1. Introduction
In recent years, coal has accounted for 74% of China’s total primary energy consumption and will continue to be the major energy source in the next decades [
1]. However, environmental hazards were caused as a result of the release and dispersal of harmful trace elements contained in coal, coal gangue and coal combustion residues [
2,
3,
4,
5]. The rapid development of coal mining in China over the last twenty years has led to a huge coal gangue accumulation in coalfields. According to the incomplete statistics of 2010, there were more than 4.5 billion tons of coal gangue, which covered approximately 1.5 thousands square kilometers of land in China [
6]. This could result in substantial environmental hazard and ecological risk, such as soil and water pollution and ecological deterioration, if reasonable precautions are not taken [
7,
8].
Coal gangue, a mixture of rocks derived from coal bed’s roof, floor, partings and coal itself, is mainly produced from coal mining and, in some cases, is enriched in some toxic trace elements [
9,
10,
11]. Trace elements are defined as elements with concentrations lower than 0.1% in coal gangue [
12]; they have become a hot topic due to the complex changes of their particles, inability to decompose in natural processes and high toxicity to ecosystems following their release and dispersal into the atmosphere, soils, water and vegetation through the pathways of leaching, weathering and spontaneous combustion [
6,
9,
13,
14]. Leaching has proven to be one of the primary pathways for trace elements entering into the ecosystem. Extensive studies on the leaching behavior of trace elements from coal, coal fly ash and bottom ash have been conducted [
13,
15,
16,
17]. However, studies focused on the leaching behavior of trace elements from coal gangue are relatively rare [
6,
18], especially from coal gangue piles. Nevertheless, “leaching behavior” and its impact factors are not clearly defined in these current investigations.
Time is one of the important impact factors on the leaching behavior of trace elements from coal gangue [
4,
19,
20]. The integrative efforts to determine the leaching behavior of the selected trace elements from coal gangue piles indicated that it is a very complex process in terms of the leaching pathways, which might be influenced by various factors. Moreover, the potential environmental impacts of the resulting leachates from coal gangue should be given more attention and studied extensively using quantitative assessment methods due to their emergent potential pollution and toxicity.
Therefore, this study was conducted to focus on investigations on the leaching behavior of trace elements from coal gangue piles and their environmental effects. Based on the comparative column leaching experiments, the environmental and ecological risks generated by the trace elements of the resulting leachates from coal gangue piles were evaluated in detail by different semi-quantitative methods. The leaching behavior of the trace elements from coal gangue piles, in this study, mainly refers to the leached concentrations of trace elements, leached amount, leachability, maximum leached amount (Lam) and maximum leachability (Lrm). The roles of the impact factors, including leaching time, pH of the leaching solution and sample amount, were simultaneously analyzed.
Twenty-six trace elements in coal, proposed by Swaine [
12], could lead to potential environmental impacts, including As, Cr, Cd, Hg, Pb, Se, B, Mn, Ni, Cu, V, Zn, Co, Sn, Cl, F, Mo, Bo, P, Th, U, Ba, I, Ra, Sb and Tl. Particularly, elements As, Cd, Cr, Hg, Pb and Se are of most environmental importance [
12], while elements Ni, Cu, V, Zn, Co and Sn have generally been analyzed in other leaching experiments [
6,
8]. Twelve trace elements, including As, V, Cr, Co, Ni, Cu, Zn, Se, Cd, Sn, Pb and Hg, were therefore selected for investigation in this study, to assess their leaching behavior in coal gangue piles and the corresponding environmental and ecological hazards levels.
2. Materials and Methods
2.1. Study Area
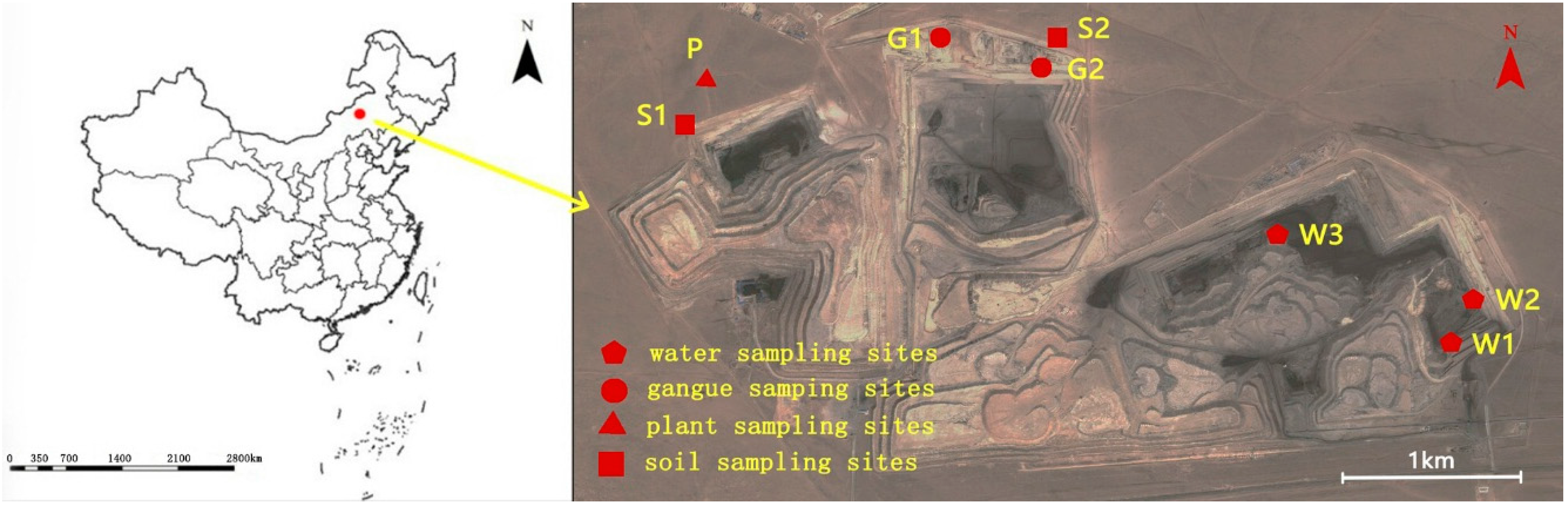
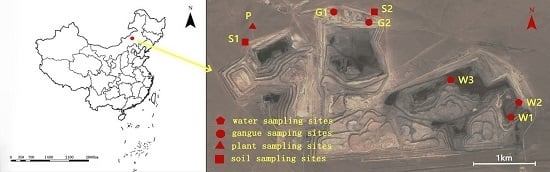
The Wulantuga open-cast germanium coal mine area (43°56′57.86″ N, 115°54′37.36″ E), covering an area of 2.2 km
2 [
21,
22], is located in the southwest of the Shengli Coalfield (with a total area of 342 km
2 [
21]) in northeastern Inner Mongolia, northern China (
Figure 1). The germanium-rich coal in the open-cast mine has been mined since 1997. The production of raw coal was 7.3 million tons in 2014, resulting in approximately 0.7 million tons of coal gangue accumulation [
23]. The coal properties and the geological setting have previously been described in a great detail [
21,
22,
24,
25,
26].
The study area has a semi-arid continental climate of the middle temperate zone. The annual average temperature is 0–3 °C, and the mean annual rainfall is approximately 276.3 mm. The soil type is chestnut soil, and the vegetation type is typical grassland, with 50% coverage. There is a seasonal river named the Xilin River flowing northward, 12 km from the east of the open-cast coal mine area, which has no direct hydraulic connection to the coal mine. The terrain of the Shengli Coalfield is gentle, with a slope of no more than 7° and an elevation of 1061–1196 m. The germanium coal mine area belongs to an approximate level-slight inclination monocline structure, with a formation dip of less than 5°.
2.2. Sample Collection
To investigate the mineral and chemical compositions in coal gangue and their potential environmental effects, coal gangue samples were collected in the open-cast germanium coal mine in July 2014, as well as the soils, water and plant samples around the mine area (
Figure 1).
Coal gangue samples were selected from two sites of coal gangue piles in study area (
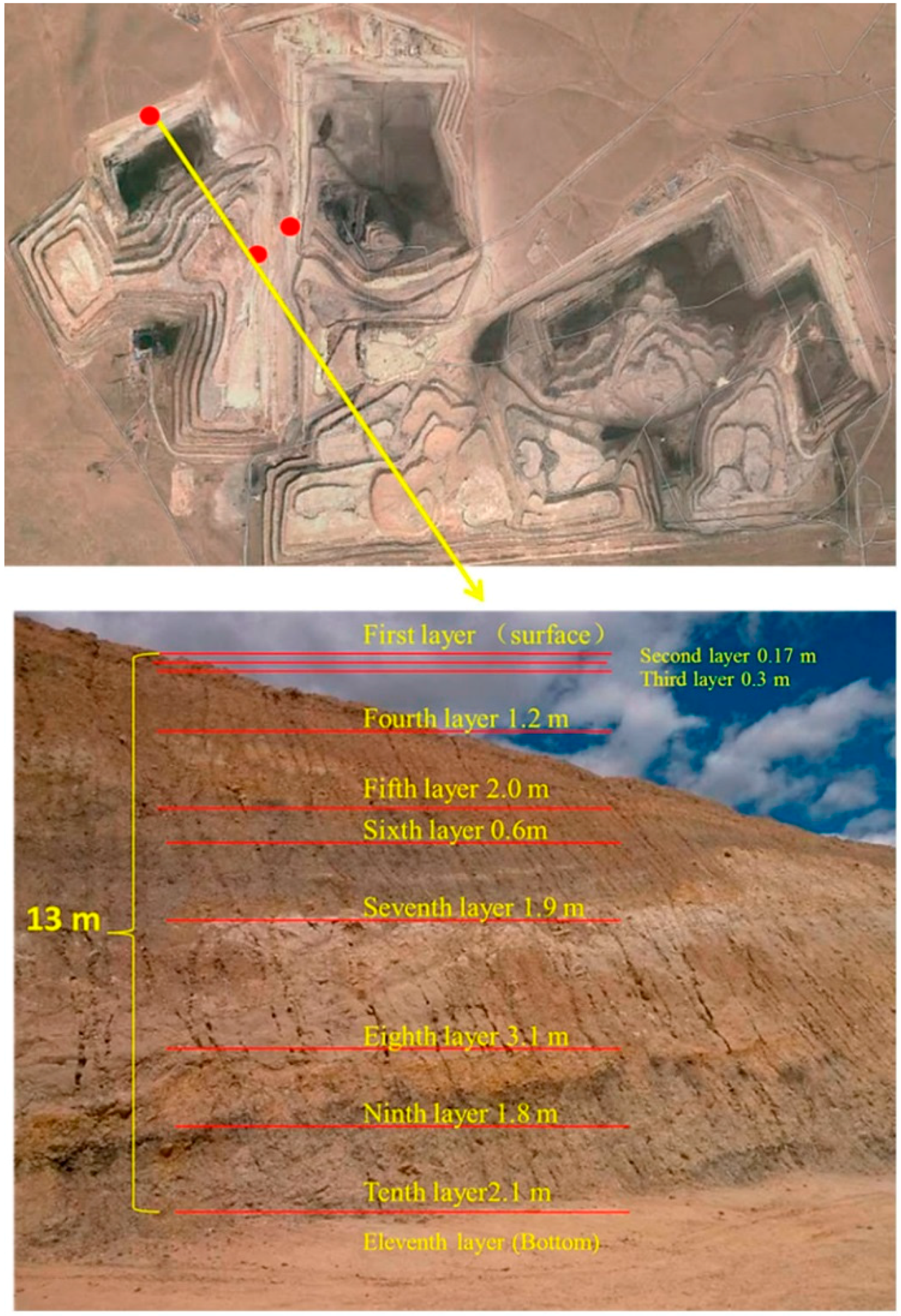
Figure 1). Three samples were taken by hand and were immediately stored in individual sealed plastic bags in order to avoid any contamination and oxidation. Soil samples were collected from two sites in the north of the open-cast coal mine area using a geotome at a depth of 0–15 cm of each layer (
Figure 2), and three sampling points were set in each layer, according to the sample collection methods described in detail by Jia
et al. [
23]. Background soil samples were taken from the grassland located approximately 15 km to the northeast of Xilinhaote city. All of the soil samples were also stored in sealed plastic bags in a portable freezer to minimize possible changes and contaminations. Three water samples were taken using a glass water sampler with 2 L capacity from the water pools in the open-cast germanium coal mine area. Two background samples were collected from the Jiuquwan Reservoir, which is situated approximately 20 km from the southeast of the coal mine area. The samples were immediately put into polyethylene terephthalate (PET) bottles and stored in the portable freezer. Four plant samples (
Filifolium Kitam,
Artemisia lavandulaefolia,
Allium tuberosum Rottler and
Leymus chinensis (Trin.) Tzvel) were selected in the grassland located in the north of the coal mine area. The leaves and trunks of plant samples were collected and immediately stored in sealed plastic bags.
2.3. Analytical Methods
The contents of major-element oxides, including SiO
2, TiO
2, Al
2O
3, Fe
2O
3, MgO, CaO, MnO, Na
2O, K
2O and P
2O
5, in coal gangue samples were analyzed by X-ray fluorescence spectrometry (XRF, ARL ADVANT′XP+, ThermoFisher, Waltham, MA, USA) as outlined by Dai
et al. [
27]. The mineralogical compositions were determined on a D/max-2500/PC powder diffractometer with Ni-filtered Cu-Kα radiation and a scintillation detector. Each XRD pattern was recorded over a 2θ interval of 2.6°–70°, with a step size of 0.01° [
27]. The selected environmentally-sensitive trace elements were As, V, Cr, Co, Ni, Cu, Zn, Se, Cd, Sn, Pb and Hg. The trace element contents of V, Cr, Co, Ni, Cu, Zn, Cd, Sn and Pb in the coal gangue, soil, water and plant samples and those in the resulting leachates of the following experiments of coal gangue were all determined by inductively-coupled plasma mass spectrometry (X series II ICP-MS, ThermoFisher), according to the procedures described in detail by Dai
et al. [
28]. Arsenic and Se were determined by ICP-MS using collision cell technology (CCT), as described by Li
et al. [
29]. The concentration of Hg was determined by a Milestone DMA-80 Hg analyzer (Milestone, Sorisole, Italy). The detection limit of Hg is 0.005 ng; the relative standard deviation from eleven runs on Hg standard reference is 1.5%; and the linearity of the calibration is in the range 0–1000 ng [
30]. The handling methods of soil samples were described in detail by Jia
et al. [
24]. Four plant samples were mixed and were cleaned by deionized water. They were dried in a drying oven (60 °C) and crushed to 100 mesh size for testing.
2.4. Leaching Experiments
Coal gangue samples were air-dried and crushed to 200 mesh. They were blended by the method of repeated division into four equal portions to mix thoroughly (four times) and prepared for the leaching experiments (5 kg). Then, three 30-g sub-samples and one 45-g sub-sample were obtained by an analytical balance of 0.01-mg precision, accurate to four decimal places.
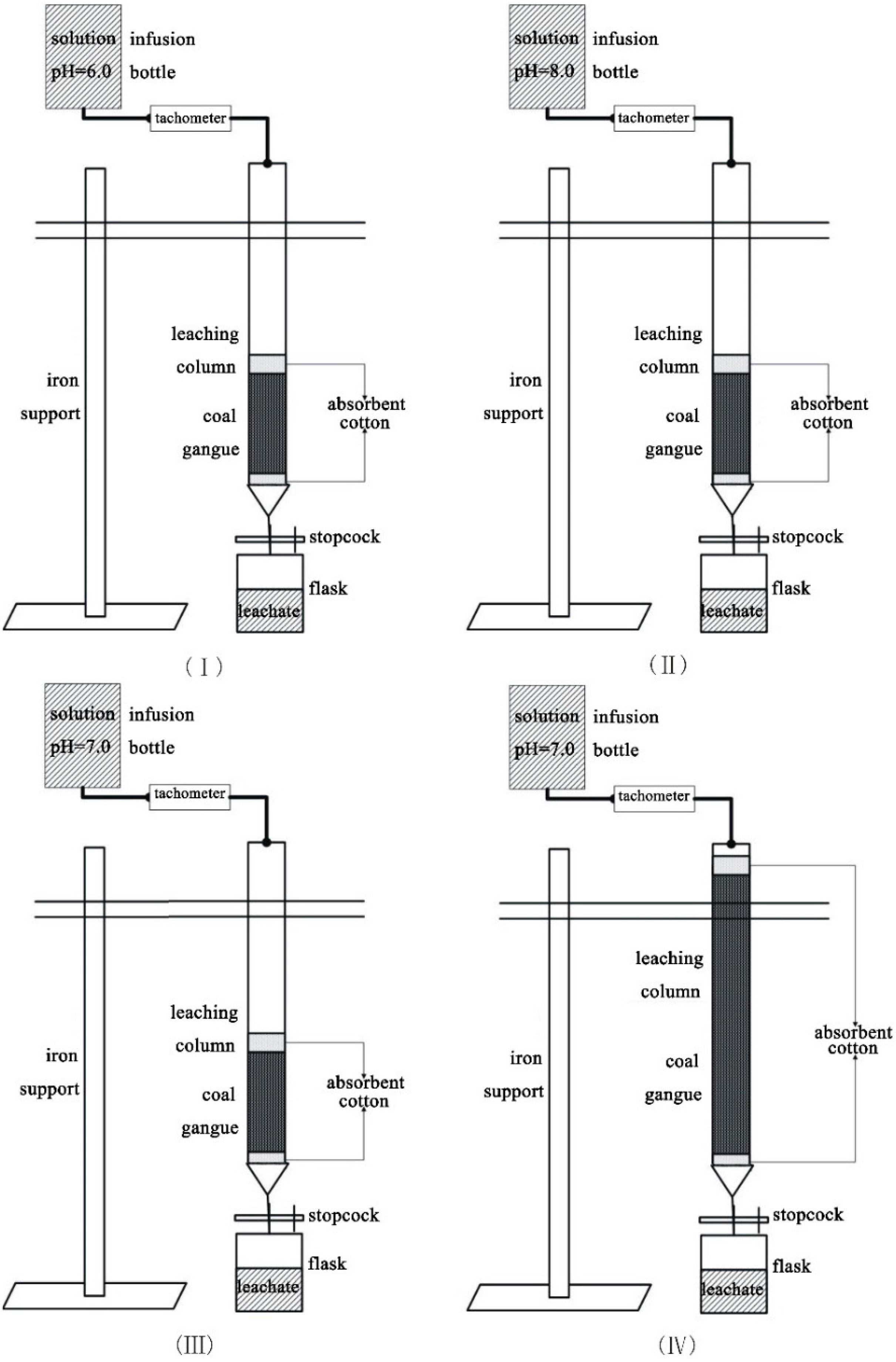
To investigate the impacts of different pH values and sample quantities on the leaching behavior of the selected trace elements in the coal gangue, four column leaching experimental groups were determined in this study (
Table 1). The pH of the rainfall in the study area is approximately 6.60–8.19, slightly alkaline, with no acid rain. The concentration ratio between SO
42− and NO
3− in the rainfall is approximately 2.3–29.0, suggesting sulfate precipitation [
19,
31]. According to the rainfall characteristics, distilled water (pH = 7.0 ± 0.3), acidic solution (pH = 6.0 ± 0.3, using distilled water with H
2SO
4) and alkaline solution (pH = 8.0 ± 0.3, using distilled water with NaOH) were prepared for the leaching experimental groups. In this study, Experiments I, II and III were set for detecting the effects of solution pH on leaching behavior, with the same sample weight of 30-g, and different solutions of acid, alkaline and neutral pH, respectively. All of the test utensils were soaked in a 14% HNO
3 solution for 24 h and rinsed by distilled water before the leaching experiments [
19].
Each coal gangue sample was transferred into a fixed glass column, which was 30 mm in internal diameter and 50 cm in length (
Figure 3). Quartz sand (10 g, particle size < 0.83 mm) was packed into the bottom of the column in Experiments I, III and IV, respectively, to prevent fine particle loss during leaching. It was also packed at the top of the sample to make the solution disperse uniformly. However, a small amount of absorbent cotton, instead of quartz sand, was used at the top and bottom of the column in Experiment II. This was to prevent the quartz sand from reacting with the alkaline solution, in which absorbent cotton could not be dissolved. The four solutions were controlled in terms of influx into the columns at room temperature. The experiments lasted for 90 h. The resulting leachates were sampled once every 3 h and then put into 50-mL volumetric tubes for element analysis. Thirty samples were obtained from each experimental group. As a matter of convenience for displaying and analyzing the results, the concentrations of the 30 samples were averaged over 10 time units,
i.e., 0–9 h, 9–18 h, 18–27 h, 27–36 h, 36–45 h, 45–54 h, 54–63 h, 63–72 h, 72–81 h and 81–90 h.
The “leachability (
Lr)”, “leached concentration (
Cl)” and “maximum leached concentration (
Clm)” were used to analyze the leaching behavior of the coal gangue during the four experimental groups.
Lr (%) is calculated by Equation (1), and
Clm (μg/L) was calculated by Equation (2):
where
Clx (μg/L) and
Vx (L) represent the concentration of an element and the volume of the resulting leachates, respectively, during a period of leaching time.
Mx (μg) is the total mass of an element in the coal gangue samples.
2.5. Environmental Effect Indicators
To compare the concentrations of an element in coal gangue with black shales, Clarke values were used as a practical method of assessing trace element enrichment [
6]. The enrichment factor (EF) was then an important parameter to evaluate the pollution level of an element [
6,
32,
33,
34]. EF values of elements in coal gangue, coal, soil, water and vegetation of the Wulantuga open-cast coal mine were applied to assess the pollution degrees of the 12 trace elements. The EF value is expressed as follows:
where
Ai (μg/g) is an element’s concentration;
Bi (μg/g) is the background value of an element (Clarke value);
C (μg/g) is the reference element concentration;
D (μg/g) is the Clarke value of the reference element. Scandium is usually used as the reference element due to its stable concentration, and it was also applied in this study.
“Maximum leached amount” (
Lam) and “maximum leachability” (
Lrm) are important indicators for trace element environmental risk assessment, which could provide valuable information of the maximum extent of element migrating ability [
4,
33]. The value of
Lam is defined here as the ratio of all amounts of an element in the leachates of 90 h to the mass of the coal gangue sample. The value of
Lrm is based on the ratio of the amount of the trace element in the leachates after 90 h to the mass of the trace element in the coal gangue sample.
Aimed at further evaluating the environmental threat of the 12 environmentally-sensitive elements, the concentration limits of the trace elements in groundwater, soil and food were also compared in this research. Furthermore, the effects range low (ERL) and effects range median (ERM), defined in international sediment quality guidelines (SQGs), were adopted in this study to assess the ecological risk, namely the biological toxicity of trace elements in coal gangue. ERL values are the concentrations below which adverse effects on sediment-dwelling fauna would be unlikely, and ERM values were, in contrast, the concentrations above which adverse effects are likely [
5,
34]. The existence of biological toxicity could be divided into three levels,
i.e., <ERL, very low toxicity, >ERL and <ERM, middle toxicity, and >ERM, probable toxicity [
35].
The environmental quality standard for soils, the quality standard for groundwater and limits of contaminants in foods were also applied to be compared with the Lam values of the trace elements in soil samples, water samples and vegetation samples, respectively, in the study. Unfortunately, there are no specific limits, standards or references of the trace element concentrations for plants. Therefore, the concentrations in vegetation samples had to be compared to the limits of contaminants in foods, the alternative comparing reference, for evaluating the environmental impacts of coal gangue leaching on vegetation.
5. Conclusions
The changes of concentrations, leached amount, leachability, Lam and Lrm of the selected 12 trace elements from coal gangue in the Wulantuga open-cast coal mine area, Inner Mongolia, China, were investigated in this study. Based on the results of leaching behavior, the potential environmental and ecological hazards were also evaluated through different methods.
Leaching time and sample mass play important roles in determining the trace element concentrations, the leached amounts and leachabilities. pH values do not exhibit an obvious effect on the leaching behavior in this study. The coal gangue is enriched in As, Co, Se and Pb, and the EF values of As and Se indicate higher environmental pollution levels. All of the selected trace elements, namely, As, V, Cr, Co, Ni, Cu, Zn, Se, Cd, Sn, Pb and Hg, show no potential risk to soils and vegetation, but high potential risk to groundwater, based on the analysis of Lam values. Simultaneously, according to the ERL and ERM values in the SQGs, Ni from the coal gangue is inclined to have high biological toxicity, and As, Cr and Zn show moderate ecological risk to the environment. Further research of the environment-sensitive trace element leaching behavior should be investigated through more quantitative methods with the aid of GIS to identify environmental pollution and effectively make decisions regarding prevention and reclamation.