Abstract
Roasting and flotation are common techniques used in mineral processing, and they have increasingly been combined for the pre-concentration of muscovite from stone coal. The research was mainly to study flotation properties of muscovite after roasting at 200, 400, 600, 800 and 1000 °C, respectively. The changes of chemical and physical properties of muscovite during the roasting process were investigated by thermogravimetric analysis (TGA), Fourier transform infrared spectrum (FTIR), X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), Zeta potential measurements, particle size analysis, and the BET surface area measurements. The results indicated that the dehydroxylation of crystal structure took place at temperatures over 600 °C. A large number of hydroxyl groups were removed from the crystal structure of muscovite at 600–1000 °C. The layer structure, surface element distribution, and electrical properties of muscovite remained after roasting. The flotation recovery of roasted muscovite samples increased with the increase in roasting temperature in the same flotation system, because the specific surface and the adsorption capacity of dodecylamine (DDA) were reduced when roasting temperature was over 600 °C. A suitable roasting temperature and dosage of reagents can be provided for the roasting-flotation of muscovite.
1. Introduction
In China, the vanadium-bearing stone coal is a somewhat important vanadium resource [1,2], and muscovite is the primary vanadium-bearing mineral in the stone coal because the V3+ readily replaces Al3+ from the dioctahedral structure as an isomorphism in muscovite [3]. However, the mineral composition of stone coal is extremely complex, and the grade of V2O5 is relatively low—generally 0.13%–1.2%. In order to enhance the resources for vanadium-bearing stone coal use efficiently, many studies have been devoted to the pre-concentration of vanadium-bearing muscovite from stone coal, and roasting–flotation was found to be an effective pre-concentration method for the concentration of muscovite [4,5,6,7]. The carbon in stone coal is usually removed via roasting to avoid its negative impact on the separation process of muscovite from stone coal, but the physical and chemical properties of minerals in stone will change after roasting in such ways as calcite decomposition, pyrite oxidation, and crystal distortion of muscovite [8,9,10], which have an important effect on the flotation properties of muscovite. Therefore, it is necessary to study the changes in the physical and chemical properties of roasted muscovite with respect to the flotation behavior of muscovite, by which a suitable roasting temperature can be selected for the following flotation process.
Numerous studies have been carried out on the flotation properties of muscovite, but there are little studies on the flotation properties of roasted muscovite. It has been acknowledged that the flotation properties of muscovite are related to its crystal structure characteristics. Muscovite is a kind of 2:1 layer silicate formed from an octahedral layer sandwiched between two identical tetrahedral (SiO4) layers. The octahedral layer has a gibbsite-like structure, with some coordination by OH groups. Interlayer cations (K+) compensate the overall negative charge due to substitution of Al3+ for Si4+ in the tetrahedron. Muscovite has been shown to have permanently negatively charged basal planes, which is not dependent on the pH in aqueous solutions [11,12]. For this reason, muscovite is expected to show much greater affinity for cationic collectors than anionic collectors [13,14], and the dodecylamine (DDA) is the cationic collector that is most widely used in the flotation of muscovite [15].
The objective of this research is to study the flotation properties of muscovite after roasting and provide a suitable roasting temperature and dosage of reagents in the pre-concentration of muscovite from stone coal by roasting-flotation. The floatability of roasted muscovite was investigated by froth flotation of pure muscovite with DDA, and the differences in the floatability of roasted muscovite will be explained by studying the crystal structures and surface properties.
2. Experimental Section
2.1. Materials
Naturally pure muscovite was obtained from Lingshou town of the Hebei province in China. The sample was ground to −75 μm in a pottery ball mill for roasting and flotation experiments. The sample was further ground to about −5 μm for X-ray diffraction (XRD), Fourier transform infrared spectrum (FTIR), and Zeta potentials measurement. The chemical composition of the muscovite sample is listed in Table 1, which was similar to the theoretical chemical composition of muscovite (KAl2(AlSi3O10)(OH)2, SiO2 45.2%, Al2O3 38.5%, K2O 11.8%, H2O 4.5%) [15]. Dodecylamine (DDA) was obtained from Sinopharm Chemical Reagent Co., Ltd. (Wuhan, China). Sodium hydroxide (NaOH) and hydrochloric acid (HCl) were purchased from Tianjin Kemiou Chemical Reagent Co., Ltd. (Tianjin, China). The collector was prepared by mixing equimolar amounts of the DDA and HCl. HCl and NaOH were prepared as 10% solutions for pH adjustment of the pulp; meanwhile, the pH of the pulp was monitored using a digital pH meter. All of the chemicals were analytical grade, and deionized water (18.25 MΩ·cm) was used for all of the experiments.

Table 1.
Chemical composition of pure minerals (wt %).
2.2. Processes and Methods
Muscovite (50 g) was roasted for an hour at 200, 400, 600, 800 and 1000 °C in a muffle furnace with a heating rate of 8 °C/min.
Micro-flotation experiments of roasted muscovite (2 g) were conducted using a XFG5-35 flotation machine (Wuhan Rock Crush and Grand Equipment Manufacture Co., Wuhan, China) with a spindle speed of 2000 r/min. The reagents were added in the following order: (a) HCl or NaOH conditioning for 3 min; (b) mixed DDA (30 g/L) conditioning for 3 min; and (c) a flotation separation period for 3 min. Froth products and tailings were weighed separately after drying, and the average recovery value of duplicated flotation experiments were regarded as the experimental result.
Thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC) were carried out in air by STA409C/PC thermal analyzer (NETZSCH Co., Selb, German,) at a heating rate of 10 °C/min (40−1000 °C).
FTIR spectra of the roasted samples were recorded on a Nicolet 6700 spectrometer (Thermo Fisher Scientific Co., Waltham, MA, USA) at room temperature in the wavenumber range from 4000 to 400 cm−1. Spectra of the solids were taken with KBr pellets.
Mineral phase analysis of the roasted samples was conducted by XRD with RU-200B/D/MAX-RB RU-200B (Rigaku Co., Tokyo, Japan) with Cu-Kα radiation, voltage 40 kV, current 30 mA, and the scanning rate of 15°/min from 3° to 70°. The phases were identified by comparison of the peak positions and d values with data published by the International Centre for Diffraction Data (ICDD).
The X-ray photoelectron spectroscopy (XPS) spectra of roasted muscovite powders untreated and treated with DDA were recorded with a Kα 1063 spectrometer (Thermo Fisher Scientific Co.). The instrument uses Al Kα as a sputtering source at 12 kV and 6 mA. A value of 286.0 eV was adopted as the standard C (1s) binding energy.
The zeta potential of minerals was measured by Zetasizer Nano ZS90 (Malvern Instrument Co., Malvern, UK). The measurement was carried out at 20 °C. The sample (2 mg) was added into 100 mL deionized water, and the mineral suspension was conditioned over the pH range of 2–12. The average zeta potential value was recorded at least six individual measurements. Repeated tests showed a measurement error of ±5 mV.
Surface area of roasted muscovite was compared with an F-Sorb 3400 specific area and a pore size analysis tester. The samples were dried for 3 h at 115 °C in a vacuum environment prior to the test.
The adsorption density of DDA on muscovite was determined with an UV-VIS spectrophotometer (Shanghai Metash Instrument Co., Shanghai, China). First, the absorption spectroscopy of DDA at a series of given concentrations were determined to obtain the calibration curve. Then, muscovite sample (2 g) was mixed with DDA (300 mg/L) at a given pH in a XFG5-35 flotation machine (Wuhan Rock Crush and Grand Equipment Manufacture Co.) with a spindle speed of 2000 r/min for 3 min. Last, the solid particles were separated by centrifuging for 12 min. The concentration of the DDA in the supernatant was analyzed based on the calibration curve. The adsorption on the mineral surface was calculated by Equation (1),
where Γ is the unit mass mineral adsorption amount of DDA, C0 is the initial concentration of the collector, Ceq. is the collector concentration in the supernatant, V is the volume of the collector, and m is the quality of muscovite.
3. Results
3.1. Structural Characterization of Roasted Muscovite
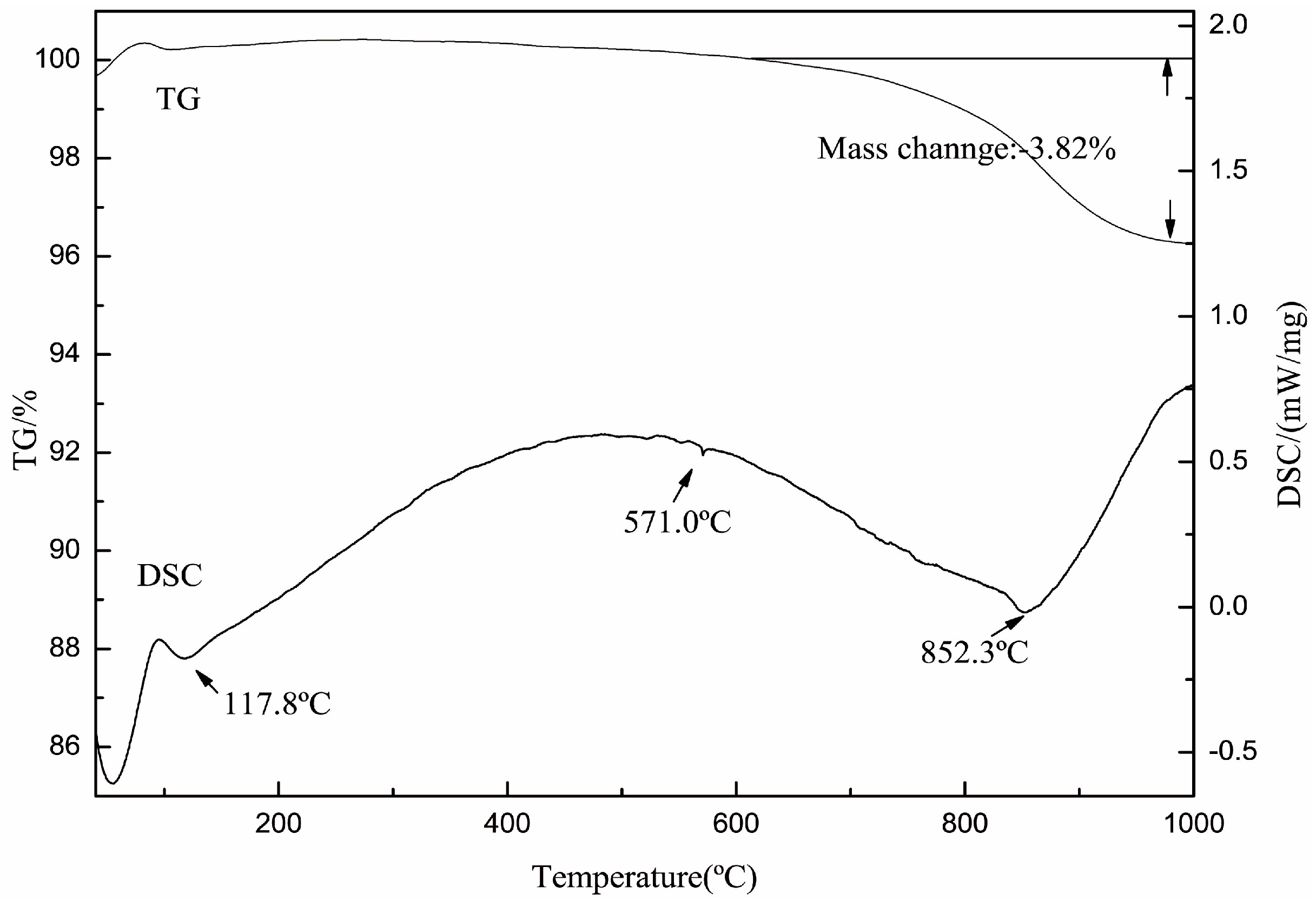
The transformations of crystal structure of muscovite after roasting were carried out by TG-DSC, FTIR, and XRD. Figure 1 shows that the sample weight lost 3.82 wt % from 600 °C up to 1000 °C. The weight of the loss was very close to the expected value (4.5 wt %) of dehydroxylation of mica. This confirmed the initial temperature of dehydroxylation was about 600 °C. In the DSC curve, the peak found at 852.3 °C indicated the dehydroxylation of muscovite was most strong at 852.3 °C.
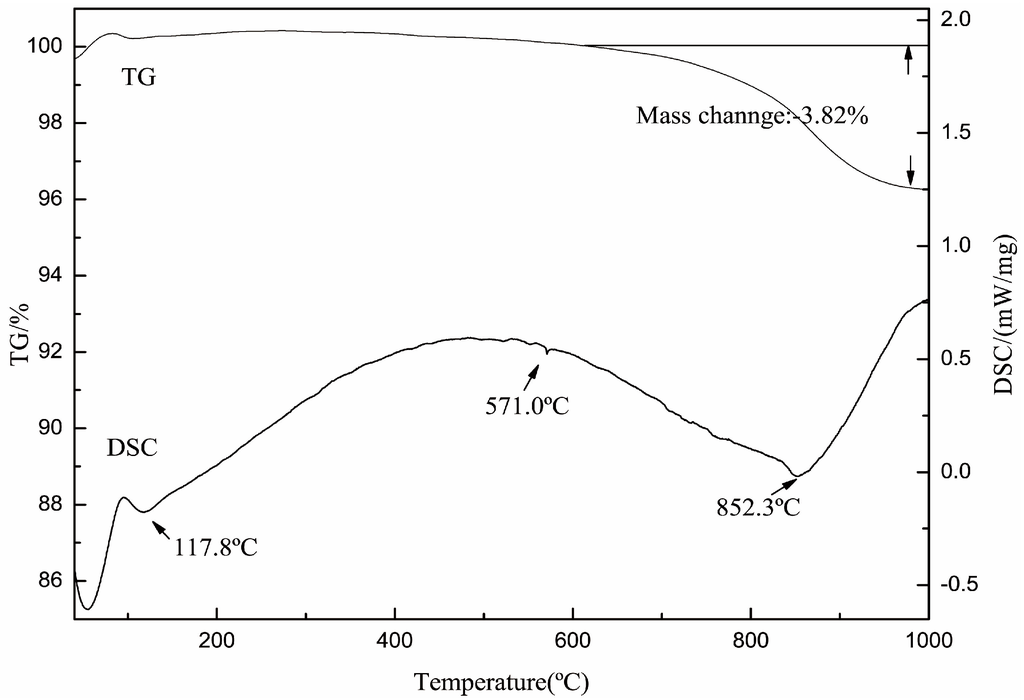
Figure 1.
The thermogravimetric differential scanning calorimetry (TG-DSC) curve of muscovite.
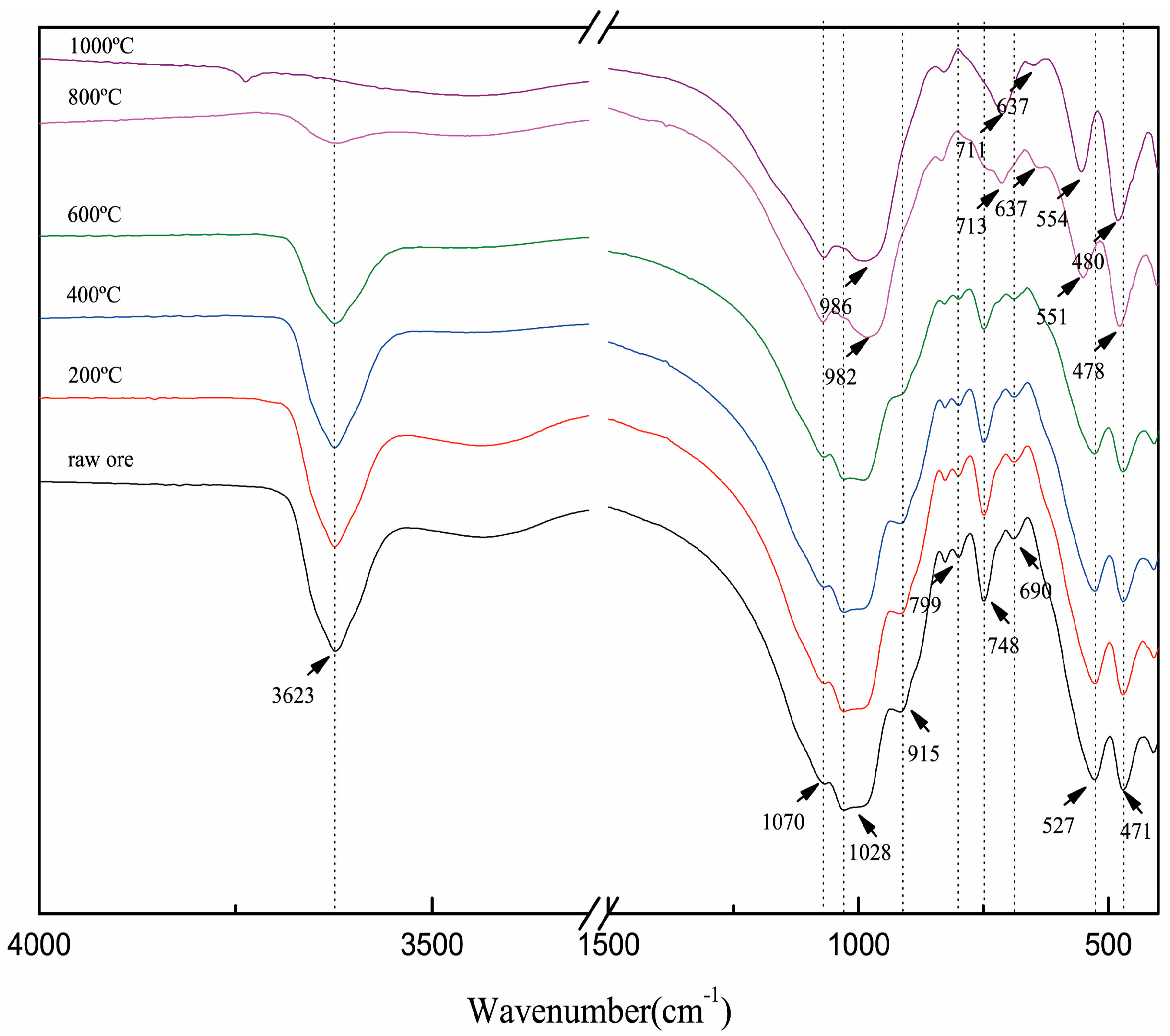
The dehydroxylation of muscovite was also confirmed by FTIR. As shown in Figure 2, the absorption peaks at 3623 and 915 cm−1 are attributed to O–H, and they gradually decreased with the increase in temperature form 600 to 1000 °C and even disappeared at 1000 °C. The temperature range of dehydroxylation was consistent with the results of TGA.
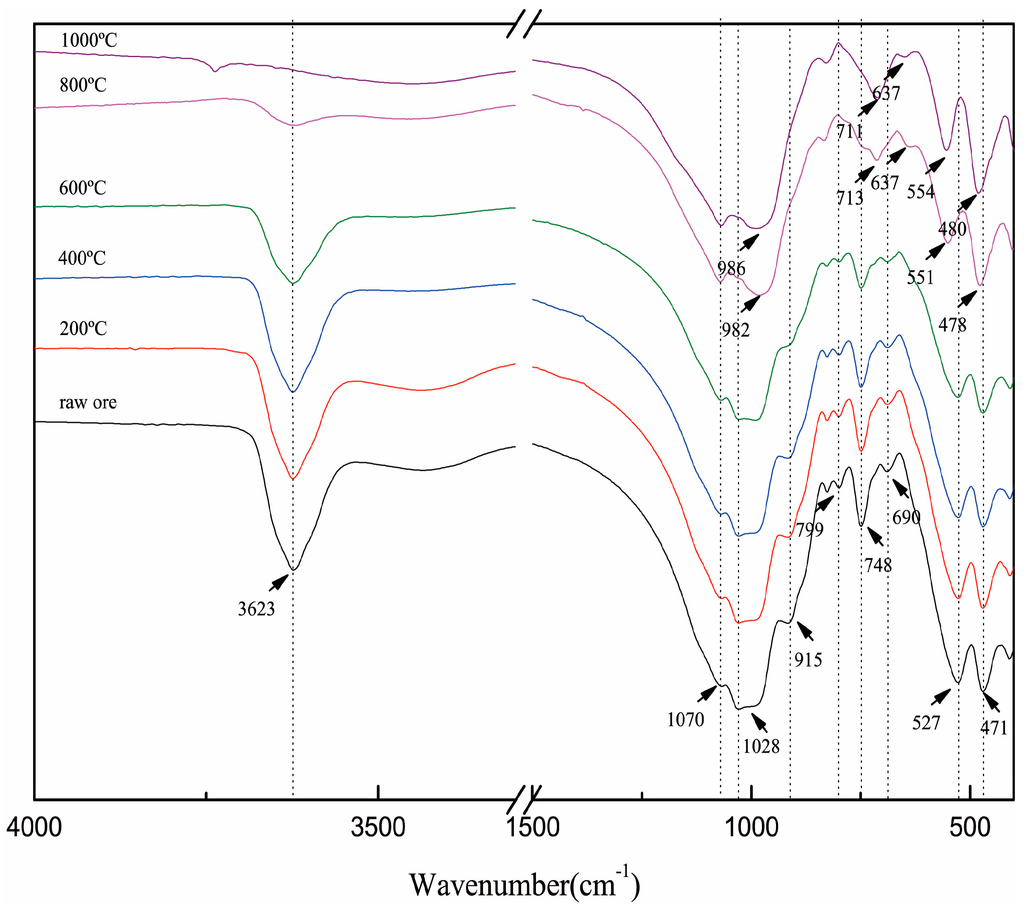
Figure 2.
Fourier transform infrared spectrum (FTIR) spectrum of roasted muscovite at different temperatures.
The absorption peaks at 1028, 799 and 748 cm−1 are attributed to Si (Al)–O and Si–O–Si (Al). At 800 °C, the shoulder absorption peak at 1029 cm−1 shifted to about 990 cm−1. At the same time, the weak absorption peak at 799 cm−1 disappeared, and the weak absorption peak at 748 cm−1 appeared to shift to 713 cm−1. These changes are related to the distortions of the (AlO6) octahedron and (SiO4) tetrahedron caused by dehydroxylation in (AlO6) octahedral.
Because of the layered structure of muscovite, the Si (Al)–O bending vibration was presented as a set of split peaks—527 and 471 cm−1 [16]. On the other hand, the existence of a set of split peaks can also be verified by the layered structure of muscovite. At 800 °C, the split peaks shifted from 527 to 551 cm−1 and from 471 to 478 cm−1, respectively, and further shifted to 554 and 480 cm−1 at 1000 °C. The migration of the split peaks showed the changes of the Si (Al)–O band; meanwhile, the existence of the split peaks confirmed that the layered structure of muscovite was not destroyed after roasting.
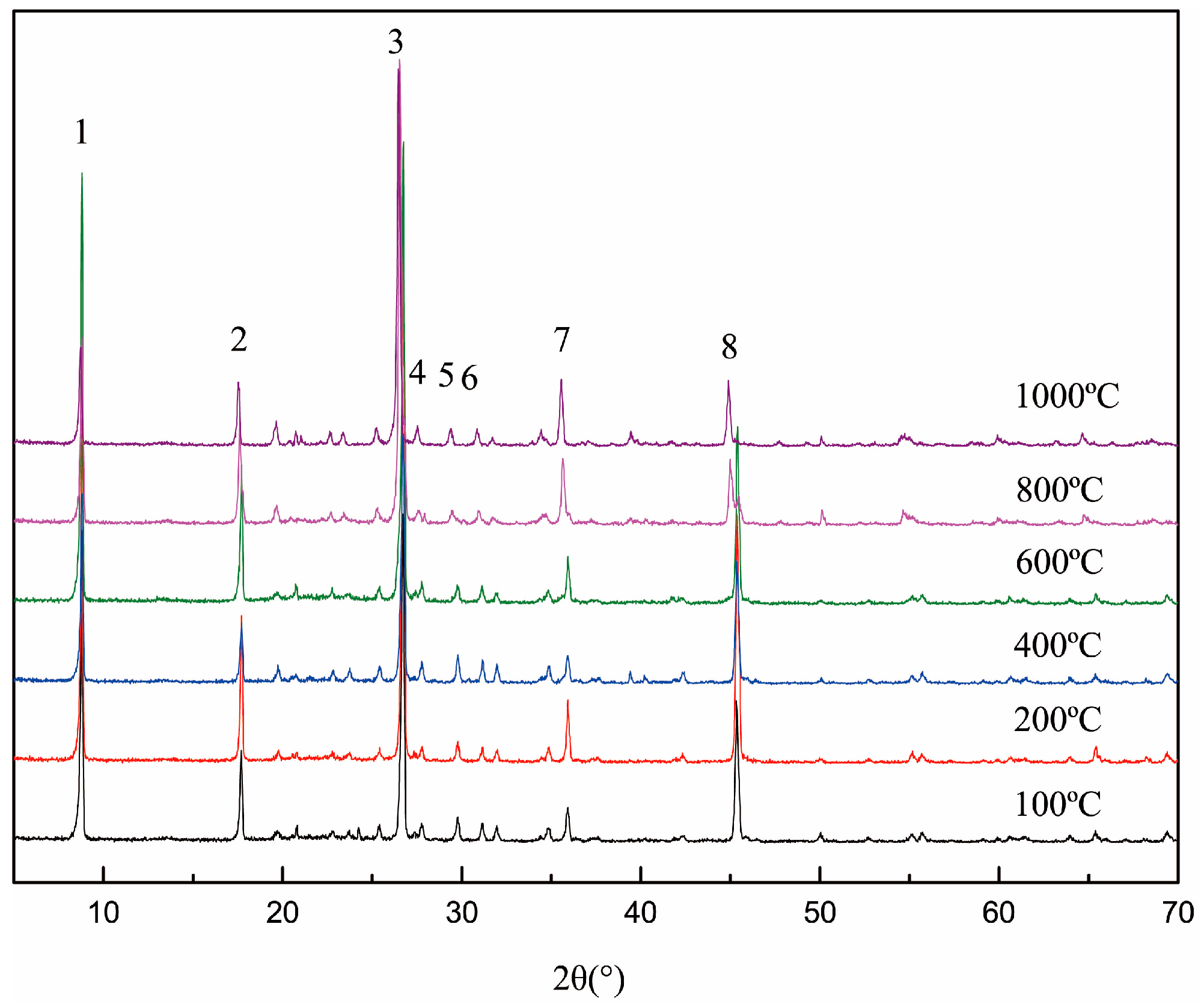
Figure 3 indicates that there was no phase transformation during the whole roasting temperature range. The d value of each crystal face, shown in Table 2, increased significantly when the temperature was over 600 °C. Based on the analysis of the above, the increase of the d value was mainly due to the linear expansion in the roasting process. Due to the dehydroxylation, the aluminum coordination environment in the (AlO6) octahedron gradually changed, leading to the relaxation of the crystal structure. Meanwhile, the layer force of the muscovite was the weakest, so the structural adjustment mainly concentrated on the c-axis, such as 002, 004, 006 and 008. This stage, the d value of the crystal face on the c-axis increased with the increase in temperature, which can be called the dehydroxylation expansion stage [17].
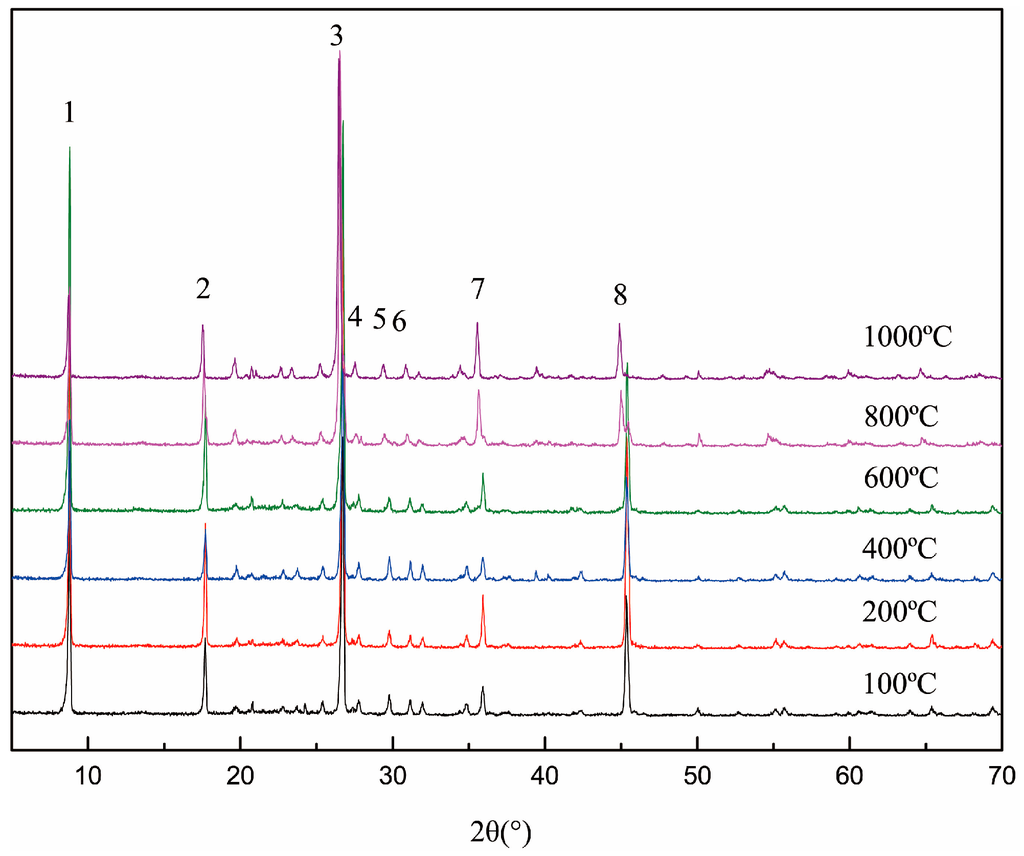
Figure 3.
X-ray diffraction (XRD) patterns of roasted muscovite at different temperatures.

Table 2.
The d value comparison of the different crystal face in the roasted muscovite.
The crystal structure of the muscovite had to be adjusted for dehydroxylation. The six-coordination of the aluminum gradually changed to five-coordination, and the (AlO6) octahedron gradually formed (AlO5) double-triangle vertebral bodies [18].
3.2. Surface Properties
XPS analysis was employed to explore the surface properties of muscovite via roasting. Table 3 shows the binding energies of the elements for roasted muscovite and roasted muscovite treated with DDA. The relative concentrations of the elements are shown in Table 4.

Table 3.
Binding energy of elements on surface of raw ore and muscovite roasted at 1000 °C before and after interaction with dodecylamine (DDA).

Table 4.
Relative content of elements on the roasted muscovite surface.
Fe3+, Al3+, Si4+, Mg2+, and K+ were the main metal cations, and O2− was the main anion on the surface of the muscovite. In addition to elements belonging to the mineral or reagents, the presence of adventitious carbon was observed on the mineral surface. The electron binding energy of Fe3+, Al3+, Si4+, Mg2+, and O2− decreased at 1000 °C, but the changes of chemical shift were smaller, which indicates that the valence state of the surface element did not change. From the above analysis, the changes in chemical shift are believed to be caused by dehydroxylation. Because the electronegative of O is stronger than Al, the charge density of Al should increase from a six-coordination (AlO6) octahedron to five-coordination (AlO5) double triangle vertebral bodies. The increase in charge density is equivalent to the process of reduction, so the electron binding energy should decrease. By the same token, the charge density of the other elements in the muscovite structure will also increase due to the removal of hydroxyl groups. Thus, the electron binding energy will decrease.
When muscovite was treated with DDA, N appeared on the surface, and there was no chemical shift on the surface, indicating that DDA was adsorbed on the muscovite surface, and the adsorption was physical. The N content on the surface of the muscovite roasted at 1000 °C was lower than the sample roasted at 100 °C, which indicated that the DDA adsorption capacity of muscovite roasted at 1000 °C was smaller.
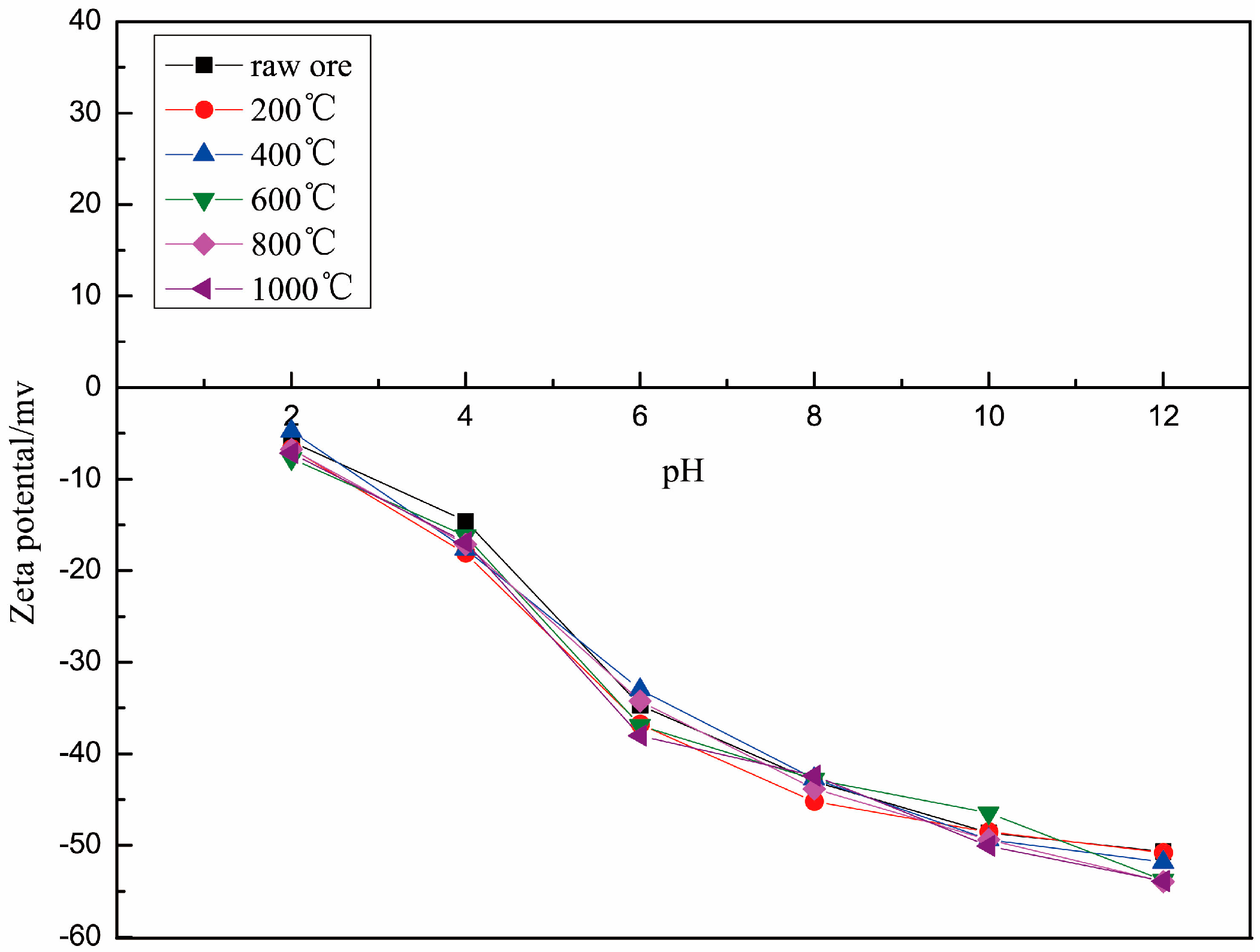
The zeta potentials of roasted muscovite are shown in Figure 4. The zeta potentials of samples with different roasting temperatures saw no obvious changes in the same pH solution, and the zeta potential of all samples was negative in a pH range from 2 to 14 and decreased with the increase in pH, which is consistent with previous research results [11,19,20]. In conclusion, the zeta-potential of muscovite is related to its cleavage plane and its element properties. The layer structure of the muscovite was not destroyed, and the element distribution on the surface also was not changed by the roasting. Therefore, the electric properties of muscovite will not change, nor thus will the electrostatic physical adsorption mechanisms of muscovite and DDA.
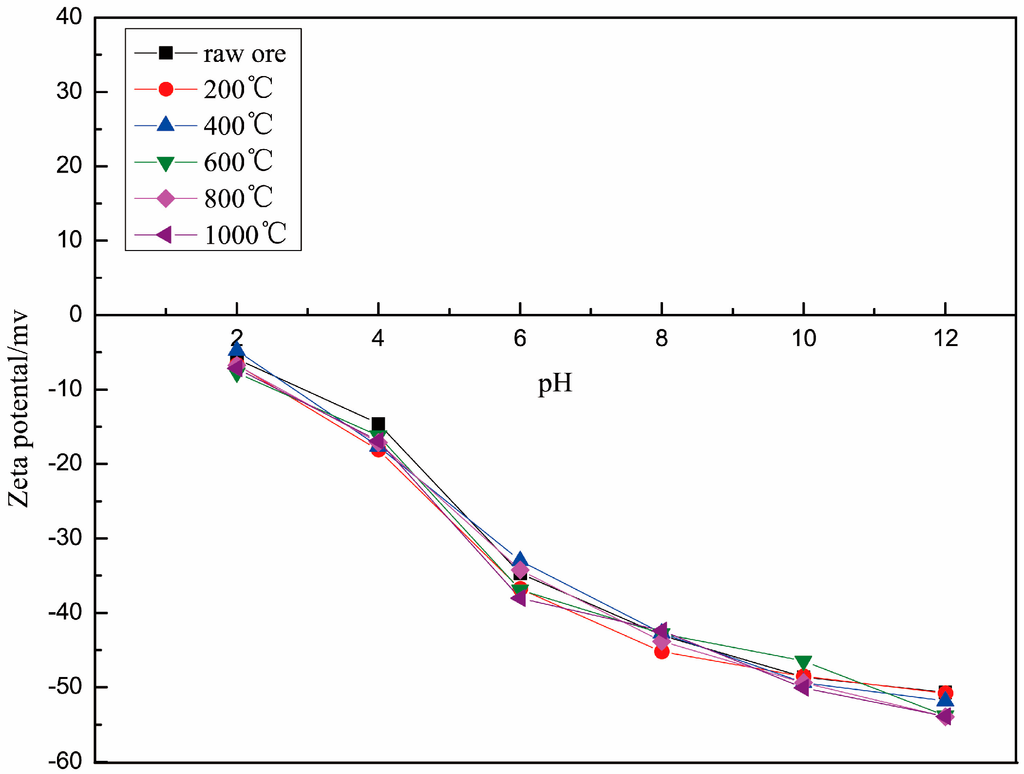
Figure 4.
Zeta potential of muscovite roasted at different temperatures.
3.3. Flotation Properties
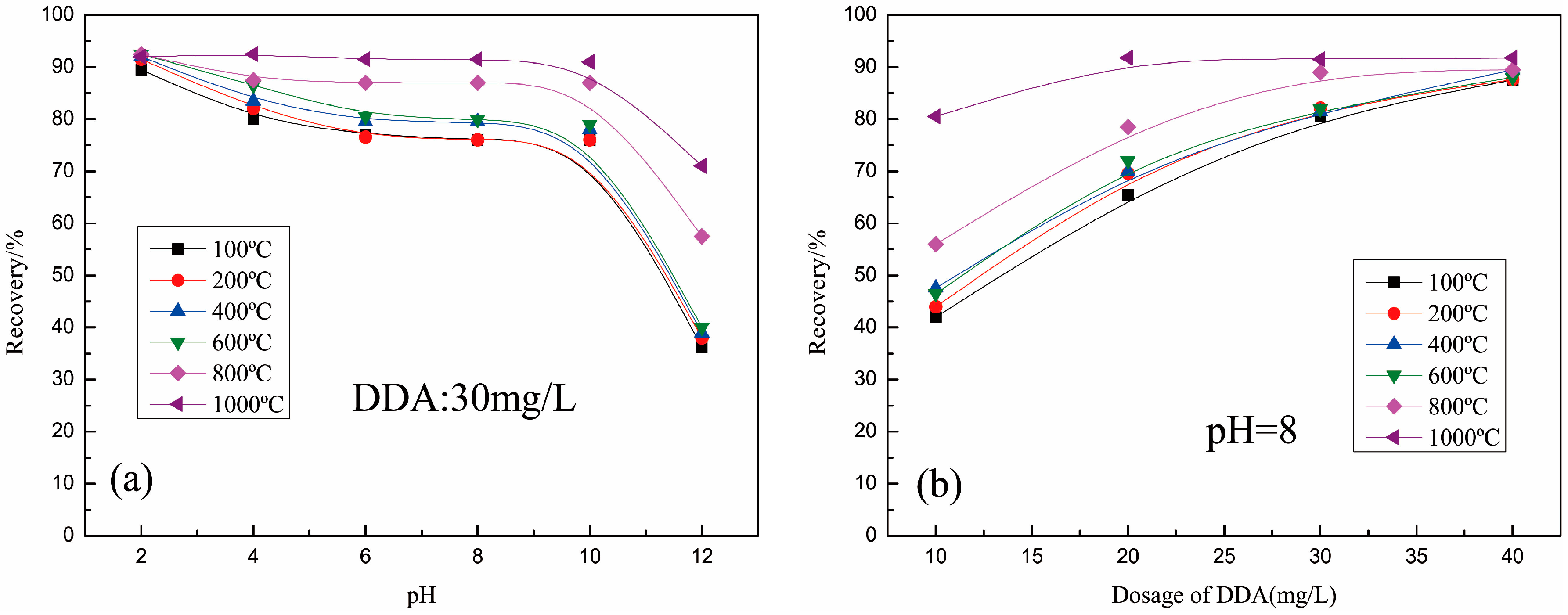
The samples roasted at different temperatures showed different flotability with 30 mg/L DDA (Figure 5a). At pH = 2, the roasted samples had similar flotation recovery (90%). When the roasting temperature was below 600 °C, the samples had a similar flotation phenomenon, with a pH range from 4 to 14, and the flotation recovery of the sample increased with the increase in roasting temperature when it was over 600 °C in the same pH. As shown in Figure 5b, the recoveries of all samples are close to 90% with the increase in dosage of DDA, but the samples with different roasting temperatures needed different dosages of DDA. The sample roasted at 1000 °C only needed 20 mg/L DDA to make the recovery reach 90%, but the sample roasted at 800 °C needed about 30 mg/L DDA, and other samples need about 40 mg/L DDA.
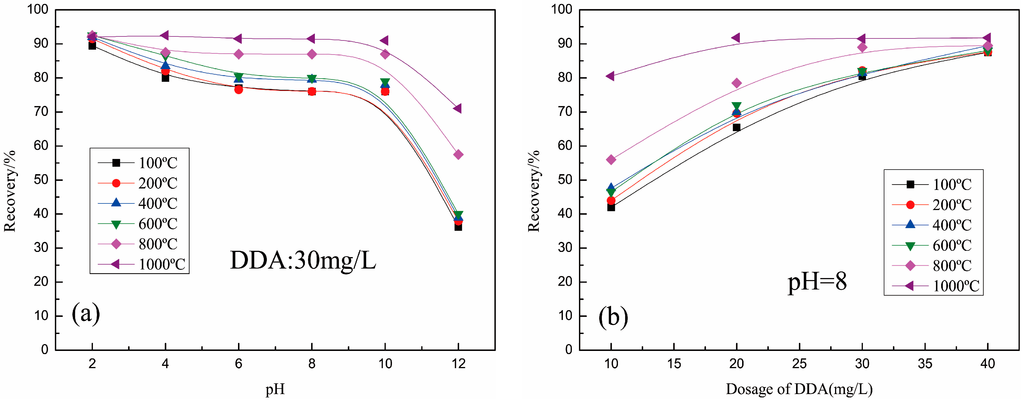
Figure 5.
Recoveries of muscovite roasted as a function of (a) pH; (b) dosage of DDA.
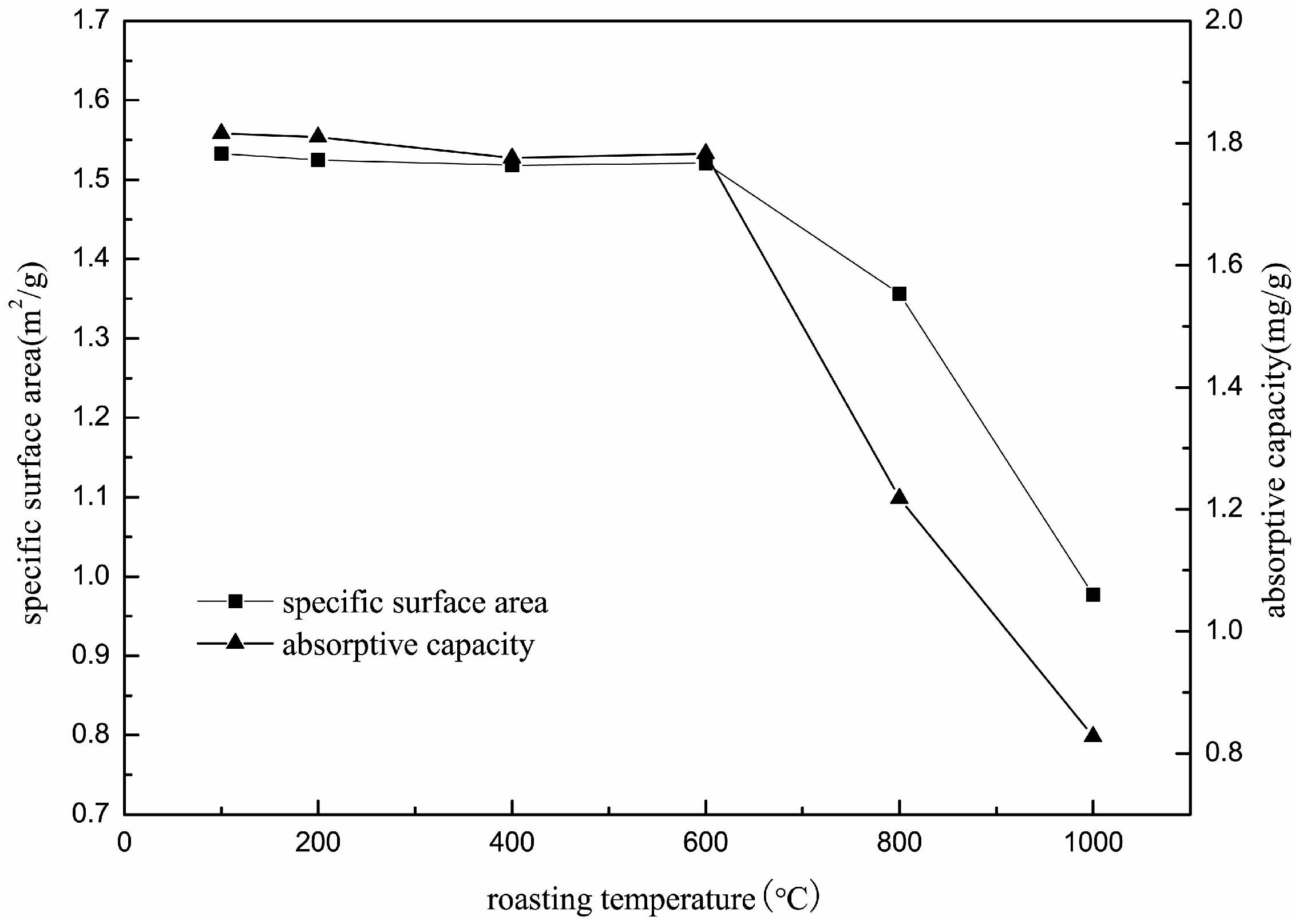
The surface area and DDA adsorption capacity of roasted muscovite samples were tested, and the results are shown in Figure 6. The specific surface was stable at about 1.5 m2/g when the roasting temperature was below 600 °C, and the specific surface of roasted muscovite decreased when the roasting temperature was over 600 °C. At 800 °C, the specific surface was 1.35 m2/g, and, at 1000 °C, the specific surface dropped to 0.97 m2/g. The DDA adsorption capacity of muscovite was stable at about 1.80 mg/g when roasting temperature was below 600 °C. The DDA adsorption capacity of muscovite roasted at 800 °C decreased from 1.80 to 1.22 mg/g, and the DDA adsorption capacity of muscovite roasted at 1000 °C dropped to 0.82 mg/g. The downward trends of specific surfaces are consistent with the adsorption capacity, indicating that the decrease in specific surface area was the main reason for the decrease in adsorption capacity.
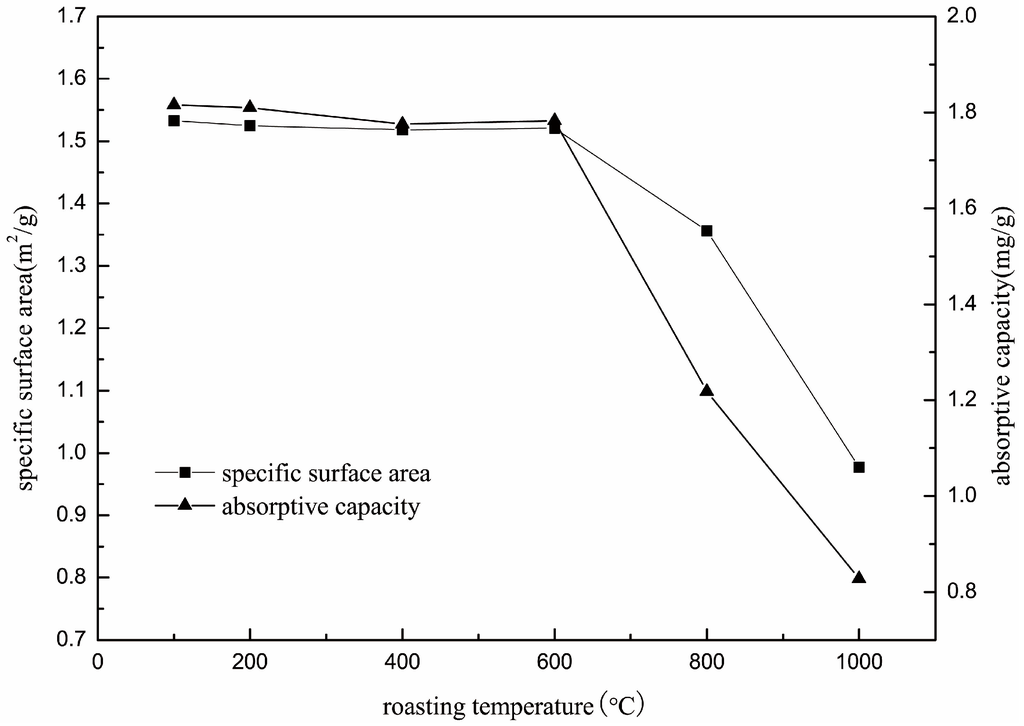
Figure 6.
The specific surface area of roasted muscovite at different temperature.
4. Discussion
The crystal structure, surface properties, and flotation properties of roasted muscovite at different temperatures saw different changes. When the roasting temperature was below 600 °C, the crystal structure, element distribution, specific surface, and zeta potential of muscovite did not change. When roasting temperature was 600 °C, dehydroxylation started, and, when roasting temperature was 1000 °C, the hydroxy was disappeared completely. The structure of the alumina octahedral was changed by dehydroxylation, resulting in a slight distortion of the crystal structure of muscovite. However, the layer structure of muscovite was not destroyed. At the same time, the influence of dehydroxylation on the surface properties of muscovite was limited. The electron binding energy of Fe3+, Al3+, Si4+, Mg2+, and O2− decreased slightly. Therefore, the electric properties of the muscovite surface in the pulp were similar. For this reason, all of the roasted samples could float via DDA, which was confirmed by the flotation experiments. However, the DDA adsorption capacity of muscovite roasted with a higher temperature was smaller, but the flotation recovery was higher. This phenomenon was not consistent with industrial flotation. In general, the specific surface of the mineral remained constant, and the higher reagents’ adsorption capacity meant a greater proportion of hydrophobic area caused by reagents on the surface of the mineral; thus, the mineral floated easily.
In this study, the specific surface of muscovite was reduced via roasting and decreased with the increase in roasting temperature when the roasting temperature reached over 600 °C. For this reason, the proportion of hydrophobic area on the roasted muscovite with smaller specific surfaces was larger at identical reagent dosages; thus, the flotation recovery increased with the increase in roasting temperature in the same flotation system.
5. Conclusions
During the roasting process, dehydroxylation of muscovite occurred when temperatures were over 600 °C. The layered structure, element distribution, and electrical properties of muscovite were basically constant such that all the roasted samples could float via DDA. However, the specific surface of muscovite was reduced when the roasting temperature was over 600 °C, and the proportion of the hydrophobic area on the roasted muscovite with a higher roasting temperature was larger with the same dosage of DDA; thus, a higher flotation recovery could be obtained. Hence, in the pre-concentration of muscovite from stone coal by roasting-flotation, the roasting temperature should be below 600 °C to retain the flotation properties of muscovite, or else should be appropriate to reduce the dosage of the collector when the roasting temperature is over 600 °C.
Acknowledgments
This research was supported by the National Key Science-Technology Support Programs of China (No. 2015BAB03B05) and the National Science Foundation of China (No. 51404177). This project was also supported by College of Resources and Environmental Engineering, Wuhan University of Technology, China.
Author Contributions
Yimin Zhang and Jiayan Tang conceived and designed the experiments, Jiayan Tang performed the experiments; Shenxu Bao and Jiayan Tang analyzed the data; Yimin Zhang contributed reagents, materials, and analysis tools; Jiayan Tang wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhu, X.B.; Zhang, Y.M.; Huang, J.; Liu, T.; Wang, Y. A kinetics study of multi-stage counter-current circulation acid leaching of vanadium from stone coal. Int. J. Miner. Process. 2012, 114, 1–6. [Google Scholar] [CrossRef]
- Ye, P.H.; Wang, X.W.; Wang, M.Y.; Fan, Y.Y.; Xiang, X.Y. Recovery of vanadium from stone coal acid leaching solution by coprecipitation, alkaline roasting and water leaching. Hydrometallurgy 2012, 117, 108–115. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Hu, Y.J.; Bao, S.X. Vanadium emission during roasting of vanadium-bearing stone coal in chlorine. Miner. Eng. 2012, 30, 95–98. [Google Scholar] [CrossRef]
- Zhao, Y.L.; Zhang, Y.M.; Liu, T.; Chen, T.J.; Bian, Y.; Bao, S.X. Pre-concentration of vanadium from stone coal by gravity separation. Int. J. Miner. Process. 2013, 121, 1–5. [Google Scholar] [CrossRef]
- Zhao, Y.L.; Zhang, Y.M.; Bao, S.X.; Liu, T.; Bian, Y.; Liu, X.; Jiang, M.F. Separation factor of shaking table for vanadium pre-concentration from stone coal. Sep. Purif. Technol. 2013, 115, 92–99. [Google Scholar] [CrossRef]
- Wang, L.; Sun, W.; Liu, R.Q.; Gu, X.C. Flotation recovery of vanadium from low-grade stone coal. Trans. Nonferrous Met. Soc. 2014, 24, 1145–1151. [Google Scholar] [CrossRef]
- Wu, H.L.; Zhao, W.; Li, M.T.; Deng, G.Z.; Ge, H.H.; Wei, C. New craft study on enriching vanadium by means of priority coal flotation from high carbon stone-coal. J. Chin. Rare Earth Soc. 2008, 26, 530–533. [Google Scholar]
- Zhao, Y.L.; Zhang, Y.M.; Song, S.X.; Chen, T.J.; Bao, S.X. Behaviors of impurity elements Ca and Fe in vanadium-bearing stone coal during roasting and its control measure. Int. J. Miner. Process. 2016, 148, 100–104. [Google Scholar] [CrossRef]
- Chen, T.J.; Qiu, G.Z.; Zhu, D.Q. Oxidation mechanism of vanadium extraction in stone coal roasting with cyclic oxidation. Met. Mine. 2008, 6, 62–65. [Google Scholar]
- Chen, T.J.; Qiu, G.Z.; Zhu, D.Q. Valence variation and oxidation kinetics of vanadium during vanadium bearing stone coal roasting. Min. Metall. Eng. 2008, 3, 64–67. [Google Scholar]
- Nosrati, A.; Addai-Mensah, J.; Skinner, W. Muscovite clay mineral particle interactions in aqueous media. Powder Technol. 2012, 219, 228–238. [Google Scholar]
- Yan, L.J.; Masliyah, J.H.; Xu, Z.H. Interaction of divalent cations with basal planes and edge surfaces of phyllosilicate minerals: Muscovite and Talc. J. Colloid Interface Sci. 2013, 404, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Pugh, R.J.; Rutland, M.W.; Manev, E.; Claesson, P.M. Dodecylamine collector—PH effect on mica flotation and correlation with thin aqueous foam film and surface force measurements. Int. J. Miner. Process. 1996, 46, 245–262. [Google Scholar] [CrossRef]
- Sekulić, Ž.; Canić, N.; Bartulović, Z.; Daković, A. Application of different collectors in the flotation concentration of feldspar, mica and quartz sand. Miner. Eng. 2004, 17, 77–80. [Google Scholar] [CrossRef]
- Wang, L.; Sun, W.; Hu, Y.H.; Xu, L.H. Adsorption mechanism of mixed anionic/cationic collectors in Muscovite–Quartz flotation system. Miner. Eng. 2014, 64, 44–50. [Google Scholar] [CrossRef]
- Wen, L.; Liang, W.X.; Zhang, Z.G. The Infrared Spectroscopy of Minerals; Chongqing Publishing House: Chongqing, China, 1998. (In Chinese) [Google Scholar]
- Zhou, Z.J.; Yang, Z.Y.; Chen, D.Z. The thermal swellability of illite from Duchuantou in Zhejiang province. J. Miner. Petrol. 1996, 65, 7–12. [Google Scholar]
- Gridi-Bennadji, F.; Beneu, B.; Laval, J.P.; Blanchart, P. Structural transformations of muscovite at high temperature by X-ray and neutron diffraction. Appl. Clay. Sci. 2008, 38, 259–267. [Google Scholar] [CrossRef]
- Nishimura, S.; Tateyama, H.; Tsunematsu, K.; Jinnai, K. Zeta potential measurement of muscovite mica basal plane-aqueous solution interface by means of plane interface technique. J. Colloid Interface Sci. 1992, 152, 359–367. [Google Scholar] [CrossRef]
- Xu, L.H.; Wu, H.Q.; Dong, F.Q.; Wang, L.; Wang, Z.; Xiao, J.H. Flotation and adsorption of mixed cationic/anionic collectors on muscovite mica. Miner. Eng. 2013, 41, 41–45. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).