Abstract
This study focused on creating a novel material by integrating ZnO and CuO nanoparticles into the structure of halloysite using a hydrothermal method. The formation of the nanocomposite was validated through X-ray diffraction and Raman analysis, which confirmed the presence of ZnO and CuO phases without compromising the structure of halloysite. Microscopic analysis revealed a well-distributed presence of metallic oxide nanoparticles within the nanotubular structure of halloysite, which adhered to both the outer and inner surfaces of the clay mineral. Optical characterization identified a substantial density of defects, which played a key role in improving the performance of the supported semiconductors. Furthermore, the narrow band gap at 3.02 eV promoted the mobility of photogenerated charges. Photocatalytic tests yielded promising results, demonstrating a synergistic effect between photocatalysis and adsorption processes that positively influenced the removal of ciprofloxacin from solutions. The material achieved up to 76% removal of the antibiotic within 120 min, utilizing a catalyst concentration of 0.5 g L−1 with a pollutant concentration of 20 mg L−1. In reuse experiments, the material exhibited high recyclability even after multiple reaction cycles. Halloysite-based nanocomposites represent a strategic advancement in environmental remediation technologies, contributing to the development of clean, effective, and reusable materials.
1. Introduction
Halloysite is a 1:1-type clay mineral with a structural composition consisting of tetrahedral (Si-O) and octahedral (Al-OH) sheets in each layer. It also contains varying amounts of water in its interlayer spaces [1]. Typically, halloysite exists in a hydrated state, with two water molecules per unit cell and a layer thickness of about 10 Å. This hydrated form is unstable and can irreversibly transition to a dehydrated state with an interlayer space of approximately 7 Å. These two forms are known as halloysite (10 Å) and halloysite (7 Å), respectively. The presence of additional water molecules weakens the bonds between the alumina and silica layers, causing them to bend and resulting in halloysite’s characteristic hollow tubular form [2]. The rolling mechanism of halloysite leads to the formation of aluminol (Al-OH) units situated in the interior of the nanotube. These units consist solely of basal oxygen atoms, which bear a positive charge. In contrast, the siloxane (Si-O-Si) groups present on the outer surface of the nanotube comprise apical oxygen atoms and exhibit a negative charge distribution [3]. The distinctive tubular shape of halloysite is drawing considerable attention, fueled by its swift integration into an ever-growing array of technological applications.
Due to its unique surface characteristics, halloysite has been extensively studied as a sustainable alternative for developing multifunctional materials across various applications [4,5,6]. Chemical modifications can enhance the halloysite surface for a broad range of uses, from biomedicine to environmental remediation. These modifications, which can involve the application of organic or inorganic compounds, may target the external surfaces, internal surfaces, and interlayer spacing [7]. In the context of environmental remediation, halloysite can be functionalized with various semiconductor nanoparticles to effectively remove contaminants from solutions. For example, Zsirka et al. [8] prepared a ZnO–halloysite nanocomposite and applied it to degrade 4-nitrophenol under UV light irradiation. Their findings indicated that the best results were achieved with samples containing 9% ZnO, which showed low toxicity. Similarly, Hu et al. [9] developed a ZnO/halloysite nanocomposite that they used for removing methylene blue. Their study concluded that halloysite-based materials were more efficient than pure ZnO, achieving up to 99.88% dye removal. Aghababaei et al. [10] created a catalyst composed of O-g-C3N4/ZnO/TiO2/halloysite for the degradation of diclofenac, observing complete degradation of the drug within 50 min at a pH of 6.83. In another study by Albuquerque et al. [11], the authors demonstrated the effectiveness of a RuO2@ZnO–alginate–halloysite nanocomposite for the photodegradation of eosin and ciprofloxacin. Notably, they observed high activity of the materials across different semiconductor regions’ concentrations. Recently, Pouthika et al. [12] prepared a halloysite-based heterostructure modified with CuO and TiO2, concluding that this material is highly stable and efficient, achieving up to 82% degradation of Congo red dye.
Heterogeneous photocatalysis has recently garnered considerable attention in the realm of environmental remediation, primarily due to its efficacy in eliminating organic pollutants [13,14,15]. To tackle challenges related to the agglomeration and recycling of bare semiconductors, researchers have been investigating the use of various supports [16,17,18,19,20]. Clay minerals stand out for their chemical and mechanical stability, biocompatibility, sorption capacity, expansive surface area, and numerous active sites for reactions [21]. Moreover, clay minerals have gained traction in photocatalytic studies, as they act as electron acceptors, thereby enhancing the photocatalytic activity of materials by facilitating charge transfer and boosting the generation of reactive oxygen species.
In this study, we developed a nanocomposite by integrating zinc and copper nanoparticles into a halloysite structure under mild conditions. The nanocomposite was synthesized using a hydrothermal method that eliminated the need for stabilizing agents and subsequent calcination steps. Notably, the process avoided harmful chemical compounds and focused on non-toxic materials to minimize environmental impact. The effectiveness of the resulting nanocomposite was assessed based on its capacity to remove ciprofloxacin, a model pollutant. Ciprofloxacin is an antibiotic from the fluoroquinolone family, widely used in human and veterinary medicine. The pollution of water bodies with antibiotics, such as ciprofloxacin, is an escalating issue due to excessive use and inadequate disposal. Among the problems caused are bacterial resistance, a reduction in biodiversity, and negative effects on the reproduction and growth of aquatic organisms, along with potential contamination of groundwater [22,23].
The integration of semiconductor oxides, such as ZnO and CuO, into halloysite nanotubes represents a novel approach for treating emerging contaminants, including ciprofloxacin. The tubular morphology, high surface area, and natural abundance of halloysite facilitate effective dispersion of metal oxides, which reduces agglomeration and enhances material stability. The combination of ZnO and CuO generates a synergistic effect that promotes the separation of photoinduced charges and increases photocatalytic activity under various light conditions. This combination supports both adsorption through halloysite surface groups and photocatalytic degradation, resulting in the efficient removal of ciprofloxacin, a persistent antibiotic frequently detected in water sources. Consequently, the development of ZnO–CuO/halloysite nanocomposites provides a sustainable and cost-effective method for the remediation of pharmaceutical pollutants. In addition to preventive measures, advanced treatment of pollutants is a highlighted solution to this growing problem. Therefore, this study is significant for the depollution of water bodies from antibiotics, emphasizing the protection of aquatic life, the preservation and safety of water supply, and the sustainability of long-term water resources.
2. Materials and Methods
2.1. Materials
For the synthesis, high-purity chemicals, namely zinc nitrate (Zn(NO3)2·6H2O) [99.0% purity], copper nitrate (II) (Cu(NO3)2·3H2O) [99.8% purity], and halloysite (Al2Si2O5(OH)4.2H2O), were used in the experiments, purchased from Sigma Aldrich (São paulo, Brazil). Distilled water was used as a solvent for washing, ethanol for washing, and NaOH (200 g L−1) solution was used for pH adjustment. All related reagent compounds were of analytical grade.
2.2. Synthesis
Using the hydrothermal method, 1 g of halloysite was combined with 0.595 g of zinc nitrate and 0.483 g of copper nitrate in 40 mL of distilled water. The mixture was stirred for 30 min to form a homogeneous solution. The pH of the resulting mixture was adjusted to pH 12 via the addition of NaOH, while stirring constantly. This value was selected to ensure complete hydrolysis of Zn2+ and Cu2+ ions and to promote the nucleation and growth of ZnO and CuO nanoparticles. Under these conditions, the formation of stable hydroxide precursors facilitates their subsequent conversion into oxides during the hydrothermal process, while simultaneously preserving the structural integrity of the halloysite matrix. Alkaline conditions at pH 12 also enhance the anchoring of the metal oxides on the nanotubular surfaces, leading to a more homogeneous dispersion of nanoparticles across the clay framework. The mixture was then transferred to a Teflon cup and placed in a hydrothermal reactor. The system was heated in a muffle furnace at 140 °C for 13 h. The resulting solid was separated by centrifugation and washed five times with distilled water and twice with ethanol to remove any residues. Finally, the solid was dried at 100 °C for 8 h. Figure 1 illustrates the general scheme of the synthesis and formation of these composites.
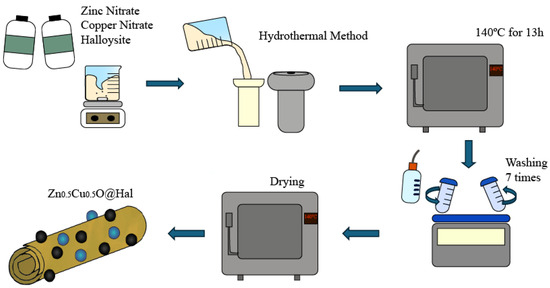
Figure 1.
Schematic representation of the synthesis and composition of the Zn0.5Cu0.5O@Hal nanocomposite (ZCHal).
2.3. Characterizations
For the structural characterization, an X-ray diffractometer (Bruker AXS GmbH, Karlsruhe, Germany), model D8 Advance from Bruker with Cu-Kα radiation (λ = 1.5406 Å), was used. Raman spectra were acquired using an XploRA confocal Raman microscope (HORIBA Jobin Yvon, Billerica, MA, USA) equipped with a 532 nm laser (10 mW power), focused onto the sample through a 100× objective (MPlanN, Olympus, NA = 0.9). Spectra were collected in the 50–1000 cm−1 range, with an integration time of 40 s per spectrum, and averaged over five accumulations to improve signal-to-noise ratio. The morphology was investigated using a Thermo Scientific Talos F200i Transmission Electron Microscope (TEM, Brno, Czech Republic), and the elemental composition was analyzed using energy-dispersive spectroscopy EDS with a TESCAN VEGA3 model. The optical properties were analyzed using a UV–VIS spectrometer, Shimadzu (Kyoto, Japan), UV-2700, and a Spectrophotometer Horiba-JobinYvon Fluorolog-3 to determine energy band gap and Room Temperature Photoluminescence (RT-PL) spectra, respectively.
2.4. Photocatalytic, Scavengers, and Recyclability Tests
The photocatalytic removal of ciprofloxacin (CIP) as a model pollutant was carried out in a borosilicate reactor connected to a thermostatic bath maintained at 24 °C ± 1 °C. A 125 W UV lamp was employed in the experimental setup. The reaction system consisted of 0.5 g L−1 of photocatalyst and 20 mg L−1 of the model pollutants. Initially, the system was kept in the dark for 30 min, after which the UV lamp was switched on. Aliquots were collected at various time intervals throughout the experiment (0, 10, 20, 30, 45, 60, 90, and 120 min). The absorbance of CIP at 273 nm was measured using a Shimadzu UV-2700 spectrophotometer in the wavelength range of 200 to 400 nm. The degradation efficiency of the model pollutants was calculated using Equation (1).
where C0 and C are the initial and final contaminant concentrations, respectively.
For scavenger tests, several inhibitor reagents were employed to determine the main species involved in the drug removal process. The irradiation tests were performed using the same parameters as the initial photocatalysis tests (0.5 g L−1 of photocatalyst and 20 mg L−1 of model pollutant). The system was left in the dark for 30 min, after which the lamp was turned on and samples were taken at intervals for a total of 120 min. During these tests, a specific amount of each inhibitor was added at the beginning of the reaction. Then, EDTA (0.0075 g), methyl alcohol (Met(OH)) (406 μL), and chloroform (CHCl3) (406 μL) were considered to trap active species such as holes (h+), hydroxyl radicals (•OH), and superoxide radicals (•O2−), respectively.
The recyclability tests were conducted over three cycles to assess the stability and reusability of the materials. The initial conditions involved maintaining 0.5 g L−1 of photocatalyst and 20 mg L−1 of model pollutants in 100 mL of solution throughout the tests. The system was kept in the dark for 30 min and then exposed to UV light for a maximum of 120 min. For each cycle, a fresh model solution was utilized, and the material was recovered through centrifugation, dried, and then reused in the subsequent cycle.
3. Results
3.1. Structural Analysis of Natural and Doping Zn0.5Cu0.5O Nanopowder of Halloysite
Figure 2 presents the X-ray diffraction (XRD) patterns of halloysite-based samples. The diffractogram in Figure 2a corresponds to dehydrated halloysite, commonly referred to as halloysite (7 Å), doping ZnO and CuO nanopowders, while Figure 2b represents natural, hydrated halloysite (10 Å). The XRD pattern of the Zn0.5Cu0.5O@Hal (ZCHal) nanocomposite, which corresponds to dehydrated halloysite (Figure 2a), was indexed according to the ICDD card 00-029-1487. This mineral has the empirical chemical formula Al2Si2O5(OH)4, exhibits a hexagonal structure, and belongs to the primitive space group. Characteristic diffraction peaks of the halloysite structure were observed at 2θ values of 12.4°, 20.0°, 24.8°, 35.6°, 39.2°, 55.2°, and 62.6°. These peaks are attributed to the (001), (100), (002), (110), (003), (210), and (300) crystallographic planes, respectively [24,25,26]. Additionally, a weaker reflection was noted at 48.0°, which is associated with secondary crystalline phases. The peak at 2θ = 12.4°, indexed as the (001) plane, corresponds to a basal spacing (d001) of approximately 7.13 Å. This is consistent with halloysite (7 Å)’s structural characteristics, which are typically associated with dehydrated tubular morphologies. In the XRD pattern of the Zn0.5Cu0.5O@Hal nanocomposite (Figure 2a), no distinct reflections of ZnO or CuO were observed. This absence is attributed to the relatively low nominal loadings of ZnO and CuO (≈12 wt% each with respect to halloysite), combined with their nanoscale dispersion within the clay matrix. These factors suppress or broaden the oxide reflections, making them indistinguishable against the dominant halloysite background. Comparable behavior has been documented in other oxide–clay systems prepared with low loadings [27].
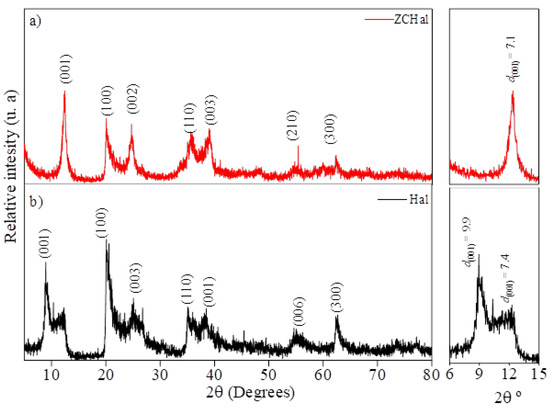
Figure 2.
X-ray diffraction (XRD) patterns of halloysite samples: (a) dehydrated halloysite, doping Zn0.5Cu0.5O nanopowder; and (b) raw hydrated halloysite.
The XRD pattern of raw hydrated halloysite (Figure 2b) corresponds to ICDD card 00-029-1489, which is associated with Al2Si2O5(OH)4·2H2O and exhibits a hexagonal primitive space group. The main diffraction peaks appear at 2θ angles of 8.9°, 20.3°, 25.4°, 35.3°, 38.5°, 54.9°, and 62.6°. These peaks are attributed to the (001), (100), (003), (110), (006), (210), and (300) planes, respectively [25]. Additionally, a weak reflection at 11.9° was detected, which corresponds to interlayer water and indicates an interlayer distance of approximately 7.4 Å [28]. The basal reflection at 2θ = 8.9°, related to the (001) plane, suggests an interlayer distance of around 9.9 Å, thus confirming the presence of halloysite (10 Å) in its hydrated state. These two expanded phases occur in samples with varying degrees of hydration [26,29].
The basal reflection of halloysite shifted from 2θ = 8.9° (d001 ≈ 9.9 Å), characteristic of hydrated halloysite (10 Å), to 2θ = 12.4° (d001 ≈ 7.1 Å), which corresponds to dehydrated halloysite (7 Å). This shift indicates a structural transformation associated with the removal of interlayer water during hydrothermal treatment and partial dehydroxylation, further influenced by the incorporation of ZnO and CuO nanoparticles that promote contraction of the interlayer spacing. Similar dehydration-induced transitions have been widely documented for halloysite systems [2,26]. In addition, a weak reflection was observed at 2θ ≈ 48.0°, which we attribute to secondary crystalline domains or minor distortions in the aluminosilicate framework. Comparable features have been reported in halloysite subjected to heating or surface modification, often linked to transitional aluminosilicate phases or disordered stacking [30]. Although no distinct ZnO or CuO reflections were detected, most likely due to their low loadings and nanoscale dispersion, the persistence of this secondary feature is consistent with earlier studies on halloysite-based nanocomposites [1].
The variation in basal spacing observed between the natural and modified halloysite samples suggests a structural transformation resulting from dehydration by thermal route treatment [26,29]. Additionally, the addition of metallic nanoparticles appears to have slightly altered the halloysite structure, resulting in minor changes in the arrangement of the materials, as observed from plane d001. However, placing these nanoparticles on the inside and outside surfaces of the clay does not alter the layered structure of halloysite, as the crystal planes remain unchanged after heating.
Figure 3 shows the Raman spectra of Zn0.5Cu0.5O@Hal (Figure 3a) and natural halloysite (Figure 3b). In Figure 3b, corresponding to hydrated halloysite (10 Å), the vibrational modes observed in the lattice region are primarily attributed to structural units composed of distorted AlO6 octahedra, Si2O4 tetrahedra, O–H–O groups, and hydroxyl bending modes, in agreement with previous studies [31,32,33,34,35]. The main Raman-active bands in this work are summarized in Table 1, with spectral assignments based on the literature [31,32,33,34,35]. The Raman spectra confirm the formation of the ZnO–CuO/halloysite nanocomposite. The emergence of Zn–O and Cu–O vibrational features, accompanied by slight shifts and intensity variations in the halloysite bands, provides evidence of strong interfacial interactions and effective dispersion of the metal oxides on the halloysite surface, consistent with previous reports on oxide–clay nanocomposites [1,36]. In the low-frequency region (50–150 cm−1), bands typically associated with halloysite morphologies were observed. These features are attributable to the structural framework of halloysite and are characteristic of its tubular morphology, in agreement with previous studies describing the vibrational signatures of halloysite nanotubes [1,37]. Notably, the band at 130 cm−1 corresponds to the symmetric bending mode of the Si2O5 unit [32,34]. Distinct bands were observed at 202, 246, and 274 cm−1, attributed to modes associated with AlO6 octahedra and symmetric and antisymmetric stretching of the O–H–O triangular group involved in hydrogen bonding between SiO4 tetrahedra and OH groups [31,33,34].
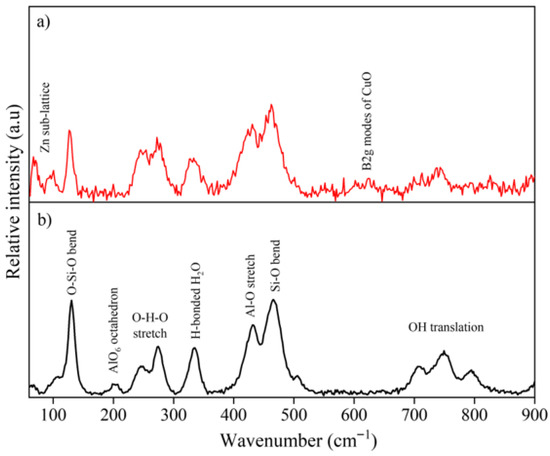
Figure 3.
Raman spectra of halloysite samples: (a) Zn0.5Cu0.5O@Hal nanocomposite (ZCHal) and (b) natural halloysite (10 Å).

Table 1.
The main Raman bands of this work are shown with spectral assignments based on the literature. The Raman bands were analyzed using the Igor Pro software 6.4.
Halloysite showcases pronounced Raman bands at 335, 432, and 466 cm−1, complemented by a more subtle band at 505 cm−1. The presence of these distinct spectral features is rooted in the diminished symmetry of the SiO4 units within the mineral’s structure. This phenomenon can be specifically attributed to the bending vibrations of the silicon–oxygen (Si–O) bonds, which play a crucial role in the vibrational characteristics observed in the Raman spectra [32,34]. The band modes at 707, 748, and 796 cm−1 were assigned to Al–OH and O-H translational modes, typical of hydrated halloysite phases. For the Zn0.5Cu0.5O@Hal sample (see Figure 3a), characteristic modes of ZnO and CuO were observed, along with peaks attributed to the clay mineral structure. The peak at approximately 97 cm−1 (E2low) corresponds to Zn sublattice vibrations in the wurtzite structure of ZnO. The peak at 582 cm−1 is associated with the A1 (LO) mode of ZnO [11,39,43]. A prominent peak at around 622 cm−1, assigned to the B2g mode of CuO, confirms the formation of a ZnO–CuO heterojunction during synthesis. This indicates structural and electronic modifications resulting from Cu doping [39,44,45]. Some overlap may occur, such as the mode at approximately 430 cm−1 (E2high), which reflects vibrations of oxygen atoms within the lattice, effects typically enhanced by Cu2+ incorporation in the ZnO lattice. Furthermore, the mode at about 333 cm−1 represents the combination of E2high and E2low, associated with two-phonon processes in ZnO [39,46].
The slight shifts and intensity variations observed in the Raman spectra of ZnO–CuO@Hal compared to pristine halloysite are attributed to interfacial interactions with the metal oxides, which induce local strain and lattice distortions in the aluminosilicate framework. These effects, together with possible charge-transfer processes, have also been reported in other oxide–clay nanocomposites [1,36]. In addition, partial orbital hybridization at the Zn-O-Si and Cu-O-Al interfaces may also contribute to the band displacements, providing further evidence of strong coupling between the nanoparticles and the halloysite matrix. Notably, the band at 202 cm−1 (A1g (υ1) of AlO6 in halloysite) was absent in the Zn0.5Cu0.5O@Hal spectrum, suggesting structural modification due to doping. The coexistence of Raman modes for both ZnO and CuO in the nanocomposite further corroborates the formation of ZnO-CuO nanohybrids and the incorporation of Cu within the ZnO lattice, supported by the halloysite matrix. The detailed Raman spectra and the main band assignments are shown in Figure 3 and Table 1.
3.2. Morphological Investigation
Figure 4 shows the Transmission Electron Microscopy (TEM) images of the Zn0.5Cu0.5O@Hal nanocomposite. Figure 4a displays an image of the characteristic tubular morphology of halloysite nanotubes, with Zn0.5Cu0.5O nanoparticles non-homogeneously distributed and anchored on the external surfaces of the nanotubes. Dimensional analysis based on the TEM micrograph revealed that the doped halloysite nanotubes exhibited an average internal diameter of approximately 22 nm, an external diameter of 82 nm, and an average length of around 600 nm. Figure 4b presents a high-resolution TEM (HRTEM) image, highlighting the well-defined lattice fringes of Zn0.5Cu0.5O nanoparticles, with an average particle size of approximately 5 nm. Notably, these nanoparticles are also observed within the inner cavity of the halloysite nanotubes, confirming effective internal and external decoration of the nanotubular structures [12,47,48,49]. Figure 5 presents the energy-dispersive X-ray spectroscopy (EDS) analysis of the Zn0.5Cu0.5O@Hal composite. The EDS spectrum shows not only the expected Al, Si, and O signals from halloysite, but also distinct Lα (low energy) and Kα (high energy) emission lines for both Cu and Zn. The detection of multiple characteristic transitions, acting as elemental fingerprints, provides robust confirmation of the presence of these metallic species in the nanocomposite. These signals suggest the effective anchoring of ZnO and CuO nanoparticles onto the external surfaces of the clay nanotube.
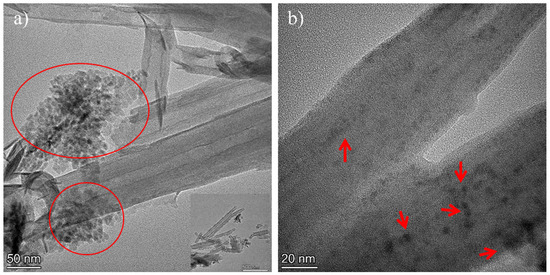
Figure 4.
TEM images of the Zn0.5Cu0.5O@Hal nanocomposite. The arrows and circles (a) indicate ZnO–CuO nanoparticles located on the external surface of halloysite in a bar scale of 50 nm and in (b), inside the cavity of the clay mineral nanotubes, represented in 20 nm.
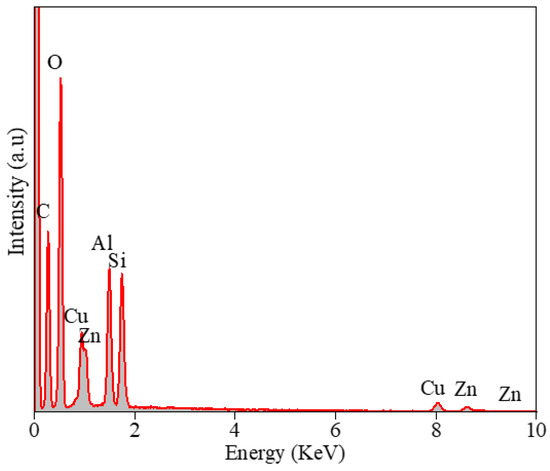
Figure 5.
Energy-dispersive X-ray spectroscopy (EDS) of the Zn0.5Cu0.5O@halloysite.
3.3. Optical Properties
Figure 6 displays the photoluminescence (PL) spectra of the Zn0.5Cu0.5O@Hal nanocomposite (ZCHal), revealing multiple overlapping emission characteristics of complex defect states within the system. In ZnO, deep-level emissions (DLEs) are commonly observed, manifesting as a broad band in the visible region. These emissions are typically attributed to defect-mediated recombination processes, such as the recombination of photogenerated holes with electrons trapped in ionized oxygen vacancies (VO+) [39,50,51,52]. In addition, zinc-related defects, including zinc vacancies (VZn) and zinc interstitials (Zni), are known to generate bluish luminescence, whereas neutral oxygen vacancies (VO0) and oxygen interstitials tend to emit at longer wavelengths [53]. For the Zn0.5Cu0.5O@Hal nanocomposite, it is expected that the optical response is predominantly influenced by ZnO defect emissions, as CuO, having a narrower band gap (~1.4–1.5 eV), exhibits intrinsically weak luminescence in the visible range [44]. In ZnO–CuO heterojunctions, CuO acts as a p-type semiconductor and effectively functions as an electron acceptor, while ZnO traps photogenerated holes. This facilitates charge carrier separation at the interface and suppresses radiative recombination. Indeed, previous studies have reported significant quenching of ZnO near-band-edge luminescence upon CuO incorporation, with the PL intensity decreasing substantially [54].
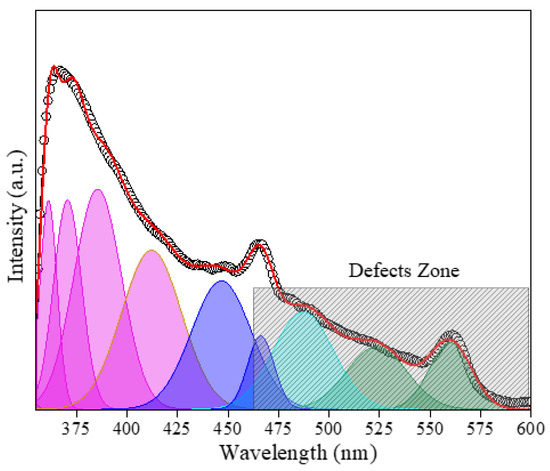
Figure 6.
PL spectra of Zn0.5Cu0.5O@Hal nanocomposite and deconvolutions using the Gaussian function, revealing multiple overlapping emission characteristics of defect states. Blue color represents zinc vacancies and green color represents oxygen vacancies.
Moreover, the presence of halloysite nanotubes, which are rich in surface hydroxyl groups and Al/Si sites, introduces additional complexity to the defect-related emissions. Halloysite-supported ZnO systems have demonstrated markedly reduced PL intensities compared to pure ZnO, indicating a lower rate of radiative recombination, likely due to enhanced charge separation [55]. Similarly, ZnO-CuO composites incorporated within montmorillonite matrices also exhibit attenuated PL relative to pure ZnO [56]. These observations suggest that in the ZnO–CuO@Hal system, the synergistic effects of heterojunction formation and clay mineral interfaces promote charge carrier separation. Fixed negative charges on the halloysite surface may act as traps for ZnO vacancies at the composite interface, thereby suppressing bulk recombination [55]. Conversely, halloysite’s hydroxyl groups may introduce additional surface recombination centers, further modulating the visible emissions associated with ZnO defects [57]. Although the PL spectrum of the Zn0.5Cu0.5O@Hal composite can be deconvoluted into several visible bands, the multicomponent nature of the system prevents unambiguous assignment of each emission to a specific defect. Instead, the broad visible luminescence is attributed to the overlap of ZnO-related defect states (oxygen vacancies and zinc interstitials), modulated by CuO domains and by interfacial interactions with halloysite surfaces.
To estimate the energy band gap (Eg) of the Zn0.5Cu0.5O@Hal nanocomposite, Tauc plot analysis was performed using diffuse reflectance spectroscopy (DRS) data (Figure 7). The reflectance (R(λ)) was converted into the apparent absorption coefficient via the Kubelka–Munk function, defined as F(R) = (1 − R)2/(2R), enabling approximation of optical absorption in powder samples. Assuming a direct allowed transition, consistent with the electronic band structure of ZnO and often employed for CuO, the plot of [F(R)·hν]2 versus photon energy (hν) was constructed. The linear segment of the highest slope was extrapolated to intersect the energy axis, yielding an estimated Eg [53]. Bulk ZnO typically exhibits band-gap values in the range of ~3.2–3.3 eV, while CuO shows narrower gaps of ~1.4–1.5 eV, reflecting its strong p-type character. In the present study, the Zn0.5Cu0.5O@Hal composite exhibited an Eg of 3.02 eV (Figure 7). This value lies below that of pristine ZnO but remains higher than CuO, suggesting that the composite’s optical response arises from a synergistic interaction between ZnO and CuO domains. The heterojunction interface likely facilitates charge transfer and introduces electronic coupling effects that modify the intrinsic electronic structure of each oxide. Additionally, halloysite nanotubes exert a significant influence on the composite’s band gap. This is because the ZnO-CuO–halloysite interfaces can generate intermediate electronic states within the band gap due to partial electronic hybridization and strain-induced defects. Similar effects have been reported in ZnO-CuO–montmorillonite systems, where additional absorption features near ~2.50 eV were attributed to interfacial states [56]. At the molecular level, the formation of M–O–Si or M–O–Al bonds (M = Zn, Cu) at halloysite surfaces, confirmed by X-ray photoelectron spectroscopy (XPS) studies detecting Zn–O–Si contributions at ~533 eV, supports the existence of hybridized orbitals (Zn 4s/O 2p with Si 3p) [58]. These hybrid states could act either as deep traps, limiting charge carrier mobility, or as moderate energy levels, improving sub-band-gap optical transitions. Additionally, the mismatch between the hexagonal ZnO lattice, the monoclinic CuO lattice, and the tubular layered silicate structure of Hal introduces interfacial stresses. Such lattice distortions can lead to structural defects, including dislocations and interfacial oxygen vacancies, which further modulate the band structure. A comparable reduction in band gap was observed in ZnO/kaolinite composites, where Eg decreased from 3.21 eV (pure ZnO) to ~2.44 eV upon addition of only 1.25 wt% kaolinite [59]. In the ZnO@Hal nanocomposite, a slight redshift (from 3.053 to 3.026 eV) was also observed, which was attributed to the increase in oxygen vacancies and surface interactions [55].
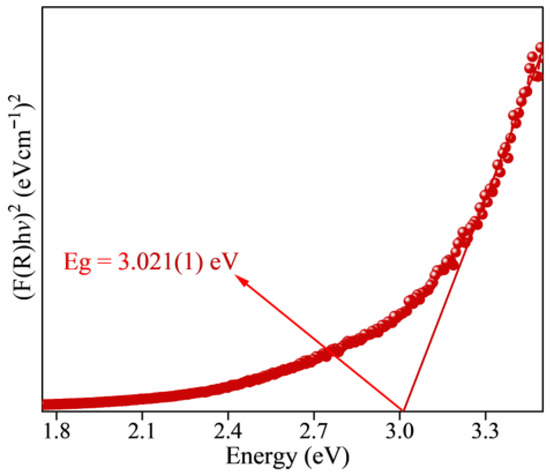
Figure 7.
Energy band gap for the Zn0.5Cu0.5O@Hal nanocomposite obtained by the hydrothermal method. The arrow indicates the direct band gap value.
The dual role of halloysite’s –OH groups also merits consideration. These functional groups may passivate ZnO surface oxygen vacancies and reduce non-radiative recombination centers, while simultaneously acting as chemically active sites capable of introducing additional shallow trap states [57]. Collectively, these interfacial phenomena, electronic hybridization, strain-induced defect formation, and surface chemical interactions, contribute to the observed modifications in both the photoluminescence (PL) and band-gap properties of the Zn0.5Cu0.5O@Hal nanocomposite (Figure 6 and Figure 7).
4. Photocatalytic Experiments
Photocatalytic tests were conducted to evaluate the performance of a halloysite-based nanocomposite supported by a ZnO-CuO heterojunction for the removal of ciprofloxacin from a solution. For these tests, 0.5 g L−1 of the photocatalyst and a solution containing 20 mg L−1 of the model pollutant were utilized. Figure 8 illustrates the time at which the system reached adsorption/desorption equilibrium in dark conditions (Figure 8a) and the relationship between C/C0 and irradiation time (Figure 8b). The results indicate a total removal rate of up to 76% for the drug. Initially, ciprofloxacin was adsorbed onto the surface of the nanocomposite, achieving a removal rate of up to 34% in the first 30 min of the reaction. Following the irradiation of the material with a UV source, an additional 42% removal via oxidative degradation of the drug was observed in 120 min. It can be concluded that a synergistic effect of adsorption and photodegradation occurred with the use of the Zn0.5Cu0.5O@Hal nanocomposite. These findings are consistent with previous studies suggesting that the use of clay mineral supports enhances the interaction between the target pollutant and the material’s surface [60,61,62,63]. This improved interaction allows for greater contact between the adsorbed pollutant molecules and the semiconductor nanoparticles incorporated into the clay mineral support. The combination of adsorption and photocatalysis effectively facilitates the removal of organic compounds from the solution. Electrostatic and hydrophobic interactions may have taken place between the ciprofloxacin molecules and the surface of halloysite modified with ZnO and CuO nanoparticles. This phenomenon likely aided in the pre-adsorption of the drug, thereby improving the overall photocatalytic process [60]. Several studies have indicated that a combination of adsorptive and photocatalytic processes can occur in systems utilizing clay minerals, and these systems have proven to be efficient and stable for the removal of various pollutants, including dyes and pharmaceuticals [64,65,66].
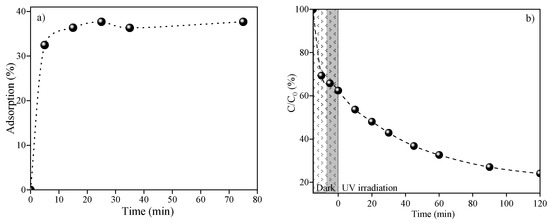
Figure 8.
(a) Adsorption rate versus time reaction in dark conditions and (b) (C/C0) versus time irradiation using Zn0.5Cu0.5O@Hal for ciprofloxacin removal under UV light.
Inhibitor tests were performed to investigate the mechanism by which the nanocomposite eliminates ciprofloxacin. These tests are vital for identifying the primary reactive species involved in the degradation of the molecule [27]. Gaining insight into these species is essential for elucidating the reaction mechanism and understanding the role of reactive agents such as radicals or electron intermediates. In summary, these tests clarify the reaction mechanism and contribute to the advancement of more efficient and selective materials. In Figure 9, the outcomes of the imprisonment tests are presented, demonstrating that the addition of EDTA does not influence the catalyst’s efficiency. This implies that holes do not have a direct role in the degradation of ciprofloxacin when using the catalyst.
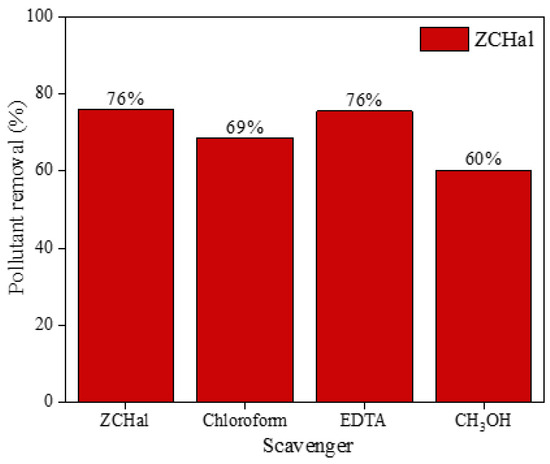
Figure 9.
Scavenger experiments using Zn0.5Cu0.5O@Hal nanocomposite for ciprofloxacin removal under UV light. EDTA (0.0075 g), methyl alcohol (Met(OH)) (406 μL), and chloroform (CHCl3) (406 μL) were considered to trap active species such as holes (h+), hydroxyl radicals (•OH), and superoxide radicals (•O2−), respectively.
In contrast, the incorporation of benzoquinone and methanol has been shown to reduce the material’s activity when exposed to UV light. These observations suggest that both superoxide and hydroxyl radicals are integral to the reaction mechanism, with hydroxyl radicals playing a predominant role. These findings are consistent with existing research in the field [11,60,67,68,69]. Ciprofloxacin undergoes photodegradation primarily through cleavage of the piperazine ring, defluorination, deamination, and decarboxylation, resulting in smaller molecular fragments. The quinolone ring is also susceptible to oxidation and chain scission, particularly at the carboxyl group and adjacent double bond. Several intermediate products, such as alcohols, acids, phenols, ketones, amines, and amides, have been identified [67,70,71,72,73,74,75]. These fragments can be degraded by oxygen radicals, leading to the formation of smaller, less toxic molecules.
Additionally, Table 2 provides a comparison of various studies from the literature that have explored the use of nanocomposites derived from different clays and clay minerals for the photocatalytic removal of ciprofloxacin.

Table 2.
Comparative studies using different semiconductors supported in clay minerals for ciprofloxacin degradation have been conducted.
As shown in Table 2, the photocatalytic efficiency of the materials is strongly influenced by multiple factors, including catalyst loading, pollutant concentration, irradiation source, and reaction time. For instance, increasing the dosage of ZnO/BENT led to 97% ciprofloxacin removal within 180 min under sunlight irradiation, whereas a lower concentration of g-C3N4/REC achieved only 70% degradation after 360 min under visible light. Likewise, in the cases of Ag-TiO2/rGO/HAL and ZnO/CuO/HAL, UV irradiation played a decisive role in enhancing the photocatalytic activity of the materials.
4.1. Degradation Reaction Mechanism
The photocatalytic removal process of pollutants using heterogeneous photocatalysts typically involves several steps: (i) transfer of the pollutant to the catalyst surface after initial contact; (ii) adsorption and interfacial interaction of pollutants on the catalyst; (iii) photoactivation of the material; (iv) decomposition of the adsorbed molecules through redox reactions; (v) disposal of the reaction by-products; and (vi) removal of by-products from the catalyst surface. The general mechanism, as outlined in Equations (2)–(10), is well established in the literature [79,80,81,82,83,84]. When a semiconductor is exposed to light with energy equal to or greater than its band-gap energy, electrons are excited from the valence band to the conduction band, generating electron–hole pairs. The electrons and photogenerated holes then participate in reduction and oxidation reactions involving the adsorbed contaminant molecules on the catalyst surface, leading to the degradation of the pollutants. Electrons reduce oxygen to form superoxide radicals, while holes react with water or hydroxide ions to generate hydroxyl radicals. The recombination of these electron–hole pairs can decrease the efficiency of the material. To enhance efficiency and reduce recombination rates of the photogenerated charges, various strategies are employed, such as doping, forming heterojunctions, or using supports.
Photocatalyst + hv → e−(CB) + h+(VB)
H2O(ads) + h+ → •OH(ads) + H+(ads)
h+ + OH− → •OH(ads)
O2 + e− → •O2−(ads)
•O2−(ads) + H+ → HO2•(ads)
HO2•(ads) → O2 + H2O2(ads)
H2O2 + •O2− → OH− + •OH + O2
H2O2(ads) + hv → 2 •OH(ads)
Ciprofloxacin (•OH or •O2−) → drug degradation
The ZnO-CuO heterojunction is particularly effective in charge separation. Due to the differences in the Fermi levels of the two materials, electrons from ZnO migrate to CuO through the contact interface, creating an excess of positive charges at the ZnO interface and negative charges at the CuO interface, in turn forming an internal electric field [85]. Consequently, the electrons in the conduction band of CuO migrate to the conduction band of ZnO, while the holes move in the opposite direction. This process occurs under irradiation and is influenced by the internal electric field. Following this, the electrons in the ZnO conduction band react with oxygen molecules to produce superoxide radicals, while the holes in the valence band of CuO react with water to form hydroxyl radicals. These photogenerated radicals are responsible for the oxidative degradation of the target pollutants. Halloysite serves as an effective functional support for nanoparticles, increasing the availability of distinguishable active sites due to its high surface reactivity, large surface area, and porosity. This enhances the interfacial contact between ciprofloxacin molecules and the ZnO-CuO heterojunction [86]. Recent studies also highlight the benefits of using clay minerals to support photocatalysts, indicating that these materials not only act as supports but also help reduce the recombination rate of photogenerated charges. This leads to increased concentration and mobility of the charges, further enhancing the photocatalytic performance of nanocomposites [87,88,89,90]. Another major advantage of using clay minerals in these systems is that the material can be reused, making it easier to recover the catalyst from the reaction environment and increasing its practicality in real-world applications.
4.2. Exploring Nanocomposite Reusability
Evaluating the reusability and long-term stability of photocatalysts is crucial for assessing their practical applicability and cost-efficiency in large-scale operations. Figure 10 displays the photocatalytic performance of the ZCHal nanocomposite over three consecutive degradation cycles. Notably, the pollutant removal efficiency remained statistically consistent, ranging from 75% to 77%, with minimal fluctuation well within the experimental error margins. This stable behavior indicates that the nanocomposite exhibits excellent recyclability, with minimal loss of photocatalytic activity.
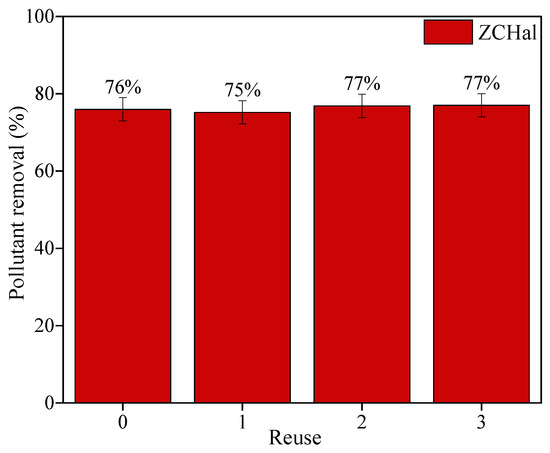
Figure 10.
Reuse tests using Zn0.5Cu0.5O@Hal nanocomposite in ciprofloxacin removal under UV light. The initial conditions involved maintaining 0.5 g L−1 of photocatalyst and 20 mg L−1 of model pollutants in 100 mL of solution throughout the tests.
The stability and recyclability of the dehydrated halloysite doped with Zn0.5Cu0.5O nanopowder were evaluated through reuse experiments and confirmed by XRD results (Figure 11). Figure 11a shows the nanocomposite before regeneration tests. As shown in Figure 11b, the composite exhibits only a slight and gradual reduction in photocatalytic efficiency over three consecutive runs. This decline can be attributed to the accumulation of intermediate by-products and minor structural modifications induced during reuse. The incorporation of Zn0.5Cu0.5O into the halloysite matrix enhances material stability, making the nanocomposite a promising candidate for sustainable photocatalytic applications. To further confirm structural robustness, XRD analyses were performed on the composite recovered after three reuse cycles. The diffraction patterns (Figure 11) display reflections at (001), (100), (002), (110), (003), (210), and (300), which are characteristic of dehydrated halloysite (7 Å), typically associated with tubular morphologies. Additionally, a peak at 26.6° (assigned to quartz (011)) was detected, accompanied by a relative decrease in the intensity of the halloysite (001) reflection [91]. This effect may result from partial structural rearrangements caused by repeated photocatalytic processes and subsequent drying at 100 °C. Nevertheless, the persistence of the main halloysite reflections demonstrates that the tubular framework remains largely preserved after multiple reuse cycles, confirming the composite’s structural integrity.
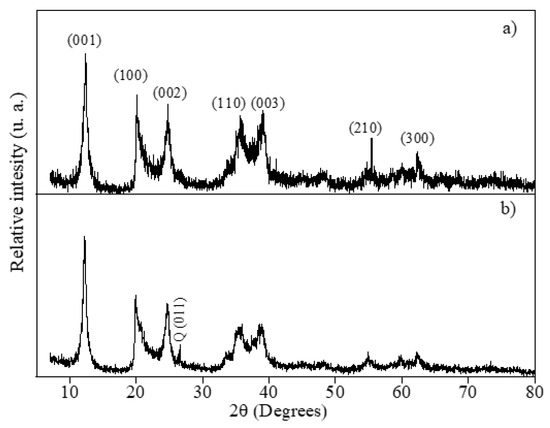
Figure 11.
XRD patterns of the irradiation tests using Zn0.5Cu0.5O@Hal nanocomposite (a) before and (b) after reuse tests in ciprofloxacin removal under UV light.
The observed stability can be attributed to several physicochemical factors. First, the structural integrity of the composite appears to be preserved during the photocatalytic cycles, suggesting resistance to photo-corrosion and leaching of active components, which are often major limitations in transition metal-doped systems. The strong interaction between ZnO and the halloysite matrix likely contributes to this stability, as the tubular morphology of halloysite may offer anchoring sites that prevent nanoparticle agglomeration and degradation under irradiation. Furthermore, the retention of activity suggests that no significant surface fouling or blocking of active sites occurred during the reactions, maintaining the accessibility of photoactive regions. The ease of recovery, through simple sedimentation or centrifugation, without the need for thermal or chemical reactivation, further underscores the material’s robustness. This not only reduces operational costs but also minimizes secondary waste generation, aligning with sustainable wastewater treatment strategies. Taken together, the ability of ZCHal to sustain its photocatalytic performance across multiple cycles reinforces its potential for practical applications in environmental remediation and supports its viability for upscaling.
5. Conclusions
The nanocomposite Zn0.5Cu0.5O@Hal was successfully synthesized using the hydrothermal method. The resulting material demonstrated structural, morphological, and optical properties that significantly enhance its photocatalytic performance. The interface between halloysite and the semiconductor improved electron transfer and facilitated charge separation. Furthermore, pre-adsorption increased the semiconductor’s efficiency by promoting the diffusion of the supported material. The findings of this study highlight the potential of the halloysite-based nanocomposite. The effective integration of the nanotubular structure of halloysite with the incorporated ZnO and CuO nanoparticles resulted in a substantial degradation of ciprofloxacin, achieving up to 76%. Consequently, this material stands out for its high efficiency, stability, and low environmental impact, effectively combining nanotechnology with sustainability. The utilization of halloysite as an intercalating matrix in photocatalytic processes can transform environmental remediation strategies, making a significant contribution to reducing pollutants in aquatic ecosystems.
Author Contributions
W.A.A., A.J.N.F., Y.R.-B., S.M.-C. and, M.d.M.O. contributed to conceptualization, data curation, investigation, methodology, visualization, writing—original draft, and writing—review and editing; P.T. and R.R.P.-G. contributed to conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, resources, supervision, validation, visualization, writing—original draft, and writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
The authors are grateful to the Brazilian Agencies: National Council for Scientific and Technological Development (CNPq), Coordination for the Improvement of Higher Education Personnel (CAPES), Financing Agency for Studies and Projects (FINEP), and Foundation for Science and Technology Support of Pernambuco (FACEPE).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Papoulis, D. Halloysite based nanocomposites and photocatalysis: A Review. Appl. Clay Sci. 2019, 168, 164–174. [Google Scholar] [CrossRef]
- Drits, V.A.; Sakharov, B.A.; Hillier, S. Phase and structural features of tubular halloysite (7 Å). Clay Miner. 2018, 53, 691–720. [Google Scholar] [CrossRef]
- Gianni, E.; Pospíšil, M.; Scholtzová, E. Halloysite as a carrier/sorbent of diclofenac: A molecular simulations study. Surf. Interfaces 2025, 70, 106710. [Google Scholar] [CrossRef]
- Fahimizadeh, M.; Wong, L.W.; Baifa, Z.; Sadjadi, S.; Auckloo, S.A.B.; Palaniandy, K.; Pasbakhsh, P.; Tan, J.B.L.; Singh, R.K.R.; Yuan, P. Halloysite clay nanotubes: Innovative applications by smart systems. Appl. Clay Sci. 2024, 251, 107319. [Google Scholar] [CrossRef]
- Filice, S.; Bongiorno, C.; Libertino, S.; Compagnini, G.; Gradon, L.; Iannazzo, D.; La Magna, A.; Scalese, S. Structural characterization and adsorption properties of dunino raw halloysite mineral for dye removal from water. Materials 2021, 14, 3676. [Google Scholar] [CrossRef]
- Filice, S.; Bongiorno, C.; Libertino, S.; Gradon, L.; Iannazzo, D.; Scalese, S. Photo-Fenton Degradation of Methyl Orange with Dunino Halloysite as a Source of Iron. Catalysts 2022, 12, 257. [Google Scholar] [CrossRef]
- Danyliuk, N.; Tomaszewska, J.; Tatarchuk, T. Halloysite nanotubes and halloysite-based composites for environmental and biomedical applications. J. Mol. Liq. 2020, 309, 113077. [Google Scholar] [CrossRef]
- Zsirka, B.; Vágvölgyi, V.; Horváth, E.; Juzsakova, T.; Fónagy, O.; Szabó-bárdos, E.; Kristóf, J. Halloysite-Zinc Oxide Nanocomposites as Potential Photocatalysts. Minerals 2022, 12, 476. [Google Scholar] [CrossRef]
- Yu, H.; Xu, H.; Hao, T.; Yuan, Y.; Zhang, B.; Wang, H.; Shao, G.; Fan, B.; Lu, H. Facile synthesis of ZnO/halloysite nanotube composite with greatly enhanced photocatalytic performance. Colloids Surf. A Physicochem. Eng. Asp. 2024, 688, 133633. [Google Scholar] [CrossRef]
- Aghababaei, N.; Abdouss, M.; Hosseini-Monfared, H.; Ghanbari, F. Photocatalytic degradation of diclofenac using a novel double Z-scheme catalyst (O-g-C3N4/ZnO/TiO2@halloysite nanotubes): Degradation mechanism, identification of by-products and environmental implementation. J. Water Process Eng. 2023, 53, 103702. [Google Scholar] [CrossRef]
- Albuquerque, W.; Trigueiro, P.; Silva, B.V.; Neves, L.; Almeida, L.C.; Peña-Garcia, R.R. A novel RuO2@ZnO-Alginate-Halloysite composite for the effective degradation of Eosin Yellow dye and Ciprofloxacin drug. Mater. Res. Bull. 2024, 182, 113178. [Google Scholar] [CrossRef]
- Pouthika, K.; Madhumitha, G. Tailoring interfacial charge separation in Z-Scheme CuO@TiO2@halloysite heterostructure for efficient photocatalytic removal of Congo red. J. Taiwan Inst. Chem. Eng. 2024, 166, 105752. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, L.; Alshammari, D.A.; Hessien, M.M.; Yu, W.; Cui, L.; Ren, J.; El-Bahy, Z.M.; Guo, Z. Z-scheme Ag2O/ZnO heterostructure on carbon fibers for efficient photocatalysis of tetracycline. Sep. Purif. Technol. 2025, 354, 129414. [Google Scholar] [CrossRef]
- Shoran, S.; Dahiya, S.; Rani, M.; Nehra, S.; Sharma, A.; Chaudhary, S. Synergistic photocatalysis of VO2-A/g-C3N4 composites for efficient degradation of anionic and cationic dyes: Towards a sustainable environmental solution. Appl. Surf. Sci. 2025, 684, 161852. [Google Scholar] [CrossRef]
- Xue, Q.; Lin, H.; Feng, Q.; Yang, Y.; Dong, M.; Hu, K.; Song, B.; Goh, P.S.; Shen, X. Synergistic photocatalysis and fenton-like process driven by a biochar-supported biochar/iron hydroxide oxide/bismuth molybdate S-type heterojunction for tetracycline degradation: Mechanistic insights and degradation pathways. Appl. Surf. Sci. 2025, 679, 161277. [Google Scholar] [CrossRef]
- Dalhatou, S.; Sali, M.; Tetteh, S.; Boubakari, A.; Talami, B.; Zeghioud, H.; Kane, A.; El Jery, A.; Assadi, A.A.; Obada, D.O. Sorbent and Photocatalytic Potentials of Local Clays for the Removal of Organic Xenobiotic: Case of Crystal Violet. Catalysts 2022, 12, 899. [Google Scholar] [CrossRef]
- Kowalczyk, A.; Rutkowska, M.; Gnyla, S.; Pacia, M.; Chmielarz, L. Synergistic Effect of Co and Ni Co-Existence on Catalytic Decomposition of Ammonia to Hydrogen—Effect of Catalytic Support and Mg-Al Oxide Matrix. ChemEngineering 2024, 8, 55. [Google Scholar] [CrossRef]
- Mylarappa, M.; Raghavendra, N.; Bhumika, N.R.; Chaithra, C.H.; Nagalaxmi, B.N.; Kumara, K.N.S. Study of ZnO nanoparticle-supported clay minerals for electrochemical sensors, photocatalysis, and antioxidant applications. ChemPhysMater 2024, 3, 83–93. [Google Scholar] [CrossRef]
- de Moraes, N.P.; Pereira, R.A.; da Silva, T.V.C.; da Silva, B.H.B.; de Assis, G.P.; Campos, T.M.B.; Thim, G.P.; de Vasconcelos Lanza, M.R.; de Freitas, L.; Rodrigues, L.A. Cross-linked cellulose beads as an eco-friendly support for ZnO/SnO2/carbon xerogel hybrid photocatalyst: Exploring the synergy between adsorption and photocatalysis under simulated sunlight. Int. J. Biol. Macromol. 2024, 254, 127826. [Google Scholar] [CrossRef]
- Nadeem, M.S.; Munawar, T.; Mukhtar, F.; Rabbani, A.W.; Rehman, N.U.; Mahmood, K.; Iqbal, F. Facile synthesis of PANI and rGO supported Y/Pr co-doped ZnO: Boosted solar light-driven photocatalysis. Appl. Phys. A Mater. Sci. Process. 2023, 129, 450. [Google Scholar] [CrossRef]
- Trigueiro, P.; Albuquerque, W.A.; Jerônimo, A.G.; Rodrigues, M.S.; França, E.L.T.; Peña-Garcia, R.R. CuO-TiO2–Saponite Ternary Nanocomposite for Efficient Removal of Bromocresol Green Dye. Minerals 2024, 14, 1268. [Google Scholar] [CrossRef]
- Lv, H.; Wang, P.; Lv, Y.; Dong, L.; Li, L.; Xu, M.; Fu, L.; Yue, B.; Yu, D. Piezo-Photocatalytic Degradation of Ciprofloxacin Based on Flexible BiVO4 PVDF Nanofibers Membrane. Catalysts 2025, 15, 163. [Google Scholar] [CrossRef]
- Monroy, L.H.; Tavares, J.R.; Dumont, M.-J. Photodegradation of ciprofloxacin using an alginate/TiO2 hydrogel for water remediation. J. Environ. Chem. Eng. 2025, 13, 115868. [Google Scholar] [CrossRef]
- Pham, X.N.; Nguyen, M.B.; Doan, H.V. Direct synthesis of highly ordered Ti-containing Al-SBA-15 mesostructured catalysts from natural halloysite and its photocatalytic activity for oxidative desulfurization of dibenzothiophene. Adv. Powder Technol. 2020, 31, 3351–3360. [Google Scholar] [CrossRef]
- Bayliss, P. Unit-Cell Dimensions of Two-Dimensionai Clay Minerals. Powder Diffr. 1989, 4, 19–20. [Google Scholar] [CrossRef]
- Siranidi, E.; Hillier, S.; Chryssikos, G.D. Structure of tubular halloysite-(10 Å) and its transition to halloysite-(7 Å) by infrared spectroscopy and X-ray diffraction. Clays Clay Miner. 2024, 72, e33. [Google Scholar] [CrossRef]
- Talami, B.; Zeghioud, H.; Dalhatou, S.; Bonnet, P.; Caperaa, C.; Ligny, R.; Assadi, A.A.; Massai, H.; Kane, A. A New Sunlight Active Photocatalyst Based on CuO-TiO2-Clay Composite for Wastewater Remediation: Mechanistic Insights and Degradation Optimization. Water Air Soil Pollut. 2024, 235, 104. [Google Scholar] [CrossRef]
- Ouyang, J.; Mu, D.; Zhang, Y.; Yang, H. Mineralogy and Physico-Chemical Data of Two Newly Discovered Halloysite in China and Their Contrasts with Some Typical Minerals. Minerals 2018, 8, 108. [Google Scholar] [CrossRef]
- Frost, R.L.; Kristof, J.; Horvath, E.; Kloprogge, J.T. Rehydration and Phase Changes of Potassium Acetate-Intercalated Halloysite at 298 K. J. Colloid Interface Sci. 2000, 226, 318–327. [Google Scholar] [CrossRef]
- Frost, R.L.; Xi, Y.; He, H. Synthesis, characterization of palygorskite supported zero-valent iron and its application for methylene blue adsorption. J. Colloid Interface Sci. 2010, 341, 153–161. [Google Scholar] [CrossRef]
- Kloprogge, J.T.; Frost, R.L. Raman microprobe spectroscopy of hydrated halloysite from a Neogene cryptokarst from Southern Belgium. J. Raman Spectrosc. 1999, 30, 1079–1085. [Google Scholar] [CrossRef]
- Frost, R.L.; Shurvell, H.F. Raman Microprobe Spectroscopy of Halloysite. Clays Clay Miner. 1997, 45, 68–72. [Google Scholar] [CrossRef]
- Le Ba, T.; Alkurdi, A.Q.; Lukács, I.E.; Molnár, J.; Wongwises, S.; Gróf, G.; Szilágyi, I.M. A novel experimental study on the rheological properties and thermal conductivity of halloysite nanofluids. Nanomaterials 2020, 10, 1834. [Google Scholar] [CrossRef] [PubMed]
- Frost, R.L.; Kristof, J. Intercalation of Halloysite: A Raman Spectroscopic Study. Clays Clay Miner. 1997, 45, 551–563. [Google Scholar] [CrossRef]
- Loh, E. Optical vibrations in sheet silicates. J. Phys. C Solid State Phys. 1973, 6, 1091–1104. [Google Scholar] [CrossRef]
- Liu, X.; Duan, Y.; Yang, X.; Huang, L.; Gao, M.; Wang, T. Enhancement of magnetic properties in FeCoNiCr0.4CuX high entropy alloys through the cocktail effect for megahertz electromagnetic wave absorption. J. Alloys Compd. 2021, 872, 159602. [Google Scholar] [CrossRef]
- Joussein, E.; Petit, S.; Churchman, J.; Theng, B.; Righi, D.; Delvaux, B. Halloysite clay minerals—A review. Clay Miner. 2005, 40, 383–426. [Google Scholar] [CrossRef]
- López, R.; Villa-Sánchez, G.; de la Cruz, I.V.; Encarnación-Gómez, C.; Castrejón-Sánchez, V.H.; Coyopol, A.; Mastache, J.E.; Leyva-Porras, C. Cupric oxide (CuO)/zinc oxide (ZnO) heterojunction diode with low turn-on voltage. Results Phys. 2021, 22, 103891. [Google Scholar] [CrossRef]
- Soares, A.S.; Araujo, F.P.; França, R.; Osajima, J.A.; Guerra, Y.; Castro-Lopes, S.; Silva-Filho, E.C.; Santos, F.E.; Almeida, L.C.; Viana, B.C.; et al. Effect of pH on the growth and ibuprofen photocatalytic response of Zn1−xCoxO compound synthesized by the co-precipitation method. J. Mater. Res. 2023, 38, 2439–2452. [Google Scholar] [CrossRef]
- Kloprogge, J.T.; Frost, R.L. Infrared emission spectroscopy of Al-pillared beidellite. Appl. Clay Sci. 1999, 15, 431–445. [Google Scholar] [CrossRef]
- Decremps, F.; Pellicer-Porres, J.; Saitta, A.M.; Chervin, J.-C.; Polian, A. High-pressure Raman spectroscopy study of wurtzite ZnO. Phys. Rev. B Condens. Matter Mater. Phys. 1998, 65, 92101. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, Q.; Fei, X.; He, Y.; Zhang, P.; Zhang, G.; Peng, L.; Xie, W. Synthesis of CuO nano- and micro-structures and their Raman spectroscopic studies. CrystEngComm 2010, 12, 2232. [Google Scholar] [CrossRef]
- Silva, M.; Trigueiro, P.; Jerônimo, A.; Barbosa, R.; Lins, A.; Albuquerque, W.; Araujo, F.P.; Osajima, J.A.; Peña-Garcia, R.R. Eco-friendly synthesis of Zn0.97La0.03O compound with natural polysaccharide and its application in methylene blue and eosin dyes discoloration. Mater. Lett. 2024, 363, 136256. [Google Scholar] [CrossRef]
- Kuriakose, S.; Satpati, B.; Mohapatra, S. Highly efficient photocatalytic degradation of organic dyes by Cu doped ZnO nanostructures. Phys. Chem. Chem. Phys. 2015, 17, 25172–25181. [Google Scholar] [CrossRef]
- Castro-Lopes, S.; Guerra, Y.; Silva-Sousa, A.; Oliveira, D.M.; Gonçalves, L.A.P.; Franco, A.; Padrón-Hernández, E.; Peña-Garcia, R. Influence of pH on the structural and magnetic properties of Fe-doped ZnO nanoparticles synthesized by sol gel method. Solid State Sci. 2020, 109, 106438. [Google Scholar] [CrossRef]
- França, R.; Araujo, F.P.; Neves, L.; Melo, A.; Lins, A.; Soares, A.S.; Osajima, J.A.; Guerra, Y.; Almeida, L.C. Photoresponsive Activity of the Zn0.94Er0.02Cr0.04O Compound with Hemisphere-like Structure Obtained by Co-Precipitation. Materials 2023, 16, 1446. [Google Scholar] [CrossRef]
- Pouthika, K.; Madhumitha, G. Synergistic synthesis of Carrisa edulis fruit extract capped heterogeneous CuO-ZnO-HNT composite for photocatalytic removal of organic pollutants. Inorganica Chim. Acta 2023, 551, 121457. [Google Scholar] [CrossRef]
- Geng, S.; Chen, D.; Guo, Z.; Li, Q.; Wen, M.; Wang, J.; Guo, K.; Wang, J.; Wang, Y.; Yu, L.; et al. Halloysite-Nanotube-Mediated High-Flux γ-Al2O3 Ultrafiltration Membranes for Semiconductor Wastewater Treatment. Membranes 2025, 15, 130. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Y.; Tang, X.; Sun, Y.; Li, F. Photocatalytic decomposition of toxic phosphine gas over halloysite nanotubes co-doped by Ni and Fe3O4 and theory calculation of its mechanism. Chem. Eng. J. 2025, 506, 159994. [Google Scholar] [CrossRef]
- Lins, A.; Jerônimo, A.G.; Barbosa, R.; Neves, L.; Trigueiro, P.; Almeida, L.C.; Osajima, J.A.; Pereira, F.A.; Peña-Garcia, R.R. Facile Synthesis of Ni-Doped ZnO Nanoparticles Using Cashew Gum: Investigation of the Structural, Optical, and Photocatalytic Properties. Molecules 2023, 28, 7772. [Google Scholar] [CrossRef] [PubMed]
- Martins, E.; Jerônimo, A.G.; Barbosa, R.; Neves, L.; Santos, E.; Meira, T.; Osajima, J.A.; Trigueiro, P.; Soares, A.S.; Peña-Garcia, R.R. Influence of Al cations insertion on the structural, morphological, optical properties, and methyl orange photocatalytic remotion of Pr-doped ZnO system. Mater. Chem. Phys. 2024, 318, 129300. [Google Scholar] [CrossRef]
- Jerônimo, A.G.; Barbosa, R.; Neves, L.; Trigueiro, P.; Guerra, Y.; Santos, E.; Almeida, L.C.; Osajima, J.A.; Araujo, F.P.; Peña-Garcia, R.R. Simultaneous La3+ and Cu2+ cations insertion in the ZnO crystal structure and its effect on the structural, optical, and photocatalytic properties. J. Mater. Sci. 2024, 59, 1280–1297. [Google Scholar] [CrossRef]
- França, R.; Araujo, F.P.; Castro-Lopes, S.; Neves, L.; Melo, A.; Jerônimo, A.G.; Osajima, J.A.; Guerra, Y.; Almeida, L.C.; Peña-Garcia, R. Effect of Cr cations addition on the structural, morphological, optical, and photocatalytic properties of Er-doped ZnO structures. Mater. Today Commun. 2023, 37, 107419. [Google Scholar] [CrossRef]
- Alsulmi, A.; Mohammed, N.N.; Soltan, A.; Messih, M.F.A.; Ahmed, M.A. Engineering S-scheme CuO/ZnO heterojunctions sonochemically for eradicating RhB dye from wastewater under solar radiation. RSC Adv. 2023, 13, 13269–13281. [Google Scholar] [CrossRef]
- Peng, H.; Liu, X.; Tang, W.; Ma, R. Facile synthesis and characterization of ZnO nanoparticles grown on halloysite nanotubes for enhanced photocatalytic properties. Sci. Rep. 2017, 7, 2250. [Google Scholar] [CrossRef]
- Suppaso, C.; Pongkan, N.; Intachai, S.; Rattanawongsa, W.; Baoulan, A.; Yamauchi, Y.; Asakura, Y.; Khaorapapong, N. Enhancement of photocatalytic efficiency of copper oxide/zinc oxide-montmorillonite photocatalyst under visible light irradiation. Sci. Technol. Adv. Mater. 2025, 26, 2469484. [Google Scholar] [CrossRef]
- Nagpal, K.; Rauwel, E.; Estephan, E.; Soares, M.R.; Rauwel, P. Significance of Hydroxyl Groups on the Optical Properties of ZnO Nanoparticles Combined with CNT and PEDOT:PSS. Nanomaterials 2022, 12, 3546. [Google Scholar] [CrossRef] [PubMed]
- Khoddam, M.A.; Norouzbeigi, R.; Velayi, E.; Cavallaro, G. Statistical-based optimization and mechanism assessments of Arsenic (III) adsorption by ZnO-Halloysite nanocomposite. Sci. Rep. 2024, 14, 21629. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, H.; Saleem, R.; Halepoto, I.A.; Barhaam, M.S.; Soomro, M.Y.; Abbasi, M.A.; Shaikh, N.M.; Bhatti, M.A.; Wassan, S.H.; Dawi, E.; et al. Facile and Low-Cost Fabrication of ZnO/Kaolinite Composites by Modifying the Kaolinite Composition for Efficient Degradation of Methylene Blue Under Sunlight Illumination. Catalysts 2025, 15, 566. [Google Scholar] [CrossRef]
- Trigueiro, P.; Jerônimo, A.G.; Albuquerque, W.A.; da Silva, W.L.; Osajima, J.A.; Jaber, M.; Peña-Garcia, R.R. Shaping a ZnO-alginate-hectorite nanocomposite for improved photocatalytic drug removal. Appl. Mater. Today 2025, 44, 102680. [Google Scholar] [CrossRef]
- Jiang, Q.; Han, Z.; Qu, N.; Sun, L.; Yuan, Y.; Ren, Y.; Cheng, Z. Low-cost magnetic clay derivants from palygorskite/MIL-101(Fe) for high-performance adsorption-photocatalysis. Appl. Clay Sci. 2022, 218, 106427. [Google Scholar] [CrossRef]
- Damaceno, D.H.; Trigueiro, P.; Lima, L.C.B.; Honorio, L.M.; Peña-Garcia, R.; Furtini, M.B.; Guerra, Y.; Fonseca, M.G.; da Silva-Filho, E.C.; Jaber, M.; et al. ZnO-Saponite Nanocomposite: Input of Adsorption and Photocatalysis for Removal of Rhodamine B Dye. Water Air Soil Pollut. 2024, 235, 656. [Google Scholar] [CrossRef]
- Sacco, O.; Vaiano, V.; Matarangolo, M. ZnO supported on zeolite pellets as efficient catalytic system for the removal of caffeine by adsorption and photocatalysis. Sep. Purif. Technol. 2018, 193, 303–310. [Google Scholar] [CrossRef]
- Qi, Y.; Zhao, S.; Shen, Y.; Jiang, X.; Lv, H.; Han, C.; Liu, W.; Zhao, Q. A Critical Review of Clay Mineral-Based Photocatalysts for Wastewater Treatment. Catalysts 2024, 14, 575. [Google Scholar] [CrossRef]
- Li, X.; Simon, U.; Bekheet, M.F.; Gurlo, A. Mineral-Supported Photocatalysts: A Review of Materials, Mechanisms and Environmental Applications. Energies 2022, 15, 5607. [Google Scholar] [CrossRef]
- Freitas, W.; Trigueiro, P.; Marinho, T.; Honorio, L.M.; Silva-Filho, E.C.; Furtini, M.B.; Cecília, J.A.; Fonseca, M.G.; Osajima, J. The Role of Clay Mineral-Derived Photocatalysts in Insights of Remediation. Ceramics 2022, 5, 862–882. [Google Scholar] [CrossRef]
- Van Thuan, D.; Nguyen, T.B.H.; Pham, T.H.; Kim, J.; Chu, T.T.H.; Nguyen, M.V.; Nguyen, K.D.; Al-onazi, W.A.; Elshikh, M.S. Photodegradation of ciprofloxacin antibiotic in water by using ZnO-doped g-C3N4 photocatalyst. Chemosphere 2022, 308, 136408. [Google Scholar] [CrossRef] [PubMed]
- Hassaan, M.A.; Meky, A.I.; Fetouh, H.A.; Ismail, A.M.; El Nemr, A. Central composite design and mechanism of antibiotic ciprofloxacin photodegradation under visible light by green hydrothermal synthesized cobalt-doped zinc oxide nanoparticles. Sci. Rep. 2024, 14, 9144. [Google Scholar] [CrossRef]
- Freitas, W.A.; Soares, B.E.C.F.; Rodrigues, M.S.; Trigueiro, P.; Honorio, L.M.C.; Peña-Garcia, R.; Alcântara, A.C.S.; Silva-Filho, E.C.; Fonseca, M.G.; Furtini, M.B.; et al. Facile synthesis of ZnO-clay minerals composites using an ultrasonic approach for photocatalytic performance. J. Photochem. Photobiol. A Chem. 2022, 429, 113934. [Google Scholar] [CrossRef]
- Meng, L.; Zhao, C.; Wang, T.; Chu, H.; Wang, C.-C. Efficient ciprofloxacin removal over Z-scheme ZIF-67/V-BiOIO3 heterojunctions: Insight into synergistic effect between adsorption and photocatalysis. Sep. Purif. Technol. 2023, 313, 123511. [Google Scholar] [CrossRef]
- Jayeola, K.D.; Sipuka, D.S.; Sebokolodi, T.I.; Nkwachukwu, O.V.; Muzenda, C.; Koiki, B.A.; Babalola, J.O.; Zhou, M.; Arotiba, O.A. The design and characterisation of a Z-scheme Bi2O2S/ZnO heterojunction photoanode for the photoelectrochemical removal of ciprofloxacin in synthetic and real wastewater. Chem. Eng. J. 2024, 479, 147482. [Google Scholar] [CrossRef]
- Sarkhosh, M.; Sadani, M.; Abtahi, M.; Mohseni, S.M.; Sheikhmohammadi, A.; Azarpira, H.; Najafpoor, A.A.; Atafar, Z.; Rezaei, S.; Alli, R.; et al. Enhancing photo-degradation of ciprofloxacin using simultaneous usage of eaq− and OH over UV/ZnO/I− process: Efficiency, kinetics, pathways, and mechanisms. J. Hazard. Mater. 2019, 377, 418–426. [Google Scholar] [CrossRef]
- Wen, X.-J.; Niu, C.-G.; Zhang, L.; Liang, C.; Guo, H.; Zeng, G.-M. Photocatalytic degradation of ciprofloxacin by a novel Z-scheme CeO2–Ag/AgBr photocatalyst: Influencing factors, possible degradation pathways, and mechanism insight. J. Catal. 2018, 358, 141–154. [Google Scholar] [CrossRef]
- Ahamad, T.; Naushad, M.; Alshehri, S.M. Analysis of degradation pathways and intermediates products for ciprofloxacin using a highly porous photocatalyst. Chem. Eng. J. 2021, 417, 127969. [Google Scholar] [CrossRef]
- Ngo, H.S.; Nguyen, T.L.; Tran, N.T.; Le, H.C. Experimental Study on Kinetics and Mechanism of Ciprofloxacin Degradation in Aqueous Phase Using Ag-TiO2/rGO/Halloysite Photocatalyst. Catalysts 2023, 13, 225. [Google Scholar] [CrossRef]
- Hassani, A.; Khataee, A.; Karaca, S.; Karaca, C.; Gholami, P. Sonocatalytic degradation of ciprofloxacin using synthesized TiO2 nanoparticles on montmorillonite. Ultrason. Sonochem. 2017, 35, 251–262. [Google Scholar] [CrossRef]
- Nasiri, H.; Golmohammadi, M.; Sazegaran, H. Photocatalysts Capability of Bentonite-ZnO Nanocomposite Synthesized by Solution Combustion in Ciprofloxacin Degradation. J. Ultrafine Grained Nanostruct. Mater. 2023, 56, 1–10. [Google Scholar] [CrossRef]
- Sun, Z.; Zhang, X.; Zhu, R.; Dong, X.; Xu, J.; Wang, B. Facile synthesis of visible light-induced g-C3N4/rectorite composite for efficient photodegradation of ciprofloxacin. Materials 2018, 11, 2452. [Google Scholar] [CrossRef]
- Saeed, M.; Muneer, M.; Haq, A.U.; Akram, N. Photocatalysis: An effective tool for photodegradation of dyes—A review. Environ. Sci. Pollut. Res. 2022, 29, 293–311. [Google Scholar] [CrossRef]
- Kabra, K.; Chaudhary, R.; Sawhney, R.L. Treatment of Hazardous Organic and Inorganic Compounds through Aqueous-Phase Photocatalysis: A Review. Ind. Eng. Chem. Res. 2004, 43, 7683–7696. [Google Scholar] [CrossRef]
- Kumari, H.; Sonia; Suman; Ranga, R.; Chahal, S.; Devi, S.; Sharma, S.; Kumar, S.; Kumar, P.; Kumar, S.; et al. A Review on Photocatalysis Used For Wastewater Treatment: Dye Degradation. Water Air Soil Pollut. 2023, 234, 349. [Google Scholar] [CrossRef]
- Mishra, S.; Sundaram, B. A review of the photocatalysis process used for wastewater treatment. Mater. Today Proc. 2024, 102, 393–409. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Zhao, X.; Li, C.; Song, X.; Zhang, P.; Huo, P.; Li, X. A review on heterogeneous photocatalysis for environmental remediation: From semiconductors to modification strategies. Chin. J. Catal. 2022, 43, 178–214. [Google Scholar] [CrossRef]
- Rueda-Marquez, J.J.; Levchuk, I.; Ibañez, P.F.; Sillanpää, M. A critical review on application of photocatalysis for toxicity reduction of real wastewaters. J. Clean. Prod. 2020, 258, 120694. [Google Scholar] [CrossRef]
- Ahmad, I.; Bousbih, R.; Mahal, A.; Khan, W.Q.; Aljohani, M.; Amin, M.A.; Jafar, N.N.A.; Jabir, M.S.; Majdi, H.; Alshomrany, A.S.; et al. Recent progress in ZnO-based heterostructured photocatalysts: A review. Mater. Sci. Semicond. Process. 2024, 180, 108578. [Google Scholar] [CrossRef]
- Papoulis, D.; Komarneni, S.; Panagiotaras, D.; Stathatos, E.; Toli, D.; Christoforidis, K.C.; Fernández-García, M.; Li, H.; Yin, S.; Sato, T.; et al. Halloysite–TiO2 nanocomposites: Synthesis, characterization and photocatalytic activity. Appl. Catal. B Environ. 2013, 132–133, 416–422. [Google Scholar] [CrossRef]
- Zhou, D.; Jiang, D.; Jing, H.; Yin, C.; Li, C. Natural aluminosilicate nanoclay mineral for photocatalytic applications: Influence of the surface properties in photocatalysis. Appl. Clay Sci. 2024, 249, 107240. [Google Scholar] [CrossRef]
- Trigueiro, P.; Albuquerque, W.A.; Jerônimo, A.G.; Barbosa, R.; Jaber, M.; Peña-Garcia, R.R. Tailoring Y-doped ZnO loaded onto eco-friendly support alginate-hectorite for azo dye removal. Appl. Surf. Sci. 2025, 704, 163461. [Google Scholar] [CrossRef]
- Fatimah, I.; Syu’aib, Y.; Ramanda, G.D.; Kooli, F.; Sagadevan, S.; Oh, W.-C. Facile synthesis of highly active and reusable NiO/montmorillonite photocatalyst for tetracycline removal by photocatalytic oxidation. Inorg. Chem. Commun. 2025, 172, 113731. [Google Scholar] [CrossRef]
- Haounati, R.; Ighnih, H.; Malekshah, R.E.; Alahiane, S.; Alakhras, F.; Alabbad, E.; Alghamdi, H.; Ouachtak, H.; Addi, A.A.; Jada, A. Exploring ZnO/Montmorillonite photocatalysts for the removal of hazardous RhB Dye: A combined study using molecular dynamics simulations and experiments. Mater. Today Commun. 2023, 35, 105915. [Google Scholar] [CrossRef]
- Falcón, J.M.; Sawczen, T.; Aoki, I.V. Dodecylamine-Loaded Halloysite Nanocontainers for Active Anticorrosion Coatings. Front. Mater. 2015, 2, 1–13. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).