Vanadium Bioleaching Behavior by Acidithiobacillus ferrooxidans from a Vanadium-Bearing Shale
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Microorganism and Culture Medium
2.3. Bioleaching Experiment
2.4. Analytical Methods
3. Results and Discussion
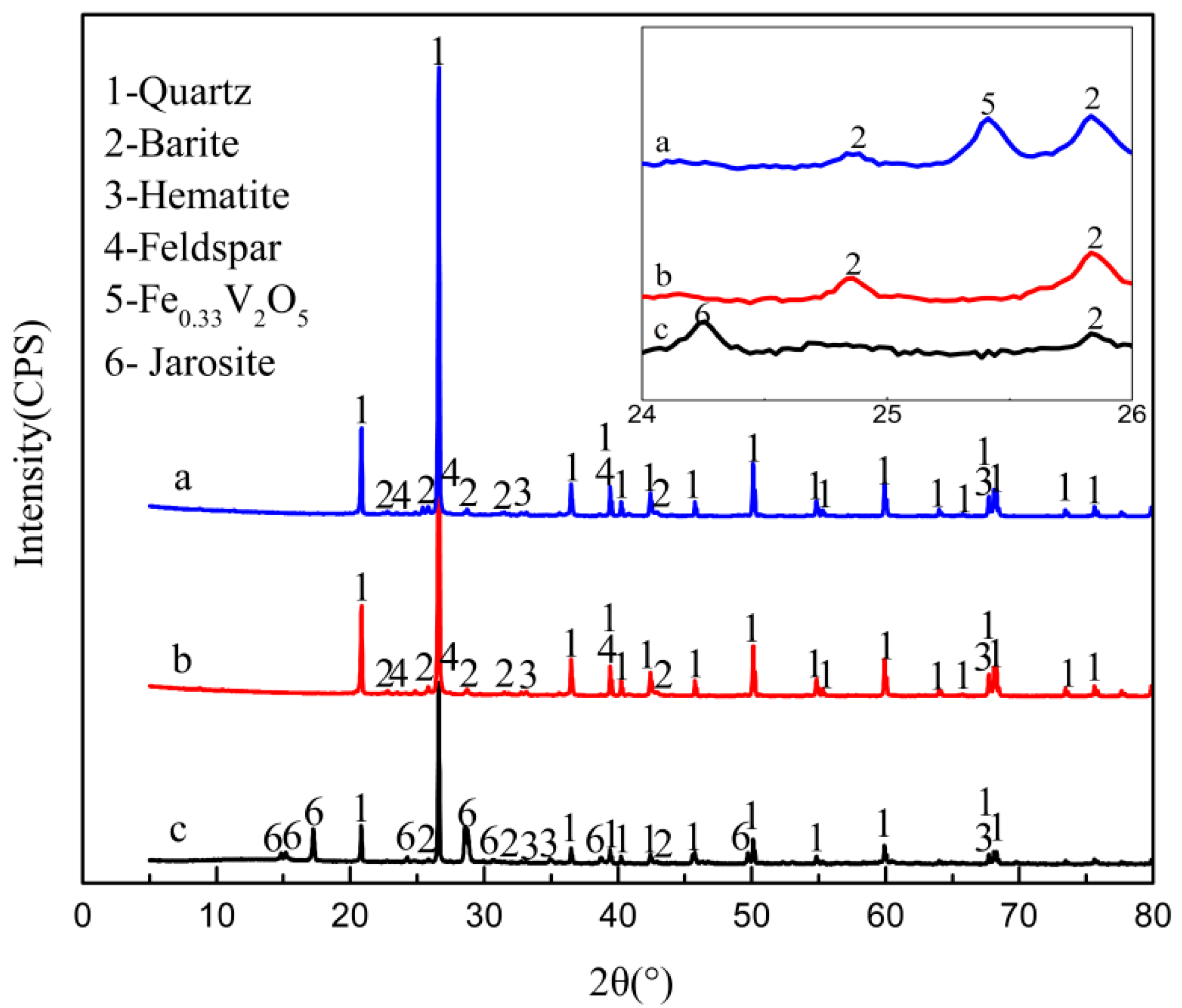
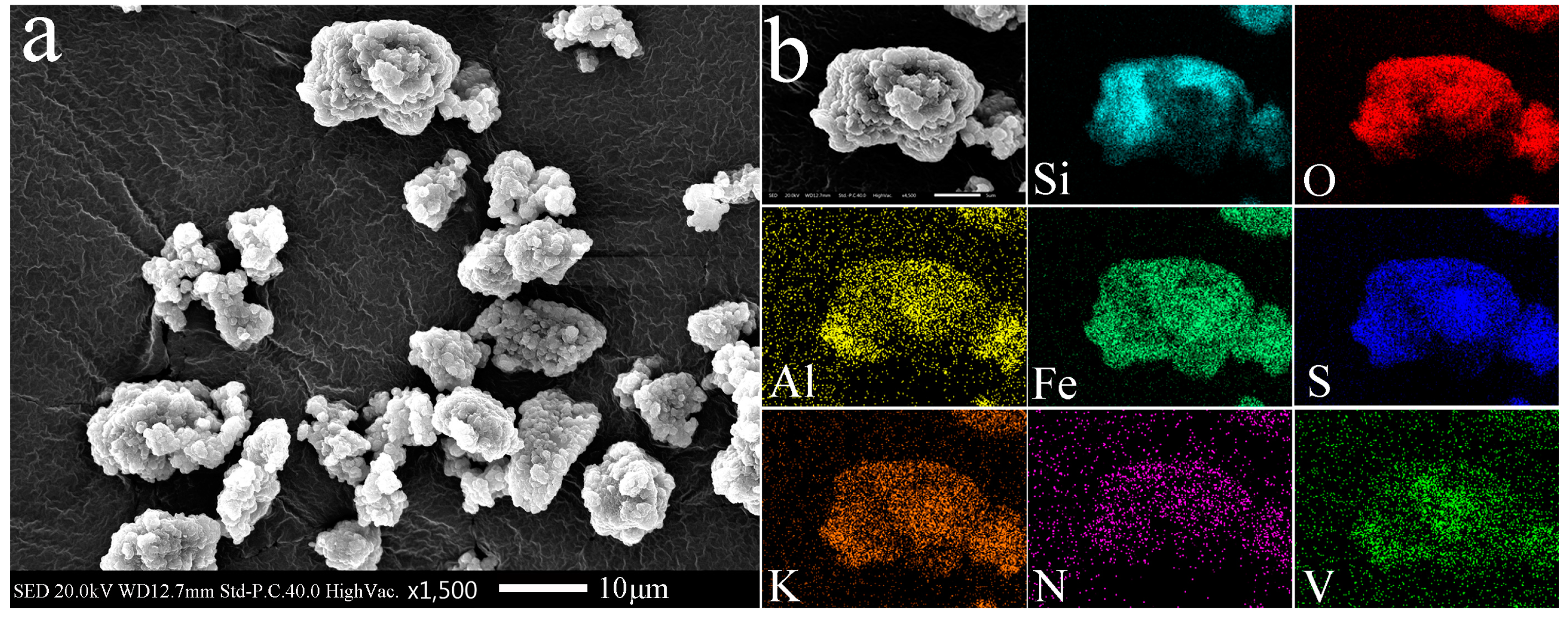
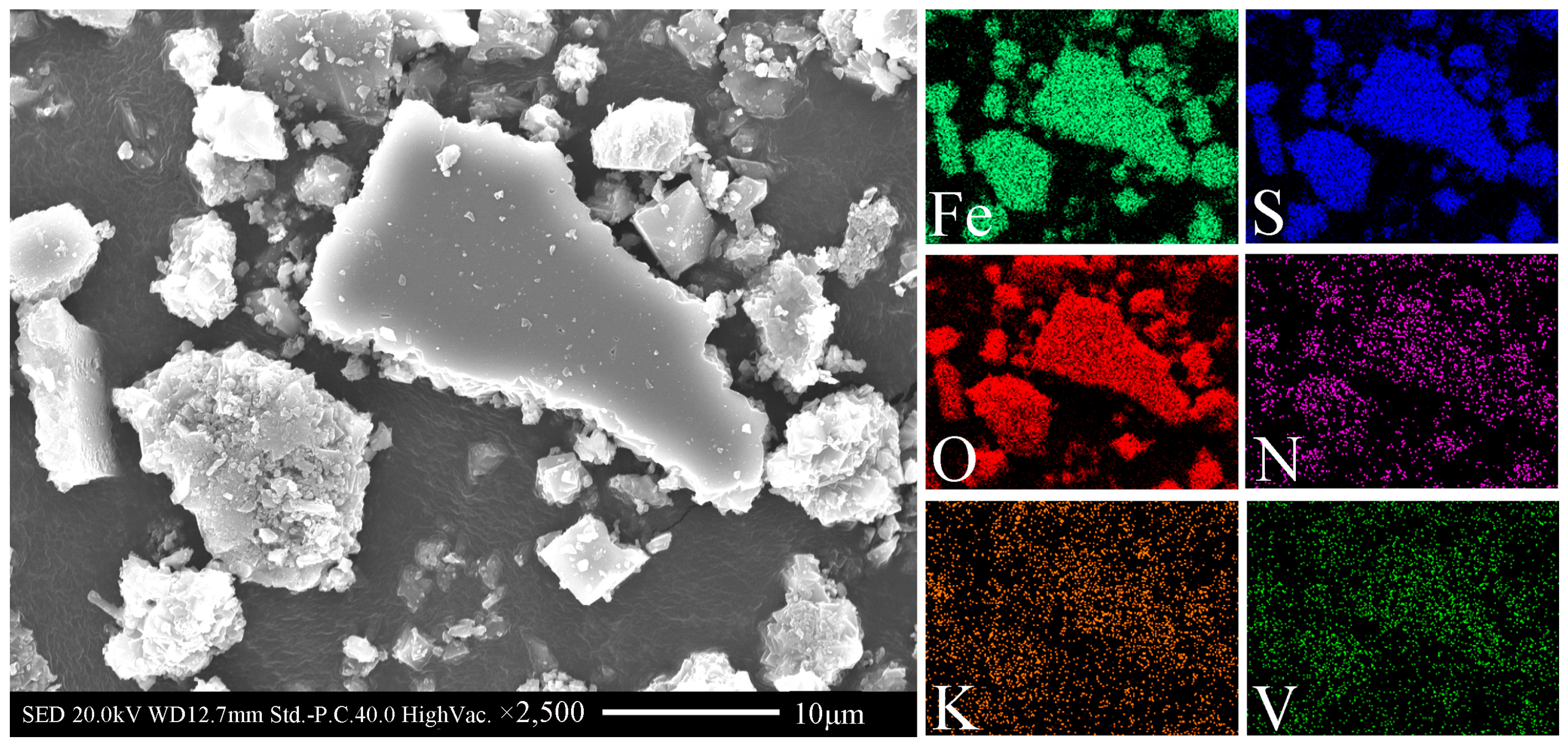
3.1. Material Characterization
3.2. Main Affecting Factors
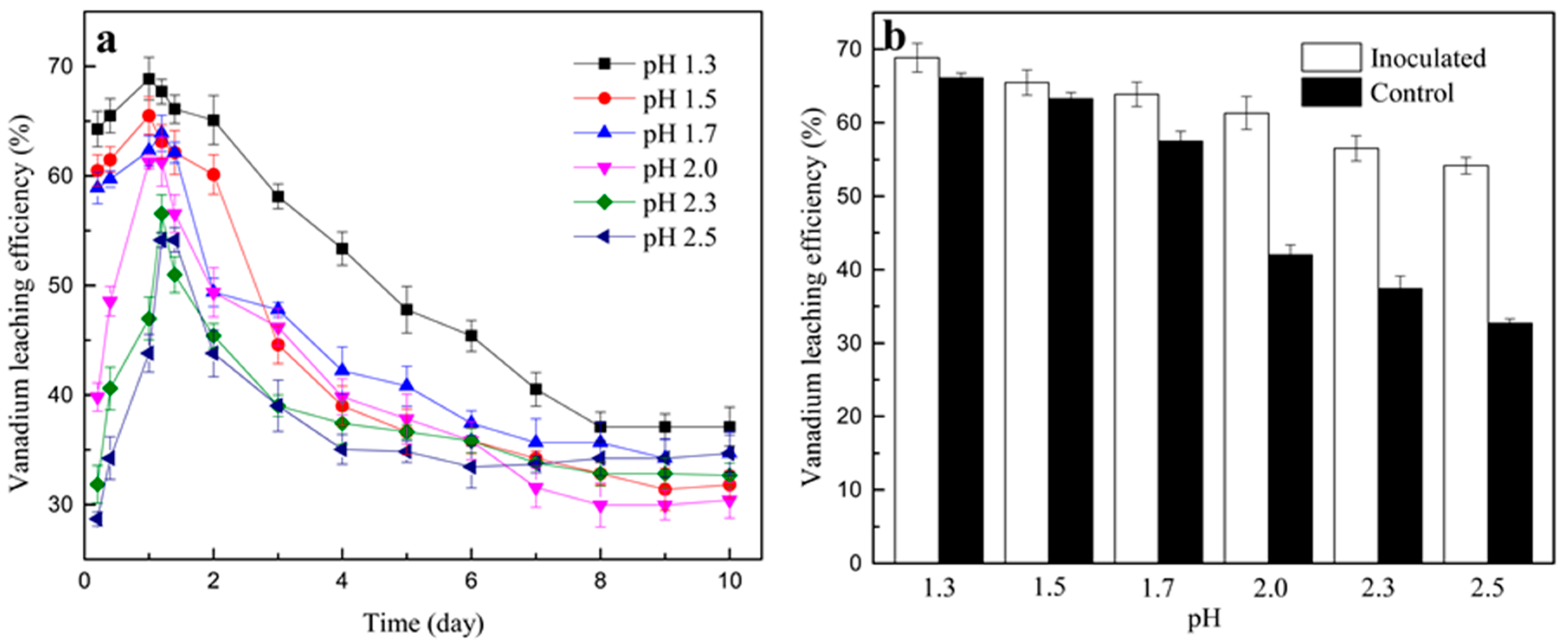
3.2.1. Effect of Initial pH
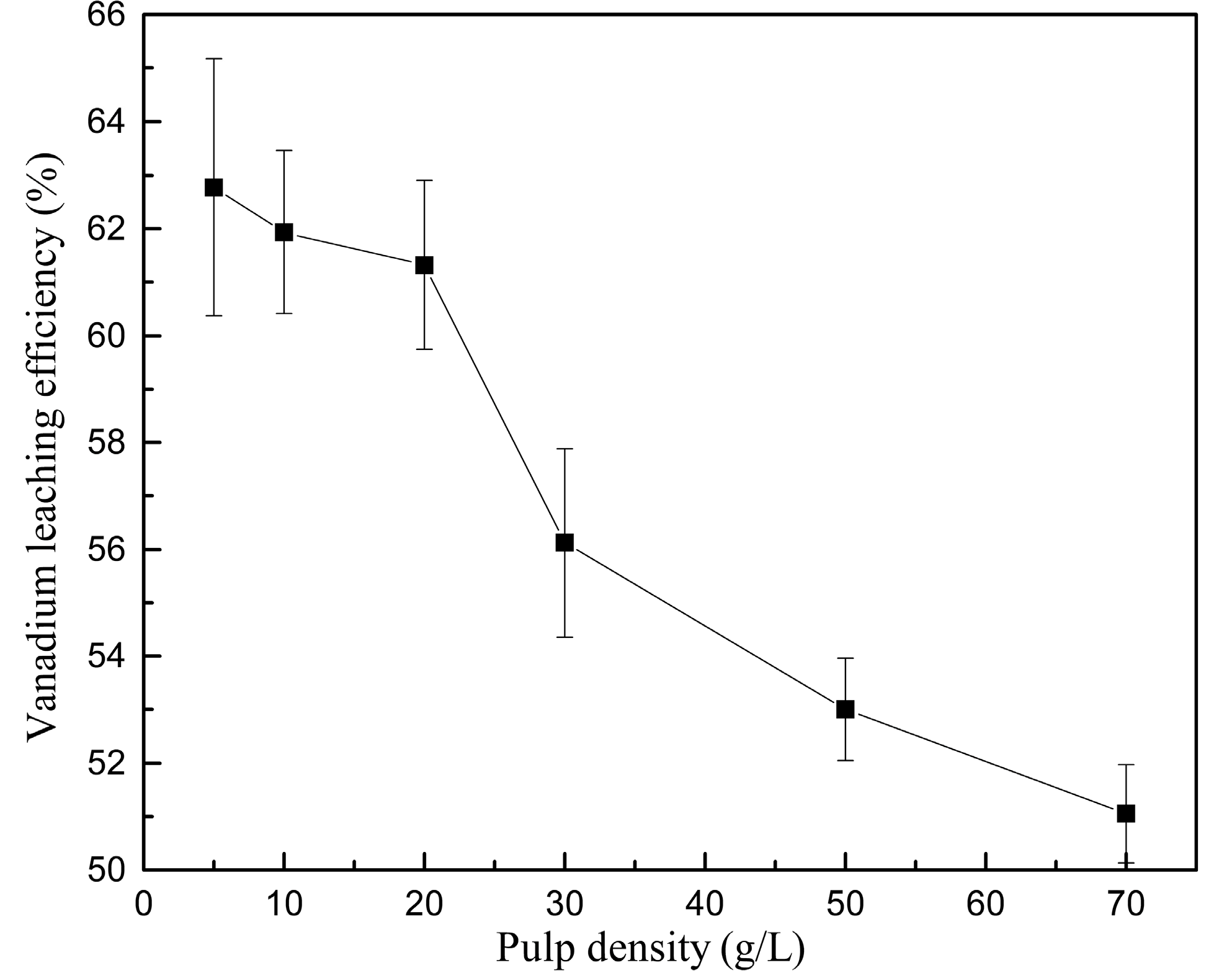
3.2.2. Effect of Pulp Density
3.2.3. Effect of Initial Fe2+ Concentration
3.2.4. Effect of Inoculum Percentage
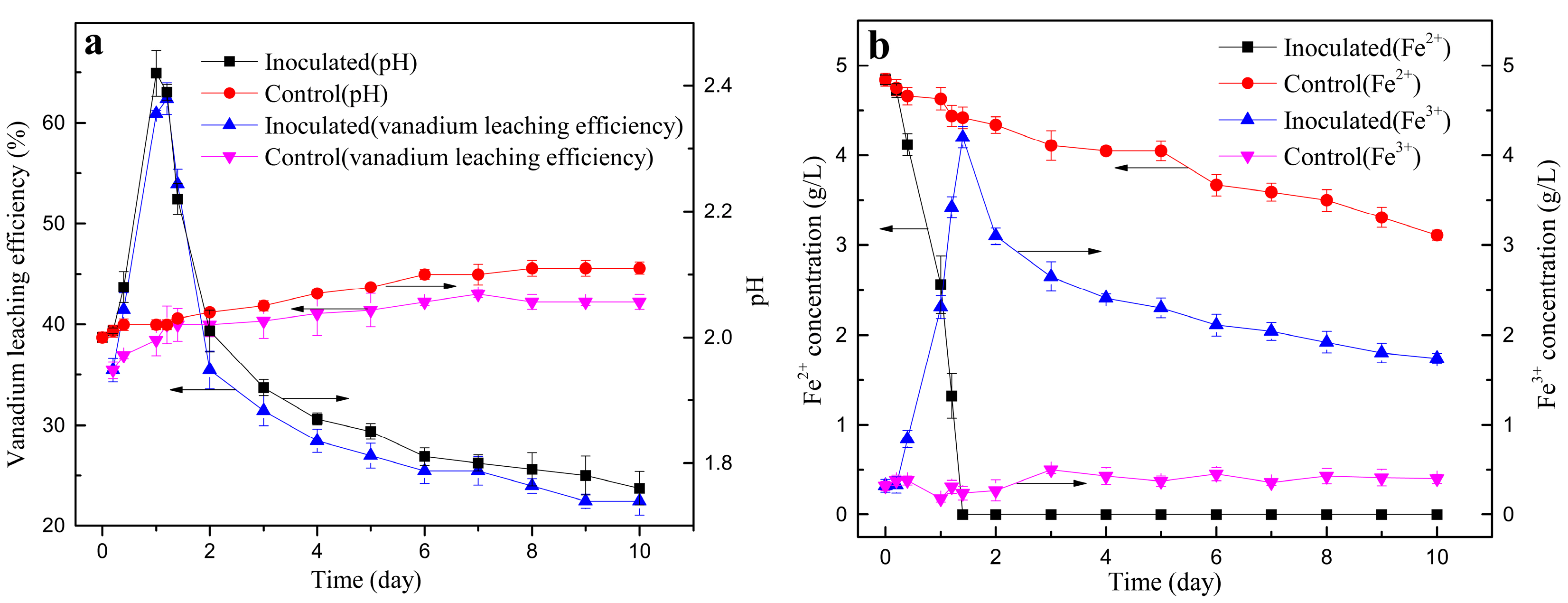
3.2.5. Effect of Leaching Time
3.3. Vanadium Bioleaching Behavior
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yamamura, T.; Wu, X.; Ohta, S.; Shirasaki, K.; Sakuraba, H.; Satoh, I.; Shikama, T. Vanadium solid-salt battery: Solid state with two redox couples. J. Power Sources 2011, 196, 4003–4011. [Google Scholar] [CrossRef]
- Imtiaz, M.; Rizwan, M.S.; Xiong, S.; Li, H.; Ashraf, M.; Shahzad, S.M.; Shahzad, M.; Rizwan, M.; Tu, S. Vanadium, recent advancements and research prospects: A review. Environ. Int. 2015, 80, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.M.; Bao, S.X.; Liu, T.; Chen, T.J.; Huang, J. The technology of extracting vanadium from stone coal in China: History, current status and future prospects. Hydrometallurgy 2011, 109, 116–124. [Google Scholar] [CrossRef]
- Cai, Z.; Zhang, Y.; Liu, T.; Huang, J. Mechanisms of Vanadium Recovery from Stone Coal by Novel BaCO3/CaO Composite Additive Roasting and Acid Leaching Technology. Minerals 2016, 6, 26. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, Y.M.; Liu, T.; Huang, J.; Zhao, J.; Zhang, B.-G.; Liu, J. Comparison of direct acid leaching process and blank roasting acid leaching process in extracting vanadium from stone coal. Int. J. Miner. Process. 2014, 128, 40–47. [Google Scholar] [CrossRef]
- He, D.; Feng, Q.; Zhang, G.; Ou, L.; Lu, Y. An environmentally-friendly technology of vanadium extraction from stone coal. Miner. Eng. 2007, 20, 1184–1186. [Google Scholar] [CrossRef]
- Xue, N.; Zhang, Y.; Liu, T.; Huang, J.; Liu, H.; Chen, F. Mechanism of vanadium extraction from stone coal via hydrating and hardening of anhydrous calcium sulfate. Hydrometallurgy 2016, 166, 48–56. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, Y.; Liu, T.; Chen, T. Comparison of the mechanisms of microwave roasting and conventional roasting and of their effects on vanadium extraction from stone coal. Int. J. Miner. Metall. Mater. 2015, 22, 476–482. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, J.; Gan, X.; Zheng, X.; Tao, L.; Hu, M.; Li, Y.; Qin, W.; Qiu, G. Effects of pyrite and bornite on bioleaching of two different types of chalcopyrite in the presence of Leptospirillum ferriphilum. Bioresour. Technol. 2015, 194, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhong, H.; Hu, Y.; Zhao, J.; He, Z.; Gu, G. Bioleaching of a low-grade nickel-copper sulfide by mixture of four thermophiles. Bioresour. Technol. 2014, 153, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Gou, M.; Tang, Y.Q.; Li, G.Y.; Sun, Z.Y.; Kida, K. Effective bioleaching of chromium in tannery sludge with an enriched sulfur-oxidizing bacterial community. Bioresour. Technol. 2016, 218, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Urík, M.; Bujdoš, M.; Milová-Žiaková, B.; Mikušová, P.; Slovák, M.; Matúš, P. Aluminium leaching from red mud by filamentous fungi. J. Inorg. Biochem. 2015, 152, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Rastegar, S.O.; Mousavi, S.M.; Shojaosadati, S.A.; Sarraf Mamoory, R. Bioleaching of V, Ni, and Cu from residual produced in oil fired furnaces using Acidithiobacillus ferrooxidans. Hydrometallurgy 2015, 157, 50–59. [Google Scholar] [CrossRef]
- Kermer, R.; Hedrich, S.; Bellenberg, S.; Brett, B.; Schrader, D.; Schönherr, P.; Köpcke, M.; Siewert, K.; Günther, N.; Gehrke, T.; et al. Lignite ash: Waste material or potential resource—Investigation of metal recovery and utilization options. Hydrometallurgy 2017, 168, 141–152. [Google Scholar] [CrossRef]
- Li, M.; Wei, C.; Fan, G.; Wu, H.; Li, C.; Li, X. Acid leaching of black shale for the extraction of vanadium. Int. J. Miner. Process. 2010, 95, 62–67. [Google Scholar] [CrossRef]
- Dopson, M.; Lövgren, L.; Boström, D. Silicate mineral dissolution in the presence of acidophilic microorganisms: Implications for heap bioleaching. Hydrometallurgy 2009, 96, 288–293. [Google Scholar] [CrossRef]
- Abhilash; Pandey, B.D. Bioreactor leaching of uranium from a low grade Indian silicate ore. Biochem. Eng. J. 2013, 71, 111–117. [Google Scholar] [CrossRef]
- Anjum, F.; Shahid, M.; Akcil, A. Biohydrometallurgy techniques of low grade ores: A review on black shale. Hydrometallurgy 2012, 117–118, 1–12. [Google Scholar] [CrossRef]
- Štyriaková, I.; Bhatti, T.M.; Bigham, J.M.; Štyriak, I.; Vuorinen, A.; Tuovinen, O.H. Weathering of phlogopite by Bacillus cereus and Acidithiobacillus ferrooxidans. Can. J. Microbiol. 2004, 50, 213. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, T.M.; Bigham, J.M.; Vuorinen, A.; Tuovinen, O.H. Weathering of phlogopite in simulated bioleaching solutions. Int. J. Miner. Process. 2011, 98, 30–34. [Google Scholar] [CrossRef]
- Bhatti, T.M.; Bigham, J.M.; Vuorinen, A.; Tuovinen, O.H. Weathering of biotite in Acidithiobacillus ferrooxidans cultures. Geomicrobiol. J. 2011, 28, 130–134. [Google Scholar] [CrossRef]
- Mishra, D.; Kim, D.J.; Ralph, D.E.; Ahn, J.G.; Rhee, Y.H. Bioleaching of spent hydro-processing catalyst using acidophilic bacteria and its kinetics aspect. J. Hazard. Mater. 2008, 152, 1082–1091. [Google Scholar] [CrossRef] [PubMed]
- Rasoulnia, P.; Mousavi, S.M. Maximization of organic acids production by Aspergillus niger in a bubble column bioreactor for V and Ni recovery enhancement from power plant residual ash in spent-medium bioleaching experiments. Bioresour. Technol. 2016, 216, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Mirazimi, S.M.J.; Abbasalipour, Z.; Rashchi, F. Vanadium removal from LD converter slag using bacteria and fungi. J. Environ. Manag. 2015, 153, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Liao, M.; Zhang, X.; Zhang, M.; Deng, R.; Zeng, Z. Study on the bioleaching conditions of vanadium from vanadium-bearing stone coal by Acidthiobacillus ferrooxidans. Fine Chem. 2015, 32, 144–148. (In Chinese) [Google Scholar]
- Hu, Y.J.; Zhang, Y.M.; Bao, S.X.; Liu, T. Effects of the mineral phase and valence of vanadium on vanadium extraction from stone coal. Int. J. Miner. Metall. Mater. 2012, 19, 893–898. [Google Scholar] [CrossRef]
- Chinese National Standard: GB/T 8704.5; Ferrovanadium–Determination of Vanadium Content–The Ammonium Ferrous Sulfate Titrimetric Method and the Potentiometric Titrimetric Method; Standard Press of China: Beijing, China, 2007. (In Chinese)
- Chinese National Standard: HJ/T 345; Water Quality Betermination of Iron-Phenanthroline Specterophotometry; Standard Press of China: Beijing, China, 2007. (In Chinese)
- Pradhan, D.; Mishra, D.; Kim, D.J.; Ahn, J.G.; Chaudhury, G.R.; Lee, S.W. Bioleaching kinetics and multivariate analysis of spent petroleum catalyst dissolution using two acidophiles. J. Hazard. Mater. 2010, 175, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, T.M. Bioleaching of organic carbon rich polymetallic black shale. Hydrometallurgy 2015, 157, 246–255. [Google Scholar] [CrossRef]
- Leiviskä, T.; Khalid, M.K.; Sarpola, A.; Tanskanen, J. Removal of vanadium from industrial wastewater using iron sorbents in batch and continuous flow pilot systems. J. Environ. Manag. 2017, 190, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Blackmore, D.P.T.; Ellis, J.; Riley, P.J. Treatment of a vanadium-containing effluent by adsorption/coprecipitation with iron oxyhydroxide. Water Res. 1996, 30, 2512–2516. [Google Scholar] [CrossRef]
- Roccaro, P.; Vagliasindi, F.G.A. Coprecipitation of vanadium with iron(III) in drinking water: A pilot-scale study. Desalin. Water Treat. 2015, 55, 799–809. [Google Scholar] [CrossRef]
- Ahoranta, S.H.; Kokko, M.E.; Papirio, S.; Özkaya, B.; Puhakka, J.A. Arsenic removal from acidic solutions with biogenic ferric precipitates. J. Hazard. Mater. 2016, 306, 124–132. [Google Scholar] [CrossRef] [PubMed]
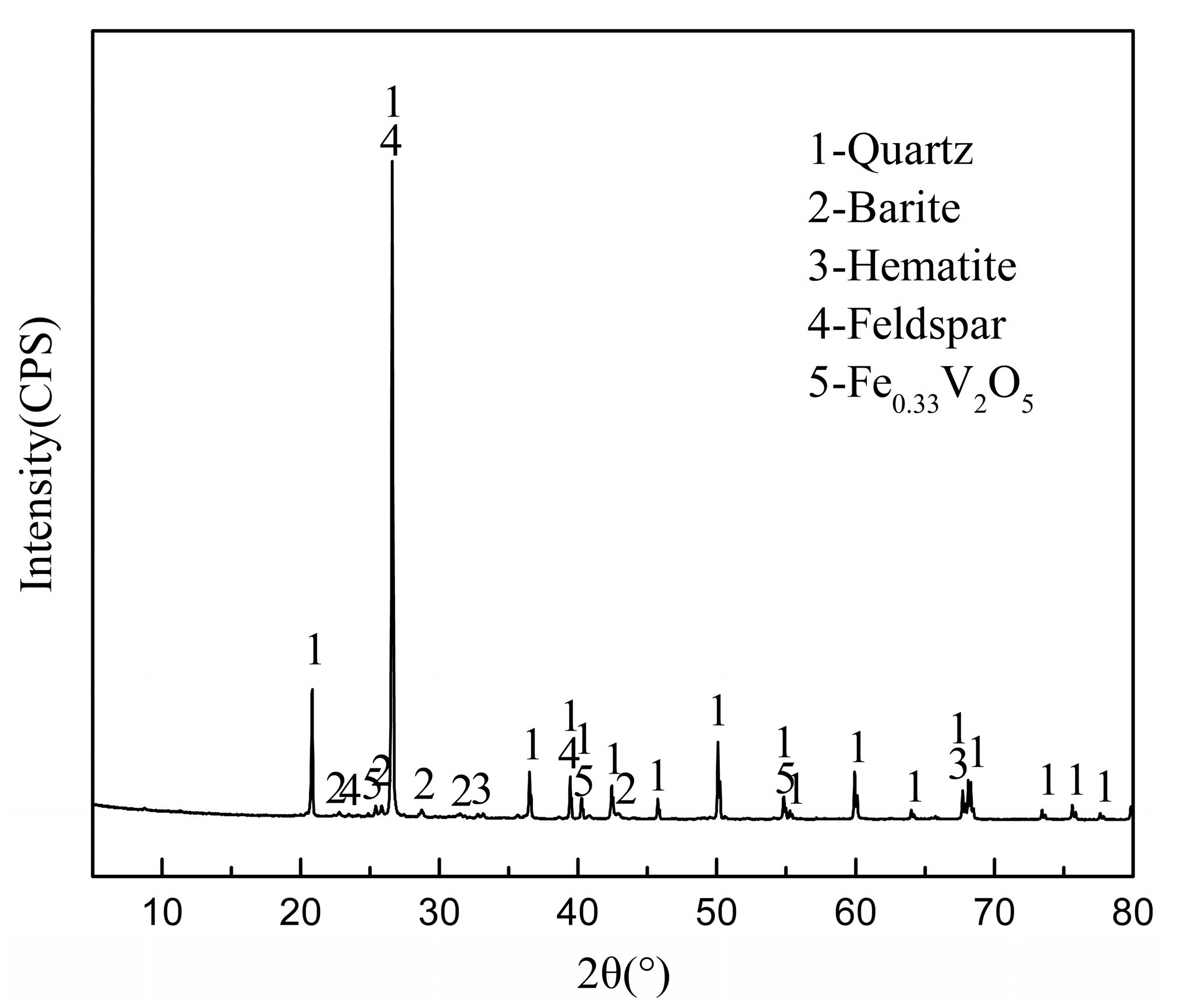

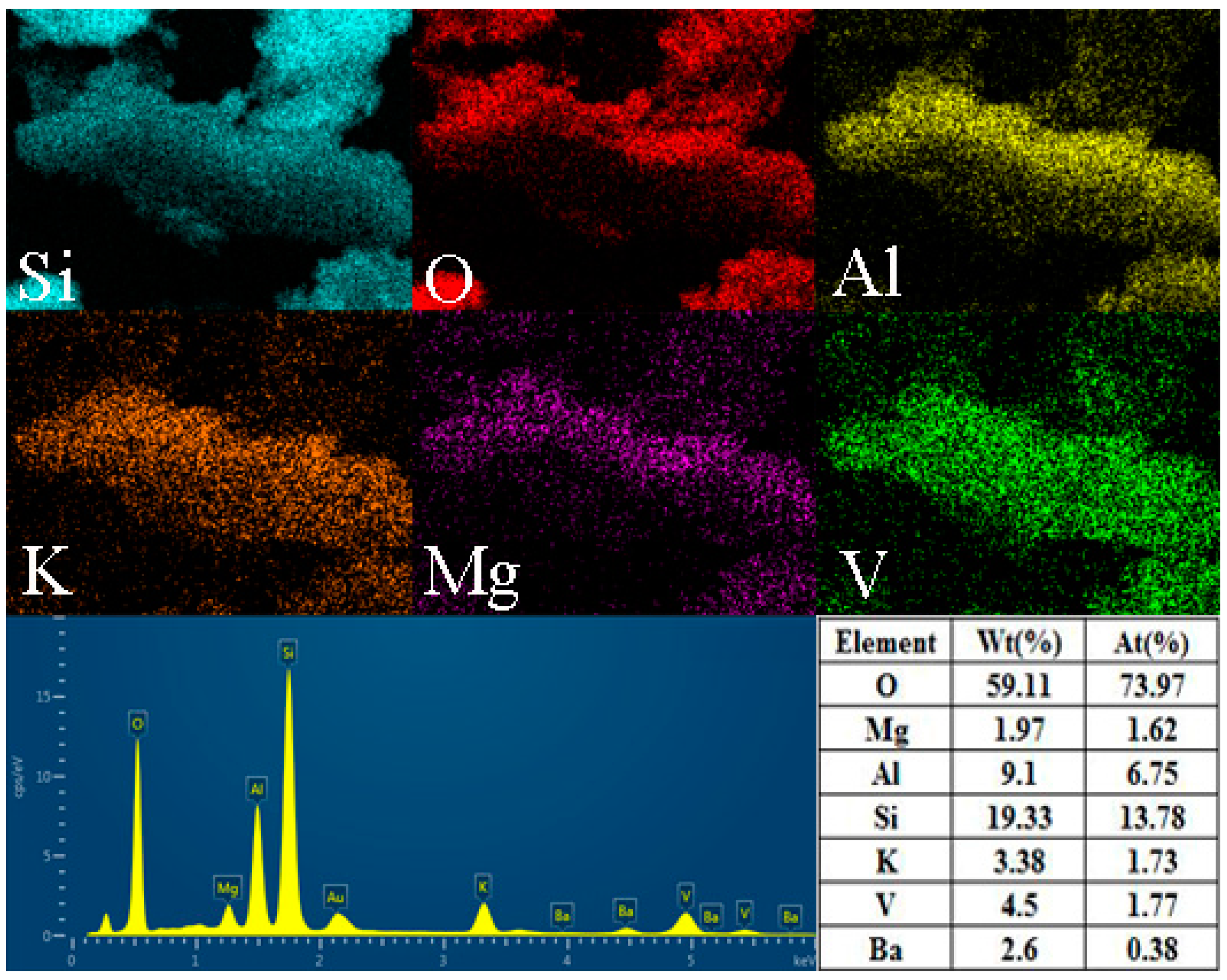


| Element | V | Fe | Si | Al | K | S | Ca | Mg |
|---|---|---|---|---|---|---|---|---|
| Content, wt % | 0.73 | 1.14 | 41.82 | 1.45 | 0.67 | 0.55 | 1.04 | 0.37 |
| Total Vanadium | Bound to Free Oxides | Silico-Aluminate | Bound to Organic |
|---|---|---|---|
| 100 | 63.87 | 32.35 | 3.78 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, D.; Liu, T.; Zhang, Y.; Cai, Z.; He, J.; Xu, C. Vanadium Bioleaching Behavior by Acidithiobacillus ferrooxidans from a Vanadium-Bearing Shale. Minerals 2018, 8, 24. https://doi.org/10.3390/min8010024
Wei D, Liu T, Zhang Y, Cai Z, He J, Xu C. Vanadium Bioleaching Behavior by Acidithiobacillus ferrooxidans from a Vanadium-Bearing Shale. Minerals. 2018; 8(1):24. https://doi.org/10.3390/min8010024
Chicago/Turabian StyleWei, Dunpei, Tao Liu, Yimin Zhang, Zhenlei Cai, Jingtao He, and Chengbao Xu. 2018. "Vanadium Bioleaching Behavior by Acidithiobacillus ferrooxidans from a Vanadium-Bearing Shale" Minerals 8, no. 1: 24. https://doi.org/10.3390/min8010024
APA StyleWei, D., Liu, T., Zhang, Y., Cai, Z., He, J., & Xu, C. (2018). Vanadium Bioleaching Behavior by Acidithiobacillus ferrooxidans from a Vanadium-Bearing Shale. Minerals, 8(1), 24. https://doi.org/10.3390/min8010024




