Mineralogical Composition of Urinary Stones and Their Frequency in Patients: Relationship to Gender and Age
Abstract
:1. Introduction
- Intrinsic factors: sex, age, ethnic, racial and familial background and inherited physiological or anatomical predisposition to urinary calculi.
2. Experimental
2.1. Samples
2.2. Analytical Procedures
3. Results and Discussion
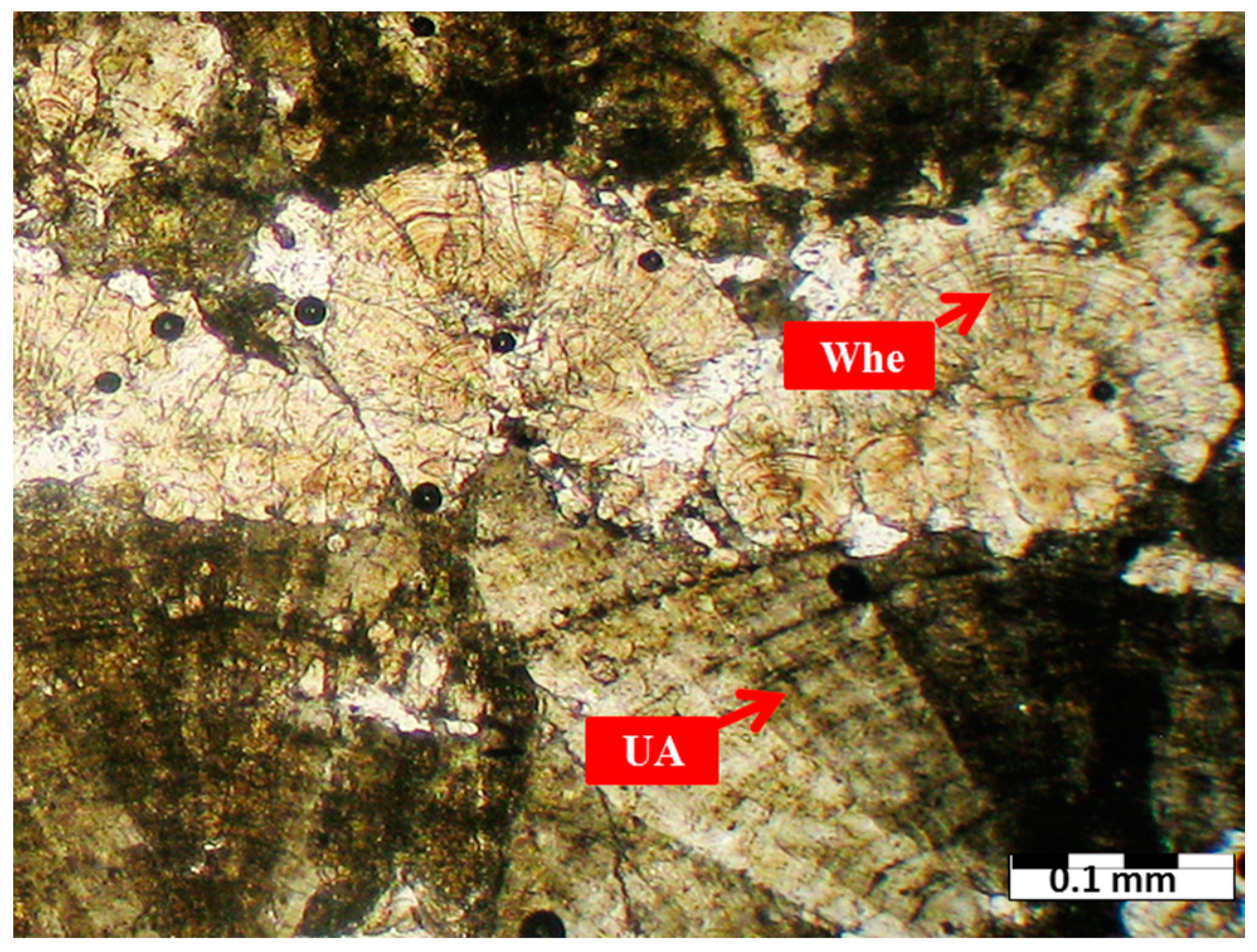
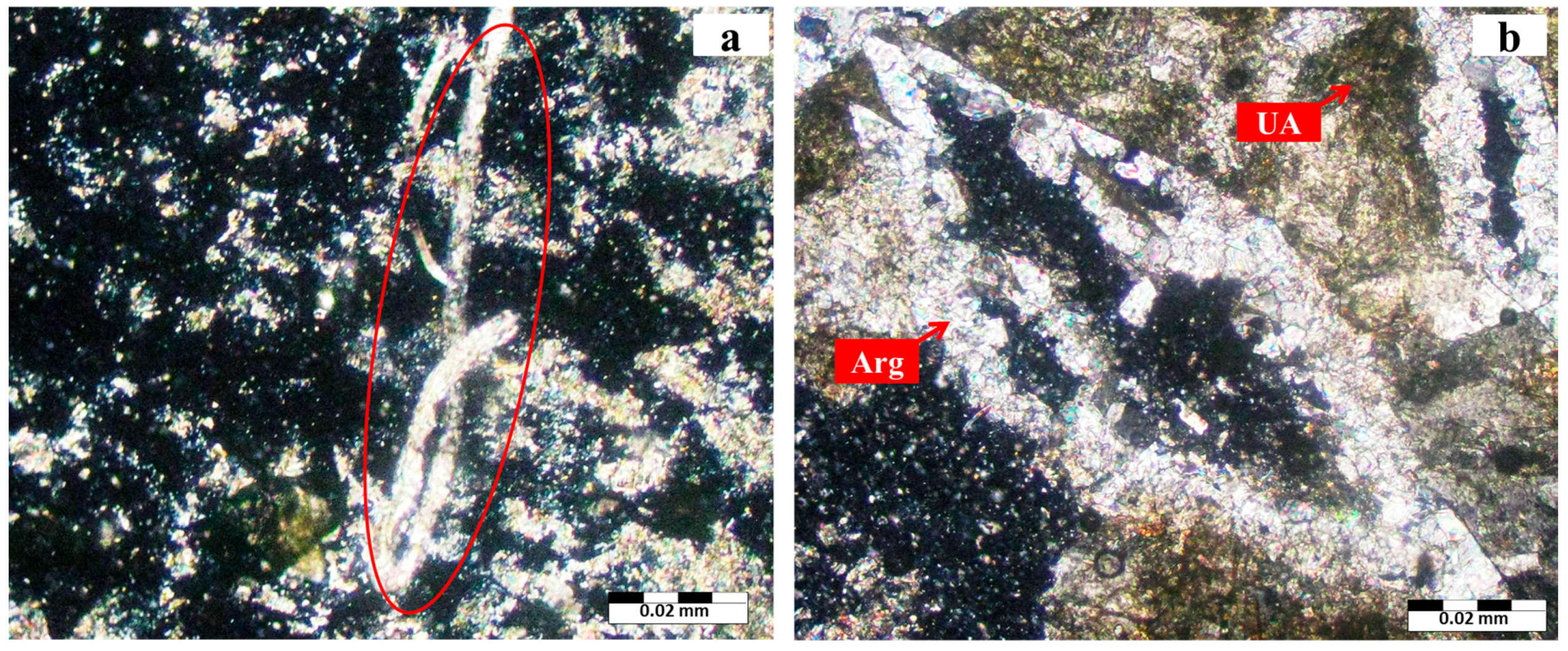
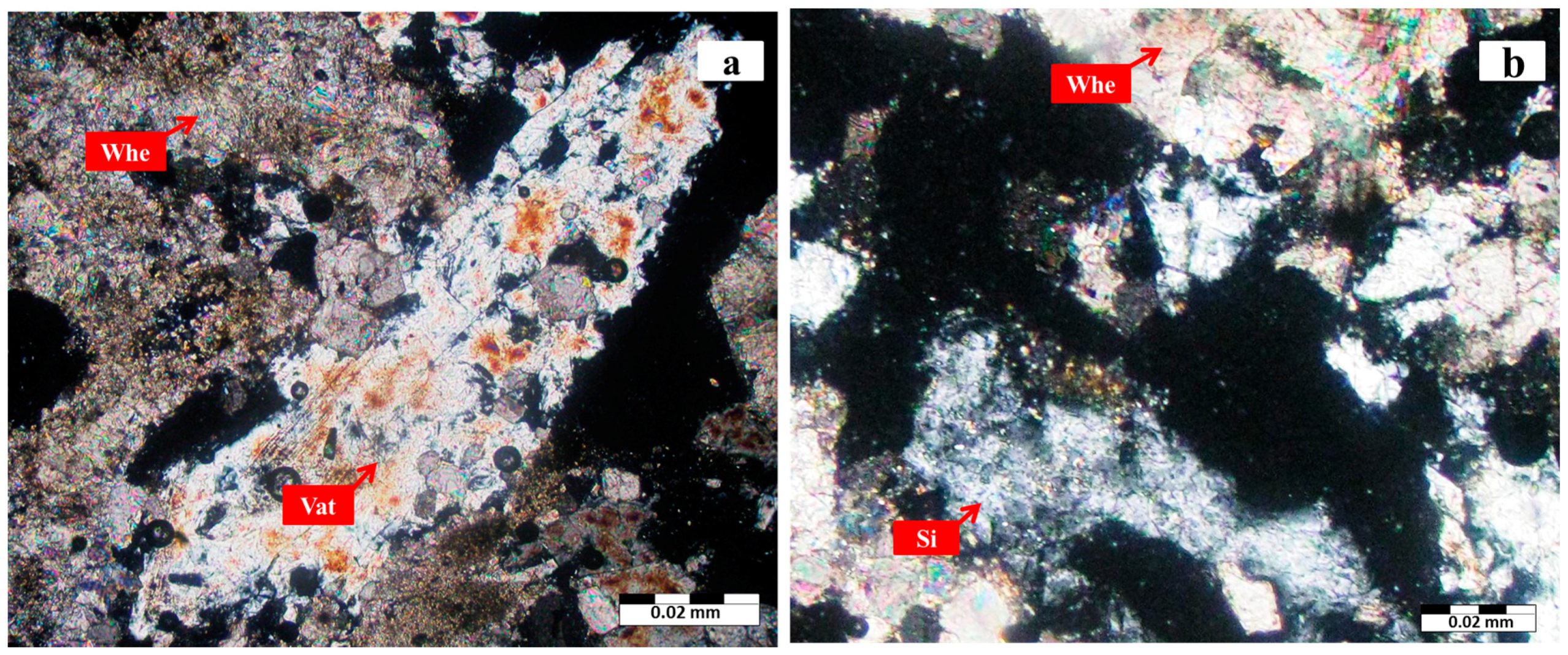
3.1. Mineralogy
3.2. Distribution of Urinary Stone by Age and Sex
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sahai, N. Modeling apatite nucleation in the human body and in the geochemical environment. Am. J. Sci. 2005, 305, 661–672. [Google Scholar] [CrossRef]
- Bazin, D.; Daudon, M.; Combes, C.; Rey, C. Characterization and some physicochemical aspects of pathological microcalcifications. Chem. Rev. 2012, 112, 5092–5120. [Google Scholar] [CrossRef] [PubMed]
- Giannossi, M.L.; Summa, V. A Review of Pathological Biomineral Analysis Techniques and Classification Schemes; INTECH Open Access Publisher: Rijeka, Croatia, 2012. [Google Scholar]
- Selvaraju, R.; Raja, A.; Thiruppathi, G. FT-IR spectroscopic, thermal analysis of human urinary stones and their characterization. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 137, 1397–1402. [Google Scholar] [CrossRef] [PubMed]
- Kuta, J.; Smetanová, S.; Benová, D.; Kořistková, T.; Machát, J. Urinary stones as a novel matrix for human biomonitoring of toxic and essential elements. Environ. Geochem. Health 2016, 38, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Schubert, G. Stone analysis. Urol. Res. 2006, 34, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, M.T. Urology, 2nd ed.; William & Wilkins: Baltimore, MD, USA, 1995. [Google Scholar]
- Singh, V.K.; Rai, A.K.; Rai, P.K.; Jindal, P.K. Cross-sectional study of kidney stones by laser-induced breakdown spectroscopy. Lasers Med. Sci. 2009, 24, 749–759. [Google Scholar] [CrossRef] [PubMed]
- Master, V.A.; Meng, M.M.; Stoller, M.L. Urinary Stone Disease: The Practical Guide to Medical and Surgical Management; Humana Press: Totowa, NJ, USA, 2007. [Google Scholar]
- Giannossi, M.L.; Summa, V.; Mongelli, G. Trace element investigations in urinary stones: A preliminary pilot case in Basilicata (Southern Italy). J. Trace Elem. Med. Biol. 2013, 27, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Mandel, I.; Mandel, N. Structure and compositional analysis of kidney stones. In Urinary Stone Disease; Humana Press: Totowa, NJ, USA, 2007; pp. 69–81. [Google Scholar]
- Gray, D.; Laing, M.; Nel, F.; Naudé, J.H. The Composition of Urinary Calculi Collected in the Durban Area. S. Afr. Med. J. 1982, 61, 121–125. [Google Scholar] [PubMed]
- Scales, C.D.; Smith, A.C.; Hanley, J.M.; Saigal, C.S.; Urologic Diseases in America Project. Urologic Diseases in America Project. Prevalence of kidney stones in the United States. Eur. Urol. 2012, 62, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Pietrow, P.K.; Karellas, M.K. Medical management of common urinary calculi. S. Afr. Fam. Pract. 2007, 49, 44–48. [Google Scholar]
- Golub, E.E. Biomineralization and matrix vesicles in biology and pathology. In Seminars in Immunopathology; Springer: New York, NY, USA, 2011; Volume 33, pp. 409–417. [Google Scholar]
- Golovanova, O.A.; Frank-Kamenetskaya, O.V.; Punin, Y.O. Specific features of pathogenic mineral formation in the human body. Russ. J. Gen. Chem. 2011, 81, 1392–1406. [Google Scholar] [CrossRef]
- Cheng, C.L.; Chang, H.H.; Huang, P.J.; Chu, Y.T.; Lin, S.Y. Composition and distribution of elements and ultrastructural topography of a human cardiac calculus. Biol. Trace Elem. Res. 2013, 152, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Giannossi, M.L.; Summa, V. An observation on the composition of urinary calculi: Environmental influence. In Medical Geochemistry; Springer Netherlands: Dordrecht, The Netherlands, 2013; pp. 67–90. [Google Scholar]
- Raman, C.V.; Krishnan, K.S. A new type of secondary radiation. Nature 1928, 121, 501–502. [Google Scholar] [CrossRef]
- Abboud, I.A. Mineralogy and chemistry of urinary stones: Patients from North Jordan. Environ. Geochem. Health 2008, 30, 445–463. [Google Scholar] [CrossRef] [PubMed]
- Doebelin, N.; Kleeberg, R. Profex: A graphical user interface for the Rietveld refinement program BGMN. J. Appl. Crystallogr. 2015, 48, 1573–1580. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, J.; Friedel, P.; Kleeberg, R. BGMN—A new fundamental parameters based Rietveld program for laboratory X-ray sources, its use in quantitative analysis and structure investigations. CPD Newsl. 1998, 20, 5–8. [Google Scholar]
- Mukherjee, A.K. Human kidney stone analysis using X-ray powder diffraction. J. Indian Inst. Sci. 2014, 94, 35–44. [Google Scholar]
- Shamema, A.A.; Arul, K.T.; Kumar, R.S.; Kalkura, S.N. Physicochemical analysis of urinary stones from Dharmapuri district. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 134, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Hesse, A.; Hicking, W.; Bach, D.; Vahlensieck, W. Characterisation of urinary crystals and thin polished sections of urinary calculi by means of an optical microscopic and scanning electron microscopic arrangement. Urol. Int. 1981, 36, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Hyacinth, P.; Rajamohanan, K.; Marckar, F.Y.; Koshy, P.; Krishnamurthy, S. A study of the ultrastructure of urinary calculi by scanning electron microscopy. Urol. Res. 1984, 12, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Afaj, A.H.; Sultan, M.A. Mineralogical composition of the urinary stones from different provinces in Iraq. Sci. World J. 2005, 5, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Hesse, A.; Tiselius, H.G.; Siener, R.; Hoppe, B. Urinary Stones: Diagnosis, Treatment, and Prevention of Recurrence, 3rd revised and enlarged ed.; S. Karger: Basel, Switzerland, 2009; p. 232. [Google Scholar]
- Zarasvandi, A.; Carranza, E.J.; Heidari, M.; Mousapour, E. Environmental factors of urinary stones mineralogy, Khouzestan Province, Iran. J. Afr. Earth Sci. 2014, 97, 368–376. [Google Scholar] [CrossRef]
- Keshavarzi, B.; Yavarashayeri, N.; Irani, D.; Moore, F.; Zarasvandi, A.; Salari, M. Trace elements in urinary stones: A preliminary investigation in Fars province, Iran. Environ. Geochem. Health. 2015, 37, 377–389. [Google Scholar] [CrossRef] [PubMed]
- Bazin, D.; Chevallier, P.; Matzen, G.; Jungers, P.; Daudon, M. Heavy elements in urinary stones. Urol. Res. 2007, 35, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Moe, O.W. Kidney stones: Pathophysiology and medical management. Lancet 2006, 367, 333–344. [Google Scholar] [CrossRef]
- Robertson, W.G. Urinary tract stones. In Medical Therapy in Urology; Springer: London, UK, 2010; pp. 147–162. [Google Scholar]
- Gibson, R.I. Descriptive human pathological mineralogy. Am. Mineral. 1974, 59, 1177–1182. [Google Scholar]
- Ma, M.G.; Sun, R.C. Biomineralization and Biomimetic Synthesis of Biomineral and Nanomaterials; INTECH Open Access Publisher: Rijeka, Croatia, 2011. [Google Scholar]
- Potapov, S.S.; Parshina, N.V.; Moroz, T.N.; Lyutoev, V.P. Presented at the International Workshop “Quartz. Silica”, Syktyvkar, Russia, 2004. (In Russian)
- Pal’chik, N.A.; Moroz, T.N.; Maksimova, N.V.; Dar’in, A.V. Mineral and microelement compositions of urinary stones. Russ. J. Inorg. Chem. 2006, 51, 1098–1105. [Google Scholar] [CrossRef]
- Knoll, T. Epidemiology, pathogenesis, and pathophysiology of urolithiasis. Eur. Urol. Suppl. 2010, 9, 802–806. [Google Scholar] [CrossRef]
- Romero, V.; Akpinar, H.; Assimos, D.G. Kidney stones: A global picture of prevalence, incidence, and associated risk factors. Rev. Urol. 2010, 12, e86–e96. [Google Scholar] [PubMed]
- Pourmand, G.; Pourmand, B. Epidemiology of Stone Disease in Iran. In Urolithiasis; Springer: London, UK, 2012; pp. 85–87. [Google Scholar]
- Baker, P.W.; Coyle, P.; Bais, R.; Rofe, A.M. Influence of season, age, and sex on renal stone formation in South Australia. Med. J. Aust. 1993, 159, 390–392. [Google Scholar] [PubMed]
- Hosseini, M.M.; Eshraghian, A.; Dehghanian, I.; Irani, D.; Amini, M. Metabolic abnormalities in patients with nephrolithiasis: Comparison of first-episode with recurrent cases in Southern Iran. Int. Urol. Nephrol. 2010, 42, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Daudon, M.; Doré, J.C.; Jungers, P.; Lacour, B. Changes in stone composition according to age and gender of patients: A multivariate epidemiological approach. Urol. Res. 2004, 32, 241–247. [Google Scholar] [CrossRef] [PubMed]
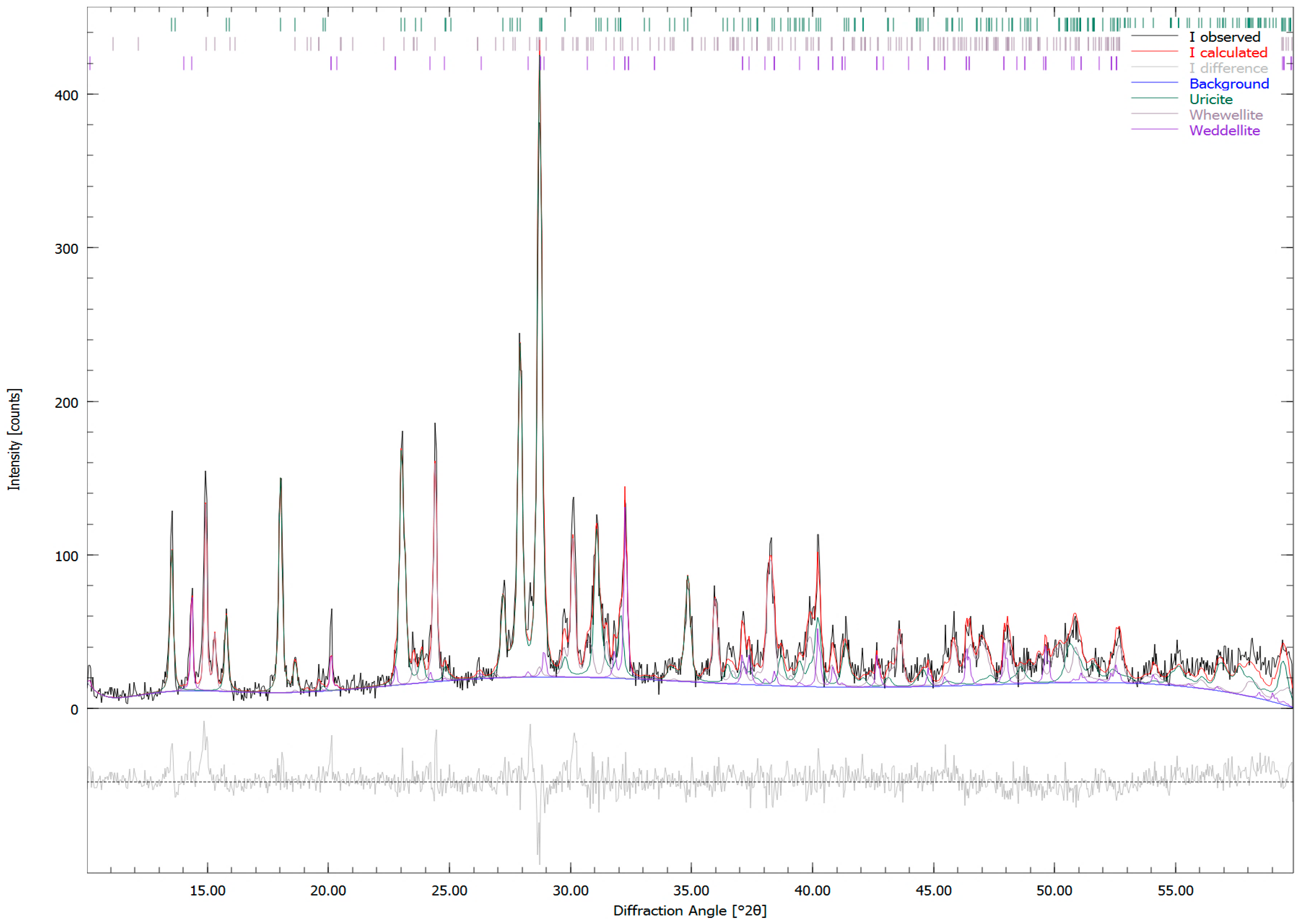
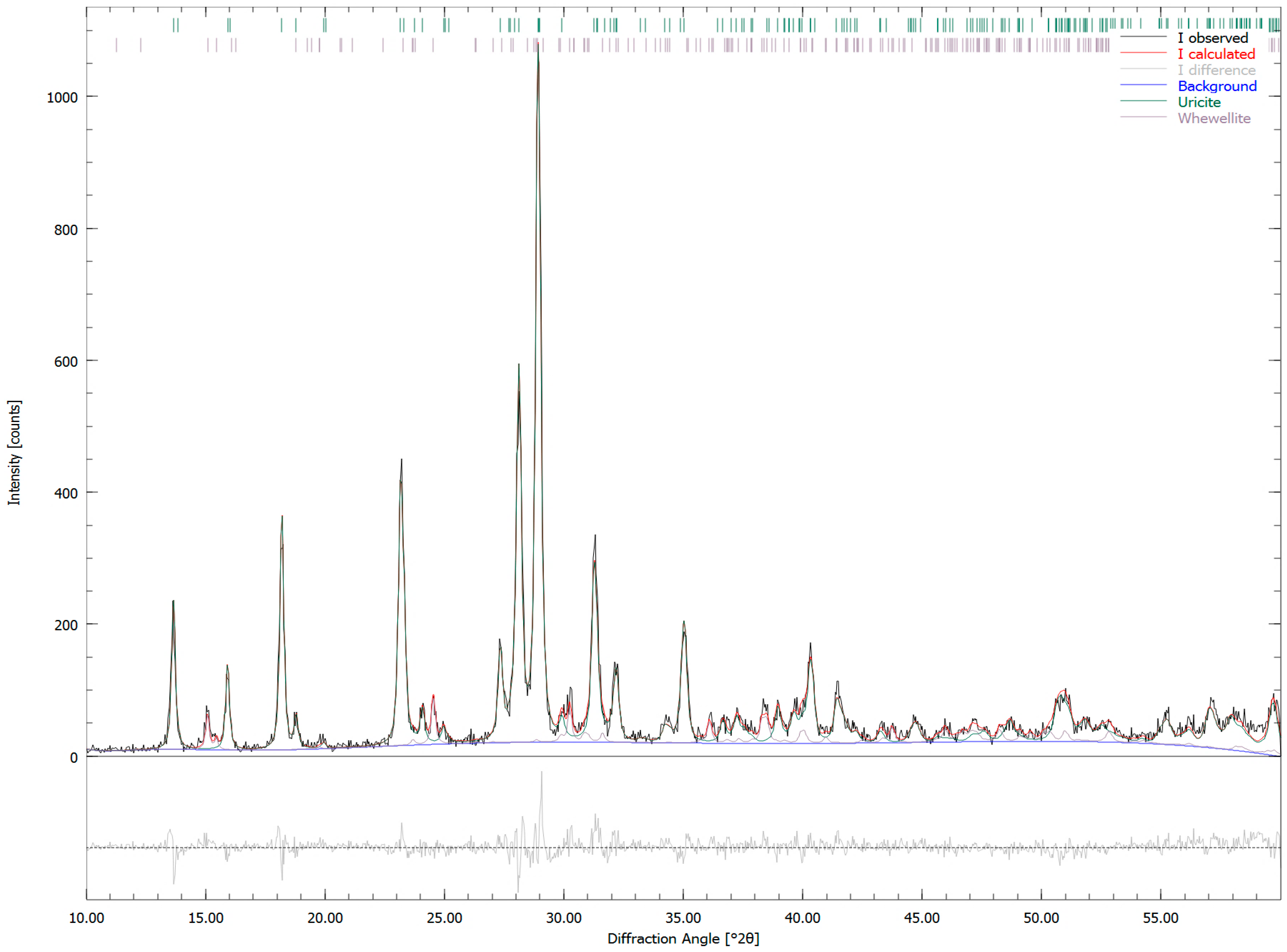
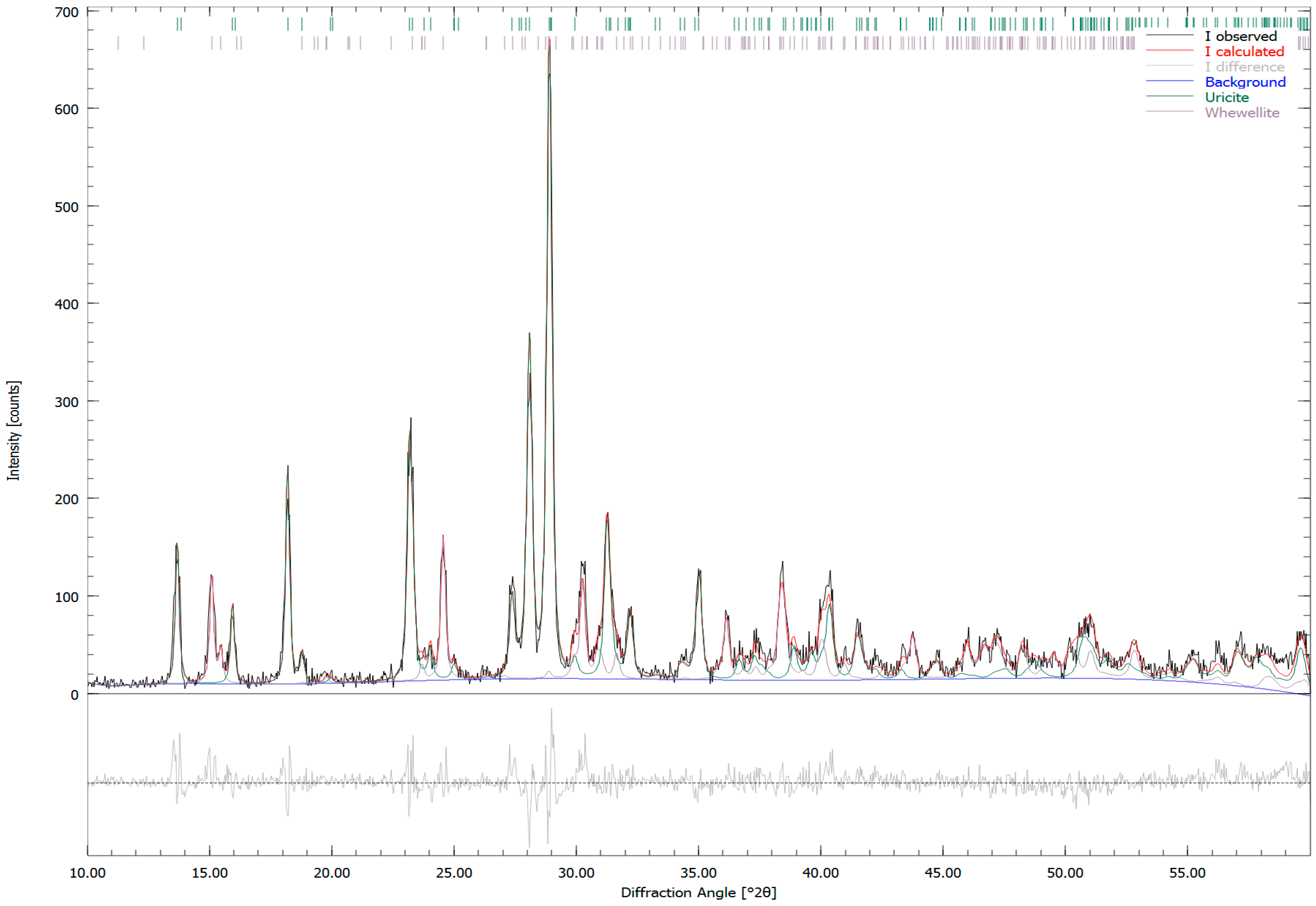
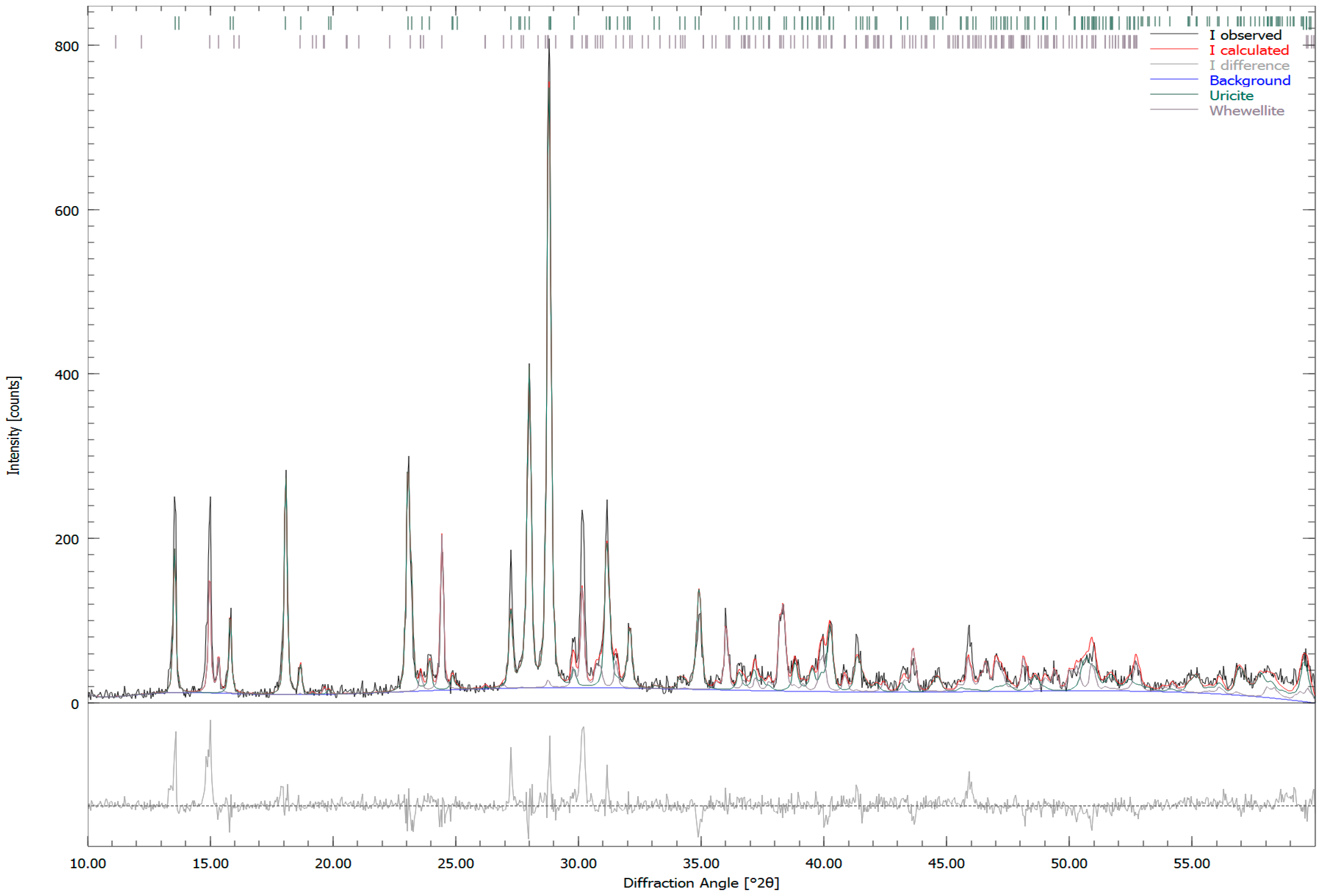

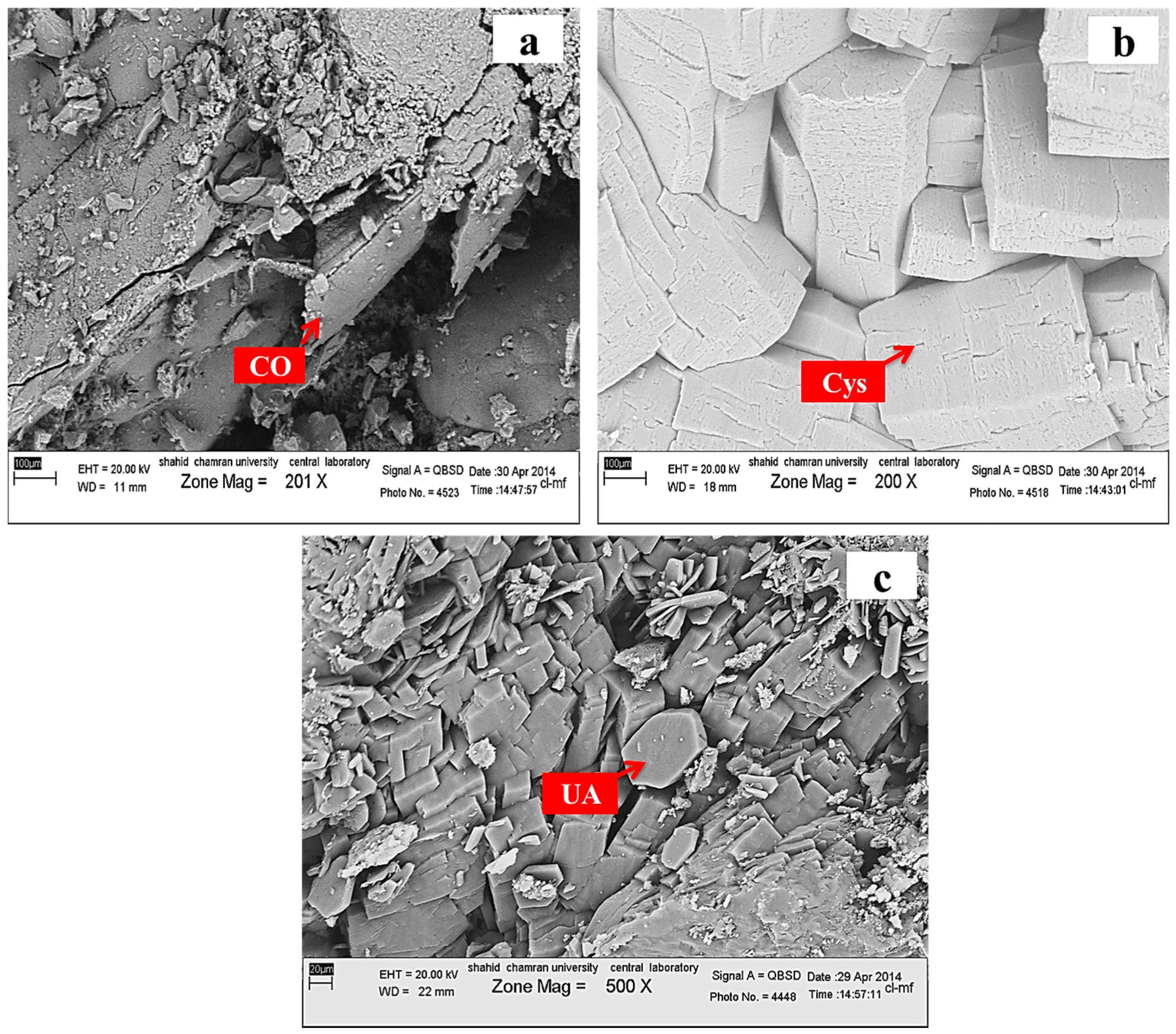
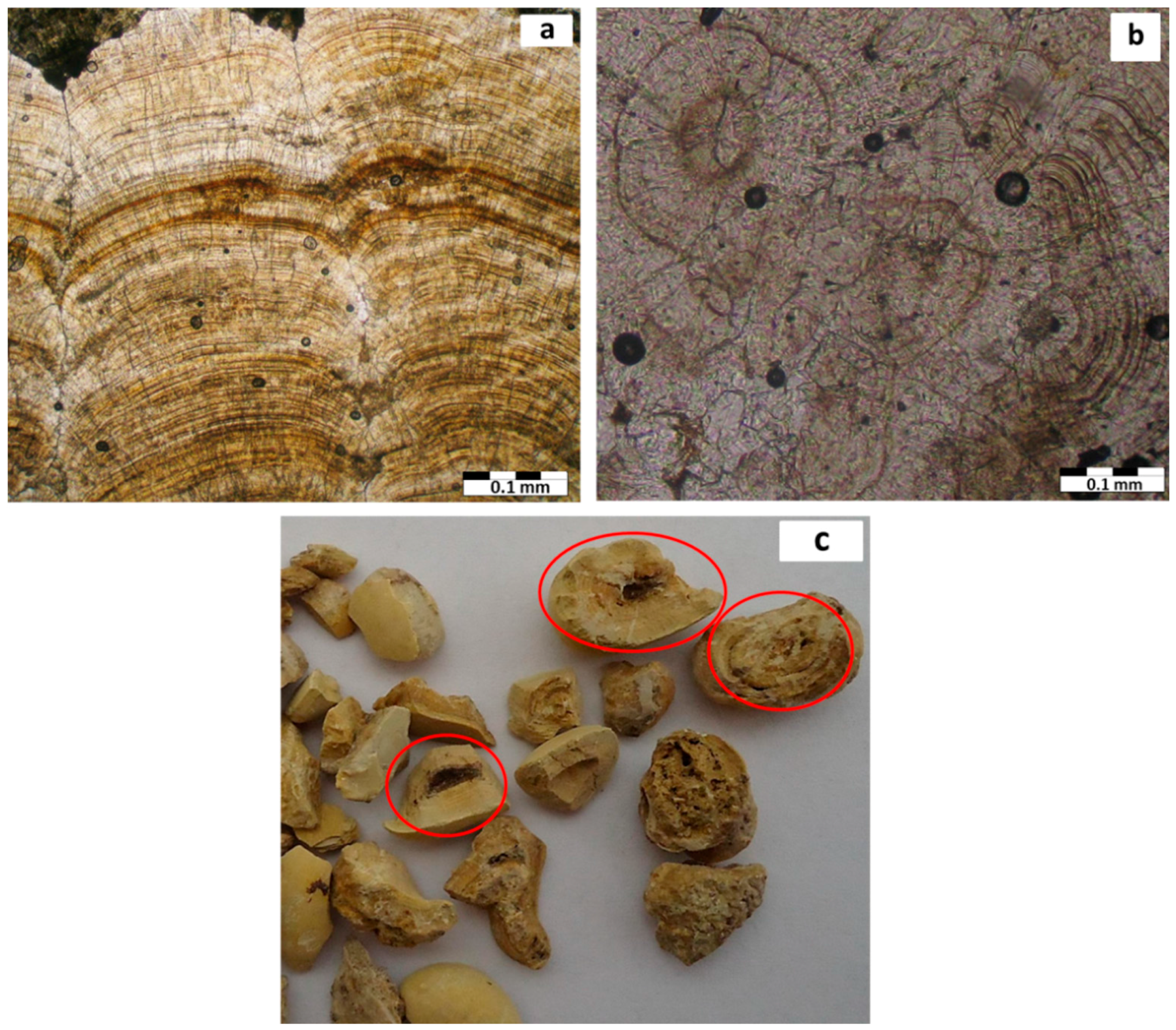
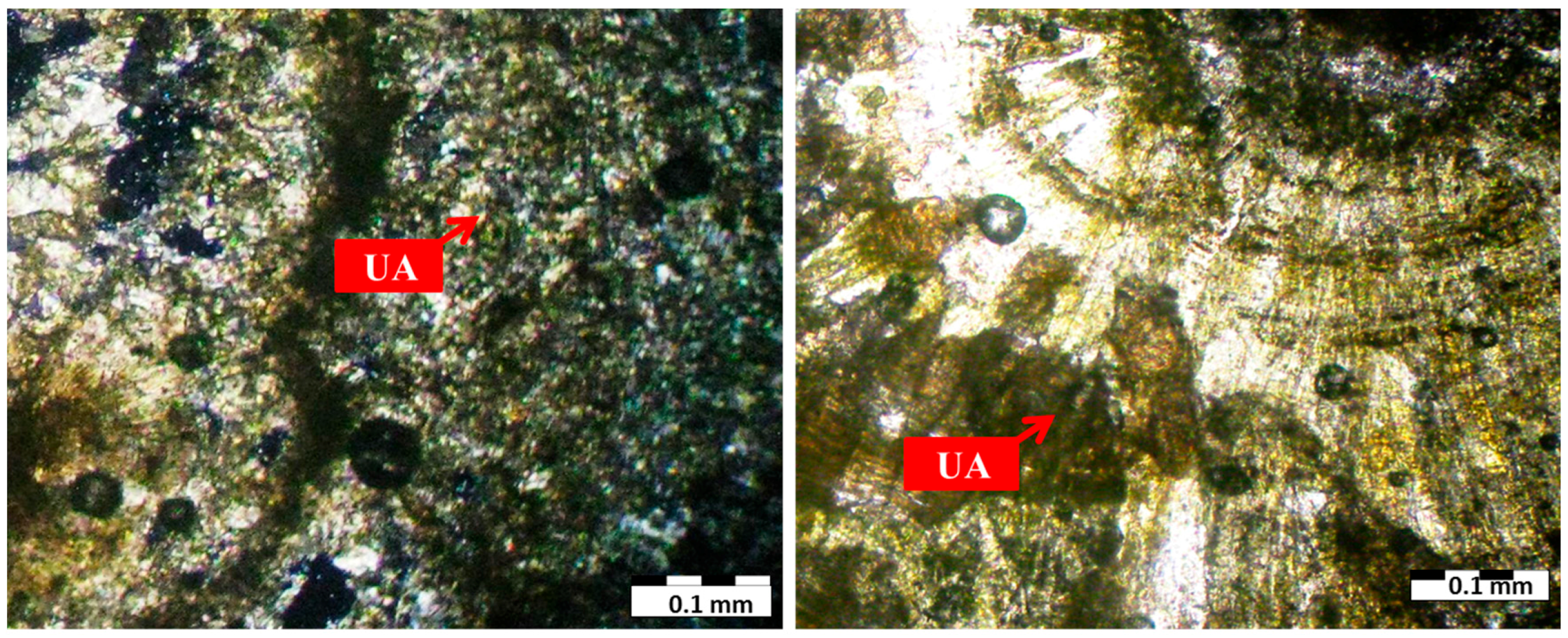
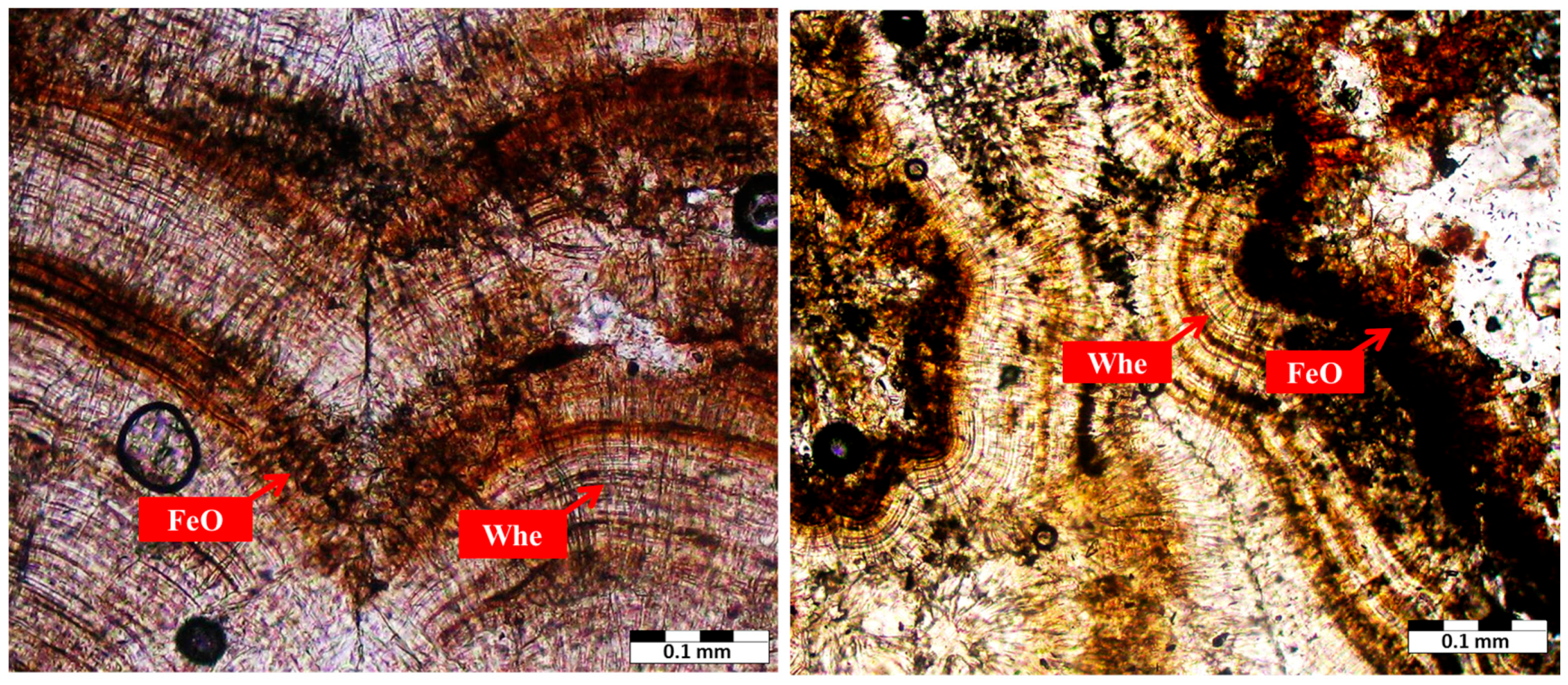
| Sample Number | Sample Type | Age | Gender | Minerals | wt. % |
|---|---|---|---|---|---|
| MP-1 | Renal | 54 | Male | Whewellite | 100 |
| MP-2 | Renal | 43 | Male | Whewellite | 100 |
| MK-1 | Renal | 52 | Male | Whewellite | 100 |
| MPo-1 | Renal | 55 | Male | Whewellite | 100 |
| MPo-2 | Renal | 54 | Male | Whewellite | 100 |
| MK-2 | Renal | 43 | Male | Whewellite | 100 |
| MP-3 | Renal | 69 | Male | Whewellite | 100 |
| MP-4 | Renal | 40 | Male | Uricite | 100 |
| FP-1 | Renal | 63 | Female | Uricite | 100 |
| MP-5 | Renal | 39 | Male | Uricite | 100 |
| FK-1 | Renal | 56 | Female | Uricite | 100 |
| MK-3 | Renal | 45 | Male | Uricite | 100 |
| MK-4 | Renal | 58 | Male | Uricite | 100 |
| MK-5 | Renal | 70 | Male | Uricite | 100 |
| MB-3 | Bladder | 45 | Male | Uricite | 100 |
| FK-2 | Renal | 24 | Female | L-Cystine | 100 |
| FU | Ureter | 25 | Female | L-Cystine | 100 |
| FP-2 | Renal | 29 | Female | Hydroxylapatite | 100 |
| MPo-3 | Renal | 50 | Male | Whewellite | 51.28 |
| Uricite | 48.72 | ||||
| MPo-4 | Renal | 69 | Male | Uricite | 78.51 |
| Whewellite | 21.49 | ||||
| FPo-1 | Renal | 57 | Female | Uricite | 91.88 |
| Whewellite | 8.12 | ||||
| MK-6 | Renal | 82 | Male | Uricite | 82.05 |
| Whewellite | 17.95 | ||||
| MK-7 | Renal | 52 | Male | Uricite | 52.28 |
| Whewellite | 47.72 | ||||
| MP-6 | Renal | 47 | Male | Uricite | 82.03 |
| Whewellite | 17.97 | ||||
| MP-7 | Renal | 48 | Male | Uricite | 83.71 |
| Whewellite | 16.29 | ||||
| MPo-5 | Renal | 52 | Male | Whewellite | 100 |
| MPo-6 | Renal | 43 | Male | Uricite | 74.54 |
| Whewellite | 25.46 | ||||
| FP-3 | Renal | 51 | Female | Whewellite | 76.90 |
| Uricite | 23.10 | ||||
| MP-8 | Renal | 48 | Male | Uricite | 73.20 |
| Whewellite | 26.80 | ||||
| FK-3 | Renal | 45 | Female | Uricite | 75.88 |
| Whewellite | 24.12 | ||||
| MP-9 | Renal | 27 | Male | Whewellite | 76.30 |
| Uricite | 23.70 | ||||
| FPo-2 | Renal | 37 | Female | Uricite | 94.29 |
| Whewellite | 5.71 | ||||
| MP-10 | Renal | 31 | Male | Uricite | 60.40 |
| Weddellite | 31.92 | ||||
| Whewellite | 7.68 | ||||
| MB-15 | Bladder | 54 | Male | Uricite | 71.21 |
| Whewellite | 20.92 | ||||
| Weddellite | 7.88 | ||||
| MPo-7 | Renal | 46 | Male | Whewellite | 84.50 |
| Weddellite | 15.50 | ||||
| MK-8 | Renal | 62 | Male | Whewellite | 76.40 |
| Weddellite | 18.40 | ||||
| FK-4 | Renal | 38 | Female | Whewellite | 90.59 |
| Weddellite | 9.41 | ||||
| MP-11 | Renal | 67 | Male | Hydroxylapatite | 48.49 |
| Whewellite | 32.41 | ||||
| Weddellite | 19.10 | ||||
| FP-4 | Renal | 65 | Female | Uricite | 49.07 |
| Whewellite | 43.87 | ||||
| Calcium Urate | 7.06 |
| Age Group | Calcium Oxalate | Uric Acid | Cystine | Calcium Phosphate | Mixed Stone | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Female | Male | Female | Male | Female | Male | Female | Male | Female | Male | |
| <40 | 1 | - | - | 1 | 2 | - | 1 | - | 1 | 2 |
| 40–50 | - | 3 | - | 3 | - | - | - | - | 1 | 4 |
| 50–60 | 4 | 2 | 1 | - | - | - | - | - | 1 | 4 |
| >60 | - | 2 | 1 | 1 | - | - | - | 1 | 1 | 2 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keshavarzi, B.; Yavar Ashayeri, N.; Moore, F.; Irani, D.; Asadi, S.; Zarasvandi, A.; Salari, M. Mineralogical Composition of Urinary Stones and Their Frequency in Patients: Relationship to Gender and Age. Minerals 2016, 6, 131. https://doi.org/10.3390/min6040131
Keshavarzi B, Yavar Ashayeri N, Moore F, Irani D, Asadi S, Zarasvandi A, Salari M. Mineralogical Composition of Urinary Stones and Their Frequency in Patients: Relationship to Gender and Age. Minerals. 2016; 6(4):131. https://doi.org/10.3390/min6040131
Chicago/Turabian StyleKeshavarzi, Behnam, Nasrin Yavar Ashayeri, Farid Moore, Dariush Irani, Sina Asadi, Alireza Zarasvandi, and Mehrdad Salari. 2016. "Mineralogical Composition of Urinary Stones and Their Frequency in Patients: Relationship to Gender and Age" Minerals 6, no. 4: 131. https://doi.org/10.3390/min6040131
APA StyleKeshavarzi, B., Yavar Ashayeri, N., Moore, F., Irani, D., Asadi, S., Zarasvandi, A., & Salari, M. (2016). Mineralogical Composition of Urinary Stones and Their Frequency in Patients: Relationship to Gender and Age. Minerals, 6(4), 131. https://doi.org/10.3390/min6040131






