3.4.1. Influence of the Initial Pb2+ Concentration
Since it is well known that natural zeolite may interact with the hydrogen or hydroxyl ions from water solutions [
33], checking of the immutability of all the adsorbents has been of interest. For that purpose, the stability of the NZA and FeA in distilled water was tested and compared to stabilities of NZ and FeZ, and the results are shown in
Table 5.
Results given in
Table 5 showed much lower amounts of the released cations (K
+, Na
+, Mg
2+, Ca
2+) than those obtained by determination of the CEC, for all the adsorbents. That means that all of the samples have high stabilities in water solution. However, the stability of the FeZ is slightly higher than NZ, while modification with Na-alginate had no significant influence on stability of NZA and FZA. Also, the amounts of released iron, aluminium, and silica were very low, supporting the previous conclusion that all of the samples are very stable in water.
Further, Pb
2+ adsorption on NZA and FeA at different initial concentrations (18–320 mg Pb
2+/g) was investigated. During these experiments increase of pH of all the suspensions was observed, and the final pH for both adsorbents was between ~6.1 (for 18 mg Pb
2+/g) and 4.5 (for 320 mg Pb
2+/g), meaning that lead was predominantly in cationic-Pb
2+ form [
2]. The obtained results are shown in
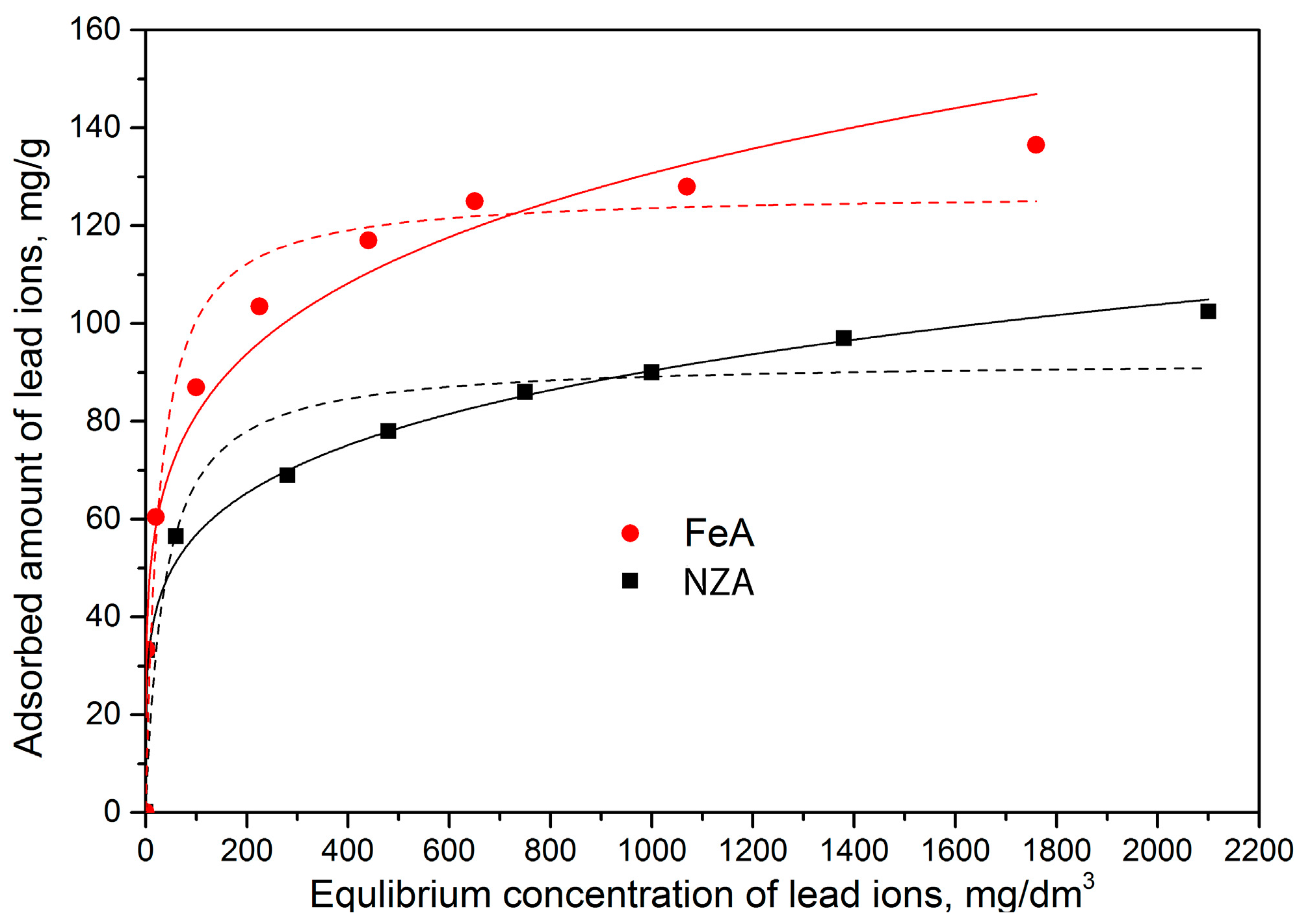
Figure 5.
From
Figure 5 it can be seen that, for both adsorbents, adsorption of Pb
2+ increased with increasing its initial concentration. However, a significantly higher adsorption was achieved for FeA. For the highest initial concentration, maximum adsorbed amounts of Pb
2+ were: 102 mg/g for NZA and 136 mg/g for FeA, respectively. Our previous studies [
7] showed adsorption capacities of 66 for NZ and 133 mg/g for FeZ. By comparing results for NZ and NZA, or FeZ and FeA, it can be concluded that modification with Na-alginate, in addition to encapsulation, also have a positive effect on adsorption capacities of both adsorbents. However, a higher positive effect on adsorption capacity was obtained for the NZ. That could be indication that the adsorption properties of the starting materials (NZ and FeZ) are also important for the removal of Pb
2+ by NZA and FeA.
The experimental results were fitted to two the most commonly used adsorption models: Langmuir (Equation (2)) and Freundlich (Equation (3)) [
10]:
where
qmax is maximal adsorption capacity, (mg/g);
b is Langmuir constant that is related to heat of the adsorption (dm
3/mg);
Ce is equilibrium concentration of Pb
2+ (mg/dm
3) and
qe (mg/g) is adsorbed amount of Pb
2+,
KF (dm
3/mg); and,
n are Freundlich constant related to the adsorption capacity and factor of heterogeneity of surface, respectively. The characteristic parameters of these two models are given in
Table 6.
From
Table 6 it can be seen that, for both adsorbents, due to the higher values of R
2, Freundlich isotherm better fits the experimental data. That means, that adsorption of Pb
2+ on both adsorbents takes place over complex mechanism, and/or surfaces of the adsorbents are heterogeneous and/or adsorption is not limited only to monolayer [
34]. For both of the adsorbents, Freundlich parameter,
n, was <1, indicating that adsorption intensity was good (or favourable) over the entire range of studied concentrations [
35]. Mahmoud and Mohamed (2012) [
36] also found the best fitting of the experimental results with Freundlich model for removal of Pb
2+ from water solutions by using sodium alginate/itaconic acid hydrogel. However, the obtained value of the
n parameter was >1, meaning that the adsorption intensity was favourable at a high concentration, but much less at lower concentrations. On the other side, Stewart et al. (2009) [
37] found that Langmuir model better describes the adsorption of Pb
2+ from water solutions on Ca-alginate. Cheraghali et al. (2013) [
38] also found the best fit of the experimental data with Langmuir model for the removal of Pb
2+ from water solutions by alginate-nanoporous silica composite.
For a better explanation of differences in adsorption capacities between NZ and NZA, FeZ and FeA, or NZA and FeA, and understanding of the adsorption mechanism, the concentrations of Ca
2+, Mg
2+, Na
+, and K
+ released from NZA and FeA, were measured, and their sum, for each initial concentration, when compared to the amounts of Pb
2+ removed from solutions. The obtained results are shown in
Figure 6.
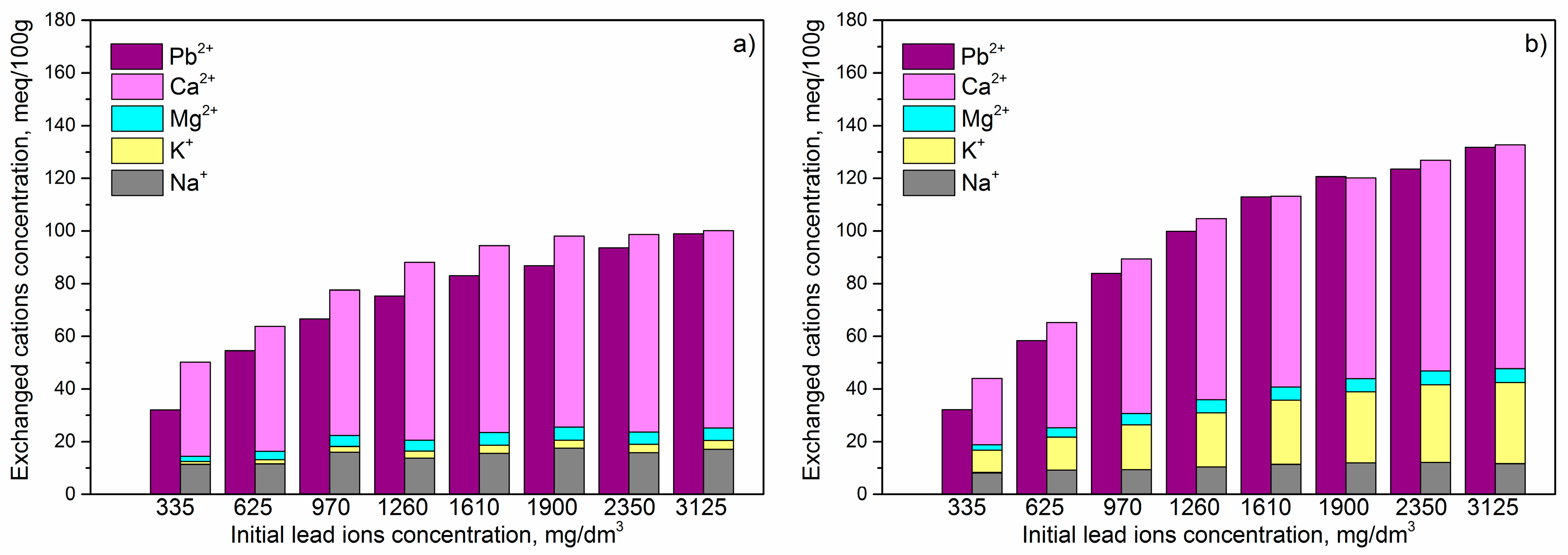
Results that are given in
Figure 6 showed, for both adsorbents, increase of the adsorbed amount of Pb
2+, as well as the total amount of released cations with increasing of the initial Pb
2+ concentration. For NZA and FeA, the ratio between released and adsorbed equivalents of positive charges were stoichiometric for higher initial concentrations, while for lower initial concentrations, the total amount of exchanged cations was higher than the amount of adsorbed Pb
2+. These results suggest that ion exchange was the only mechanism included in the removal of Pb
2+ by NZA and FeA. Also, we observed a direct correlation between the amount of Pb
2+ adsorbed and amounts of exchangeable cations released. For NZA, Pb
2+ were mainly exchanged with calcium, then sodium, while the contribution of potassium and magnesium was considerably lower. For FeA, Pb
2+ were also mainly exchanged with calcium and potassium, while the ion exchange with sodium and magnesium was significantly smaller. The largest accessibility of calcium for ion exchange can be explained with the modification procedure by using CaCl
2 for preparation of composite samples. On the other side, releasing of the potassium and magnesium during the adsorption process indicate that ion exchange with Pb
2+, beside cations from alginate, also includes cations from the surface of NZ in NZA or FeZ in FeA. These results are considerable different in comparison with results for NZ and FeZ [
6], where adsorption process beside ion exchange include chemisorption. Additionally, due to the presence of iron, much higher amount of Pb
2+ was chemisorbed on the surface of FeZ than NZ. Changed adsorption mechanism after encapsulation could be explained with trapping and unavailability of the active centres where chemisorption occurs due to the presence of big polysaccharide molecule in NZA and FeA. Finally, because a higher number of active centres for chemisorption were trapped in FeA, after encapsulation, a much lower increase of adsorption capacity was obtained than for NZA (
Figure 5).
3.4.2. Influence of the Initial pH
Solution pH is a critical factor significantly affecting the metal ions adsorption [
39]. At different pH values, lead can be in different forms in water solutions [
2], and also, pH can influence the ionization of chemically active sites on the adsorbent surface. It was therefore of interest to investigate the effect of solution pH on the Pb
2+ adsorption on NZA and FeA. Since the primary goal of this study was the removal of Pb
2+ from solutions, experiments were carried out for initial pH (pH
i) from 2.5 to 5.2, and the results are shown in
Figure 7a.
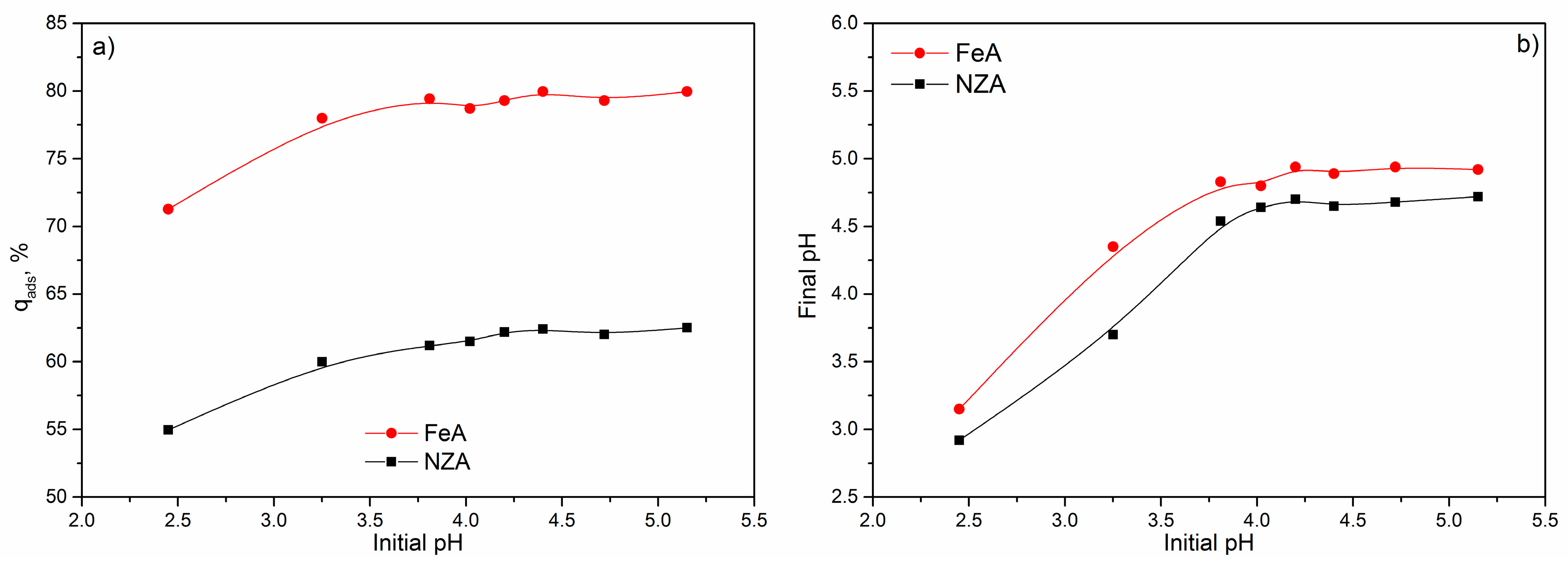
As it is shown in
Figure 7a, the removal of Pb
2+ from solutions slightly increased (from ~55% to ~61% for the NZA and from ~72% to ~79% for FeA) with an increasing of the pH
i from 2.5 to 3.8. Further increase of the pH
i from 3.8 to 5.2, for both of the adsorbents, had no significant influence on the removal of Pb
2+, and adsorption reached a maximum (~63% for NZA and ~80% for FeA). Increasing of the adsorbed amount of Pb
2+ for ~8%, for both adsorbents, when pH
i increased from 2.5 to 5.2, indicated that initial pH does not have a significant influence on the adsorption capacities of adsorbents. However, slightly higher removal of Pb
2+ was obtained for pH
i higher than 3.8, for NZA and FeA. These results are in accordance with our previous results [
40], where it was shown that initial pH had no significant influence on the removal of Pb
2+ by NZ and FeZ, and the highest removal is achieved for initial pH > 4.0.
During these experiments, final pH values (pH
f) were also measured and results are shown at
Figure 7b. For both adsorbents, an increase of pH
i from 2.5 to 3.8 increased pH
f. After that, curves pH
f = f(pH
i) reached equilibrium, and a further increase of pH
i up to 5.2 had no significant influence on pH
f. Also, for 2.5 ≤ pH
i ≤ 4.7, pH
f values were higher, while for pH
i > 4.7, the pH
f values were lower than pH
i.
Higher values of pH
f than pH
i obtained for 2.5 ≤ pH
i ≤ 4.7 can be explained by the competitive removal of H
+ from solution caused by a high concentration of H
+ in solution. Higher pH
f values obtained for FeA than NZA for same pH
i indicated that a higher amount of H
+ were removed by the FeA. Competitive bounding of the H
+ leads to a decrease in the amount of Pb
2+ immobilized onto NZA and FeA [
39]. Furthermore, at lower initial pHs, the alkaline groups at surface of the adsorbents were protonated, thus electrostatic repulsion of Pb
2+ caused decrease of Pb
2+ adsorption capacity [
33]. After increase of pH
i, due to decrease of H
+ concentration, the competition of Pb
2+ and H
+ becomes weak, resulting in more Pb
2+ and lower H
+ immobilization onto two adsorbents. Consequently, a lower increase of the pH
f was observed. For the highest pH
i, pH of solutions decreased, as the result of removal of protons from surface Brønsted acidic sites, or from zeolitic water that coordinate exchangeable cations in adsorbents [
33].
In order to confirm competitive bounding of the H
+, for all of the initial pH, amounts of released cations were measured and compared with amounts of removed Pb
2+ from solutions, and results are shown at
Figure 8.
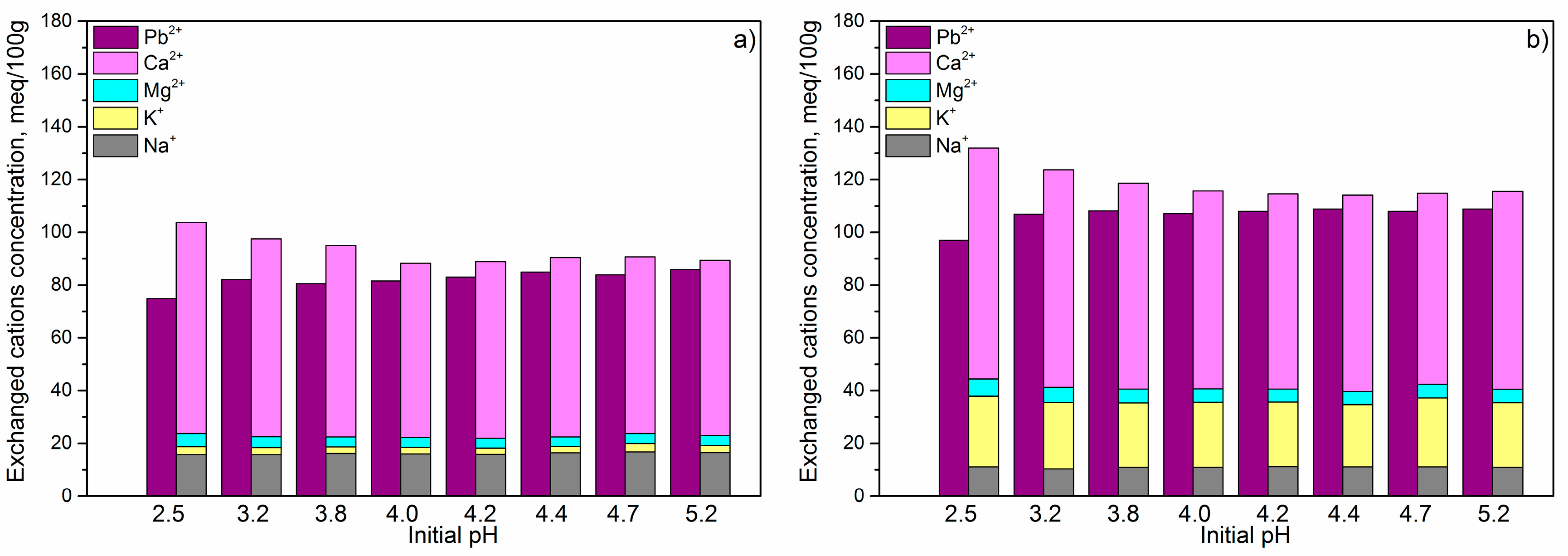
Results from
Figure 8 showed that, for all the initial pHs, the total amounts of exchanged cations was higher than the amount of removed Pb
2+. That means that pH had no influence on the adsorption mechanism, and ion exchange was the only mechanism included in removal of the Pb
2+ for all initial pHs. For both the adsorbents, for the pH
i = 2.5, the total amount of the released cations was much higher than removed Pb
2+ concentration, confirming that H
+ together with Pb
2+ from solutions participate in ion exchange with exchangeable cations. Due to decrease of H
+ concentration, and therefore their competitiveness, increasing of the pH
i up to 4.0 decreased the difference between amounts of the total exchanged cations and removed Pb
2+. For the pH
i > 4.0 concentration of H
+ was lower than 0.1 mmol/dm
3, which is much lower than concentration of the Pb
2+ in solution (~7 mmol/dm
3), and consequently, their competitiveness was negligible. For that reason, almost stoichiometric ratio of the total concentration of the exchanged cations and removed Pb
2+ was observed. Also, for all the initial pHs, for both of the adsorbents, the contribution of each individual exchangeable cation in ion exchange with Pb
2+ (
Figure 8) was the same as it was described in
Figure 6, indicating that pH
i had no influence on the accessibility of individual exchangeable cations.
Good removals obtained by NZA and FeA, for applied experimental conditions, which are presented in this study, indicated that both alginate composites may be used as potential purifiers of waters contaminated with lead. Possible application may be in mining industry, as materials for collector filters on tailings. Namely, according to the Environmental Protection Agency (2005), in the “Report of the State of the Environment in Republic of Serbia”, it is estimated that there are around 700 million tons of flotation and separation tailings, between 1.4 and 1.7 billion tons of tailing wastes from opening pits and around 170 million tons of ashes from thermal power plants on deposit spots and landfills in Serbia [
41]. From that reason, experiments on real samples were done. For that purpose, preliminary experiments on removal of heavy metals from wastewater from facility of lead and zinc mine were performed, and the obtained results are shown in
Table 7.
As can be seen, different elements are presented in investigated water, whereby the concentration of some of them is very high and dangerous to human health. For example, the concentration of lead was ~60, mercury ~2500 or manganese ~615 times higher than the maximum allowed concentration in drinking water. On the other side, concentration of cadmium, barium, beryllium, and copper was very low and below the detection limit of the instrument.
After treatment of wastewater with NZA and FeA, the content of all heavy metal cations was reduced. For both adsorbents, after treatment, the concentration of lead in wastewater was lower than the detection limit of the instrument. On the other side, NZA showed a better removal of zinc and alumina, while FeA better adsorbed manganese and mercury. Higher amounts of Mg, Ca, and Si after the treatment process may be a consequence of ion exchange (Ca and Mg) or very small dissolution of natural or Fe(III)-modified zeolite from NZA or FeA, respectively (Si). However, concentrations of all three elements, as well as Fe, were lower than the maximum allowed amounts for drinking waters, and therefore do not pose a risk to human health and nature in general. From these results, it may be concluded that both samples, and especially FeA, could be used for the production of collector filters for tailings. However, for practical applications, additional investigations must be done, and that will be the aim of our further research.