4.2. Petrology
The coal at the Parrott 7½’-quadrangle site is high volatile A bituminous, with a maximum vitrinite reflectance in the 0.8%–0.9% R
max range (
Table 5). This is generally higher than the rank of the previously studied samples (
Table 3). The current study location is further to the southeast and closer to the locus of the high-rank region on the northwest side of the Pine Mountain thrust fault [
29,
32]. Maceral assemblages are dominated by collotelinite, telinite and vitrodetrinite; fusinite and semifusinite with lesser amounts of micrinite and macrinite; and sporinite with lesser amounts of cutinite (
Table 5).
Table 3.
As-determined moisture, ash and forms of sulfur; mineral-free-basis maceral groups, and maximum vitrinite reflectance of Gray Hawk coal samples from early 1980s Kentucky Geological Survey and Center for Applied Energy Research collections.
Table 3.
As-determined moisture, ash and forms of sulfur; mineral-free-basis maceral groups, and maximum vitrinite reflectance of Gray Hawk coal samples from early 1980s Kentucky Geological Survey and Center for Applied Energy Research collections.
| Sample | Proximate Analysis (%) | Sulfur and Forms of Sulfur (%) | HV (MJ/kg) | Maceral (vol %) | Ro,max |
|---|
| M | Ash | St | Spy | Ssulf | Sorg | V | I | L |
|---|
| 5139 | 6.00 | 2.20 | 0.60 | 0.14 | 0.01 | 0.45 | 32.05 | 82.6 | 11.0 | 6.4 | 0.73 |
| 5028 | 4.69 | 5.79 | 3.98 | 2.07 | 0.01 | 1.90 | 30.77 | 73.1 | 17.9 | 9.0 | 0.73 |
| 5091 | 5.28 | 1.88 | 0.65 | 0.10 | 0.01 | 0.54 | 31.89 | 79.8 | 11.5 | 8.7 | 0.77 |
| 5023 | 4.11 | 3.44 | 0.60 | 0.08 | 0.00 | 0.52 | 30.87 | 81.1 | 11.9 | 7.0 | 0.72 |
| 5029 | 5.22 | 1.68 | 0.63 | 0.35 | 0.01 | 0.27 | 31.87 | 77.3 | 12.2 | 10.5 | 0.81 |
| 5048 | 6.15 | 1.99 | 0.55 | 0.23 | 0.00 | 0.32 | 31.66 | 79.5 | 13.2 | 7.3 | 0.78 |
| 5049 | 5.47 | 2.28 | 0.58 | 0.30 | 0.00 | 0.28 | 31.70 | 77.1 | 11.9 | 11.0 | 0.78 |
| 5050 | 5.61 | 2.74 | 1.09 | 0.30 | 0.01 | 0.78 | 31.35 | 70.4 | 21.7 | 7.9 | 0.75 |
| 5185 | 5.90 | 1.74 | 0.64 | 0.01 | 0.02 | 0.61 | 32.15 | 78.3 | 14.9 | 6.8 | 0.83 |
Table 4.
As-determined proximate and ultimate analyses; forms of sulfur, as-determined heating value of Gray Hawk benches. Unit for moisture, ash yield, volatile matter, fixed carbon, C, H, N, O, and sulfur content, is wt %. Unit for heating value is MJ/kg.
Table 4.
As-determined proximate and ultimate analyses; forms of sulfur, as-determined heating value of Gray Hawk benches. Unit for moisture, ash yield, volatile matter, fixed carbon, C, H, N, O, and sulfur content, is wt %. Unit for heating value is MJ/kg.
| Sample | Bench | Thick (cm) | M | Ash | VM | FC | C | H | N | O | St | Spy | Ssulf | Sorg | HV |
|---|
| 5508 | 1 of 3 (top) | 12.2 | 2.77 | 3.04 | 38.49 | 55.69 | 79.63 | 5.71 | 1.64 | 8.43 | 1.55 | 0.99 | 0.02 | 0.54 | 32.93 |
| 5509 | 2 of 3 | 11.8 | 3.25 | 1.42 | 39.03 | 56.29 | 80.46 | 5.76 | 1.66 | 9.98 | 0.72 | 0.10 | 0.01 | 0.61 | 33.41 |
| 5510 | 3 of 3 | 8.5 | 2.76 | 4.07 | 40.44 | 52.73 | 78.77 | 5.78 | 1.61 | 9.08 | 0.69 | 0.08 | 0.01 | 0.60 | 32.84 |
Table 5.
Petrological compositions and vitrinite maximum reflectance of samples 5508, 5509 and 5510 (%).
Table 5.
Petrological compositions and vitrinite maximum reflectance of samples 5508, 5509 and 5510 (%).
| Sample | T | CT | VD | CG | T-V | F | SF | Mic | Mac | ID | T-I | Sp | Cut | Res | LipD | T-L | Ro,max |
|---|
| 5508 | 12.6 | 42.9 | 6.7 | 0.8 | 63.0 | 14.0 | 8.1 | 2.6 | 1.4 | 0.4 | 26.5 | 7.7 | 2.6 | 0.0 | 0.2 | 10.5 | 0.89 |
| 5509 | 14.0 | 56.5 | 6.6 | 0.6 | 77.8 | 2.2 | 4.8 | 1.6 | 1.6 | 0.2 | 10.4 | 10.6 | 1.2 | 0.0 | 0.0 | 11.8 | 0.86 |
| 5510 | 12.8 | 38.5 | 12.2 | 3.2 | 66.7 | 9.8 | 5.0 | 1.2 | 0.8 | 0.2 | 17.0 | 14.2 | 1.0 | 0.4 | 0.6 | 16.2 | 0.82 |
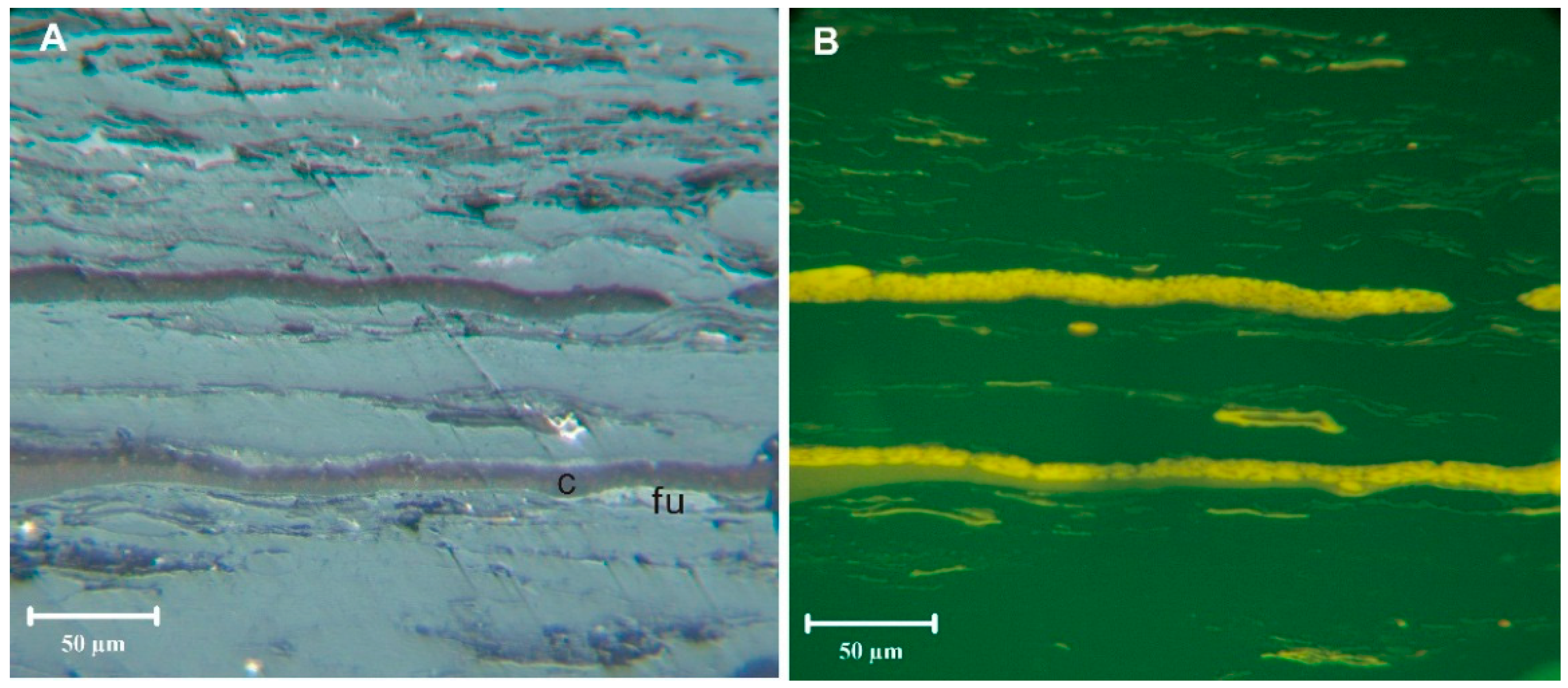
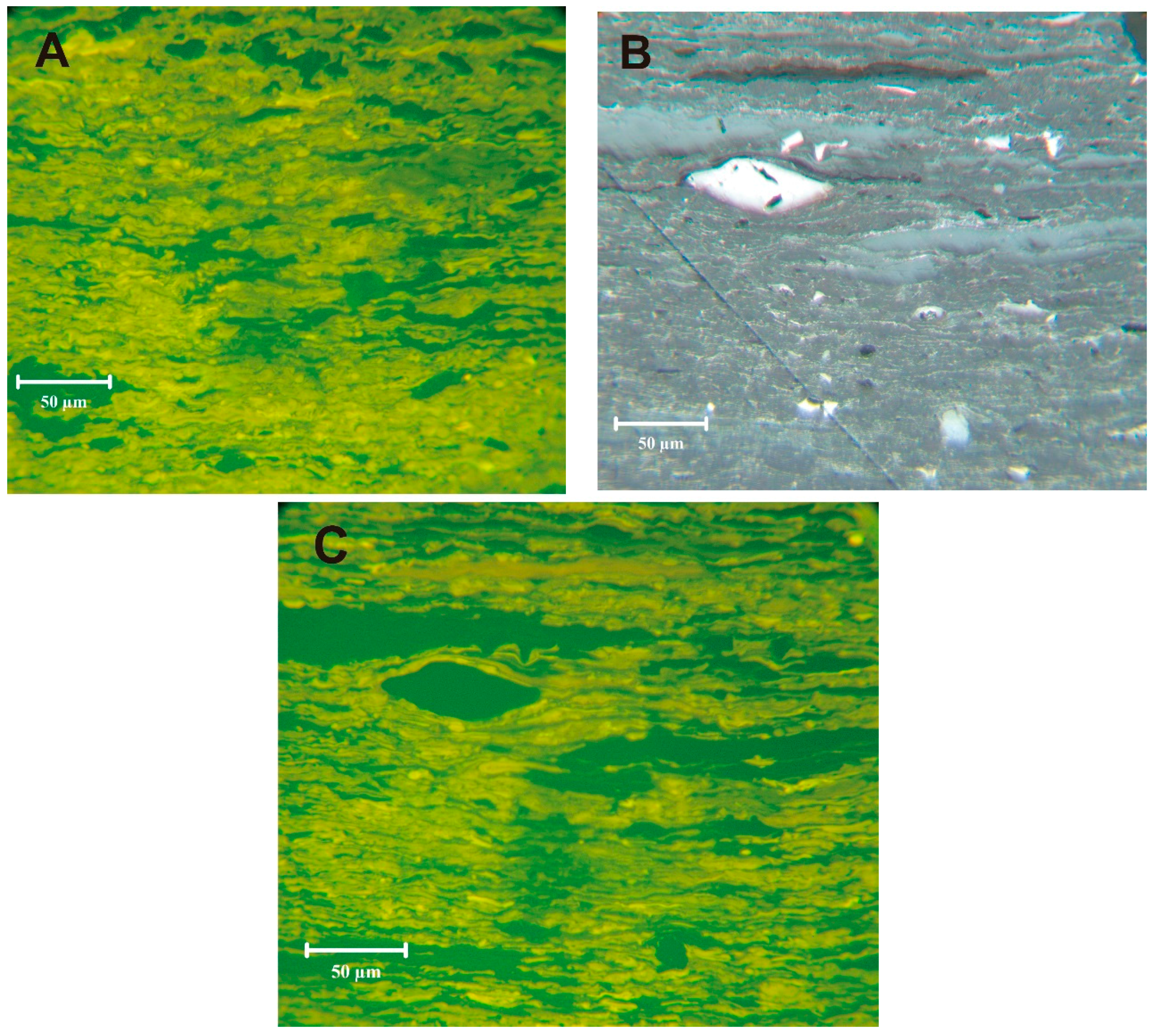
Cutinite was noted to occur in association with possible epiphyllous fungi (funginite) (
Figure 3). While such forms are not well known before the Cretaceous, Taylor
et al. [
33] and Burlington [
34] did note epiphyllous fungi in association with Stephanian pteridosperms.
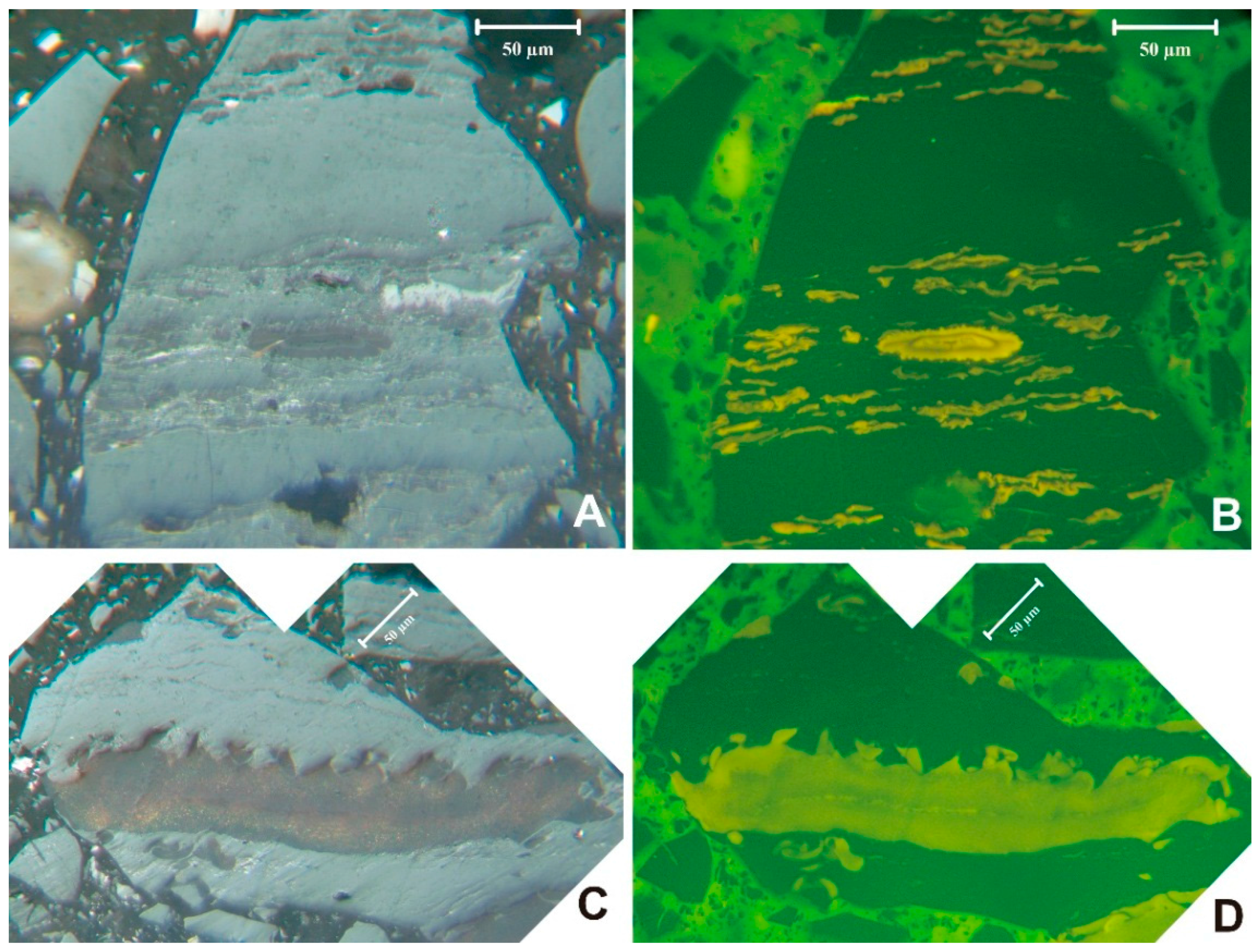
Verrucosporites, not observed in the palynomorph counts, was noted in the bottom bench during petrography studies (sample 5510) (
Figure 4A,B). The megaspore
Triletes globosus (
Figure 4C,D) would have been excluded from the miospore slides due to the screening step during processing. Thin vitrinertoliptite cannel bands were noted in the bottom bench (sample 5510) (
Figure 5). Without oriented block samples, we cannot establish the location of the cannel within the bench.
Figure 3.
Cutinite in sample 5510. Epiphyllous fungi (fu) growing on cutinite (c). (A) Reflected light, oil immersion; (B) Blue-light.
Figure 3.
Cutinite in sample 5510. Epiphyllous fungi (fu) growing on cutinite (c). (A) Reflected light, oil immersion; (B) Blue-light.
Figure 4.
Sporinite in sample 5510. (A) and (B) Verrucosporites (larger ornamented spore in lower center) with smaller Lycospora in mixed maceral matrix. (C) and (D) megaspore, probably Triletes globosus. (A) and (C) reflected light, oil immersion. (B) and (D) blue-light.
Figure 4.
Sporinite in sample 5510. (A) and (B) Verrucosporites (larger ornamented spore in lower center) with smaller Lycospora in mixed maceral matrix. (C) and (D) megaspore, probably Triletes globosus. (A) and (C) reflected light, oil immersion. (B) and (D) blue-light.
Figure 5.
Cannel in sample 5510. (A) cannel; (B) and (C) cannel with vitrinite bands and inertinite fragments; (A) and (C) blue-light; (B) reflected light, oil immersion.
Figure 5.
Cannel in sample 5510. (A) cannel; (B) and (C) cannel with vitrinite bands and inertinite fragments; (A) and (C) blue-light; (B) reflected light, oil immersion.
Fusinite and semifusinite occur as both primary, direct fire-derived varieties and forms which were degraded and then charred. The primary, fire-derived origin of fusinite has long been established [
35,
36], with more recent advocacy on the part of Scott and his co-workers, among others [
37,
38,
39,
40,
41,
42,
43,
44,
45,
46,
47,
48]. Certain inertinite macerals with a fusinite and semifusinite reflectance took a more convoluted path, undergoing varying degrees of degradation prior to charring [
49,
50], and likely charring at a range of temperatures [
47,
48,
51].
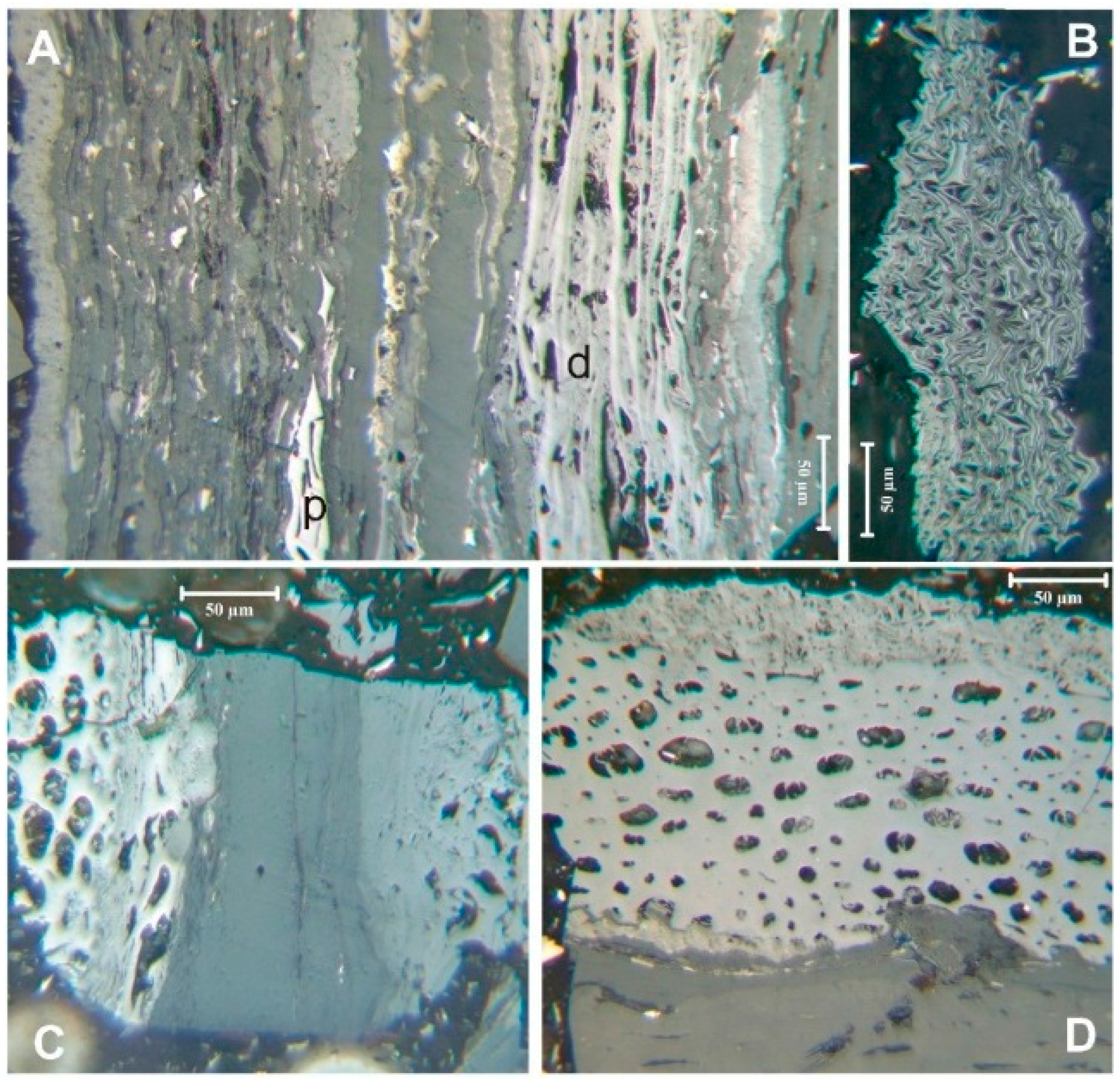
A variety of primary fusinite (such as in
Figure 6A,B) and fusinite- and semifusinite-levels of reflectances occur in inertinites subjected to charring after degradation (
Figure 6A,C,D). Both the fusinite- and semifusinite-reflectance macerals in
Figure 6C and, in particular, the semifusinite- reflectance maceral in
Figure 6D have swollen cell walls typically associated with fungal or bacterial degradation [
52,
53].
Macrinite, at least in part, is a product of the ingestion and excretion of woody material with possible (or likely) successive cycles of fungal and bacterial colonization and/or coprophagous recycling [
49,
50,
54,
55,
56,
57]. In part, the complexity of macrinite structure, particularly the inclusion of fusinite and liptinite within the macrinite, underwent significant changes from the Devonian [
58,
59,
60] to the Pennsylvanian [
4,
61,
62], the Cretaceous and later [
5,
49,
54,
63]. Much of this was due to the expansion and evolution of insects and other arthropods. Raymond
et al. [
64] note that, while the three extant digestive schemes employed by modern detritivores (external rumen, facultative mutualism and symbiosis) existed in the Pennsylvanian, the faunal participants were different. This difference is exemplified in the difficulty of assigning fossil arthropod coprolites to their producers [
58,
59,
65,
66,
67,
68]. Further, the rate of fungal, bacterial, and detritivore decomposition was slower in the Pennsylvanian compared to younger settings [
64]. In the latter context, the inclusions in the macrinite on the right side of
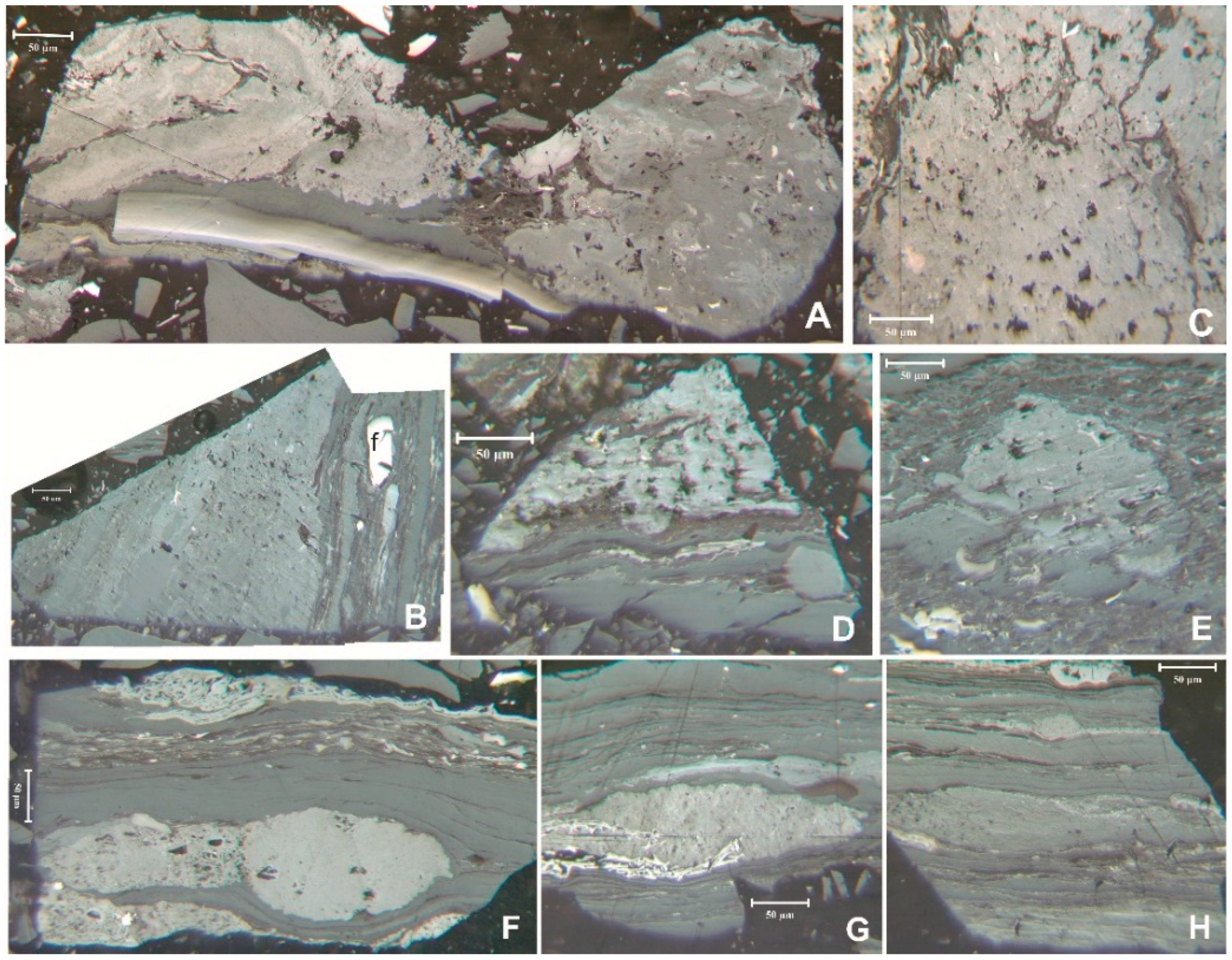
Figure 7A demonstrate that non-discriminating feeding activity was part of the Langsettian ecosystem. Much more common in Pennsylvanian coals is relatively inclusion-free macrinite (
Figure 7A,B,F,G and the macrinite across the center of
Figure 7H). Fecal pellet-derived macrinite (
Figure 7C–E,H), common in younger coals [
49], was previously noted in a survey of Pennsylvanian macrinite [
4]. This should not be a surprise; if something was ingesting the wood, excretion would be a necessary function. The Cretaceous forms are dominated by individual pellets while the Gray Hawk forms appear to be merged into larger masses, consistent with Type 1 coprolites as defined by Taylor and Scott [
62], albeit with some indication that the origin was from individual pellets. The compaction and merger may be a function of the moisture content of the original pellets, the vegetal composition of the diet and the subsequent fecal pellet, the wet
vs. dry nature of the depositional (and subsequent) environment, or a combination of all of the above plus coalification and other unaccounted for influences. Given the small size of the individual pellets forming the coprolite masses, collembola or perhaps an extinct group of herbivores or detritovores, may have produced them [
49].
Figure 6.
Fusinite and semifusinite in the coal. (A) mix of primary (p) and degraded (d) fusinite and semifusinite; (B) primary fusinite; (C) degraded fusinite and semifusinite; (D) degraded semifusinite. Reflected light, oil immersion.
Figure 6.
Fusinite and semifusinite in the coal. (A) mix of primary (p) and degraded (d) fusinite and semifusinite; (B) primary fusinite; (C) degraded fusinite and semifusinite; (D) degraded semifusinite. Reflected light, oil immersion.
Figure 7.
Macrinite in the coal. (
A) macrinite with inertodetrinite inclusions on right; macrinite on left may have replaced sporinite, as noted in the Hower
et al. [
4] publication of the image, or it may just have a shape coincident with sporinite; note that Hower and Ruppert [
55] showed an example of funginite replacing a megaspore in a Pennsylvanian coal; (
B) assemblage of macrinite (ma), semifusinite (sf), and fusinite (f); (
C) agglomerated rounded macrinite reminiscent of fecal pellet macrinite seen in younger coals [
49]; from Hower
et al. [
4]; (
D) agglomerated rounded macrinite reminiscent of fecal pellet macrinite; (
E) macrinite with inertodetrinite inclusions but also with agglomerated rounded macrinite reminiscent of fecal pellet macrinite; (
F) macrinite (ma) with semifusinite (sf); (
G) macrinite (ma) with fusinite; (
H) fecal pellet macrinite (fp), Reflected light, oil immersion; (A–E) sample 5510; (F) sample 5028; (G) and (H) sample 5023. Scale bar: 50 μm.
Figure 7.
Macrinite in the coal. (
A) macrinite with inertodetrinite inclusions on right; macrinite on left may have replaced sporinite, as noted in the Hower
et al. [
4] publication of the image, or it may just have a shape coincident with sporinite; note that Hower and Ruppert [
55] showed an example of funginite replacing a megaspore in a Pennsylvanian coal; (
B) assemblage of macrinite (ma), semifusinite (sf), and fusinite (f); (
C) agglomerated rounded macrinite reminiscent of fecal pellet macrinite seen in younger coals [
49]; from Hower
et al. [
4]; (
D) agglomerated rounded macrinite reminiscent of fecal pellet macrinite; (
E) macrinite with inertodetrinite inclusions but also with agglomerated rounded macrinite reminiscent of fecal pellet macrinite; (
F) macrinite (ma) with semifusinite (sf); (
G) macrinite (ma) with fusinite; (
H) fecal pellet macrinite (fp), Reflected light, oil immersion; (A–E) sample 5510; (F) sample 5028; (G) and (H) sample 5023. Scale bar: 50 μm.
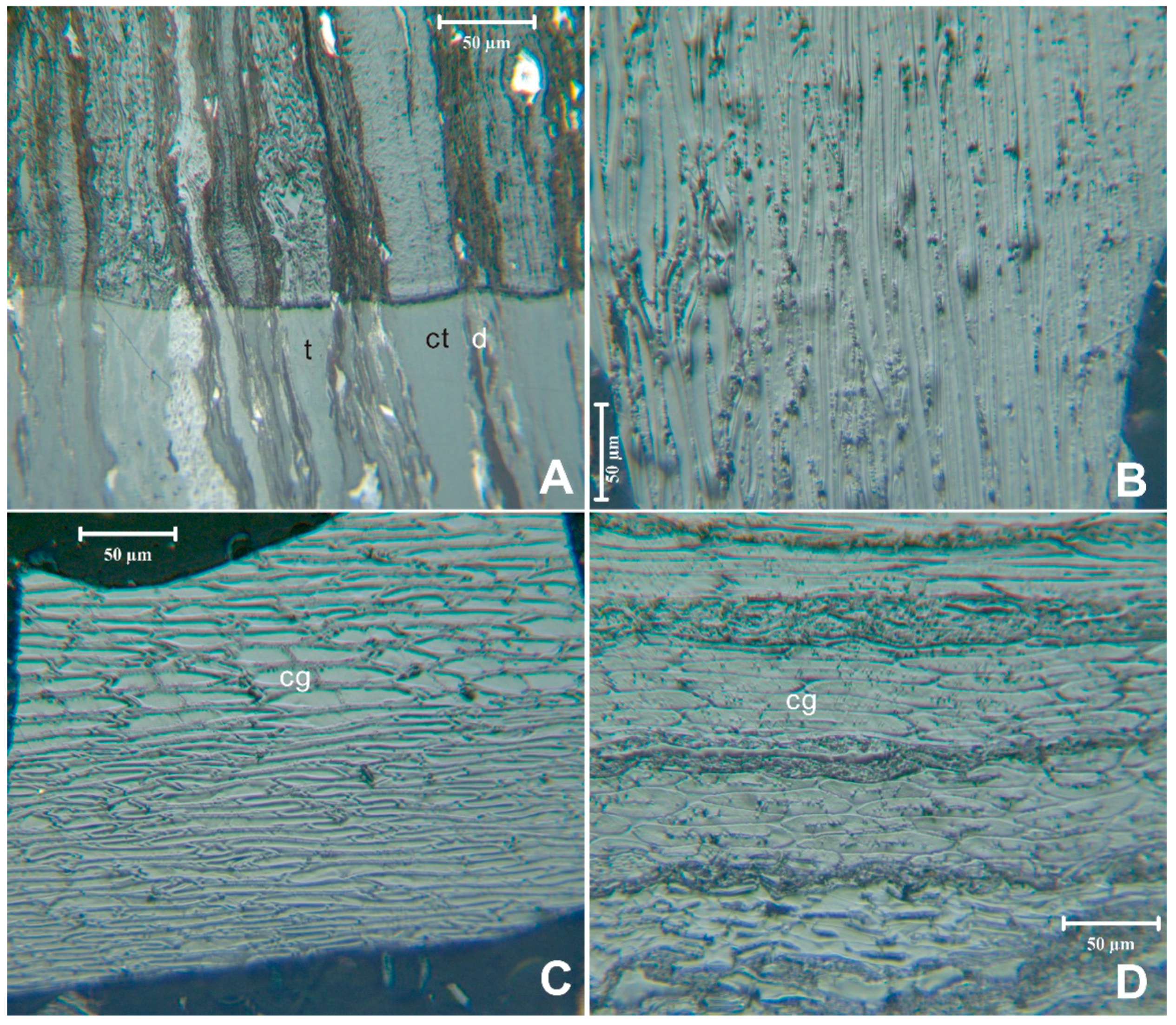
Vitrinite counts were made on the unetched pellets; the observations here are based on the details revealed in the etching process. Based on the etched pellets the telinite and collotelinite have superficial similarities in the unetched portion of
Figure 8A. Etching emphasizes the fundamental differences in maceral structure, with the telinite band shown to largely consist of cell walls and the collotelinite band showing a more uniform structure. Telinite is also seen in
Figure 8B and in the lower half of
Figure 8C. The upper half of the
Figure 8C vitrinite particle consists of corpogelinite cell fillings surrounded by telinite cell walls. Similarly, that in
Figure 8D has bands of corpogelinite with thin cell walls. The latter bands are separated by
ca. 10-μm bands, probably originating from detrovitrinite.
Petrographically, the relatively high vitrinite contents, especially telovitrinite which peaks in the middle bench, are likely a function of the same wet conditions that promoted arborescent lycopods, pteridospermalean and cordaitalean gymnosperms, as well as the relatively slower rates of decomposition present in the Pennsylvanian coal bed. A direct correspondence between high vitrinite contents and high percentages of arborescent lycopod spores, especially
Lycospora, is a common feature in many Early and Middle Pennsylvanian coal beds in the Appalachian and Eastern Interior, USA basins [
69,
70,
71,
72,
73,
74]. The decrease in fusinite + semifusinite from the bottom bench to the middle bench, followed by a large increase to the top bench, is a further indication of the cycle of relative wet and dry conditions in the Gray Hawk coal.
Figure 8.
Vitrinite in the coal. (A) etched vitrinite, inertinite, and liptinite showing contrast between telinite (t), collotelinite (ct), and detrovitrinite (d) bands; (B) etched telinite; (C) etched corpogelinite (cg) cell filling with telinite cell walls; (D) etched corpogelinite (cg) cell filling with telinite cell walls, Reflected light, oil immersion; (A) sample 5509; (B) sample 5510; (C) and (D) 5509.
Figure 8.
Vitrinite in the coal. (A) etched vitrinite, inertinite, and liptinite showing contrast between telinite (t), collotelinite (ct), and detrovitrinite (d) bands; (B) etched telinite; (C) etched corpogelinite (cg) cell filling with telinite cell walls; (D) etched corpogelinite (cg) cell filling with telinite cell walls, Reflected light, oil immersion; (A) sample 5509; (B) sample 5510; (C) and (D) 5509.
4.3. Palynology
Palynomorph data is reported as percentages of the total count in
Table 6, according to parent plant affinity. Palynomorph/parent plant affinities are based on excellent summaries by [
75,
76,
77]. Forms identified in addition to those observed in the point counts are marked with an X in
Table 6.
The occurrence of a few, stratigraphically constrained, spore taxa indicate a late Early Pennsylvanian (late Langsettian) age for the Gray Hawk coal bed. These taxa include
Schulzospora rara,
Radiizonates aligerans,
R. striatus,
Secarisporites remotus,
Endosporites globifomis,
Laevigatosporites spp. and
Granasporites medius [
74]
. The Gray Hawk coal palynoflora is strongly dominated by
Lycospora spp. (>85% in all three samples), which is typical of coal beds in this stratigraphic interval. Indeed, many, if not most, Early Pennsylvanian coal beds in the Appalachian Basin are dominated by
Lycospora spp., which is the dispersed spore genus of several of the large lycopsid trees (e.g.,
Lepidodendron,
Lepidophloios) that were important elements of Early Pennsylvanian mire floras [
71,
72,
73,
74,
78].
Lycospora pellucida and
L. pusilla are abundant in all three benches (
Table 6).
Lycospora granulate is relatively abundant in the bottom two benches, exceeding the concentration of
L. pusilla in the bottom bench, but undergoes a precipitous drop in concentration to the top bench, sample 5508. Small ferns, particularly represented by
Granulatisporites parvus, and calamites (
Calamospora breviradiata) in the bottom bench, are present in lesser abundance than the lycopsids. Cordaites, primarily
Florinites spp., occur in all three benches, but reach significant abundances in the bottom and middle benches. Common palynomorph taxa recovered from the Gray Hawk coal bed are shown in
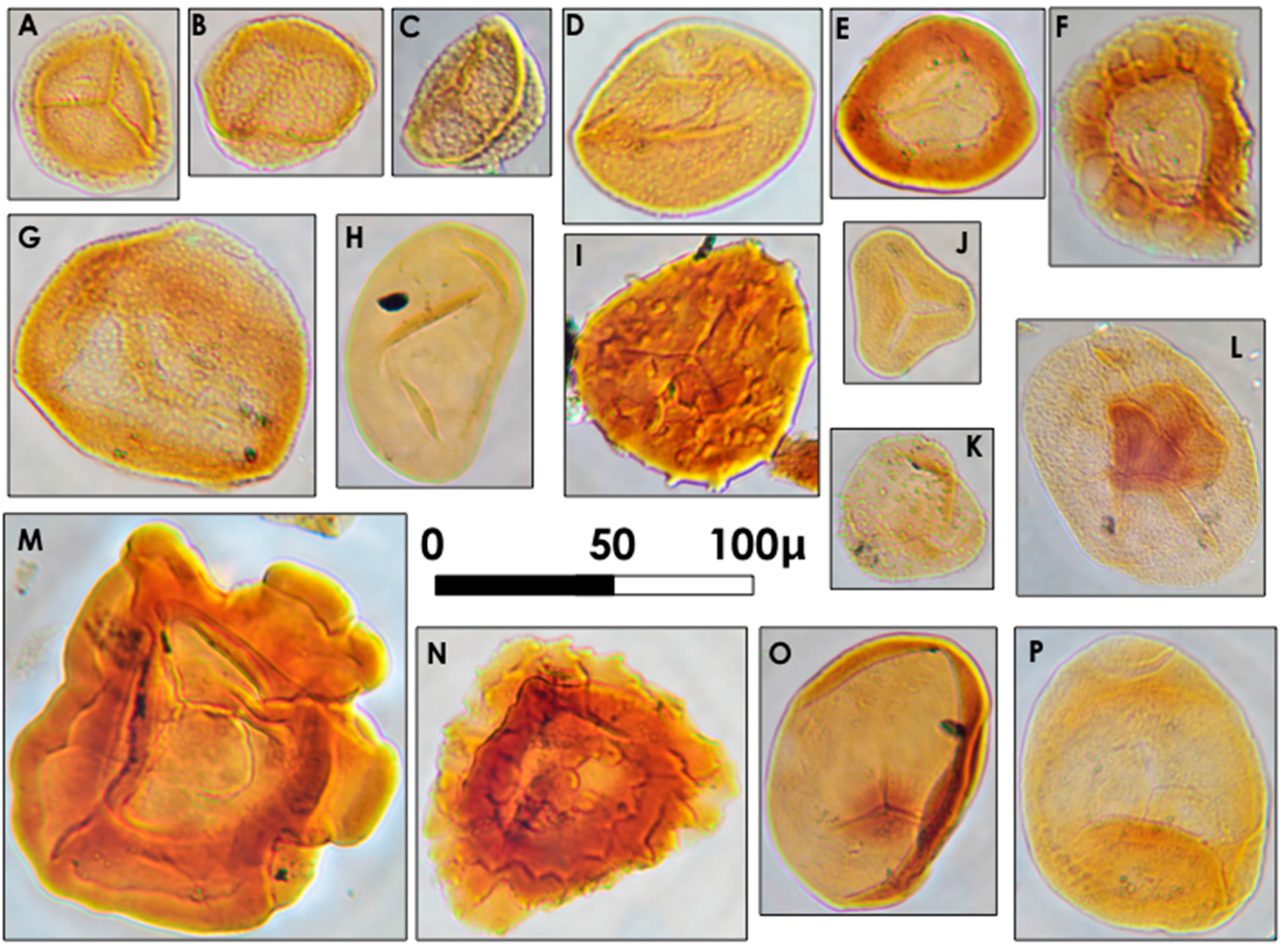
Figure 9.
Figure 9.
Common palynomorphs in the Gray Hawk coal bed. (A) Lycospora pellucida; (B) Lycospora pusilla; (C) Lycospora granulata; (D) Granasporites medius; (E) Densosporites annulatus; (F) Radiizonates aligerans; (G) Crassispora kosankei; (H) Laevigatosporites minor; (I) Camptotriletes bucculentus; (J) Granulatisporites adnatoides; (K) Pilosisporites triquetrus; (L) Florinites similis; (M) Reticulatisporites reticulatus; (N) Cristatisporites connexus; (O) Calamospora breviradiata; (P) Schulzospora rara. All images were collected using Nomarski interference contrast illumination (DIC).
Figure 9.
Common palynomorphs in the Gray Hawk coal bed. (A) Lycospora pellucida; (B) Lycospora pusilla; (C) Lycospora granulata; (D) Granasporites medius; (E) Densosporites annulatus; (F) Radiizonates aligerans; (G) Crassispora kosankei; (H) Laevigatosporites minor; (I) Camptotriletes bucculentus; (J) Granulatisporites adnatoides; (K) Pilosisporites triquetrus; (L) Florinites similis; (M) Reticulatisporites reticulatus; (N) Cristatisporites connexus; (O) Calamospora breviradiata; (P) Schulzospora rara. All images were collected using Nomarski interference contrast illumination (DIC).
Table 6.
Palynology of Gray Hawk benches. Note: X indicates that the palynomorph was observed to occur in trace amounts.
Table 6.
Palynology of Gray Hawk benches. Note: X indicates that the palynomorph was observed to occur in trace amounts.
| Sample | 5508 | 5509 | 5510 | Sample | 5508 | 5509 | 5510 | Sample | 5508 | 5509 | 5510 |
|---|
| Lycospora pellucida | 46.8 | 37.2 | 41.2 | Granulatisporites parvus | 5.6 | 2.4 | 2.4 | Schulzospora rara | | 1.6 | |
| L. pusilla | 32.8 | 30.4 | 22.4 | G. adnatoides | X | X | X | Total Seed Ferns | 0.0 | 1.6 | 0.0 |
| L. granulata | 3.2 | 22.0 | 25.6 | G. piroformis | X | X | X | | | | |
| L. orbicula | X | X | X | G. granulatus | X | | | Calamospora breviradiata | 0.8 | X | 0.4 |
| L. micropapillata | 3.2 | 0.4 | 3.2 | Cyclogranisporites microgranus | X | | | C. microrugosa | X | X | |
| L. rotunda | X | X | X | C. minutus | | X | | C. pallida | X | | X |
| L. torquifer | X | X | | Lophotriletes microsaetosus | 0.8 | 0.4 | | C. parva | | X | |
| Granasporites medius | 3.2 | 3.2 | X | L. commissuralis | X | X | X | Laevigatosporites minor | 0.4 | 0.8 | 2.0 |
| Crassispora kosankei | X | X | X | L. granoornatus | | X | | L. vulgaris | | | 0.4 |
| Total Lycopsid Trees | 89.2 | 93.2 | 92.4 | Leiotriletes subadnatoides | 1.2 | X | X | Reticulatisporites reticulatus | | X | 0.4 |
| | | | | L. adnatus | X | X | | R. muricatus | X | X | |
| Densosporites annulatus | X | X | 0.4 | L. pridyii | X | | | Total Calamites | 1.2 | 0.8 | 3.2 |
| D. triangularis | X | | | Pilosisporites triquetrus | X | X | | | | | |
| D. sphaerotriangularis | 0.8 | 0.4 | X | P. aculeolatus | X | | X | Florinites florini | 0.8 | 0.4 | X |
| Cristatisporites indignabumndus | X | X | X | Savitrisporites majus | X | X | | F. mediapudens | X | X | |
| C. connexus | X | X | | Camptotriletes bucculentus | X | X | X | F. similis | X | | |
| Cingulizonates loricatus | X | X | | C. corrugatus | X | X | | F. milotti | | | X |
| Radiizonates aligerans | X | X | X | Verrucosisporites verrucosus | X | X | X | Total Cordaites | 0.8 | 0.4 | 0.0 |
| R. striatus | | X | | V. donarii | X | X | | | | | |
| Cirratriradites saturni | X | | X | Convolutispora florida | X | X | | | | | |
| Endosporites globiformis | X | X | X | Reticulitriletes reticulocingulum | | X | | Potonieisporites elegans | | | X |
| E. ornatus | | X | | R. falsus | X | | | Total Conifers | 0.0 | 0.0 | 0.0 |
| Spencerisporites radiatus | X | X | X | Microreticulatisporites concavus | | 0.8 | 0.4 | | | | |
| Total Small Lycopsids | 0.8 | 0.4 | 0.4 | Raistrickia abdita | X | X | X | Ahrensisporites guerickei | X | | |
| | | | | R. saetosa | | | X | Secarisporites remotus | X | X | |
| Punctatisporites minutus | 0.4 | X | 1.2 | Apiculatisporis variocorneus | X | X | X | Reinschospora magnifica | X | | |
| Laevigatosporites minimus | | X | | Apiculatasporites spinulistratus | X | X | | Dictyotriletes bireticulatus | X | X | X |
| Total Tree Ferns | 0.4 | 0.0 | 1.2 | Punctatisporites punctatus | X | | | Grumosisporites varireticulatus | X | X | |
| | | | | P. aerarius | X | X | | Total Unknown Affinity | 0.0 | 0.0 | 0.0 |
| | | | | Total Small Ferns | 7.6 | 3.6 | 2.8 | | | | |
Abundant
Lycospora spp. spores indicate that arborescent lycopsids, notably
Lepidophloios and
Lepidodendron, were contributors to the peat that formed the Gray Hawk coal bed. The proportion of
Granasporites medius in the samples indicates that
Diaphorodendron and/or
Synchysidendron, was also present, though likely in proportionally fewer numbers than
Lepidophloios or
Lepidodendron. Mire-centered lycopsids had developed reproductive and vegetative strategies for growth and development in very wet areas. The megasporangium of
Lepidophloios (
Lepidocarpon) and
Lepidodendron (
Achlamydocarpon) were both boat-shaped, facilitating dispersal in areas of standing water. Lycopsid root systems (
Stigmaria) radiated laterally from the base of the plant, rather than downward, to provide stability. This is a common feature of many modern tree-size plants growing in wet, soft substrates. Furthermore, the rootlets emanating from
Stigmarian axes were very slender in design, and would have been incapacitated by firm substrates [
78].
Other plant groups, ferns, seed ferns, calamites and cordaites, are represented by minor quantities of palynomorphs in the Gray Hawk coal bed. The same supersaturated substrates that would have favored the proliferation of arborescent lycopods may have also favored pteridospermatophytean and cordaitalean gymnospermous trees [
79]. Both taxa produced fewer palynomorphs than the lycopsid taxa, and may have been, at least to a degree, arthropod pollenated [
68]. The presence of coprolitic macrinite in the samples supports the supposition that saprophytic and/or grazing arthropods occurred in the mire ecosystem; these and others may have served as pollinators for the early gymnosperms.
4.4. Geochemistry
Table 7 lists the percentages of major-element oxides and concentrations of trace elements in the newly-collected samples (5508, 5509 and 5510). Due to the low ash yields, the major-element oxide contents are very low relative to those in coals of other areas (e.g., Dai
et al. [
80]). Iron content is highest in the upper bench (48.24 wt % Fe
2O
3) falling to 5.70 wt % in the lowermost bench with concomitant increases in silica, alumina and titanium. Calcium concentration is highest in the middle bench.
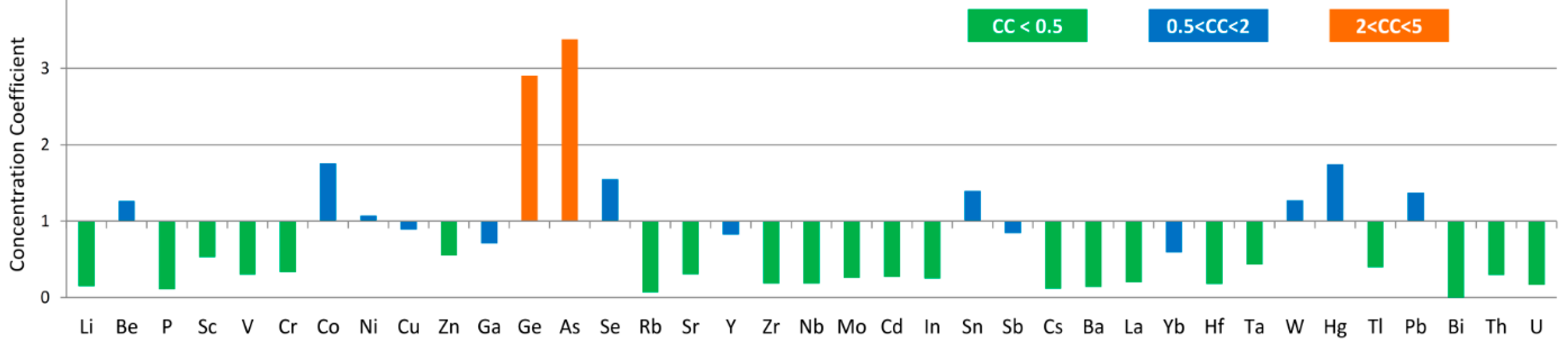
Compared to average values for world hard coals reported by Ketris and Yudovich [
81], Ge and As are slightly enriched in the coal with concentration coefficients between 2-5 (CC, ratio of trace element concentration in studied samples
vs. averages for world hard coals); however, the concentrations of other trace elements are either close to or much lower than world averages (
Figure 10).
With the exception of As (28.04 μg/g), most elements of environmental concern in the coal are low in concentrations, such as Be (2.53 μg/g on average for the three benches), Cr (5.65 μg/g), As (28.04 μg/g), Se (2.02 μg/g), Cd (0.05 μg/g), Mo (0.54 μg/g), Hg (174 ng/g), Tl (0.23 μg/g), Pb (12.34 μg/g) and U (0.31 μg/g). Arsenic and Hg are also enriched in the top bench (sample 5508). This is also the bench with the highest pyritic sulfur content (
Table 1), and, as discussed more fully below, the highest proportion of pyrite both within the LTA and the whole-coal material. Such an occurrence suggests an association with the sulfide minerals, in accordance with the observations of many other authors [
82,
83,
84,
85,
86,
87,
88,
89,
90].
A number of elements (Li, Be, Sc, V, Cr, Co, Ni, Cu, Zn, Ga, Ge, Se, Zr, Nb, Th, U and REY) are relatively enriched in the lower bench. Enrichment of Zr + TiO
2 in the basal lithotype of eastern Kentucky coals has been noted in other studies, for example Hower and Bland [
8]. Considering the known association of U and Th with zircons, more abundant zircon in the basal bench may explain the greater abundance of U and Th in the coal. The lowermost bench (sample 5510) has the highest ash yield (
Table 4) and also (as described below) the highest proportion of illite and illite/smectite among the clay minerals. Vanadium and Cr are known to be associated with clay minerals [
91,
92], and in some cases with organic matter [
93]. These two elements in the present study are thus probably associated with the more abundant clay minerals, especially illite and illite/smectite, in the lower bench sample.
Germanium, enriched in both the top and, in particular, the bottom bench, has been found to be enriched in the same position in many other coals [
94,
95]. The element is generally associated with organic matter [
96], and its enrichment is usually attributed to the leaching of granite by hydrothermal solutions and the Ge-rich solutions discharged into the peat swamp [
80,
96]. Copper and Zn are associated with traces of chalcopyrite and sphalerite, respectively, as described more fully below.
Figure 10.
Concentration coefficients (CC) of trace elements in the Gray Hawk coals, normalized by average trace element concentrations in world hard coals [
81].
Figure 10.
Concentration coefficients (CC) of trace elements in the Gray Hawk coals, normalized by average trace element concentrations in world hard coals [
81].
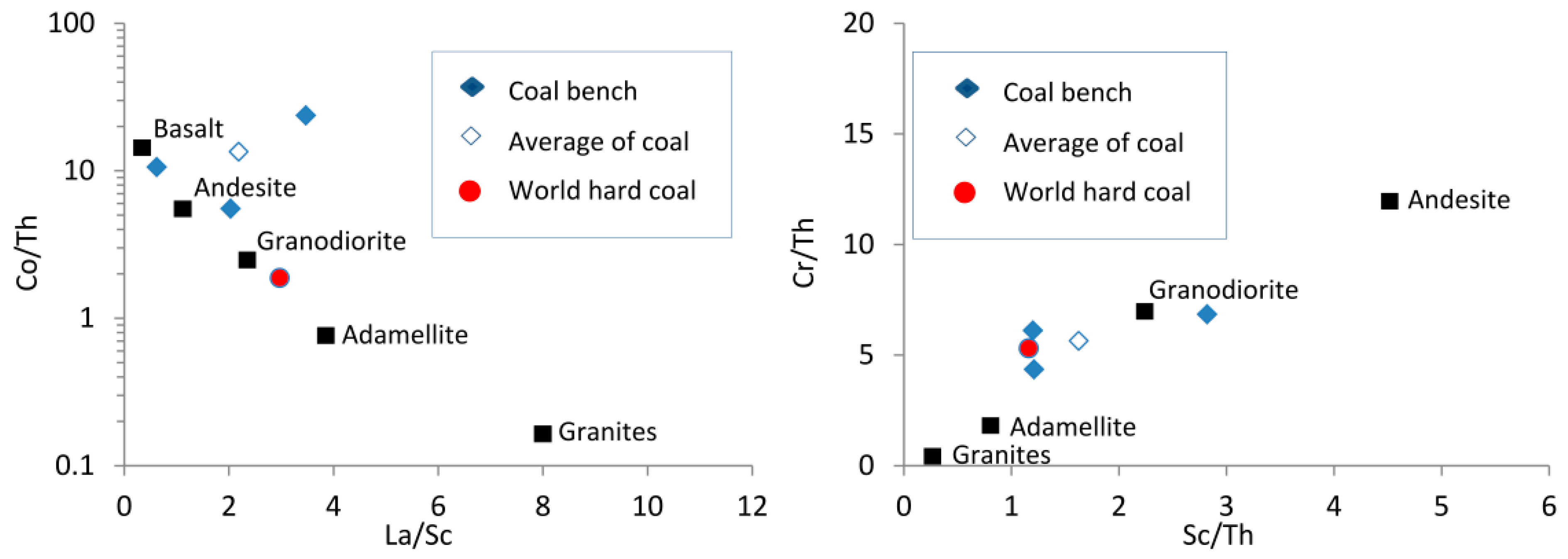
The Al
2O
3/TiO
2 ratio is a useful provenance indicator for sedimentary rocks [
97] and sediments associated with coal deposits [
63]. Typical Al
2O
3/TiO
2 ratios are 3-8, 8-21, and 21-70 for sediments derived from mafic, intermediate, and felsic igneous rocks, respectively [
97]. The three benches of the Gray Hawk coals have Al
2O
3/TiO
2 ratios of 16.8 (sample 5508), 22.1 (sample 5509), and 17.7 (sample 5510), indicating a sediment-source region with intermediate and felsic compositions; this is also supported by the relationship of Cr/Th-Sc/Th (
Figure 11). However, the relationship of Co/Th
vs. La/Sc plot (
Figure 11) indicates a minor input of intermediate-mafic materials.
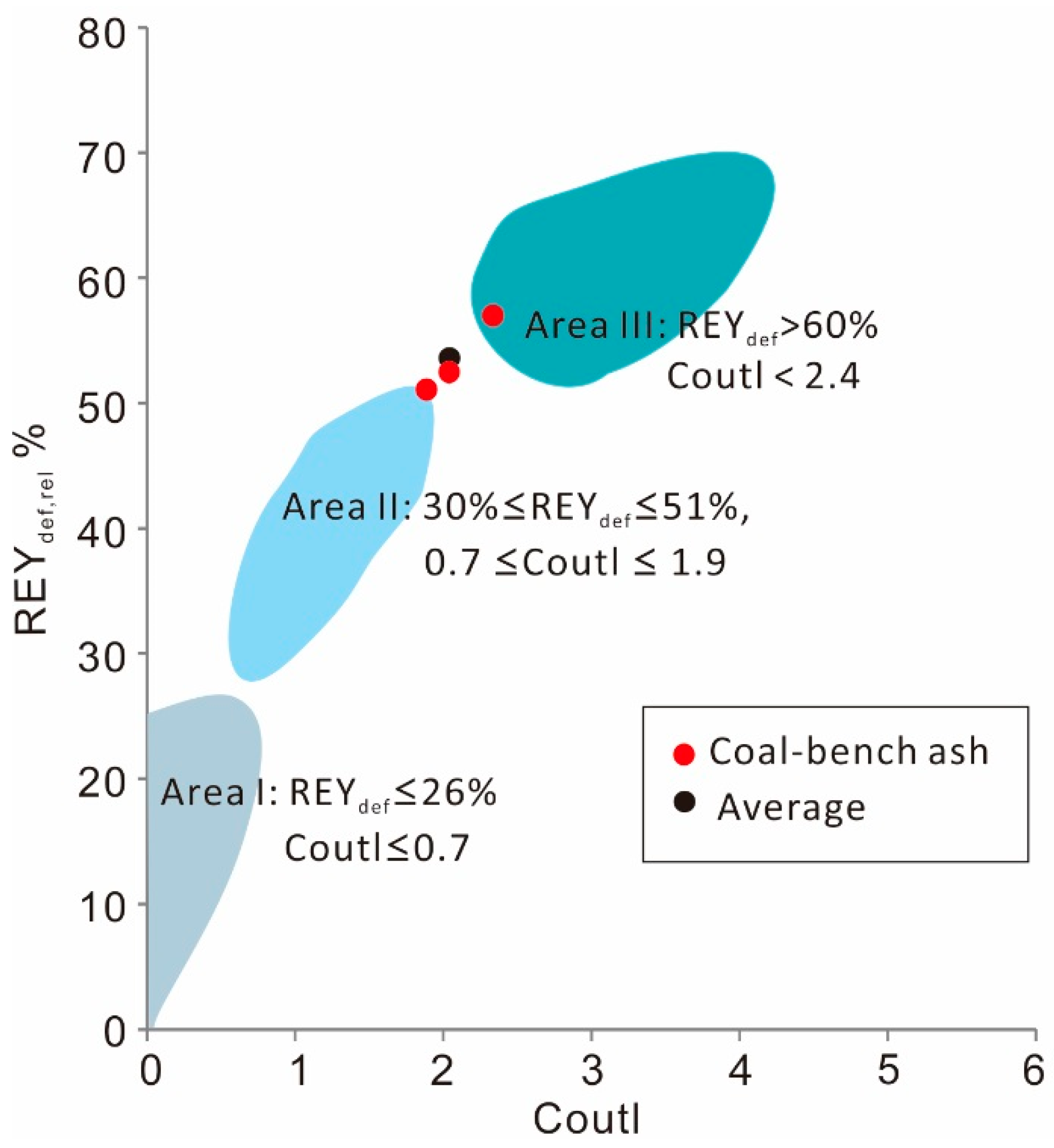
Although the rare metals (Ga; rare earth elements and yttrium, REY or REE if Y is not included) in the coal samples are relatively low in concentration compared to world averages, they are, as along with Ge, highly enriched in the coal ashes (
Table 8). Based on the cut-off grade (higher than 1000 μg/g) for beneficial recovery of REY from coal combustion products as proposed by Seredin and Dai [
98], the Gray Hawk coals have great potential for industrial use. The REY
def,
rel-Coutl graph, which was proposed by Seredin and Dai [
98] to evaluate of REY-rich ashes as raw materials (
Figure 12), where the X-axis is the outlook coefficient (Coutl, ratio of the relative amount of critical REY metals in the REY sum to the relative amount of excessive REY) and the Y-axis is the percentage of critical elements in total REY (REY
def,
rel, %), shows that the REY in the three coal bench samples (ash basis) fall in Area III or between Areas II (promising) and III (highly promising).
Table 7.
Concentrations of major-element oxides and trace elements in the samples 5508, 5509 and 5510, as well as their comparison with averages for world hard coals (Unit for loss-on-ignition and major-element oxides is %; Unit for trace elements is μg/g and for Hg is ng/g).
Table 7.
Concentrations of major-element oxides and trace elements in the samples 5508, 5509 and 5510, as well as their comparison with averages for world hard coals (Unit for loss-on-ignition and major-element oxides is %; Unit for trace elements is μg/g and for Hg is ng/g).
| Sample | SiO2 | TiO2 | Al2O3 | Fe2O3 | MgO | CaO | Na2O | K2O | P2O5 | SO3 | LOI | Li | Be | Cl | Sc | V | Cr | Co | Ni | Cu |
|---|
| 5508 | 27.97 | 0.92 | 15.4 | 47.64 | 0.54 | 2.91 | 0.37 | 0.98 | 0.23 | 2.08 | 96.96 | 1.39 | 1.83 | 2608 | 0.92 | 4.61 | 3.31 | 4.22 | 9.56 | 6.44 |
| 5509 | 36.16 | 1.35 | 29.85 | 18.18 | 0.88 | 6.43 | 1.08 | 0.78 | 0.33 | 3.91 | 98.58 | 1.22 | 1.76 | 3571 | 0.49 | 3.63 | 2.51 | 9.73 | 9.32 | 11.18 |
| 5510 | 52.98 | 1.74 | 30.9 | 5.7 | 0.78 | 2.36 | 0.88 | 2.41 | 0.18 | 1.31 | 95.93 | 4.35 | 4.60 | 2743 | 5.49 | 20.54 | 13.36 | 20.71 | 43.1 | 29.74 |
| Average | 37.48 | 1.29 | 24.70 | 25.97 | 0.73 | 4.04 | 0.76 | 1.28 | 0.25 | 2.54 | 97.16 | 2.1 | 2.53 | 2993 | 1.96 | 8.42 | 5.65 | 10.53 | 18.24 | 14.25 |
| World | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 14 | 2 | 340 | 3.7 | 28 | 17 | 6 | 17 | 16 |
| Sample | Zn | Ga | Ge | As | Se | Rb | Sr | Y | Zr | Nb | Mo | Cd | In | Sn | Sb | Cs | Ba | La | Ce | Pr |
| 5508 | 7.66 | 3.12 | 1.72 | 71.11 | 2.12 | 0.83 | 30.47 | 4.9 | 4.81 | 0.47 | 0.8 | 0.03 | 0.01 | 1.21 | 0.38 | 0.09 | 24.69 | 1.87 | 3.66 | 0.54 |
| 5509 | 8.08 | 1.98 | 0.24 | 1.93 | 1.07 | bdl | 28.45 | 4.62 | 3.83 | 0.3 | 0.5 | 0.01 | 0.01 | 3.42 | 0.06 | 0.02 | 14.46 | 1.7 | 4.17 | 0.62 |
| 5510 | 36.73 | 9.03 | 23.83 | 2.48 | 3.18 | 3.44 | 33.44 | 13.03 | 13.02 | 1.72 | 0.24 | 0.15 | 0.01 | 0.99 | 2.6 | 0.33 | 25.28 | 3.41 | 7.21 | 1.04 |
| Average | 15.42 | 4.25 | 6.97 | 28.04 | 2.02 | 1.21 | 30.51 | 6.92 | 6.6 | 0.74 | 0.54 | 0.05 | 0.01 | 1.95 | 0.84 | 0.13 | 21.13 | 2.21 | 4.77 | 0.7 |
| World | 28 | 6 | 2.4 | 8.3 | 1.3 | 18 | 100 | 8.4 | 36 | 4 | 2.1 | 0.2 | 0.04 | 1.4 | 1 | 1.1 | 150 | 11 | 23 | 3.4 |
| Sample | Nd | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | Hf | Ta | W | Hg | Tl | Pb | Bi | Th | U |
| 5508 | 2.39 | 0.56 | 0.14 | 0.64 | 0.12 | 0.68 | 0.15 | 0.47 | 0.06 | 0.35 | 0.06 | 0.15 | 0.06 | 1.68 | 425 | 0.61 | 15.67 | bdl | 0.76 | 0.16 |
| 5509 | 2.86 | 0.7 | 0.15 | 0.75 | 0.11 | 0.65 | 0.12 | 0.37 | 0.04 | 0.27 | 0.03 | 0.1 | bdl | 1.3 | 22 | bdl | 3.91 | bdl | 0.41 | 0.11 |
| 5510 | 4.74 | 1.25 | 0.32 | 1.54 | 0.31 | 2.23 | 0.46 | 1.47 | 0.19 | 1.39 | 0.18 | 0.46 | 0.41 | 0.6 | 26 | bdl | 19.28 | bdl | 1.95 | 0.82 |
| Average | 3.18 | 0.79 | 0.19 | 0.92 | 0.17 | 1.07 | 0.22 | 0.7 | 0.09 | 0.59 | 0.08 | 0.21 | 0.13 | 1.26 | 174 | 0.23 | 12.34 | 0 | 0.94 | 0.31 |
| World | 12 | 2.2 | 0.43 | 2.7 | 0.31 | 2.1 | 0.57 | 1 | 0.3 | 1 | 0.2 | 1.2 | 0.3 | 0.99 | 100 | 0.58 | 9 | 1.1 | 3.2 | 1.9 |
Table 8.
Rare metals (Ga, Ge and REY) in the ash of Gray Hawk coal (μg/g).
Table 8.
Rare metals (Ga, Ge and REY) in the ash of Gray Hawk coal (μg/g).
| Sample | Ga | Ge | La | Ce | Pr | Nd | Sm | Eu | Gd | Tb | Dy | Y | Ho | Er | Tm | Yb | Lu | REY | REO |
|---|
| 5508 | 99.8 | 54.9 | 59.6 | 117 | 17.2 | 76.3 | 17.8 | 4.31 | 20.4 | 3.77 | 21.9 | 157 | 4.89 | 15.0 | 1.88 | 11.1 | 1.76 | 529 | 635 |
| 5509 | 135 | 16.1 | 116 | 284 | 42.1 | 195 | 47.4 | 10.1 | 51.2 | 7.45 | 44.0 | 315 | 8.33 | 24.9 | 2.64 | 18.6 | 2.37 | 1168 | 1402 |
| 5510 | 215 | 569 | 81.4 | 172.11 | 24.9 | 113 | 29.7 | 7.70 | 36.8 | 7.34 | 53.2 | 311 | 11.0 | 35.1 | 4.50 | 33.2 | 4.26 | 925 | 1110 |
| Average | 143 | 175 | 85.77 | 192 | 28.26 | 129 | 31.7 | 7.30 | 35.9 | 6.04 | 38.1 | 254 | 7.75 | 23.8 | 2.84 | 19.6 | 2.64 | 865 | 1038 |
Figure 11.
Relationship of Co/Th against La/Sc [
99] and Cr/Th against Sc/Th [
100] for the Gray Hawk coal samples. Data of different igneous rocks are from Condie [
99].
Figure 11.
Relationship of Co/Th against La/Sc [
99] and Cr/Th against Sc/Th [
100] for the Gray Hawk coal samples. Data of different igneous rocks are from Condie [
99].
Figure 12.
The REYdef, rel-Coutl plot for Gray Hawk coal ashes. Area I, unpromising, Area II, promising, and Area III, highly promising.
Figure 12.
The REYdef, rel-Coutl plot for Gray Hawk coal ashes. Area I, unpromising, Area II, promising, and Area III, highly promising.
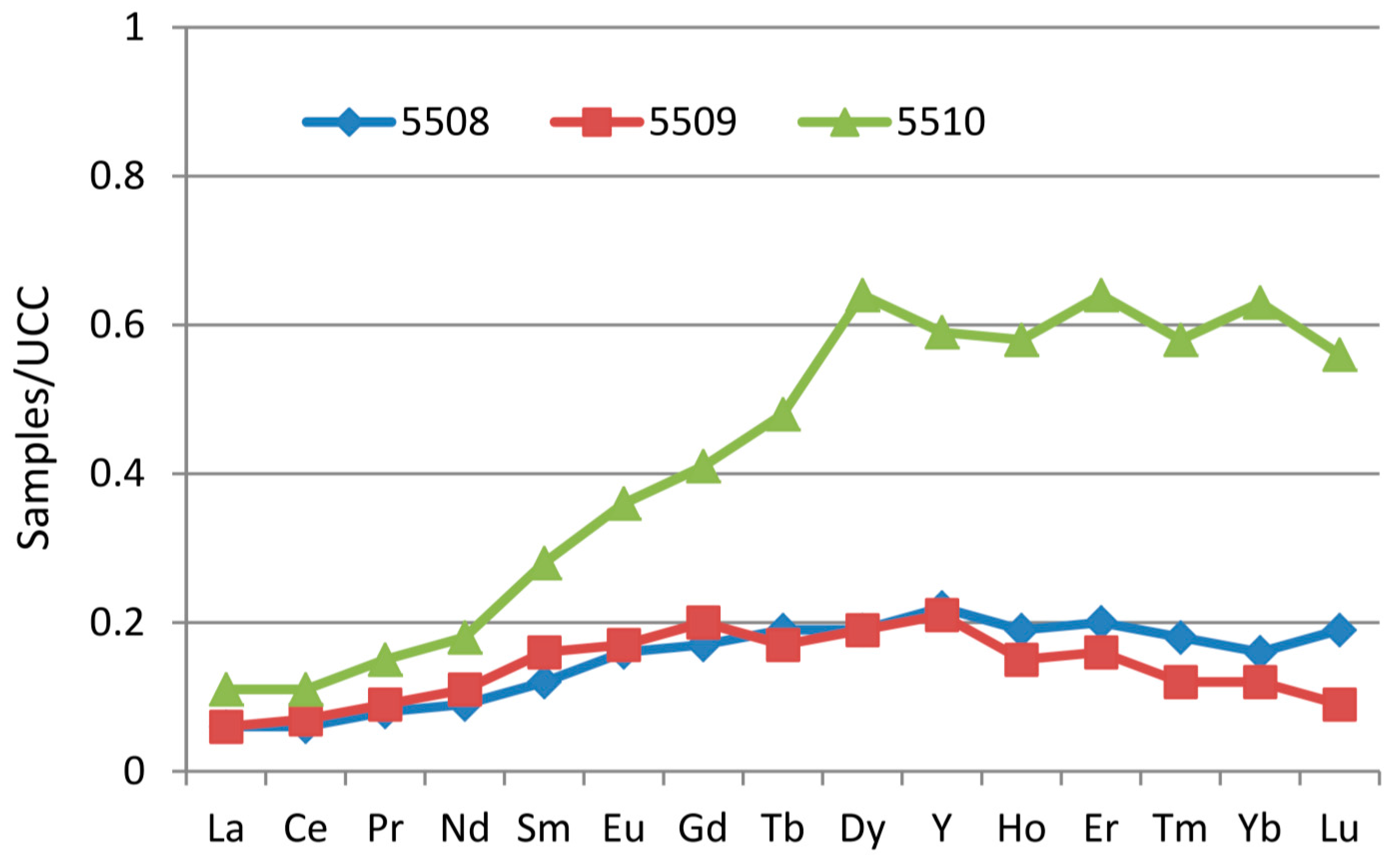
The classification of REY used in the present study is based on Seredin and Dai [
98] and includes light (LREY: La, Ce, Pr, Nd and Sm), medium (MREY: Eu, Gd, Tb, Dy and Y), and heavy (HREY: Ho, Er, Tm, Yb and Lu) REY. Accordingly, in comparison with the upper continental crust (UCC) [
101], three enrichment types are identified: L-type (light-REY; La
N/Lu
N > 1), M-type (medium-REY; La
N/Sm
N < 1, Gd
N/Lu
N > 1), and H-type (heavy-REY; La
N/Lu
N < 1) [
98]. The REY in samples 5508 and 5509, however, have similar distribution patterns; both are weakly fractionated (
Figure 13) but are slightly enriched in medium- and heavy-REY. Sample 5510 is distinctively characterized by a heavy-REY enrichment type (
Figure 13). The three samples do not show anomalies of Eu, Ce and Y (
Figure 13). However, the REE distribution patterns of the coal samples present in this study would have been expected to have a light-REY enrichment type because the sediment-source region is mainly of felsic to intermediate composition. The REY in the present coal samples may have been subjected to acid natural waters or epithermal solutions, which can lead to the medium-REY [
98,
102] and heavy-REY [
98,
103] enrichment types, respectively.
Figure 13.
Distribution patterns of REY in the Gray Hawk coals. REY are normalized by Upper Continental Crust [
101].
Figure 13.
Distribution patterns of REY in the Gray Hawk coals. REY are normalized by Upper Continental Crust [
101].
The concentration of Ge in the Gray Hawk coal ash is 18-times higher than that in the world hard coal ashes reported by Ketris and Yudovich [
81]. For comparison, the Ge concentration in the ashes of coal-hosted Ge ore deposits including Wulantuga, Lincang, and Spetzugli, are 2820, 3902 and 4906 μg/g, respectively [
104]. Germanium is currently being industrially extracted as a raw material from these three Ge-bearing coal deposits. Gallium concentrations in the coal-ash samples are 103, 139 and 222 μg/g, respectively, much higher than the average value for world hard coal ash (36 μg/g) [
81]. The average concentration of Ga in fly ash derived from the coal-hosted Al-Ga ore deposit in Jungar, Inner Mongolia, China, is 92 μg/g [
105].
4.5. Mineralogy
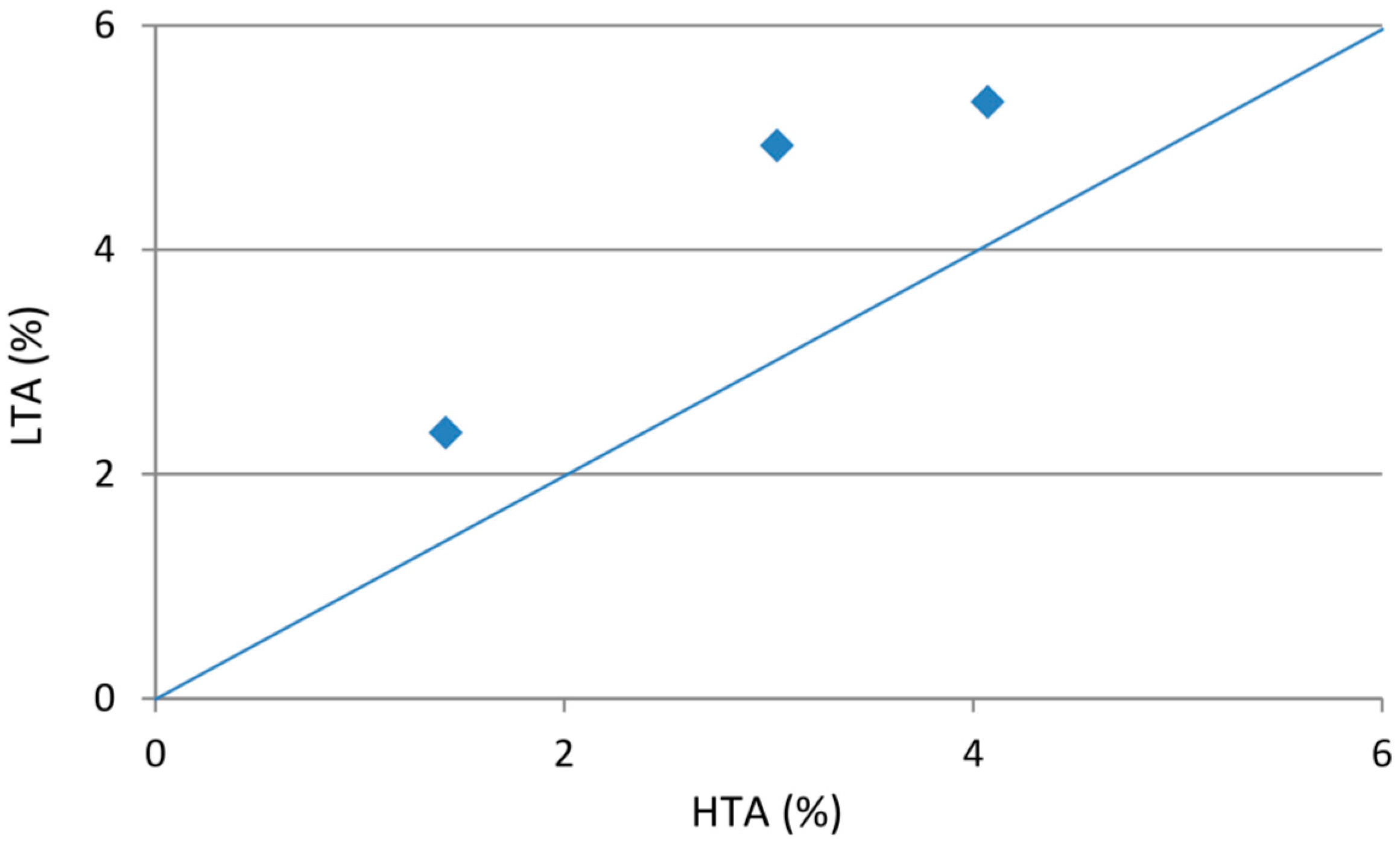
The proportion of LTA for each of the coal bench samples studied is slightly higher than the respective high-temperature ash yield (
Figure 14). The difference is in part due to dehydration of the clay minerals, oxidation of the pyrite, and conversion of the bassanite-forming components to either anhydrite or lime during the (high-temperature) ashing process. Different mineral assemblages occur in the LTA residues of the three coal bench samples (
Table 9). The LTA of the uppermost bench mainly contains kaolinite, pyrite, illite and iron III hydroxyl-sulfate, with minor proportions of quartz, and szomolnokite, and traces of jarosite, anatase and bassanite. Minerals in the LTA of the middle bench are mainly kaolinite, with minor proportions of illite, pyrite, quartz, chlorite and bassanite, along with traces of iron III hydroxyl-sulfate, jarosite, anatase and calcite. The LTA of the lower bench is composed of kaolinite, illite, mixed-layer illite/smectite, quartz, and traces of iron III hydroxyl-sulfate, pyrite, basanite and anatase.
Figure 14.
Relationship between low-temperature and high-temperature ash yields.
Figure 14.
Relationship between low-temperature and high-temperature ash yields.
Table 9.
Minerals in the coal low-temperature ashes (LTA) (A) and in whole-coal samples (B) wt %.
Table 9.
Minerals in the coal low-temperature ashes (LTA) (A) and in whole-coal samples (B) wt %.
| Sample | LTA | Quartz | Kaolinite | Illite | I/S | Chlorite | Pyrite | Iron-HS | Jarosite | Szomolnokite | Anatase | Calcite | Bassanite |
|---|
| A-Low-Temperature Ashes |
| 5508 | 5.3 | 6.7 | 29.2 | 15.6 | | | 27.2 | 11.8 | 1.6 | 4.5 | 0.6 | | 2.7 |
| 5509 | 2.4 | 5.3 | 63.6 | 9.3 | | 5.1 | 6.0 | 1.7 | 0.8 | | 1.2 | 0.3 | 6.7 |
| 5510 | 4.9 | 17.1 | 45.1 | 19.2 | 12.8 | | 0.3 | 1.6 | | | 0.8 | | 3.0 |
| B-Whole Coal |
| 5508 | - | 0.36 | 1.55 | 0.83 | 0.00 | 0.00 | 1.44 | 0.63 | 0.08 | 0.24 | 0.03 | 0.00 | 0.14 |
| 5509 | - | 0.13 | 1.53 | 0.22 | 0.00 | 0.12 | 0.14 | 0.04 | 0.02 | 0.00 | 0.03 | 0.01 | 0.16 |
| 5510 | - | 0.84 | 2.21 | 0.94 | 0.63 | 0.00 | 0.01 | 0.08 | 0.00 | 0.00 | 0.04 | 0.00 | 0.15 |
The bassanite in coal LTAs is commonly thought to be formed as an artifact of the plasma-ashing process, derived from interaction of non-mineral Ca and sulfur released from the maceral components during oxidation [
106,
107,
108]. In some cases, however, the bassanite may be produced when sulfuric acid from oxidation of pyrite during storage of the coal reacts with calcite (if present) to form gypsum, which is then partly dehydrated during the low-temperature ashing process [
109].
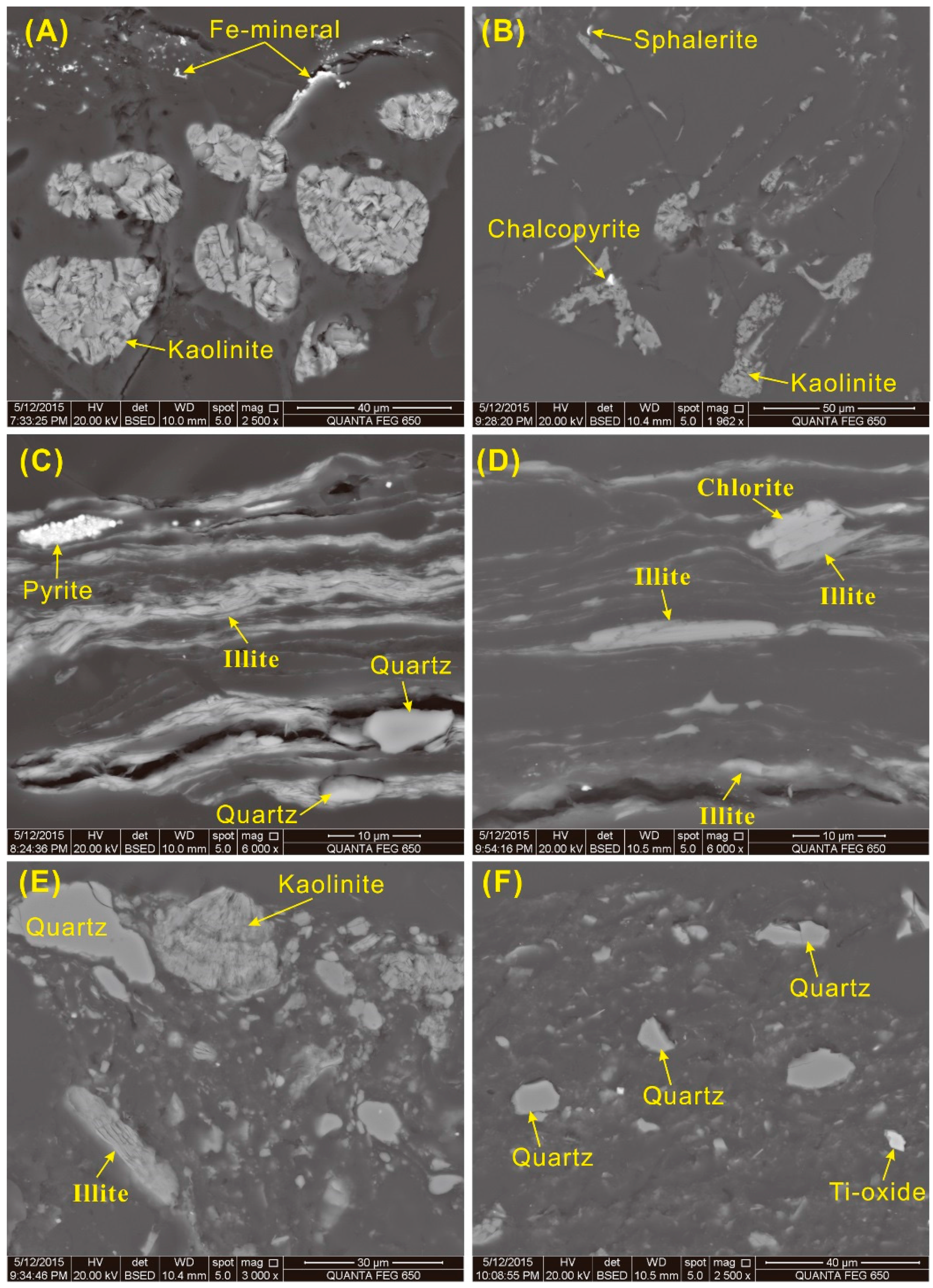
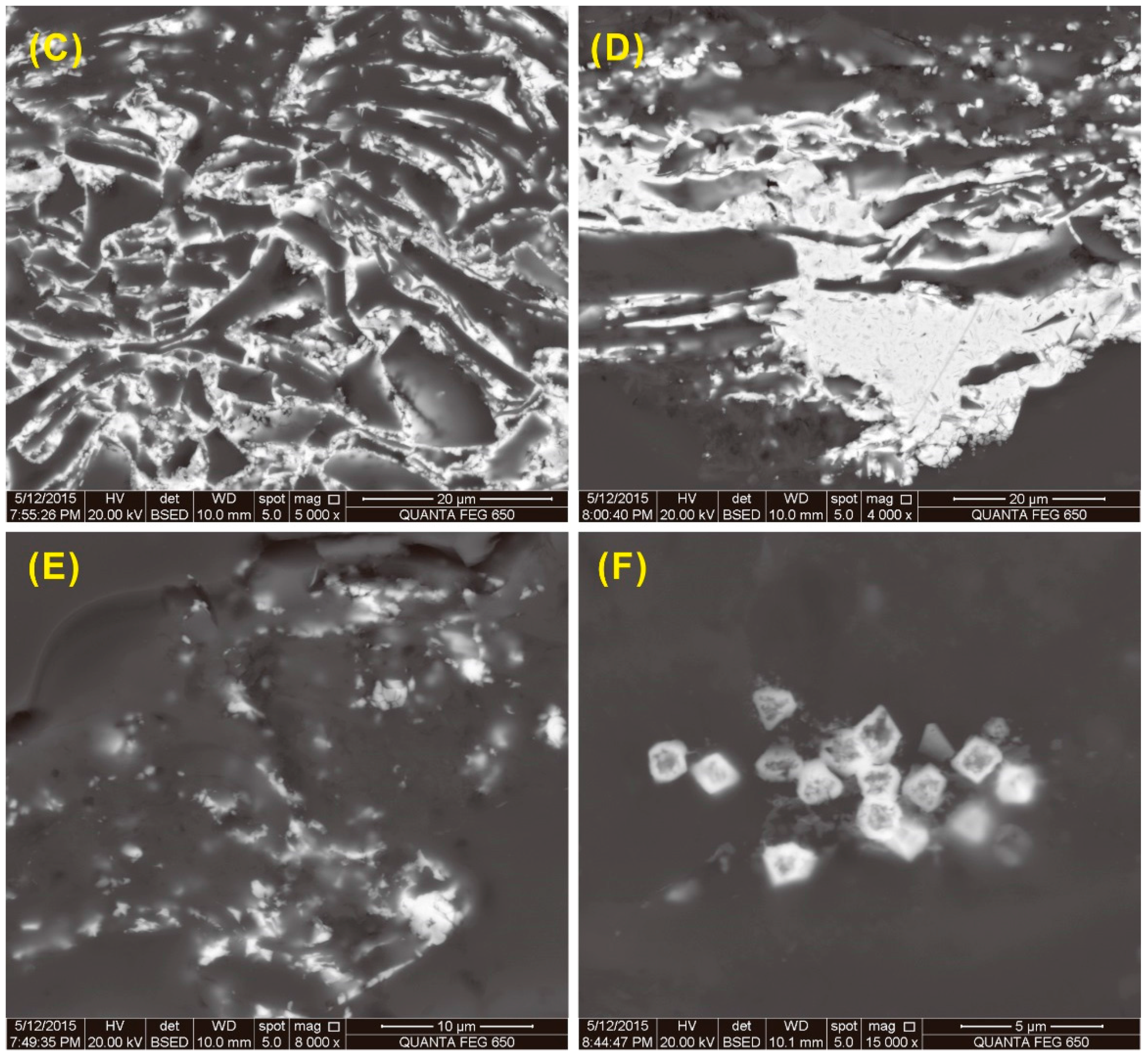
SEM-EDS studies show that the kaolinite mainly occurs in cell-fillings, indicating an authigenic origin (
Figure 15A,B), although in some cases, it also occurs as massive material in the collodetrinite, most likely of detrital origin. (
Figure 15E). Illite is distributed along bedding planes (
Figure 15C,D), or occurs in lath form (
Figure 15D,E), respectively indicating an authigenic origin and derivation from detrital materials of terrigenous origin [
110,
111]. Chlorite occurs in association with illite along bedding planes, suggesting a detrital origin. Quartz occurs as detrital grains in collodetrinite (
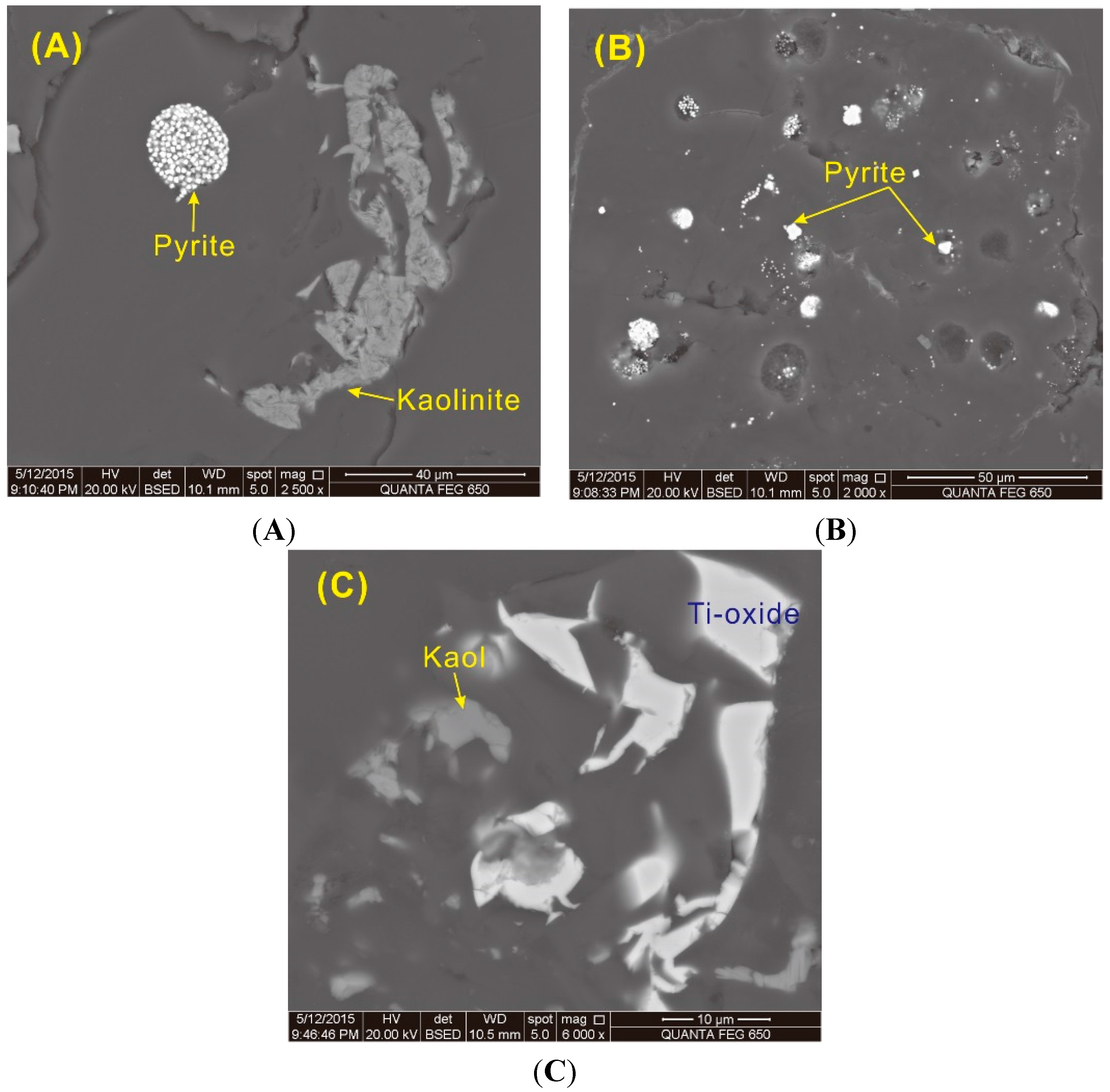
Figure 15E,F). Pyrite, mainly concentrated in the top bench, occurs as framboidal and dispersed fine-grained particles in the collodetrinite (
Figure 16A,B), both occurrences indicative of an authigenic origin. Anatase occurs in cell-fillings of the coal-forming plants (
Figure 16C), indicating an authigenic origin. Traces of sphalerite and chalcopyrite, which are the carriers of relatively high Zn and Cu concentrations in the samples, occur in the collodetrinite of sample 5510 (
Figure 15B).
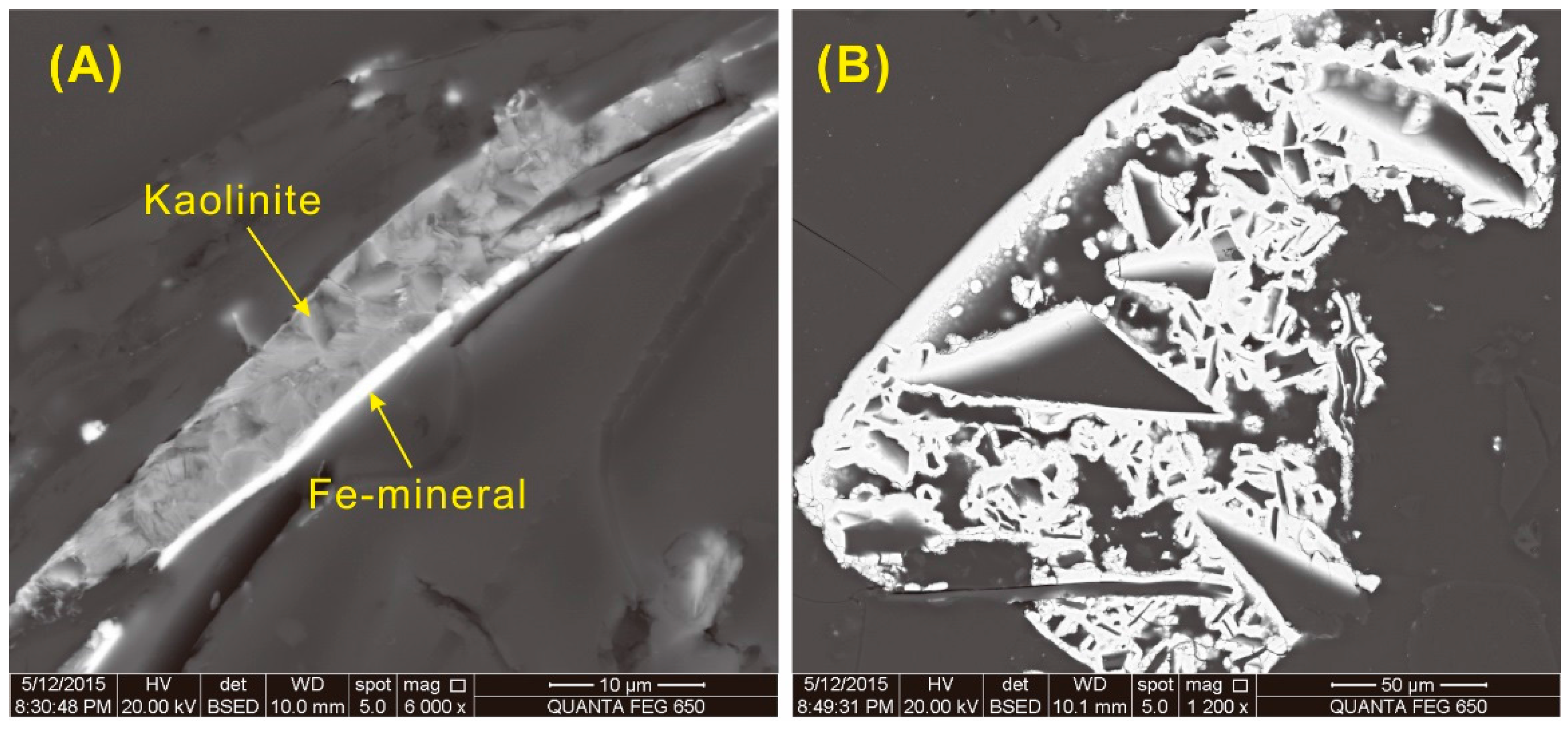
A trace of an Fe-bearing phase without S, possibly an iron oxide or hydroxy-oxide (such as hematite or goethite), was detected by SEM-EDS in sample 5510 but is below the detection limit of the XRD technique. The modes of occurrence of this Fe-bearing phase, including fracture-fillings (
Figure 17A), cell-fillings (
Figure 17C,D), and fine particles distributed in the collodetrinite (
Figure 17D,E), indicate an epigenetic origin.
Figure 15.
Back-scattered electron images of kaolinite, illite and quartz in the coal. (A) Kaolinite in cell-fillings, sample 5508; (B) Kaolinite in cell-fillings, and chalcopyrite and sphalerite in collodetrinite of sample 5510; (C) Illite distributed along bedding planes, as well as pyrite and quartz in collodetrinite, sample 5508; (D) Illite and chlorite occurring in lath form in collodetrinite, sample 5510; (E) Discrete quartz, massive kaolinite, and lath-like illite in collodetrinite, sample 5510; (F) Discrete quartz in collodetrinite, sample 5510.
Figure 15.
Back-scattered electron images of kaolinite, illite and quartz in the coal. (A) Kaolinite in cell-fillings, sample 5508; (B) Kaolinite in cell-fillings, and chalcopyrite and sphalerite in collodetrinite of sample 5510; (C) Illite distributed along bedding planes, as well as pyrite and quartz in collodetrinite, sample 5508; (D) Illite and chlorite occurring in lath form in collodetrinite, sample 5510; (E) Discrete quartz, massive kaolinite, and lath-like illite in collodetrinite, sample 5510; (F) Discrete quartz in collodetrinite, sample 5510.
Figure 16.
Back-scattered electron images of pyrite, rutile, and kaolinite in the coal. (A) Framboidal pyrite and cell-filling pyrite in sample 5509; (B) Cell-filling and dispersed pyrite in sample 5509; (C) Cell-filling anatase and kaolinite in sample 5510.
Figure 16.
Back-scattered electron images of pyrite, rutile, and kaolinite in the coal. (A) Framboidal pyrite and cell-filling pyrite in sample 5509; (B) Cell-filling and dispersed pyrite in sample 5509; (C) Cell-filling anatase and kaolinite in sample 5510.
Figure 17.
Back-scattered electron images of iron oxide or hydroxy-oxide (such as hematite or goethite; light-colored) in sample 5508. (A) Fe-mineral and kaolinite in fractures. (B–D), Fe-mineral in fractures. (E–F), Fe-mineral organic matter matrix.
Figure 17.
Back-scattered electron images of iron oxide or hydroxy-oxide (such as hematite or goethite; light-colored) in sample 5508. (A) Fe-mineral and kaolinite in fractures. (B–D), Fe-mineral in fractures. (E–F), Fe-mineral organic matter matrix.