Nitric Acid Purification of Molybdenite Concentrate: Copper-Iron Removal and Development of a Comprehensive Dissolution Kinetics Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results and Discussion
3.1. Thermodynamic Evaluation
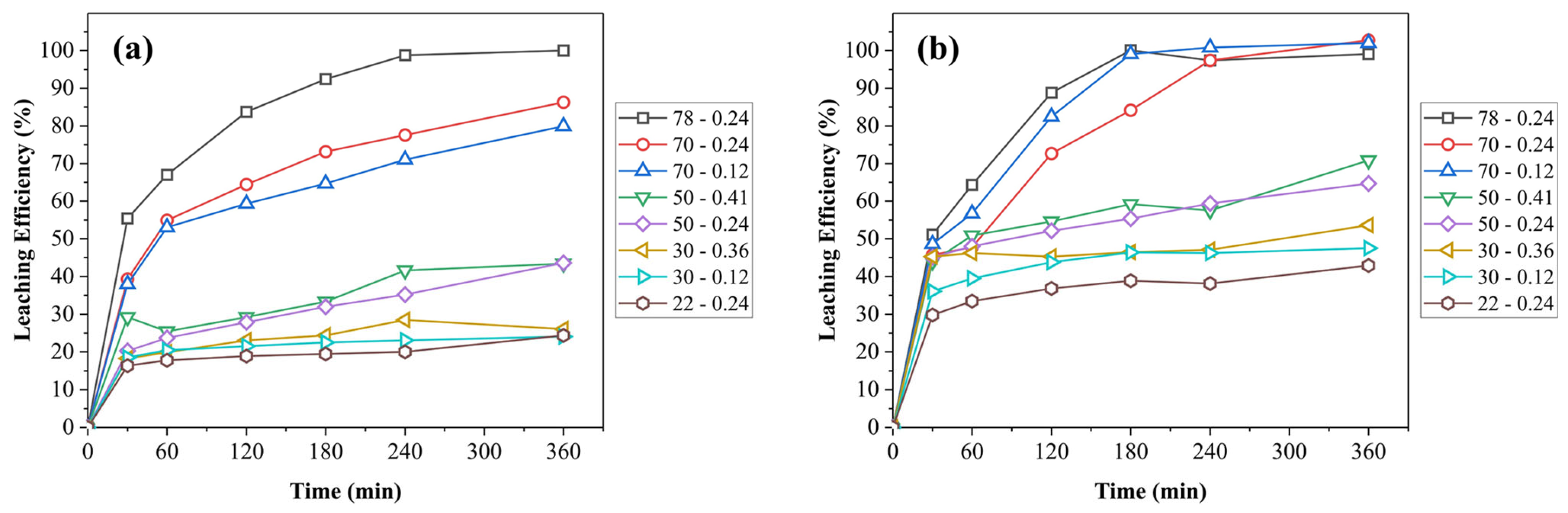
3.2. Leaching Results
3.3. Kinetics Study
3.3.1. Shrinking Core Model Fitting
3.3.2. Model Development
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lunk, H.-J.; Hartl, H. Discovery, properties and applications of molybdenum and its compounds. ChemTexts 2017, 3, 13. [Google Scholar] [CrossRef]
- Wang, M.; Wang, X.; Liu, W. A novel technology of molybdenum extraction from low grade Ni–Mo ore. Hydrometallurgy 2009, 97, 126–130. [Google Scholar] [CrossRef]
- Saji, V.S.; Lee, C.W. Molybdenum, molybdenum oxides, and their electrochemistry. ChemSusChem 2012, 5, 1146–1161. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, C.; Chandrasekar, S.; Li, Y.; Xu, F. Preparation of sodium molybdate from molybdenum concentrate by microwave roasting and alkali leaching. Int. J. Miner. Metall. Mater. 2024, 31, 91–105. [Google Scholar] [CrossRef]
- Hu, X.-T.; Qian, J.G.; Yin, Y.; Li, X.; Li, T.J.; Li, J. Approaches to electrodeposit molybdenum from ionic liquid. Rare Met. 2023, 42, 2439–2446. [Google Scholar] [CrossRef]
- Kim, B.-S.; Jha, M.K.; Jeong, J.; Lee, J.C. Leaching of impurities for the up-gradation of molybdenum oxide and cementation of copper by scrap iron. Int. J. Miner. Process. 2008, 88, 7–12. [Google Scholar] [CrossRef]
- Lasheen, T.A.; El-Ahmady, M.E.; Hassib, H.B.; Helal, A.S. Molybdenum metallurgy review: Hydrometallurgical routes to recovery of molybdenum from ores and mineral raw materials. Miner. Process. Extr. Metall. Rev. 2015, 36, 145–173. [Google Scholar] [CrossRef]
- Jiang, Z.-Y.; Pan, M.; Ren, F.M.; Chen, R.; Sun, Z.X.; Yang, Z.C.; Liu, Z.H.; Chen, W. Boosting stability of inverted perovskite solar cells with magnetron-sputtered molybdenum rear electrodes. Rare Met. 2023, 42, 3741–3754. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, W.; Park, J.H.; Mu, W. Effect of hafnium and molybdenum addition on inclusion characteristics in Co-based dual-phase high-entropy alloys. Int. J. Miner. Metall. Mater. 2024, 31, 1639–1650. [Google Scholar] [CrossRef]
- Tumen-Ulzii, N.; Batnasan, A.; Gunchin, B. Selective dissolution of copper and iron from molybdenite concentrate using acidic sodium nitrate solution. Miner. Eng. 2022, 185, 107715. [Google Scholar] [CrossRef]
- Shalchian, H.; Birloaga, I.; Moghaddam, M.B.; Nasiri, H.; Vegliò, F. A hydrometallurgical process flowsheet for recovering MoO3 from Molybdenite. Hydrometallurgy 2024, 228, 106355. [Google Scholar] [CrossRef]
- Abdollahi, M.; Bahrami, A.; Mirmohammadi, M.S.; Kazemi, F.; Danesh, A.; Ghorbani, Y. A process mineralogy approach to optimize molybdenite flotation in copper–molybdenum processing plants. Miner. Eng. 2020, 157, 106557. [Google Scholar] [CrossRef]
- Padilla, R.; Letelier, H.; Ruiz, M.C. Kinetics of copper dissolution in the purification of molybdenite concentrates by sulfidation and leaching. Hydrometallurgy 2013, 137, 78–83. [Google Scholar] [CrossRef]
- Lv, X.; Luo, A.; Tong, X.; Chen, J.; Jian, S. Experimental and Mechanistic Study on Flotation Separation of Chalcopyrite and Molybdenite Using the Novel Depressant 2-Mercapto-6-Methylpyrimidin-4-ol. Molecules 2025, 30, 1396. [Google Scholar] [CrossRef]
- Nam, Y.I.; Seo, S.Y.; Kang, Y.C.; Kim, M.J.; Senanayake, G.; Tran, T. Purification of molybdenum trioxide calcine by selective leaching of copper with HCl–NH4Cl. Hydrometallurgy 2011, 109, 9–17. [Google Scholar] [CrossRef]
- Zamani, M.A.; Vaghar, R.; Oliazadeh, M. Selective copper dissolution during bioleaching of molybdenite concentrate. Int. J. Miner. Process. 2006, 81, 105–112. [Google Scholar] [CrossRef]
- Romano, P.; Blazquez, M.L.; Alguacil, F.J.; Munoz, J.A.; Ballester, A.; Gonzalez, F. Comparative study on the selective chalcopyrite bioleaching of a molybdenite concentrate with mesophilic and thermophilic bacteria. FEMS Microbiol. Lett. 2001, 196, 71–75. [Google Scholar] [CrossRef]
- Ruiz, M.; Padilla, R. Copper removal from molybdenite concentrate by sodium dichromate leaching. Hydrometallurgy 1998, 48, 313–325. [Google Scholar] [CrossRef]
- Behmadi, R.; Mirzaei, M.; Afshar, M.R.; Najafi, H. Investigation of chalcopyrite removal from low-grade molybdenite using response surface methodology and its effect on molybdenum trioxide morphology by roasting. RSC Adv. 2023, 13, 14899–14913. [Google Scholar] [CrossRef]
- Padilla, R.; Letelier, H.; Ruiz, M.C. Copper Removal from Molybdenite by Sulfidation-Leaching Process. In Materials Processing Fundamentals; John Wiley & Sons: Hoboken, NJ, USA, 2013; pp. 217–223. [Google Scholar]
- Oke, E.A.; Fedai, Y.; Potgieter, J.H. Hydrometallurgical Leaching of Copper and Cobalt from a Copper–Cobalt Ore by Aqueous Choline Chloride-Based Deep Eutectic Solvent Solutions. Minerals 2025, 15, 815. [Google Scholar] [CrossRef]
- Abbas, Z.; Jung, S.M. Green and selective recovery process of Mo, V, and Ni from spent hydrodesulfurization catalysts via novel ionic liquids and deep eutectic solvents technology. Sep. Purif. Technol. 2024, 346, 127450. [Google Scholar] [CrossRef]
- Oke, E.A.; Potgieter, H.; Mondlane, F.; Skosana, N.P.; Teimouri, S.; Nyembwe, J.K. Concurrent leaching of copper and cobalt from a copper–cobalt ore using sulfuric and organic acids. Miner. Eng. 2024, 216, 108853. [Google Scholar] [CrossRef]
- Shalchian, H.; Khaki, J.V.; Babakhani, A.; De Michelis, I.; Veglio, F.; Parizi, M.T. An enhanced dissolution rate of molybdenite and variable activation energy. Hydrometallurgy 2018, 175, 52–63. [Google Scholar] [CrossRef]
- Shalchian, H.; Khaki, J.V.; Babakhani, A.; Taglieri, G.; De Michelis, I.; Daniele, V.; Veglio, F. On the mechanism of molybdenite exfoliation during mechanical milling. Ceram. Int. 2017, 43, 12957–12967. [Google Scholar] [CrossRef]
- Kadιoğlu, Y.Y.; Karaca, S.; Bayrakceken, S. Kinetics of pyrite oxidation in aqueous suspension by nitric acid. Fuel Process. Technol. 1995, 41, 273–287. [Google Scholar] [CrossRef]
- Vizsolyi, A.; Peters, E. Nitric acid leaching of molybdenite concentrates. Hydrometallurgy 1980, 6, 103–119. [Google Scholar] [CrossRef]
- Copur, M. Solubility of ZnS concentrate containing pyrite and chalcopyrite in HNO~ 3 solutions. Chem. Biochem. Eng. Q. 2001, 15, 181–184. [Google Scholar]
- Shalchian, H.; Hajizadeh Navakh, M.; Birloaga, I.; Babakhani, A.; Vegliò, F.A. Comparison Study on the Recovery of REEs from Red Mud by Sulfation Roasting–Water Leaching and Citric Acid Leaching. Minerals 2024, 14, 1044. [Google Scholar] [CrossRef]
- Shalchian, H.; Khalili, M.; Kiani-Rashid, A.; Nateq, B.; Vegliò, F. Selective Leaching of Lithium and Beyond: Sustainable Eggshell-Mediated Recovery from Spent Li-Ion Batteries. Minerals 2024, 14, 1120. [Google Scholar] [CrossRef]
- Shalchian, H.; Romano, P.; Rahmati, S.; Birloaga, I.; Innocenzi, V.; Vegliò, F. Innovative sulfation strategy for efficient recovery of rare earth elements from spent fluorescent lamp powder. Resour. Conserv. Recycl. 2025, 222, 108495. [Google Scholar] [CrossRef]
- Rahmati, S.; Shalchian, H.; Adavodi, R.; Birloaga, I.; Romano, P. Evaluation of a hybrid pyro–hydrometallurgical process for the selective leaching of rare earth elements from spent NdFeB magnets. Chem. Eng. J. 2025, 521, 167003. [Google Scholar] [CrossRef]
- Levenspiel, O. Chemical Reaction Engineering; John Wiley & Sons: Hoboken, NJ, USA, 1998. [Google Scholar]
- Habashi, F. Chalcopyrite, Its Chemistry and Metallurgy; McGraw-Hill: New York, NY, USA; London, UK, 1978. [Google Scholar]
- Bafghi, M.S.; Emami, A.H.; Zakeri, A.; Khaki, J.V. Effect of mechanical activation on the kinetics of leaching of chalcopyrite in the ferric sulfate media. Iran. J. Mater. Sci. Eng. 2010, 7, 30–35. [Google Scholar]
- Brittan, M. Variable activation energy model for leaching kinetics. Int. J. Miner. Process. 1975, 2, 321–331. [Google Scholar] [CrossRef]
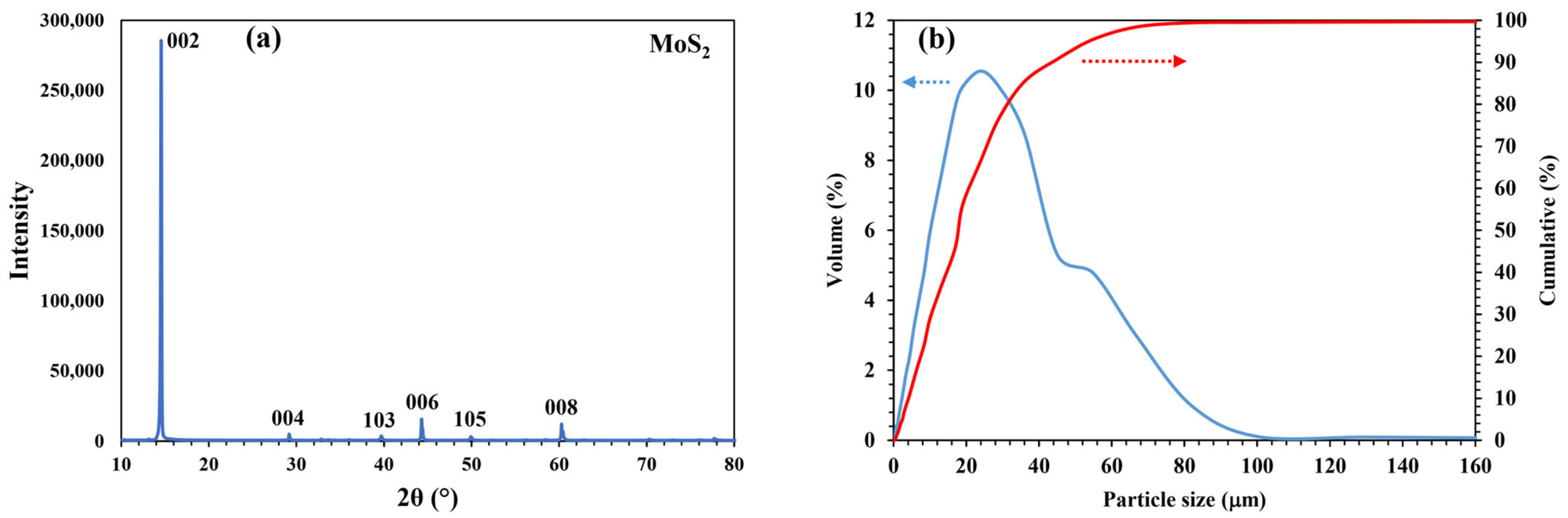
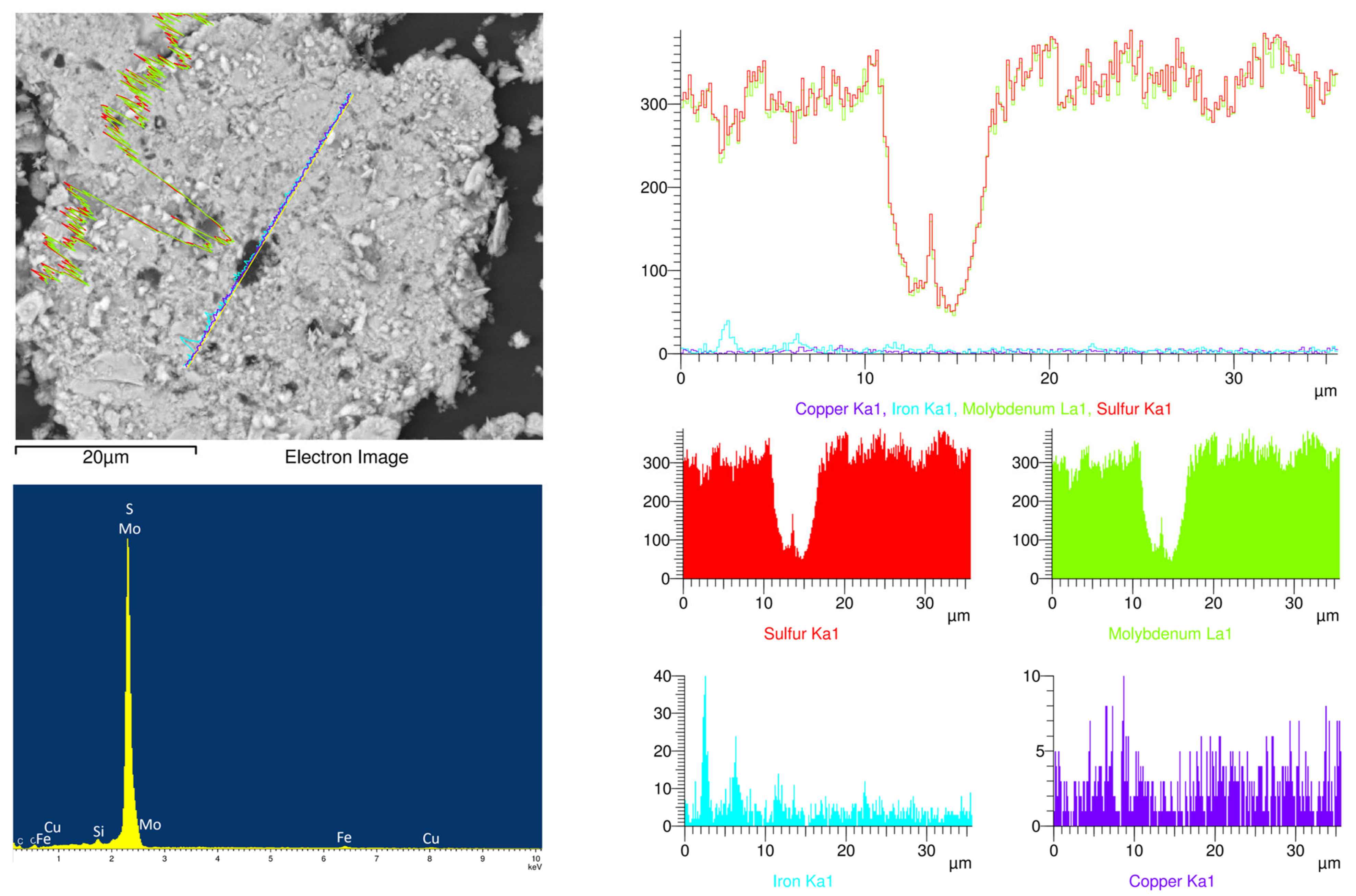




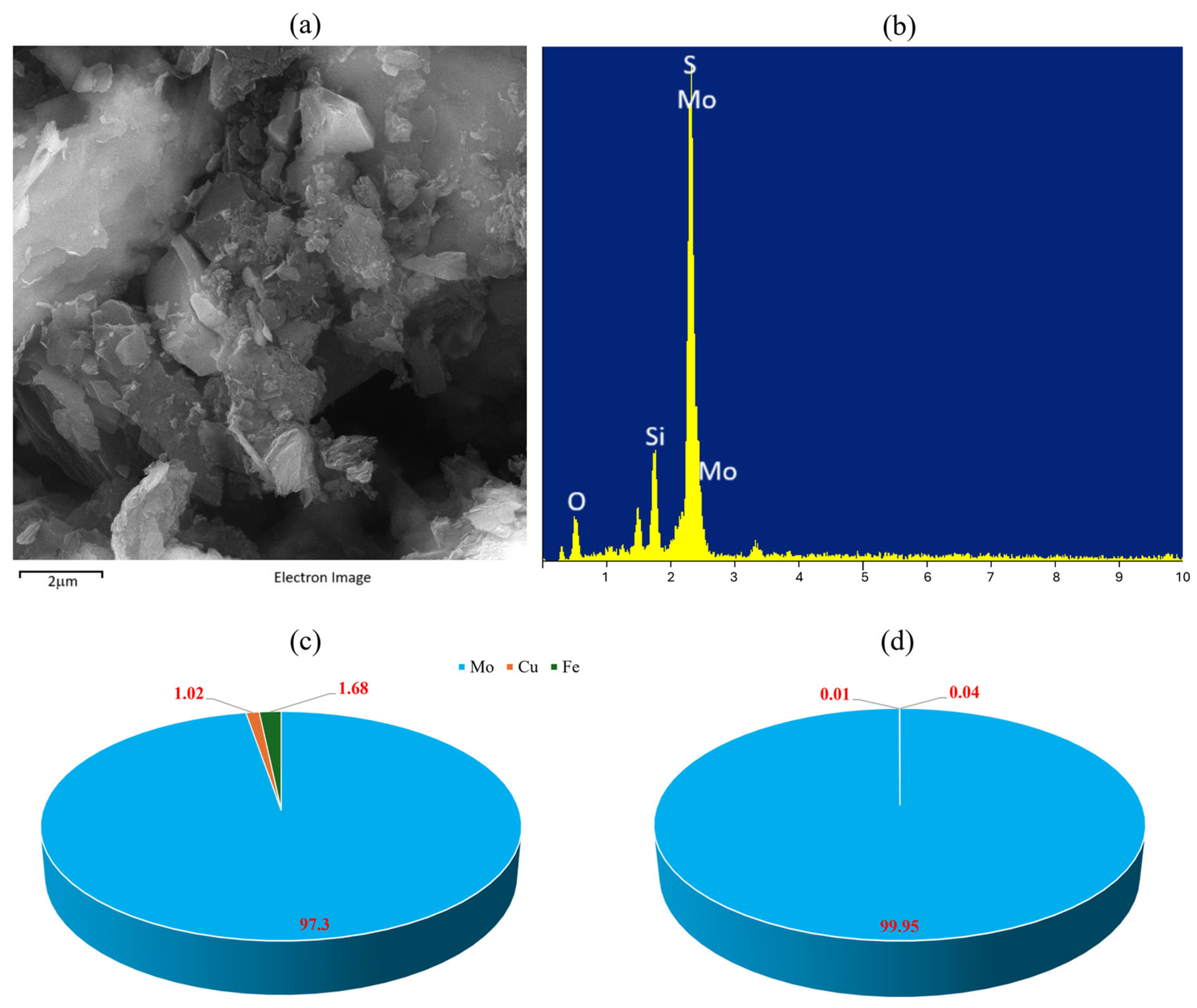
| Component | Mo | Fe | Cu | S | Silica |
|---|---|---|---|---|---|
| Wt. % | 55.6 | 0.96 | 0.58 | 38.23 | 4.63 |
| Mineral | Model Parameters | Temp. - Conc. | R2 | |
|---|---|---|---|---|
| FeS2 | b | 14.2 | 22 – 0.24 | 0.9204 |
| m | 0.35 | 30 – 0.12 | 0.9203 | |
| n | 6 | 30 – 0.36 | 0.8592 | |
| E (J/mol) | 43,500 | 50 – 0.24 | 0.9895 | |
| 50 – 0.41 | 0.9805 | |||
| b | 11.5 | 70 – 0.24 | 0.9583 | |
| m | 0.4 | |||
| n | 1 | 78 – 0.24 | 0.9900 | |
| E (J/mol) | 43,500 | |||
| CuFeS2 | b | 16.5 | 22 – 0.24 | 0.8942 |
| m | 0.3 | 30 – 0.12 | 0.8940 | |
| n | 13.5 | 30 – 0.36 | 0.9596 | |
| E (J/mol) | 51,000 | 50 – 0.24 | 0.9389 | |
| 50 – 0.41 | 0.9322 | |||
| b | 14.1 | 70 – 0.24 | 0.9721 | |
| m | 0.4 | |||
| n | 1.5 | 78 – 0.24 | 0.9838 | |
| E (J/mol) | 51,000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shalchian, H.; Ghorbanpour, P.; Nateq, B.; Passadoro, M.; Romano, P.; Vegliò, F.; Ippolito, N.M. Nitric Acid Purification of Molybdenite Concentrate: Copper-Iron Removal and Development of a Comprehensive Dissolution Kinetics Model. Minerals 2025, 15, 982. https://doi.org/10.3390/min15090982
Shalchian H, Ghorbanpour P, Nateq B, Passadoro M, Romano P, Vegliò F, Ippolito NM. Nitric Acid Purification of Molybdenite Concentrate: Copper-Iron Removal and Development of a Comprehensive Dissolution Kinetics Model. Minerals. 2025; 15(9):982. https://doi.org/10.3390/min15090982
Chicago/Turabian StyleShalchian, Hossein, Payam Ghorbanpour, Behzad Nateq, Marco Passadoro, Pietro Romano, Francesco Vegliò, and Nicolò Maria Ippolito. 2025. "Nitric Acid Purification of Molybdenite Concentrate: Copper-Iron Removal and Development of a Comprehensive Dissolution Kinetics Model" Minerals 15, no. 9: 982. https://doi.org/10.3390/min15090982
APA StyleShalchian, H., Ghorbanpour, P., Nateq, B., Passadoro, M., Romano, P., Vegliò, F., & Ippolito, N. M. (2025). Nitric Acid Purification of Molybdenite Concentrate: Copper-Iron Removal and Development of a Comprehensive Dissolution Kinetics Model. Minerals, 15(9), 982. https://doi.org/10.3390/min15090982












