Abstract
The reductive leaching of manganese from a low-grade manganese oxide ore was investigated by using pretreated sawdust as the reductant in a sulfuric acid medium. The effects of stirring speed, liquid/solid ratio, sawdust/ore mass ratio, sulfuric acid concentration, reaction temperature, and time on the manganese extraction were examined. It was found that the leaching efficiency is strongly dependent on temperature and acid concentration. The leaching efficiency of manganese reached 94.1% under the optimal conditions: stirring speed of 300 rpm, liquid/solid ratio of 8:1, mass ratio of sawdust to ore 0.25, sulfuric acid concentration of 1 mol/L and a temperature of 363 K for 180 min. The kinetic analysis was carried out based on the shrinking core model, which indicated that the reductive leaching process was controlled by the chemical reaction. The reaction orders with respect to the sulfuric acid concentration and mass ratio of sawdust are 1.66 and 0.57, respectively. The apparent activation energy for the leaching process has been calculated using the Arrhenius expression and was found to be 51.7 kJ/mol.
1. Introduction
Manganese, one of the important strategic metals, is widely used in metallurgy, the chemical industry, batteries, electronics, and many other fields. The need for manganese is greatest in the steel industry, accounting for more than 90% of the total production [1]. With the rapidly growing demand for manganese and the continuous mining of high-grade manganese ore, considerable research attention has been directed at the recovery of manganese from low-grade ores [2,3,4,5]. However, these low-grade ores are mainly high-valence oxides, such as pyrolusite, which are stable in both acid and alkaline media, so the extraction of manganese from such sources must be carried out under reductive conditions [1,2]. When using the conventional reduction roasting method, plenty of smoke is produced in the roasting process, which is harmful to the environment. To alleviate these problems, hydrometallurgical processes have been studied. Manganese can be treated by direct acid leaching under reducing conditions. Several inorganic reductants generally employed in acidic media are FeSO4 [3,4], H2O2 [5], SO2 [6], and sulfide [7,8]. However, some of these reducing agents, such as SO2, may be harmful to the environment, and FeSO4 may result in a high content of iron in the leaching solution.
Organics (oxalate, sucrose, alcohols, etc.) have been considered as efficient and simple reducing agents. There are a large number of reports about using organics as reductants to leach manganese oxide ores [9,10]. Su et al. [9], on the reductive leaching of manganese ore in the presence of alcohol wastewater, showed that the leaching efficiency of manganese increases with the temperature, H2SO4 concentration, organic matter in alcohol wastewater, and decreasing ore particle size. Although using these organics has some advantages, there has been little application value in industry due to the high cost and consumption rate.
Recently it has been demonstrated that biomass, such as sawdust, corncob, and straw, are low-cost, renewable, and non-hazardous reducing agents [11,12,13], which are oxidized to carbon dioxide (CO2) in the process of manganese ore reduction. The amount of CO2 released during the reducing process is equal to the amount absorbed during biomass growth, so this is a zero emission process [14]. Biomass can be regenerated in a short natural cycle of 5–10 years compared to fossil coal, which takes a long cycle of more than 100 million years to form in nature from the same vegetal matter, which is more advantageous [14].
Cellulose, one of the main components in many plants with reducing ability, is susceptible to acid catalyzed hydrolysis. In the hydrolysis process, some soluble sugars are released from cellulose and the final hydrolysis product is glucose, which can act as a reductant. The overall reaction which takes place during the reductive leaching of manganese ores by using cellulose as a reductant can be described as follows:
Several authors have evaluated the use of cellulose as a reductant in manganese leaching [11,12,13,15]. Tang et al. [12] reported that the leaching efficiency of Xiangxi manganese oxide ore reached 99.8% under the optimal conditions: mass ratio of manganese ore to waste tea 10:1, 1.7 mol/L sulfuric acid, a liquid/solid ratio of 7.5:1, and a leaching temperature of 368 K for 8 h. However, when using biomass as a reducing agent, the process often requires long reaction times, high temperature, and acid strength. To solve this problem, in this experiment, the sawdust was pretreated with concentrated sulfuric acid, which carbonizes the sawdust by removing moisture from it. This carbonization process may facilitate the reaction between manganese oxide ore and sawdust.
This work seeks to investigate the leaching process for low-grade manganese ores in sulfuric acid by using sawdust pretreated with sulfuric acid as a reducing agent. The effects of parameters, such as stirring speed, liquid/solid ratio, reductant/ore mass ratio, sulfuric acid concentration, reaction temperature, and time on the leaching efficiency of manganese were examined. In addition, kinetics analysis was carried out.
2. Experiments
2.1. Materials
The low-grade manganese ore used in this experiment was obtained from Liaoning Province, China. The ore was crushed and ground to provide ore with a particle size of less than 75 μm. The mineral phases were determined by X-ray diffraction (XRD, Bruker, Karlsruhe, Germany) analysis, which is shown in Figure 1. It can be seen that manganese exists as Mn2O3∙H2O, and occurs together with Fe2O3, CaMgC(O3)2, and SiO2. The chemical composition of ore samples presented in Table 1 show that manganese content is approximately 25%.
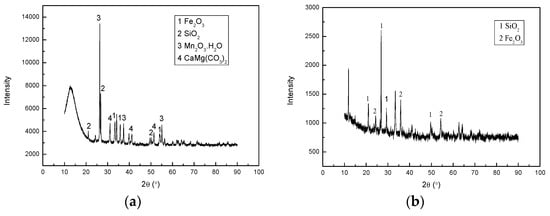
Figure 1.
X-ray diffraction (XRD) pattern (a) manganese ore; and (b) leaching residue.

Table 1.
Chemical analysis of the manganese ore.
The sawdust obtained from a timber processing plant is made up of cellulose, hemicellulose, and lignin. All of the reagents used were of analytical grade.
2.2. Experiment Procedure
Samples of manganese ore and sawdust were prepared after crushing and milling. Before reductive leaching, the sawdust was pretreated with concentrated sulfuric acid in advance. The main purpose of pretreatment is to remove moisture from the sawdust, and it takes advantage of the dehydration property of concentrated sulfuric acid. In this process, certain amounts of concentrated sulfuric acid were added into the reactor containing the sawdust, stirring constantly until the sawdust was carbonized. The ratio of sawdust to sulfuric acid is 1 g/1 mL.
Leaching experiments were performed in a 500 mL reaction vessel, equipped with a mechanical stirrer and a thermostatic water bath. Each time, 20 g of ore and calculated amounts of reductant based on the sawdust/ore mass ratio were weighed and added to the reaction vessel which contains 1–5 mol/L sulfuric acid solution with a pre-set temperature from 333.15 K to 363.15 K and a stirring speed from 150 revolutions per minute (rpm) to 400 rpm for 50–240 min. Samples were taken at appropriate time intervals to determine the content of manganese. Manganese contents were determined by the titrimetry method with ammonium ferrous sulfate, using sodium diphenylaminesulfonate as the indicator.
3. Results and Discussions
3.1. Effect of Stirring Speed
To study the effect of stirring speed on the leaching efficiency of manganese, different experiments were carried out in the range of 150–400 rpm, under the conditions of a sawdust/ore mass ratio of 0.25, sulfuric acid of 0.85 mol/L, a liquid/solid ratio of 8:1, and a temperature of 363.15 K for 240 min. As shown in Figure 2, the leaching efficiency of manganese increased with stirring speed due to the enhanced diffusion. When stirring speed is over 250 rpm, there was no significant increase in leaching efficiency. The experimental results indicated that the effect of external diffusion could be ignored when the stirring speed exceeded 250 rpm. Therefore, a stirring speed of 300 rpm was chosen for the subsequent experiments.
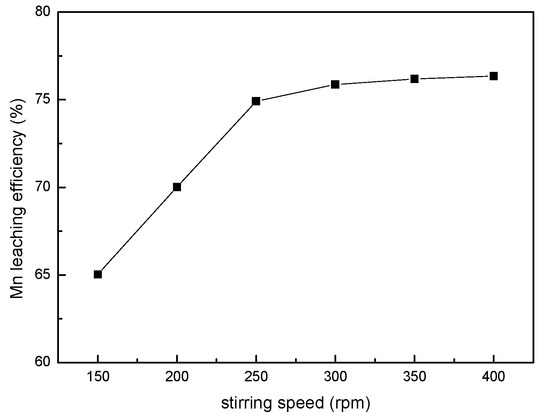
Figure 2.
The effect of the stirring speed on the manganese leaching efficiency.
3.2. Effect of the Liquid/Solid Ratio
The effect of the liquid/solid ratio on the leaching efficiency of manganese was investigated in the range of 2:1–10:1. The other leaching conditions were fixed at a sawdust/ore mass ratio of 0.25, 0.85 mol/L sulfuric acid, a stirring speed of 300 rpm, and a temperature of 353.15 K for 240 min. The results obtained (see Figure 3) show that the leaching efficiency increases with the liquid/solid ratio. However, above a ratio of 8:1, the effect of the liquid to solid ratio is negligible. Therefore, a liquid/solid ratio of 8:1 is recommended in this study.
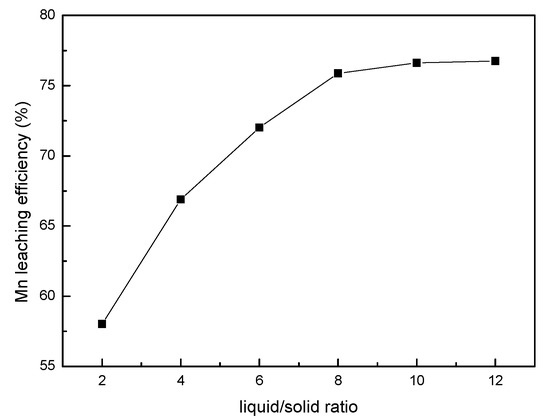
Figure 3.
The effect of the liquid/solid ratio on the manganese leaching efficiency.
3.3. Effect of the Sawdust/Ore Mass Ratio
The effects of the sawdust/ore mass ratio on the leaching efficiency of manganese were examined by varying the sawdust/ore mass ratio from 0.05 to 0.35, and other conditions were fixed at a sulfuric acid concentration of 0.85 mol/L, a liquid/solid ratio of 8:1, a stirring speed of 300 rpm, and a temperature of 353.15 K. The results presented in Figure 4 indicated that with the increasing mass ratio of sawdust to ore, the leaching efficiency of manganese gradually increased. At the mass ratio of 0.25, 75.9% manganese was extracted after leaching for 240 min. When the mass ratio was over 0.25, there was no significant increase in manganese recovery. Therefore, the mass ratio of pretreated sawdust to ore may be fixed at 0.25.
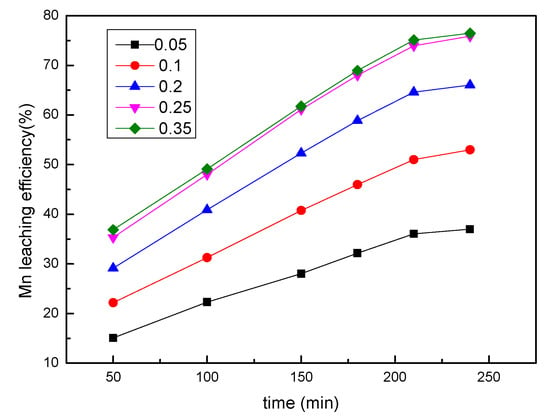
Figure 4.
The effect of sawdust/ore mass ratio on the manganese leaching efficiency.
3.4. Effect of the Sulfuric Acid Concentration
A series of leaching experiments were performed at different sulfuric acid concentrations of 0.65–1.2 mol/L, and the other conditions were fixed at a sawdust/ore mass ratio of 0.25, a liquid/solid ratio of 8:1, a stirring speed of 300 rpm, and a temperature of 353.15 K. As shown in Figure 5, the leaching efficiency of manganese increased with the sulfuric acid concentration. With the acid concentration of 1 mol/L and a leaching time of 240 min, approximately 90% manganese was extracted. There was no noticeable difference of both the rate and efficiency at acid concentrations above 1 mol/L. In consideration of the leaching cost, an acid concentration of 1 mol/L is suggested.
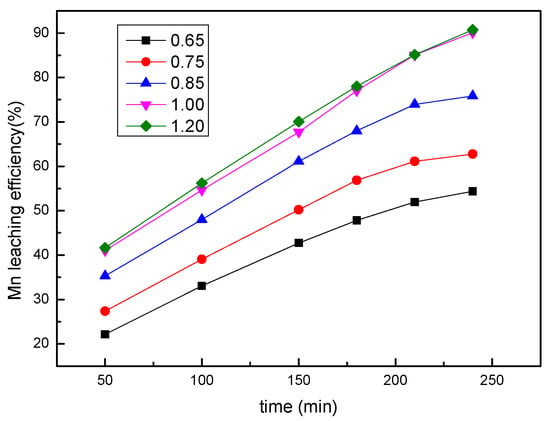
Figure 5.
The effect of the sulfuric acid concentration on the manganese leaching efficiency.
3.5. Effect of Temperature
In order to investigate the effect of temperature on the leaching efficiency of manganese, experiments were performed in the temperature range of 333.15–363.15 K and keeping other conditions constant: a mass ratio of sawdust to ore 0.25, a sulfuric acid concentration of 1 mol/L, a liquid/solid ratio of 8:1, and a stirring speed of 300 rpm. From the obtained results, it was observed from Figure 6 that temperature plays an important role in manganese leaching. For example, when the temperature increased from 333.15 K to 363.15 K, the leaching efficiency increased sharply from 34.1% to 94.1% after leaching for 180 min.
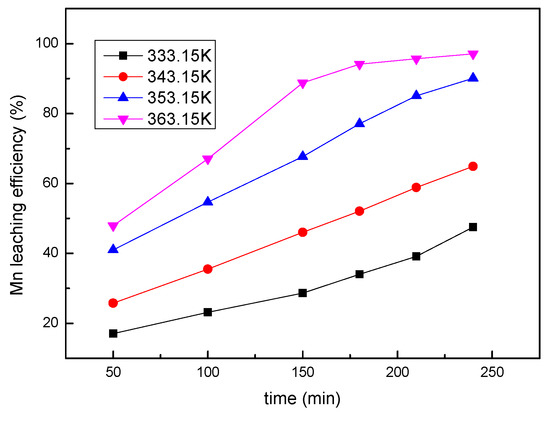
Figure 6.
The effect of the temperature on the manganese leaching efficiency.
Figure 1b presents the XRD pattern of the leaching residue and Table 2 shows the leaching efficiency of the main elements of the ore, which are obtained under the following conditions: a sawdust/ore mass ratio 0.25, a sulfuric acid concentration of 1 mol/L, a liquid/solid ratio of 8:1, a temperature of 363.15 K for a period of 180 min. It is evident that after the reduction leaching, most of the manganese was removed, leaving behind a residue consisting mainly of Fe2O3 and SiO2.

Table 2.
Leaching efficiency of main elements in ores.
3.6. Kinetics Analysis
The reaction rate depends on the slowest step in the process, which is called the rate-limiting step. In order to determine the rate-limiting step and obtain the kinetic equation, experimental data were processed and correlated to different kinetic models for solid–liquid reactions. Based on these models, the reaction rate may be controlled by the following steps: chemical reactions at the surface or diffusion through the product layer.
When the reductive leaching process is controlled by surface chemical reaction, the kinetic equation can be expressed as [16]:
When the reductive leaching process is controlled by internal diffusion, the kinetic equation can be expressed as [16]:
where x is the extraction efficiency of manganese and t is the leaching time (min); kr and kd are the apparent reaction rate constant (min−1). To determine the rate-limiting step, the experimental data were transformed and fitted to Equations (2) and (3), and the fitting degree was evaluated by correlation coefficient (R2) values. Since the experimental conditions were constant, the slopes estimated from the plots are directly proportional to the rate constant and diffusion coefficient, respectively.
Table 3 presents the correlation coefficients for the chemical reaction controlled model and the diffusion through the product layer. It was observed that the chemical reaction controlled model was more suitable for this leaching process.

Table 3.
Correlation coefficients (R2) of the kinetic models in different conditions.
The activation energy can be calculated by the Arrhenius formula, as follows:
where k is the rate constant; T is the temperature (K); Ea is the apparent activation energy (kJ/mol); R is the gas constant (8.314 J/K·mol); and A is the frequency factor.
The Arrhenius plot of the process is shown in Figure 7. A plot of ln(k) versus (1/T) presents a linear relationship, where the slope is (−Ea/R), and the intercept is lnA. According to the slope (−Ea/R), the activation energy (Ea) was calculated to be 51.7 kJ/mol. Generally, chemically-controlled processes are strongly dependent on temperature, and the activation energy is usually in the range of 40–100 kJ/mol, while the diffusion controlled processes are slightly dependent on the temperature, with the activation energy usually less than 21 kJ/mol−1 [10], which further proved that in this process, the chemical reaction is the rate-limiting step.
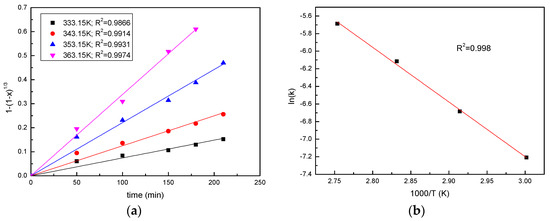
Figure 7.
Analysis of activation energy (a) Plots of 1 − (1 − x)1/3 vs. time for manganese; (b) Arrhenius plot of ln(k) vs. 1000/T at different temperatures.
In order to determine the reaction order, the results on the effect of the sawdust/ore mass ratio and the sulfuric acid concentration were applied to the chemical reaction kinetic model, as shown in Figure 8 and Figure 9. According to the slopes of the straight lines, the values of rate constant kr were determined, and a good linear relationship was presented. The plots of ln(k) versus ln(H2SO4) and ln(sawdust/ore) were constructed to determine the respective reaction orders. The results shown in Figure 8 and Figure 9 indicate a reaction order of 0.57 with respect to the sawdust/ore mass ratio and a reaction order of 1.66 with respect to the H2SO4 concentration.
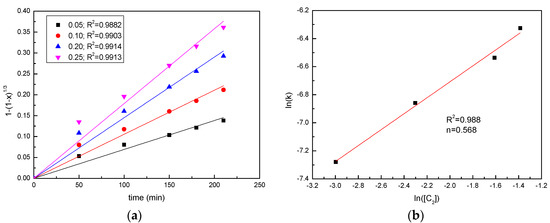
Figure 8.
Analysis of reaction order with respect to sawdust/ore mass ratio (a) Plots of 1 − (1 − x)1/3 vs. time for manganese at various sawdust/ore mass ratios; (b) the determination of the reaction order with respect to the sawdust/ore mass ratio.
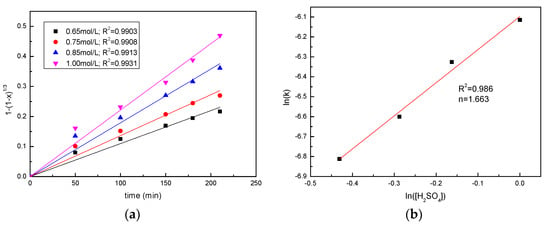
Figure 9.
Analysis of reaction order with respect to H2SO4 concentration (a) Plots of 1 − (1 − x)1/3 vs. time for manganese leaching at various sulfuric acid concentrations; (b) the determination of reaction order with respect to sulfuric acid concentration.
4. Conclusions
The reductive leaching of manganese oxide ore with pre-treated sawdust as a reducing agent in a sulfuric acid solution was investigated. Through the present work, the following results have been obtained:
(1) It is possible to carry out the leaching process by using pretreated sawdust as a reducing agent. Many factors influencing the manganese extraction are studied, and the results indicate that the leaching efficiency of manganese increased with the liquid/solid ratio, sawdust amount, sulfuric acid concentration, and temperature. Among these factors, sulfuric acid concentration and temperature have the most significant influence. The optimal conditions are as follows: a stirring speed of 300 rpm, a liquid/solid ratio of 8:1, a mass ratio of sawdust to ore of 0.25, a sulfuric acid concentration of 1 mol/L, and a temperature of 363.15 K for 180 min.
(2) The results of the kinetic analysis show that the leaching process is controlled by chemical reaction, and the activation energy is calculated to be 51.7 kJ/mol. The results also show a reaction order of 1.66 with respect to the sulfuric acid concentration and a reaction order of 0.57 with respect to the sawdust/ore mass ratio.
Acknowledgments
We thank Dequan Wang for providing help for the design of this experiment.
Author Contributions
Gaofeng Fu, Yang Sun and Lan Jiang contributed to the formulation of overarching research goals and aims; Yang Sun and Lan Jiang contributed to the experiment; Yang Sun and Gaofeng Fu analyzed the data; Yang Sun wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dwivedi, D.; Randhawa, N.S.; Saroj, S.; Jana, R.K. An Overview of Manganese Recovery by Hydro and Pyro-Metallurgical Routes. J. Inst. Eng. 2017, 98, 147–154. [Google Scholar] [CrossRef]
- Zhang, W.; Cheng, C.Y. Manganese metallurgy review. Part I: Leaching of ores/secondary materials and recovery of electrolytic/chemical manganese dioxide. Hydrometallurgy 2007, 89, 137–159. [Google Scholar] [CrossRef]
- Tekin, T.; Bayramoǧlu, M. Kinetics of the reduction of MnO2, with Fe2+, ions in acidic solutions. Hydrometallurgy 1993, 32, 9–20. [Google Scholar] [CrossRef]
- Das, S.C.; Sahoo, P.K.; Rao, P.K. Extraction of manganese from low-grade manganese ores by FeSO4 leaching. Hydrometallurgy 1982, 8, 35–47. [Google Scholar] [CrossRef]
- Nayl, A.A.; Ismail, I.M.; Aly, H.F. Recovery of pure MnSO4. H2O by reductive leaching of manganese from pyrolusite ore by sulfuric acid and hydrogen peroxide. Int. J. Miner. Proc. 2011, 100, 116–123. [Google Scholar] [CrossRef]
- Naik, P.K.; Sukla, L.B.; Das, S.C. Aqueous SO2 leaching studies on Nishikhal manganese ore through factorial experiment. Hydrometallurgy 2000, 54, 217–228. [Google Scholar] [CrossRef]
- Vračar, R.Ž.; Cerović, K.P. Manganese leaching in the FeS2–MnO2–O2–H2O system at high temperature in an autoclave. Hydrometallurgy 2000, 55, 79–92. [Google Scholar] [CrossRef]
- Li, C.X.; Zhong, H.; Wang, S.; Xue, J.R.; Wu, F.F.; Zhang, Z.Y. Manganese extraction by reduction–acid leaching from low-grade manganese oxide ores using CaS as reductant. Trans. Nonferr. Met. Soc. China 2015, 25, 1677–1684. [Google Scholar] [CrossRef]
- Su, H.F.; Liu, H.K.; Wang, F.; Lv, X.Y.; Wen, Y.X. Kinetics of Reductive Leaching of Low-grade Pyrolusite with Molasses Alcohol Wastewater in H2SO4. Chin. J. Chem. Eng. 2010, 18, 730–735. [Google Scholar] [CrossRef]
- Alaoui, A.; Kacemi, K.E.; Ass, K.E.; Darmane, Y.; Kitane, S. Kinetic study of the leaching of manganese mine tailings by organic reductant in sulphuric acid solution. Miner. Process. Extr. Metall. IMM Trans. 2016, 125, 109–116. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, G.; Yan, H.; Zhao, Y.; Li, T. Reduction of low-grade manganese dioxide ore pellets by biomass wheat stalk. Acta Metall. Sin. 2013, 26, 167–172. [Google Scholar] [CrossRef]
- Tang, Q.; Zhong, H.; Wang, S.; Li, J.Z.; Liu, G.Y. Reductive leaching of manganese oxide ores using waste tea as reductant in sulfuric acid solution. Trans. Nonferr. Met. Soc. China 2014, 24, 861–867. [Google Scholar] [CrossRef]
- Feng, Y.L.; Zhang, S.Y.; Li, H.R. Reductive leaching of manganese from low-grade pyrolusite ore in sulfuric acid using pyrolysis-pretreated sawdust as a reductant. Int. J. Miner. Metall. Mater. 2016, 23, 241–246. [Google Scholar] [CrossRef]
- Seetharaman, S. Treatise on Process Metallurgy, Volume 3: Industrial Processes; Elsevier Ltd.: Kidlington, UK, 2013. [Google Scholar]
- Hariprasad, D.; Dash, B.; Ghosh, M.K.; Anand, S. Leaching of manganese ores using sawdust as a reductant. Miner. Eng. 2007, 20, 1293–1295. [Google Scholar] [CrossRef]
- Free, M.L. Hydrometallurgy: Fundamentals and Applications; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; Volume 1, pp. 86–122. [Google Scholar]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).