Biosynthesis of Schwertmannite and Goethite in a Bioreactor with Acidophilic Fe(II)-Oxidizing Betaproteobacterium Strain GJ-E10
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Microorganism and Culture Conditions
2.2. Bioreactor Experiments
2.3. Characterization of Iron Mineral Products
2.4. Sorption Experiments with Metal Oxyanions
3. Results and Discussion
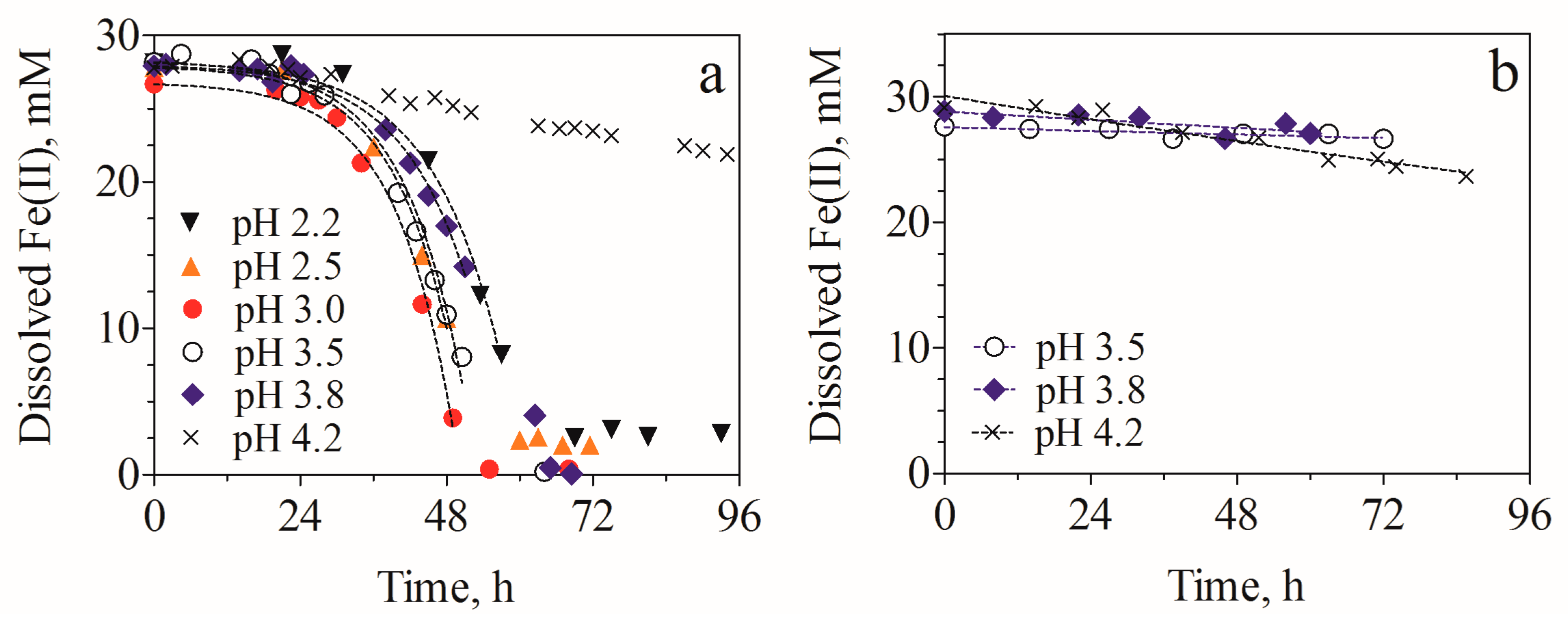
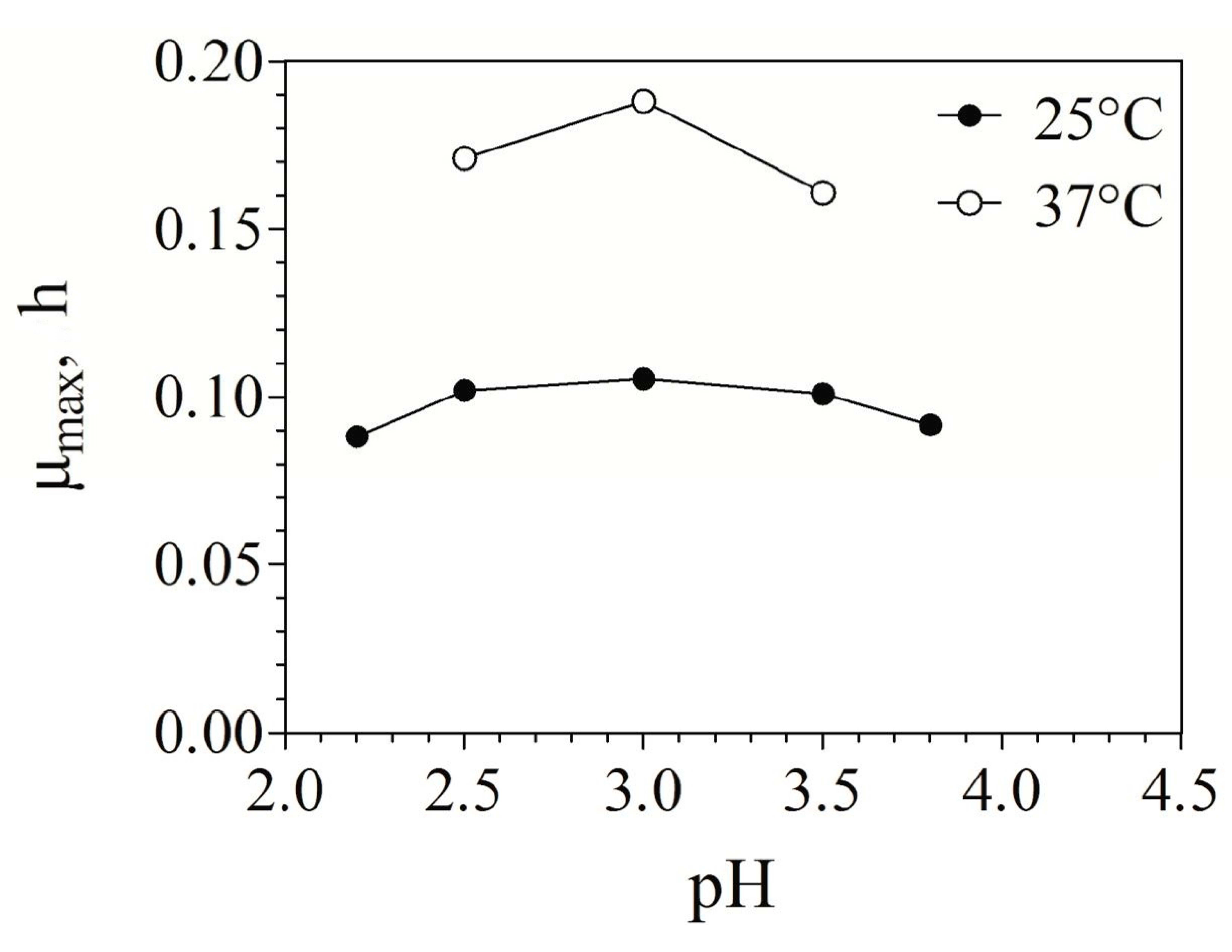
3.1. Fe(II) Oxidation Kinetics in Batch Cultures
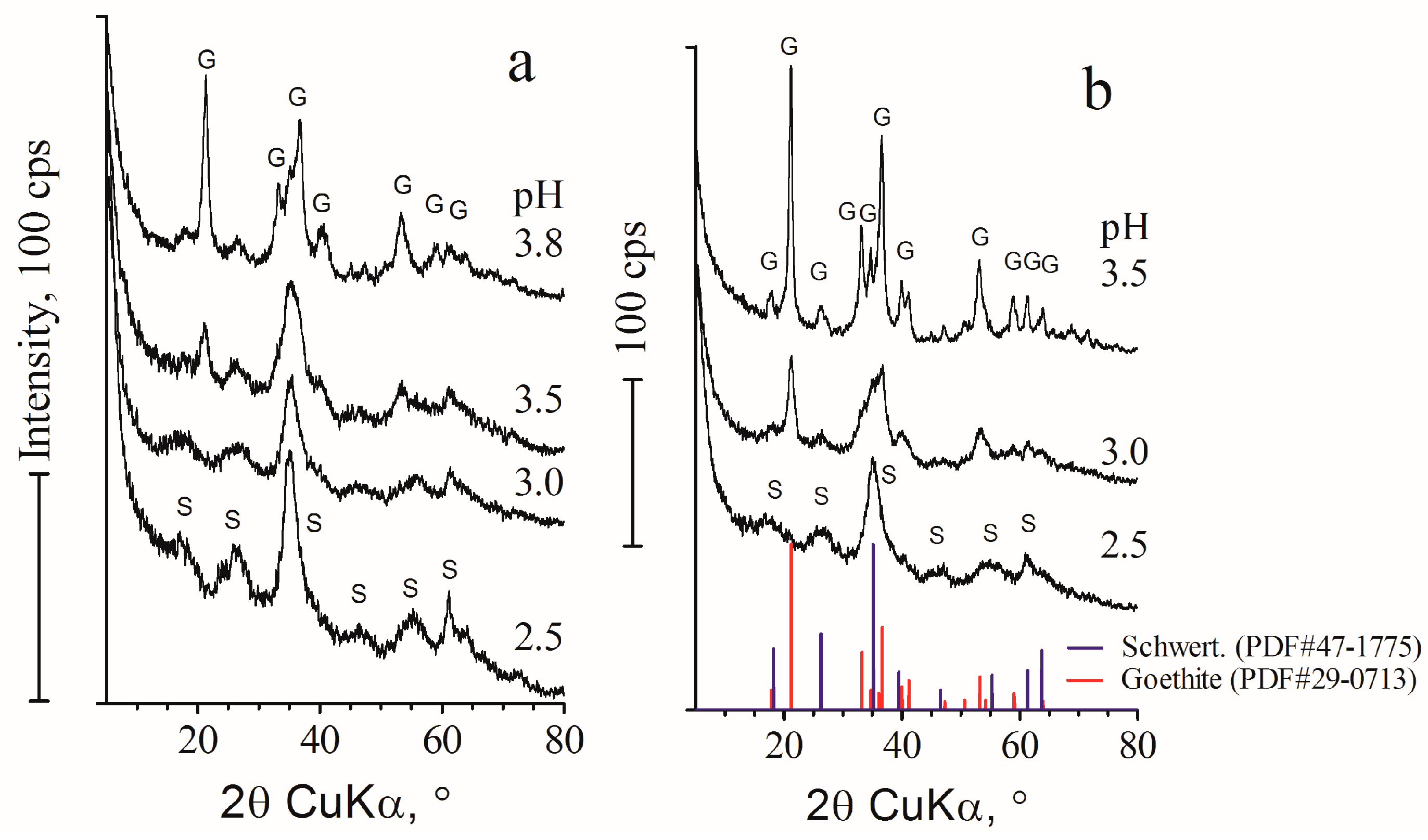
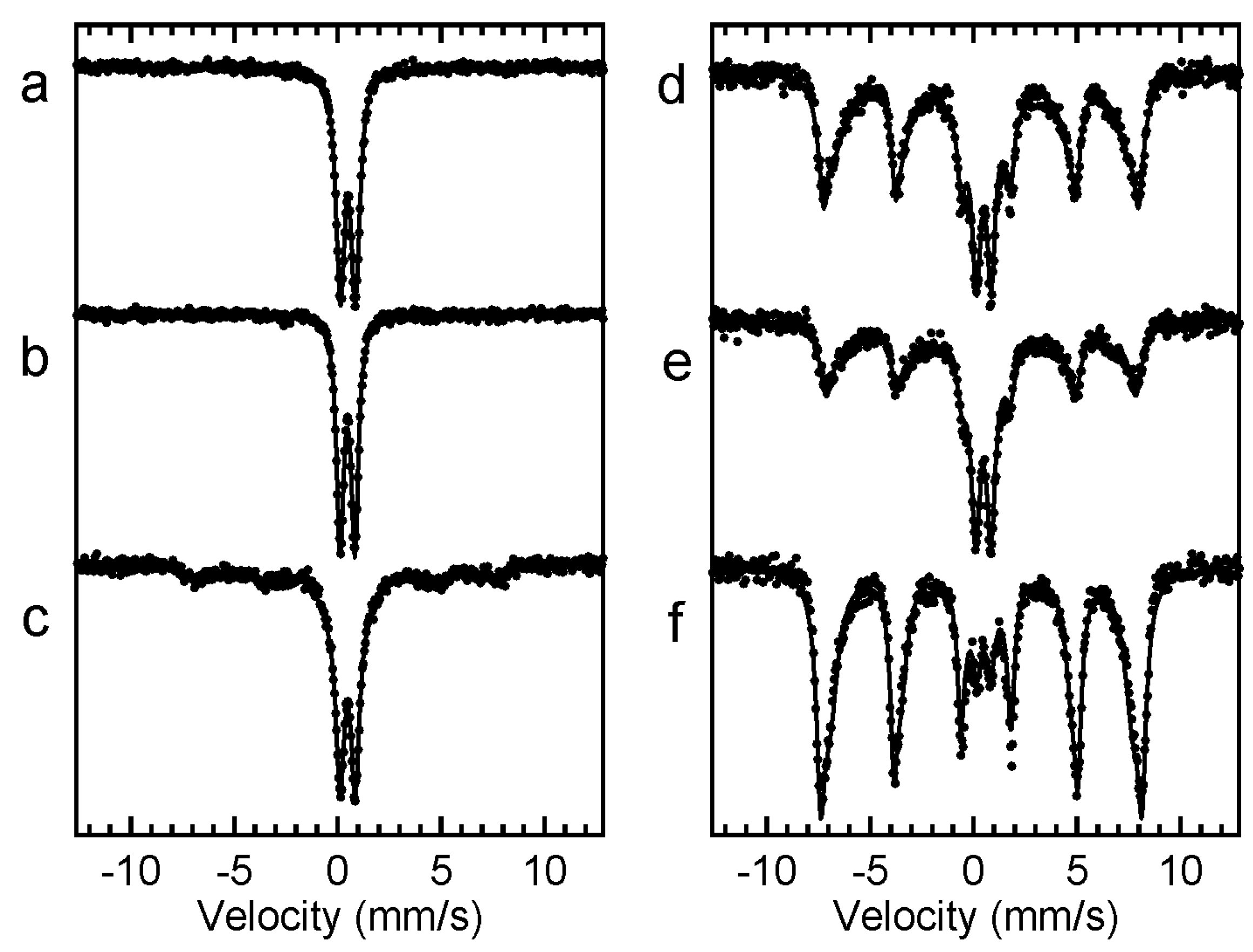
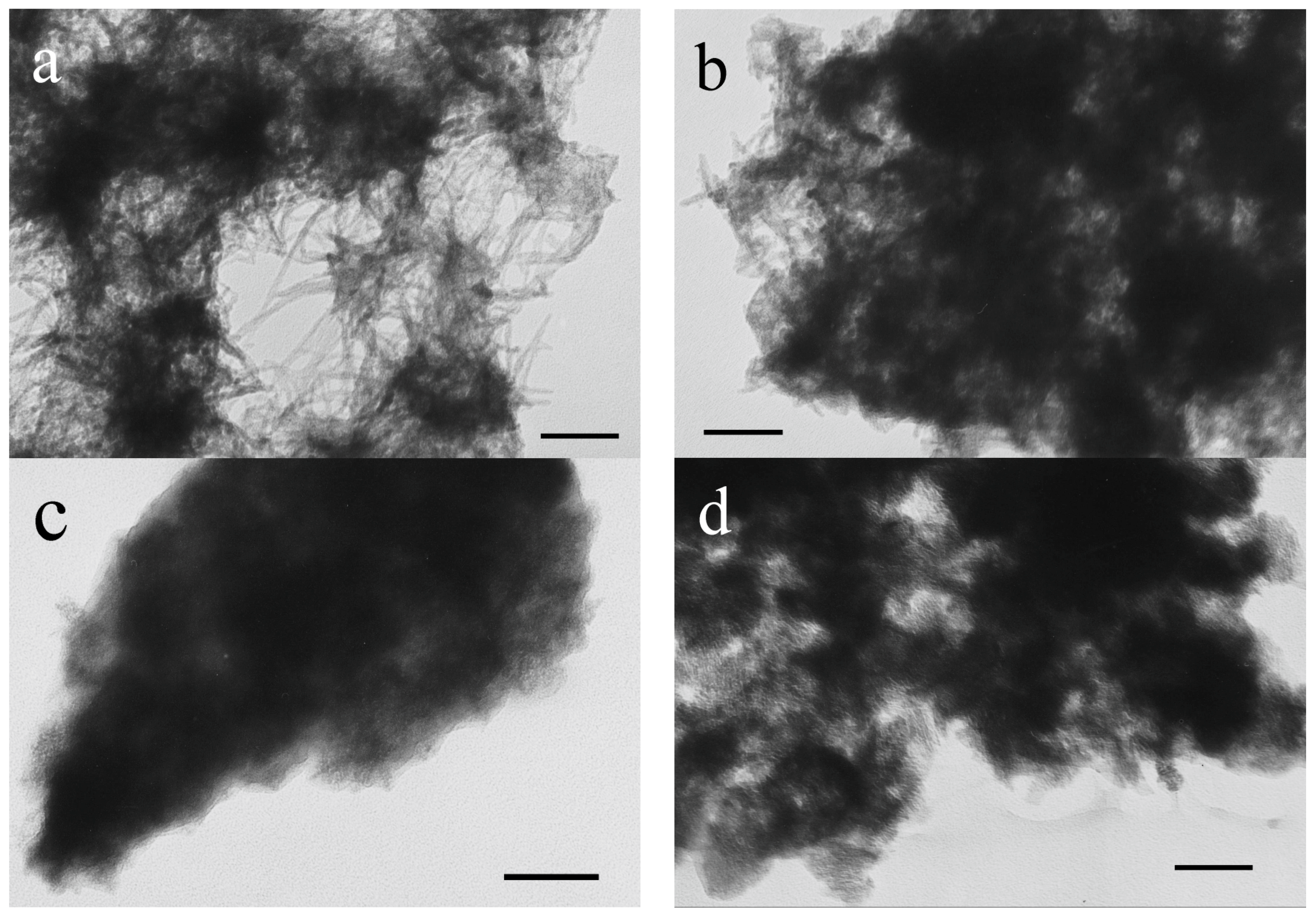
3.2. Characteristics of Bioreactor Products
3.3. Sorption of Metal Oxyanions to Bioreactor Products
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kawano, M.; Tomita, K. Geochemical modeling of bacterially induced mineralization of schwertmannite and jarosite in sulfuric acid spring water. Am. Mineral. 2001, 86, 1156–1165. [Google Scholar] [CrossRef]
- Hedrich, S.; Schlömann, M.; Johnson, D.B. The iron-oxidizing proteobacteria. Microbiology 2011, 157, 1551–1564. [Google Scholar] [CrossRef] [PubMed]
- Bigham, J.M.; Schwertmann, U.; Traina, S.J.; Winland, R.L.; Wolf, M. Schwertmannite and the chemical modeling of iron in acid sulfate waters. Geochim. Cosmochim. Acta 1996, 60, 2111–2121. [Google Scholar] [CrossRef]
- Regenspurg, S.; Brand, A.; Peiffer, S. Formation and stability of schwertmannite in acidic mining lakes. Geochim. Cosmochim. Acta 2004, 68, 1185–1197. [Google Scholar] [CrossRef]
- Schwertmann, U.; Carlson, L. The pH-dependent transformation of schwertmannite to goethite at 25 °C. Clay Miner. 2005, 40, 63–66. [Google Scholar] [CrossRef]
- Knorr, K.-H.; Blodau, C. Controls on schwertmannite transformation rates and products. Appl. Geochem. 2007, 22, 2006–2015. [Google Scholar] [CrossRef]
- Burton, E.D.; Johnston, S.G. Impact of silica on the reductive transformation of schwertmannite and the mobilization of arsenic. Geochim. Cosmochim. Acta 2012, 96, 134–153. [Google Scholar] [CrossRef]
- Schoepfer, V.A.; Burton, E.D.; Johnston, S.G.; Kraal, P. Phosphate-imposed constraints on schwertmannite stability under reducing conditions. Environ. Sci. Technol. 2017, 51, 9739–9746. [Google Scholar] [CrossRef] [PubMed]
- Bertel, D.; Peck, J.; Quick, T.J.; Senko, J.M. Iron transformation induced by an acid-tolerant Desulfosporosinus species. Appl. Environ. Microbiol. 2012, 78, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Carlson, L.; Bigham, J.M.; Schwetmann, U.; Kyek, A.; Wagner, F. Scavenging of As from acid mine drainage by schwertmannite and ferrihydrite: A comparison with synthetic analogues. Environ. Sci. Technol. 2002, 36, 1712–1719. [Google Scholar] [CrossRef] [PubMed]
- Fukushi, K.; Sato, T.; Yanase, N. Solid-solution reactions in As(V) sorption by schwertmannite. Environ. Sci. Technol. 2003, 37, 3581–3586. [Google Scholar] [CrossRef] [PubMed]
- Burton, E.D.; Bush, R.T.; Johnston, S.G.; Watling, K.M.; Hocking, R.K.; Sullivan, L.A.; Parker, G.K. Sorption of arsenic(V) and arsenic(III) to schwertmannite. Environ. Sci. Technol. 2009, 43, 9202–9207. [Google Scholar] [CrossRef] [PubMed]
- Antelo, J.; Fiol, S.; Gondar, D.; López, R.; Arce, F. Comparison of arsenate, chromate and molybdate binding on schwertmannite: Surface adsorption vs. anion-exchange. J. Colloid Interface Sci. 2012, 386, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Jia, S.-Y.; Ren, H.-T.; Wu, S.-H.; Han, X. Application of high-surface-area schwertmannite in the removal of arsenate and arsenite. Int. J. Environ. Sci. Technol. 2015, 12, 1559–1568. [Google Scholar] [CrossRef]
- Liao, Y.; Liang, J.; Zhou, L. Adsorptive removal of As(III) by biogenic schwertmannite from simulated As-contaminated groundwater. Chemosphere 2011, 83, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Hua, M.; Zhang, S.; Pan, B.; Zhang, W.; Lv, L.; Zhang, Q. Heavy metal removal from water/wastewater by nanosized metal oxides: A review. J. Hazard. Mater. 2012, 211–212, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, A.; Banerjee, S.; Mani, R.; Chattopadhyaya, M.C. Synthesis, characterization and application of goethite mineral as an adsorbent. J. Environ. Chem. Eng. 2013, 1, 281–289. [Google Scholar] [CrossRef]
- Liu, H.; Chen, T.; Frost, R.L. An overview of the role of goethite surfaces in the environment. Chemosphere 2014, 103, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loan, M.; Cowley, J.M.; Hart, R.; Parkinson, G.M. Evidence on the structure of synthetic schwertmannite. Am. Mineral. 2004, 89, 1735–1742. [Google Scholar] [CrossRef]
- Wang, H.; Bigham, J.M.; Tuovinen, O.H. Formation of schwertmannite and its transformation to jarosite in the presence of acidophilic iron-oxidizing microorganisms. Mater. Sci. Eng. C 2006, 26, 588–592. [Google Scholar] [CrossRef]
- Xiong, H.; Liao, Y.; Zhou, L. Influence of chloride and sulfate on formation of akaganéite and schwertmannite through ferrous biooxidation by Acidithiobacillus ferrooxidans cells. Environ. Sci. Technol. 2008, 42, 8681–8686. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Zhou, L.; Liang, J.; Xiong, H. Biosynthesis of schwertmannite by Acidithiobacillus ferrooxidans cell suspensions under different pH condition. Mater. Sci. Eng. C 2009, 29, 211–215. [Google Scholar] [CrossRef]
- Zhu, J.; Gan, M.; Zhang, D.; Hu, Y.; Chai, L. The nature of schwertmannite and jarosite mediated by two strains of Acidithiobacillus ferrooxidans with different ferrous oxidation ability. Mater. Sci. Eng. C 2013, 33, 2679–2685. [Google Scholar] [CrossRef] [PubMed]
- Gan, M.; Zheng, Z.; Sun, S.; Zhu, J.; Liu, X. The influence of aluminum chloride on biosynthetic schwertmannite and Cu(II)/Cr(VI) adsorption. RSC Adv. 2015, 5, 94500–94512. [Google Scholar] [CrossRef]
- Chai, L.; Tang, J.; Liao, Y.; Yang, Z.; Liang, L.; Li, Q.; Wang, H.; Yang, W. Biosynthesis of schwertmannite by Acidithiobacillus ferrooxidans and its application in arsenic immobilization in the contaminated soil. J. Soils Sediments 2016, 16, 2430–2438. [Google Scholar] [CrossRef]
- Mukherjee, C.; Jones, F.S.; Bigham, J.M.; Tuovinen, O.H. Synthesis of argentojarosite with simulated bioleaching solutions produced by Acidithiobacillus ferrooxidans. Mater. Sci. Eng. C 2016, 66, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Shi, J.; Zhang, S.; Zhou, L.; Xu, J.; Ge, Y.; Fan, W.; Liu, F. Schwertmannite adherence to the reactor wall during the bio-synthesis process and deterioration of its structural characteristics and arsenic(III) removal efficiency. Minerals 2017, 7, 64. [Google Scholar] [CrossRef]
- Bigham, J.M.; Schwertmann, U.; Pfab, G. Influence of pH on mineral speciation in a bioreactor simulating acid mine drainage. Appl. Geochem. 1996, 11, 845–849. [Google Scholar] [CrossRef]
- Hedrich, S.; Lünsdorf, H.; Kleeberg, R.; Heide, G.; Seifert, J.; Schlömann, M. Schwertmannite formation adjacent to bacterial cells in a mine water treatment plant and in pure cultures of Ferrovum myxofaciens. Environ. Sci. Technol. 2011, 45, 7685–7692. [Google Scholar] [CrossRef] [PubMed]
- Hedrich, S.; Johnson, D.B. A modular continuous flow reactor system for the selective bio-oxidation of iron and precipitation of schwertmannite from mine-impacted waters. Bioresour. Technol. 2012, 106, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, J.; Tojo, F.; Asano, R.; Kobayashi, Y.; Shimura, Y.; Okano, K.; Miyata, N. Complete genome sequence of the unclassified iron-oxidizing, chemolithoautotrophic Burkholderiales bacterium GJ-E10, isolated from an acidic river. Genome Announc. 2015, 3, e01455-14. [Google Scholar] [CrossRef] [PubMed]
- Bryan, C.G.; Johnson, D.B. Dissimilatory ferrous iron oxidation at a low pH: A novel trait identified in the bacterial subclass Rubrobacteridae. FEMS Microbiol. Lett. 2008, 288, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Japan Sewage Works Association. Sewage Analytical Methods; Japan Sewage Works Association: Tokyo, Japan, 1997; pp. 271–274. [Google Scholar]
- Simkins, S.; Alexander, M. Models for mineralization kinetics with the variables of substrate concentration and population density. Appl. Environ. Microbiol. 1984, 47, 1299–1306. [Google Scholar] [PubMed]
- Bigham, J.M.; Carlson, L.; Murad, E. Schwertmannite, a new iron oxyhydroxysulphate from Pyhäsalmi, Finland, and other localities. Mineral. Mag. 1994, 58, 641–648. [Google Scholar] [CrossRef]
- Frandsen, C.; Madsen, D.E.; Boothroyd, C.B.; Mørup, S. Anomalous particle size dependence of magnetic relaxation phenomena in goethite nanoparticles. Croat. Chem. Acta 2015, 88, 481–485. [Google Scholar] [CrossRef]
- Gan, M.; Song, Z.; Jie, S.; Zhu, J.; Zhu, Y.; Liu, X. Biosynthesis of bifunctional iron oxyhydrosulfate by Acidithiobacillus ferrooxidans and their application to coagulation and adsorption. Mater. Sci. Eng. C 2016, 59, 990–997. [Google Scholar] [CrossRef] [PubMed]
- Fukushi, K.; Sato, T.; Yanase, N.; Minato, J.; Yamada, H. Arsenate sorption on schwertmannite. Am. Mineral. 2004, 89, 1728–1734. [Google Scholar] [CrossRef]
- Asta, M.P.; Cama, J.; Martínez, M.; Giménez, J. Arsenic removal by goethite and jarosite in acidic conditions and its environmental implications. J. Hazard. Mater. 2009, 171, 956–972. [Google Scholar] [CrossRef] [PubMed]
- Rovira, M.; Giménez, J.; Martínez, M.; Martínez-Lladó, X.; De Pablo, J.; Martí, V.; Duro, L. Sorption of selenium(IV) and selenium(VI) onto natural iron oxides: Goethite and hematite. J. Hazard. Mater. 2008, 150, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.; Ding, J.; Wang, H.; Huang, X.; Gan, J. Goethite-mediated transformation of bisphenol A. Chemosphere 2012, 89, 789–795. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-M.; Song, J.; Han, X. Schwertmannite as a new Fenton-like catalyst in the oxidation of phenol by H2O2. J. Hazard. Mater. 2013, 262, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.C.C.; Huang, S.-C.; Wang, C.-L.; Jen, Y.-S. Degradation of phthalate esters and acetaminophen in river sediments using the electrokinetic process integrated with a novel Fenton-like process catalyzed by nanoscale schwertmannite. Chemosphere 2016, 159, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Yan, S.; Wu, W.; Zheng, G.; Zhou, L. Heterogeneous Fenton-like degradation of phenanthrene catalyzed by schwertmannite biosynthesized using Acidithiobacillus ferrooxidans. RSC Adv. 2017, 7, 21638–21648. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Xie, Y.; Zeng, Y.; Li, P.; Xie, T.; Wang, Y. Ultrasonic-enhanced Fenton-like degradation of bisphenol A using a bio-synthesized schwertmannite catalyst. J. Hazard. Mater. 2018, 344, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Yu, H.; Wang, X.; Gao, N.; Geng, L.; Ai, L. Enhanced anode performance of microbial fuel cells by adding nanosemiconductor goethite. J. Power Sources 2013, 223, 94–99. [Google Scholar] [CrossRef]

| Culture Conditions | Full Width at Half Maximum (Degree) 1 | Crystallite Size (nm) | Relative Content in Solid Iron (%) 2 |
|---|---|---|---|
| 25 °C, pH 3.5 | 0.771 | 10.3 | 24 |
| 25 °C, pH 3.8 | 0.569 | 14.0 | 77 |
| 37 °C, pH 3.0 | 0.588 | 13.5 | 64 |
| 37 °C, pH 3.5 | 0.350 | 22.7 | 90.5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miyata, N.; Takahashi, A.; Fujii, T.; Hashimoto, H.; Takada, J. Biosynthesis of Schwertmannite and Goethite in a Bioreactor with Acidophilic Fe(II)-Oxidizing Betaproteobacterium Strain GJ-E10. Minerals 2018, 8, 98. https://doi.org/10.3390/min8030098
Miyata N, Takahashi A, Fujii T, Hashimoto H, Takada J. Biosynthesis of Schwertmannite and Goethite in a Bioreactor with Acidophilic Fe(II)-Oxidizing Betaproteobacterium Strain GJ-E10. Minerals. 2018; 8(3):98. https://doi.org/10.3390/min8030098
Chicago/Turabian StyleMiyata, Naoyuki, Ayato Takahashi, Tatsuo Fujii, Hideki Hashimoto, and Jun Takada. 2018. "Biosynthesis of Schwertmannite and Goethite in a Bioreactor with Acidophilic Fe(II)-Oxidizing Betaproteobacterium Strain GJ-E10" Minerals 8, no. 3: 98. https://doi.org/10.3390/min8030098
APA StyleMiyata, N., Takahashi, A., Fujii, T., Hashimoto, H., & Takada, J. (2018). Biosynthesis of Schwertmannite and Goethite in a Bioreactor with Acidophilic Fe(II)-Oxidizing Betaproteobacterium Strain GJ-E10. Minerals, 8(3), 98. https://doi.org/10.3390/min8030098





