Kinetic Study of the Leaching of Low-Grade Manganese Ores by Using Pretreated Sawdust as Reductant
Abstract
:1. Introduction
2. Experiments
2.1. Materials
2.2. Experiment Procedure
3. Results and Discussions
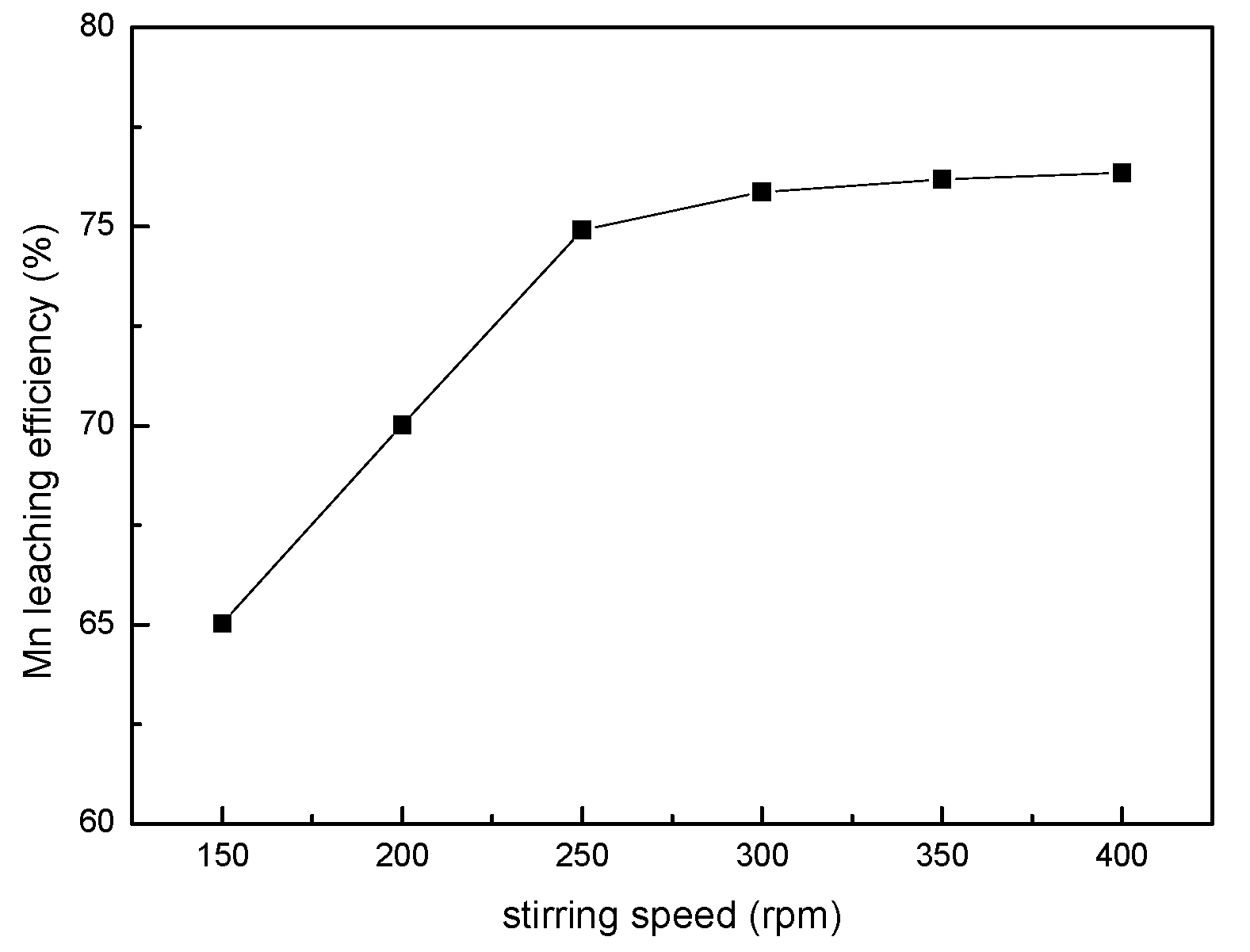
3.1. Effect of Stirring Speed
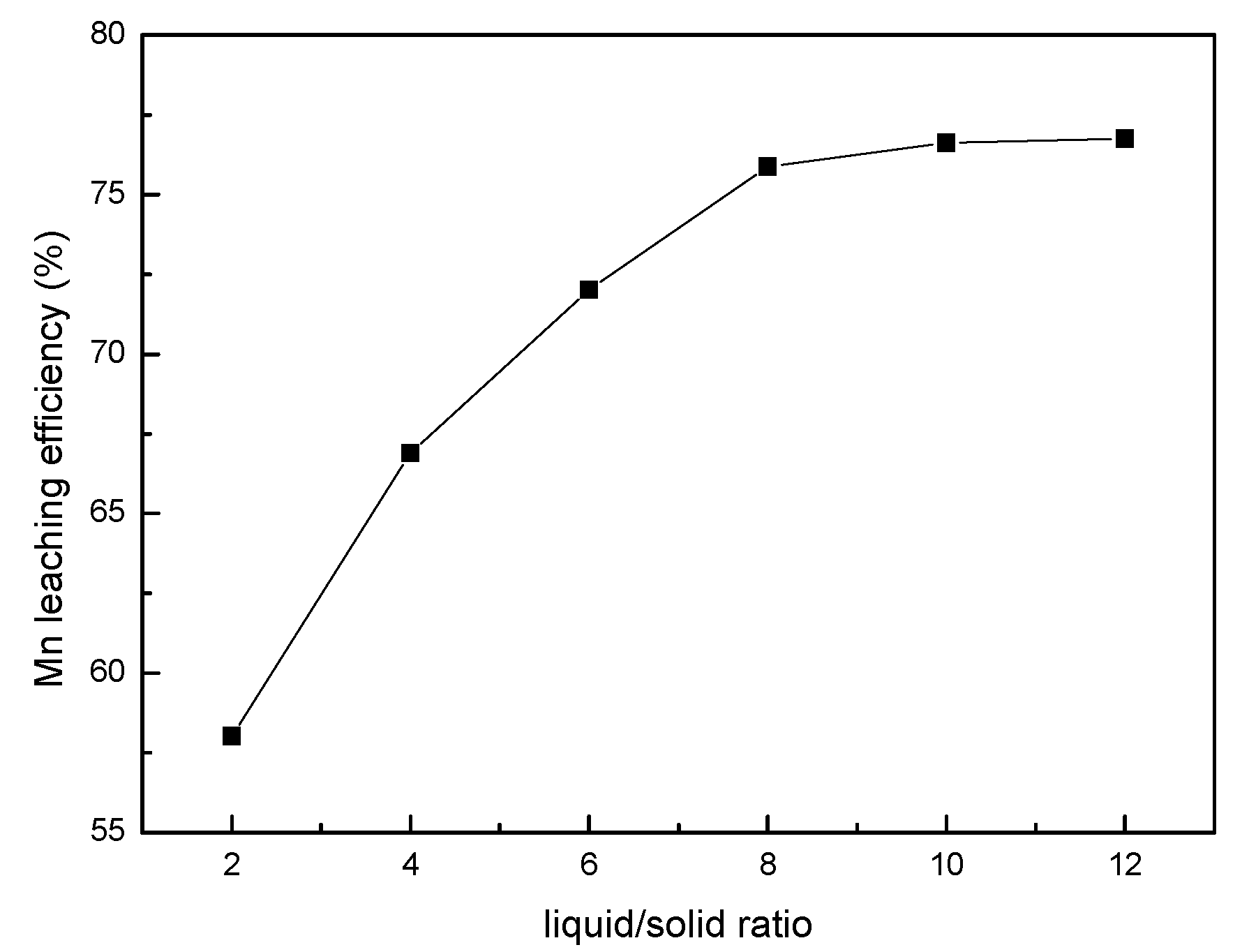
3.2. Effect of the Liquid/Solid Ratio
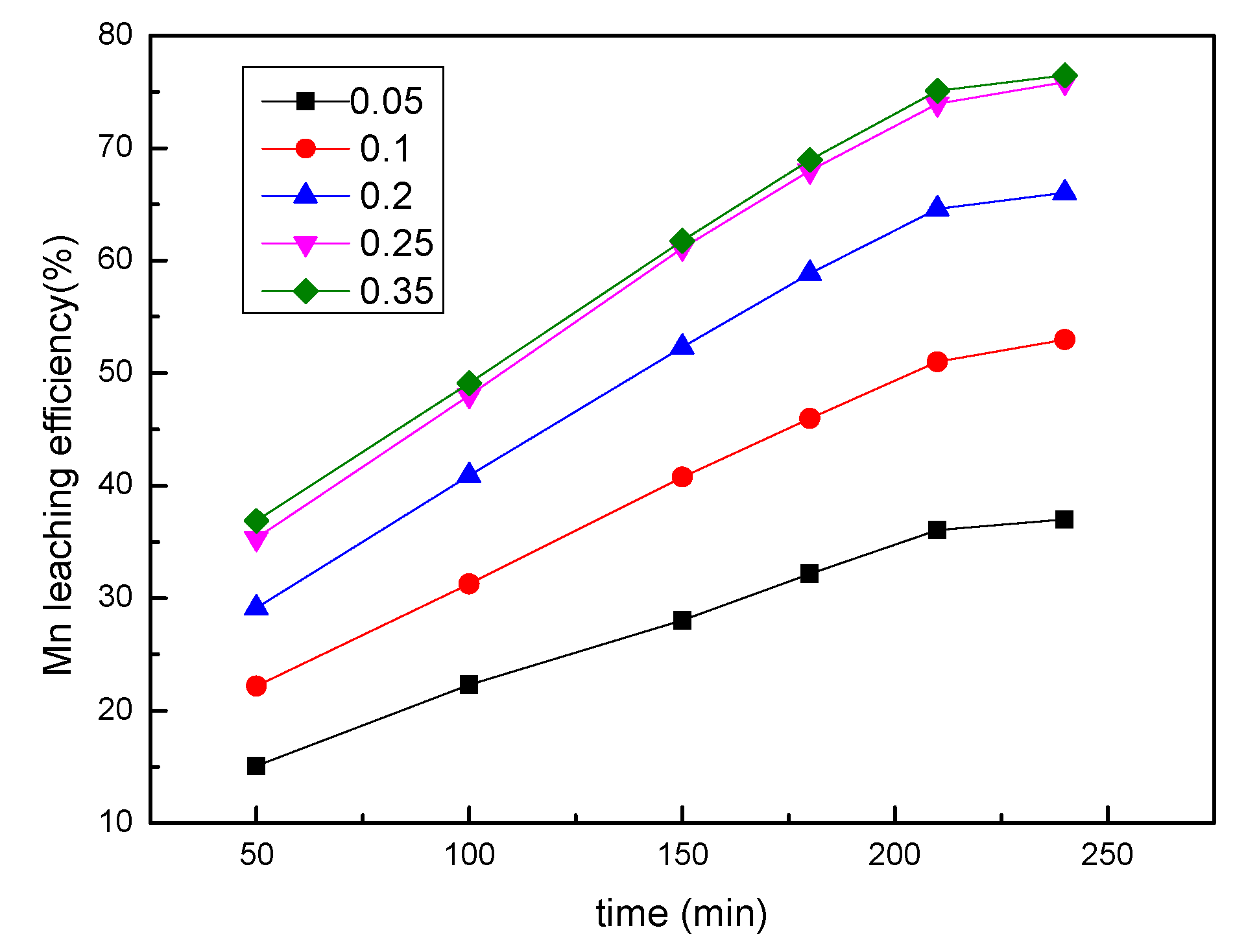
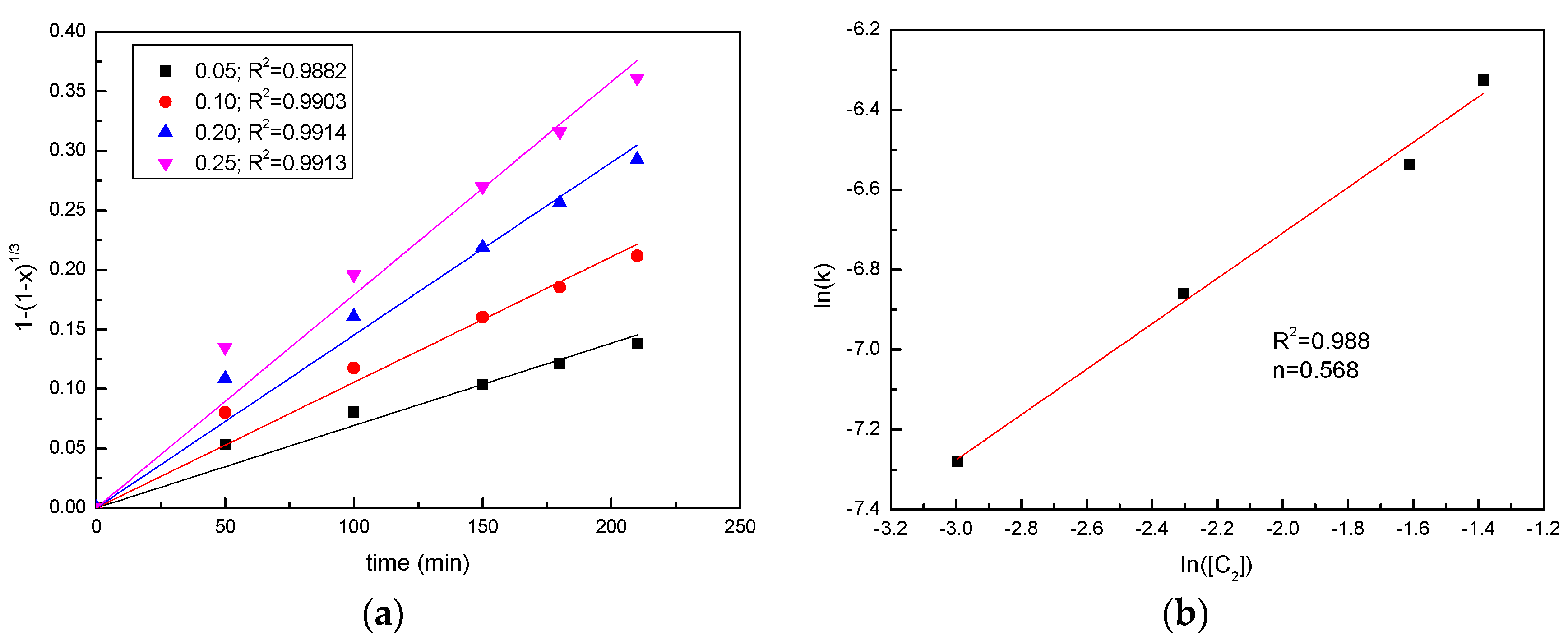
3.3. Effect of the Sawdust/Ore Mass Ratio
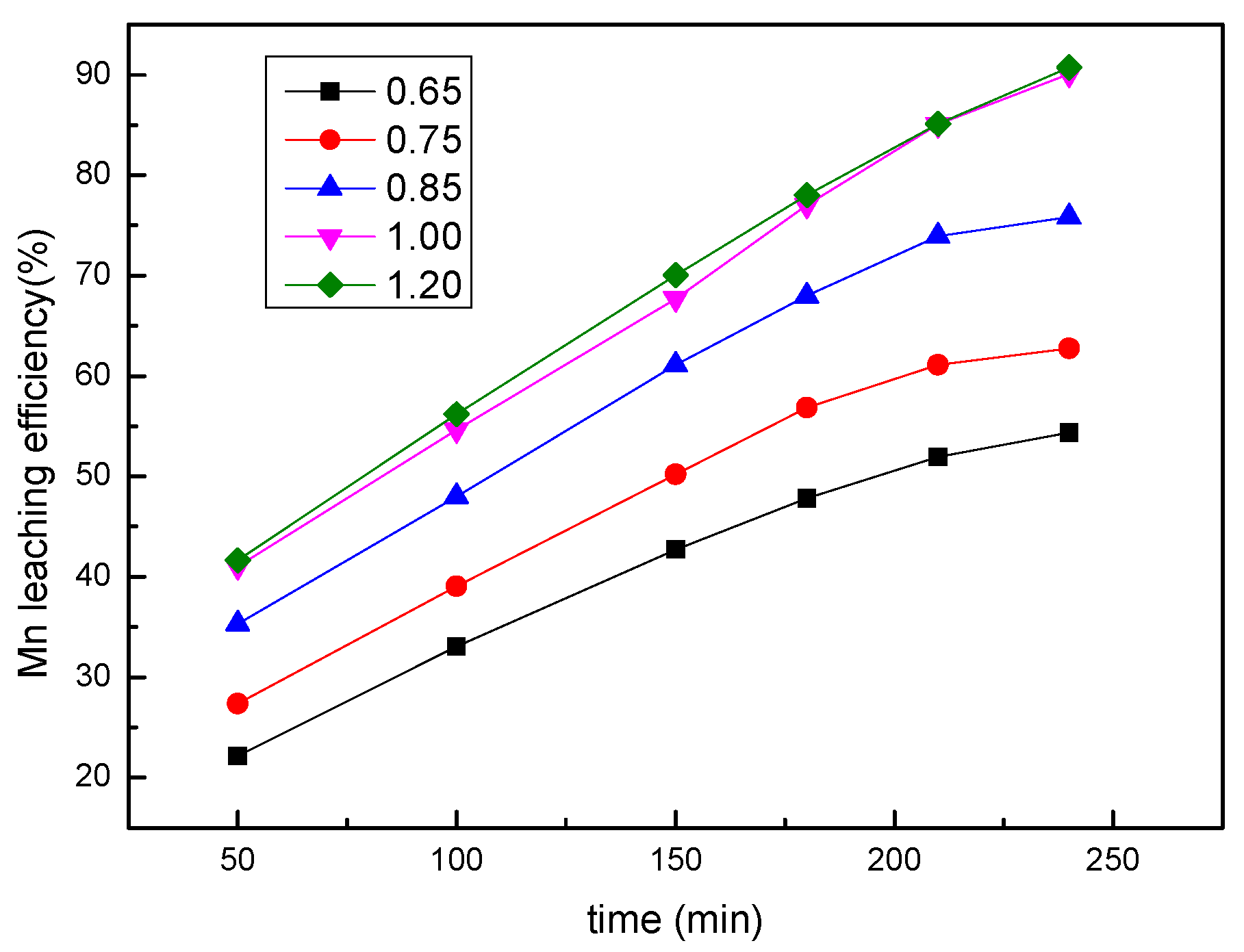
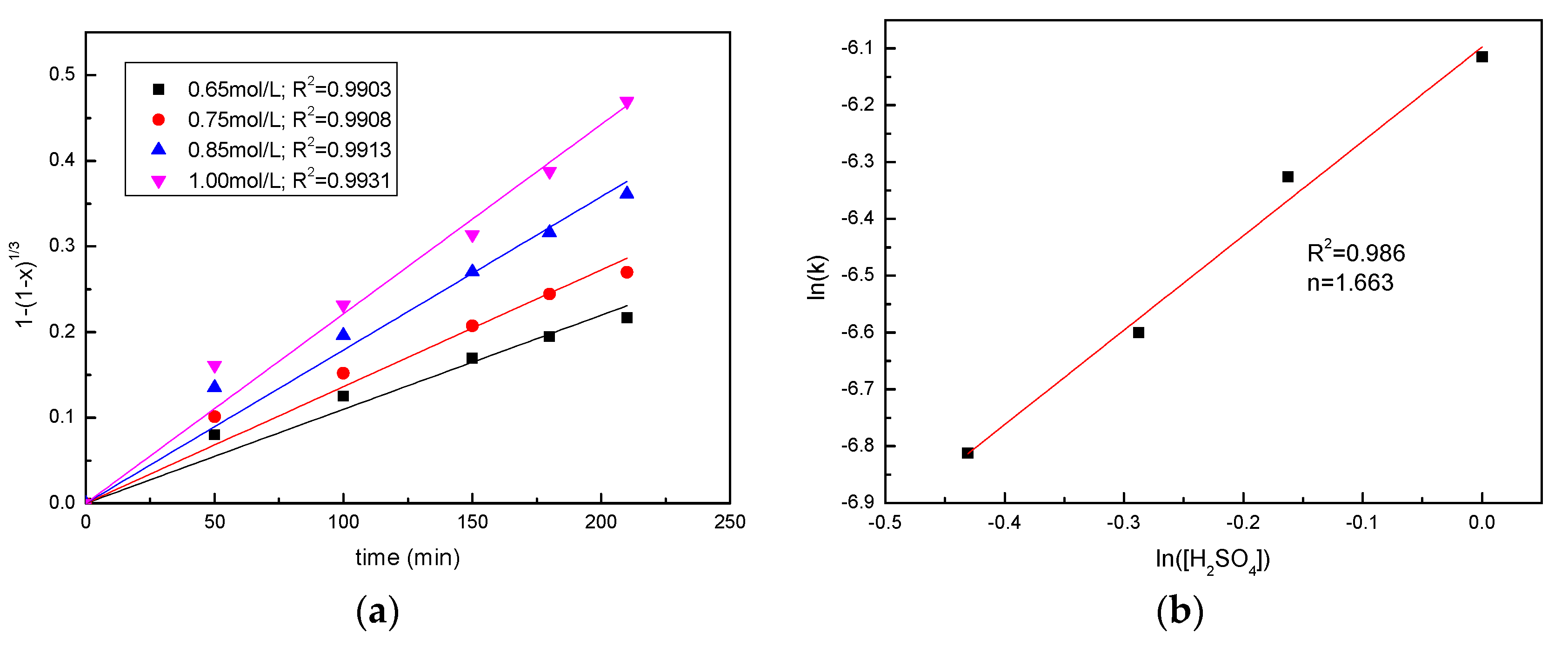
3.4. Effect of the Sulfuric Acid Concentration
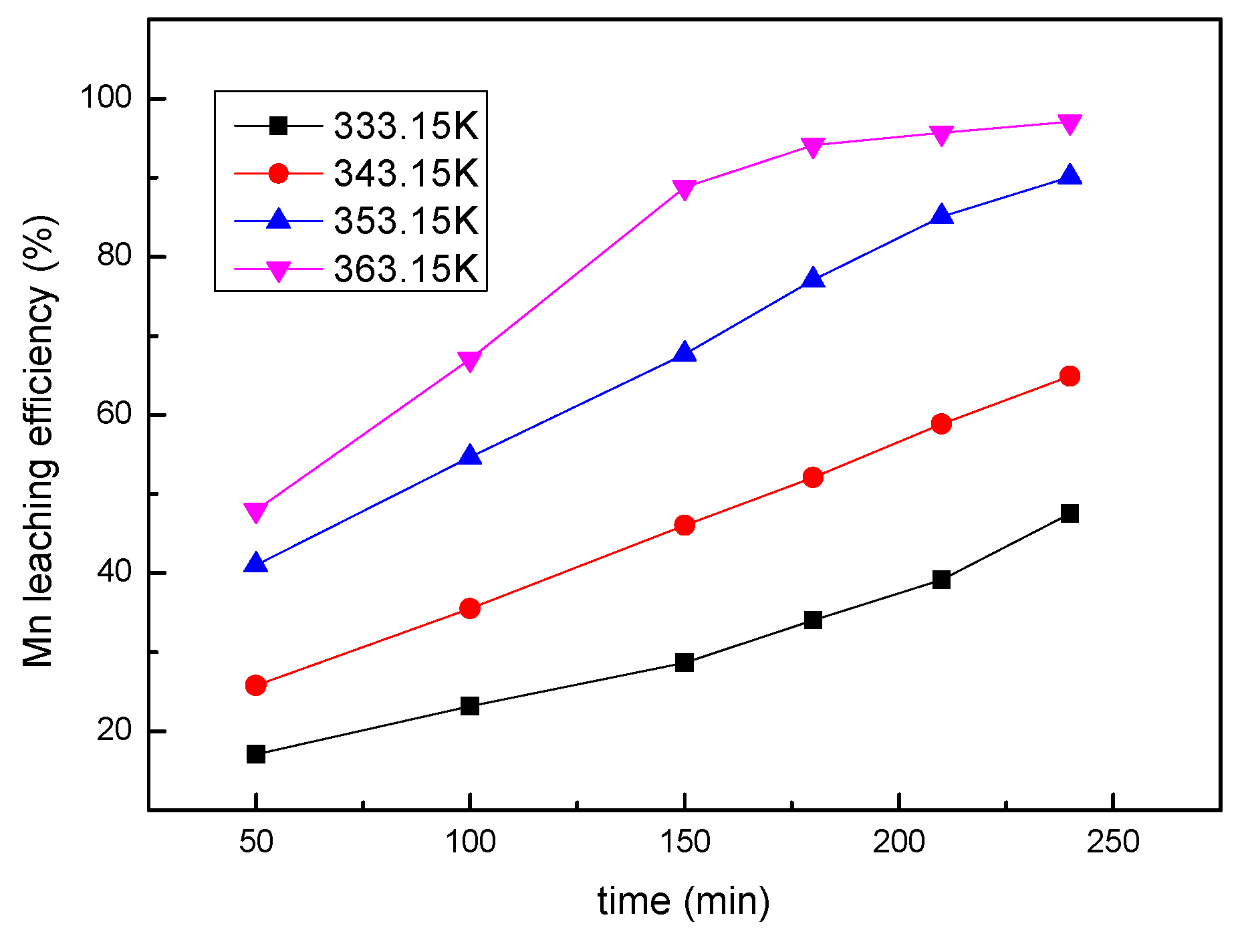
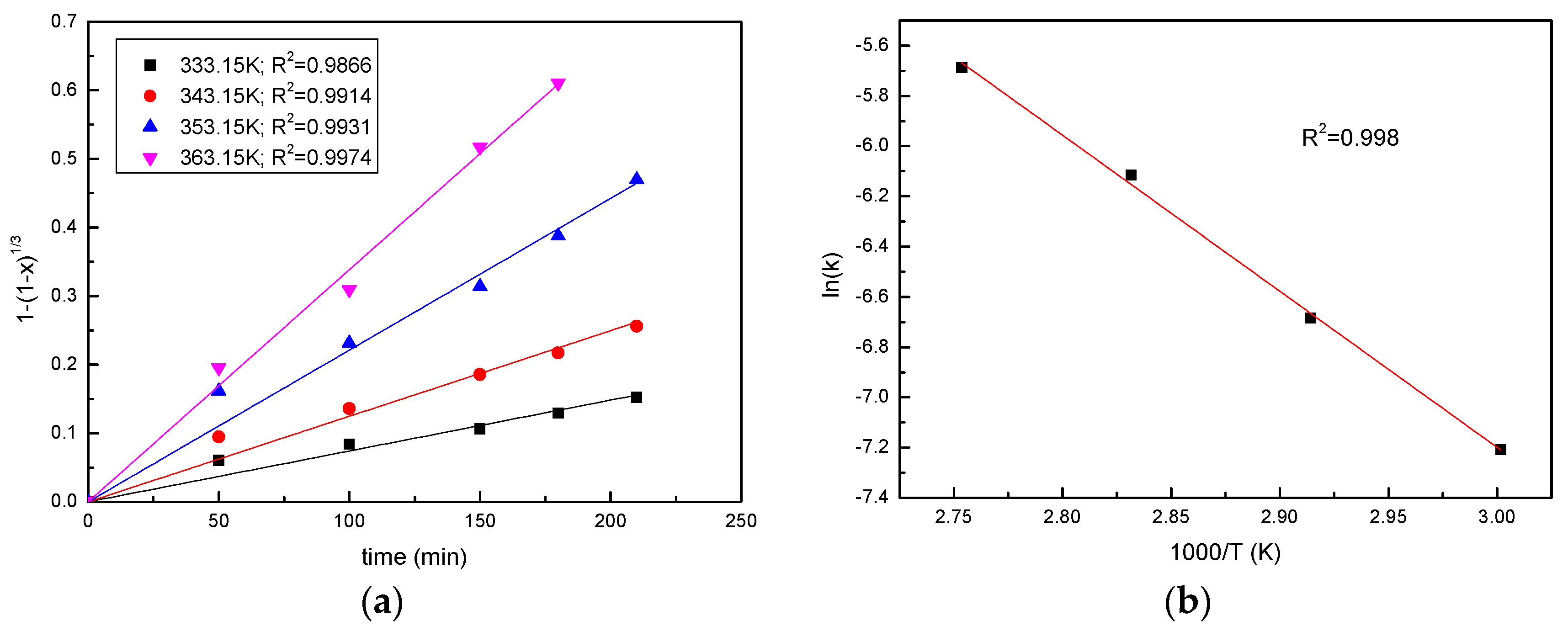
3.5. Effect of Temperature
3.6. Kinetics Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dwivedi, D.; Randhawa, N.S.; Saroj, S.; Jana, R.K. An Overview of Manganese Recovery by Hydro and Pyro-Metallurgical Routes. J. Inst. Eng. 2017, 98, 147–154. [Google Scholar] [CrossRef]
- Zhang, W.; Cheng, C.Y. Manganese metallurgy review. Part I: Leaching of ores/secondary materials and recovery of electrolytic/chemical manganese dioxide. Hydrometallurgy 2007, 89, 137–159. [Google Scholar] [CrossRef]
- Tekin, T.; Bayramoǧlu, M. Kinetics of the reduction of MnO2, with Fe2+, ions in acidic solutions. Hydrometallurgy 1993, 32, 9–20. [Google Scholar] [CrossRef]
- Das, S.C.; Sahoo, P.K.; Rao, P.K. Extraction of manganese from low-grade manganese ores by FeSO4 leaching. Hydrometallurgy 1982, 8, 35–47. [Google Scholar] [CrossRef]
- Nayl, A.A.; Ismail, I.M.; Aly, H.F. Recovery of pure MnSO4. H2O by reductive leaching of manganese from pyrolusite ore by sulfuric acid and hydrogen peroxide. Int. J. Miner. Proc. 2011, 100, 116–123. [Google Scholar] [CrossRef]
- Naik, P.K.; Sukla, L.B.; Das, S.C. Aqueous SO2 leaching studies on Nishikhal manganese ore through factorial experiment. Hydrometallurgy 2000, 54, 217–228. [Google Scholar] [CrossRef]
- Vračar, R.Ž.; Cerović, K.P. Manganese leaching in the FeS2–MnO2–O2–H2O system at high temperature in an autoclave. Hydrometallurgy 2000, 55, 79–92. [Google Scholar] [CrossRef]
- Li, C.X.; Zhong, H.; Wang, S.; Xue, J.R.; Wu, F.F.; Zhang, Z.Y. Manganese extraction by reduction–acid leaching from low-grade manganese oxide ores using CaS as reductant. Trans. Nonferr. Met. Soc. China 2015, 25, 1677–1684. [Google Scholar] [CrossRef]
- Su, H.F.; Liu, H.K.; Wang, F.; Lv, X.Y.; Wen, Y.X. Kinetics of Reductive Leaching of Low-grade Pyrolusite with Molasses Alcohol Wastewater in H2SO4. Chin. J. Chem. Eng. 2010, 18, 730–735. [Google Scholar] [CrossRef]
- Alaoui, A.; Kacemi, K.E.; Ass, K.E.; Darmane, Y.; Kitane, S. Kinetic study of the leaching of manganese mine tailings by organic reductant in sulphuric acid solution. Miner. Process. Extr. Metall. IMM Trans. 2016, 125, 109–116. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, G.; Yan, H.; Zhao, Y.; Li, T. Reduction of low-grade manganese dioxide ore pellets by biomass wheat stalk. Acta Metall. Sin. 2013, 26, 167–172. [Google Scholar] [CrossRef]
- Tang, Q.; Zhong, H.; Wang, S.; Li, J.Z.; Liu, G.Y. Reductive leaching of manganese oxide ores using waste tea as reductant in sulfuric acid solution. Trans. Nonferr. Met. Soc. China 2014, 24, 861–867. [Google Scholar] [CrossRef]
- Feng, Y.L.; Zhang, S.Y.; Li, H.R. Reductive leaching of manganese from low-grade pyrolusite ore in sulfuric acid using pyrolysis-pretreated sawdust as a reductant. Int. J. Miner. Metall. Mater. 2016, 23, 241–246. [Google Scholar] [CrossRef]
- Seetharaman, S. Treatise on Process Metallurgy, Volume 3: Industrial Processes; Elsevier Ltd.: Kidlington, UK, 2013. [Google Scholar]
- Hariprasad, D.; Dash, B.; Ghosh, M.K.; Anand, S. Leaching of manganese ores using sawdust as a reductant. Miner. Eng. 2007, 20, 1293–1295. [Google Scholar] [CrossRef]
- Free, M.L. Hydrometallurgy: Fundamentals and Applications; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; Volume 1, pp. 86–122. [Google Scholar]
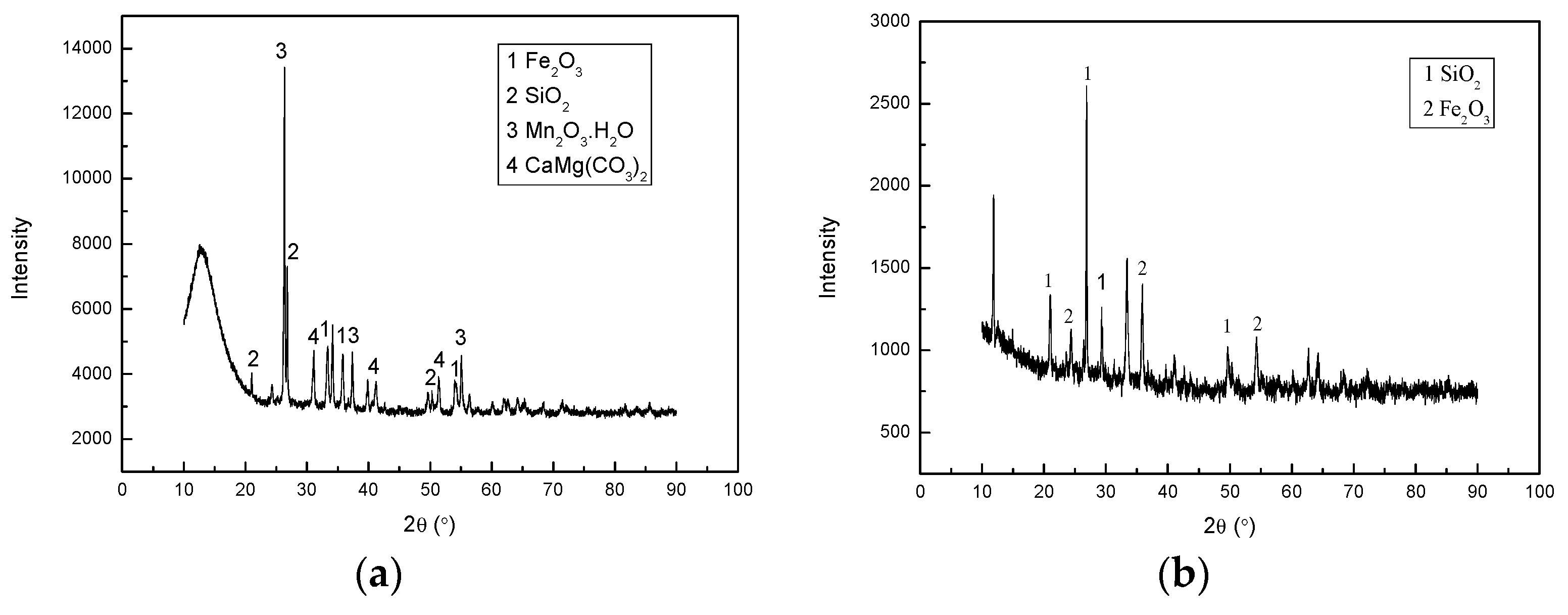
| Component | Mass % |
|---|---|
| Mn | 25.0 |
| Fe | 12.6 |
| C | 1.7 |
| CaO | 4.0 |
| MgO | 3.7 |
| Al2O3 | 1.7 |
| SiO2 | 20.1 |
| Element | Leaching Efficiency % |
|---|---|
| Mn | 94.1 |
| Fe | 6.15 |
| Ca | 18.3 |
| Mg | 29.5 |
| Conditions | ||||
|---|---|---|---|---|
| R2 | R2 | R2 | R2 | |
| 1 − (1 − x)1/3 vs. Time | R2 ln(kr) vs. ln(c1) | 1 − 2x/3 − (1 − x)2/3 vs. Time | ln(kd) vs. ln(c2) | |
| Sawdust/ore mass ratio | ||||
| 0.05 | 0.988 | - | 0.965 | - |
| 0.1 | 0.990 | 0.988 | 0.971 | 0.925 |
| 0.2 | 0.991 | - | 0.919 | - |
| 0.25 | 0.991 | - | 0.926 | - |
| H2SO4 concentration | ||||
| 0.65 mol/L | 0.990 | - | 0.920 | - |
| 0.75 mol/L | 0.991 | 0.986 | 0.882 | 0.896 |
| 0.85 mol/L | 0.991 | - | 0.926 | - |
| 1.0 mol/L | 0.993 | - | 0.954 | - |
| Temperature | ||||
| 333.15 K | 0.987 | - | 0.972 | - |
| 343.15 K | 0.991 | 0.998 | 0.926 | 0.902 |
| 353.15 K | 0.993 | - | 0.908 | - |
| 363.15 K | 0.997 | - | 0.896 | - |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Fu, G.; Jiang, L. Kinetic Study of the Leaching of Low-Grade Manganese Ores by Using Pretreated Sawdust as Reductant. Minerals 2017, 7, 83. https://doi.org/10.3390/min7050083
Sun Y, Fu G, Jiang L. Kinetic Study of the Leaching of Low-Grade Manganese Ores by Using Pretreated Sawdust as Reductant. Minerals. 2017; 7(5):83. https://doi.org/10.3390/min7050083
Chicago/Turabian StyleSun, Yang, Gaofeng Fu, and Lan Jiang. 2017. "Kinetic Study of the Leaching of Low-Grade Manganese Ores by Using Pretreated Sawdust as Reductant" Minerals 7, no. 5: 83. https://doi.org/10.3390/min7050083
APA StyleSun, Y., Fu, G., & Jiang, L. (2017). Kinetic Study of the Leaching of Low-Grade Manganese Ores by Using Pretreated Sawdust as Reductant. Minerals, 7(5), 83. https://doi.org/10.3390/min7050083





