoven, R. Production of magnesium carbonates from serpentinite for long-term storage of CO2. Int. J. Miner. Process. 2007, 85, 1–15. [Google Scholar] [CrossRef]
- Li, J.; Hitch, M. Structural and chemical changes in mine waste mechanically-activated invarious milling environments. Powder Technol. 2017, 308, 13–19. [Google Scholar] [CrossRef]
- Styles, M.T.; Sanna, A.; Lacinska, A.M.; Naden, J.; Maroto-Valer, M. The variation in composition of ultramfic rocks and the effect on their suitability for carbon dioxide sequestration by mineralization following acid leaching. Greenh. Gases Sci. Technol. 2012, 2, 408–418. [Google Scholar]
- Larachi, F.; Gravel, J.-P.; Grandjean, B.P.A.; Beaudoin, G. Role of steam, hydrogen and pretreatment in chrysotile gas–solid carbonation: Opportunities for pre-combustion CO2 capture. Int. J. Greenh. Gas Control 2012, 6, 69–76. [Google Scholar] [CrossRef]
- Declercq, J.; Bosc, O.; Oelkers, E.H. Do organic ligands affect forsterite dissolution rates? Appl. Geochem. 2013, 39, 69–77. [Google Scholar] [CrossRef]
- Li, J. Mechanical Activation of Ultramafic Mine Waste Materials for Enhanced Mineral Carbonation. Ph.D. Thesis, University of British Columbia, Vancouver, BC, Canada, 2017. [Google Scholar]
- Li, J.; Hitch, M. Economic analysis on the application of mechanical activation in an integrated mineral carbonation process. Int. Biodeterior. Biodegrad. 2016, 128, 63–71. [Google Scholar] [CrossRef]
- Li, J.; Hitch, M. Carbon dioxide sorption isotherm study on pristine and acid-treated olivine and its application in the vacuum swing adsorption process. Minerals 2015, 5, 259–275. [Google Scholar] [CrossRef]
- Kemache, N.; Pasquier, L.-C.; Cecchi, E.; Mouedhen, I.; Blais, J.-F.; Mercier, G. Aqueous mineral carbonation for CO2 sequestration: From laboratory to pilot scale. Fuel Process. Technol. 2017, 166, 209–216. [Google Scholar] [CrossRef]
- Mouedhen, I.; Kemache, N.; Pasquier, L.C.; Cecchi, E.; Blais, J.F.; Mercier, G. Effect of pCO2 on direct flue gas mineral carbonation at pilot scale. J. Environ. Manag. 2017, 198, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kemache, N.; Pasquier, L.-C.; Mouedhen, I.; Cecchi, E.; Blais, J.F.; Mercier, G. Aqueous mineral carbonation of serpentinite on a pilot scale: The effect of liquid recirculation on CO2 sequestration and carbonate precipitation. Appl. Geochem. 2016, 67, 21–29. [Google Scholar] [CrossRef]
- Bobicki, E.R.; Liu, Q.; Xu, Z. Microwave heating of ultramafic nickel ores and mineralogical effects. Miner. Eng. 2014, 58, 22–25. [Google Scholar] [CrossRef]
- Bobicki, E.R.; Liu, Q.; Xu, Z. Effect of microwave pre-treatment on ultramafic nickel ore slurry rheology. Miner. Eng. 2014, 61, 97–104. [Google Scholar] [CrossRef]
- Giannoulakis, S.; Volkart, K.; Bauer, C. Life cycle and cost assessment of mineral carbonation for carbon capture and storage in European power generation. Int. J. Greenh. Gas Control 2014, 21, 140–157. [Google Scholar] [CrossRef]
- Azapagic, A.; Cue, R.M. Carbon capture, storage and utilisation technologies: A critical analysis and comparison of their life cycle environmental impacts. J. CO2 Util. 2015, 9, 82–102. [Google Scholar]
- Hitch, M.; Dipple, G.M. Economic feasibility and sensitivity analysis of integrating industrial-scale mineral carbonation into mining operations. Miner. Eng. 2012, 39, 268–275. [Google Scholar] [CrossRef]
- Pasquier, L.-C.; Mercier, G.; Cecchi, E.; Kentish, S. Technical & economic evaluation of a mineral carbonation process using southern Québec mining wastes for CO2 sequestration of raw flue gas with by-product recovery. Int. J. Greenh. Gas Control 2016, 50, 147–157. [Google Scholar]
- Rubin, E.S.; Davison, J.E.; Herzog, H.J. The cost of CO2 capture and storage. Int. J. Greenh. Gas Control 2015, 40, 378–400. [Google Scholar] [CrossRef]
- Khoo, H.H.; Sharratt, P.N.; Bu, J.; Yeo, T.Y.; Borgna, A.; Highfield, J.G.; Björklöf, T.G.; Zevenhoven, R. Carbon capture and mineralization in singapore: Preliminary environmental impacts and costs via LCA. Ind. Eng. Chem. Res. 2011, 50, 11350–11357. [Google Scholar] [CrossRef]

| Mining Deposits | Majority Minerals | CO2 Source | Rock Pretreat | Carbonation Method | Ref. |
|---|---|---|---|---|---|
| American Chrome, QC | Serpentine | Flue gas | TA | DC gas–solid | [99] |
| Old ilmenite mine, QC | Anorthite | Flue gas | NA | DC gas–solid DC aqueous | [100] |
| Okanogan nickel deposit, WA; Thompson nickel bell, MB | Serpentine | Pure CO2 | TA, CA | DC aqueous | [64] |
| American Chrome, QC | Serpentine | Flue gas | TA | DC aqueous | [67,101] |
| Black Lake mine, QC | Serpentine | Flue gas | TA, MS | DC gas–solid | [102] |
| Lonmin Platnum mine, South Africa | Enstatite, plagioclase feldspar | Pure CO2 | CA | IDC aqueous | [70] |
| Nickel Slag, New Caledonia | Olivine, serpentine | Pure CO2 | TA, CA, MA | DC aqueous | [74] |
| Turnagain deposit, BC | Olivine, serpentine | Pure CO2 | NA | DC aqueous | [103] |
| Turnagain deposit, BC | Olivine, serpentine | Pure CO2 | MA | DC aqueous, DC gas–solid | [104,105] |
| Troodos ophiolite complex, Cyprus | clinopyroxene, anorthite | Pure CO2 | MA | DC gas–solid | [106] |
| Black Lake mine, QC; Dumont Nickel project QC | Chrysotile, lizardite | Flue gas | NA | DC gas–solid | [66] |
| Thetford Mines, QC | Chrysotile | CO2 mix | TA | DC gas–solid | [63] |
| Hitura nickel mine, Finland | Serpentine | Pure CO2 | CA | IDC aqueous | [107] |
| CCS Component | Technology | Cost ($/t CO2) | Ref. |
|---|---|---|---|
| CO2 capture a | Post-combustion (coal-fired) | 34 | [7] |
| Pre-combustion (coal-fired) | 23 | [7] | |
| Oxy-fuel (coal-fired) | 36 | [7] | |
| Post-combustion (gas-fired) | 58 | [7] | |
| Pre-combustion (gas-fired) | 112 | [7] | |
| Oxy-fuel (gas-fired) | 102 | [7] | |
| CO2 transportation | Railway | 12.64 | [7] |
| Ship | 7.48 | [7] | |
| Pipeline | 7.05 | [7] | |
| CO2 storage & utilization | Geological storage b,c | 8 | [21] |
| In-situ mineral carbonation c | 17 | [21] | |
| Ex-situ mineral carbonation | 50–300 | [21] |
| CO2 Source | CO2 Capture | CO2 Transportation | CO2 Storage | Cost ($/t CO2 Avoided) | Ref. |
|---|---|---|---|---|---|
| NGCC a | Post-combustion | Pipeline | Geological storage | 59–143 | [124] |
| SCPC b | Post-combustion | Pipeline | Geological storage | 46–99 | [124] |
| IGCC c | Pre-combustion | Pipeline | Geological storage | 38–84 | [124] |
| NGCC | Post-combustion | Pipeline | EOR d | 10–112 | [124] |
| SCPC | Post-combustion | Pipeline | EOR | (5)–58 | [124] |
| IGCC | Pre-combustion | Pipeline | EOR | (16)–46 | [124] |
| Oil & gas | Not given | Pipeline | Mineral carbonation | 28–238 | [122] |
| NGCC | Not applied | Pipeline | Mineral carbonation | 120–159 | [125] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Hitch, M.; Power, I.M.; Pan, Y. Integrated Mineral Carbonation of Ultramafic Mine Deposits—A Review. Minerals 2018, 8, 147. https://doi.org/10.3390/min8040147
Li J, Hitch M, Power IM, Pan Y. Integrated Mineral Carbonation of Ultramafic Mine Deposits—A Review. Minerals. 2018; 8(4):147. https://doi.org/10.3390/min8040147
Chicago/Turabian StyleLi, Jiajie, Michael Hitch, Ian M. Power, and Yueyi Pan. 2018. "Integrated Mineral Carbonation of Ultramafic Mine Deposits—A Review" Minerals 8, no. 4: 147. https://doi.org/10.3390/min8040147
APA StyleLi, J., Hitch, M., Power, I. M., & Pan, Y. (2018). Integrated Mineral Carbonation of Ultramafic Mine Deposits—A Review. Minerals, 8(4), 147. https://doi.org/10.3390/min8040147







