Modes of Occurrence of Fluorine by Extraction and SEM Method in a Coal-Fired Power Plant from Inner Mongolia, China
Abstract
:1. Introduction
2. Method and Sampling
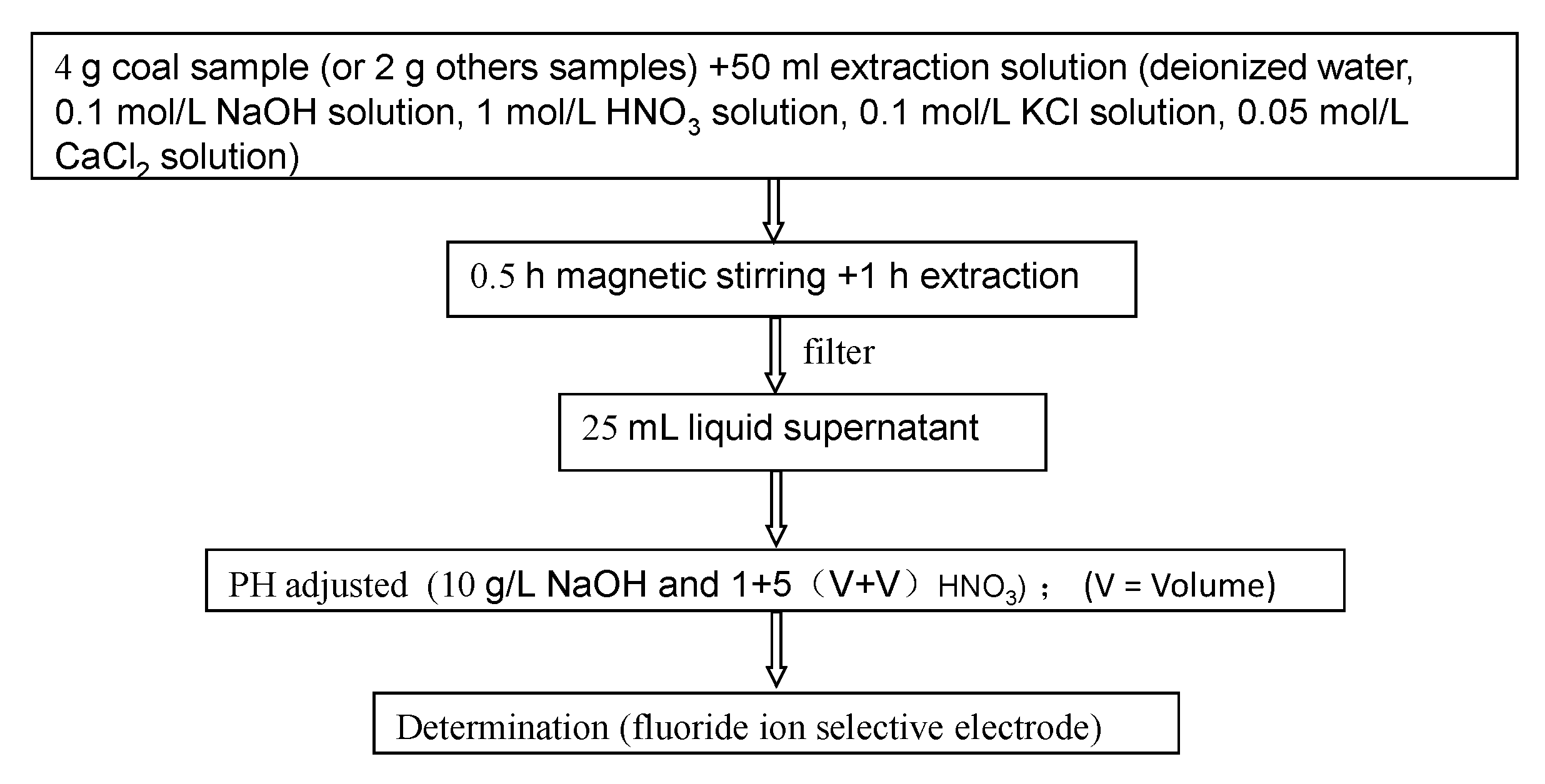
3. Results and Discussion
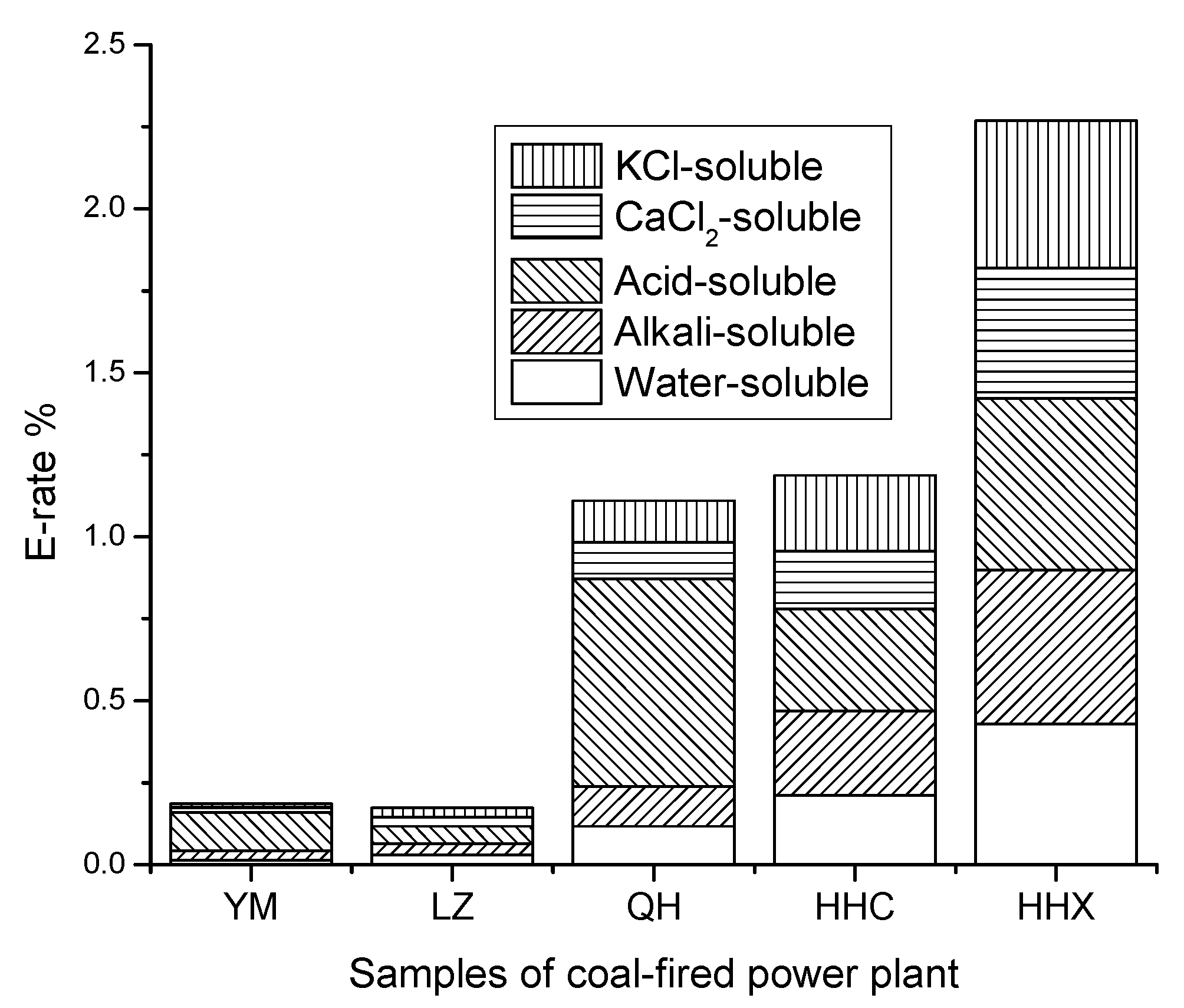
3.1. Occurrence Modesof Fluorine in Coal
| Items | Water-Soluble | Alkali-Soluble | Acid-Soluble | CaCl2-Soluble | KCl-Soluble | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Con (μg/g) | E-Rate (%) | Con (μg/g) | E-Rate (%) | Con (μg/g) | E-Rate (%) | Con (μg/g) | E-Rate (%) | Con (μg/g) | E-Rate (%) | |
| YM | 4.51 | 1.33 | 9.74 | 2.88 | 39.92 | 11.81 | 4.16 | 1.23 | 4.89 | 1.45 |
| LZ | 2.45 | 3.03 | 2.77 | 3.42 | 4.33 | 5.34 | 2.36 | 2.91 | 2.17 | 2.68 |
| QH | 6.36 | 11.78 | 6.49 | 12.02 | 34.22 | 63.38 | 6.06 | 11.22 | 6.76 | 12.52 |
| HHC | 57.97 | 21.08 | 71.0 | 25.82 | 85.21 | 30.99 | 48.69 | 17.71 | 63.64 | 23.14 |
| HHX | 349.37 | 42.87 | 383.52 | 47.06 | 426.15 | 52.29 | 324.70 | 39.85 | 365.30 | 44.82 |
| Proximate (wt %) | Ultimate (wt %) | ||||||
|---|---|---|---|---|---|---|---|
| Moisture | Ash | Volatile | C | H | O | N | S |
| 16.83 | 13.57 | 48.65 | 68.84 | 5.12 | 22.73 | 1.51 | 1.32 |
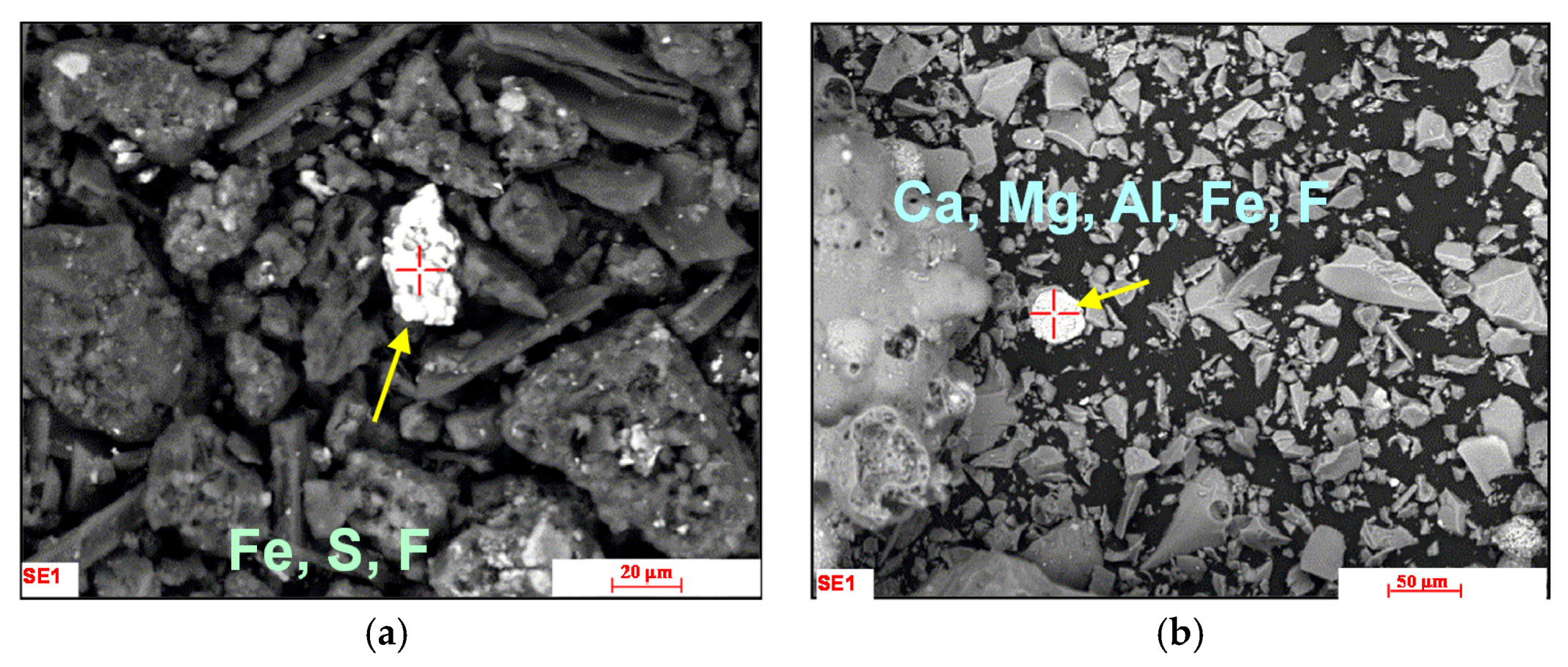

3.2. Occurrence Modesof Fluorine in LZ
3.3. Occurrence Modesof Fluorine in QH
3.4. Occurrence Modes of Fluorine in HHC and HHX
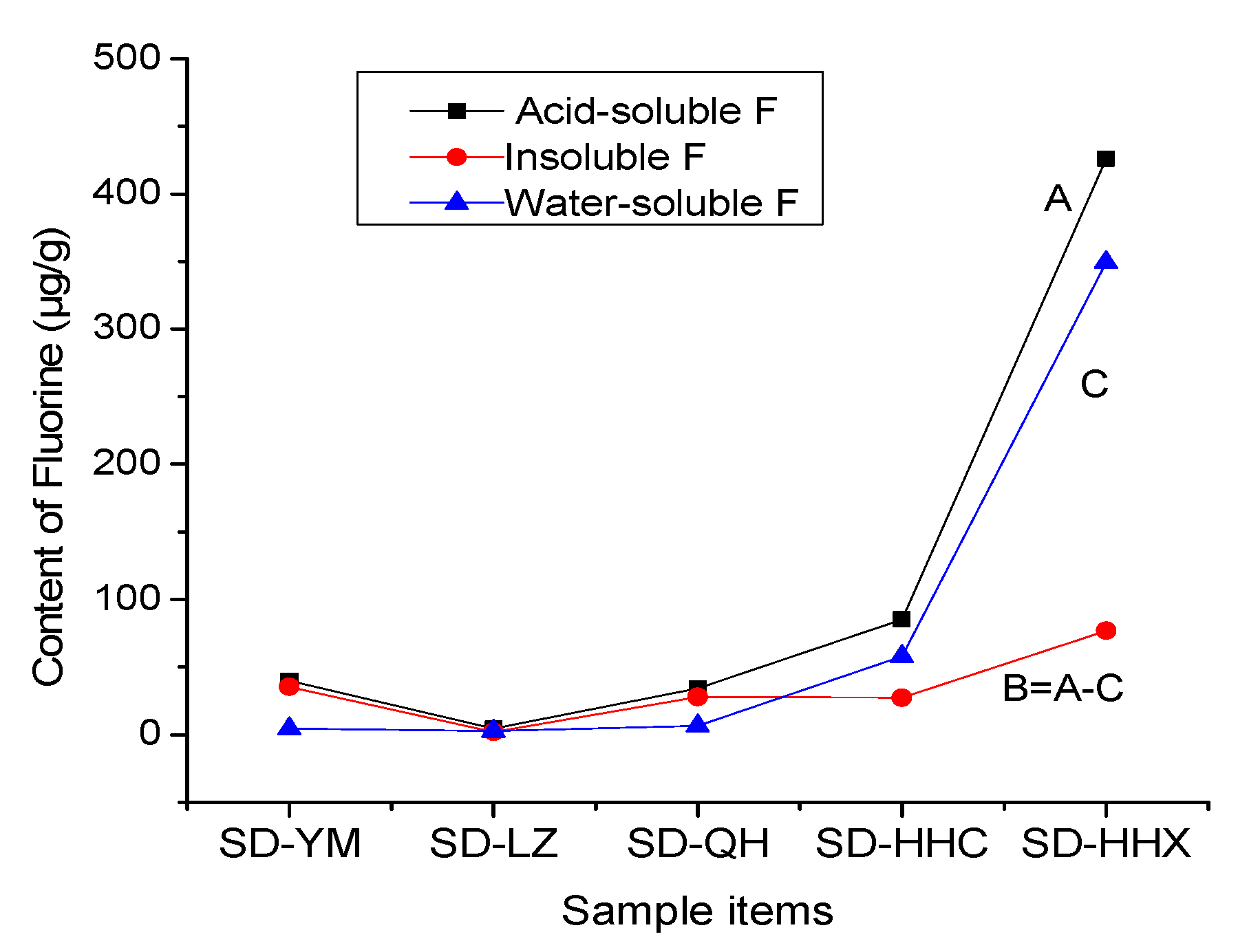
3.5. The Changes of Fluorine in the Power Plant
| Sample | YM | LZ | QH | HHC | HHX | SG | Gas |
|---|---|---|---|---|---|---|---|
| P (%) | 100 | 3.02 | 8.05 | 14.96 | 1.84 | 72.74 | 1.24 |
| S (ppm) | 169 | 81 | 54 | 275 | 815 | 2730 | - |

4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dai, S.; Ren, D.; Chou, C.L.; Finkelman, R.B.; Seredin, V.V.; Zhou, Y. Geochemistry of trace elements in chinese coals: A review of abundances, genetic types, impacts on human health, and industrial utilization. Int. J. Coal Geol. 2012, 94, 3–21. [Google Scholar] [CrossRef]
- Ketris, M.P.; Yudovich, Y.E. Estimations of Clarkes for Carbonaceous biolithes: World averages for trace element contents in black shales and coals. Int. J. Coal Geol. 2009, 78, 135–148. [Google Scholar] [CrossRef]
- Greta, E.; Dai, S.; Li, X. Fluorine in Bulgarian coals. Int. J. Coal Geol. 2013, 105, 16–23. [Google Scholar] [CrossRef]
- Martinez-Tarazona, M.R.; Suarez-Fernandez, G.P.; Cardin, J.M. Fluorine in Asturian coals. Fuel 1994, 73, 1209–1213. [Google Scholar] [CrossRef]
- Dai, S.; Li, D.; Chou, C.L.; Zhao, L.; Zhang, Y.; Ren, D.; Ma, Y.; Sun, Y. Mineralogy and geochemistry of boehmite-rich coals: New insights from the Haerwusu Surface Mine, Jungar Coalfield, Inner Mongolia, China. Int. J. Coal Geol. 2008, 74, 185–202. [Google Scholar] [CrossRef]
- Dai, S.; Jiang, Y.; Ward, C.R.; Gu, L.; Seredin, V.V.; Liu, H.; Zhou, D.; Wang, X.; Sun, Y.; Zou, J.; et al. Mineralogical and geochemical compositions of the coal in the Guanbanwusu Mine, Inner Mongolia, China: Further evidence for the existence of an Al (Ga and REE) ore deposit in the Jungar Coalfield. Int. J. Coal Geol. 2012, 98, 10–40. [Google Scholar] [CrossRef]
- Dai, S.; Zhang, W.; Seredin, V.V.; Ward, C.R.; Hower, J.C.; Wang, X.; Li, X.; Song, W.; Zhao, L.; Kang, H.; et al. Factors controlling geochemical and mineralogical compositions of coals preserved within marine carbonate successions: A case study from the Heshan Coalfield, southern China. Int. J. Coal Geol. 2013, 109–110, 77–100. [Google Scholar] [CrossRef]
- Dai, S.; Hower, J.C.; Ward, C.R.; Guo, W.; Song, H.; O’Keefe, J.M.K.; Xie, P.; Hood, M.M.; Yan, X. Elements and phosphorus minerals in the middle Jurassic inertinite-rich coals of the Muli Coalfield on the Tibetan Plateau. Int. J. Coal Geol. 2015, 144–145, 23–47. [Google Scholar] [CrossRef]
- Wang, X.; Dai, S.; Sun, Y.; Li, D.; Zhang, W.; Zhang, Y. Modes of occurrence of fluorine in the late paleozoic No. 6 coal from the Haerwusu surface mine, Inner Mongolia, China. Fuel 2011, 90, 248–254. [Google Scholar] [CrossRef]
- Liu, X.; Zheng, C.; Liu, J.; Zhang, J.Y.; Song, D.Y. Analysis on fluorine speciation in coals from Guizhou province. Chin. J. Electr. Eng. 2008, 28, 46–51. (In Chinese) [Google Scholar]
- Derher, G.B.; Finkelman, R.B. Selenium mobilization in a surface coal mine. Environ. Geol. Water Sci. 1992, 19, 155–167. [Google Scholar] [CrossRef]
- Cavender, P.F.; Spears, D.A. Analysis of forms of sulfur within coal, and minor and trace element associations with pyrite by ICP analysis of extraction solutions. Coal Sci. Technol. 1995, 24, 1653–1656. [Google Scholar]
- Spears, D.A. The determination of trace element distributions in coals using sequential chemical leaching—A new approach to an old method. Fuel 2013, 114, 31–37. [Google Scholar] [CrossRef]
- Liu, J.; Yang, Z.; Yan, X.; Ji, D.; Yang, Y.; Hu, L. Modes of occurrence of highly-elevated trace elements in superhigh-organic-sulfur coals. Fuel 2015, 156, 190–197. [Google Scholar] [CrossRef]
- Lessing, R. Fluorine in coal. Nature 1934, 134, 699–700. [Google Scholar] [CrossRef]
- Beijing Research Institute of Coal Chemistry. GB/T 4633-1997, Determination of Fluorine in Coal; Standards Press of China: Beijing, China, 1997. [Google Scholar]
- Qi, Q.J.; Liu, J.Z.; Wang, J.R.; Cao, X.Y.; Zhou, J.H.; Cen, K.F. Extracting experimental research on occurrence modes of fluorine in coal. J. Liaoning Tech. Univ. 2003, 22, 577–579. (In Chinese) [Google Scholar]
- Elrashidi, M.A.; Lindsay, W.L. Chemical equilibria of fluorine in soils. Soil Sci. 1986, 141, 274–280. [Google Scholar] [CrossRef]
- Larsen, S.; Widdowson, A.E. Soil fluorine. J. Soil Sci. 1971, 22, 210–221. [Google Scholar] [CrossRef]
- Elrashidi, M.A.; Lindsay, W.L. Solubility of aluminum fluoride, fluorite, and fluorophlogopite minerals in soils. Soil Sci. Soc. Am. J. 1986, 50, 594–598. [Google Scholar] [CrossRef]
- Koukouzas, N.; Ketikidis, C.; Itskos, G. Heavy metal characterization of CFB-derived coal fly ash. Fuel Process. Technol. 2011, 92, 441–446. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, G.; Luo, Z.; Zhang, J.; Zhao, Y. Modes of Occurrence of Fluorine by Extraction and SEM Method in a Coal-Fired Power Plant from Inner Mongolia, China. Minerals 2015, 5, 863-869. https://doi.org/10.3390/min5040530
Wang G, Luo Z, Zhang J, Zhao Y. Modes of Occurrence of Fluorine by Extraction and SEM Method in a Coal-Fired Power Plant from Inner Mongolia, China. Minerals. 2015; 5(4):863-869. https://doi.org/10.3390/min5040530
Chicago/Turabian StyleWang, Guangmeng, Zixue Luo, Junying Zhang, and Yongchun Zhao. 2015. "Modes of Occurrence of Fluorine by Extraction and SEM Method in a Coal-Fired Power Plant from Inner Mongolia, China" Minerals 5, no. 4: 863-869. https://doi.org/10.3390/min5040530
APA StyleWang, G., Luo, Z., Zhang, J., & Zhao, Y. (2015). Modes of Occurrence of Fluorine by Extraction and SEM Method in a Coal-Fired Power Plant from Inner Mongolia, China. Minerals, 5(4), 863-869. https://doi.org/10.3390/min5040530






