The Use of Infrared Spectroscopy to Determine the Quality of Carbonate-Rich Diatomite Ores
Abstract
:1. Introduction
2. Materials and Methods
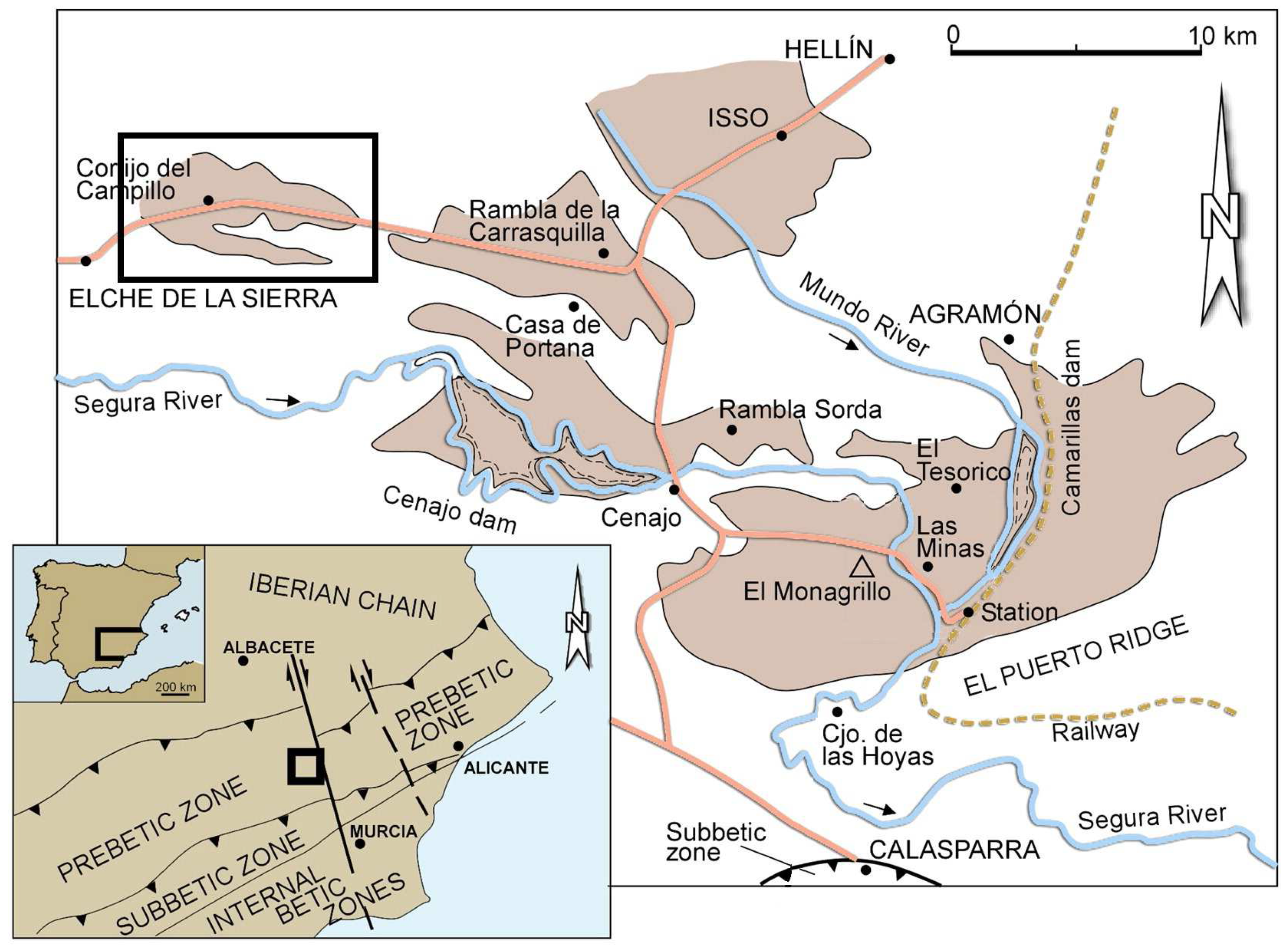
2.1. Geological Background and Samples
2.2. Analytical Methods
3. Results
3.1. Ore ESEM Microscopy
3.2. XRF Characterisation
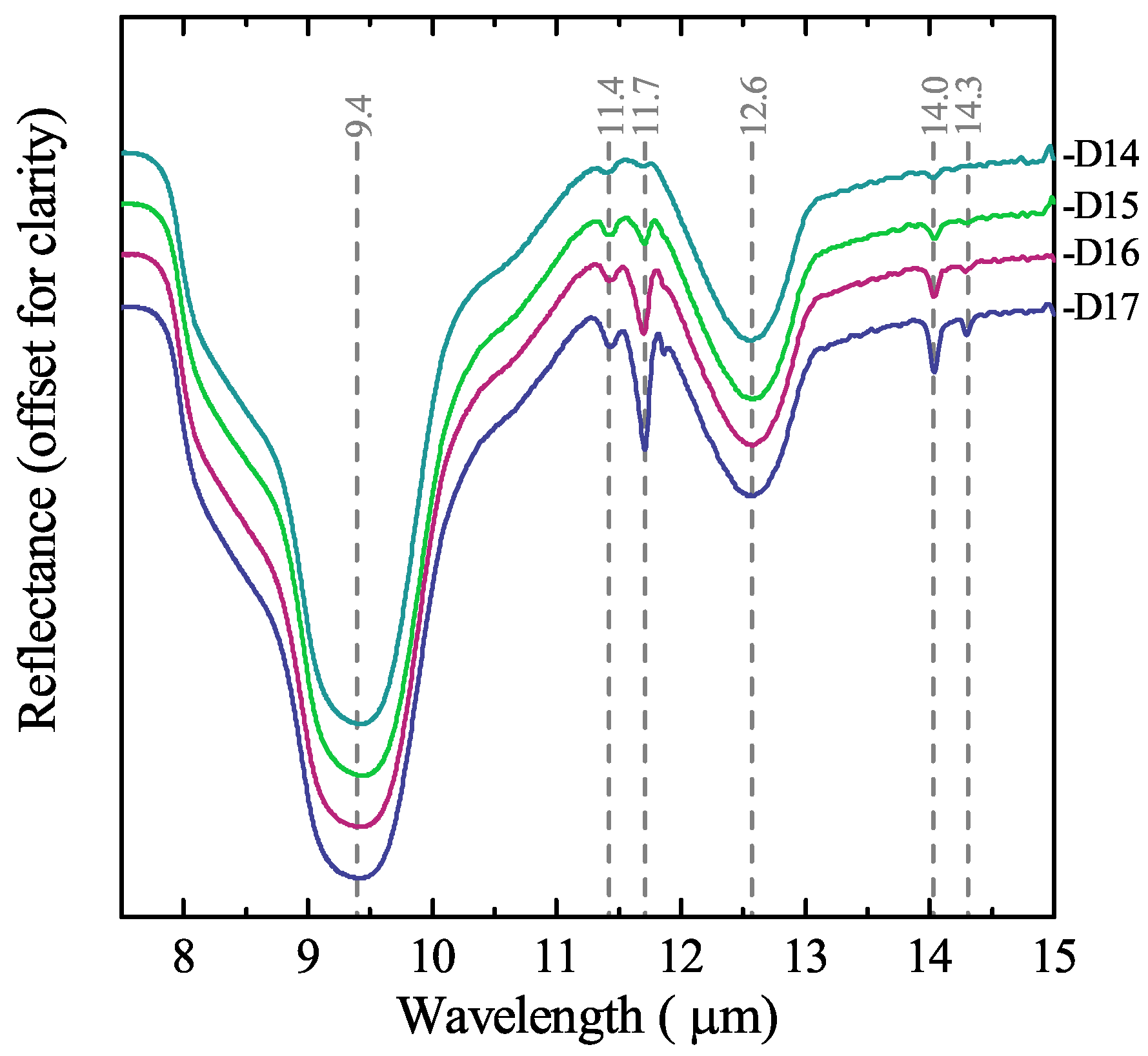
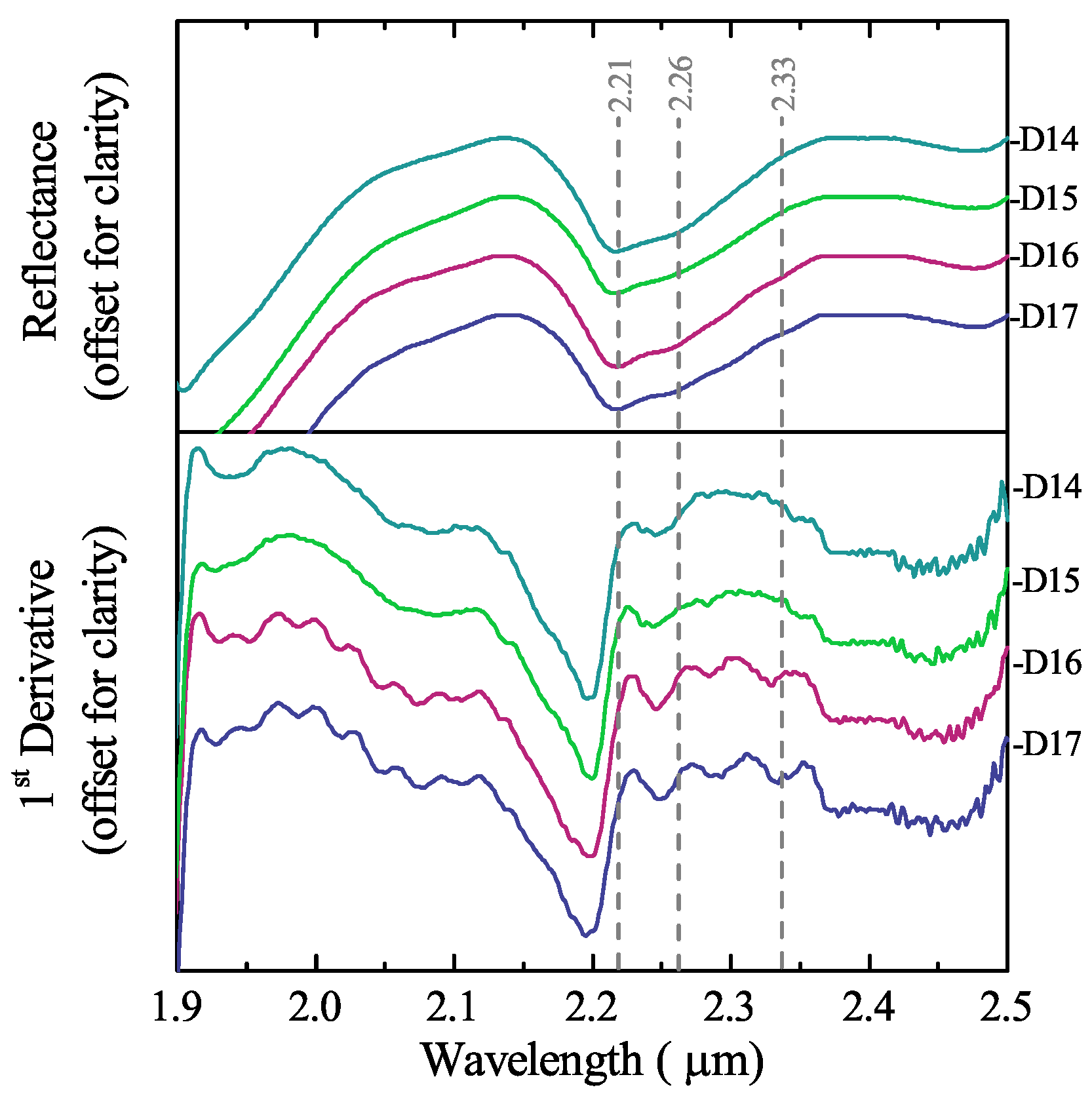
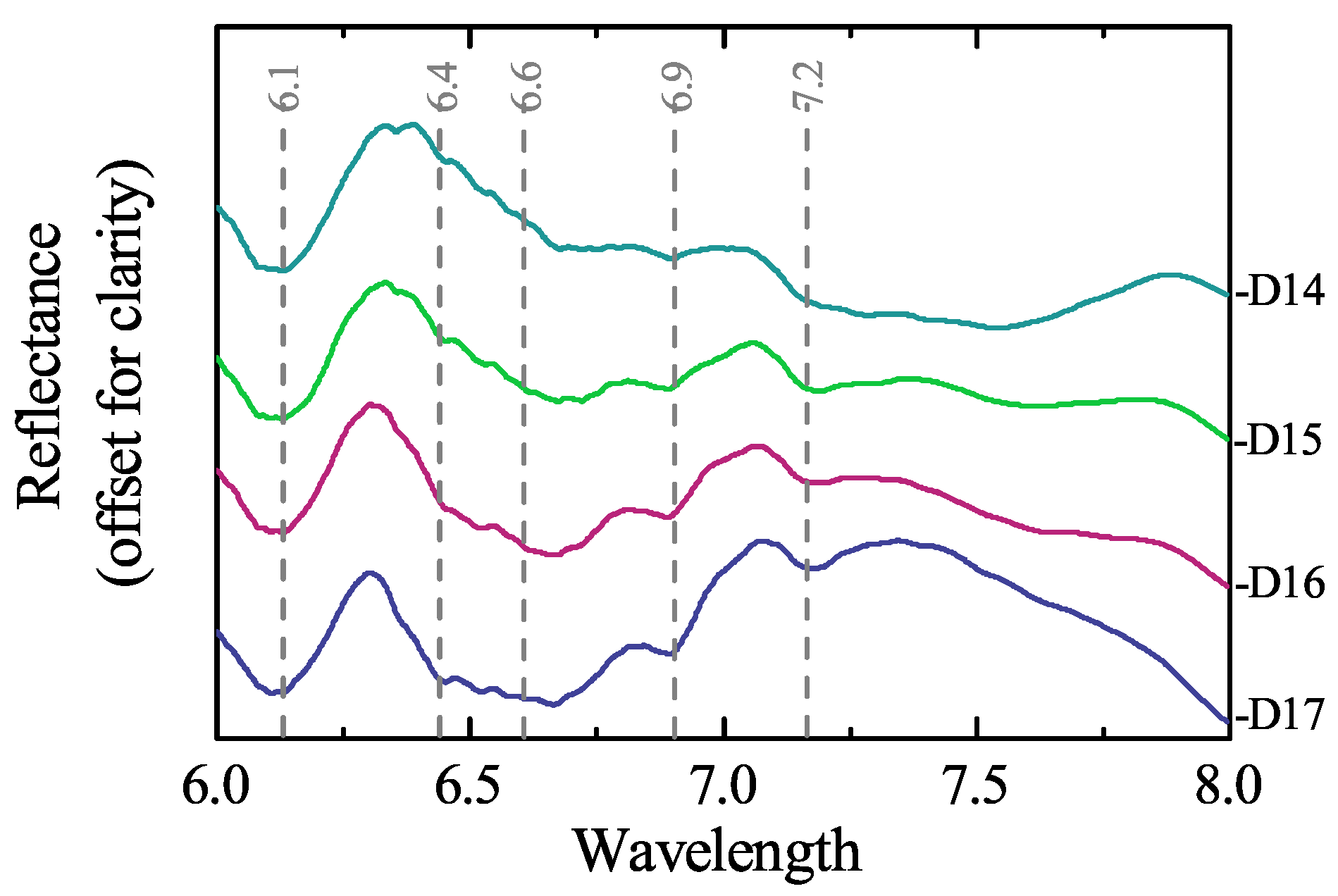
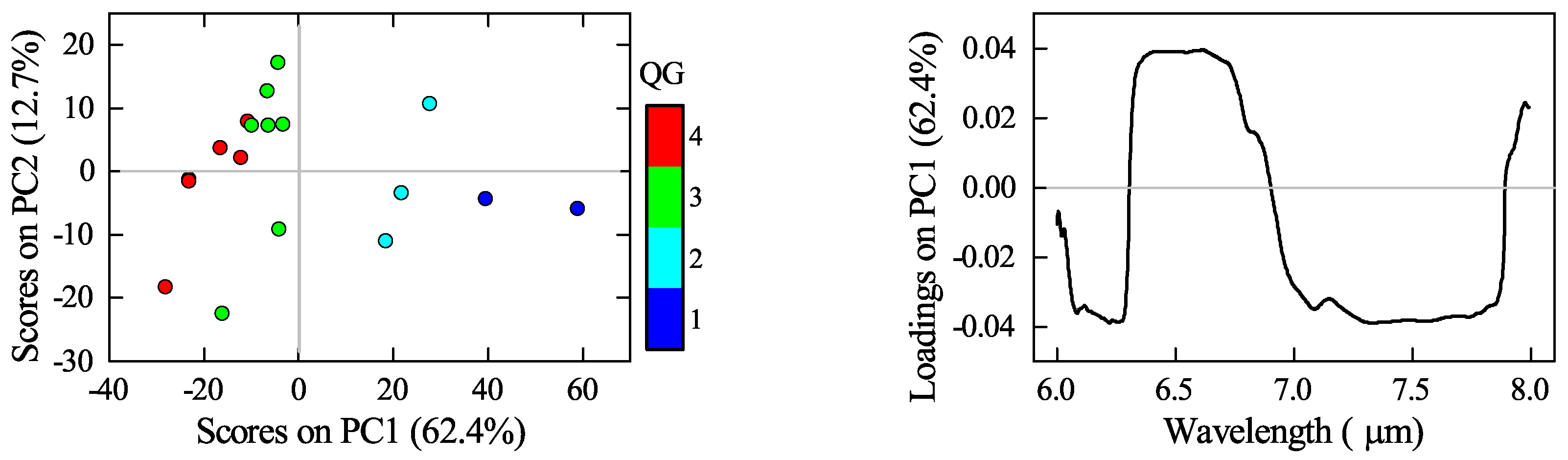
3.3. Mineralogical Characterisation Using Laboratory Infrared Spectroscopy
3.4. Mineral Identification Using Portable Infrared Devices
4. Discussion
4.1. Influence of the Ore Characteristics in Mineral Processing
4.2. Potential for the Use of Infrared Sensors as a Tool for Optimising Mining and Mineral Processing
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Korunic, Z. Review—Diatomaceous earths, a group of natural insecticides. J. Stored Prod. Res. 1998, 34, 87–97. [Google Scholar] [CrossRef]
- Breese, R.O.Y.; Bodycomb, F.M. Diatomite. In Industrial Minerals and Rocks, 7th ed.; Kogel, J.E., Trivedi, N.C., Barker, J.M., Krukowski, S.T., Eds.; Society for Mining, Metallurgy, and Exploration: Englewood, CO, USA, 2006; pp. 433–450. [Google Scholar]
- Crangle, R.D. 2015 Minerals Year Book–Diatomite [Advance Release]. 2016. Available online: https://minerals.usgs.gov/minerals/pubs/commodity/diatomite/myb1-2015-diato.pdf (accessed on 30 January 2018).
- USPC. Food Chemicals Codex. 2016. Available online: https://app.knovel.com/hotlink/toc/id:kpFCCE0031/food-chemicals-codex/food-chemicals-codex (accessed on 14 March 2018).
- Braun, F.; Hildebrand, N.; Wilkinson, S.; Back, W.; Krottenthaler, M.; Becker, T. Large-Scale Study on Beer Filtration with Combined Filter Aid Additions to Cellulose Fibres. J. Inst. Brew. 2011, 117, 314–328. [Google Scholar] [CrossRef]
- Bellanca, A.; Calvo, J.P.; Censi, P.; Elizaga, E.; Neri, R. Evolution of Lacustrine Diatomite Carbonate Cycles of Miocene Age, Southeastern Spain: Petrology and Isotope Geochemistry. J. Sediment. Petrol. 1989, 59, 45–52. [Google Scholar]
- Martinovic, S.; Vlahovic, M.; Boljanac, T.; Pavlovic, L. Preparation of filter aids based on diatomites. Int. J. Miner. Process. 2006, 80, 255–260. [Google Scholar] [CrossRef]
- Ediz, N.; Bentli, İ.; Tatar, İ. Improvement in filtration characteristics of diatomite by calcination. Int. J. Miner. Process. 2010, 94, 129–134. [Google Scholar] [CrossRef]
- Moffat, W.; Walmsley, M.R.W. Understanding lime calcination kinetics for energy cost reduction. In Proceedings of the 59th Appita Conference, Auckland, New Zealand, 16–19 May 2005. [Google Scholar]
- Al-Wakeel, M.I. Characterization and process development of the Nile diatomaceous sediment. Int. J. Miner. Process. 2009, 92, 128–136. [Google Scholar] [CrossRef]
- Jung, K.W.; Jang, D.; Ahn, K.H. A novel approach for improvement of purity and porosity in diatomite (diatomaceous earth) by applying an electric field. Int. J. Miner. Process. 2014, 131, 7–11. [Google Scholar] [CrossRef]
- Moradi, S.; Moseley, D.; Hrach, F.; Gupta, A. Electrostatic beneficiation of diatomaceous earth. Int. J. Miner. Process. 2017, 169, 142–161. [Google Scholar] [CrossRef]
- Sun, Z.; Mao, J.; Hu, Z.; Zheng, S. Study on pilot-scale centrifugal separator for low-grade diatomite purification using response surface methodology. Part. Sci. Technol. 2017, 35, 119–126. [Google Scholar] [CrossRef]
- Rezai, B. The beneficiation studies of diatomite by flotation and hydrocyclone. In Mineral Processing Technology Mpt-2005; Venugopal, R., Sharma, T., Saxena, V.K., Mandre, N.R., Eds.; McGraw Hill: New York, NY, USA, 2005; pp. 214–227. [Google Scholar]
- Şan, O.; Gören, R.; Özgür, C. Purification of diatomite powder by acid leaching for use in fabrication of porous ceramics. Int. J. Miner. Process. 2009, 93, 6–10. [Google Scholar] [CrossRef]
- Zhang, G.; Cai, D.; Wang, M.; Zhang, C.; Zhang, J.; Wu, Z. Microstructural modification of diatomite by acid treatment, high-speed shear, and ultrasound. Microporous Mesoporous Mater. 2013, 165, 106–112. [Google Scholar] [CrossRef]
- Guatame-García, A.; Buxton, M. Detection of mineral impurities in diatomite ores. In Proceedings of the 2nd International Conference on Applied Mineralogy & Advanced Materials AMAM-ICAM 2017, Taranto, Italy, 5–9 June 2017; Fiore, S., Ed.; Digilabs: Bari, Italy, 2017; Volume 4, pp. 19–24. [Google Scholar]
- Elizaga, E.; Calvo, J.P. Evolución sedimentaria de las cuencas lacustres neógenas de la zona prebética (Albacete, España). Relación, posicióny efectos del vulcanismo durante la evolución. Interés minero. Boletín Geológicoy Minero 1988, 99, 837–846. [Google Scholar]
- Foucault, A.; Calvo, J.; Elizaga, E.; Rouchy, J.; Servant-Vildary, S. Situation of the late Miocene lacustrine deposits from Hellin—Province of Albacete, Spain—in the geodynamic evolution of the Betic Cordilleras. [Place des depots lacustres d’age miocene superieur de la region de Hellin (Province de Albacete, Espagne) dans l’evolution geodynamique des Cordilleres betiques.]. Comptes Rendus Acad. Sci. Ser. II 1987, 305, 1163–1166. [Google Scholar]
- Servant-Vildary, S.; Rouchy, J.; Pierre, C.; Foucault, A. Marine and continental water contributions to a hypersaline basin using diatom ecology, sedimentology and stable isotopes: An example in the Late Miocene of the Mediterranean (Hellin Basin, southern Spain). Palaeogeogr. Palaeoclimatol. Palaeoecol. 1990, 79, 189–204. [Google Scholar] [CrossRef]
- Ortí, F.; Rosell, L.; Gibert, L.; Moragas, M.; Playà, E.; Inglès, M.; Rouchy, J.M.; Calvo, J.P.; Gimeno, D. Evaporite sedimentation in a tectonically active basin: The lacustrine Las Minas Gypsum unit (Late Tortonian, SE Spain). Sediment. Geol. 2014, 311, 17–42. [Google Scholar] [CrossRef]
- Chester, R.; Elderfield, H. The Infrared Determination of Opal in Siliceous Deep-sea Sediments. Geochim. Cosmochim. Acta 1968, 32, 1128–1140. [Google Scholar] [CrossRef]
- Wehrens, R. Chemometrics with R; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Gopi, S.P.; Subramanian, V. Polymorphism in CaCO3—Effect of temperature under the influence of EDTA (di sodium salt). Desalination 2012, 297, 38–47. [Google Scholar] [CrossRef]
- Gendron-Badou, A.; Coradin, T.; Maquet, J.; Fröhlich, F.; Livage, J. Spectroscopic characterization of biogenic silica. J. Non-Cryst. Solids 2003, 316, 331–337. [Google Scholar] [CrossRef]
- Moenke, H.H.W. Silica, the Three-dimensional Silicates, Borosilicates and Beryllium Silicates. In The Infrared Spectra of Minerals; Monograph 4, Book Section 16; Farmer, V.C., Ed.; Mineralogical Society: Chantilly, VA, USA, 1974; pp. 365–382. [Google Scholar]
- Goryniuk, M.C. The reflectance spectra of opal-A (0.5–25 microns) from the Taupo Volcanic Zone: Spectra that may identify hydrothermal systems on planetary surfaces. Geophys. Res. Lett. 2004, 31. [Google Scholar] [CrossRef]
- Andersen, F.A.; Brečević, L. Infrared Spectra of Amorphous and Crystalline Calcium Carbonate. Acta Chem. Scand. 1991, 45, 1018–1024. [Google Scholar] [CrossRef]
- Gunasekaran, S.; Anbalagan, G.; Pandi, S. Raman and infrared spectra of carbonates of calcite structure. J. Raman Spectrosc. 2006, 37, 892–899. [Google Scholar] [CrossRef]
- Gaffey, S. Spectral reflectance of carbonate minerals in the visible and near infrared (0.35–2.55 microns): Calcite, aragonite, and dolomite. Am. Mineral. 1986, 71, 151–162. [Google Scholar]
- Cooper, B.L.; Salisbury, J.W.; Killen, R.M.; Potter, A.E. Mid-infrared spectral features of rocks and their powders. J. Geophys. Res. E Planets 2002, 107. [Google Scholar] [CrossRef]
- Meyer-Jacob, C.; Vogel, H.; Boxberg, F.; Rosén, P.; Weber, M.E.; Bindler, R. Independent measurement of biogenic silica in sediments by FTIR spectroscopy and PLS regression. J. Paleolimnol. 2014, 52, 245–255. [Google Scholar] [CrossRef]
- Zaini, N.; van der Meer, F.; van Ruitenbeek, F.; de Smeth, B.; Amri, F.; Lievens, C. An Alternative Quality Control Technique for Mineral Chemistry Analysis of Portland Cement-Grade Limestone Using Shortwave Infrared Spectroscopy. Remote Sens. 2016, 8, 950. [Google Scholar] [CrossRef]


| Sample ID | Al2O3 | CaCO3 | Cl | Fe2O3 | K2O | MgO | Na2O | P2O5 | SiO2 | SO3 | SrO | TiO2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D1 | 0.56 | 19.9 | 0.06 | 0.43 | 0.10 | 0.19 | 0.12 | 0.07 | 68.4 | 0.04 | 0.17 | 0.03 |
| D2 | 0.42 | 18.6 | 0.07 | 0.14 | 0.06 | 0.16 | 0.08 | 0.07 | 70.9 | 0.03 | 0.17 | 0.04 |
| D3 | 0.46 | 10.9 | 0.09 | 0.33 | 0.08 | 0.14 | 0.12 | 0.04 | 82.2 | 0.08 | 0.08 | 0.05 |
| D4 | 0.64 | 16.4 | 0.07 | 0.34 | 0.11 | 0.19 | 0.09 | 0.06 | 73.7 | 0.05 | 0.10 | 0.03 |
| D5 | 0.45 | 22.1 | 0.07 | 0.14 | 0.07 | 0.16 | 0.09 | 0.06 | 65.5 | 0.04 | 0.17 | 0.06 |
| D6 | 0.54 | 22.1 | 0.04 | 0.22 | 0.09 | 0.16 | 0.08 | 0.06 | 65.3 | 0.05 | 0.22 | 0.06 |
| D7 | 0.77 | 22.2 | 0.03 | 0.34 | 0.13 | 0.28 | 0.06 | 0.08 | 64.7 | 0.04 | 0.14 | 0.07 |
| D8 | 0.67 | 20.8 | 0.04 | 0.69 | 0.14 | 0.21 | 0.08 | 0.08 | 66.7 | 0.03 | 0.13 | 0.05 |
| D9 | 0.67 | 24.3 | 0.06 | 0.25 | 0.12 | 0.20 | 0.14 | 0.04 | 61.8 | 0.04 | 0.19 | 0.08 |
| D10 | 0.96 | 23.1 | 0.12 | 0.40 | 0.18 | 0.45 | 0.13 | 0.07 | 62.7 | 0.05 | 0.21 | 0.04 |
| D11 | 0.38 | 9.61 | 0.03 | 0.47 | 0.04 | 0.16 | 0.04 | 0.04 | 84.3 | 0.02 | 0.07 | 0.03 |
| D12 | 0.68 | 10.4 | 0.02 | 0.32 | 0.09 | 0.25 | 0.06 | 0.05 | 82.8 | 0.02 | 0.02 | 0.05 |
| D13 | 0.84 | 21.5 | 0.03 | 0.50 | 0.15 | 0.32 | 0.05 | 0.08 | 65.5 | 0.03 | 0.13 | 0.06 |
| D14 | 0.51 | 6.29 | 0.03 | 0.28 | 0.07 | 0.17 | 0.05 | 0.04 | 89.3 | 0.02 | 0.02 | 0.04 |
| D15 | 0.73 | 10.9 | 0.02 | 0.45 | 0.11 | 0.25 | 0.06 | 0.07 | 81.8 | 0.02 | 0.05 | 0.06 |
| D16 | 0.74 | 16.4 | 0.05 | 0.50 | 0.11 | 0.29 | 0.11 | 0.07 | 73.3 | 0.03 | 0.12 | 0.07 |
| D17 | 0.72 | 24.4 | 0.03 | 0.42 | 0.10 | 0.28 | 0.05 | 0.07 | 61.4 | 0.05 | 0.17 | 0.01 |
| Mean | 0.63 | 17.6 | 0.05 | 0.36 | 0.10 | 0.23 | 0.08 | 0.06 | 80.6 | 0.04 | 0.13 | 0.05 |
| SD | 0.16 | 5.9 | 0.03 | 0.14 | 0.03 | 0.08 | 0.03 | 0.01 | 6.08 | 0.02 | 0.06 | 0.02 |
| Min | 0.38 | 6.3 | 0.02 | 0.14 | 0.04 | 0.14 | 0.04 | 0.04 | 73.6 | 0.02 | 0.02 | 0.01 |
| Max | 0.96 | 24.2 | 0.12 | 0.69 | 0.18 | 0.45 | 0.14 | 0.08 | 92.4 | 0.08 | 0.22 | 0.08 |
| Error | 0.02 | 0.08 | 0.006 | 0.02 | 0.009 | 0.01 | 0.008 | 0.007 | 0.10 | 0.006 | 0.009 | 0.007 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guatame-Garcia, A.; Buxton, M. The Use of Infrared Spectroscopy to Determine the Quality of Carbonate-Rich Diatomite Ores. Minerals 2018, 8, 120. https://doi.org/10.3390/min8030120
Guatame-Garcia A, Buxton M. The Use of Infrared Spectroscopy to Determine the Quality of Carbonate-Rich Diatomite Ores. Minerals. 2018; 8(3):120. https://doi.org/10.3390/min8030120
Chicago/Turabian StyleGuatame-Garcia, Adriana, and Mike Buxton. 2018. "The Use of Infrared Spectroscopy to Determine the Quality of Carbonate-Rich Diatomite Ores" Minerals 8, no. 3: 120. https://doi.org/10.3390/min8030120
APA StyleGuatame-Garcia, A., & Buxton, M. (2018). The Use of Infrared Spectroscopy to Determine the Quality of Carbonate-Rich Diatomite Ores. Minerals, 8(3), 120. https://doi.org/10.3390/min8030120





