Geoenvironmental Characterisation of Heap Leach Materials at Abandoned Mines: Croydon Au-Mines, QLD, Australia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description and Sampling
2.2. Mineralogical Investigations
2.3. Geochemical Analyses
3. Results
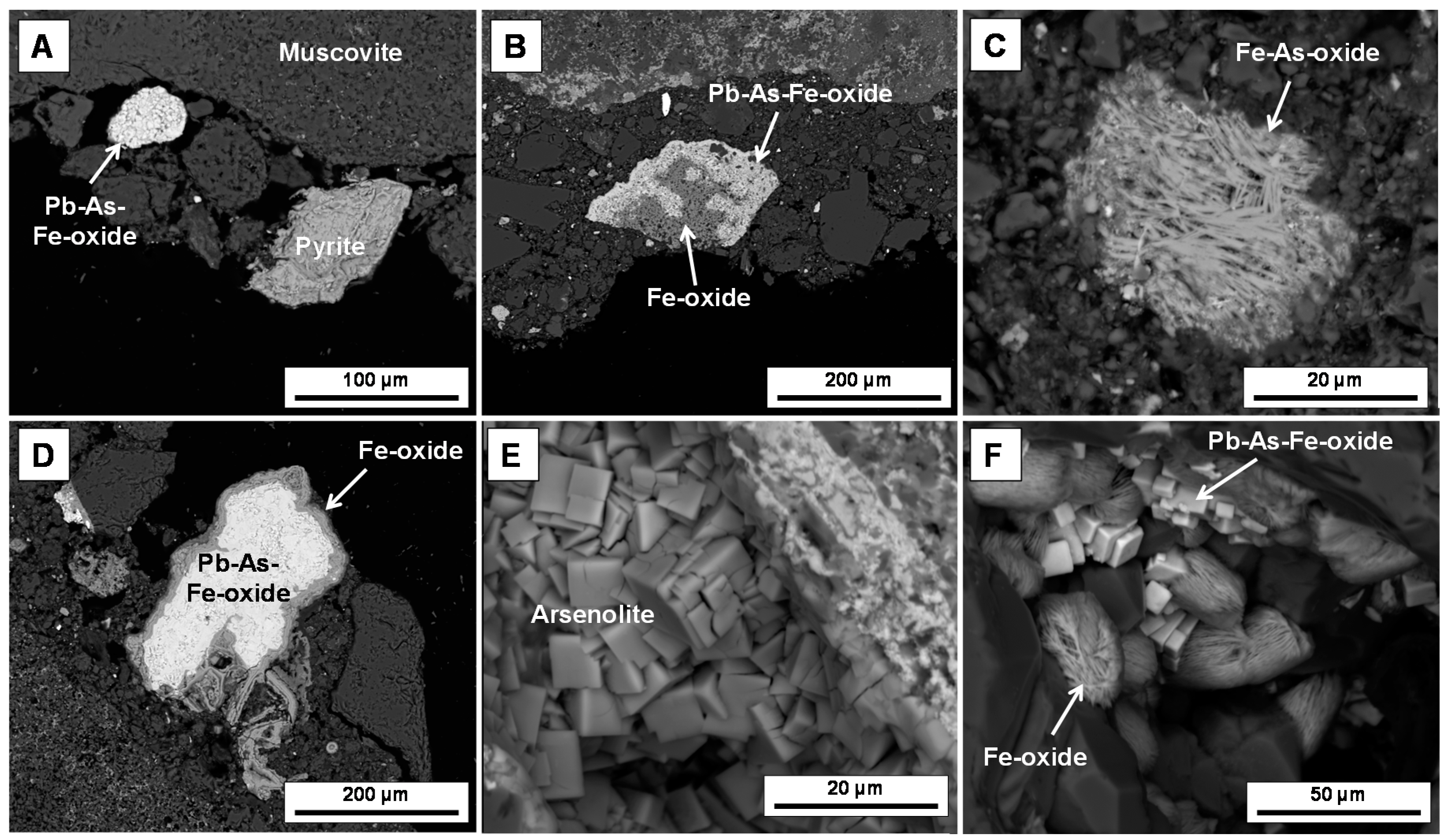
3.1. Mineralogical Characteristics
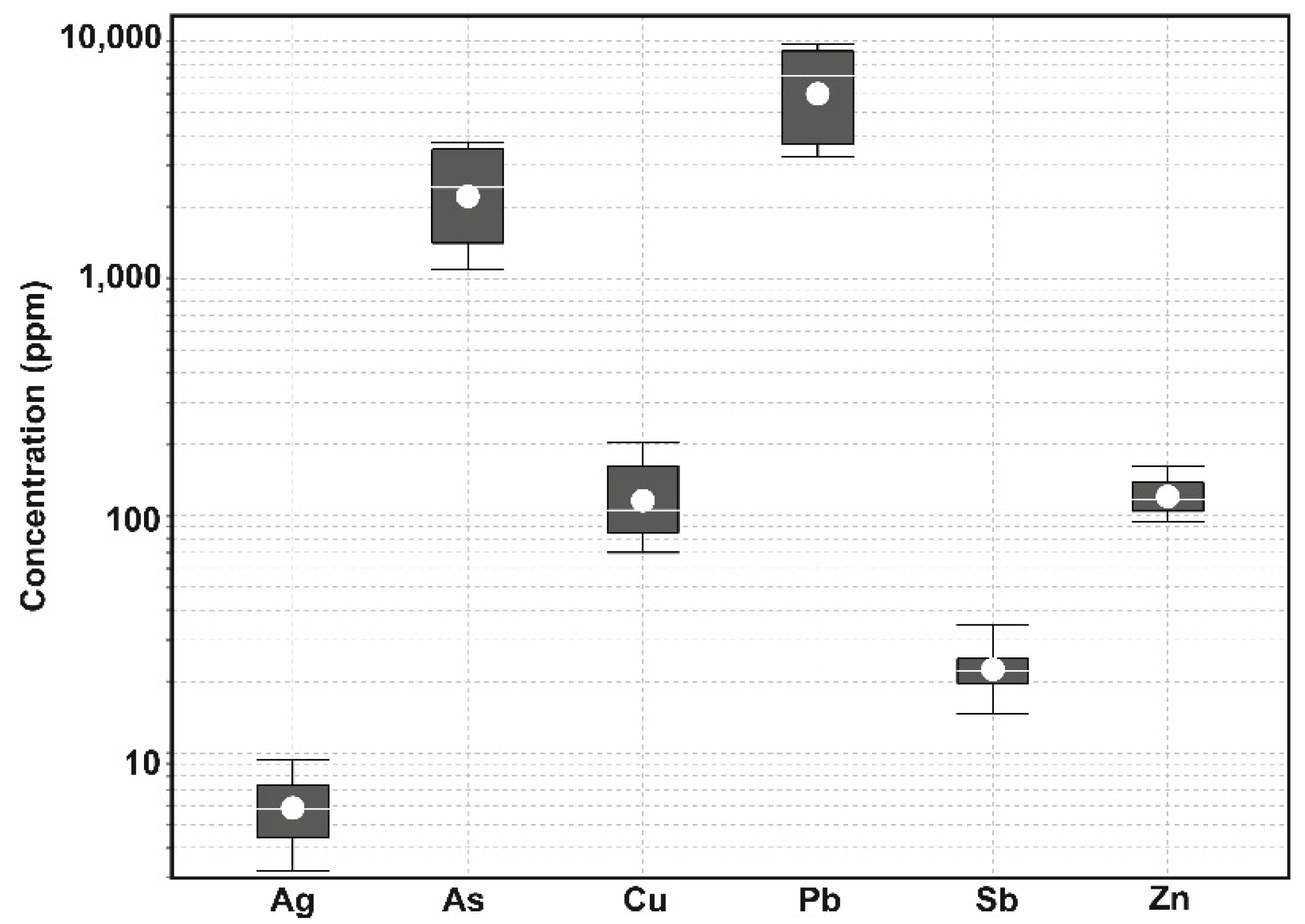
3.2. Major and Trace Element Chemistry
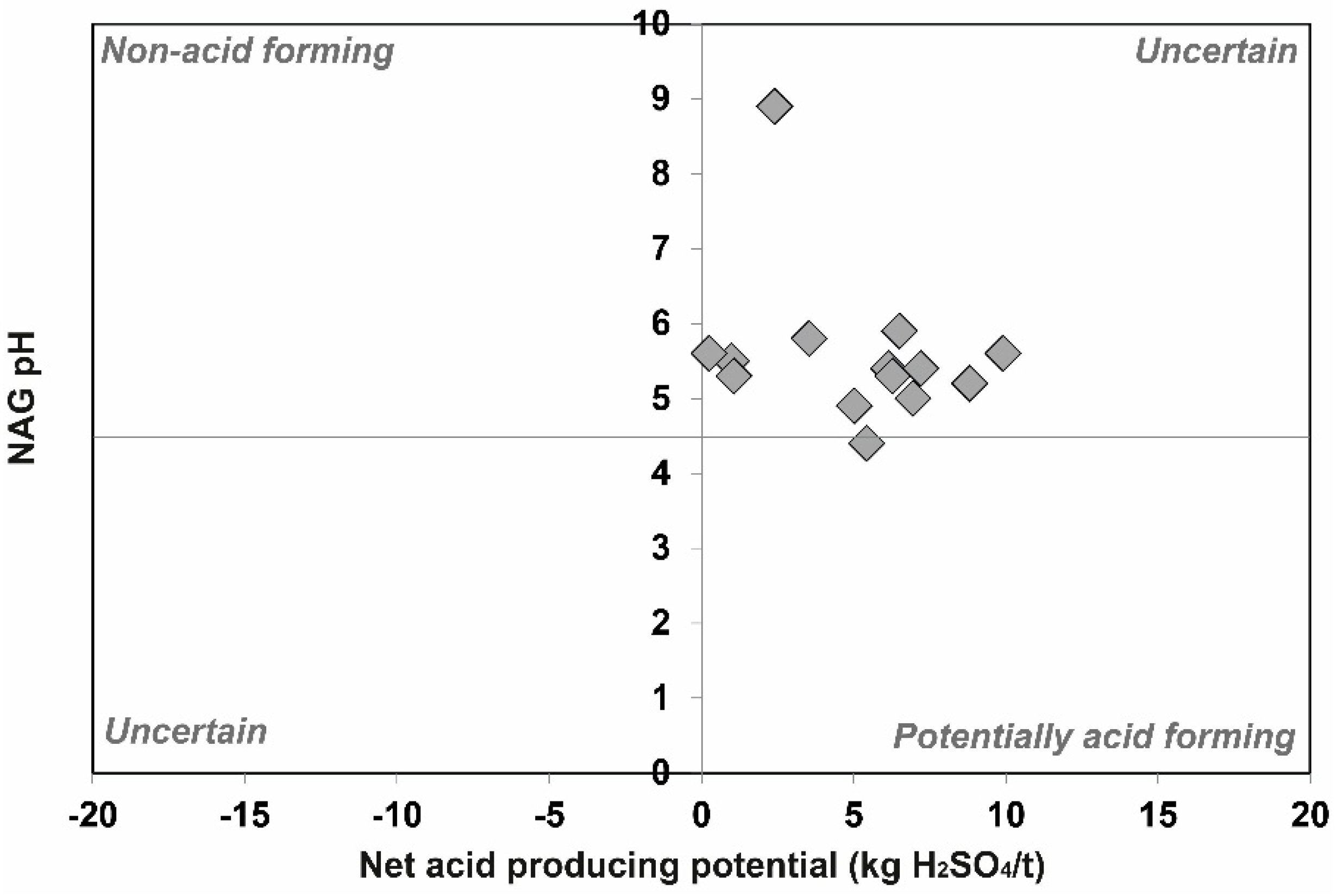
3.3. Acid-Base Accounting
4. Discussion
4.1. Heap Leach Pile Evolution
4.2. Rehabilitation Options
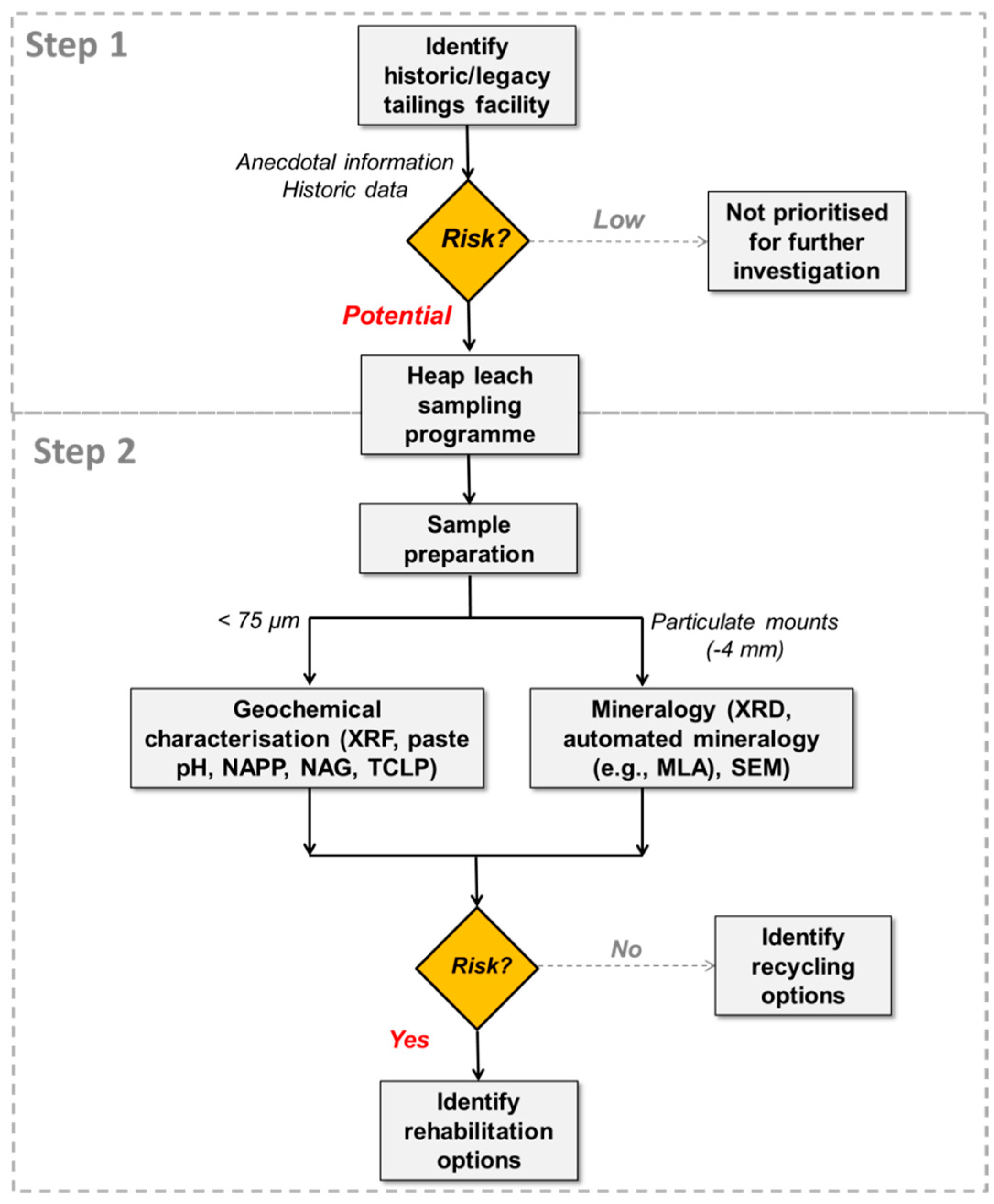
4.3. Risk Assessment Framework
5. Conclusions
- Fine-grained (i.e., >20 μm diameter) sulphides were encapsulated in quartz and have remained fresh. In contrast, larger primary sulphides (e.g., pyrite, arsenopyrite and galena) particles experienced extensive oxidation. Consequently a diverse range of secondary mineral phases from the alunite supergroup, as well as other Pb-As-Fe oxide phases can are now observed.
- Minerals of the alunite supergroup are not observed in adjacent waste rock piles at this site, suggesting that the lixiviant has chemically preconditioned the gangue sulphides to oxidize via different reaction pathways, a hypothesis which requires further experimental clarification.
- Far from being inert waste landforms these heap leach piles are sources of Pb, with concentrations in their basal leachates exceeding WHO (2006) values by 6 times. Considering this, they represent a moderate geoenvironmental risk and should be included in future rehabilitation strategies developed for this site.
- Whilst costly, these materials could be rehandled and chemically pre-treated (i.e., oxidized) to remove sulphides and then recycled (i.e., for use as aggregate/construction fill), if TCLP assessments classify them as non-hazardous.
- Development of a global spent heap leach characterisation framework is required, and will be beneficial for rehabilitating such abandoned sites, and determining if indeed these materials have a place in the circular economy model.
Acknowledgments
Conflicts of Interest
Abbreviations
| AMD | acid mine drainage |
| BSE | backscattered electron |
| CVG | Croydon Volcanic Group |
| ISQG | interim sediment quality guideline |
| MLA | mineral liberation analyser |
| NAG | net acid generation |
| NAPP | net acid producing potential |
| SEM | scanning electron microscopy |
| TCLP | toxicity characteristic leaching procedure |
| WHO | World Health Organisation |
References
- Petersen, J. Heap leaching as a key technology for recovery of values from low-grade ores—A brief overview. Hydrometallurgy 2015. [Google Scholar] [CrossRef]
- Ghorbani, Y.; Kuan, S.H. A review of sustainable development in the Chilean mining sector: Past, present and future. Int. J. Min. Reclam. Environ. 2016. [Google Scholar] [CrossRef]
- Ghorbani, Y.; Becker, M.; Mainza, A.; Franzidis, J.-P.; Franzidis, J.P.; Petersen, J. Large particle effects in chemical/biochemical heap leach processes—A review. Miner. Eng. 2011, 24, 1172–1184. [Google Scholar] [CrossRef]
- Dhawan, N.; Sadegh Safarzadeh, M.; Miller, J.D.; Moats, M.S.; Rajamani, R.K. Crushed ore agglomeration and its control for heap leaching operations. Miner. Eng. 2013, 41, 53–70. [Google Scholar] [CrossRef]
- Oxley, A.; Smith, M.E.; Caceres, O. Why heap leach nickel laterites? Miner. Eng. 2016, 88, 53–60. [Google Scholar] [CrossRef]
- Kumar, P.A.; Vengtasalam, R. Mineral beneficiation by heap leaching technique in mining. Procedia Earth Planet. Sci. 2015, 11, 140–148. [Google Scholar] [CrossRef]
- Groznov, I.N. Heap leaching. Computer simulation as an alternative technology. Resour. Effic. Technol. 2015, 1, 3–9. [Google Scholar] [CrossRef]
- Parbhakar-Fox, A.; Lottermoser, B.G. A critical review of acid rock drainage prediction methods and practices. Miner. Eng. 2015, 82, 107–124. [Google Scholar] [CrossRef]
- Van Veen, E.M.; Lottermoser, B.G.; Parbhakar-Fox, A.; Fox, N.; Hunt, J. A new test for plant bioaccessibility in sulphidic wastes and soils: A case study from the Wheal Maid historic tailings repository in Cornwall, UK. Sci. Total Environ. 2016. [Google Scholar] [CrossRef] [PubMed]
- Paldyna, J.; Krasnodebska-Ostrega, B.; Kregielwska, K.; Kowalska, J.; Jedynak, L.; Golimowski, J.; Grobelski, T.; Farbiszewska-Kiczma, J.; Farbiszewska, T. The assessment of environmental pollution caused by mining and metallurgical wastes from highly polluted post-industrial regions in South Poland. Environ. Earth Sci. 2012, 68, 439–450. [Google Scholar] [CrossRef] [Green Version]
- Mudd, G.; Patterson, J. Continuing pollution from the Rum Jungle U-Cu project: A critical evaluation of environmental monitoring and rehabilitation. Environ. Pollut. 2010, 158, 1252–1260. [Google Scholar] [CrossRef] [PubMed]
- Bowell, R.J.; Declerq, J.; Warrender, R.; Prestia, A.; Barber, J.R.; Parshley, J.V. The role of natural attenuation for arsenic in heap leach drainage. In Proceedings of the 27th International Applied Geochemistry Symposium, Tucson, AZ, USA, 20–24 April 2015.
- Parbhakar-Fox, A.; Edraki, M.; Hardie, K.; Kadletz, O.; Hall, T. Identification of acid rock drainage sources through mesotextural classification at abandoned mines of Croydon, Australia: Implications for the rehabilitation of waste rock repositories. J. Geochem. Explor. 2014, 137, 11–28. [Google Scholar] [CrossRef]
- Van Eck, M.; Child, R. Croydon gold deposits. In Monograph 14—Geology and Mineral Deposits of Australia and Papua New Guinea; Hughes, F.E., Ed.; Australian Institute of Mining and Metallurgy: Melbourne, Australia, 1990; pp. 979–982. [Google Scholar]
- Federation La Perouse Mine (Croydon) Remediation Project. Available online: https://www.qld.gov.au/environment/land/abandoned-mines/projects/federation-la-perouse/ (accessed on 13 March 2016).
- Fandrich, R.; Gu, Y.; Burrows, D.; Moeller, K. Modern SEM-based mineral liberation analysis. Int. J. Miner. Proc. 2007, 84, 310–320. [Google Scholar] [CrossRef]
- Parbhakar-Fox, A.; Edraki, M.; Walters, S.; Bradshaw, D.J. Development of a textural index for the prediction of acid rock drainage. Miner. Eng. 2011, 24, 1277–1287. [Google Scholar] [CrossRef]
- White, W.W.; Lapakko, K.A.; Cox, R.L. Static test methods most commonly used to predict acid mine drainage: Practical guidelines for use and interpretation. In The Environmental Geochemistry of Mineral Deposits Part A: Processes, Techniques, and Health Issues, Reviews of Economic Geology; Plumlee, G.S., Lodgson, M.J., Eds.; Society of Economic Geologists: Littleton, CO, USA, 1999; Volume 6A, pp. 325–338. [Google Scholar]
- Smart, R.; Skinner, W.M.; Levay, G.; Gerson, A.R.; Thomas, J.E.; Sobieraj, H.; Schumann, R.; Weisener, C.G.; Weber, P.A.; Miller, S.D. ARD Test Handbook: Project P387. A Prediction and Kinetic Control of Acid Mine Drainage; AMIRA, International Ltd.: Melbourne, Australia; Ian Wark Research Institute: Melbourne, Australia, 2002. [Google Scholar]
- Yu, Z.; Norman, M.D.; Robinson, P. Major and trace element analysis of silicate rocks by XRF and laser ablation ICP-MS using lithium borate fused glasses: Matrix effects, instrument response and results for international reference materials. Geostandard. Newsl. 2003, 27, 67–89. [Google Scholar] [CrossRef]
- Danyushevsky, L.; Robinson, P.; Gilbert, S.; Norman, M.; Large, R.; McGoldrick, P.; Shelley, M. Routine quantitative multi-element analysis of sulphide minerals by laser ablation ICP-MS: Standard development and consideration of matrix effects. Geochem. Explor. Environ. Anal. 2011, 11, 51–60. [Google Scholar] [CrossRef]
- Li, J.; Smart, R.S.C.; Schumann, R.C.; Gerson, A.R.; Levay, G. A simplified method for estimation of jarosite and acid-forming sulfates in acid mine wastes. Sci. Total Environ. 2007, 373, 391–403. [Google Scholar] [CrossRef] [PubMed]
- Jambor, J.L. Mine-waste mineralogy and mineralogical perspectives of acid-base accounting. In Environmental Aspects of Mine Wastes; Canada Short Course Series; Jambor, J.L., Blowes, D.W., Ritchie, A.I.M., Eds.; Mineralogical Association of Canada: Québec, QC, Canada, 2003; Volume 31, pp. 117–145. [Google Scholar]
- Simpson, S.L.; Batley, G.E.; Chariton, A.A.; Stauber, J.L.; King, C.K.; Chapman, J.C.; Hyne, R.V.; Gale, S.A.; Roach, A.C.; Maher, W.A. Handbook for Sediment Quality Assessment; CSIRO: Canberra, Australia, 2005; Available online: https://publications.csiro.au/rpr/pub?list=BRO&pid=procite:9b5d8b41-e8e1-4602-b58c-13bd21e96e73 (accessed on 27 May 2016).
- Breuer, P.L.; Hewitt, D.M.; Meakin, R.L. Does pre-oxidation or lead(II) addition reduce the impact of iron sulfides in cyanidation? In Hydrometallurgy 2008: Proceedings of the 6th International Symposium; Society for Mining, Metallurgy and Exploration, Inc.: Englewood, Co, USA, 2008; pp. 750–757. [Google Scholar]
- McFarlane, A.; (Commonwealth Scientific and Industrial Research Organisation, Mineral Resources, Kensington, Perth, Australia). Personal communication, 2016.
- Marsden, J.; House, I. The Chemistry of Gold Extraction, 2nd ed.; Society for Mining, Metallurgy, and Exploration: Englewood, CO, USA, 2006; pp. 251–260. [Google Scholar]
- Souza-Fagundes, E.; Rosa, L.H.; Gomes, N.C.M.; Santos, M.H.; Pimentel, P.F. Thiocyanide degradation by pure and mixed cultures of microorganisms. Braz. J. Microbiol. 2004, 35, 333–336. [Google Scholar] [CrossRef]
- Treatment of Cyanide Heap Leaches and Tailings. United States Environmental Protection Agency. Available online: https://fs.ogm.utah.gov/pub/MINES/Minerals_Related/EPApublications/Cyanide.PDF (accessed on 30 May 2016).
- Hsieh, Y.H.; Huang, C.P. The dissolution of PbS(s) in dilute aqueous solutions. J. Colloid Interface Sci. 1989, 131, 537–549. [Google Scholar] [CrossRef]
- Mular, A.; Halbe, D.N.; Barratt, D.J. Mineral Processing Plant Design, Practice and Control; Society for Mining, Metallurgy, and Exploration: Englewood, CO, USA, 2002; Volume 2, pp. 1619–1622. [Google Scholar]
- Heinen, H.J.; Petersen, D.G.; Lindstrom, R.E. Processing Gold Ore Using Heap Leach-Carbon Adsorption Methods. U.S. Department of the Interior, Bureau of Mines, 1978. Available online: http://anfacal.org/media/Biblioteca_Digital/Mineria_Metalica/JM-ore_proces_gold.pdf (accessed on 27 May 2016).
- Hawkins, B.A. Implications of Pyrite Oxidation for Engineering Works; Springer Science and Business Media: Berlin, Germany, 2013; p. 307. [Google Scholar]
- Acero, P.; Cama, J.; Ayora, C. Rate law for galena dissolution in acidic environment. Chem. Geol. 2007, 245, 219–229. [Google Scholar] [CrossRef]
- Jambor, J.L. Nomenclature of the alunite supergroup. Can. Mineral. 1999, 37, 1323–1341. [Google Scholar]
- Bayliss, P.; Kolitsch, U.; Nickel, E.H.; Pring, A. Alunite supergroup: Recommended nomenclature. Mineral. Mag. 2010, 74, 919–927. [Google Scholar] [CrossRef]
- Handbook of Mineralogy. Available online: http://www.handbookofmineralogy.org/ (accessed on 26 May 2016).
- Golebeiowoska, B.; Wodek, A.; Pieczka, A.; Borkiewicz, O.; Polak, M. The philipsbornite-segnetite solid solution series from Redziny, eastern metamorphic cover of the karkonsze granite (SW Poland). Ann. Soc. Geol. Pol. 2016, 86, 73–83. [Google Scholar]
- Smith, A.M.L.; Dubbin, W.E.; Wright, K.; Hudson-Edwards, K.A. Dissolution of lead- and lead-arsenic-jarosites at pH 2 and 8 and 20 C: Insights from batch experiments. Chem. Geol. 2006, 229, 344–361. [Google Scholar] [CrossRef]
- United States Congress, Office of Technology Assessment. Mining Waste. In Managing Industrial Solid Wastes from Manufacturing, Mining, Oil and Gas Production, and Utility Coal Combustion; United States Congress, Office of Technology Assessment: Washington, D.C., USA, 1992. [Google Scholar]
- Cyanide Management. Available online: http://www.industry.gov.au/resource/Documents/LPSDP/LPSDP-CyanideHandbook.pdf (accessed on 27 March 2016).
- Smith, M.E. Potential Problems in Copper Dump Leaching. Available online: http://www.ausenco.com/uploads/pages/1444279171-Potential_Problems_in_Copper_Dump_Leaching.pdf (accessed on 27 May 2016).
- Edraki, M.; Baumgartl, T.; Haymont, R. Investigating the Linkage between Water fluxes, Geochemistry and Water Quality in the Post-Closure Landscape of the Mt Leyshon Mine, Queensland. In Proceedings of the First International Seminar on Mine Closure, Perth, Australia, 13–15 September 2016; Fourie, A., Tibbett, T., Eds.; pp. 647–656.
- Round Mountain Expansion. Available online: http://www.blm.gov/style/medialib/blm/nv/field_offices/battle_mountain_field/blm_information/nepa/round_mountain_expansion.Par.23204.File.dat/2.0_Alternatives.pdf (accessed on 30 May 2016).
- Major Heap Leach Operations 2015. Available online: http://www.infomine.com/maps/posters/heapleach/ (accessed on 27 March 2016).
- Blengini, G.A.; Garbarino, E.; Solar, S.; Sheilds, D.J.; Hamor, T.; Vinai, R.; Agioutantis, Z. Life cycle assessment guideline for the sustainable production and recycling of aggregates: The sustainable aggregates resource management project (SARMa). J. Clean. Prod. 2012, 27, 177–181. [Google Scholar] [CrossRef]
- Giurco, D.; Littleboy, A.; Boyle, T.; Fyfe, J.; White, S. Circular economy: Questions for responsible minerals, additive manufacturing and recycling of metals. Resources 2014, 3, 432–453. [Google Scholar] [CrossRef]



| Mineral Phase | Formula | 222 | 225 | 227 | 231 | 233 |
|---|---|---|---|---|---|---|
| Alunite | KAl3(SO4)2(OH)6 | A | • | • | • | • |
| Anglesite | PbSO4 | • | • | • | • | • |
| Arsenolite | As4O6 | • | • | • | • | • |
| Bernalite | Fe(OH)3 | • | • | • | • | • |
| Beudantite | PbFe3(OH)6SO4AsO4 | • | • | • | • | • |
| Carminite | PbFe2(AsO4)2(OH)2 | A | • | • | • | • |
| Fe-oxide | FeOOH | • | A | • | • | A |
| Ferrihydrite | Fe2O3·0.5(H2O) | • | • | • | • | • |
| Finnemanite | Pb5Cl(AsO3)3 | A | • | A | A | A |
| Hematite | Fe2O3 | • | • | • | • | A |
| Hidalgoite | PbAl3(AsO4)(SO4)(OH)4 | • | • | • | • | • |
| Kintoreite | Pb(Fe)3(PO4)2(OH,H2O)6 | A | A | • | • | • |
| Pitticite | Fe2(AsO4)(SO4)·(H2O) | • | • | • | • | • |
| Schultenite | PbHAsO4 | A | • | • | A | • |
| Segnitite | PbFe3H(AsO4)2(OH)6 | • | • | • | • | • |
| Surite | (Pb,Ca)3(Al,Fe,Mg)2((Si,Al)4O10)(CO3)2(OH)2 | • | • | • | • | • |
| Sample | SiO2 | TiO2 | Al2O3 | Fe2O3 | MgO | CaO | Na2O | K2O | P2O5 | PbO | As2O3 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 220 | 82.0 | 0.2 | 8.4 | 3.1 | 0.2 | 0.0 | 0.0 | 2.7 | 0.1 | 0.4 | 0.2 |
| 221 | 80.3 | 0.2 | 8.4 | 4.5 | 0.2 | 0.0 | <0.03 | 2.4 | 0.1 | 0.4 | 0.2 |
| 222 | 77.8 | 0.3 | 10.1 | 4.0 | 0.2 | 0.1 | 0.0 | 2.8 | 0.1 | 0.4 | 0.2 |
| 223 | 77.1 | 0.3 | 10.6 | 4.0 | 0.2 | 0.1 | 0.1 | 3.2 | 0.1 | 0.4 | 0.2 |
| 224 | 78.1 | 0.3 | 10.4 | 3.5 | 0.2 | 0.0 | 0.1 | 3.1 | 0.1 | 0.4 | 0.2 |
| 225 | 78.3 | 0.2 | 9.7 | 3.3 | 0.3 | 0.1 | <0.03 | 2.8 | 0.1 | 1.1 | 0.6 |
| 226 | 81.7 | 0.2 | 8.3 | 3.2 | 0.2 | 0.0 | <0.03 | 2.7 | 0.1 | 0.5 | 0.3 |
| 227 | 78.3 | 0.2 | 9.4 | 3.6 | 0.2 | 0.1 | 0.1 | 2.9 | 0.1 | 0.9 | 0.5 |
| 228 | 79.1 | 0.2 | 8.2 | 4.5 | 0.3 | 0.1 | <0.03 | 2.2 | 0.1 | 1.0 | 0.6 |
| 229 | 79.6 | 0.2 | 8.5 | 4.6 | 0.2 | 0.2 | 0.0 | 2.2 | 0.1 | 0.7 | 0.3 |
| 230 | 81.3 | 0.2 | 7.5 | 3.9 | 0.3 | 0.1 | <0.03 | 2.2 | 0.1 | 1.0 | 0.6 |
| 231 | 78.3 | 0.2 | 9.7 | 4.0 | 0.2 | 0.1 | <0.03 | 2.5 | 0.1 | 0.9 | 0.5 |
| 232 | 75.5 | 0.2 | 10.3 | 5.0 | 0.3 | 0.4 | <0.03 | 2.4 | 0.1 | 1.0 | 0.5 |
| 233 | 77.6 | 0.2 | 9.0 | 4.5 | 0.2 | 0.1 | <0.03 | 2.4 | 0.1 | 0.9 | 0.5 |
© 2016 by the author; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parbhakar-Fox, A. Geoenvironmental Characterisation of Heap Leach Materials at Abandoned Mines: Croydon Au-Mines, QLD, Australia. Minerals 2016, 6, 52. https://doi.org/10.3390/min6020052
Parbhakar-Fox A. Geoenvironmental Characterisation of Heap Leach Materials at Abandoned Mines: Croydon Au-Mines, QLD, Australia. Minerals. 2016; 6(2):52. https://doi.org/10.3390/min6020052
Chicago/Turabian StyleParbhakar-Fox, Anita. 2016. "Geoenvironmental Characterisation of Heap Leach Materials at Abandoned Mines: Croydon Au-Mines, QLD, Australia" Minerals 6, no. 2: 52. https://doi.org/10.3390/min6020052
APA StyleParbhakar-Fox, A. (2016). Geoenvironmental Characterisation of Heap Leach Materials at Abandoned Mines: Croydon Au-Mines, QLD, Australia. Minerals, 6(2), 52. https://doi.org/10.3390/min6020052






