Quartz-Amethyst Hosted Hydrocarbon-Bearing Fluid Inclusions from the Green Ridge Breccia in the Snoqualmie Granite, North Cascades, WA, USA
Abstract
1. Introduction
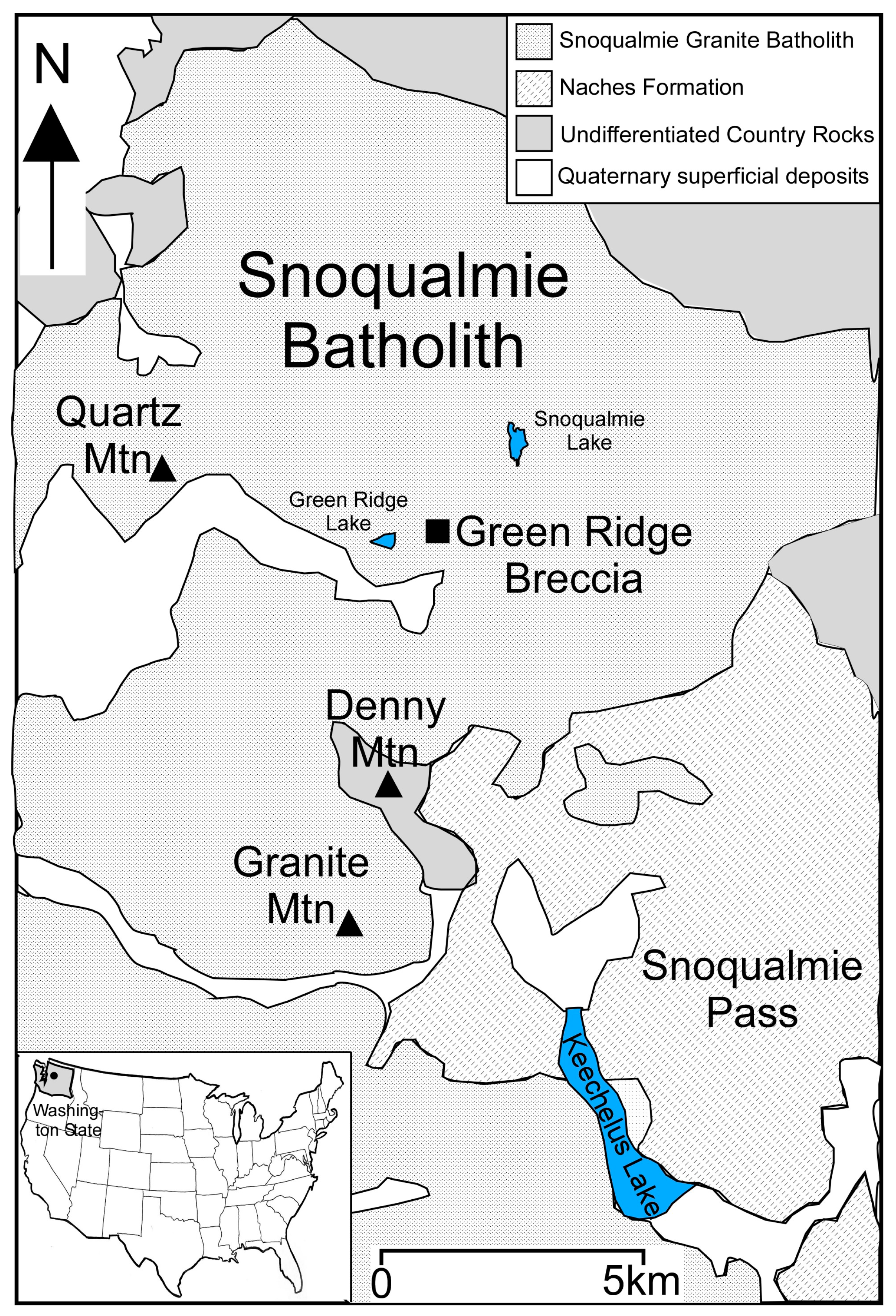
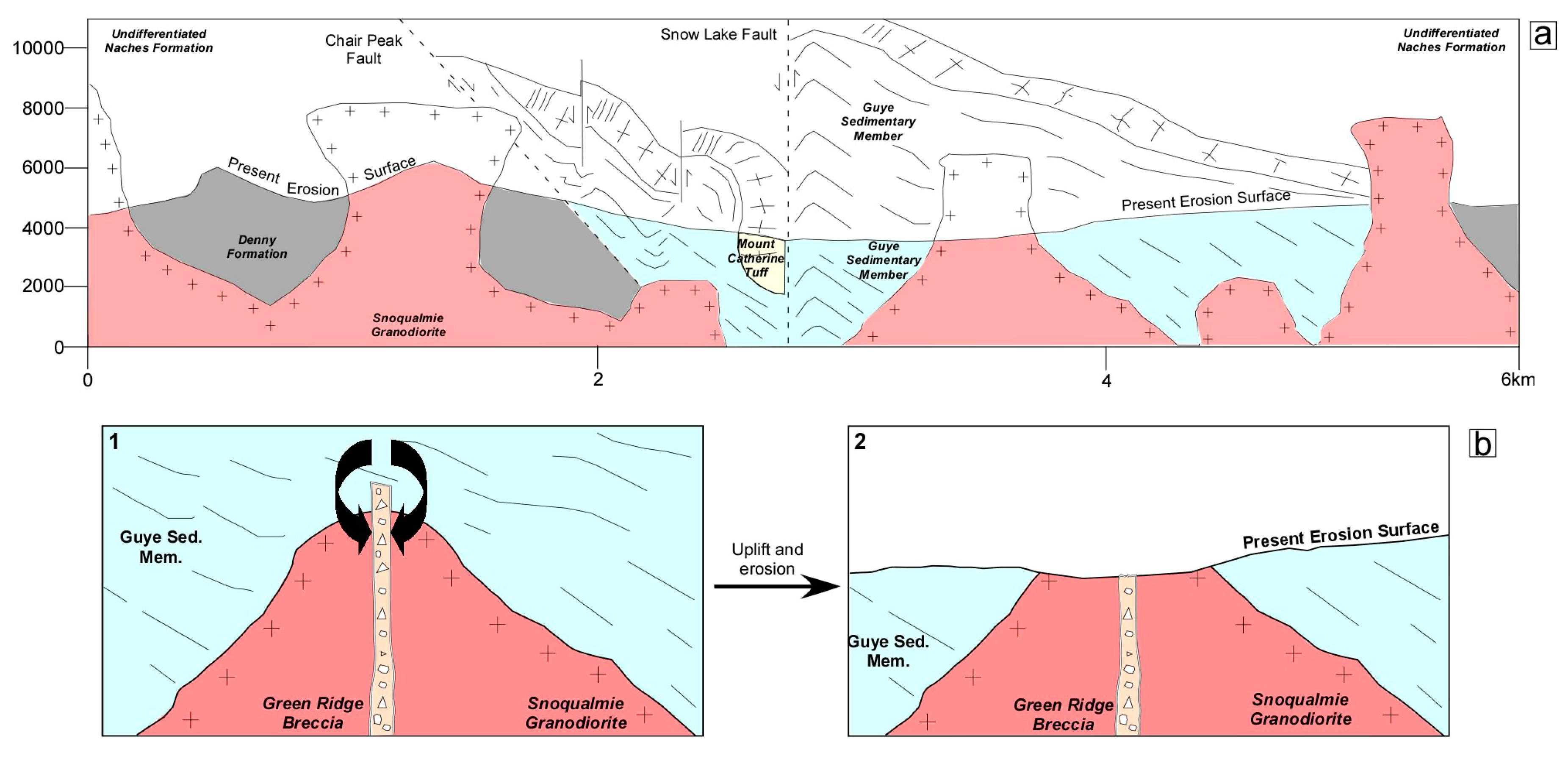
2. Geological Background
3. Analytical Techniques
4. Results
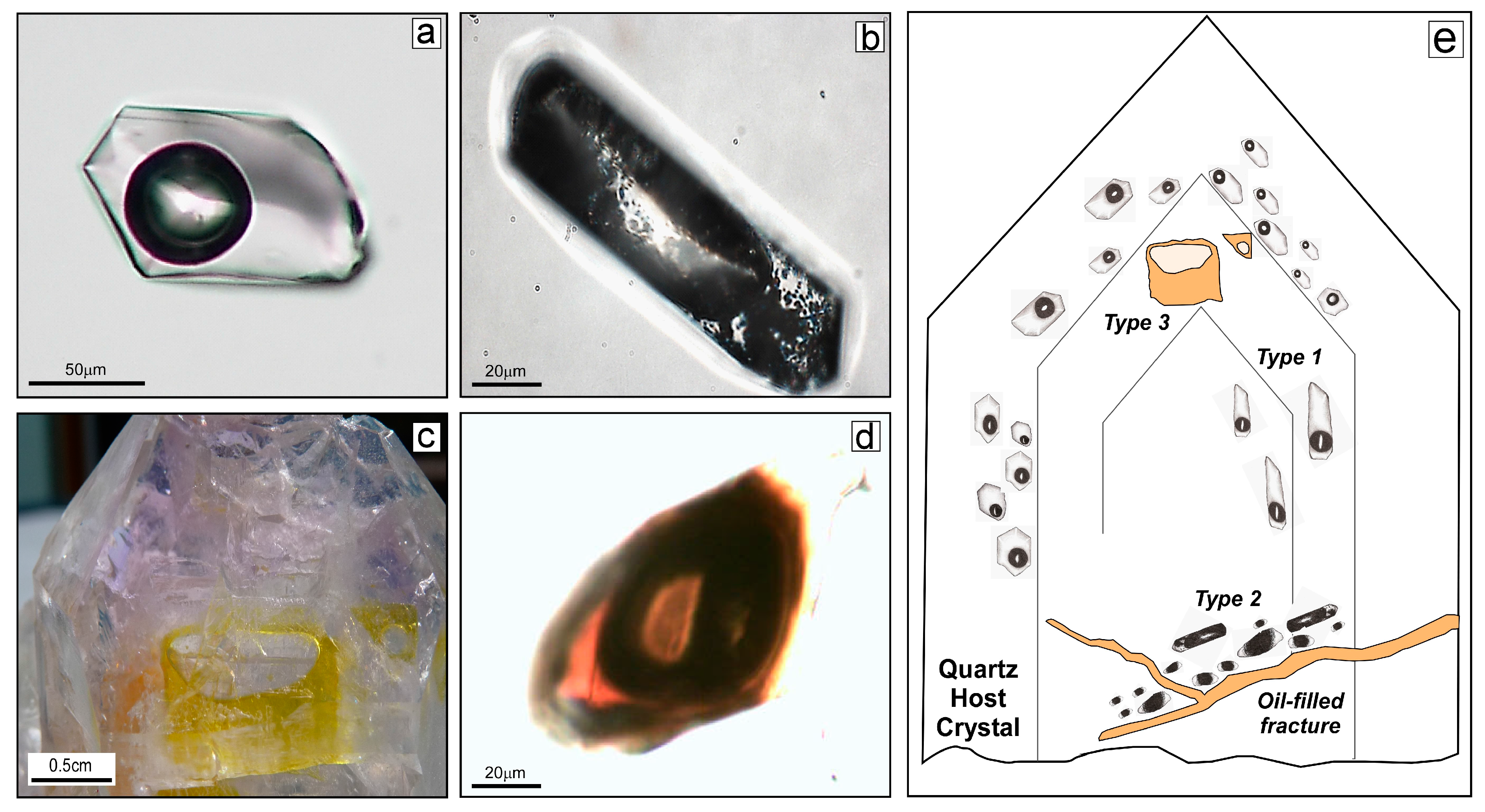
4.1. Fluid Inclusion Petrography
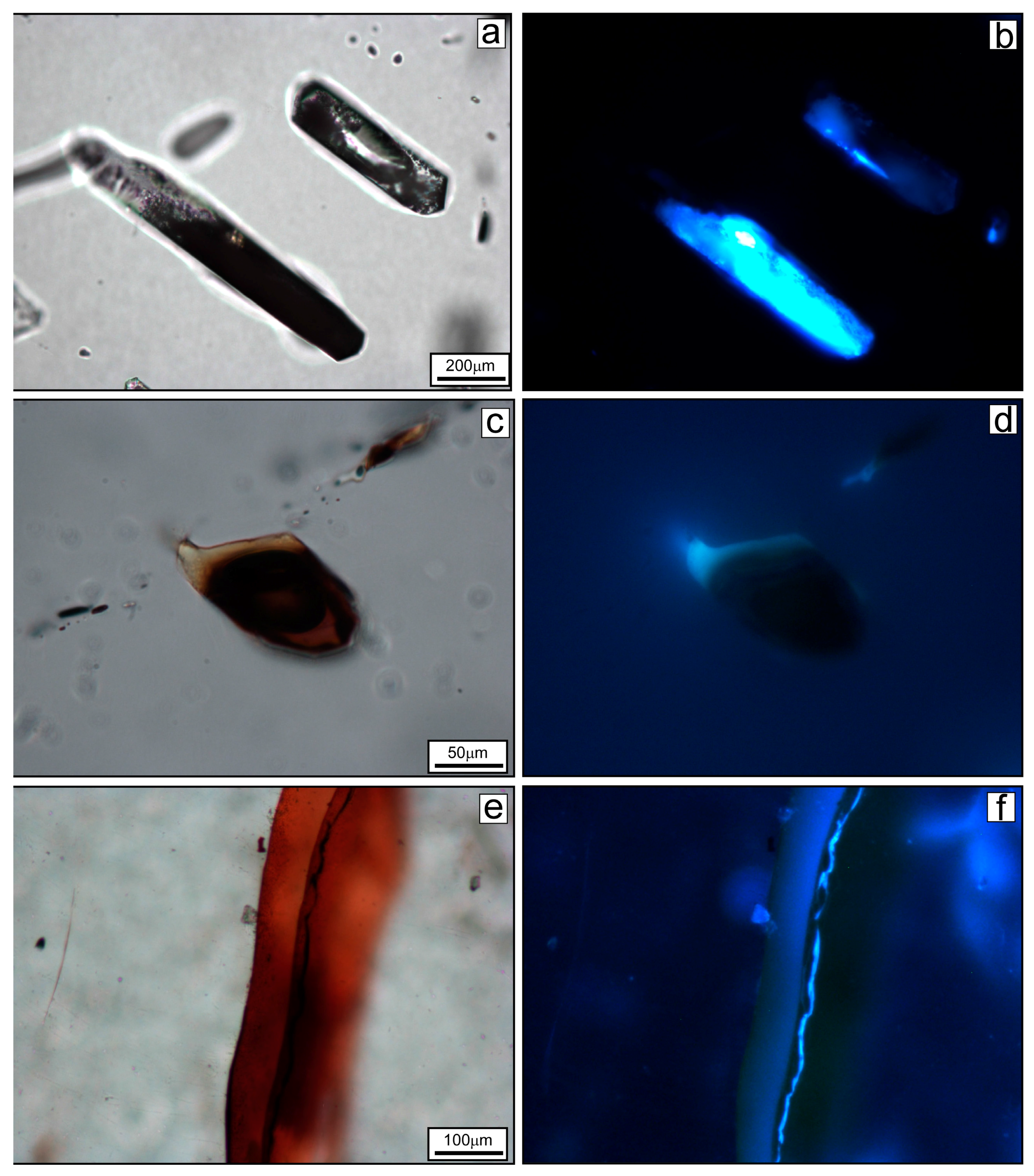
4.2. UV Light Microscopy
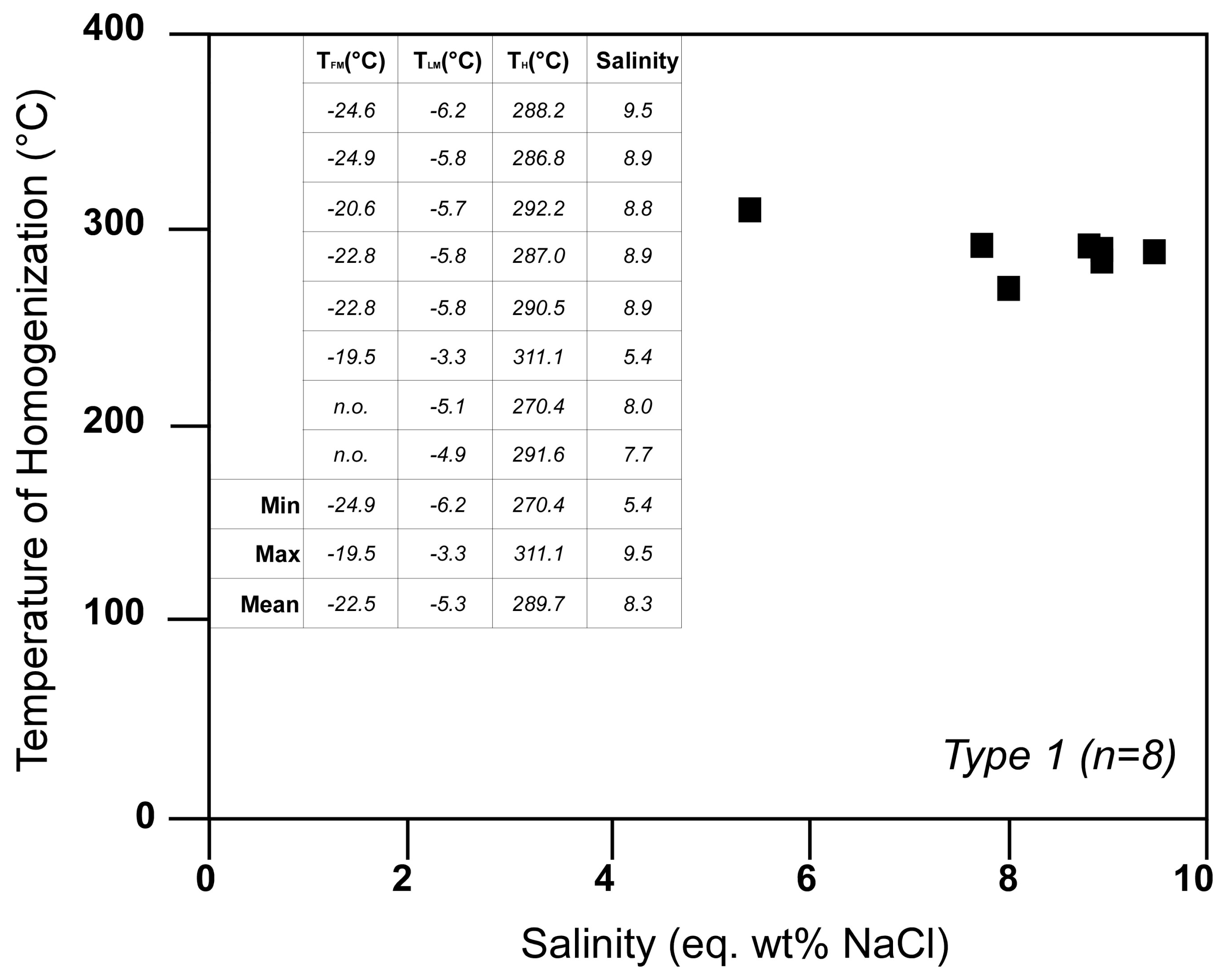
4.3. Microthermometry and LRM of Type 1 Fluid Inclusions
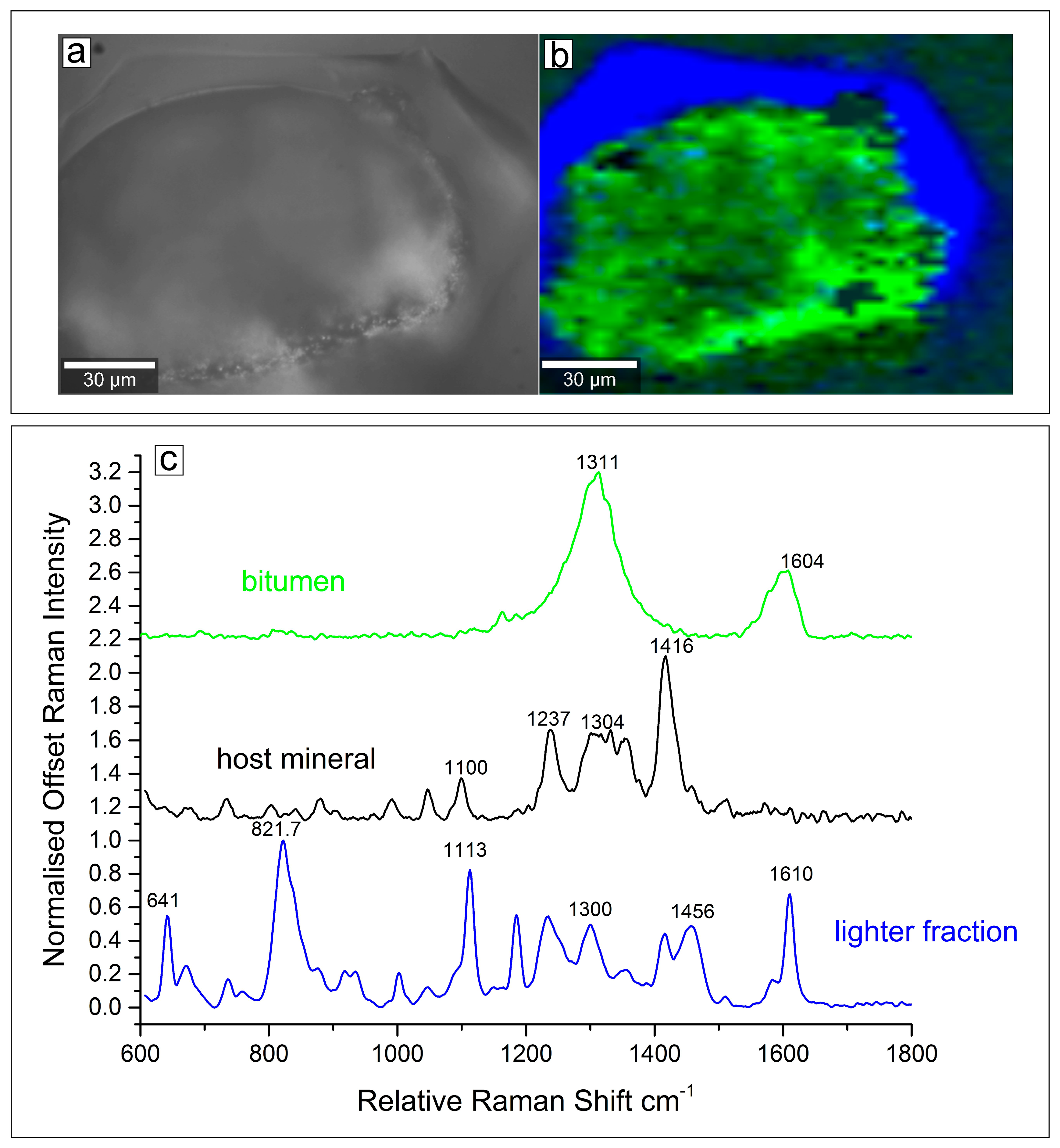
4.4. Confocal Laser Raman Microscopy (CLRM) of Type 2 Fluid Inclusions
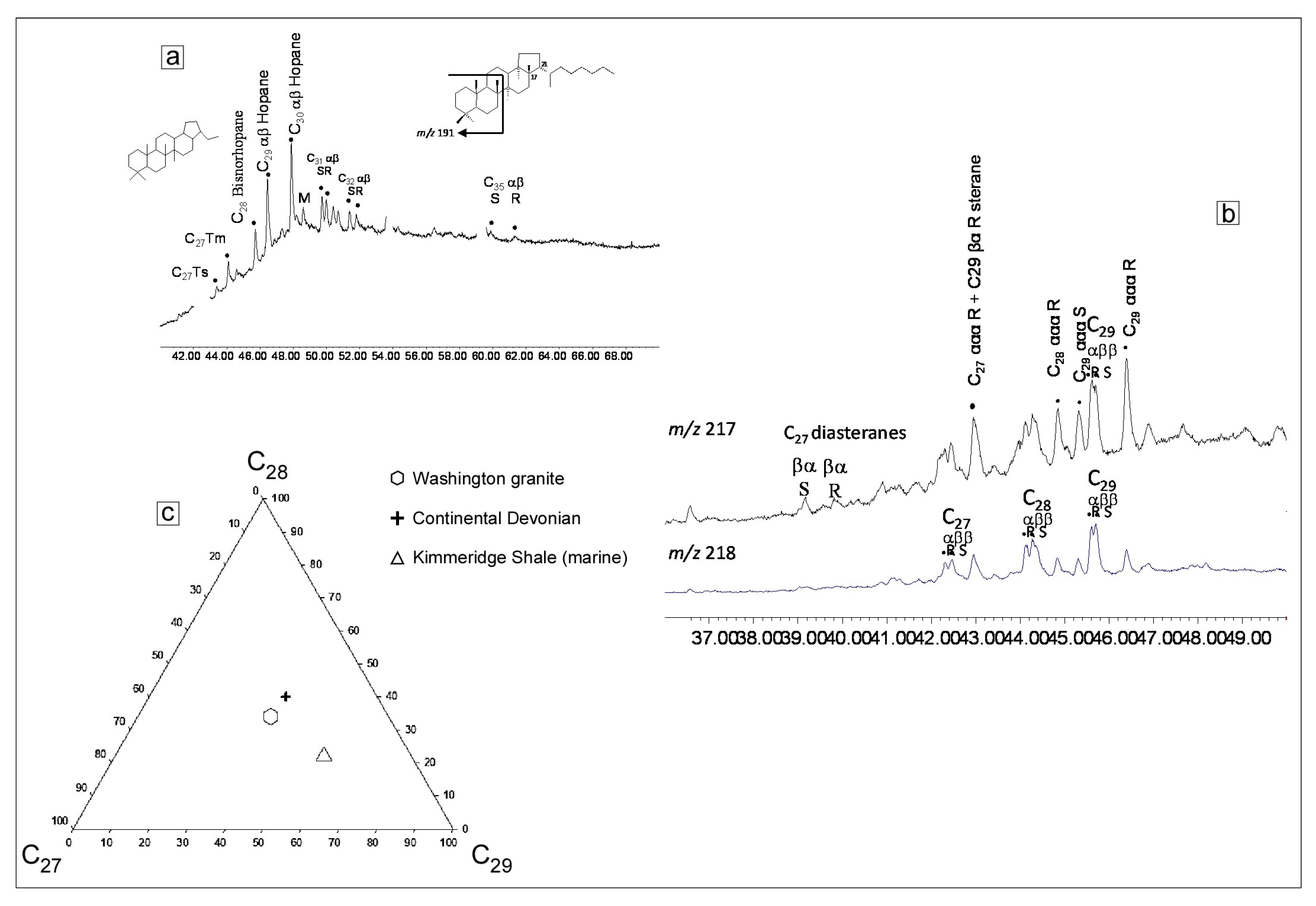
4.5. Gas Chromatograhy-Mass Spectrometry (GC-MS) of the Amber Coloured Oil in Type 3 Inclusions
5. Interpretation of Results
5.1. Source of Type 1 Fluids
5.2. The Bitumen Bearing Type 2 Inclusions
5.3. Source of Amber Coloured Oil in Type 3 Inclusions
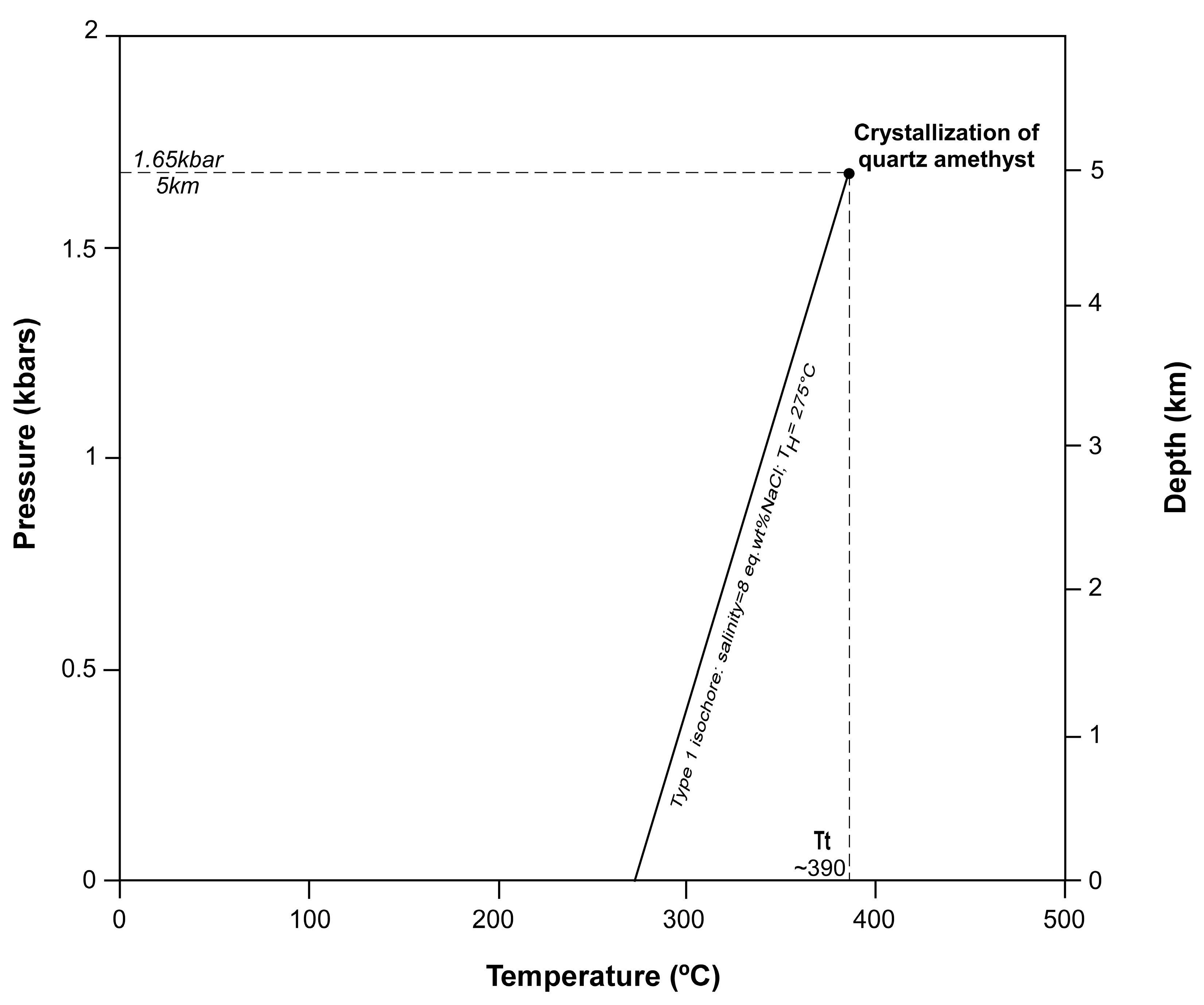
5.4. Pressure–Temperature Modelling of Type 1 Fluid Inclusions
5.5. Biogenic Hydrocarbon Migration into the Quartz-Amethyst Euhedra of the Green Ridge Breccia
6. Summary and Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dutkiewicz, A.; Volk, H.; Ridley, J.; George, S.C. Geochemistry of oil in fluid inclusions in a middle Proterozoic igneous intrusion: Implications for the source of hydrocarbons in crystalline rocks. Org. Geochem. 2004, 35, 937–957. [Google Scholar] [CrossRef]
- Konnerup-Madsen, J.; Larsen, E.; Rose-Hansen, J. Hydrocarbon-rich fluid inclusions in minerals from the alkaline Ilimaussaq intrusion, South Greenland. Bull. Miner. 1979, 102, 642–653. [Google Scholar]
- Abrajano, T.A.; Sturchio, N.C.; Bohlke, J.K.; Lyon, G.L.; Poreda, R.J.; Stevens, C.M. Methane–hydrogen gas seeps, Zambales Ophiolite, Philippines: Deep or shallow origin? Chem. Geol. 1988, 71, 211–222. [Google Scholar] [CrossRef]
- Salvi, S.; Williams-Jones, A.E. Fischer–Tropsch synthesis of hydrocarbons during sub-solidus alteration of the Strange Lake peralkaline granite, Quebec/Labrador, Canada. Geochim. Cosmochim. Acta 1997, 61, 83–99. [Google Scholar] [CrossRef]
- Potter, J.; Rankin, A.H.; Treloar, P.J.; Nivin, V.A.; Ting, W.; Ni, P. The preliminary study of methane inclusions in alkaline igneous rocks of the Kola igneous province, Russia: Implications for the origin of methane in igneous rocks. Eur. J. Miner. 1998, 10, 1167–1180. [Google Scholar] [CrossRef]
- Sherwood Lollar, B.; Westgate, T.D.; Ward, J.A.; Slater, G.F.; Lacrampe-Couloume, G. Abiogenic formation of alkanes in the Earth’s crust as a minor source of global hydrocarbon reservoirs. Nature 2002, 416, 522–524. [Google Scholar] [CrossRef] [PubMed]
- Parnell, J. Migration of biogenic hydrocarbons into granites: A review of hydrocarbons in British plutons. Mar. Pet. Geol. 1988, 5, 385–396. [Google Scholar] [CrossRef]
- Petford, N.; McCaffrey, K. Hydrocarbons in crystalline rocks: An introduction; Geological Society: London, UK, Special Publications; 2003; Volume 214, pp. 1–5. [Google Scholar]
- Trice, R. Basement exploration, West of Shetlands: Progress in opening a new play on the UKCS. In Hydrocarbon Exploration to Exploitation West of Shetlands; Cannon, S.J.C., Ellis, D., Eds.; Geological Society: London, UK, Special Publications; 2014; Volume 397, pp. 81–105. [Google Scholar]
- Thomson, M.L.; Mastalerz, M.; Sinclair, A.J.; Bustin, R.M. Fluid source and thermal history of an epithermal vein deposit, Owen Lake, central British Columbia: Evidence from bitumen and fluid inclusions. Miner. Depos. 1992, 27, 219–225. [Google Scholar] [CrossRef]
- Rossman, G.R. Colored varieties of the silica minerals. Rev. Miner. 1994, 29, 433–467. [Google Scholar]
- Gilg, H.A.; Morteani, G.; Kostitsyn, Y.; Preinfalk, C.; Gatter, I.; Strieder, A.J. Genesis of amethyst geodes in basaltic rocks of the Serra Geral Formation (Ametista do Sul, Rio Grande do Sul, Brazil): A fluid inclusion, REE, oxygen, carbon, and Sr isotope study on basalt, quartz, and calcite. Miner. Depos. 2003, 38, 1009–1025. [Google Scholar] [CrossRef]
- Thomas, R.; Blankenburg, H.J. Erste Ergebnisse uÅN ber Einschlussuntersuchungen an Quarzen aus Achatmandeln und Kugeln basischer und sauerer Vulkanite. Z. Geol. Wiss. 1981, 9, 625–633. (In German) [Google Scholar]
- Juchem, P.L.; Fallick, A.E.; Bettencourt, J.S.; Svisero, D.P. Geoquímica isotó pica de oxigênio em geodos mineralizados a ametista da região do Alto Uruguaí, RS—Um estudo preliminar. 1. In Símposio Sobre Vulcanismo e Ambientes Asociados; Cidade Universitária: Gramado, Brazil, 1999. [Google Scholar]
- Fallick, A.E.; Jocelyn, J.; Donnelly, T.; Guy, M.; Behan, C. Origin of agates in volcanic rocks from Scotland. Nature 1985, 313, 672–674. [Google Scholar] [CrossRef]
- Fallick, A.E.; Jocelyn, J.; Hamilton, P.J. Oxygen and hydrogen stable isotope systematics in Brazilian agates. In Geochemistry and Mineral Formation in the Earth Surface; Rodriguez-Clemente, R., Tardy, Y., Eds.; Consejo Superiór de Investigaciónes Científicas: Madrid, Spain, 1987; pp. 99–117. [Google Scholar]
- McArthur, J.R.; Jennings, E.A.; Kissin, S.A.; Sherlock, R.L. Stable isotope, fluid-inclusion and mineralogical studies relating to the genesis of amethyst, Thunder Bay, Amethyst Mine, Ontario. Can. J. Earth Sci. 1993, 30, 1955–1969. [Google Scholar] [CrossRef]
- Balitsky, V.S. Les conditions de formation des améthystes et leur croissance artificielle. Bull Minér. 1978, 101, 383–386. (In French) [Google Scholar]
- Robinson, R.W.; Norman, D.I. Mineralogy and fluid inclusion study of the Southern Amethyst vein system, Creede mining district, Colorado. Econ. Geol. 1984, 79, 439–447. [Google Scholar] [CrossRef]
- Gatter, I. Fluid inclusion studies in the polymetallic ores of Gyo ngyo soroszi (North Hungary)—Spatial and temporal evolution of ore-forming fluids. Chem. Geol. 1987, 61, 169–181. [Google Scholar] [CrossRef]
- Yang, K.H.; Yun, S.H.; Lee, J.D. A fluid inclusion study of an amethyst deposit in the Cretaceous Kyongsan Basin, South Korea. Miner. Mag. 2001, 64, 477–487. [Google Scholar] [CrossRef]
- Dragovich, J.D.; Logan, R.L.; Schasse, H.W.; Walsh, T.J.; Lingley, W.S., Jr.; Norman, D.K.; Gerstel, W.J.; Lapen, T.J.; Schuster, J.E. Geological map of Washington-Northwest Quadrant. In Washington Division of Geology and Earth Resources Geologic Map; GM-50, 72 p. Pamphlet, 3 Sheets, Scale 1:250,000; Washinton State Department of Natural Resources: Olympia, WA, USA, 2002. [Google Scholar]
- Foster, R.J. Tertiary Geology of a portion of the Central Cascade Mountains, Washington. Bull. Geol. Soc. Am. 1960, 71, 99–126. [Google Scholar] [CrossRef]
- Chitwood, L.A. Stratigraphy, Structure and Petrology of the Snoqualmie Pass Area, Washington. Ph.D. Thesis, Portland State University, Portland, OR, USA, 1976. [Google Scholar]
- Erikson, E.H. Petrology of the Composite Snoqualmie Batholith, Central Cascade Mountains, Washington. Geol. Soc. Am. Bull. 1969, 80, 2213–2236. [Google Scholar] [CrossRef]
- Tabor, R.W.; Frizzell, V.A., Jr.; Booth, D.B.; Waitt, R.B. Geologic map of the Snoqualmie Pass 30–60 Min Quadrangle, Washington, U.S. In Geological Survey, Geologic Investigations; Series I-2538, Online Version 1.0; DGGS: Fairbanks, AK, USA, 1995. [Google Scholar]
- Grant, A.R. Chemical and Physical Controls for Base Metal Deposition in the Cascade Range of Washington; State of Washington, Department of Natural Resources Bulletin 58; Washinton State Department of Natural Resources: Olympia, WA, USA, 1969.
- Gualtieri, J.L.; Simmons, G.C.; Thurber, H.K.; Miller, M.S.; Davis, W.E. Mineral resources of the Alpine Lakes study area, Chelan, King, and Kittitas Counties, Washington: U.S. In Geological Survey Open-File Report; United States Geological Survey: Reston, VA, USA, 1973; p. 132. [Google Scholar]
- Gualtieri, J.L.; Thurber, H.K.; Miller, M.S.; McMahan, M.C.; Federspiel, M.S. Mineral resources of additions to the Alpine Lakes Study Area, Chelan, King, and Kittitas Counties, Washington: U.S. In Geological Survey Open-File Report 75-3; United States Geological Survey: Reston, VA, USA, 1975; p. 162. [Google Scholar]
- Church, S.E.; Tabor, R.W.; Johnson, F.L. Mineral resource potential map of the Glacier Peak Roadless Area, Snohomish County, Washington: U.S. In Geological Survey Miscellaneous Field Studies Map; MF-1380-C 15p, scale 1:50,000; United States Geological Survey: Reston, VA, USA, 1983. [Google Scholar]
- Thurber, H.K.; Miller, M.S.; McMahan, A.B.; Federspiel, F.E. Economic appraisal of the alpine lakes study area and addition, Washington. U.S. In Geological Survey; Bulletin 1542-E; United States Geological Survey: Reston, VA, USA, 1989. [Google Scholar]
- McBirney, A.R. Volcanic Evolution of the Cascade Range. Ann. Rev. Earth Planet Sci. 1978, 6, 437–456. [Google Scholar] [CrossRef]
- Dillhoff, R.; George, J. The Purple Hope Claims: Green Ridge, Middle Fork of the Snoqualmie River, King County, Washington. Rocks Miner. 2016, 91, 498–517. [Google Scholar] [CrossRef]
- MacDonald, A.J.; Spooner, E.T.C. Calibration of a Linkam TH 600 programmable heating-cooling stage for microthermometric examination of fluid inclusions. Econ. Geol. 1981, 74, 1248–1258. [Google Scholar] [CrossRef]
- Dieing, T.; Hollricher, O.; Toporski, J. Confocal Raman Microscopy. In Optical Sciences; Springer: Berlin, Germany, 2011; Volume 158. [Google Scholar]
- Seifert, W.K.; Moldowan, J.M. The effect of biodegradation on steranes and terpanes in crude oils. Geochim. Cosmochim. Acta 1979, 43, 111–126. [Google Scholar] [CrossRef]
- Bennett, B.; Fustic, M.; Farrimond, P.; Huang, H.; Larter, S.R. 25-Norhopanes: Formation during biodegradation of petroleum in the subsurface. Org. Geochem. 2006, 37, 787–797. [Google Scholar] [CrossRef]
- Parnell, J.; Baba, M.; Bowden, S.; Muirhead, D. Subsurface Biodegradation of Crude Oil in a Fractured Basement Reservoir, Shropshire, UK. J. Geol. Soc. 2017, 174, 655–666. [Google Scholar] [CrossRef]
- Peters, K.E.; Moldowan, J.M. The Biomarker Guide: Interpreting Molecular Fossils in Petroleum and Ancient Sediments; Prentice Hall: Englewood Cliffs, NJ, USA, 1993. [Google Scholar]
- Shepherd, T.J.; Rankin, A.H.; Alderton, D.H.M. A Practical Guide to Fluid Inclusion Studies; Blackie Academic & Professional: New York, NY, USA, 1985. [Google Scholar]
- Bodnar, R.J. Revised equation and table for determining the freezing point depression of H2O-NaCl solutions. Geochim. Cosmochimi. Acta 1993, 57, 683–684. [Google Scholar] [CrossRef]
- Muirhead, D.K.; Parnell, J.; Spinks, S.; Bowden, S.A. Characterization of organic matter in the Torridonian using Raman spectroscopy. In Geological Society; Special Publications: London, UK, 2016; p. 448. [Google Scholar]
- Zhou, Q.; Xiao, X.; Pan, L.; Tian, H. The relationship between micro-Raman spectral parameters and reflectance of solid bitumen. Int. J. Coal Geol. 2014, 121, 19–25. [Google Scholar] [CrossRef]
- Hurai, V.; Huraiová, M.; Slobodník, M.; Thomas, R. Geofluids: Developments in Microthermometry, Spectroscopy, Thermodynamics, and Stable Isotopes; Elsevier: London, UK, 2015; p. 504. [Google Scholar]
- O’Reilly, C.; Jenkin, G.R.T.; Feely, M.; Alderton, D.H.M.; Fallick, A.E. A fluid inclusion and stable isotope study of 200 Ma of fluid evolution in the Galway Granite, Connemara, Ireland. Contrib. Miner. Petrol. 1997, 129, 120–142. [Google Scholar] [CrossRef]
- Conliffe, J.; Feely, M. Microthermometric characteristics of fluids associated with granite and greisen quartz, and vein quartz and beryl from the Rosses Granite Complex, Donegal, NW Ireland’. J. Geochem. Explor. 2006, 89, 73–77. [Google Scholar] [CrossRef]
- Feely, M.; Selby, D.; Conliffe, J.; Judge, M. Re–Os geochronology and fluid inclusion microthermometry of molybdenite mineralisation in the late-Caledonian Omey Granite, western Ireland Applied Earth Science. Trans. Inst. Min. Metall. B 2007, 116, 143–149. [Google Scholar]
- Selby, D.; Conliffe, J.; Crowley, Q.G.; Feely, M. Geochronology (Re–Os and U–Pb) and fluid inclusion studies of molybdenite mineralisation associated with the Shap, Skiddaw and Weardale granites, UK D. Applied Earth Science. Trans. Inst. Min. Metall. B 2008, 117, 11–28. [Google Scholar]
- Conliffe, J.; Feely, M. Fluid inclusions in Irish granite quartz: Monitors of fluids trapped in the onshore Irish Massif. Earth Environ. Sci. Trans. R. Soc. Edinb. 2010, 101, 53–66. [Google Scholar] [CrossRef]
- Holdsworth, B.; Dempsey, E.D.; Selby, D.; Darling, J.; Feely, M.; Costanzo, A.; Strachan, R.; Waters, P.; Finlay, A.; Porter, S.J. Silurian to Devonian magmatism, molybdenite mineralization, regional exhumation and brittle strike-slip deformation along the Loch Shin Line, NW Scotland. J. Geol. Soc. Lond. 2015, 172, 748–762. [Google Scholar] [CrossRef]
- Suchý, V.; Dobeš, P.; Sýkorová, I.; Machovič, V.; Stejskal, M.; Kroufek, J.; Chudoba, J.; Matějovský, L.; Havelcová, M.; Matysová, P. Oil bearing inclusions in vein quartz and calcite and, bitumens in veins: Testament to multiple phases of hydrocarbon migration in the Barrandian basin (lower Paleozoic), Czech Republic. Mar. Pet. Geol. 2010, 27, 285–297. [Google Scholar] [CrossRef]
- Blanc, P.; Connan, J. Preservation, degradation, and destruction of trapped oil. In The Petroleum System—From Source to Trap; Mogoon, L.B., Dow, W.G., Eds.; American Association of Petroleum Geologists Memoir: Tulsa, OK, USA, 1994; pp. 237–247. [Google Scholar]
- Mossman, D.J.; Nagy, B. Solid bitumens: An assessment of their characteristics, genesis, and role in geological processes. Terra Nova 1996, 8, 114–128. [Google Scholar] [CrossRef]
- Volk, H.; Horsfield, B.; Mann, U.; Sucký, V. Variability of petroleum inclusions in vein, fossil and vug cements—A geochemical study in the Barrandian Basin (Lower Paleozoic, Czech Republic). Org. Geochem. 2002, 33, 1319–1341. [Google Scholar] [CrossRef]
- Peters, K.E.; Walters, C.C.; Moldowan, J.M. The Biomarker Guide, 2nd ed.; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Bakker, R.J. Clathrates: Computer programs to calculate fluid inclusion V-X properties using clathrate melting temperatures. Comput. Geosci. 2003, 23, 1–18. [Google Scholar] [CrossRef]
- Selley, R.C. Elements of Petroleum Geology, 2nd ed.; Academic Press: San Diego, CA, USA, 1997; p. 470. [Google Scholar]
- Tabor, R.W.; Frizzell, V.A., Jr.; Vance, J.A.; Naeser, C.W. Ages and stratigraphy of lower and middle Tertiary sedimentary and volcanic rocks of the Central Cascades, Washington: Applications to the tectonic history of the Straight Creek Fault. Geol. Soc. Am. 1984, 95, 26–44. [Google Scholar] [CrossRef]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feely, M.; Costanzo, A.; Lindner, F.; George, J.; Parnell, J.; Bowden, S.; Baba, M.; Owens, P. Quartz-Amethyst Hosted Hydrocarbon-Bearing Fluid Inclusions from the Green Ridge Breccia in the Snoqualmie Granite, North Cascades, WA, USA. Minerals 2017, 7, 174. https://doi.org/10.3390/min7090174
Feely M, Costanzo A, Lindner F, George J, Parnell J, Bowden S, Baba M, Owens P. Quartz-Amethyst Hosted Hydrocarbon-Bearing Fluid Inclusions from the Green Ridge Breccia in the Snoqualmie Granite, North Cascades, WA, USA. Minerals. 2017; 7(9):174. https://doi.org/10.3390/min7090174
Chicago/Turabian StyleFeely, Martin, Alessandra Costanzo, Franzisca Lindner, Joe George, John Parnell, Stephen Bowden, Mas’ud Baba, and Peter Owens. 2017. "Quartz-Amethyst Hosted Hydrocarbon-Bearing Fluid Inclusions from the Green Ridge Breccia in the Snoqualmie Granite, North Cascades, WA, USA" Minerals 7, no. 9: 174. https://doi.org/10.3390/min7090174
APA StyleFeely, M., Costanzo, A., Lindner, F., George, J., Parnell, J., Bowden, S., Baba, M., & Owens, P. (2017). Quartz-Amethyst Hosted Hydrocarbon-Bearing Fluid Inclusions from the Green Ridge Breccia in the Snoqualmie Granite, North Cascades, WA, USA. Minerals, 7(9), 174. https://doi.org/10.3390/min7090174





