The Desulfurization of Magnetite Ore by Flotation with a Mixture of Xanthate and Dixanthogen
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
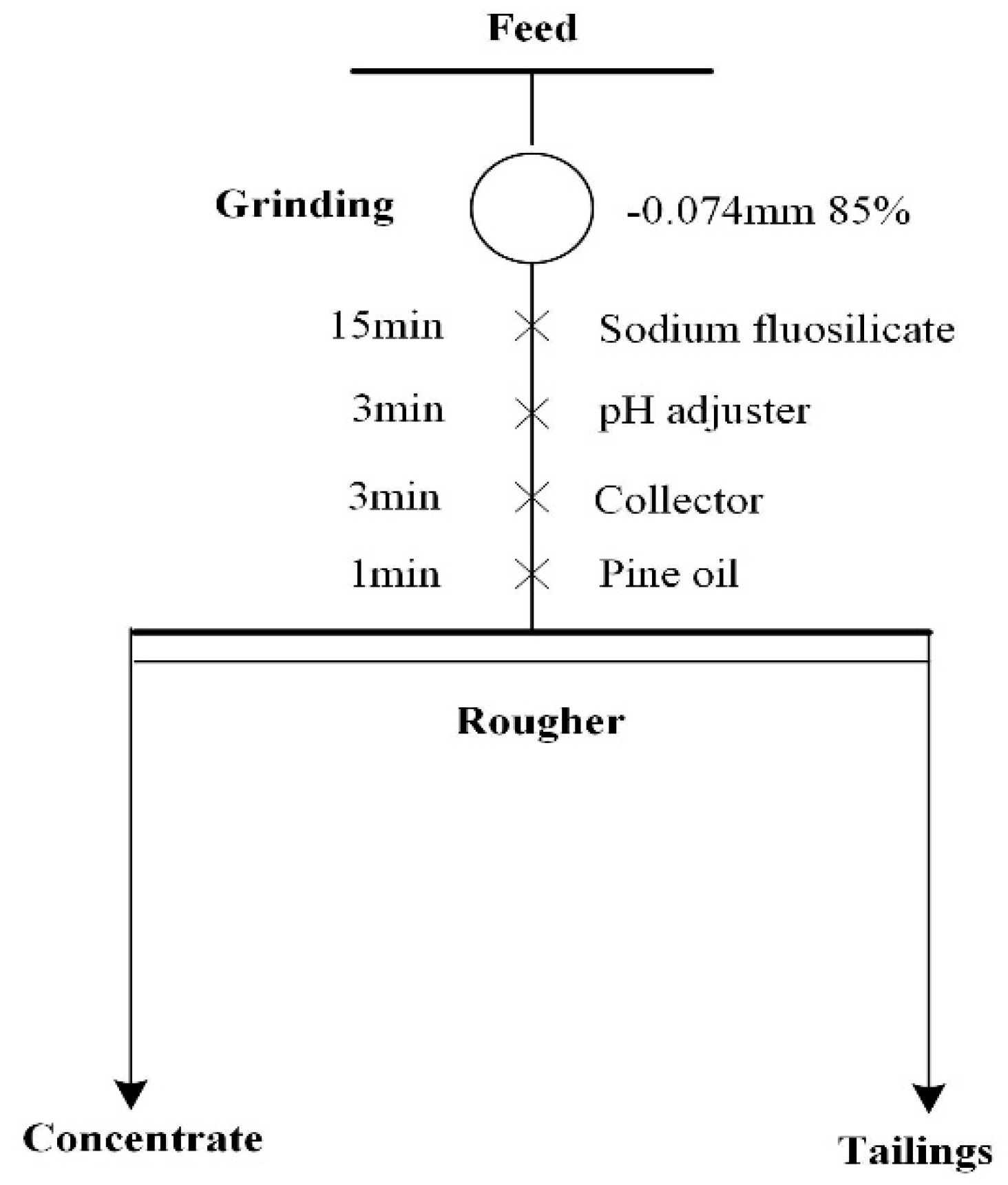
2.2.1. Grinding and Flotation Experiments
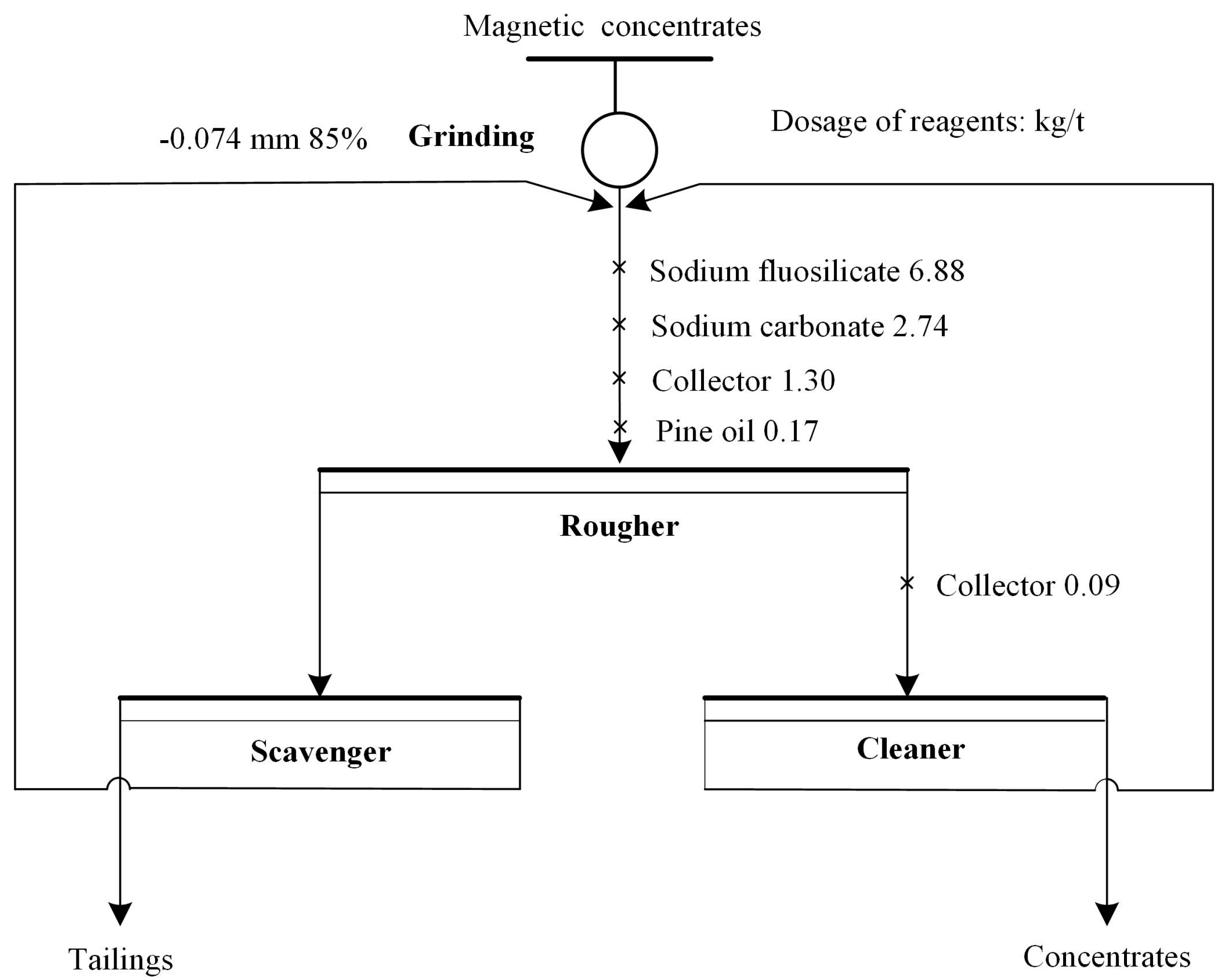
2.2.2. Industrial Tests
2.2.3. Zeta Potential Measurements
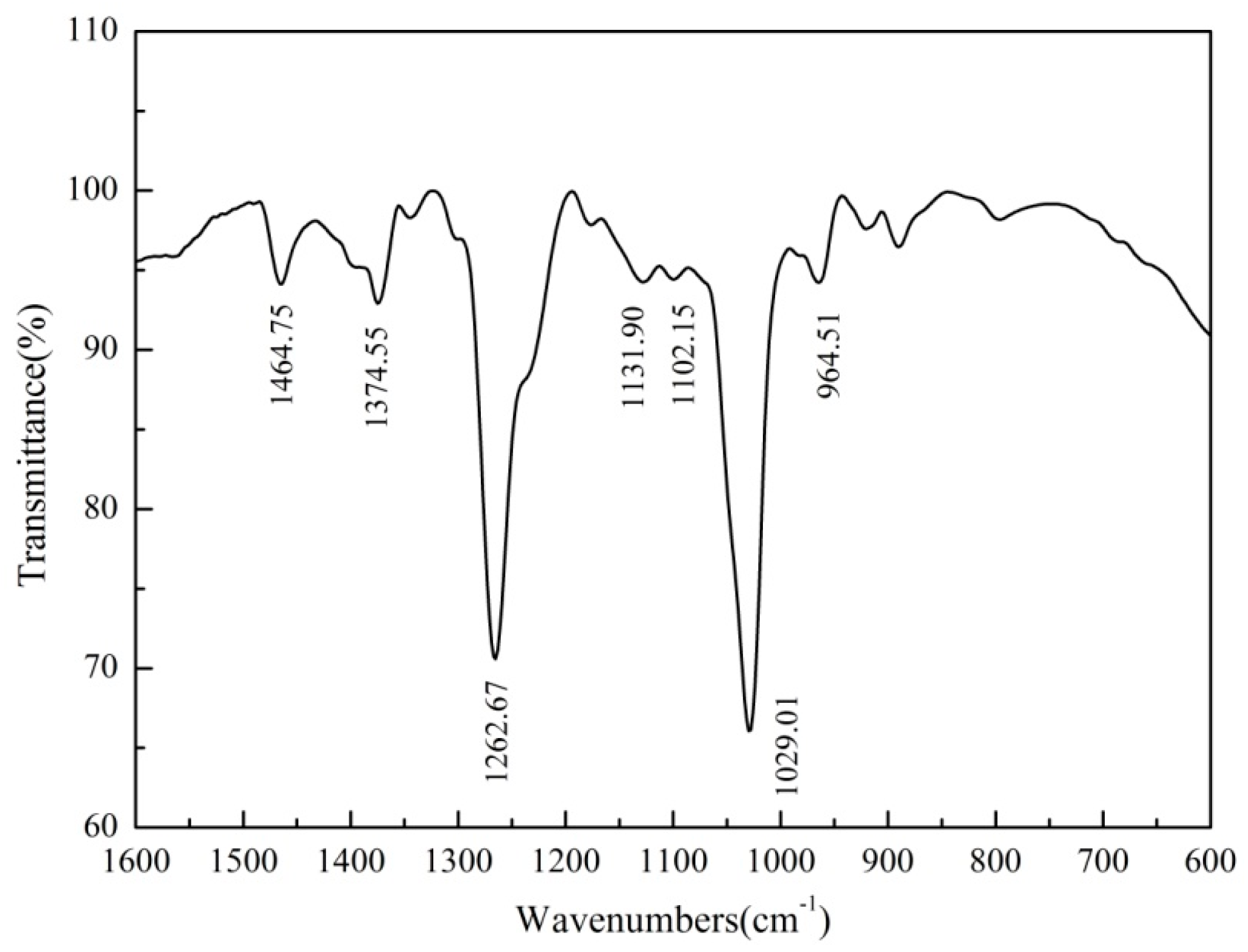
2.2.4. FTIR Measurements
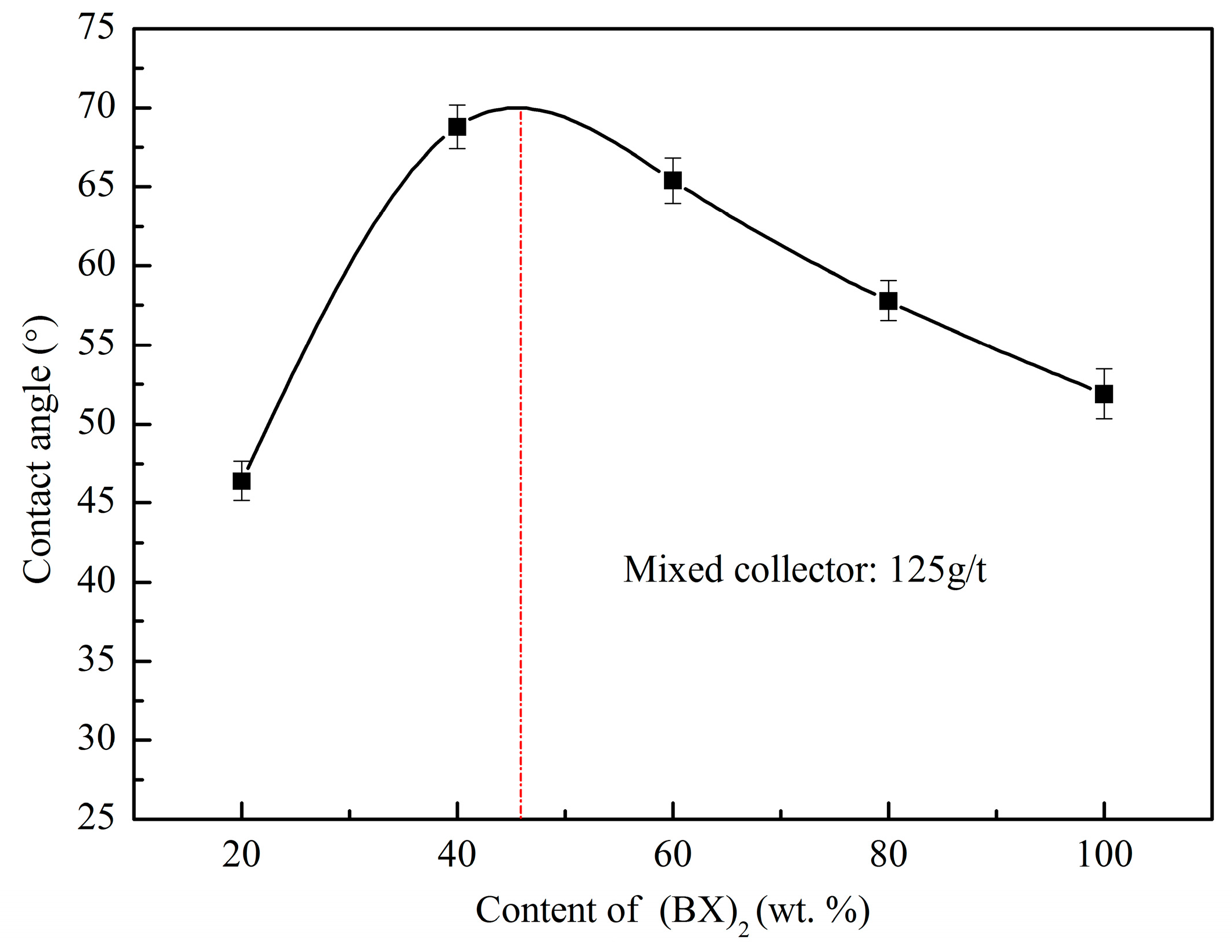
2.2.5. Contact Angle Measurements
3. Results and Discussion
3.1. Flotation
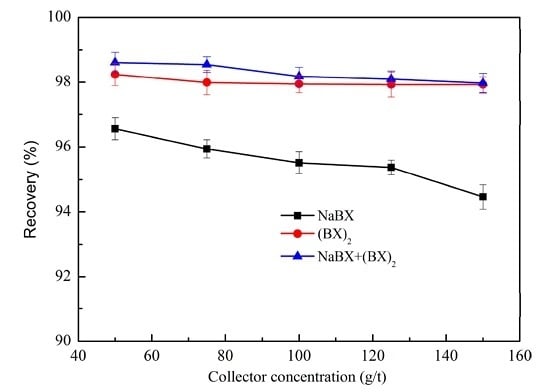
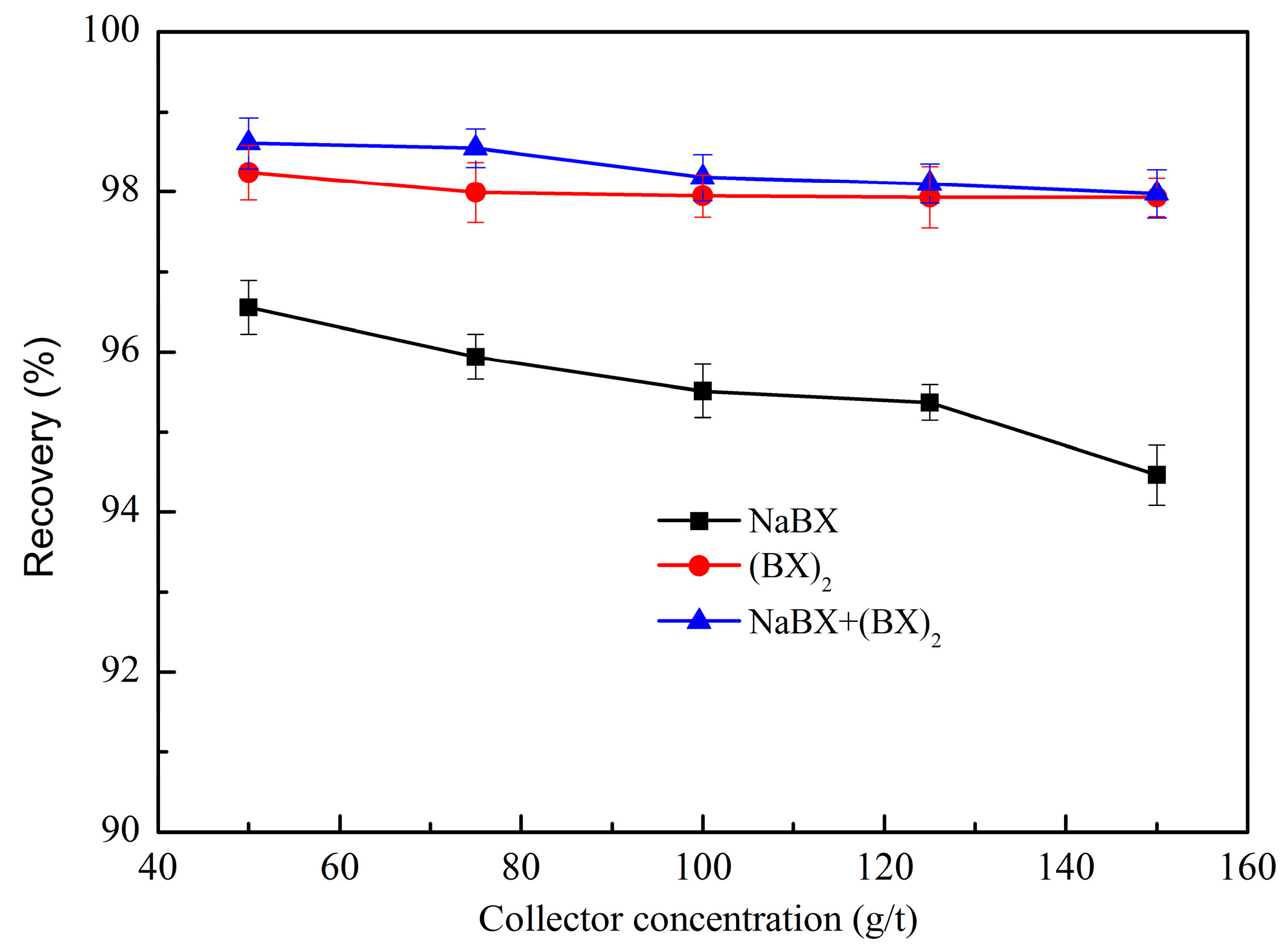
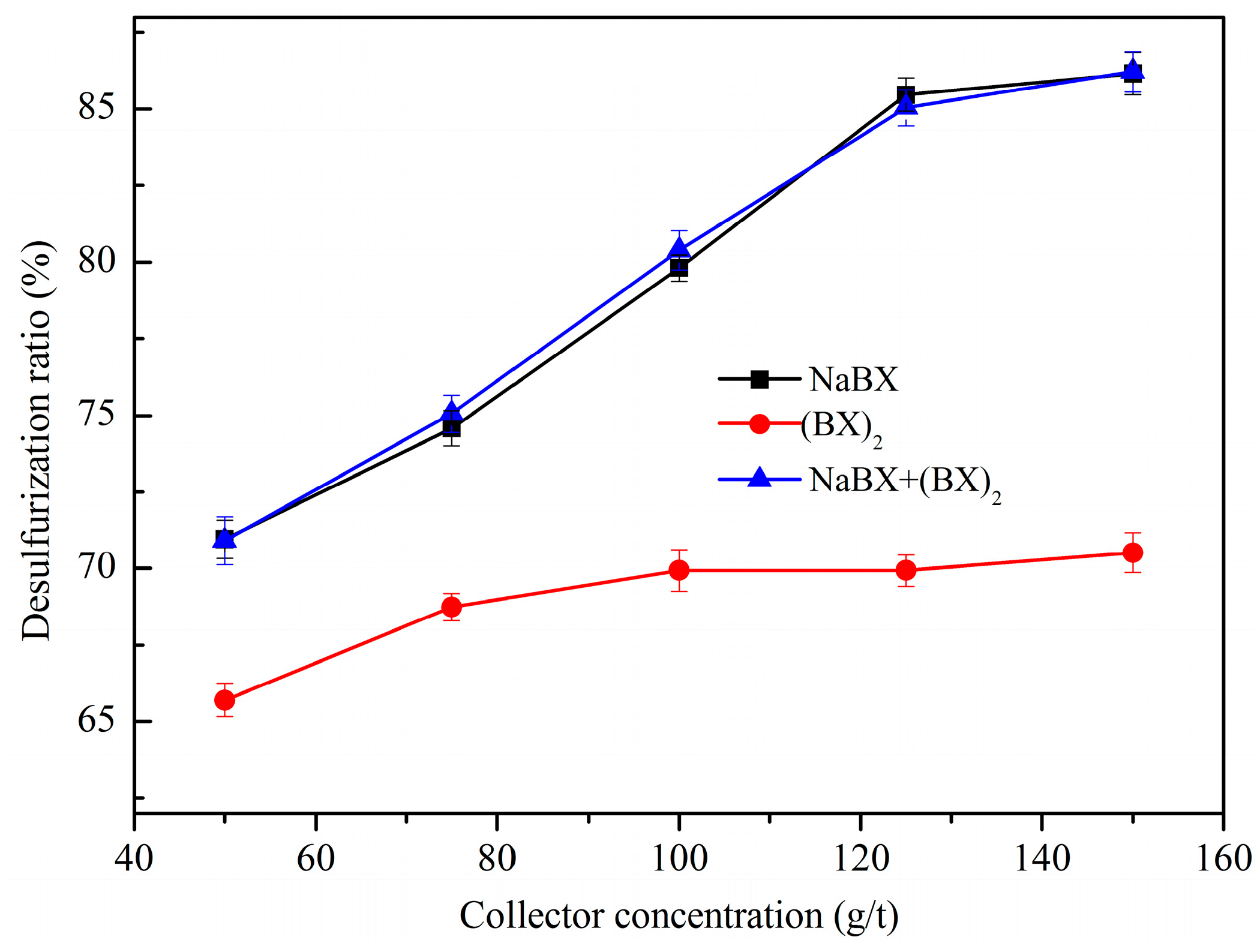
3.1.1. Effect of Collector
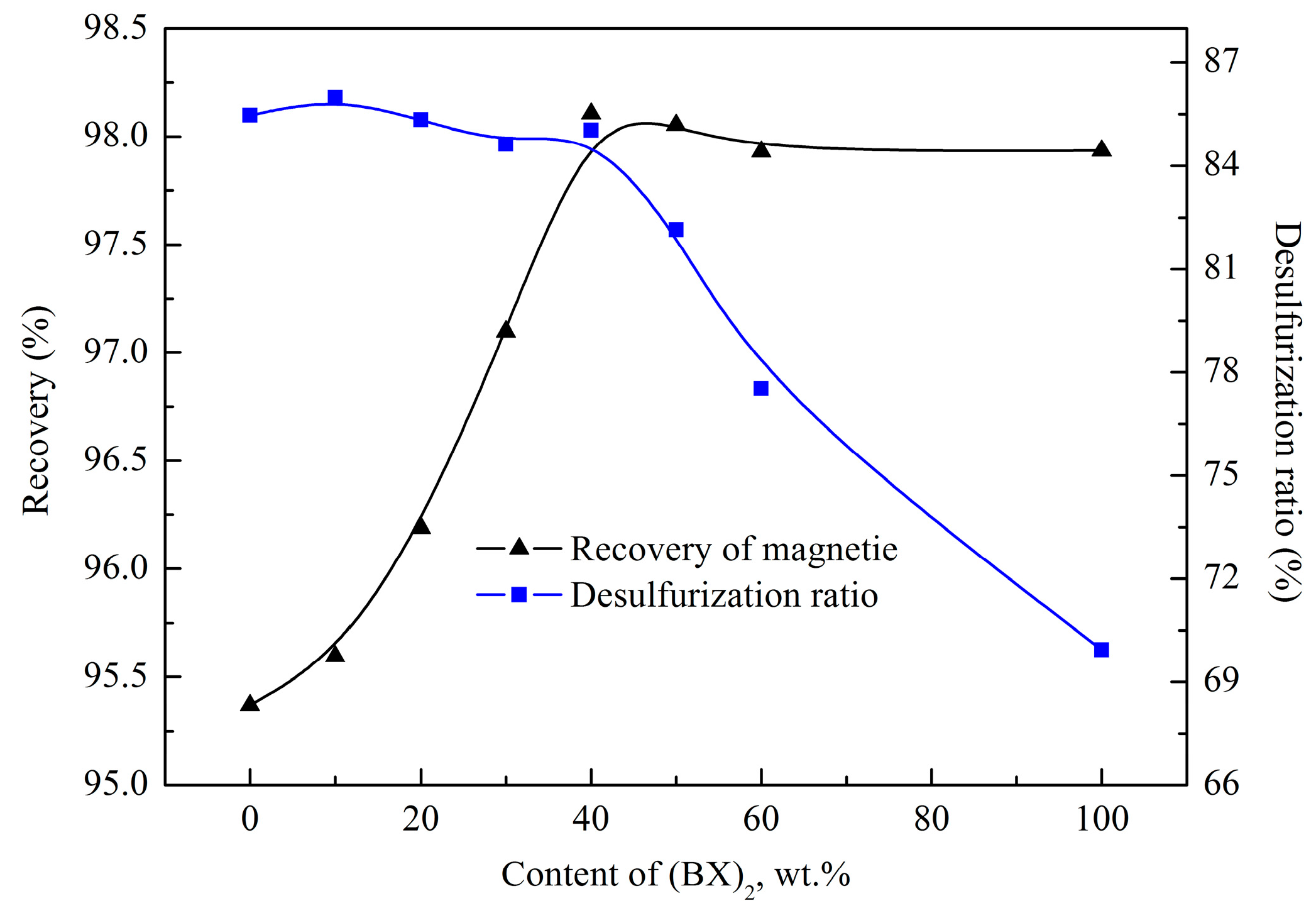
3.1.2. Effect of Mixed Collector Ratio
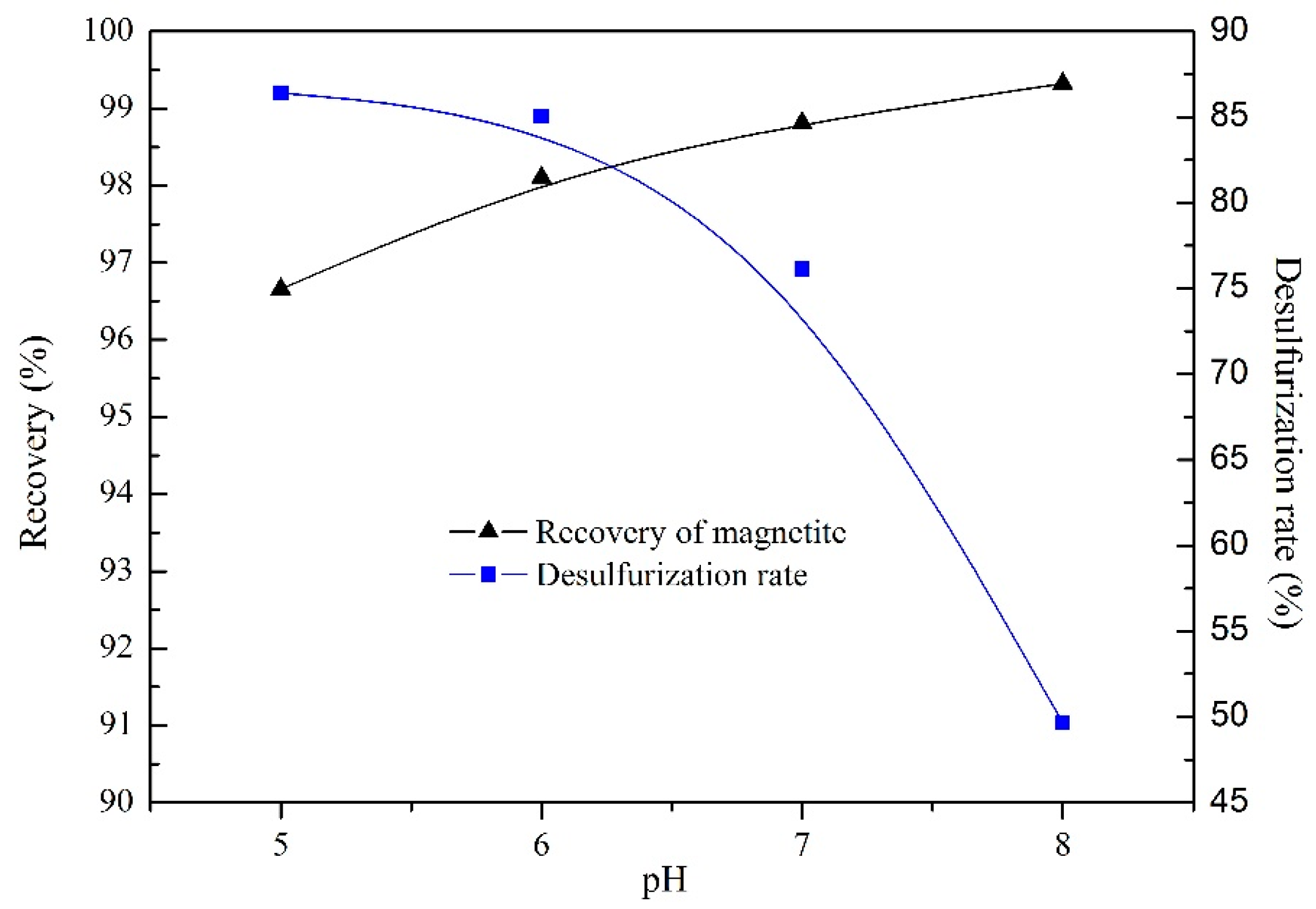
3.1.3. Effect of the pH Value
3.2. Industrial Tests
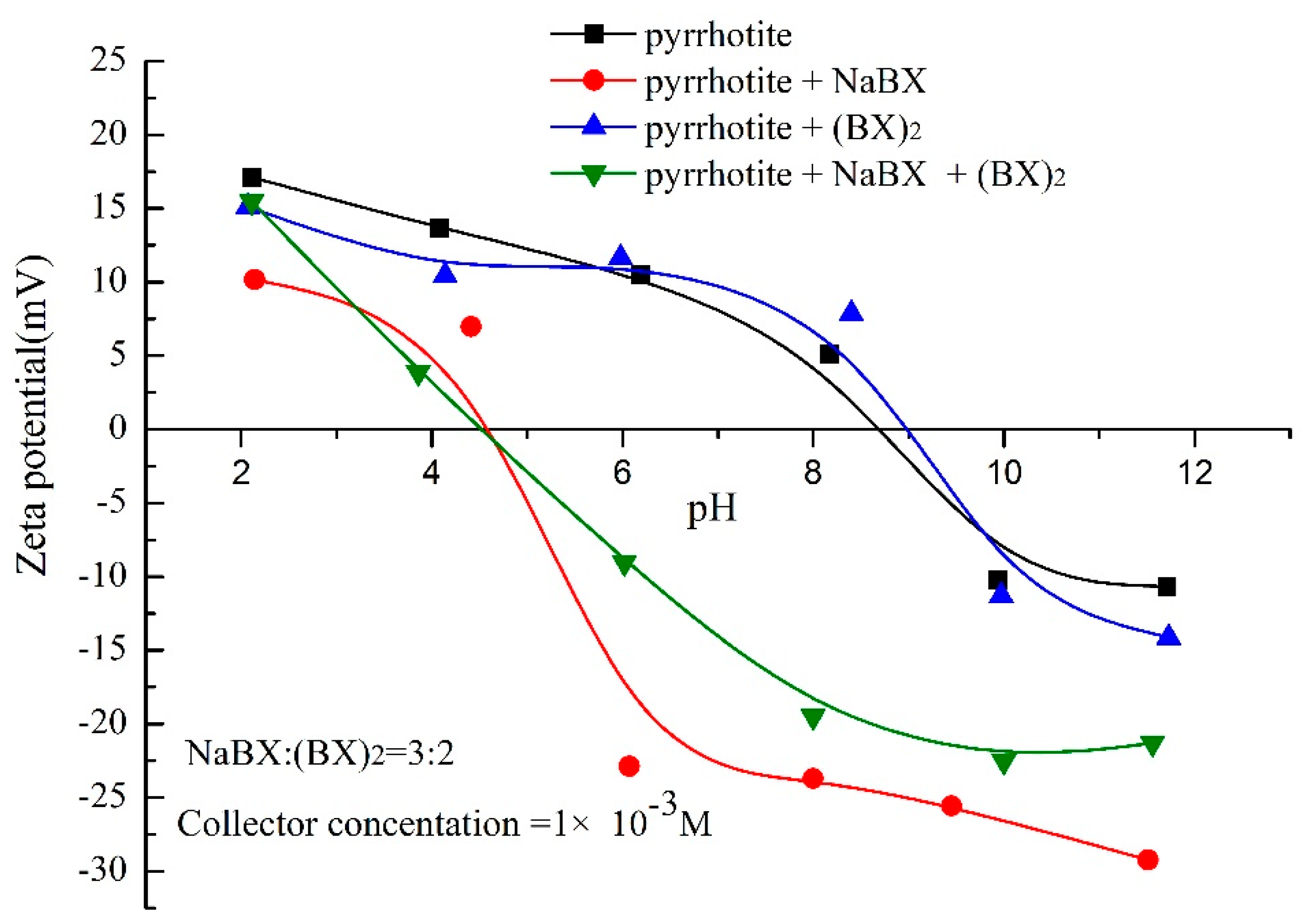
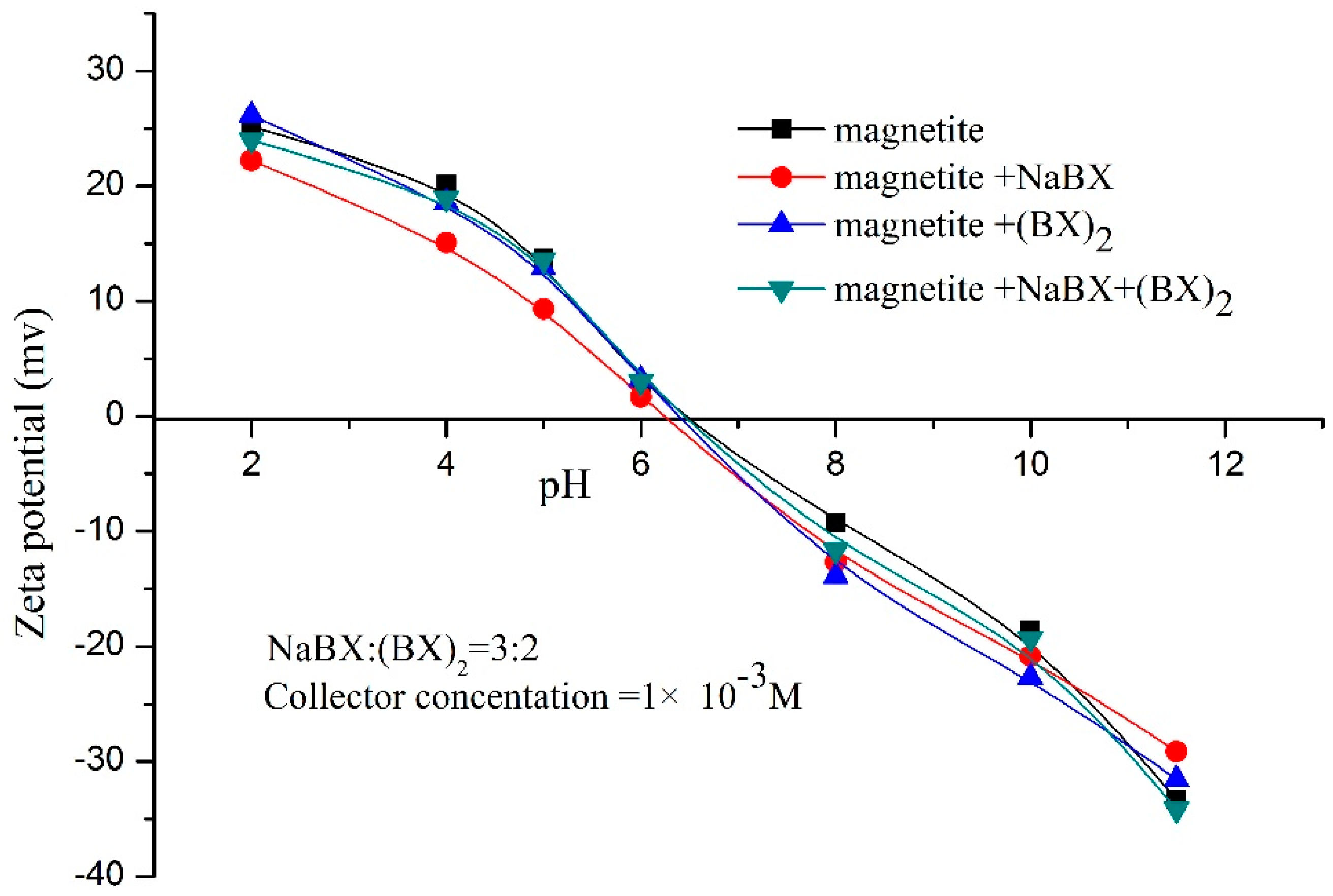
3.3. Zeta-Potential Measurements
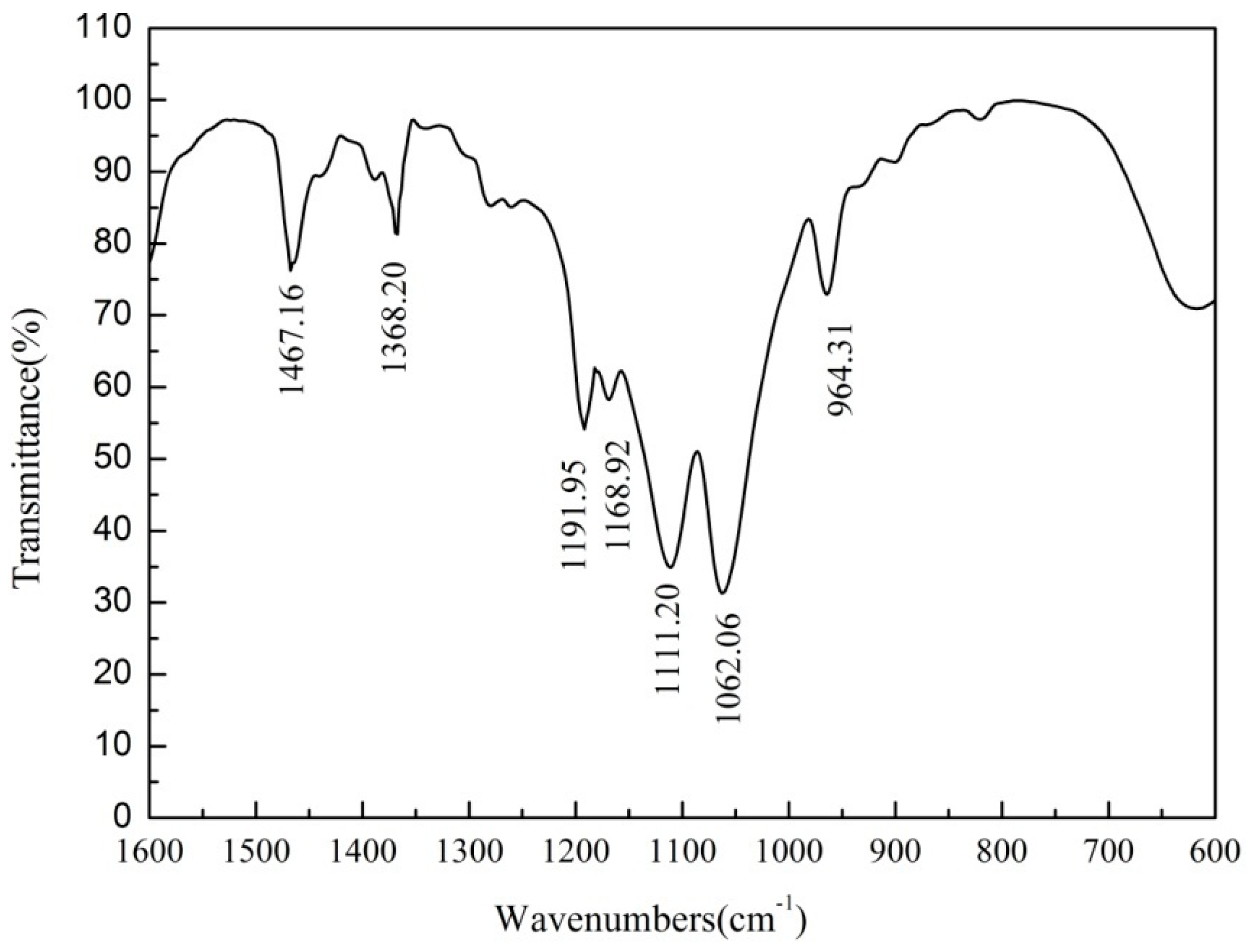
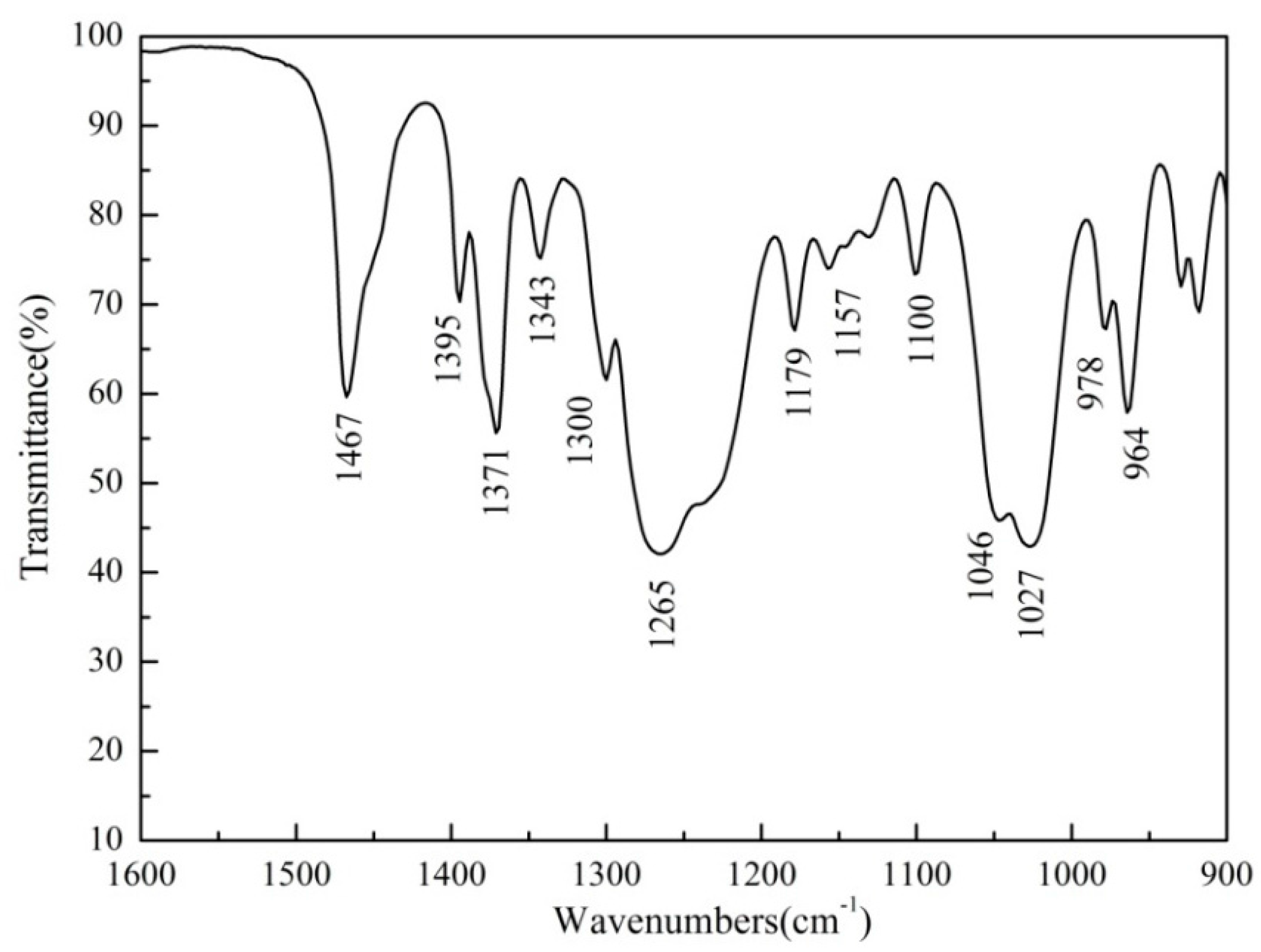
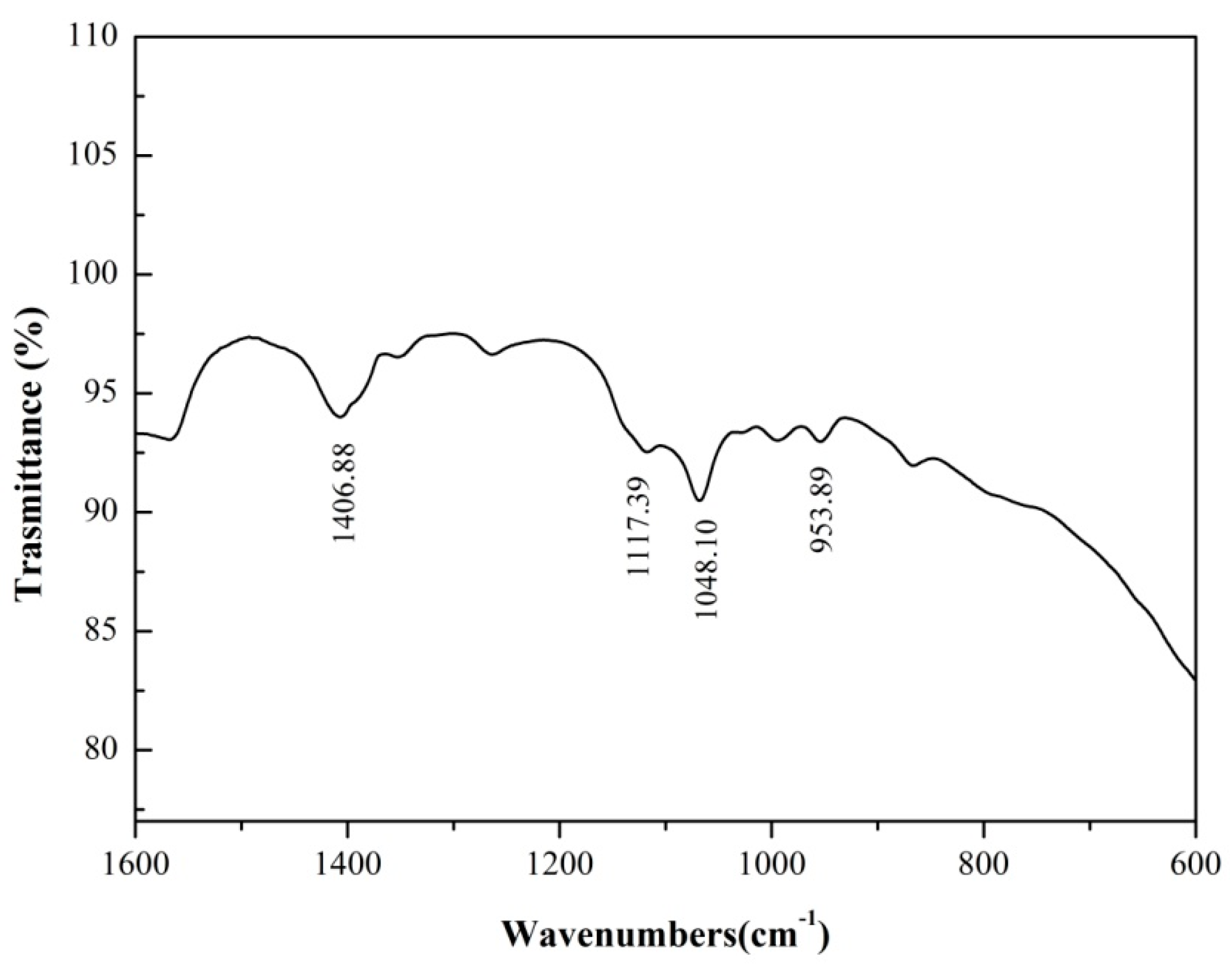
3.4. FTIR Analysis
3.5. Wetting Characteristic of Pyrrhotite wih Mixed Collectors
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yu, Y.F. Processing state and technology progress of iron ore in China. Conserv. Util. Miner. Process 2005, 6, 43–46. [Google Scholar]
- Yu, K.-P.; Yu, Y.-F.; Xu, X.-Y. Separation behavior and mechanism of hematite and collophane in the presence of collector RFP-138. Trans. Nonferr. Metals Soc. China 2013, 23, 501–507. [Google Scholar] [CrossRef]
- Liu, X.H.; Liao, Z.H.; Yan, X.H.; Chen, W. Comprehensive recovery of magnetite and pyrrhotite from a low-grade iron ore. Min. Metall. Eng. 2014, 34, 47–51. [Google Scholar]
- Arvidson, B.; Klemetti, M.; Knuutinen, T.; Kuusisto, M.; Man, Y.T.; Hughes-Narborough, C. Flotation of pyrrhotite to produce magnetite concentrates with a sulphur level below 0.05% w/w. Miner. Eng. 2013, 50, 4–12. [Google Scholar] [CrossRef]
- Allison, S.A.; O’Connor, C.T. An investigation into the flotation behaviour of pyrrhotite. Int. J. Miner. Process. 2011, 98, 202–207. [Google Scholar] [CrossRef]
- Lotter, N.O.; Bradshaw, D.J. The formulation and use of mixed collectors in sulphide flotation. Miner. Eng. 2010, 23, 945–951. [Google Scholar] [CrossRef]
- Vidyadhar, A.; Kumari, N.; Bhagat, R.P. Adsorption mechanism of mixed collector systems on hematite flotation. Miner. Eng. 2012, 26, 102–104. [Google Scholar] [CrossRef]
- Cao, Q.; Cheng, J.; Wen, S.; Li, C.; Bai, S.; Liu, D. A mixed collector system for phosphate flotation. Miner. Eng. 2015, 78, 114–121. [Google Scholar] [CrossRef]
- Rao, K.; Dwari, R.; Lu, S.; Vilinska, A.; Somasundaran, P. Mixed Anionic/Non-Ionic Collectors in Phosphate Gangue Flotation from Magnetite Fines. Open Miner. Process. J. 2011, 4, 14–24. [Google Scholar] [CrossRef]
- Rao, K.H.; Forssberg, K.S.E. Mixed collector systems in flotation. Int. J. Miner. Process. 1997, 51, 67–79. [Google Scholar]
- Gao, Z.; Bai, D.; Sun, W.; Cao, X.; Hu, Y. Selective flotation of scheelite from calcite and fluorite using a collector mixture. Miner. Eng. 2015, 72, 23–26. [Google Scholar] [CrossRef]
- Filippova, I.V.; Filippov, L.O.; Duverger, A.; Severov, V.V. Synergetic effect of a mixture of anionic and nonionic reagents: Ca mineral contrast separation by flotation at neutral pH. Miner. Eng. 2014, 66–68, 135–144. [Google Scholar] [CrossRef]
- Xu, L.; Wu, H.; Dong, F.; Wang, L.; Wang, Z.; Xiao, J. Flotation and adsorption of mixed cationic/anionic collectors on muscovite mica. Miner. Eng. 2013, 41, 41–45. [Google Scholar] [CrossRef]
- Ejtemaei, M.; Irannajad, M.; Gharabaghi, M. Influence of important factors on flotation of zinc oxide mineral using cationic, anionic and mixed (cationic/anionic) collectors. Miner. Eng. 2011, 24, 1402–1408. [Google Scholar] [CrossRef]
- Ding, D.H.; Long, X.Y.; Wang, D.Z. Mechanism of pyrite oxidation and flotation. Nonferr. Metals 1993, 45, 24–29. [Google Scholar]
- Miller, J.D.; Li, J.; Davidtz, J.C.; Vos, F. A review of pyrrhotite flotation chemistry in the processing of PGM ores. Miner. Eng. 2005, 18, 855–865. [Google Scholar] [CrossRef]
- Bunkholt, I.; Kleiv, R.A. Pyrrhotite oxidation and its influence on alkaline amine flotation. Miner. Eng. 2015, 71, 65–72. [Google Scholar] [CrossRef]
- Chen, X.; Peng, Y. The effect of regrind mills on the separation of chalcopyrite from pyrite in cleaner flotation. Miner. Eng. 2015, 83, 33–43. [Google Scholar] [CrossRef]
- Hong, Q.Y.; Tang, Y.H.; Wang, Y.H. Investigation on properties and structure of pyrrhotite and the different of its floatability. Metal Mine 2011, 415, 64–67. [Google Scholar]
- Zhang, Y.; Cao, Z.; Cao, Y.; Sun, C. FTIR studies of xanthate adsorption on chalcopyrite, pentlandite and pyrite surfaces. J. Mol. Struct. 2013, 1048, 434–440. [Google Scholar] [CrossRef]
- Bulut, G.; Atak, S. Role of dixanthogen on pyrite flotation: Solubility, adsorption studies and Eh, FTIR measurements. Miner. Metall. Process. 2002, 19, 81–86. [Google Scholar]
- Zhang, Q.; Hu, Y.H.; Gu, G.H.; Xu, J. The study on the interaction between ethyl xanthate and pyrrhotitein electrochemical flotation by FTIR spectroscopy. Min. Metall. Eng. 2004, 24, 42–44. [Google Scholar]
- Mustafa, S.; Hamid, A.; Naeem, A. Xanthate adsorption studies on chalcopyrite ore. Int. J. Miner. Process. 2004, 74, 317–325. [Google Scholar] [CrossRef]
- Xu, L.; Hu, Y.; Tian, J.; Wu, H.; Wang, L.; Yang, Y.; Wang, Z. Synergistic effect of mixed cationic/anionic collectors on flotation and adsorption of muscovite. Colloids Surf. A Physicochem. Eng. Asp. 2016, 492, 181–189. [Google Scholar] [CrossRef]
| Composition | TFe * | Fe2O3 | SiO2 | Al2O3 | K2O | CaO | MgO | P | S | TiO2 |
|---|---|---|---|---|---|---|---|---|---|---|
| wt % | 65.10 | 92.35 | 3.54 | 0.35 | 0.026 | 1.68 | 0.25 | 0.049 | 1.64 | 0.10 |
| Composition | Magnetite | Pyrrhotite | Pyrite | Quartz | Calcite | Dolomite | Phyllite | Barite |
|---|---|---|---|---|---|---|---|---|
| wt % | 86.88 | 4.32 | 0.23 | 3.56 | 2.56 | 1.22 | 0.47 | 0.75 |
| Minerals | Pyrrhotite | Chalcopyrite | Limonite | Dolomite | Quartz | Mica |
|---|---|---|---|---|---|---|
| wt % | 98.51 | 0.32 | 0.10 | 0.35 | 0.08 | 0.40 |
| Minerals | Magnetite | Quartz | Calcite | Ankerite | Phyllite | Siderite |
|---|---|---|---|---|---|---|
| wt % | 97.12 | 0.84 | 0.45 | 0.33 | 0.41 | 0.22 |
| System | Products | Yield (wt %) | Grades (%) | Recoveries (%) | ||
|---|---|---|---|---|---|---|
| Fe | S | Fe | S | |||
| S1 Mixed collector | Concentrates | 91.23 | 69.25 | 0.43 | 92.50 | 19.62 |
| Tailings | 8.77 | 58.42 | 18.75 | 7.50 | 80.38 | |
| Feed | 100.00 | 68.30 | 2.05 | 100.00 | 100.00 | |
| S2 NaBX | Concentrates | 88.92 | 69.13 | 0.44 | 90.24 | 19.32 |
| Tailings | 11.08 | 60.01 | 14.75 | 9.76 | 80.68 | |
| Feed | 100.00 | 68.12 | 2.03 | 100.00 | 100.00 | |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, J.; Ge, Y.; Cai, X. The Desulfurization of Magnetite Ore by Flotation with a Mixture of Xanthate and Dixanthogen. Minerals 2016, 6, 70. https://doi.org/10.3390/min6030070
Yu J, Ge Y, Cai X. The Desulfurization of Magnetite Ore by Flotation with a Mixture of Xanthate and Dixanthogen. Minerals. 2016; 6(3):70. https://doi.org/10.3390/min6030070
Chicago/Turabian StyleYu, Jun, Yingyong Ge, and Xinwei Cai. 2016. "The Desulfurization of Magnetite Ore by Flotation with a Mixture of Xanthate and Dixanthogen" Minerals 6, no. 3: 70. https://doi.org/10.3390/min6030070
APA StyleYu, J., Ge, Y., & Cai, X. (2016). The Desulfurization of Magnetite Ore by Flotation with a Mixture of Xanthate and Dixanthogen. Minerals, 6(3), 70. https://doi.org/10.3390/min6030070